Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microparticles by Spray Drying
2.3. Experimental Taguchi Factorial Design
2.4. Characterization of Microparticles
2.4.1. Microencapsulation Efficiency
2.4.2. Moisture Determination
2.4.3. Particle Size Distribution
2.4.4. Morphological Analysis
2.4.5. Water Activity
2.4.6. Structural Analysis
2.4.7. Thermo-Oxidative Stability
2.4.8. Thermal Properties
3. Results and Discussion
3.1. Spray-Dried L-ascorbic Acid Microparticles
3.2. Experimental Taguchi Design
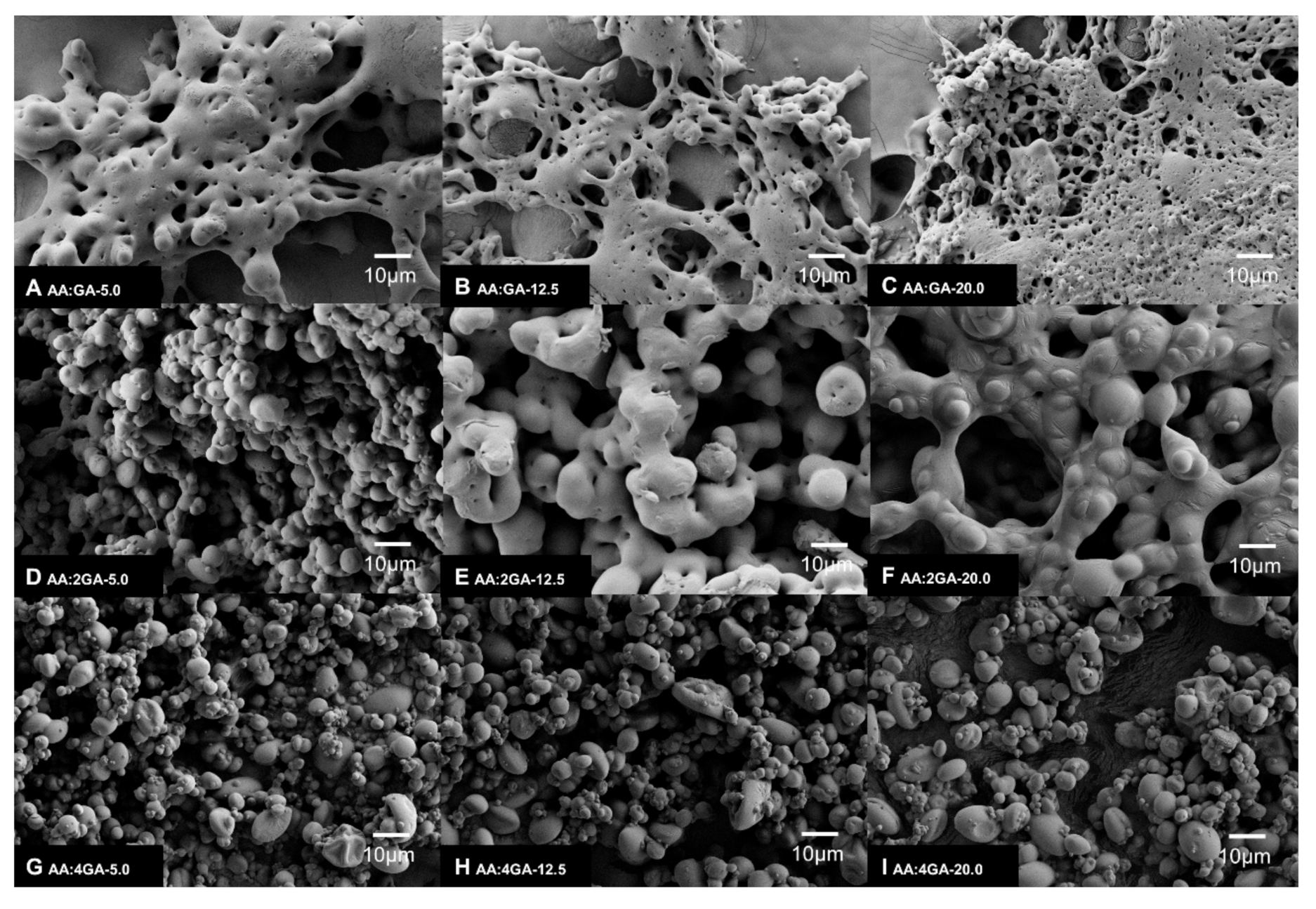
3.3. Morphological Analysis
3.4. Water Activity
3.5. Structural Characterization
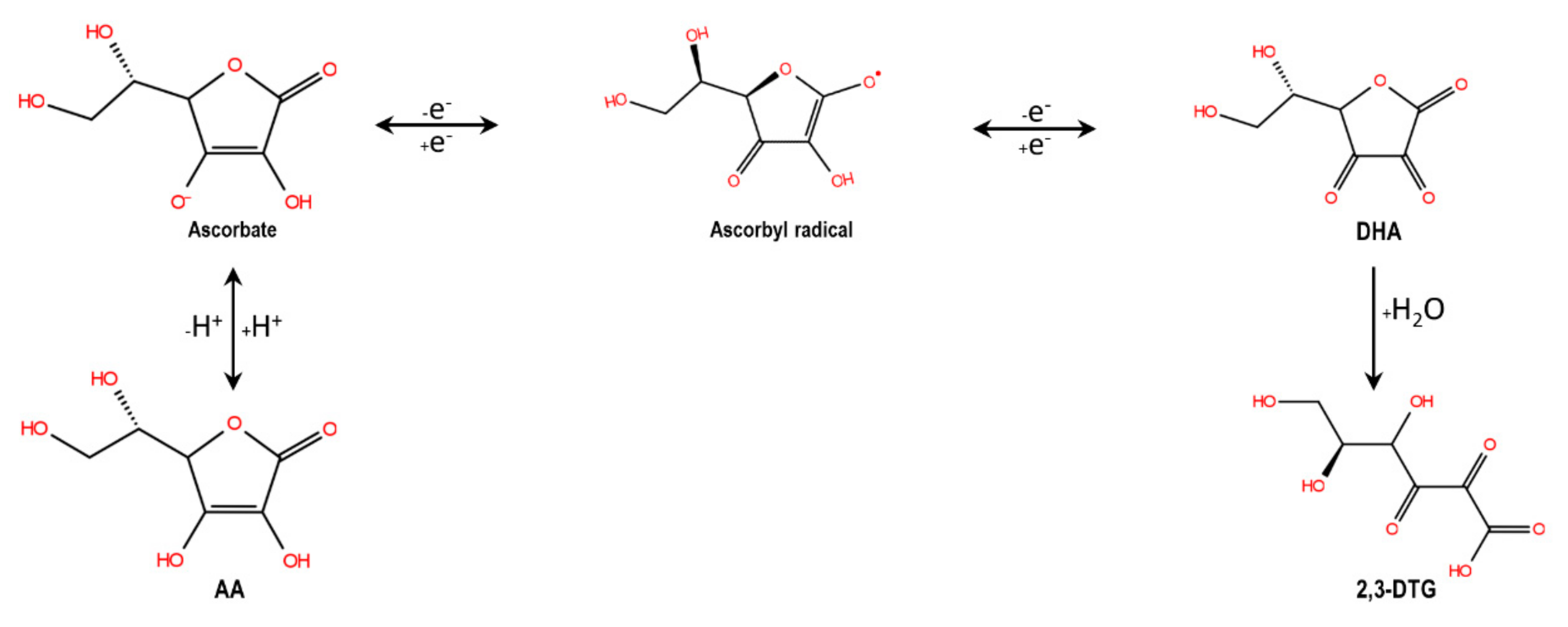
3.6. Thermo-Oxidative Stability
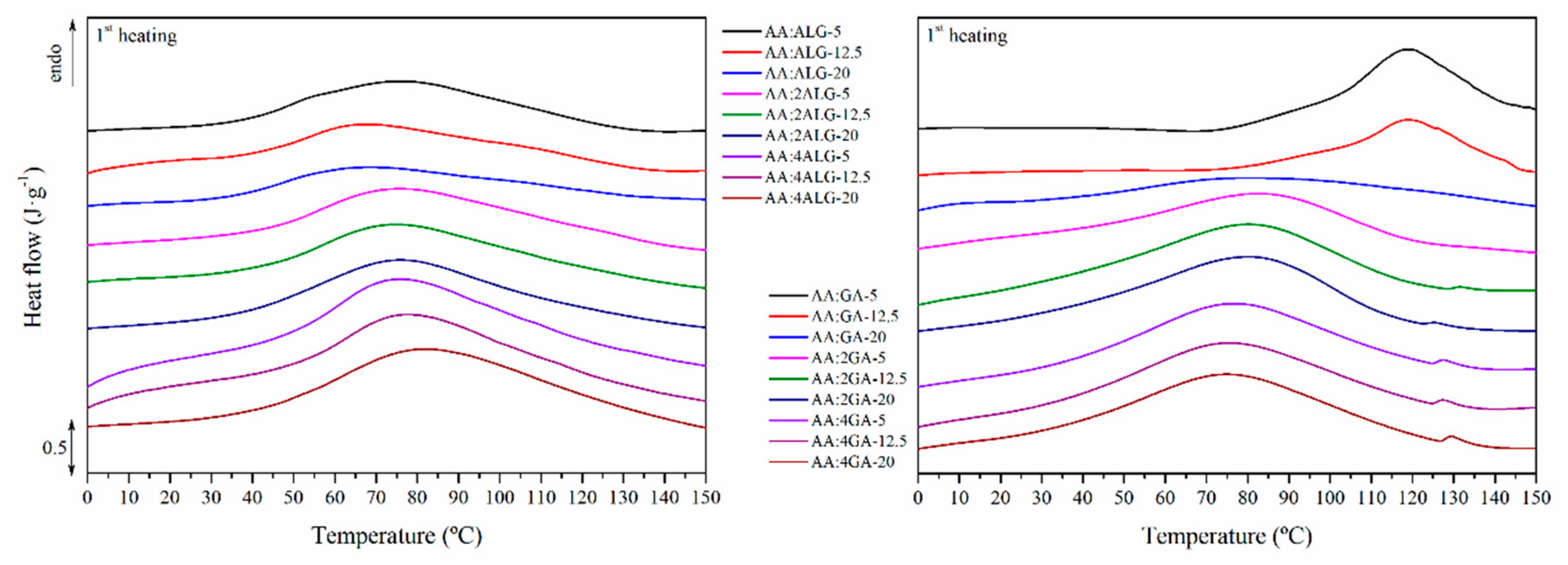
3.7. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sandnes, K. Vitamin C in fish nutrition—A review. Fiskeridirektoratets Skrifter Serie Ernaering 1991, IV, 3–32. [Google Scholar]
- John, H.; Ronald, H. Fish Nutrition, 3rd ed.; Academic Press: London, UK, 2002; ISBN 978-0-12-319652-1. [Google Scholar]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Halver, J.E. 2—The Vitamins. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 61–141. ISBN 978-0-12-319652-1. [Google Scholar]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M.; Cheruparambath, P. Effect of different levels dietary Vitamin C on growth performance, muscle composition, antioxidant and enzyme activity of freshwater prawn, Macrobrachium malcolmsonii. Aquac. Rep. 2016, 3, 229–236. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gatlin, D.M., III. Dietary Vitamin C and Vitamin E Interact to Influence Growth and Tissue Composition of Juvenile Hybrid Striped Bass (Morone chrysops × M. saxatilis) but Have Limited Effects on Immune Responses. Am. Soc. Nutr. Sci. 2002, 132, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic Acid: Microencapsulation Techniques and Trends-A Review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. Chapter 10—Vitamin C. In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 267–295. ISBN 978-0-12-802965-7. [Google Scholar]
- Berge, R.; Akerblom, N.; Maage, A.; Lavens, P.; Cecchini, S.; Saroglia, M.; Caricato, G.; Jeney, Z.; Lovell, R.; Storelli, C.; et al. Ascorbic Acid in Aquatic Organisms; Dabrowski, K., Ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781420036312. [Google Scholar]
- Sheraz, M.A.L.I.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Ahmad, I. Stability and Stabilization of Ascorbic Acid: A Review. Househ. Pers. Care Today 2015, 10, 22–26. [Google Scholar]
- Patel, A.R.; Bhandari, B. Nano- and Microencapsulation of Vitamins. In Nano- and Microencapsulation for Foods; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 223–248. [Google Scholar]
- Das, H.K.; Hattula, M.T.; Myllymäki, O.M.; Mälkki, Y. Effects of formulation and processing variables on dry fish feed pellets containing fish waste. J. Sci. Food Agric. 1993, 61, 181–187. [Google Scholar] [CrossRef]
- Anderson, J.S.; Sunderland, R. Effect of extruder moisture and dryer processing temperature on vitamin C and E and astaxanthin stability. Aquaculture 2002, 207, 137–149. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Bubancová, I.; Dlouhá, G. The stability of vitamin C and other vitamins in the diets of breeding hens. Anim. Feed Sci. Technol. 2012, 177, 253–258. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar] [CrossRef]
- Yoshitomi, B. Depletion of ascorbic acid derivatives in fish feed by the production process. Fish. Sci. 2004, 70, 1153–1156. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, F.; Liu, H.; Guo, S.; Zhu, A.; Niu, H. Gelatin-walled microencapsulated diet for larval shrimp (Penaeus japonicus Bate) manufactured using the fluidized bed coating process. Aquac. Res. 2010, 42, 65–73. [Google Scholar] [CrossRef]
- Knezevic, Z.; Gosak, D.; Hraste, M.; Jalsenjako, I. Fluid-bed microencapsulation of ascorbic acid. J. Microencapsul. 1998, 15, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lal, D. Fortification of milk with microencapsulated vitamin C and its thermal stability. J. Food Sci. Technol. 2005, 42, 191–194. [Google Scholar]
- Kirby, C.J.; Whittle, C.J.; Rigby, N.; Coxon, D.T.; Law, B.A. Stabilization of ascorbic acid by microencapsulation in liposomes. Int. J. Food Sci. Technol. 1991, 26, 437–449. [Google Scholar] [CrossRef]
- Alvim, I.D.; Stein, M.A.; Koury, I.P.; Dantas, F.B.H.; Cruz, C.L.D.C.V. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. LWT Food Sci. Technol. 2016, 65, 689–694. [Google Scholar] [CrossRef]
- Dos Santos Carvalho, J.D.; Oriani, V.B.; de Oliveira, G.M.; Hubinger, M.D. Characterization of ascorbic acid microencapsulated by the spray chilling technique using palm oil and fully hydrogenated palm oil. LWT 2019, 101, 306–314. [Google Scholar] [CrossRef]
- MUddin, M.S.; Hawlader, M.N.A.; Zhu, H.J. Microencapsulation of ascorbic acid: Effect of process variables on product characteristics. J. Microencapsul. 2001, 18, 199–209. [Google Scholar]
- Farias, M.D.P.; Albuquerque, P.B.S.; Soares, P.A.G.; de Sá, D.M.A.T.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Xyloglucan from Hymenaea courbaril var. courbaril seeds as encapsulating agent of L-ascorbic acid. Int. J. Biol. Macromol. 2018, 107, 1559–1566. [Google Scholar] [CrossRef]
- Bouquerand, P.E. Extruded Glassy Vitamin C Particles. European Patent 1836902, 26 September 2007. [Google Scholar]
- Chang, D.; Abbas, S.; Hayat, K.; Xia, S.; Zhang, X.; Xie, M.; Kim, J.M. Encapsulation of ascorbic acid in amorphous maltodextrin employing extrusion as affected by matrix/core ratio and water content. Int. J. Food Sci. Technol. 2010, 45, 1895–1901. [Google Scholar] [CrossRef]
- Stevanović, M.; Savić, J.; Jordović, B.; Uskoković, D. Fabrication, in vitro degradation and the release behaviours of poly(dl-lactide-co-glycolide) nanospheres containing ascorbic acid. Colloids Surf. B Biointerfaces 2007, 59, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Shojaosadati, S.A.; Dorkoosh, F.A.; Elsabee, M.Z. Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem. 2011, 126, 935–940. [Google Scholar] [CrossRef]
- Yoksan, R.; Jirawutthiwongchai, J.; Arpo, K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids Surf. B Biointerfaces 2010, 76, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Thomazini, M.; Alves, A.J.G.; de Matos Junior, F.E.; de Carvalho Balieiro, J.C.; Favaro-Trindade, C.S. Microencapsulation of ascorbic acid by complex coacervation: Protection and controlled release. Food Res. Int. 2013, 52, 373–379. [Google Scholar] [CrossRef]
- Rodrigues da Cruz, M.C.; Andreotti Dagostin, J.L.; Perussello, C.A.; Masson, M.L. Assessment of physicochemical characteristics, thermal stability and release profile of ascorbic acid microcapsules obtained by complex coacervation. Food Hydrocoll. 2019, 87, 71–82. [Google Scholar] [CrossRef]
- Trindade, M.A.; Grosso, C.R.F. The stability of ascorbic acid microencapsulated in granules of rice starch and in gum arabic. J. Microencapsul. 2000, 17, 169–176. [Google Scholar]
- Pierucci, A.P.T.R.; Andrade, L.R.; Baptista, E.B.; Volpato, N.M.; Rocha-Leão, M.H.M. New microencapsulation system for ascorbic acid using pea protein concentrate as coat protector. J. Microencapsul. 2006, 23, 654–662. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J. Microencapsul. 2005, 22, 179–192. [Google Scholar] [CrossRef]
- Esposito, E.; Cervellati, F.; Menegatti, E.; Nastruzzi, C.; Coretesi, R. Spray dried Eudgragit microparticles as encapsulation devices for Vitamin C. Int. J. Pharm. 2002, 242, 329–334. [Google Scholar] [CrossRef]
- Martínez-Ortiz, M.A.; Vargas-Torres, A.; Román-Gutiérrez, A.D.; Chavarría-Hernández, N.; Zamudio-Flores, P.B.; Meza-Nieto, M.; Palma-Rodríguez, H.M. Partial characterization of chayotextle starch-based films added with ascorbic acid encapsulated in resistant starch. Int. J. Biol. Macromol. 2017, 98, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C.; Ishwarya, P. Spray Drying Techniques for Food Ingredient Encapsulation; Institute of Food Technologists Series; Wiley: Oxford, UK, 2015; ISBN 9781118864197. [Google Scholar]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Comparative study of encapsulation of vitamins with native and modified soy protein. Food Hydrocoll. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Al-Ismail, K.; El-Dijani, L.; Al-Khatib, H.; Saleh, M. Effect of microencapsulation of vitamin C with gum arabic, whey protein isolate and some blends on its stability. J. Sci. Ind. Res. (India) 2016, 75, 176–180. [Google Scholar]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Kinetic models applied to soluble vitamins delivery systems prepared by spray drying. Dry. Technol. 2017, 35, 1249–1257. [Google Scholar] [CrossRef]
- Welker, T.L.; Overturf, K.; Snyder, S.; Liu, K.; Abernathy, J.; Frost, J.; Barrows, F.T. Effects of feed processing method (extrusion and expansion-compression pelleting) on water quality and growth of rainbow trout in a commercial setting. J. Appl. Aquac. 2018, 30, 97–124. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum Arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.; Blaga, A.; Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. 2016, 289, 71–78. [Google Scholar] [CrossRef]
- Bash, E. Food Analysis Laboratory Manual; Springer: West Lafayette, IN, USA, 2015; Volume 1, ISBN 9788578110796. [Google Scholar]
- Nielsen, S.S. (Ed.) Food Analysis Laboratory Manual, 2nd ed.; Food Science Texts Series; Springer US: Boston, MA, USA, 2010; ISBN 978-1-4419-1462-0. [Google Scholar]
- Desai, K.G.; Park, H.J. Effect of manufacturing parameters on the characteristics of vitamin C encapsulated tripolyphosphate-chitosan microspheres prepared by spray-drying. J. Microencapsul. 2006, 23, 91–103. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.; Fontana, A.J.; Schmidt, S.J.; Labuza, T. Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780813824086. [Google Scholar]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Yulong, Z.; Huang, Z.; Zhang, J.; Wenyu, W.U.; Wang, M.; Lili, F. Thermal Degradation of Sodium Alginate-Incorporated Soy Protein Isolate/Glycerol Composite Membranes. In Proceedings of the 17th IAPRI World Conference on Packaging, Tianjin, China, 12–15 October 2010; ISBN 9781935068365. [Google Scholar]
- Jamaludin, J.; Adam, F.; Rasid, R.A.; Hassan, Z. Thermal studies on Arabic gum—Carrageenan polysaccharides film. Chem. Eng. Res. Bull. 2017, 19, 80–86. [Google Scholar] [CrossRef]
- Mothé, C.; Rao, M. Thermal behavior of gum arabic in comparison with cashew gum. Thermochim. Acta 2000, 357–358, 9–13. [Google Scholar] [CrossRef]
- Nakamura, K.; Nishimura, Y.; Hatakeyama, T.; Hatakeyama, H. Thermal properties of water insoluble alginate films containing di- and trivalent cations. Thermochim. Acta 1995, 267, 343–353. [Google Scholar] [CrossRef]
- Mogol, B.A.; Gökmen, V.; Shimoni, E.; Author, C. Nano-encapsulation improves thermal stability of bioactive compounds Omega fatty acids and silymarin in bread. Agro Food Ind. Hi-Tech 2013, 24, 62–65. [Google Scholar]
- Lin, S.-F.; Chen, Y.-C.; Chen, R.-N.; Chen, L.-C.; Ho, H.-O.; Tsung, Y.-H.; Sheu, M.-T.; Liu, D.-Z. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Viveros-Contreras, R.; Téllez-Medina, D.I.; Perea-Flores, M.J.; Alamilla-Beltrán, L.; Cornejo-Mazón, M.; Beristain-Guevara, C.I.; Azuara-Nieto, E.; Gutiérrez-López, G.F. Encapsulation of ascorbic acid into calcium alginate matrices through coacervation coupled to freeze-drying. Rev. Mex. Ing. Química 2013, 12, 29–39. [Google Scholar]
Sample Availability: Samples of the AA-polymer microcapsules are available from the authors. |



| AA:Polymer Ratio | Total Dispersed Solids (g·L−1) | AA:ALG Label | AA:GA Label |
|---|---|---|---|
| 1:1 | 5.0 | AA:ALG-5 | AA:GA-5 |
| 1:1 | 12.5 | AA:ALG-12.5 | AA:GA-12.5 |
| 1:1 | 20.0 | AA:ALG-20 | AA:GA-20 |
| 1:2 | 5.0 | AA:2ALG-5 | AA:2GA-5 |
| 1:2 | 12.5 | AA:2ALG-12.5 | AA:2GA-12.5 |
| 1:2 | 20.0 | AA:2ALG-20 | AA:2GA-20 |
| 1:4 | 5.0 | AA:4ALG-5 | AA:4GA-5 |
| 1:4 | 12.5 | AA:4ALG-12.5 | AA:4GA-12.5 |
| 1:4 | 20.0 | AA:4ALG-20 | AA:4GA-20 |
| Encapsulation Efficiency (%) | Mean Particle Size (µm) | Encapsulation Yield (%) | Moisture (%) | ||
|---|---|---|---|---|---|
| AA:ALG-based | AA:ALG-5 | 97.9 ± 1.9 | 5.1 ± 0.4 | 75.7 ± 0.8 | 2.5 ± 0.1 |
| AA:ALG-12.5 | 98.9 ± 1.1 | 10.9 ± 1.2 | 63.1 ± 0.2 | 2.1 ± 0.3 | |
| AA:ALG-20 | 98.0 ± 1.6 | 11.0 ± 2.4 | 42.0 ± 1.1 | 2.6 ± 1.0 | |
| AA:2ALG-5 | 90.5 ± 2.8 | 8.2 ± 1.2 | 70.8 ± 0.7 | 3.9 ± 2.3 | |
| AA:2ALG-12.5 | 93.9 ± 4.4 | 7.8 ± 1.0 | 62.1 ± 0.5 | 4.3 ± 2.5 | |
| AA:2ALG-20 | 93.0 ± 2.4 | 7.8 ± 0.4 | 35.2 ± 6.7 | 4.0 ± 2.6 | |
| AA:4ALG-5 | 92.2 ± 2.8 | 9.7 ± 0.7 | 67.1 ± 3.0 | 4.1 ± 1.4 | |
| AA:4ALG-12.5 | 96.3 ± 2.3 | 7.6 ± 0.2 | 63.6 ± 0.4 | 5.5 ± 3.0 | |
| AA:4ALG-20 | 92.6 ± 1.8 | 14.1 ± 1.4 | 37.8 ± 1.4 | 4.3 ± 2.2 | |
| AA:GA-based | AA:GA-5 | 95.0 ± 4.4 | 9.5 ± 2.3 | 61.1 ± 6.7 | 2.4 ± 1.7 |
| AA:GA-12.5 | 98.3 ± 1.2 | 7.8 ± 0.6 | 68.5 ± 1.8 | 1.4 ± 0.5 | |
| AA:GA-20 | 82.6 ± 4.3 | 9.7 ± 0.5 | 67.8 ± 4.4 | 1.6 ± 0.8 | |
| AA:2GA-5 | 96.8 ± 3.4 | 2.9 ± 0.2 | 79.3 ± 1.1 | 5.0 ± 2.0 | |
| AA:2GA-12.5 | 98.4 ± 0.4 | 4.0 ± 0.1 | 83.2 ± 1.0 | 4.4 ± 2.8 | |
| AA:2GA-20 | 89.6 ± 5.6 | 5.3 ± 0.4 | 75.6 ± 3.3 | 4.5 ± 2.8 | |
| AA:4GA-5 | 91.6 ± 0.4 | 3.1 ± 0.5 | 78.4 ± 1.0 | 5.4 ± 2.7 | |
| AA:4GA-12.5 | 96.0 ± 3.0 | 5.3 ± 0.0 | 77.8 ± 4.0 | 5.4 ± 2.6 | |
| AA:4GA-20 | 93.8 ± 2.8 | 6.3 ± 0.9 | 77.0 ± 0.9 | 4.8 ± 2.7 |
| Predicted Factors by Taguchi | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Constant | C1 | C2 | C1C1 | C2C2 | R2 | Ratio | SD | ||
| AA:ALG | Encapsulation efficiency | 94.02 | −2.26 | 0.54 | 3.50 | −2.35 | 0.67 | 54.74 | 12.73 |
| Mean particle size | 7.57 | 0.72 | 1.66 | 1.79 | 0.53 | 0.44 | 15.75 | 28.34 | |
| Encapsulation yield | 61.45 | −2.04 | −16.43 | 2.20 | −8.17 | 0.96 | 1.88 | 95.05 | |
| AA:GA | Encapsulation efficiency | 98.91 | 0.91 | −2.88 | −2.04 | −5.98 | 0.53 | 5.26 | 47.94 |
| Mean particle size | 3.80 | −2.06 | 0.98 | 2.88 | 0.41 | 0.86 | 74.98 | 10.80 | |
| Encapsulation yield | 77.74 | 7.71 | −0.89 | −6.74 | −1.15 | 0.79 | 77.86 | 1.29 | |
| Characteristic Group | Wavelength (cm−1) | ||
|---|---|---|---|
| AA | ALG | GA | |
| C—O stretch | 1025 | 1030 | 1029 |
| C—O—C stretch | 1113 | 1338 | – |
| C=C stretch | 1658 | – | – |
| C=O stretch | 1750 | 1606 | 1604 |
| C—H stretch | 3350 | 2932 | 2910 |
| O—H stretch | 3523 | 3260 | 3333 |
| C(=O)—O stretch | – | 1411 | 1422 |
| (1,4), (1,6) linkage of galactose and mannose | – | – | 614 |
| Stage (i) (H2O) | Stage (ii) (AA) | Stage (iii) (ALG + AA) (GA + AA) | Stage (iv) (Char) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | Step1 | T2 | Step2 | T3 | Step3 | T4 | Step4 | ||
| (°C) | (%) | (°C) | (%) | (°C) | (%) | (°C) | (%) | ||
| AA:ALG-based | AA:ALG-5 | 64.20 | 7.45 | 188.90 | 20.86 | 239.95 | 38.98 | 335.88 | 5.78 |
| AA:ALG-12.5 | 62.30 | 5.38 | 188.00 | 13.33 | 239.50 | 34.22 | 337.51 | 5.61 | |
| AA:ALG-20 | 63.74 | 5.28 | 187.85 | 12.62 | 238.82 | 30.48 | 336.70 | 5.33 | |
| AA:2ALG-5 | 63.07 | 6.38 | – | – | 241.54 | 38.10 | 318.36 | 5.52 | |
| AA:2ALG-12.5 | 66.58 | 7.71 | – | – | 240.41 | 38.17 | 320.34 | 5.68 | |
| AA:2ALG-20 | 66.04 | 8.25 | – | – | 240.96 | 41.86 | 318.04 | 6.85 | |
| AA:4ALG-5 | 64.10 | 8.95 | – | – | 241.81 | 41.60 | 313.29 | 5.21 | |
| AA:4ALG-12.5 | 65.04 | 10.22 | – | – | 242.09 | 40.60 | 313.75 | 5.57 | |
| AA:4ALG-20 | 74.12 | 10.97 | – | – | 240.65 | 41.73 | 313.94 | 5.21 | |
| AA:GA-based | AA:GA-5 | 55.5 | 8.43 | 192.59 | 20.44 | 269.89 | 34.39 | 377.88 | 12.04 |
| AA:GA-12.5 | 51.97 | 8.65 | 195.35 | 18.83 | 288.86 | 38.48 | 388.11 | 12.02 | |
| AA:GA-20 | 49.43 | 8.37 | 195.27 | 18.96 | 283.09 | 39.19 | 400.53 | 14.02 | |
| AA:2GA-5 | 56.96 | 7.38 | 194.54 | 16.83 | 280.77 | 46.35 | 378.49 | 9.26 | |
| AA:2GA-12.5 | 63.17 | 7.08 | 195.78 | 15.56 | 287.69 | 43.19 | 404.78 | 15.34 | |
| AA:2GA-20 | 52.35 | 7.32 | 196.17 | 14.55 | 289.56 | 48.38 | 380.36 | 11.08 | |
| AA:4GA-5 | 54.28 | 7.19 | 202.84 | 11.96 | 287.71 | 55.13 | 377.86 | 7.16 | |
| AA:4GA-12.5 | 62.82 | 8.20 | 202.92 | 13.48 | 288.41 | 54.59 | 379.11 | 9.46 | |
| AA:4GA-20 | 64.19 | 7.75 | 202.44 | 12.75 | 296.93 | 55.06 | 377.27 | 7.79 | |
| AA:ALG Label | T (°C) | ∆H (J·g−1) | AA:GA Label | T (°C) | ∆H (J·g−1) |
|---|---|---|---|---|---|
| AA:ALG-5 | 76.04 | 101.74 | AA:GA-5 | 118.49 | 117.71 |
| AA:ALG-12.5 | 67.59 | 97.10 | AA:GA-12.5 | 119.27 | 121.63 |
| AA:ALG-20 | 68.81 | 91.85 | AA:GA-20 | 81.88 | 151.69 |
| AA:2ALG-5 | 76.44 | 140.08 | AA:2GA-5 | 82.31 | 158.95 |
| AA:2ALG-12.5 | 75.77 | 143.02 | AA:2GA-12.5 | 80.13 | 161.66 |
| AA:2ALG-20 | 75.98 | 145.68 | AA:2GA-20 | 80.46 | 160.57 |
| AA:4ALG-5 | 76.13 | 172.91 | AA:4GA-5 | 79.30 | 156.14 |
| AA:4ALG-12.5 | 77.56 | 159.65 | AA:4GA-12.5 | 79.32 | 151.23 |
| AA:4ALG-20 | 82.33 | 197.84 | AA:4GA-20 | 78.67 | 154.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. https://doi.org/10.3390/molecules24162872
Barra PA, Márquez K, Gil-Castell O, Mujica J, Ribes-Greus A, Faccini M. Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules. 2019; 24(16):2872. https://doi.org/10.3390/molecules24162872
Chicago/Turabian StyleBarra, Pabla A., Katherine Márquez, Oscar Gil-Castell, Javiera Mujica, Amparo Ribes-Greus, and Mirko Faccini. 2019. "Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic" Molecules 24, no. 16: 2872. https://doi.org/10.3390/molecules24162872
APA StyleBarra, P. A., Márquez, K., Gil-Castell, O., Mujica, J., Ribes-Greus, A., & Faccini, M. (2019). Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules, 24(16), 2872. https://doi.org/10.3390/molecules24162872






