Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation
Abstract
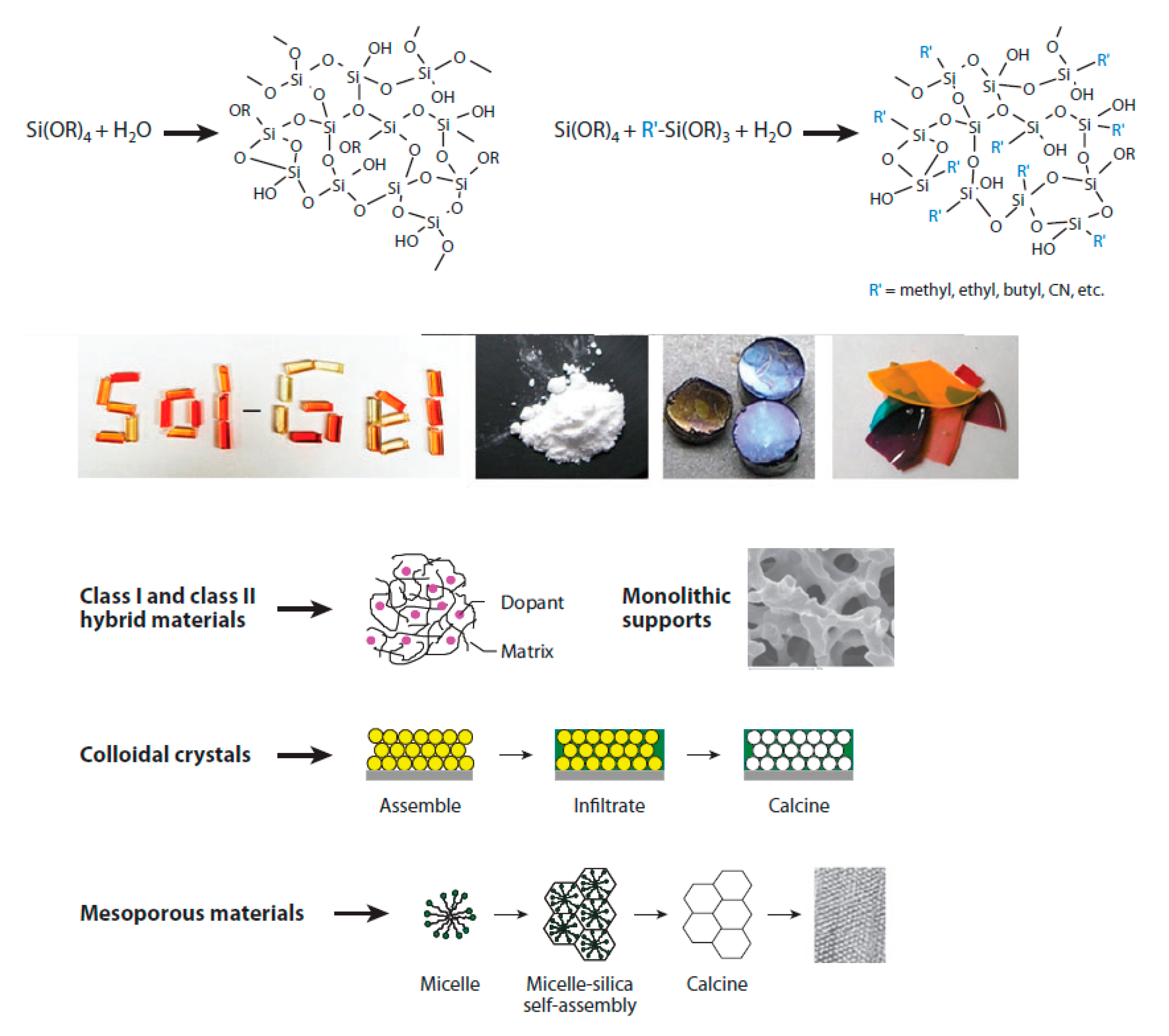
1. Introduction
2. MSG in Solid Phase Extraction (SPE)
3. MSG in Solid Phase Microextraction (SPME)
4. Monolithic MSG
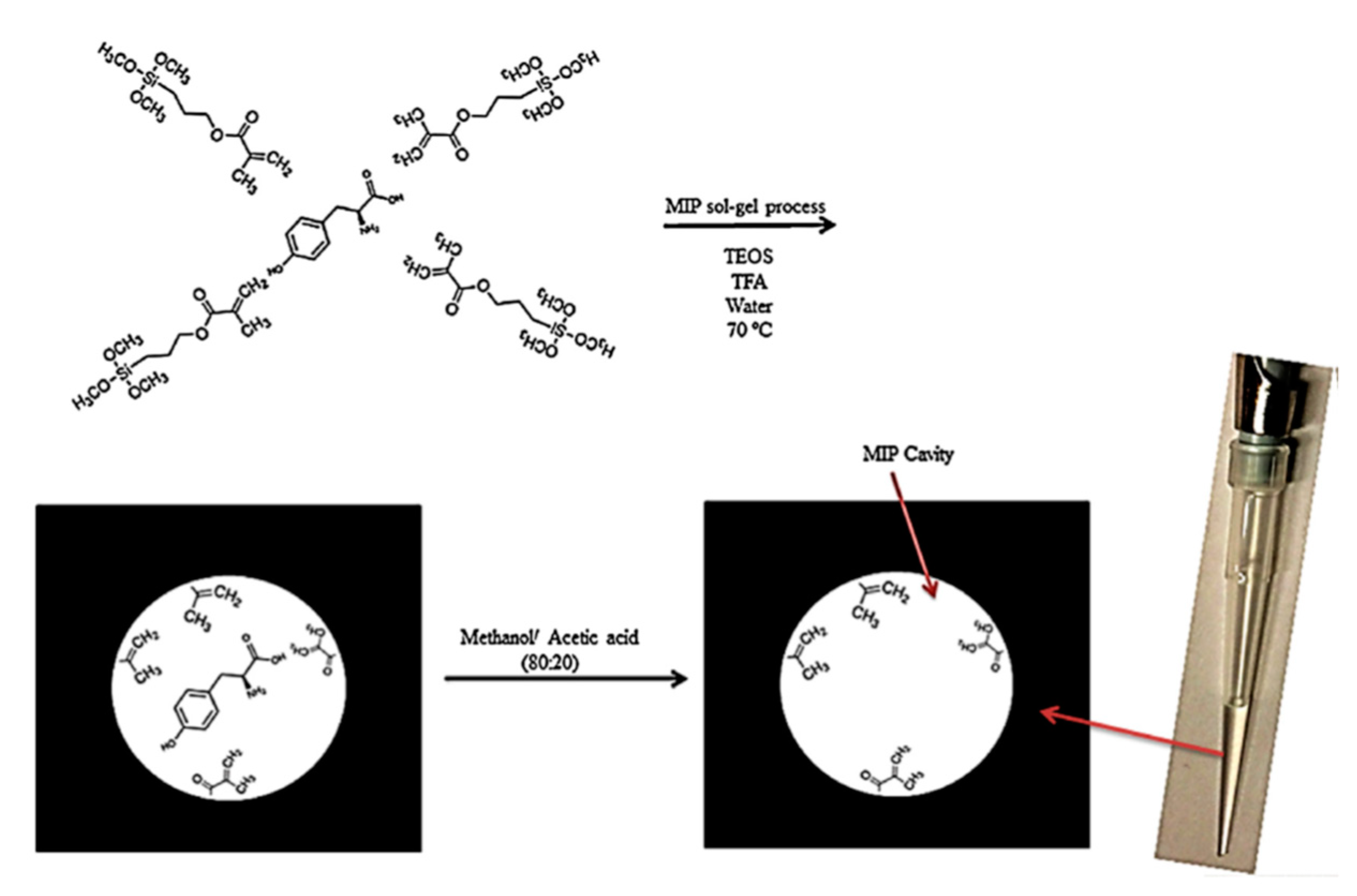
5. Hollow Fiber and Nanofiber Modification and Preparation with MSG
6. Other Novel Methods for Preparation of MSG
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Row, K.H. Solid-phase extraction of chlorophenols in seawater using a magnetic ionic liquid molecularly imprinted polymer with incorporated silicon dioxide as a sorbent. J. Chromatogr. A 2018, 1559, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Masoum, S. A multi-walled carbon nanotube-based magnetic molecularly imprinted polymer as a highly selective sorbent for ultrasonic-assisted dispersive solid-phase microextraction of sotalol in biological fluids. Analyst 2018, 143, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Selective extraction of 3,4-dihydroxybenzoic acid in Ilex chinensis Sims by meticulous mini-solid-phase microextraction using ternary deep eutectic solvent-based molecularly imprinted polymers. Anal. Bioanal. Chem. 2018, 410, 7849–7858. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Ternary deep eutectic solvent magnetic molecularly imprinted polymers for the dispersive magnetic solid-phase microextraction of green tea. J. Sep. Sci. 2018, 41, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.L.; Carlson, C.A.; Edmiston, P.L. Development and characterization of molecularly imprinted sol-gel materials for the selective detection of DDT. Anal. Chem. 2002, 74, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-H.; Zhang, S.-Q.; Yang, W.; Chen, X.-L.; Zhuang, Z.-X.; Xu, J.-G.; Wang, X.-R. Molecularly Imprinted Sol−Gel Nanotubes Membrane for Biochemical Separations. J. Am. Chem. Soc. 2004, 126, 4054–4055. [Google Scholar] [CrossRef] [PubMed]
- Fireman-Shoresh, S.; Turyan, I.; Mandler, D.; Avnir, D.; Marx, S. Chiral Electrochemical Recognition by Very Thin Molecularly Imprinted Sol−Gel Films. Langmuir 2005, 21, 7842–7847. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.R.; Linman, M.J.; Timmers, M.M.; Dean, S.L.; Burkett, C.M.; Lloyd, J.A.; Keelor, J.D.; Baughman, B.M.; Edmiston, P.L. Selective detection of gas-phase TNT by integrated optical waveguide spectrometry using molecularly imprinted sol–gel sensing films. Anal. Chim. Acta 2007, 593, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, C.; Dai, P.; Ge, S. Highly selective molecular recognition and high throughput detection of melamine based on molecularly imprinted sol–gel film. Anal. Chim. Acta 2009, 651, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Lu, F.; Li, H.; Sun, M.; Fan, L.; Qiu, H.; Li, X.; Luo, C. Flow injection chemiluminescence sensor based on core–shell magnetic molecularly imprinted nanoparticles for determination of sulfadiazine. Anal. Chim. Acta 2012, 718, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol–gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Dickert, F.L. Imprinted Oxide and MIP/Oxide Hybrid Nanomaterials for Chemical Sensors. Nanomaterials 2018, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Karimi, M. Recent progress, challenges and trends in trace determination of drug analysis using molecularly imprinted solid-phase microextraction technology. Talanta 2017, 164, 612–625. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; Van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wayne, C.; Patrick, D.; Peter, M. A comparative study of the potential of acrylic and sol–gel polymers for molecular imprinting. Anal. Chim. Acta 2005, 542, 52–60. [Google Scholar]
- Walcarius, A.; Collinson, M.M. Analytical chemistry with silica sol-Gels: Traditional routes to new materials for chemical analysis. Annu. Rev. Anal. Chem. 2009, 2, 121–143. [Google Scholar] [CrossRef]
- Xia, Y.; Gates, B.; Yin, Y.; Lu, Y. Monodispersed colloidal spheres: Old materials with new applications. Adv. Mater. 2000, 12, 693–713. [Google Scholar] [CrossRef]
- Sasaki, D.Y.; Rush, D.J.; Daitch, C.E.; Alam, T.M.; Assink, R.A.; Ashley, C.S.; Brinker, C.J.; Shea, K.J. Molecular Imprinted Receptors in Sol-Gel Materials for Aqueous Phase Recognition of Phosphates and Phosphonates. ACS Symp. Ser. 1998, 703, 314–323. [Google Scholar]
- Lasáková, M.; Jandera, P. Molecularly imprinted polymers and their application in solid phase extraction. J. Sep. Sci. 2009, 32, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Junping, W.; Mingfei, P.; Guozhen, F.; Shuo, W. Preparation of a novel molecularly imprinted polymer by a sol-gel process for on-line solid-phase extraction coupled with high performance liquid chromatography to detect trace enrofloxacin in fish and chicken samples. Microchim. Acta 2009, 166, 295–302. [Google Scholar] [CrossRef]
- Duan, Z.-J.; Fan, L.-P.; Fang, G.-Z.; Yi, J.-H.; Wang, S. Novel surface molecularly imprinted sol–gel polymer applied to the online solid phase extraction of methyl-3-quinoxaline-2-carboxylic acid and quinoxaline-2-carboxylic acid from pork muscle. Anal. Bioanal. Chem. 2011, 401, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Luo, Z.; Guo, P.; Tang, W.; Wu, N.; Zheng, P.; Du, W.; Zeng, A.; Jing, W.; Chang, C.; et al. Preparation and evaluation of a molecularly imprinted sol-gel material as the solid-phase extraction adsorbents for the specific recognition of cloxacilloic acid in cloxacillin. J. Sep. Sci. 2016, 39, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Molecularly imprinted polymer for 2, 4-dichlorophenoxyacetic acid prepared by a sol-gel method. J. Chem. Sci. 2014, 126, 1005–1011. [Google Scholar] [CrossRef]
- Sadeghi, S.; Jahani, M. Solid-Phase Extraction of Florfenicol from Meat Samples by a Newly Synthesized Surface Molecularly Imprinted Sol–Gel Polymer. Food Anal. Methods 2014, 7, 2084–2094. [Google Scholar] [CrossRef]
- Fang, G.; Feng, J.; Yan, Y.; Liu, C.; Wang, S. Highly selective determination of chrysoidine in foods through a surface molecularly imprinted sol–gel polymer solid-phase extraction coupled with HPLC. Food Anal. Methods 2014, 7, 345–351. [Google Scholar] [CrossRef]
- Kia, S.; Salavati, H.; Bohlooli, S.; Fazilati, M. Preparation of a novel molecularly imprinted polymer by the sol–gel process for solid phase extraction of vitamin D3. RSC Adv. 2016, 6, 31906–31914. [Google Scholar] [CrossRef]
- Boulanouar, S.; Combès, A.; Mezzache, S.; Pichon, V. Synthesis and application of molecularly imprinted silica for the selective extraction of some polar organophosphorus pesticides from almond oil. Anal. Chim. Acta 2018, 1018, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, X.; Xie, X.; Huang, R.; Zhang, M.; Li, J.; Zeng, G.; Fan, Y. Preparation of dual-dummy-template molecularly imprinted polymers coated magnetic graphene oxide for separation and enrichment of phthalate esters in water. Chem. Eng. J. 2019, 361, 245–255. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Tan, J.; Jiang, Z.T. Titania-based molecularly imprinted polymer for sulfonicacid dyes prepared by sol–gel method. Talanta 2013, 107, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhanga, Z.; Zhang, H.; Hu, Y.; Yao, S. Synthesis and application of multi-walled carbon nanotubes–molecularly imprinted sol–gel composite material for on-line solid-phase extraction and high-performance liquid chromatography determination of trace Sudan IV. Anal. Chim. Acta 2010, 661, 173–180. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xiao, D.; He, J.; Li, H.; He, H.; Dai, H.; Peng, J. Preparation of a core–shell magnetic ion-imprinted polymer via a sol–gel process for selective extraction of Cu(ii) from herbal medicines. Anal. 2014, 139, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chang, T.; Liu, Y.; Yan, X. One-pot synthesis of uniform and monodisperse superparamagnetic molecularly imprinted polymer nanospheres through a sol–gel process for selective recognition of bisphenol A in aqueous media. RSC Adv. 2016, 6, 66297–66306. [Google Scholar]
- Bitar, M.; Lafarge, C.; Sok, N.; Cayot, P.; Bou-Maroun, E. Molecularly imprinted sol-gel polymers for the analysis of iprodione fungicide in wine: Synthesis in green solvent. Food Chem. 2019, 293, 226–232. [Google Scholar] [CrossRef] [PubMed]
- El-Beqqali, A.; Abdel-Rehim, M. Molecularly imprinted polymer-sol-gel tablet toward micro-solid phase extraction: I. Determination of methadone in human plasma utilizing liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2016, 936, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, T.; He, J. Preparation of magnetic molecularly imprinted polymers for the rapid detection of diethylstilbestrol in milk samples. J. Sci. Food Agric. 2019, 99, 4452–4459. [Google Scholar] [CrossRef]
- Chang, T.; Yan, X.; Liu, S.; Liu, Y. Magnetic dummy template silica sol–gel molecularly imprinted polymer nanospheres as magnetic solid-phase extraction material for the selective and sensitive determination of bisphenol A in plastic bottled beverages food. Anal. Methods 2017, 10, 3980–3990. [Google Scholar] [CrossRef]
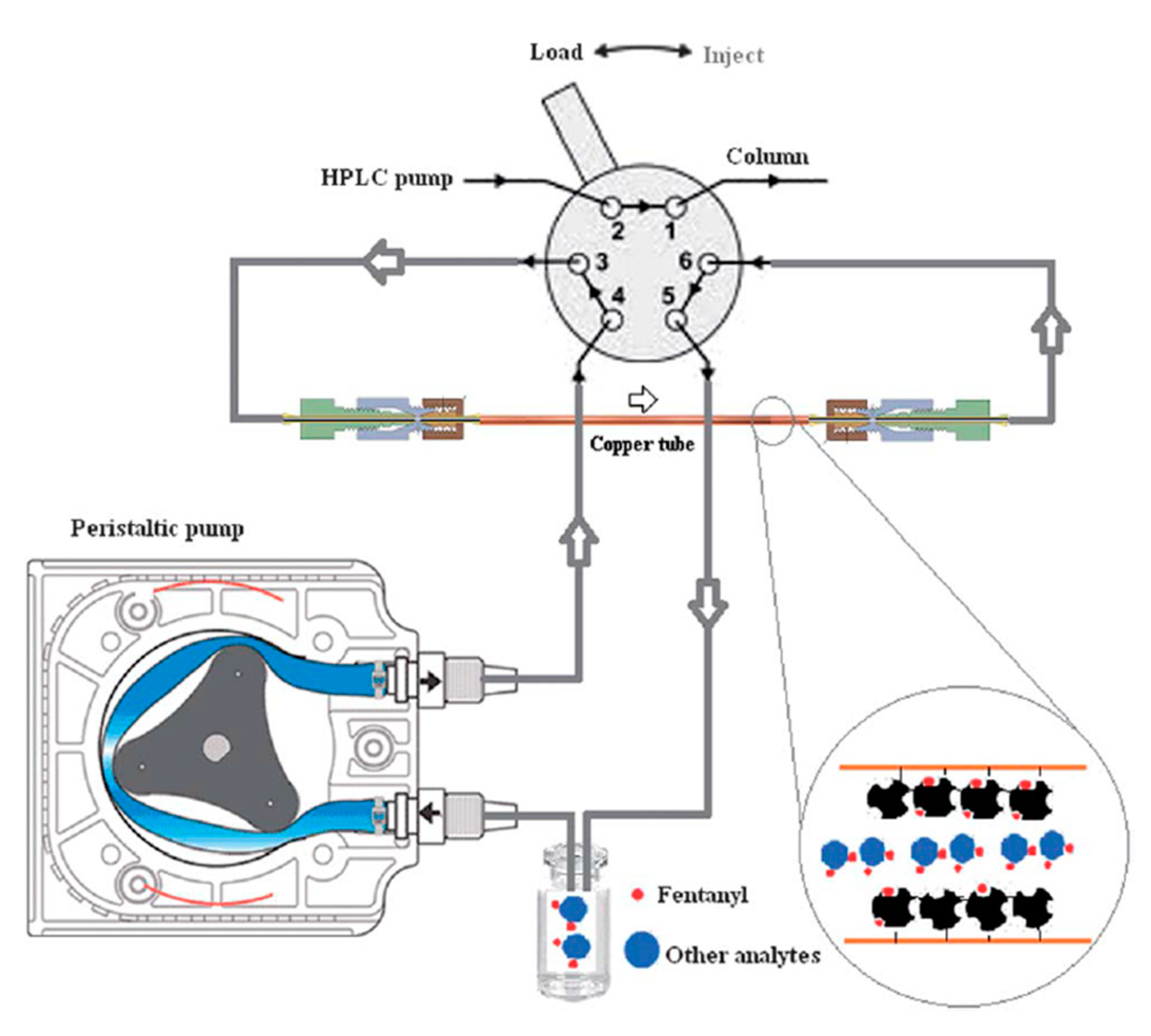
- Bagheri, H.; Piri-Moghadam, H.; Bayat, P.; Balalaie, S. Application of sol–gel based molecularly imprinted xerogel for on-line capillary microextraction of fentanyl from urine and plasma samples. Anal. Methods 2013, 5, 7096. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Gao, Y.-L.; Wang, P.-P.; Shang, H.; Pan, S.-Y.; Li, X.-J. Sol–gel molecularly imprinted polymer for selective solid phase microextraction of organophosphorous pesticides. Talanta 2013, 115, 920–927. [Google Scholar] [CrossRef]
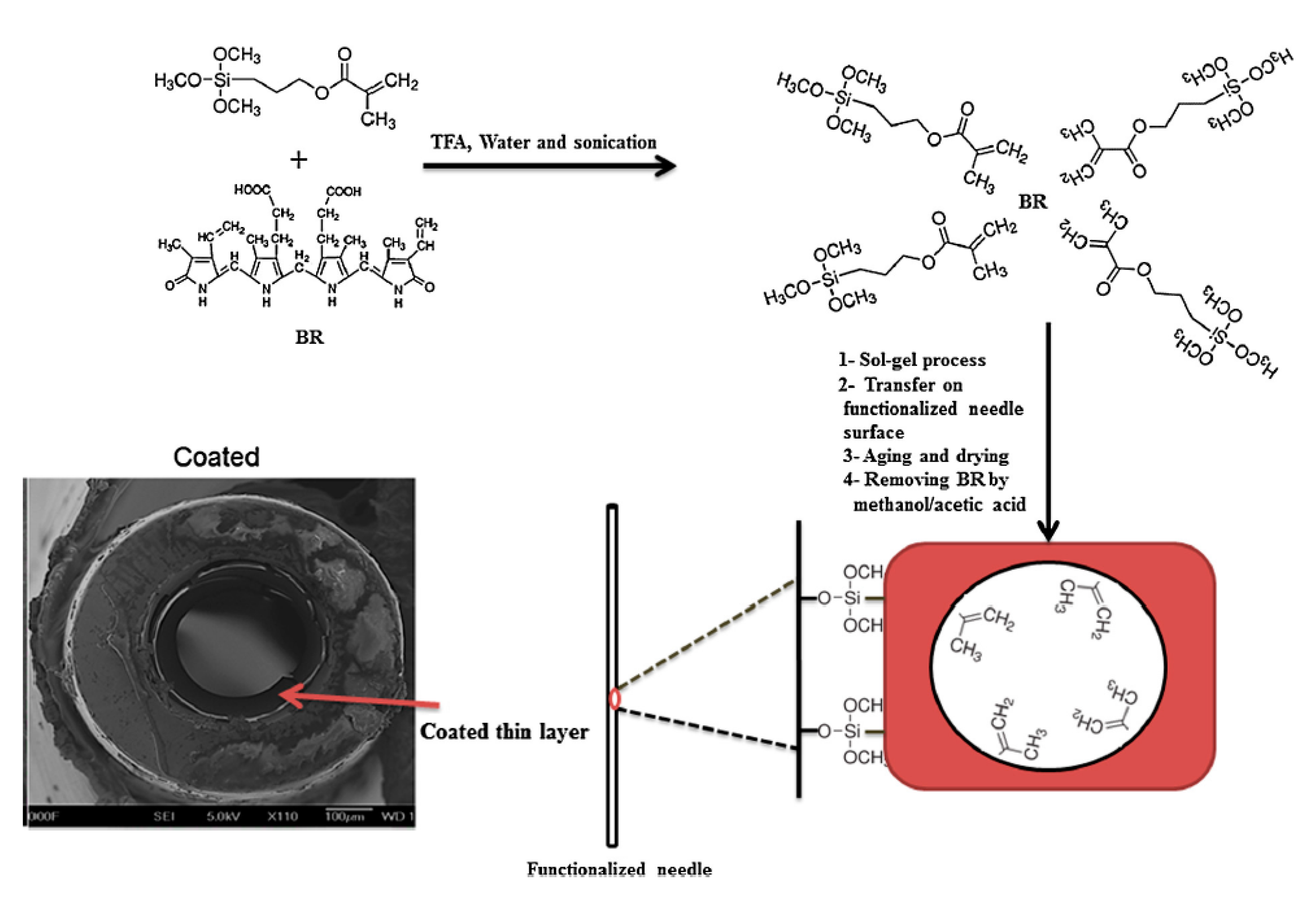
- Moein, M.M.; Jabbar, D.; Colmsjö, A.; Abdel-Rehim, M. A needle extraction utilizing a molecularly imprinted-sol–gel xerogel for on-line microextraction of the lung cancer biomarker bilirubinfrom plasma and urine samples. J. Chromatogr. A 2014, 1366, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-K.; Huang, X.-C.; Wei, S.-L. Preparation and application of chlorpyrifos molecularly imprinted solid-phase microextraction probes for the residual determination of organophosphorous pesticides in fresh and dry foods. J. Sep. Sci. 2018, 41, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; El-Beqqali, A.; Abdel-Rehim, A.; Jeppsson-Dadoun, A.; Abdel-Rehim, M. Preparation of monolithic molecularly imprinted polymer sol–gel packed tips for high-throughput bioanalysis: Extraction and quantification of l-tyrosine in human plasma and urine samples utilizing liquid chromatography and tandem mass spectrometry. J. Chromatogr. B 2014, 967, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, D.; Ai, Y.; Dang, X.; Huang, J. A dummy molecularly imprinted monolith for selective solid-phase microextraction of vanillin and methyl vanillin prior to their determination by HPLC. Microchim. Acta 2017, 184, 1161–1167. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-Adergani, B.; Abdel-Rehim, M. Three-phase molecularly imprinted sol–gel based hollow fiber liquid-phase microextraction combined with liquid chromatography–tandem mass spectrometry for enrichment and selective determination of a tentative lung cancer biomarker. J. Chromatogr. B 2015, 995, 38–45. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-Adergani, B. Molecularly imprinted sol-gel nanofibers based solid phase microextraction coupled on-line with high performance liquid chromatography for selective determination of acesulfame. Talanta 2015, 134, 340–347. [Google Scholar] [CrossRef]
- Gao, R.; Mu, X.; Zhang, J.; Tang, Y. Specific recognition of bovine serum albumin using superparamagnetic molecularly imprinted nanomaterials prepared by two-stage core–shell sol–gel polymerization. J. Mater. Chem. B 2014, 2, 783–792. [Google Scholar] [CrossRef]
- Liu, M.; Pi, J.; Wang, X.; Huang, R.; Du, Y.; Yu, X.; Tan, W.; Liu, F.; Shea, K.J. A sol-gel derived pH-responsive bovine serum albumin molecularly imprinted poly(ionic liquids) on the surface of multiwall carbon nanotubes. Anal. Chim. Acta 2016, 932, 29–40. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymer monolith containing magnetic nanoparticles for the stir-bar sorptive extraction of thiabendazole and carbendazim from orange samples. Anal. Chim. Acta 2019, 1045, 117–122. [Google Scholar] [CrossRef]
- Kadhirvel, P.; Azenha, M.; Shinde, S.; Schillinger, E.; Gomes, P.; Sellergren, B.; Silva, A.F. Imidazolium-based functional monomers for the imprinting of the anti-inflammatory drug naproxen: Comparison of acrylic and sol–gel approaches. J. Chromatogr. A 2013, 1314, 115–123. [Google Scholar] [CrossRef]
- Kadhirvel, P.; Azenha, M.; Silva, A.F.; Sellergren, B. Chromatographically efficient microspherical composites of molecularly imprinted xerogels deposited inside mesoporous silica. J. Chromatogr. A 2014, 1355, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Greaves, A.; Ribaud, C.; Manfre, F.; Haupt, K.; Bernadette, J.; Bui, B.T.S. Molecularly Imprinted Polymers of Sol-Gel Type and Their Use as Antidandruff Agent, United States LOREAL (Paris, FR) 20150342868. Available online: http://www.freepatentsonline.com/y2015/0342868.html (accessed on 2 May 2019).
- Li, B.; Xu, J.; Hall, A.; Haupt, K.; Bui, B.T.S. Water-compatible silica sol-gel molecularly imprinted polymer as a potential delivery system for the controlled release of salicylic acid. J. Mol. Recognit. 2014, 27, 559–565. [Google Scholar] [CrossRef] [PubMed]
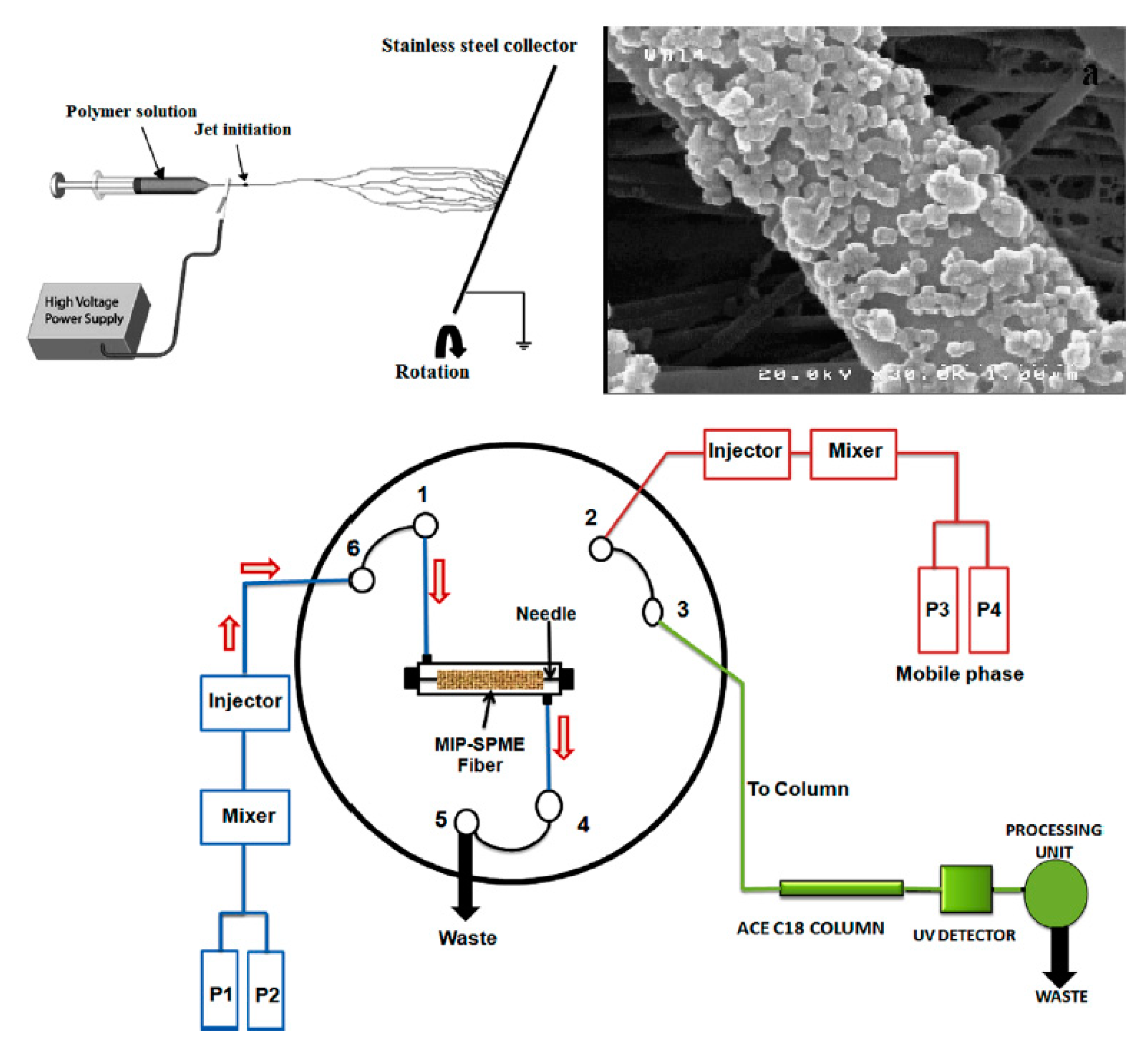
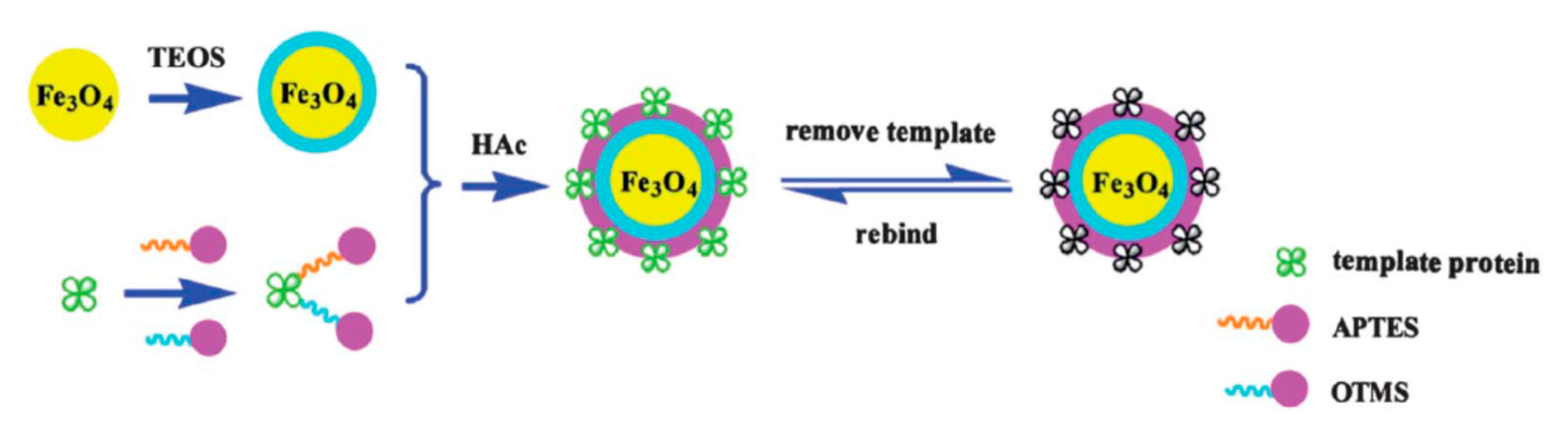
| Analyte | Sample Preparation Method | Instrumentation | Matrix | Ref |
|---|---|---|---|---|
| Enrofloxacin | SPE | HPLC | Fish and chicken samples | [22] |
| Methyl-3-quinoxaline-2-carboxylic acid and quinoxaline-2-carboxylic acid | SPE | HPLC | Pork muscle | [23] |
| Cloxacilloic acid | SPE | HPLC | Cloxacillin | [24] |
| 2,4-Dichlorophenoxyacetic | SPE | FT-IR | Aqueous media | [25] |
| Florfenicol | SPE | HPLC | Meat samples | [26] |
| Chrysoidine | SPE | HPLC | Food samples | [27] |
| Vitamin D3 | SPE | HPLC | Aqueous samples | [28] |
| Polar organophosphorus pesticides | SPE | LC-MS | Almond oil | [29] |
| Phthalate esters | SPE | GC-MS | Water sample | [30] |
| Sulfonicacid dyes | SPE | HPLC | Beverage samples | [31] |
| Sudan IV | SPE | HPLC | Chili samples | [32] |
| Cu(II) | SPE | HPLC | Herbal medicines | [33] |
| Bisphenol A | SPE | HPLC | Aqueous samples | [34] |
| Iprodione fungicide | SPE | HPLC | Wine | [35] |
| Methadone | SPE | HPLC | Human plasma | [36] |
| Diethylstilbestrol | SPE | HPLC | Milk samples | [37] |
| Bisphenol A | SPE | HPLC | Beverage samples | [38] |
| Fentanyl | Capillary-SPME | HPLC | Urine and plasma samples | [39] |
| Organophosphorous pesticides | Fiber-SPME | GC | Vegetable samples | [40] |
| Bilirubin | Needle-SMPE | LC-MS/MS | Plasma and urine samples | [41] |
| Organophosphorous pesticides | Stainless steel wire-SPME | GC | Fresh and dry foods | [42] |
| l-Tyrosine | Monolithic in tip | LC-MS/MS | Human plasma and urine samples | [43] |
| Vanillin and methyl vanillin | Monolithic in tip | HPLC | Milk powder | [44] |
| Hippuric acid | Hollow fiber liquid-phase microextraction | LC-MS/MS | Human plasma and urine samples | [45] |
| Acesulfame | Nanofiber-SPME | HPLC | Beverage samples | [46] |
| Bovine serum albumin | Magnetic nanomaterials | FT-IR | Bovine blood sample | [47] |
| Bovine serum albumin | Ionic liquid/Multiwall carbon nanotube | UV-Vis | Human serum albumin and bovine hemoglobin | [48] |
| Thiabendazole and carbendazim | Sorptive monolith nanoparticles/stir-bar | HPLC | Orange samples | [49] |
| Naproxen | Xerogel | HPLC | Aqueous samples | [50] |
| (S)-Naproxen | Xerogel composite | HPLC | Aqueous samples | [51] |
| C14-C20 fatty acid | Xerogel composite | Fluorescence spectroscopy | Hair samples | [52] |
| Salicylic acid | Xerogel composite | Fluorescence spectroscopy | Skin in vivo | [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation. Molecules 2019, 24, 2889. https://doi.org/10.3390/molecules24162889
Moein MM, Abdel-Rehim A, Abdel-Rehim M. Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation. Molecules. 2019; 24(16):2889. https://doi.org/10.3390/molecules24162889
Chicago/Turabian StyleMoein, Mohammad Mahdi, Abbi Abdel-Rehim, and Mohamed Abdel-Rehim. 2019. "Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation" Molecules 24, no. 16: 2889. https://doi.org/10.3390/molecules24162889
APA StyleMoein, M. M., Abdel-Rehim, A., & Abdel-Rehim, M. (2019). Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation. Molecules, 24(16), 2889. https://doi.org/10.3390/molecules24162889







