Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect
Abstract
1. Introduction
2. Results
2.1. Spectrophotometric Determination of Polyphenols
2.1.1. Total Anthocyanins and Tannins Determination
2.1.2. Total Anthocyanins and Tannins Determination
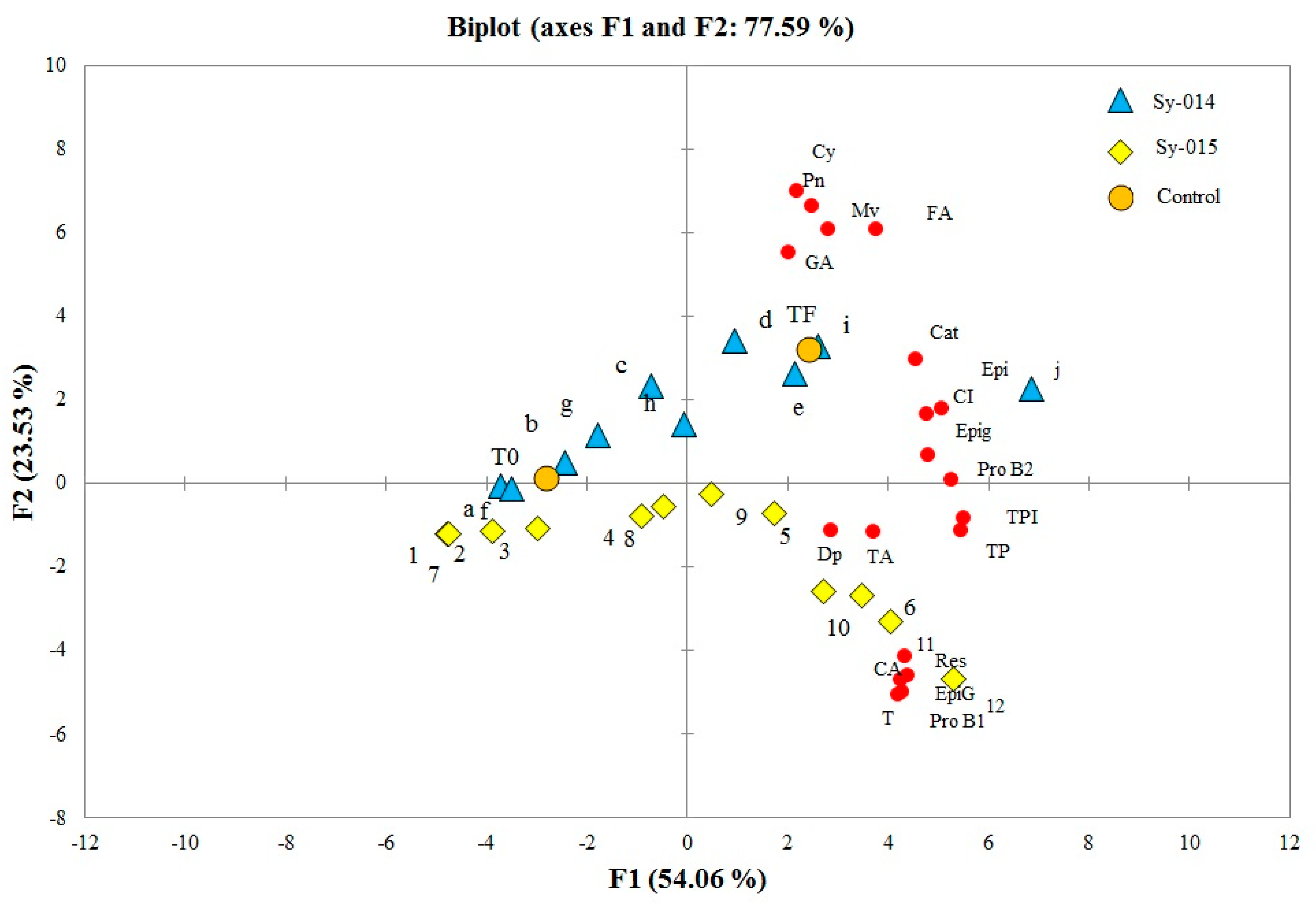
2.2. Chromatographic Determination of Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Samples
4.3. Strains and Storage Conditions
4.4. Maceration and Fermentation Procedures and Sampling
4.5. Spectrophotometric Determinations of Polyphenols
4.6. HPLC Analysis of Phenolic Compounds
4.7. Statistical Data Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Netzel, M.; Strass, G.; Bitsch, I.; Konitz, R.; Christmann, M.; Bitsch, R. Effect of grape processing on selected antioxidant phenolics in red wine. J. Food Eng. 2003, 56, 223–228. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gόmez-Plaza, E.; Lόpez-Roca, J.; Gil-Muῆoz, R.; Fernandez-Fernandez, J.; Baustita-Ortin, A. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentive maceration is affected by the enological technique. J. Agric. Food Chem. 2010, 58, 11333–11339. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.; Hanna-wakim, L.; Nehme, N.; Souchard, J.P.; Taillandier, P.; El Rayess, Y. Impact of winemaking techniques on phenolic compounds composition and content of wine. In Wine Phenolic Composition, Classification and Health Benefits; El Rayess, Y., Ed.; Nova science publishers Inc.: New York, NY, USA, 2014; pp. 104–130. [Google Scholar]
- Mateus, N.; Marques, S.; Gonçalves, A.C.; Machado, J.M.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro Valley during ripening: Influence of cultivation altitude. Am. J. Enol. Viticul. 2001, 52, 115–121. [Google Scholar]
- Orduña, R.M.D. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Galvin, C. Etude de Certaines Réactions de Dégradation des Anthocyanes et de Leur Condensation avec les Flavanols; Conséquences sur la couleur des vins; Université de Bordeaux II: Bordeaux, France, 1993. [Google Scholar]
- Morel-Salmi, C.; Souquet, J.M.; Bes, M.; Cheynier, V. The effect of flash release treatment on phenolic extraction and wine composition. J. Agric. Food Chem. 2006, 54, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.L.; Monti, J.P.; Glories, Y. Anthocyanin is a quinone method derivative resulting from malvidin 3-O-glucoside degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in anthocyanins and color characteristics of Pinot noir wines during different vinification processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Gomez Plaza, E.; Gil Munoz, R.; Lopez Roca, J.M.; Martinez Cutillas, A.; Fernandez Fernandez, J.L. Maintenance of colour composition of a red wine during storage. Influence of prefermentive practices, maceration time and storage. LWT-Food Sci. Technol. 2002, 35, 46–53. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Liazid, A.; Palma, M.; Puertas, B.; González-Barrio, R.; Gil-Izquierdo, Á.; García-Barroso, C.; Cantos-Villar, E. Phenolic characterization of red grapes autochthonous to Andalusia. Food Chem. 2009, 112, 949–955. [Google Scholar] [CrossRef]
- Atanacković, M.; Petrović, A.; Jović, S.; Gojković-Bukarica, L.; Bursać, M.; Cvejić, J. Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chem. 2012, 513–518. [Google Scholar] [CrossRef]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.C.; Bartolomé, B. Vitis vinifera L. cv., Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dolan, K.D.; Yang, L. Confidence intervals for modeling anthocyanin retention in grape pomace during nonisothermal heating. J. Food Sci. 2008, 73, E9–E15. [Google Scholar] [CrossRef] [PubMed]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts-A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Viticult. 2010, 61, 23–30. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges: 1 partie: Les équilibres des anthocyanes et des tanins. J. Int. Sci. Vigne Vin 1984, 18, 195–217. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar] [PubMed]
- Ribéreau Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Les composés phénoliques. In Traité D’œnologie 2. Chimie du vin Stabilisation et Traitements, 7th ed.; Dunod: Paris, France, 1998; pp. 163–237. [Google Scholar]
- Ribéreau-Gayon, J.; Ribéreau-Gayon, P.; Peynaud, E.; Sudraud, P. Traité d’oenologie -Ttome 1: Analyse et contrôle des Vins; Dunod: Paris, France, 1972; p. 671. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Dosage des tanins du vin rouge et détermination de leur structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Ghanem, C.; Taillandier, P.; Rizk, M.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Analysis of the impact of fining agents types, oenological tannins and mannoproteins and their concentrations on the phenolic composition of red wine. LWT-Food Sci. Technol. 2017, 83, 101–109. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
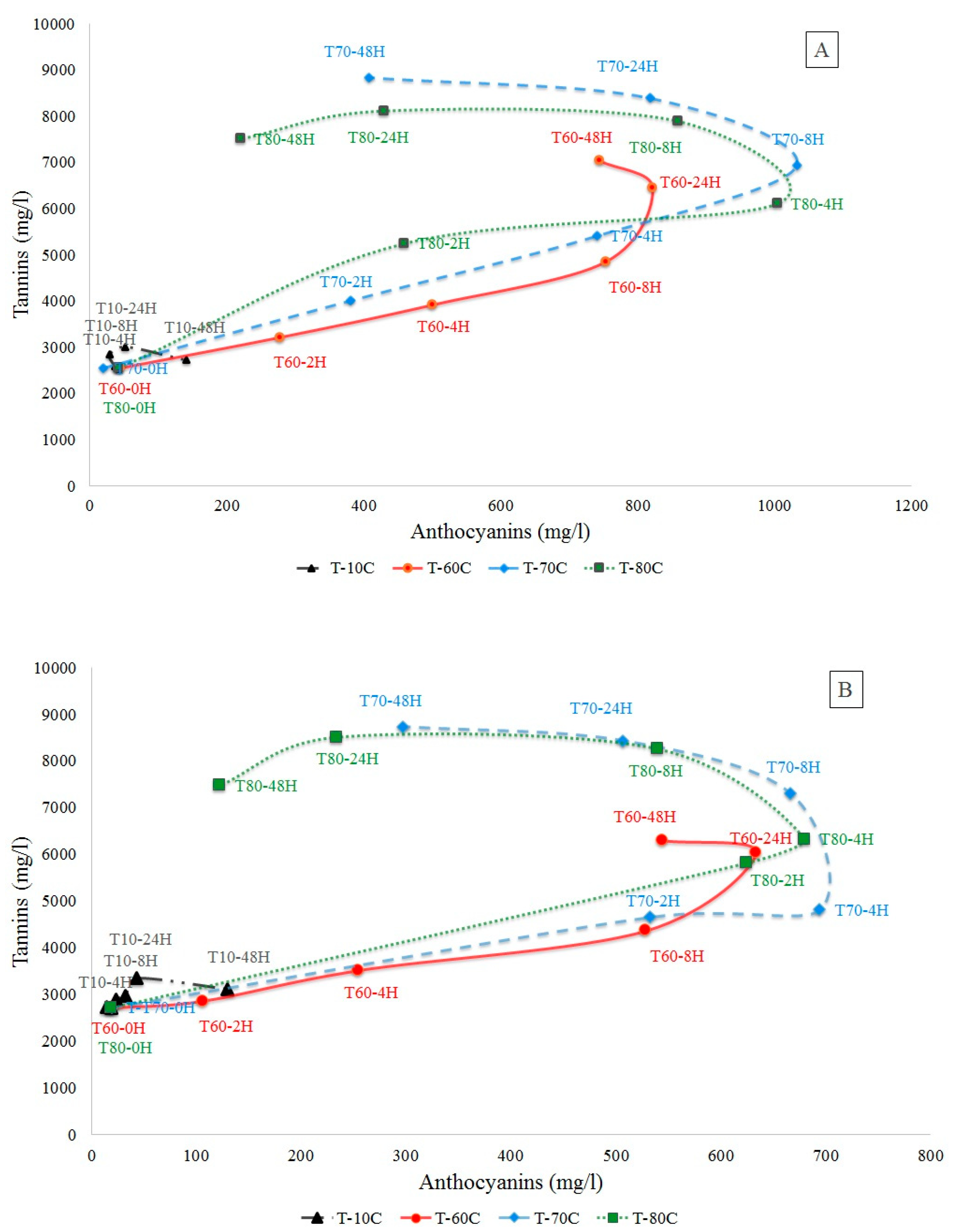
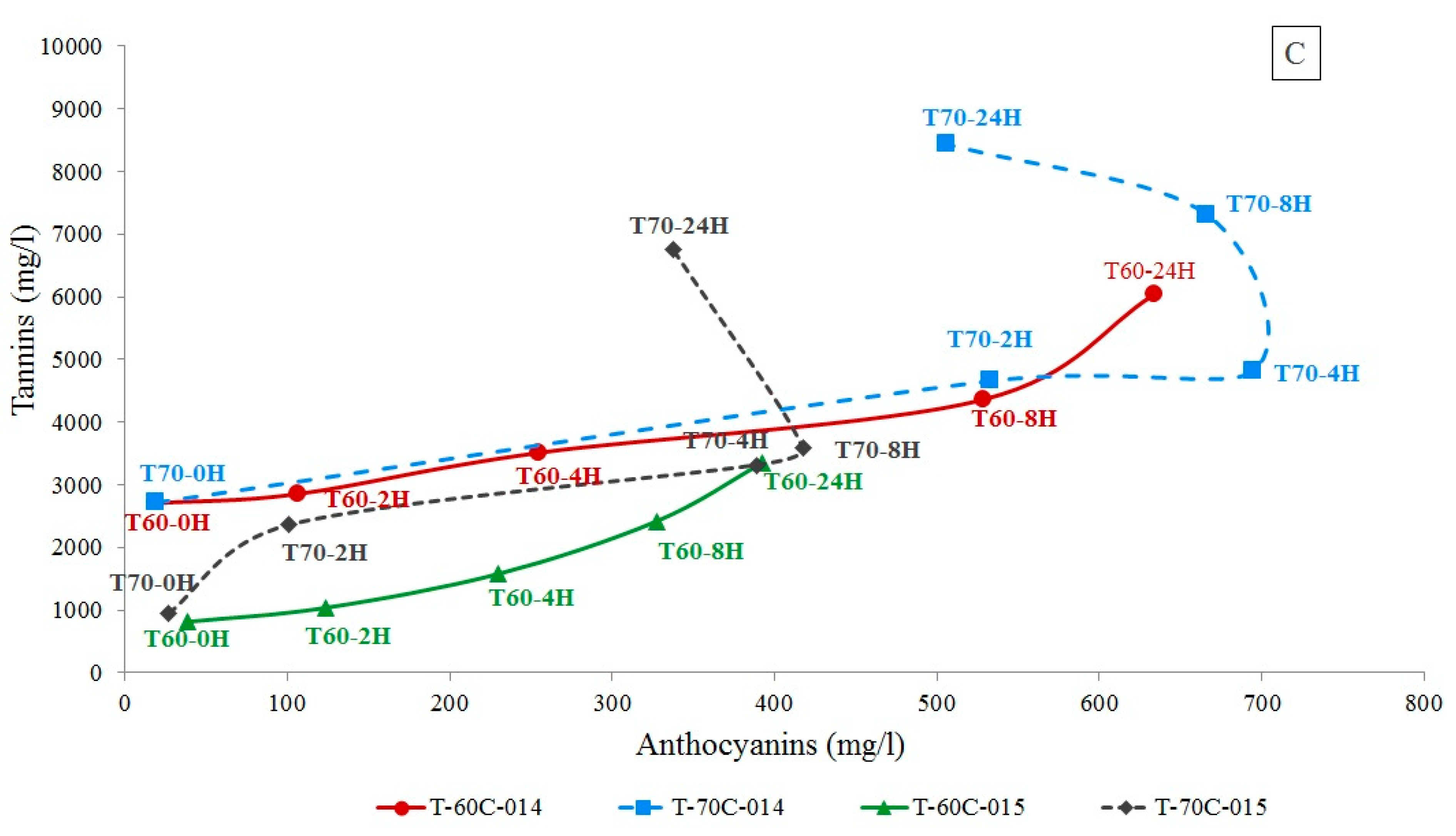

| Sy Maceration Time (Hours) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 24 | 48 | |||||||||
| Control 25 °C | F | ST | F | ST | F | ST | F | ST | F | ST | F | ST | ||
| 10 °C | CI | 1.22 ± 0.01 | 0.42 ± 0.01 a | 0.34 ± 0.01 b | 0.43 ± 0.10 a | 0.42 ± 0.21 a | 0.44 ± 0.10 a | 0.46 ± 0.01 a | 0.49 ± 0.00 a | 0.49 ± 0.00 a | 0.59 ± 0.00 a | 0.52 ± 0.00 b | 0.65 ± 0.00 a | 0.55 ± 0.00 b |
| TPI | 60.12 ± 2.57 | 16.20 ± 1.08 a | 16.27 ± 0.50 a | 16.22 ± 0.65 b | 19.10 ± 0.70 a | 16.27 ± 0.14 b | 19.77 ± 0.78 a | 16.30 ± 0.15 b | 19.30 ± 0.96 a | 15.27 ±1.62 b | 20.93 ± 0.94 a | 18.07 ± 1.70 b | 22.30 ± 0.87 a | |
| TP | 2452.25 ± 46.19 | 628.33 ± 1.21 a | 440.00 ± 0.50 b | 605.00 ± 2.89 a | 566.67 ± 0.40 b | 583.00 ± 1.73 a | 521.67± 0.81 b | 555.00 ± 2.89 a | 573.30 ± 6.07 a | 540.00 ± 3.22 b | 663.33 ± 3.09 a | 606.67 ± 2.89 b | 683.33 ± 0.20 a | |
| 60 °C | CI | 1.22 ± 0.01 | 0.42 ± 0.01 a | 0.34 ± 0.00 b | 0.80 ± 0.01 a | 0.611 ± 0.03 b | 1.20 ± 0.11 a | 0.99 ± 0.02 a | 1.67 ± 0.04 a | 1.34 ± 0.10 b | 1.81 ± 0.16 a | 1.53 ± 0.01 b | 1.80 ± 0.03 a | 1.24 ± 0.09 b |
| TPI | 60.12 ± 2.57 | 16.93 ± 0.45 a | 16.27 ± 0.25 a | 26.80 ± 0.05 a | 21.97 ± 0.50 b | 38.30 ± 0.10 a | 29.97 ± 2.90 b | 52.15 ± 0.02 a | 35.17 ± 2.80 b | 64.53 ± 1.81 a | 52.93 ± 1.62 b | 74.07 ± 1.55 a | 62.30 ± 0.63 b | |
| TP | 2452.25 ± 46.19 | 628.33 ± 0.14 a | 441.67 ± 0.81 b | 927.20 ± 3.62 a | 680.00 ± 3.41 b | 1210.80 ± 0.10 a | 873.30 ± 4.52 b | 1648.90 ± 4.87 a | 1393.33 ± 2.51 b | 2490.00 ± 0.05 a | 2266.67 ± 5.12 a | 2756.70 ± 1.66 a | 2643.30 ± 2.58 a | |
| 70 °C | CI | 1.22 ± 0.01 | 0.42 ± 0.00 a | 0.34 ± 0.00 b | 1.19 ± 0.11 a | 1.30 ± 0.04 a | 1.83 ± 0.02 a | 1.39 ± 0.10 b | 2.61 ± 0.00 a | 1.59 ± 0.04 b | 2.44 ± 0.09 a | 1.60 ± 0.02 b | 2.03 ± 0.01 a | 1.30 ± 0.06 b |
| TPI | 60.12 ± 2.57 | 16.53 ± 0.40 a | 16.70 ± 0.10 a | 30.60 ± 0.35 b | 37.43 ± 0.80 a | 45.20 ± 0.15 b | 49.93 ± 4.30 a | 62.10 ± 0.46 a | 56.00 ± 1.30 b | 71.80 ± 1.14 a | 73.73 ± 2.47 a | 88.20 ± 1.80 a | 85.20 ± 1.67 a | |
| TP | 2452.25 ± 46.19 | 628.30 ± 3.63 a | 440.00 ± 1.41 b | 1296.60 ± 3.44 b | 1526.67 ± 1.92 a | 1878.40 ± 4.78 b | 2155.00 ± 2.74 a | 2656.60 ± 3.44 b | 2758.33 ± 1.30 a | 3185.00 ± 7.55 b | 3585.00 ± 1.97 a | 4660.00 ± 0.81 a | 4380.00 ± 1.39 b | |
| 80 °C | CI | 1.22 ± 0.01 | 0.42 ± 0.01 a | 0.34 ± 0.01 | 1.45 ± 0.62 a | 1.47 ± 0.04 a | 2.63 ± 0.01 a | 1.52 ± 0.05 b | 2.40 ± 0.00 a | 1.66 ± 0.06 b | 2.01 ± 0.17 a | 1.93 ± 0.06 a | 1.95 ± 0.02 b | 2.031 ± 0.02 a |
| TPI | 60.12 ± 2.57 | 16.70 ± 0.11 a | 16.37 ± 0.28 | 42.30 ± 0.11 b | 45.47 ± 0.32 a | 58.70 ± 0.11 b | 60.87 ± 1.15 a | 72.80 ± 0.40 a | 73.17 ± 0.17 a | 80.50 ± 0.29 b | 85.80 ± 1.15 a | 86.80 ± 0.00 b | 89.20 ± 0.70 a | |
| TP | 2452.25 ± 46.19 | 628.30 ± 4.20 a | 440.00 ± 0.79 | 1852.00 ± 1.00 b | 1875.00 ± 1.22 a | 2732.60 ± 3.86 b | 2823.33 ± 0.30 a | 3108.70 ± 4.71 b | 3301.67 ± 1.05 a | 3542.80 ± 1.44 b | 3661.67 ± 0.50 a | 3329.60 ± 5.54 a | 3031.67 ± 3.05 b | |
| Sy Maceration Time (Hours) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 24 | ||||||||
| Control 25 °C | ST-014 | ST-015 | ST-014 | ST-015 | ST-014 | ST-015 | ST-014 | ST-015 | ST-014 | ST-015 | ||
| 60 °C | CI | 1.22 ± 0.01 | 0.34 ± 0.00 a | 0.15 ± 0.03 b | 0.611 ± 0.03 a | 0.39 ± 0.01 b | 0.99 ± 0.02 a | 0.79 ± 0.03 b | 1.34 ± 0.10 a | 1.06 ± 0.06 b | 1.53 ± 0.01 a | 1.08 ± 0.06 b |
| TPI | 60.12 ± 2.57 | 16.27 ± 0.25 a | 12.50 ± 0.20 b | 21.97 ± 0.50 a | 16.30 ± 0.56 b | 29.97 ± 2.90 a | 22.97 ± 1.25 b | 35.17 ± 2.80 a | 28.60 ± 0.91 b | 52.93 ± 1.62 a | 42.00 ± 2.19 b | |
| TP | 2452.25 ± 46.19 | 441.67 ± 0.81 a | 401.67 ± 2.87 b | 680.00 ± 3.41 a | 621.67 ± 5.77 b | 873.30 ± 4.52 a | 803.33 ± 79.73 b | 1393.33 ± 2.51 a | 1310.33 ± 18.92 a | 2266.67 ± 5.12 a | 2172.67 ± 28.43 b | |
| 70 °C | CI | 1.22 ± 0.01 | 0.34 ± 0.00 a | 0.16 ± 0.02 b | 1.30 ± 0.04 a | 0.66 ± 0.02 b | 1.39 ± 0.10 a | 1.16 ± 0.06 b | 1.59 ± 0.04 a | 1.51 ± 0.09 b | 1.60 ± 0.02 a | 1.46 ± 0.09 b |
| TPI | 60.12 ± 2.57 | 16.70 ± 0.10 a | 12.33 ± 0.21 b | 37.43 ± 0.80 a | 32.37 ± 0.46 b | 49.93 ± 3.30 a | 40.40 ± 0.26 b | 56.00 ± 1.30 a | 45.30 ± 0.62 b | 73.73 ± 2.47 a | 60.47 ± 1.97 b | |
| TP | 2452.25 ± 46.19 | 440.00 ± 1.41 a | 402.67 ± 12.58 a | 1526.67 ± 1.92 a | 1475.00 ± 13.23 b | 2155.00 ± 2.74 a | 2051.67 ± 2.88 a | 2758.33 ± 1.30 a | 2576.67 ± 5.77 a | 3585.00 ± 1.97 a | 3468.33 ± 2.88 a | |
| Wine-Growing Region | Wine-Producer | Soil Type | Climate Condition (2014 Data) |
|---|---|---|---|
| West Bekaa/Lebanon | Clos St. Thomas | Limestone, pebbly clay, clay-calcareous well drained, poor in humus and organic matter | The vineyards are located on the valley zones at the altitude of 950 m with a cool and semi-arid dry climate and a big difference between day and night time, with an annual rainfall of 650 mm, annual average temperature of 21.1 °C. |
| Chouf District/Lebanon | Florentine | Clay-calcareous, stony basement | The vineyards are located on the mountain’s hills at the altitude of about 1000 m with a warm, dry sub-humid and temperate climate, with an annual rainfall of 1078 mm and annual average temperature of 15.1 °C. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, C.; Taillandier, P.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect. Molecules 2019, 24, 2845. https://doi.org/10.3390/molecules24152845
Ghanem C, Taillandier P, Rizk Z, Nehme N, Souchard JP, El Rayess Y. Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect. Molecules. 2019; 24(15):2845. https://doi.org/10.3390/molecules24152845
Chicago/Turabian StyleGhanem, Chantal, Patricia Taillandier, Ziad Rizk, Nancy Nehme, Jean Pierre Souchard, and Youssef El Rayess. 2019. "Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect" Molecules 24, no. 15: 2845. https://doi.org/10.3390/molecules24152845
APA StyleGhanem, C., Taillandier, P., Rizk, Z., Nehme, N., Souchard, J. P., & El Rayess, Y. (2019). Evolution of Polyphenols during Syrah Grapes Maceration: Time versus Temperature Effect. Molecules, 24(15), 2845. https://doi.org/10.3390/molecules24152845






