An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid
Abstract
1. Introduction
2. Pharmaceutical Properties of Gaba
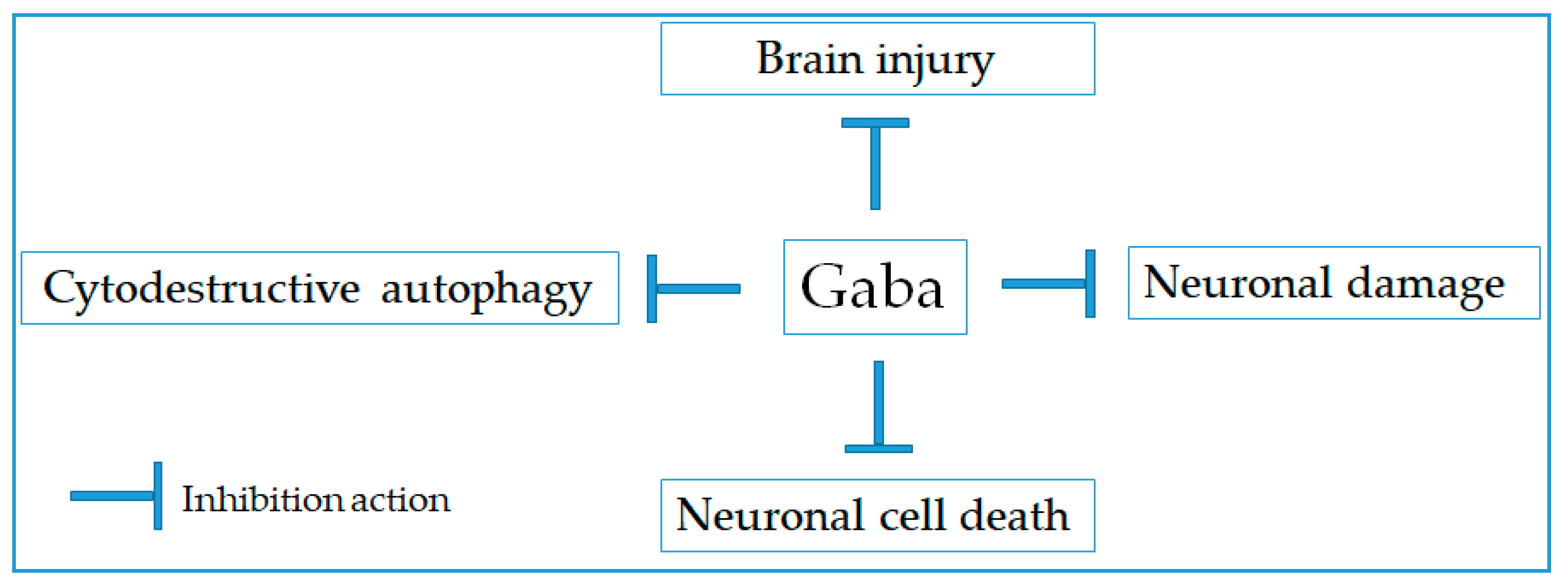
2.1. Neuroprotective Effect
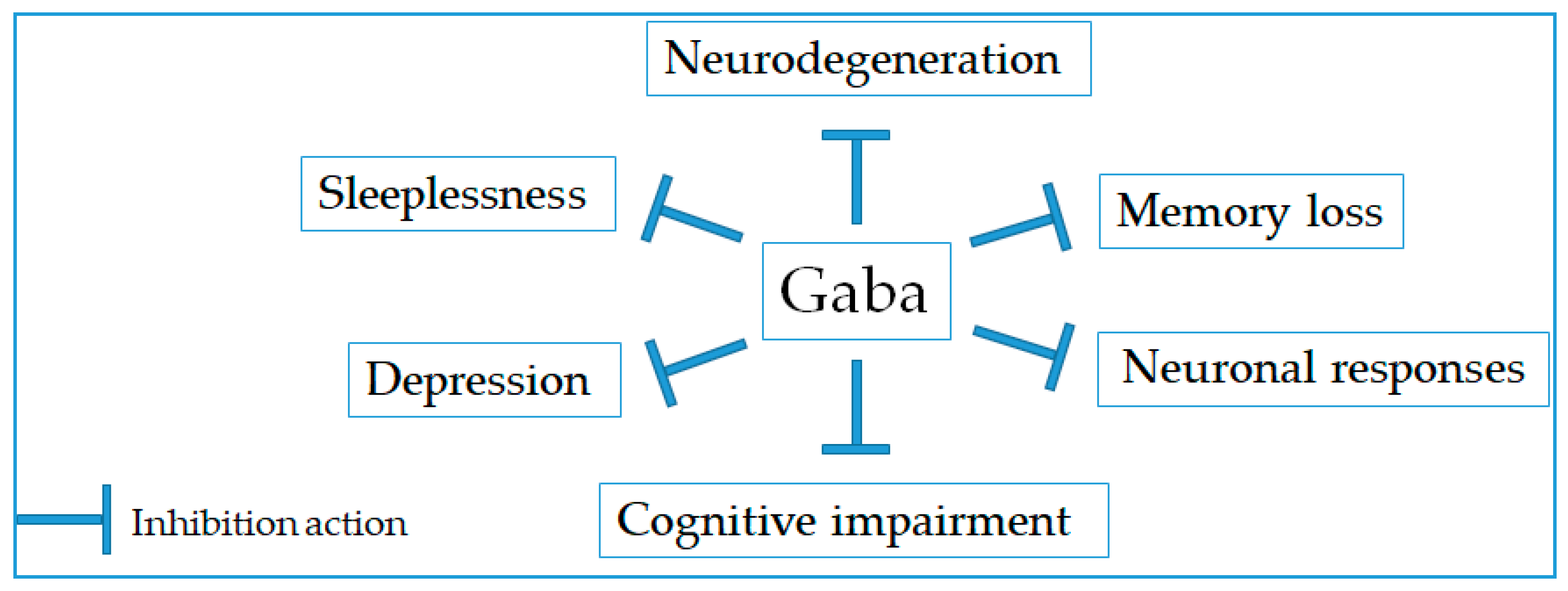
2.2. Neurological Disorder Prevention
2.3. Anti-Hypertensive Effect
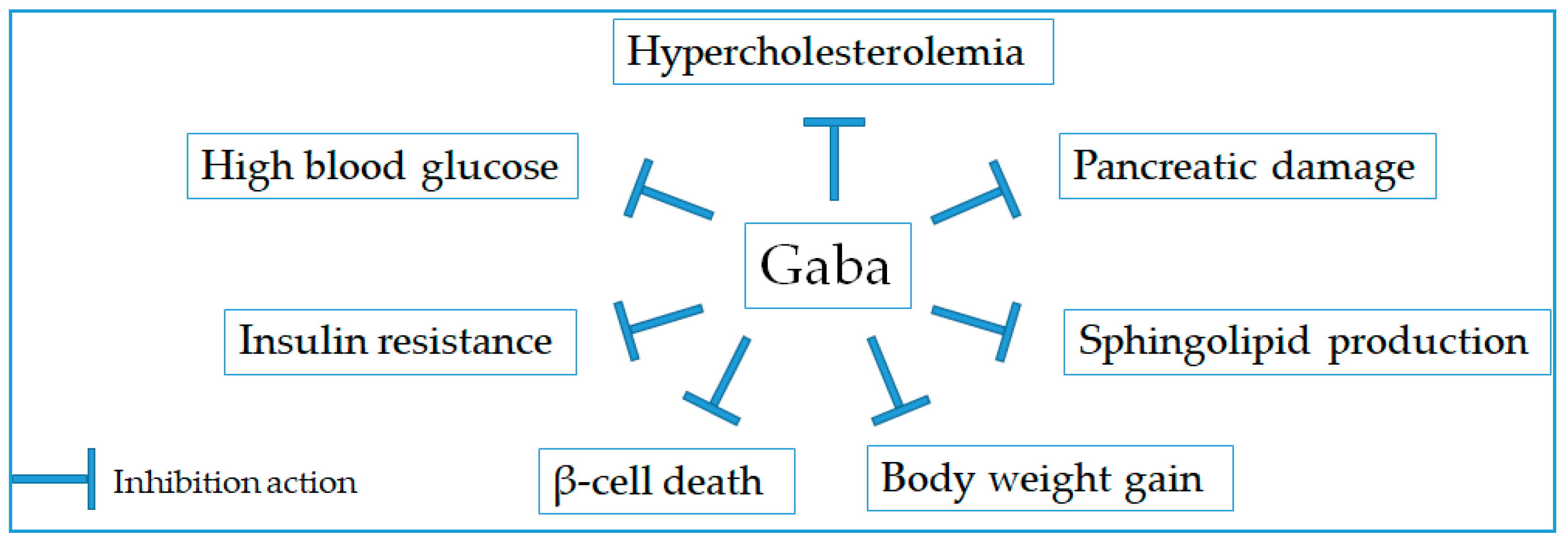
2.4. Anti-Diabetic Effect
2.5. Anti-Cancer Effect
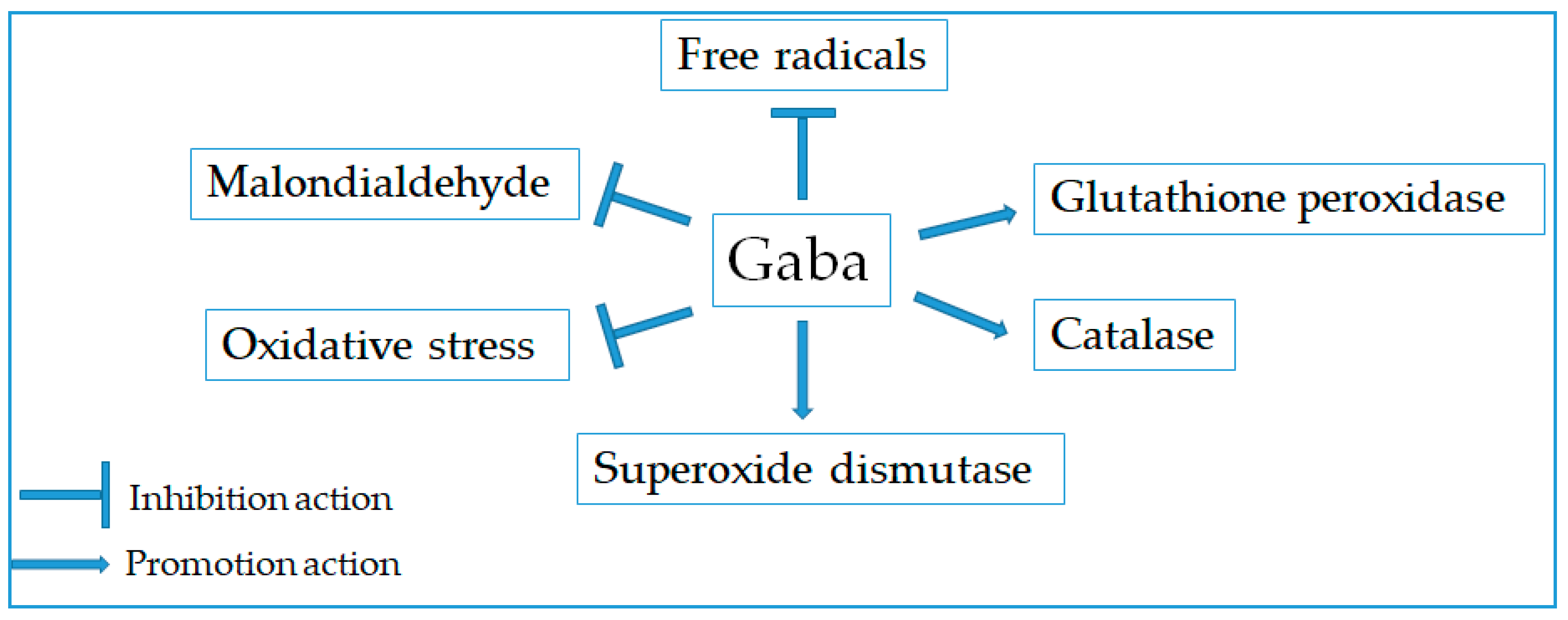
2.6. Antioxidant Effect
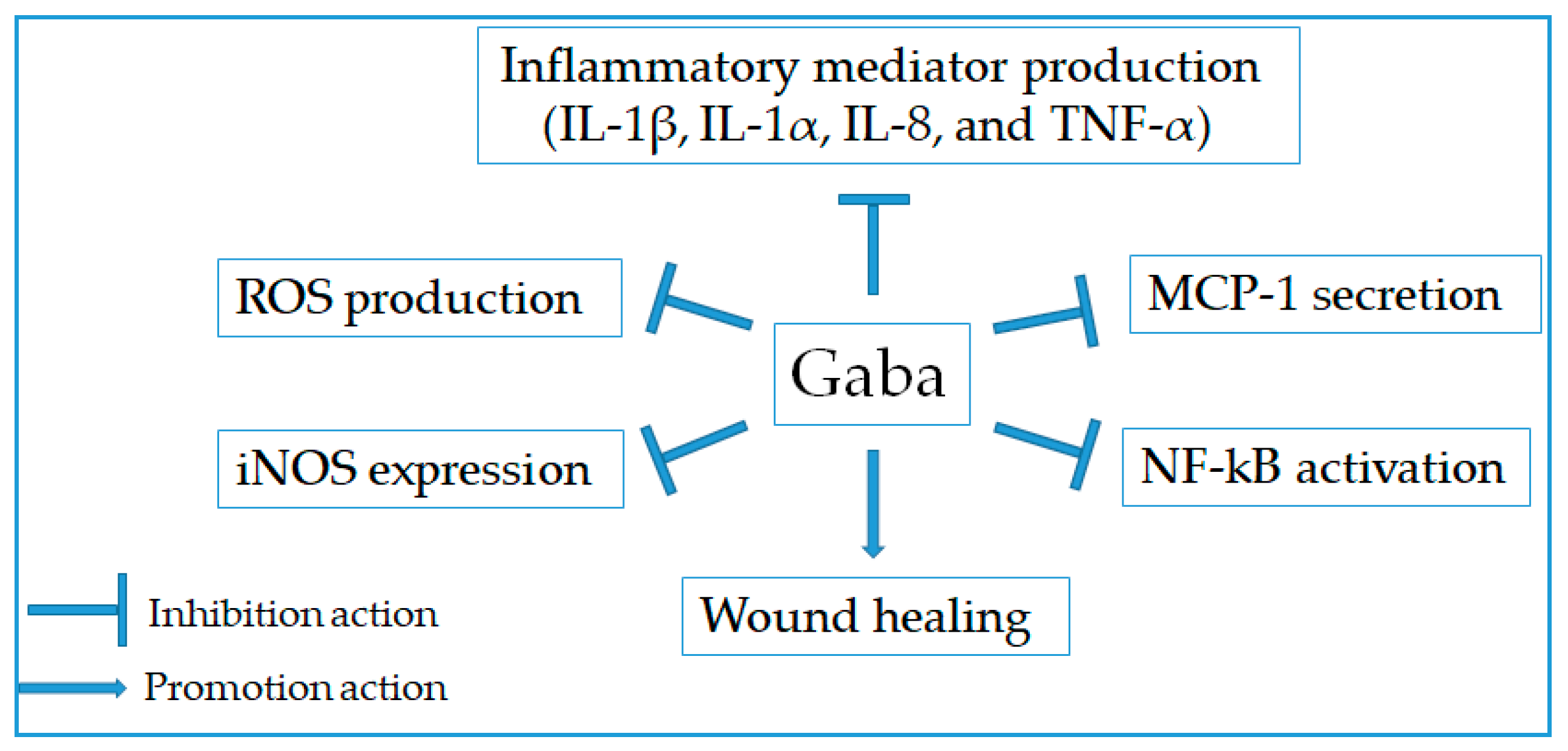
2.7. Anti-Inflammatory Effect
2.8. Anti-Microbial Effect
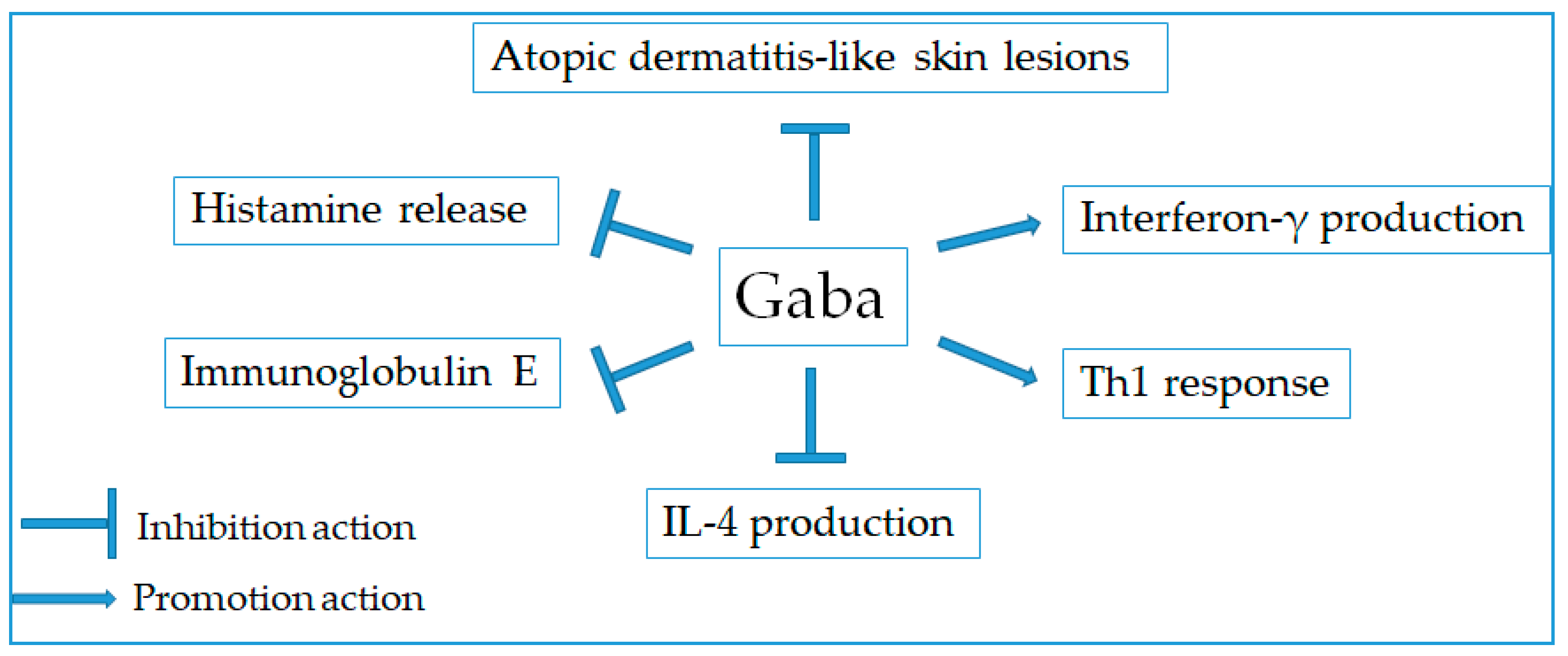
2.9. Anti-Allergic Effect
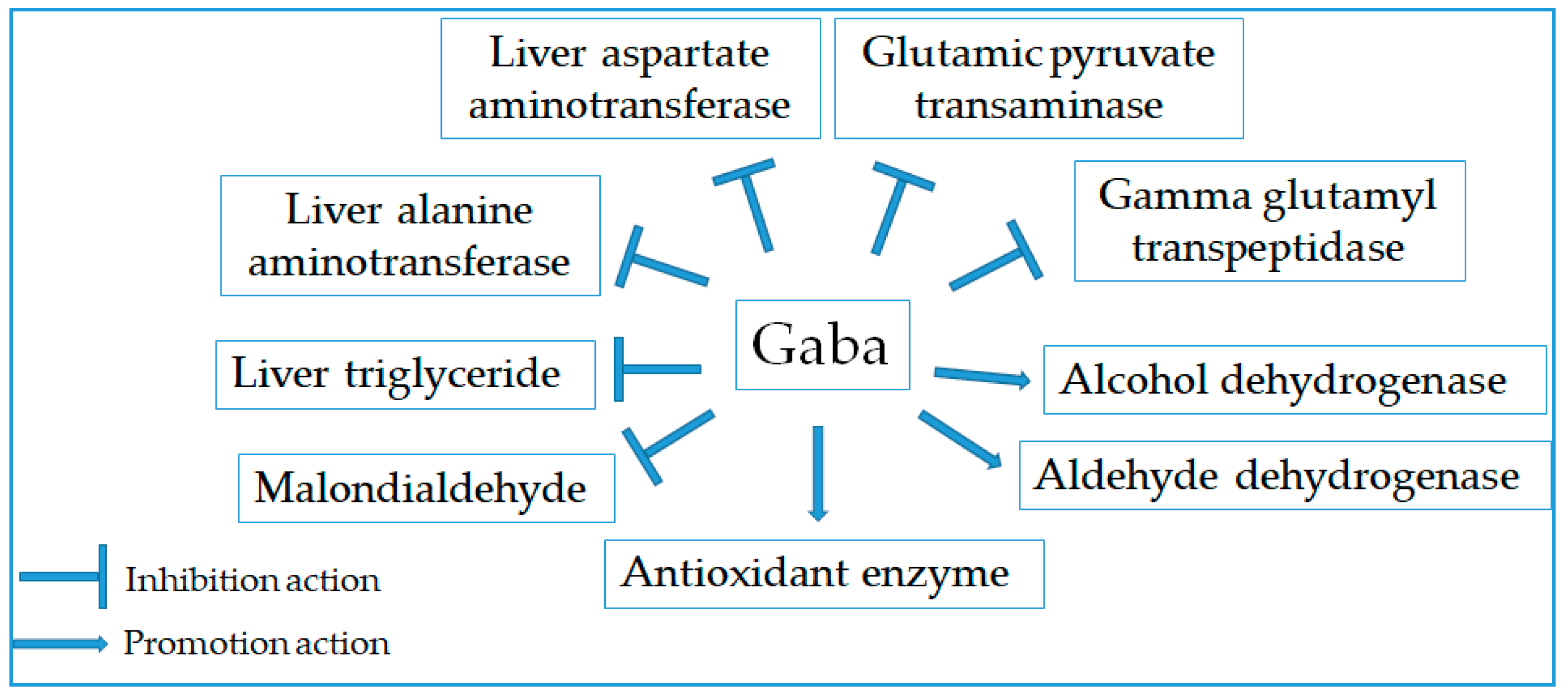
2.10. Hepatoprotective Effect
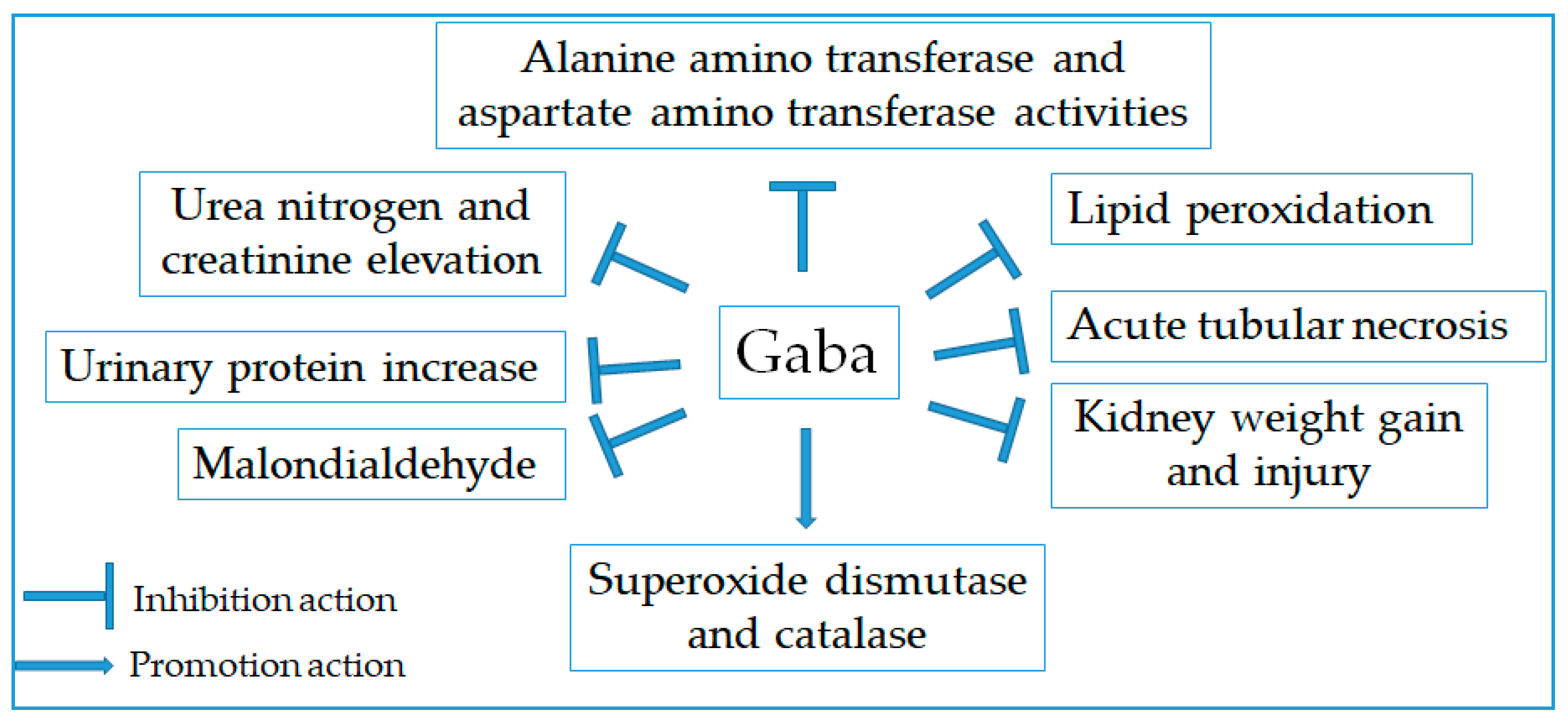
2.11. Renoprotective Effect
2.12. Intestinal Protective Effect
2.13. Other Pharmaceutical Properties
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsen, R.W.; Betz, H. GABA and glycine. In Basic Neurochemistry, 7th ed.; Siegel, G.J., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier Academic Press: Boston, MA, USA, 2006. [Google Scholar]
- Martin, D.L.; Olsen, R.W. (Eds.) GABA in the Nervous System: The View at 50 Years; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Madsen, K.K.; Clausen, R.P.; Larsson, O.M.; Krogsgaard-Larsen, P.; Schousboe, A.; White, H.S. Synaptic and extrasynaptic GABA transporters as targets for anti-epileptic drugs. J. Neurochem. 2009, 109, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.O.; Kohl, M.M.; Paulsen, O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J. Neurosci. 2009, 29, 7513–7518. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [PubMed]
- Wagner, S.; Castel, M.; Gainer, H.; Yarom, Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 1997, 387, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Plante, D.T.; Jensen, J.E.; Schoerning, L.; Winkelman, J.W. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology 2012, 37, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and application. In Study in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: 1000 AE Amsterdam, The Netherlands, 2018; Volume 57, pp. 413–452. [Google Scholar]
- Kaminsky, N.; Bihari, O.; Kanner, S.; Barzilai, A. Connecting malfunctioning glial cells and brain degenerative disorders. Genom. Proteom. Bioinform. 2016, 14, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. In Vivo 2016, 30, 535–547. [Google Scholar]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar] [PubMed]
- Li, W.; Wei, M.; Wu, J.; Rui, X.; Dong, M. Novel fermented chickpea milk with enhanced level of γ-aminobutyric acid and neuroprotective effect on PC12 cells. Peer J 2016, 4, e2292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, C.; Yu, H.M.; Zhang, F.; Han, D.; Zhang, G.Y. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J. Neurosci. Res. 2008, 86, 2973–2983. [Google Scholar] [PubMed]
- Liu, L.; Li, C.J.; Lu, Y.; Zong, X.G.; Luo, C.; Sun, J.; Guo, L.J. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Sci. Rep. 2015, 5, 14474. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Yan, H.; Xu, B.; Wu, Y.P.; Li, C.; Zhang, G.Y. Neuroprotection of co-activation of GABA receptors by preventing caspase-3 denitrosylation in KA-induced seizures. Brain. Res. Bull. 2012, 88, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K. Natural or plant products for the treatment of neurological disorders: Current knowledge. Curr. Drug. Metab. 2018, 19, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J. Jpn. Soc. Food Sci. 2000, 47, 596–603. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Shi, Y.C.; You, H.P.; Lo, Y.H.; Pan, T.M. Antidepressant effect of GABA-rich monascus-fermented product on forced swimming rat model. J. Agric. Food Chem. 2011, 59, 3027–3034. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Pandharipande, T.; Maru, I.; Kim, M. Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 2016, 25, 547–551. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effect of gamma-aminobutyric acid (GABA) administration in humans. BioFactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Seo, Y.C.; Choi, W.Y.; Kim, J.S.; Lee, C.G.; Ahn, J.H.; Cho, H.Y.; Lee, S.H.; Cho, J.S.; Joo, S.J.; Lee, H.Y. Enhancement of the cognitive effects of GABA from monosodium glutamate fermentation by Lactobacillus sakei B2-16. Food Biotechnol. 2012, 26, 29–44. [Google Scholar] [CrossRef]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evid. Based Complement. Altern. Med. 2018, 2018, 8109621. [Google Scholar]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.S.; Jeon, B.H. GABA-enriched fermented Laminaria japonica improves cognitive impairment and neuroplasticity in scopolamine- and ethanol-induced dementia model mice. Nutr. Res. Pract. 2018, 12, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.C.; Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jo, Y.H.; Jeon, B.H. Effects of γ-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae 2016, 31, 175–187. [Google Scholar] [CrossRef]
- Schellack, N.; Naicker, P. Hypertension: A review of antihypertensive medication, past and present. S. Afr. Pharm. J. 2015, 82, 17–25. [Google Scholar]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Nejati, F.; Rizzello, C.G.; Cagno, R.D.; Sheikh-Zeinoddin, M.; Diviccaro, A.; Minervini, F.; Gobbetti, M. Manufacture of a functional fermented milk enriched of angiotensin-I converting enzyme (ACE)-inhibitory peptides and γ-amino butyric acid (GABA). Food Sci. Technol. 2013, 51, 183–189. [Google Scholar] [CrossRef]
- Tung, Y.T.; Lee, B.H.; Liu, C.F.; Pan, T.M. Optimization of culture condition for ACEI and GABA production by lactic acid bacteria. J. Food Sci. 2011, 76, M585–M591. [Google Scholar] [CrossRef]
- Jang, E.K.; Kim, N.Y.; Ahn, H.J.; Ji, G.E. γ-Aminobutyric acid (GABA) production and angiotensin-I converting enzyme (ACE) inhibitory activity of fermented soybean containing sea tangle by the co-culture of Lactobacillus brevis with Aspergillus oryzae. J. Microbiol. Biotechnol. 2015, 25, 1315–1320. [Google Scholar] [CrossRef]
- Sang, V.T.; Uyen, L.P.; Hung, N.D. The increased gamma-aminobutyric acid content by optimizing fermentation conditions of bacteria from kimchi and investigation of its biological activities. EurAsian J. Biosci. 2018, 12, 369–376. [Google Scholar]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Kimura, M.; Hayakawa, K.; Sansawa, H. Involvement of gamma-aminobutyric acid (GABA) B receptors in the hypotensive effect of systemically administered GABA in spontaneously hypertensive rats. Jpn. J. Pharmacol. 2002, 89, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Kimura, M.; Kasaha, K.; Matsumoto, K.; Sansawa, H.; Yamori, Y. Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br. J. Nutr. 2004, 92, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, F.; Ueno, H.; Kentaro, T.; Tadano, K.; Suyama, T.; Imaizumi, K.; Suzuki, T.; Magata, K.; Kikuchi, N. Effects of GABA supplementation on blood pressure and safety in adults with mild hypertension. Jpn. Pharmacol. Ther. 2002, 30, 963–972. [Google Scholar]
- Akama, K.; Kanetou, J.; Shimosaki, S.; Kawakami, K.; Tsuchikura, S.; Takaiwa, F. Seed-specific expression of truncated OsGAD2 produces GABA-enriched rice grains that influence a decrease in blood pressure in spontaneously hypertensive rats. Transgenic Res. 2009, 18, 865–876. [Google Scholar] [CrossRef]
- Ebizuka, H.; Ihara, M.; Arita, M. Antihypertensive effect of pre-germinated brown rice in spontaneously hypertensive rats. Food Sci. Technol. Res. 2009, 15, 625–630. [Google Scholar] [CrossRef]
- Kawakami, K.; Yamada, K.; Yamada, T.; Nabika, T.; Nomura, M. Antihypertensive effect of γ-aminobutyric acid-enriched brown rice on spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 56–62. [Google Scholar] [CrossRef]
- Nishimura, M.; Yoshida, S.; Haramoto, M.; Mizuno, H.; Fukuda, T.; Kagami-Katsuyama, H.; Tanaka, A.; Ohkawara, T.; Sato, Y.; Nishihira, J. Effects of white rice containing enriched gamma-aminobutyric acid on blood pressure. J. Tradit. Complement. Med. 2016, 6, 66–71. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chiu, T.H.; Ho, C.Y.; Lin, P.P.; Wu, T.Y. Effects of anti-hypertension and intestinal microflora of spontaneously hypertensive rats fed gammaaminobutyric acid-enriched Chingshey purple sweet potato fermented milk by lactic acid bacteria. Afr. J. Microbiol. Res. 2013, 7, 932–940. [Google Scholar]
- Lin, P.P.; Hsieh, Y.M.; Kuo, W.W.; Lin, C.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y.; Tsai, C.C. Inhibition of cardiac hypertrophy by probiotic-fermented purple sweet potato yogurt in spontaneously hypertensive rat hearts. Int. J. Mol. Med. 2012, 30, 1365–1375. [Google Scholar] [CrossRef][Green Version]
- Suwanmanon, K.; Hsieh, P.C. Effect of γ-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Food Drug. Anal. 2014, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Furuya, Y.; Endo, Y.; Fujimoto, K. Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-Tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2003, 67, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; et al. Antihypertensive effect of a gamma-aminobutyric acid rich tomato cultivar ‘DG03-9’ in spontaneously hypertensive rats. J. Agric. Food Chem. 2010, 58, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Guasch-Ferré, M.; Quilez, J.; Merino, J.; Ferré, R.; Díaz-López, A.; Bulló, M.; Hernández-Alonso, P.; Palau-Galindo, A.; Salas-Salvadó, J. Effect of functional bread rich in potassium, γ-aminobutyric acid and angiotensin-converting enzyme inhibitors on blood pressure, glucose metabolism and endothelial function: A double-blind randomized crossover clinical trial. Medicine (Baltimore) 2015, 94, e1807. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q. The long-term effect of life style interventions to prevent diabetes in the China Da Qing diabetes prevention study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef]
- Babiker, A.; Dubayee, M.A. Anti-diabetic medications: How to make a choice? Sudan J. Paediatr. 2017, 17, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, A.; Abdo, S.; Bhattacharjee, A.; Gohil, H.; Pourasgari, F.; Ibeh, N.; Lai, M.; Batchuluun, B.; Wong, A.; Khuu, N.; et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J. 2019, 33, 3968–3984. [Google Scholar] [CrossRef]
- Liu, W.; Son, D.O.; Lau, H.K.; Zhou, Y.; Prud’homme, G.J.; Jin, T.; Wang, Q. Combined oral administration of GABA and DPP-4 inhibitor prevents beta cell damage and promotes beta cell regeneration in mice. Front. Pharmacol. 2017, 8, 362. [Google Scholar] [CrossRef]
- Bansal, P.; Wang, S.; Liu, S.; Xiang, Y.Y.; Lu, W.Y.; Wang, Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells. PLoS ONE 2011, 6, e26225. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.S.; Dizon, M.P.; Yaw, C.K.; Kaufman, D.L. Oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kuo, W.W.; Wang, H.F.; Lin, C.J.; Lin, Y.M.; Chen, J.L.; Kuo, C.H.; Chen, P.K.; Lin, J.Y. GABA tea ameliorates cerebral cortex apoptosis and autophagy in streptozotocin-induced diabetic rats. J. Funct. Foods 2014, 6, 534–544. [Google Scholar] [CrossRef]
- Imam, M.U.; Azmi, N.H.; Bhanger, M.I.; Ismail, N.; Ismail, M. Antidiabetic properties of germinated brown rice: A systematic review. J. Evid. Based Complement. Altern. Med. 2012, 2012, 816501. [Google Scholar]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A mini review on antidiabetic properties of fermented foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Seki, T.; Ariga, T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2004, 68, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Torimitsu, M.; Nagase, R.; Yanagi, M.; Homma, M.; Sasai, Y.; Ito, Y.; Hayamizu, K.; Nonaka, S.; Hosono, T.; Kise, M.; et al. Replacing white rice with pre-germinated brown rice mildly ameliorates hyperglycemia and imbalance of adipocytokine levels in type 2 diabetes model rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2010, 56, 287–292. [Google Scholar] [CrossRef]
- Adamu, H.A.; Imam, M.U.; Ooi, D.J.; Esa, N.M.; Rosli, R.; Ismail, M. Perinatal exposure to germinated brown rice and its gamma amino-butyric acid-rich extract prevents high fat diet-induced insulin resistance in first generation rat offspring. Food Nutr. Res. 2016, 60, 30209. [Google Scholar] [CrossRef]
- Chung, S.I.; Jin, X.; Kang, M.Y. Enhancement of glucose and bone metabolism in ovariectomized rats fed with germinated pigmented rice with giant embryo (Oryza sativa L. cv. Keunnunjami). Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Strappe, P.; Blanchard, C. Wheat bran with enriched gamma-aminobutyric acid attenuates glucose intolerance and hyperinsulinemia induced by a high-fat diet. Food Funct. 2018, 9, 2820–2828. [Google Scholar] [CrossRef]
- Si, X.; Shang, W.; Zhou, Z.; Shui, G.; Lam, S.M.; Blanchard, C.; Strappe, P. Gamma-aminobutyric acid enriched rice bran diet attenuates insulin resistance and balances energy expenditure via modification of gut microbiota and short-chain fatty acids. J. Agric. Food Chem. 2018, 66, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yamamoto, S.; Yoshihara, R.; Yokoyama, J. Postprandial blood glucose and insulin responses to pre-germinated brown rice in healthy subjects. J. Med. Investig. 2005, 52, 159–164. [Google Scholar] [CrossRef]
- Hsu, T.F.; Kise, M.; Wang, M.F.; Ito, Y.; Yang, M.D.; Aoto, H.; Yoshihara, R.; Yokoyama, J.; Kunii, D.; Yamamoto, S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Suzuki, S.; Kobayashi, S.; Fukutomi, T.; Ide, M.; Ohno, T.; Ohkouchi, M.; Taki, M.; Miyamoto, T.; Nimura, T. Effect of pre-germinated brown rice on metabolism of glucose and lipid in patients with diabetes mellitus type 2. J. Jpn. Assoc. Rural Med. 2009, 58, 438–446. [Google Scholar] [CrossRef]
- Tamaya, K.; Matsui, T.; Toshima, A.; Noguchi, M.; Ju, Q.; Miyata, Y.; Tanaka, T.; Tanaka, K. Suppression of blood glucose level by a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves in disaccharide-loaded Sprague-Dawley rats. J. Sci. Food Agric. 2010, 90, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.L.; Nam, Y.D.; Lee, S.H. Anti-diabetic effects of fermented green tea in KK-Ay diabetic mice. Korean J. Food Sci. Technol. 2013, 45, 488–494. [Google Scholar] [CrossRef]
- Yeap, S.K.; Ali, N.M.; Yusof, H.M.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic effects of fermented and nonfermented mung bean extracts on alloxan-induced-diabetic mice. J. Biomed. Biotechnol. 2012, 2012, 285430. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Zhang, C.; Lu, Y.; Zhu, X.; Lu, Z. γ-Aminobutyric acid-rich yogurt fermented by Streptococcus salivarius subsp. thermophiles fmb5 apprars to have anti-diabetic effect on streptozotocin-induced diabetic mice. J. Funct. Foods 2016, 20, 267–275. [Google Scholar] [CrossRef]
- Nam, H.; Jung, H.; Karuppasamy, S.; Park, Y.S.; Cho, Y.S.; Lee, J.Y.; Seong, S.I.; Suh, J.G. Anti-diabetic effect of the soybean extract fermented by Bacillus subtilis MORI in db/db mice. Food Sci. Biotechnol. 2012, 21, 1669–1676. [Google Scholar] [CrossRef]
- Song, K.; Song, I.B.; Gu, H.J.; Na, J.; Kim, S.; Yang, H.S.; Lee, S.C.; Huh, C.; Kwon, J. Anti-diabetic effect of fermented milk containing conjugated linoleic acid on type II diabetes mellitus. Korean J. Food Sci. Anim. Resour. 2016, 36, 170–177. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.H.; Oh, S.H. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J. Med. Food 2004, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Al-Wadei, H.A.; Majidi, M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 2008, 29, 1979–1985. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, C.; Wang, C.; Hu, Y.; Qiu, L.; Xu, P. Neurotransmitter γ-aminobutyric acid-mediated inhibition of the invasive ability of cholangiocarcinoma cells. Oncol. Lett. 2011, 2, 519–523. [Google Scholar] [CrossRef]
- Song, L.; Du, A.; Xiong, Y.; Jiang, J.; Zhang, Y.; Tian, Z.; Yan, H. γ-Aminobutyric acid inhibits the proliferation and increases oxaliplatin sensitivity in human colon cancer cells. Tumour. Biol. 2016, 37, 14885–14894. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Ullah, M.F.; Schuller, H.M. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. PLoS ONE 2012, 7, e43376. [Google Scholar] [CrossRef]
- Tatsuta, M.; Iishi, H.; Baba, M.; Nakaizumi, A.; Ichii, M.; Taniguchi, H. Inhibition by gamma-amino-n-butyric acid and baclofen of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1990, 50, 4931–4934. [Google Scholar]
- Chen, Z.A.; Bao, M.Y.; Xu, Y.F.; Zha, R.P.; Shi, H.B.; Chen, T.Y.; He, X.H. Suppression of human liver cancer cell migration and invasion via the GABAA receptor. Cancer Biol. Med. 2012, 9, 90–98. [Google Scholar] [PubMed]
- Zhang, M.; Gong, Y.; Assy, N.; Minuk, Y. Increased GABAergic activity inhibits a-fetoprotein mRNA expression and the proliferation activity of the HepG2 human hepatocellular carcinoma cell line. J. Hepatol. 2000, 32, 85–91. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, L.; Zeng, X.; Li, Z.; Qin, B.; He, N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: it’s interaction with malondiadehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, W.; Yu, P.; Xi, Z.; Xu, L.; Li, X.; He, N. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale Res. Lett. 2013, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yu, R.; Zhou, Q.; Jiang, S.; Le, G. Protective effects of γ-aminobutyric acid against H2O2-induced oxidative stress in RIN-m5F pancreatic cells. Nutr. Metab. (Lond.) 2018, 15, 60. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Z.; Xie, C.; Gong, W.; Hu, Z.; Peng, Y. A novel mechanism of gamma-aminobutyric acid (GABA) protecting human umbilical vein endothelial cells (HUVECs) against H2O2-induced oxidative injury. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 217, 68–75. [Google Scholar] [CrossRef]
- El-Hady, A.M.A.; Gewefel, H.S.; Badawi, M.A.; Eltahawy, N.A. Gamma-aminobutyric acid ameliorates gamma rays-induced oxidative stress in the small intestine of rats. J. Basic Appl. Zool. 2017, 78, 2. [Google Scholar] [CrossRef]
- Eltahawy, N.A.; Saada, H.N.; Hammad, A.S. Gamma amino butyric acid attenuates brain oxidative damage associated with insulin alteration in streptozotocin-treated rats. Indian J. Clin. Biochem. 2017, 32, 207–213. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Md Zamri, N.D.; Imam, M.U.; Abd Ghafar, S.A.; Ismail, M. Antioxidative effects of germinated brown rice-derived extracts on H2O2-induced oxidative stress in HepG2 cells. Evid. Based Complement. Altern. Med. 2014, 2014, 371907. [Google Scholar] [CrossRef] [PubMed]
- Phuapaiboon, P. Gamma-aminobutyric acid, total anthocyanin content and antioxidant activity of vinegar brewed from germinated pigmented rice. Pakistan J. Nutr. 2017, 16, 109–118. [Google Scholar]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, H.Y.; Lee, H.J.; Shim, I.; Hahm, D.H. Wound healing activity of gamma-aminobutyric acid (GABA) in rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar] [PubMed]
- Prud’homme, G.; Glinka, Y.; Wang, Q. GABA exerts anti-inflammatory and immunosuppressive effects (P5175). J. Immunol. 2013, 190, 68. [Google Scholar]
- Choi, J.I.; Yun, I.H.; Jung, Y.J.; Lee, E.H.; Nam, T.J.; Kim, Y.M. Effects of γ-aminobutyric acid (gaba)-enriched sea tangle Laminaria japonica extract on lipopolysaccharide-induced inflammation in mouse macrophage (RAW 264.7) cells. Fish Aquat. Sci. 2012, 15, 293–297. [Google Scholar] [CrossRef]
- Tuntipopipat, S.; Muangnoi, C.; Thiyajai, P.; Srichamnong, W.; Charoenkiatkul, S.; Praengam, K. A bioaccessible fraction of parboiled germinated brown rice exhibits a higher anti-inflammatory activity than that of brown rice. Food Funct. 2015, 6, 1480–1488. [Google Scholar] [CrossRef]
- Scandolera, A.; Hubert, J.; Humeau, A.; Lambert, C.; De Bizemont, A.; Winkel, C.; Kaouas, A.; Renault, J.H.; Nuzillard, J.M.; Reynaud, R. GABA and GABA-alanine from the red microalgae Rhodosorus marinus exhibit a significant neuro-soothing activity through inhibition of neuro-inflammation mediators and positive regulation of TRPV1-related skin sensitization. Mar. Drugs 2018, 16, 96. [Google Scholar] [CrossRef]
- Sokovic Bajic, S.; Djokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Mihajlovic, S.; Tolinacki, M. GABA-producing natural dairy isolate from artisanal zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Front. Microbiol. 2019, 10, 527. [Google Scholar] [CrossRef]
- Mau, J.L.; Chiou, S.Y.; Hsu, C.A.; Tsai, H.L.; Lin, S.D. Antimutagenic and antimicrobial activities of γ-aminobutyric acid (Gaba) tea extract. Int. Conf. Nutr. Food Sci. 2012, 39, 178–182. [Google Scholar]
- Dagorn, A.; Hillion, M.; Chapalain, A.; Lesouhaitier, O.; Duclairoir Poc, C.; Vieillard, J.; Chevalier, S.; Taupin, L.; Le Derf, F.; Feuilloley, M.G. Gamma-aminobutyric acid acts as a specific virulence regulator in Pseudomonas aeruginosa. Microbiology 2013, 159, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Lee, H.M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.H.; Min, J.J.; Sasai, M.; Jeong, J.H.; et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 2018, 9, 4184. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Hara, T.; Honma, K.; Joh, T. Suppressive effect of γ-aminobutyric acid (GABA) on histamine release in rat basophilic RBL-2H3 cells. Bull. Fac. Agric. Niigata Univ. 2008, 61, 47–51. [Google Scholar]
- Kawasaki, A.; Hara, T.; Joh, T. Inhibitory effect of γ-aminobutyric acid (GABA) on histamine release from rat basophilic leukemia RBL-2H3 cells and rat peritoneal exudate cells. Nippon Shokuhin Kagaku Kogaku Kaishi 2014, 61, 362–366. [Google Scholar] [CrossRef]
- Hokazono, H.; Omori, T.; Ono, K. Effects of single and combined administration of fermented barley extract and γ -aminobutyric acid on the development of atopic dermatitis in NC/Nga mice. Biosci. Biotechnol. Biochem. 2010, 74, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E. Molecular aspects of alcohol metabolism: Transcription factors involved in early ethanol-induced liver injury. Annu. Rev. Nutr. 2004, 24, 55–78. [Google Scholar] [CrossRef]
- Oh, S.H.; Soh, J.R.; Cha, Y.S. Germinated brown rice extract shows a nutraceutical effect in the recovery of chronic alcohol-related symptoms. J. Med. Food 2003, 6, 115–121. [Google Scholar] [CrossRef]
- Lee, B.J.; Senevirathne, M.; Kim, J.S.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Kang, Y.M.; Kim, J.I.; Nam, B.H.; Ahn, C.B.; et al. Protective effect of fermented sea tangle against ethanol and carbon tetrachloride-induced hepatic damage in Sprague-Dawley rats. Food Chem. Toxicol. 2010, 48, 1123–1128. [Google Scholar] [CrossRef]
- Cha, J.Y.; Lee, B.J.; Je, J.Y.; Kang, Y.M.; Kim, Y.M.; Cho, Y.S. GABA-enriched fermented Laminaria japonica protects against alcoholic hepatotoxicity in Sprague-Dawley rats. Fish Aquat. Sci. 2011, 14, 79–88. [Google Scholar] [CrossRef]
- Kang, Y.M.; Qian, Z.J.; Lee, B.J.; Kim, Y.M. Protective effect of GABA-enriched fermented sea tangle against ethanol-induced cytotoxicity in HepG2 Cells. Biotechnol. Bioprocess E 2011, 16, 966. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Kim, H.Y.; Yokozawa, T.; Nakagawa, T.; Sasaki, S. Protective effect of gamma-aminobutyric acid against glycerol-induced acute renal failure in rats. Food Chem. Toxicol. 2004, 42, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yokozawa, T.; Cho, E.J.; Oowada, S.; Kim, M. Protective role of gamma-aminobutyric acid against chronic renal failure in rats. J. Pharm. Pharmacol. 2006, 58, 1515–1525. [Google Scholar] [CrossRef]
- Sasaki, S.; Tohda, C.; Kim, M.; Yokozawa, T. Gamma-aminobutyric acid specifically inhibits progression of tubular fibrosis and atrophy in nephrectomized rats. Biol. Pharm. Bull. 2007, 30, 687–691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, B.H.; Al-Salam, S.; Al Za’abi, M.; Al Balushi, K.A.; AlMahruqi, A.S.; Beegam, S.; Al-Lawatia, I.; Waly, M.I.; Nemmar, A. Renoprotective effects of gamma-aminobutyric acid on cisplatin-induced acute renal injury in rats. Basic Clin. Pharmacol. Toxicol. 2015, 116, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Talebi, N.; Nematbakhsh, M.; Monajemi, R.; Mazaheri, S.; Talebi, A.; Vafapour, M. The protective effect of γ-aminobutyric acid on kidney injury induced by renal ischemia-reperfusion in ovariectomized estradiol-treated rats. Int. J. Prev. Med. 2016, 7, 6. [Google Scholar]
- Saada, H.N.; Eltahawy, N.A.; Hammad, A.S.; Morcos, N.Y.S. Gamma amino butyric acid attenuates liver and kidney damage associated with insulin alteration in γ-irradiated and streptozotocin-treated rats. Arab. J. Nucl. Sci. Appl. 2016, 49, 138–150. [Google Scholar]
- Vafapour, M.; Nematbakhsh, M.; Monajemi, R.; Mazaheri, S.; Talebi, A.; Talebi, N.; Shirdavani, S. Effect of γ-aminobutyric acid on kidney injury induced by renal ischemia-reperfusion in male and female rats: Gender-related difference. Adv. Biomed. Res. 2015, 4, 158. [Google Scholar]
- Chen, Z.; Xie, J.; Wang, B.; Tang, J. Effect of γ-aminobutyric acid on digestive enzymes, absorption function, and immune function of intestinal mucosa in heat-stressed chicken. Poult. Sci. 2014, 93, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, J.; Hu, M.Y.; Tang, J.; Shao, Z.F.; Li, M.H. Protective effects of γ-aminobutyric acid (gaba) on the small intestinal mucosa in heat-stressed wenchang chicken. J. Anim. Plant Sci. 2015, 25, 78–87. [Google Scholar]
- Chen, S.; Tan, B.; Xia, Y.; Liao, S.; Wang, M.; Yin, J.; Wang, J.; Xiao, H.; Qi, M.; Bin, P.; et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct. 2019, 10, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, H.H.; Nie, S.P.; Yin, J.Y.; Xie, M.Y. Gamma-aminobutyric acid increases the production of short-chain fatty acids and decreases pH values in mouse colon. Molecules 2017, 22, 653. [Google Scholar]
- Kubota, A.; Kobayashi, M.; Sarashina, S.; Takeno, R.; Yasuda, G.; Narumi, K.; Furugen, A.; Takahashi-Suzuki, N.; Iseki, K. Gamma-aminobutyric acid (GABA) attenuates ischemia reperfusion-induced alterations in intestinal immunity. Biol. Pharm. Bull. 2018, 41, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yue, Y.; Li, F. Improvement of gamma-aminobutyric acid on intestinal mucosalbarrier injury of colitis induced by 2,4,6-trinitrobenzene sulfonic acid and alcohol. Herald Med. 2018, 37, 931–938. [Google Scholar]
- Yang, Y.; Lian, Y.T.; Huang, S.Y.; Yang, Y.; Cheng, L.X.; Liu, K. GABA and topiramate inhibit the formation of human macrophage-derived foam cells by modulating cholesterol-metabolism-associated molecules. Cell. Physiol. Biochem. 2014, 33, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xing, R.; Liu, S.; Yu, H.; Li, P. Analysis of the protective effects of γ-aminobutyric acid during fluoride-induced hypothyroidism in male Kunming mice. Pharm. Biol. 2019, 57, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Borst, S.E.; McCoy, S.C.; Conway, R.; Yarrow, J.F. The effects of gamma aminobutyric acid on growth hormone secretion at rest and following exercise. Med. Sci. Sports Exerc. 2003, 35, S271. [Google Scholar] [CrossRef]
- Tujioka, K.; Okuyama, S.; Yokogoshi, H.; Fukaya, Y.; Hayase, K.; Horie, K.; Kim, M. Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in young rats. Amino Acids 2007, 32, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tujioka, K.; Ohsumi, M.; Horie, K.; Kim, M.; Hayase, K.; Yokogoshi, H. Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in ovariectomized female rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, M.; Nakamura, U.; Horie, N.; Yokoyama, Y.; Kim, M.; Fujita, S. Oral supplementation using gamma-aminobutyric acid and whey protein improves whole body fat-free mass in men after resistance training. J. Clin. Med. Res. 2019, 11, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food. Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
| STT | Source | Dose, Model | Time of Treatment/Administration | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | Kimchi-derived Lactobacillus buchneri | 100 µg/mL, neuronal cells | 24 h | Preventing neurotoxic-induced cell death | [13] |
| 2 | Lactobacillus plantarum-fermented chickpea milk | 537.23 mg/L, PC12 cells | 30 min | Preventing MnCl2-induced injury | [14] |
| 3 | Gaba receptor agonist | Muscimol (1 mg/kg) and baclofen (20 mg/kg), rat | 30 min | Preventing brain ischemic injury and decreasing apoptosis | [15,17] |
| 4 | Gaba receptor agonist | Baclofen (10 mL/kg), rat | Once daily/five weeks | Alleviating neuronal damage and suppressing cytodestructive autophagy | [16] |
| STT | Source | Dose/Model | Time of Treatment/Administration | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | Gaba-enriched rice germ | 26.4 mg/3 times/day, patient | N/A | Improving sleeplessness, somnipathy, and depression | [19] |
| 2 | Gaba-rich Monascus-fermented product | 2.6 mg/kg, rat | 30 days | Preventing depression | [20] |
| 3 | Gaba powder from natural fermentation using lactic acid bacteria | 100 mg Gaba/day, Japanese volunteers | 1 week | Prevention of sleep disorder | [21] |
| 4 | Gaba (90.8%) and l-theanine (99.3%) was supplied by Neo Cremar Co. Ltd. (Seoul, Korea) and BTC Co. Ltd. (Ansan, Korea), respectively | Gaba/L-theanine mixture (100/20 mg/kg)/day, mice and rat | 9 days | Decreasing sleep latency and increasing sleep duration | [22] |
| 5 | Gaba from natural fermentation using lactic acid bacteria (Pharma-GABA, Pharma Foods International Co., Japan) | Gaba/L-theanine mixture (100/200 mg/kg)/day Japanese volunteers | 7 days | Increasing relaxation, diminishing anxiety, and enhancing immunity | [23] |
| 6 | Gaba-enriched product fermented by kimchi-derived lactic acid bacteria | 46.69 mg/mL Gaba, mice and PC-12 cells | 24 h | Improving long-term memory loss and increasing neuronal cell proliferation | [24] |
| 7 | Gaba-enriched fermented Laminaria japonica product | 1.5 g/day, volunteers | 6 weeks | Preventing cognitive impairment in the elderly | [25] |
| STT | Source | Dose/Model | Time of Treatment/Administration | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | Milk fermented by Lactococcus lactis DIBCA2 and Lactobacillus plantarum PU11 | 0.70 mg/ml | 5 min | Inhibiting 50% ACE activity | [30] |
| 2 | Gaba form LAB-fermented soybean | 1.3 mg Gaba/g soybean | 10 min | Inhibiting 43% ACE activity | [33] |
| 3 | Gaba from the fermented lentils | 10.42 mg Gaba/g extract | 60 min | Inhibiting 92% ACE activity | [34] |
| 4 | Gaba from Wako Pure Chemicals (Tokyo) | 0.3 to 300 mg Gaba/kg, rat | Every 20 min for i.v. administration | Decreasing blood pressure | [35] |
| 5 | Gaba from skim cows’ milk fermented with Lactobacillus casei strain Shirota and Lactococcus lactis YIT 2027 | 5 mL (102 mg Gaba/kg) of the fermented solution/kg body weight, rat | 10 h | Lowering blood pressure | [36] |
| 6 | Gaba-enriched rice grains | 0.1 mg–0.5 mg Gaba/kg, rat | 6 weeks | Decreasing blood pressure | [38] |
| 7 | Gaba-enriched white rice | 150 g of Gaba-enriched white rice (11.2 mg Gaba/100 g rice), volunteers | 8 weeks | Decreasing blood pressure | [41] |
| 8 | Gaba-enriched Chingshey purple sweet potato-fermented milk by lactic acid bacteria | 2.5-mL dose of fermented-milk, rat | 8 weeks | Reducing both systolic blood pressure and diastolic blood pressure | [42] |
| 9 | Gaba from probiotic-fermented purple sweet potato yogurt | 1500 µg/2.5 mL/kg, rat | 8 weeks | Alleviating cardiac hypertrophy | [43] |
| 10 | Gaba-rich tomato | 2–10 g/kg, rat | 2–24 h | Decreasing blood pressure | [46] |
| 11 | Gaba-rich bread | 120 g/day, patient | 3 days | Decreasing blood pressure | [47] |
| STT | Source | Dose/Model | Time of Treatment/Administration | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | Gaba (Source: N/A) | Dose: N/A, mice | 8–15 weeks | Activating PI3-K/Akt-dependent growth and survival pathways and restoring the β-cell mass | [51] |
| 2 | Gaba (MilliporeSigma, Burlington, MA, USA) | Gaba (6 mg/mL/day), mice | 10 weeks | Up-regulating β-cell proliferation and rising a distinct subpopulation of β cells | [52] |
| 3 | Gaba (Sigma, St. Louis, USA) | Gaba (2 mg/mL/day), mice | 20 weeks | Reducing the concentrations of fasting blood glucose, improving glucose tolerance and insulin sensitivity, and inhibiting the body weight gain | [55] |
| 4 | Gaba from pre-germinated brown rice | Pre-germinated brown rice (1387–1546 g/day), rat | 7 weeks | Decreasing blood glucose, adipocytokine PAI-1 concentration, and plasma lipid peroxide | [59] |
| 5 | Gaba from germinated brown rice | Gaba (200 mg/kg/day), rat offspring | 8 weeks | Increasing adiponectin levels and reducing insulin resistance and oxidative stress | [61] |
| 6 | Gaba from blackish purple pigmented rice with a giant embryo | Diet supplemented with either 20% (w/w) germinated Keunnunjami rice powder, rat | 8 weeks | Decreasing blood glucose and plasma insulin levels, adipokine concentrations, and hepatic glucose-regulating enzyme activities | [62] |
| 7 | Gaba-enriched wheat bran | 15% Gaba-enriched bran, rat | 8 weeks | Improving glucose homeostasis | [63] |
| 8 | Gaba from pre-germinated brown rice | The test sample contained 50 g of available carbohydrate per day for each volunteer (185 g of pre-germinated brown rice), volunteers | 7 weeks | Lowering postprandial blood glucose concentration without insulin secretion increase | [65] |
| 9 | Gaba from pre-germinated brown rice | 180 g of the cooked rice/three times per day, patient | 14 weeks | Decreasing blood glucose and hypercholesterolemia | [66] |
| 10 | Fermented tea product | 50 mg/kg, rat | 120 min | Decreasing blood glucose level | [68] |
| 11 | Mung bean fermented by Rhizopus sp. | 200 mg/kg and 1000 mg/kg, mice | 240 min | Reducing blood glucose, HbA1c, cholesterol, triglyceride, and low-density lipoprotein levels | [70] |
| 12 | Yogurt fermented by Streptococcus salivarius subsp. | Gaba orally at a dose of 2 g/L or 4 g/L | 6 weeks | Reducing blood glucose, HbA1c, cholesterol, triglyceride, and low-density lipoprotein levels | [71] |
| 13 | Soybean extract fermented by Bacillus subtilis MORI | 500 mg/kg, mice | 8 weeks | Reducing blood glucose, HbA1c, cholesterol, triglyceride, and low-density lipoprotein levels | [72] |
| 14 | Milk fermented by strain YF-L812 (S. thermophilus, L. delbrueckii subsp. bulgaricus), standard strains. B. breve KCTC 3419, and L. sakei LJ011. FM | Fermented milk 0.2% and 0.6%/kg/day, mice | 6 weeks | Decreasing fasting blood glucose, serum insulin, insulin tolerance, total cholesterol, triglycerides, and LDL cholesterol | [73] |
| STT | Source | Dose/Model | Time of Treatment/Administration | Effect | Ref. |
|---|---|---|---|---|---|
| 1 | Gaba-enriched brown rice extract | 20 µL extract/well (1 × 105 cells/200 mL/well), leukemia cells and HeLa cells | 48 h | Retarding the proliferation rates of leukemia cells and enhancing apoptosis of leukemia cells | [77] |
| 2 | Gaba from Sigma Company (St. Louis, MO, USA) | Gaba (1–1000 µmol/L), cholangiocarcinoma QBC939 cells | 24 h | Inhibiting the activity and expression of MMP-2 and MMP-9 | [79] |
| 3 | Gaba was purchased from Sigma-Aldrich, Shanghai, China | Gaba (100 µM), Colon cancer cells | 72 h | Inhibiting on cell proliferation and metastasis | [80] |
| 4 | Gaba from Sigma Company (St. Louis, MO, USA) | Gaba (1000 mg/kg), rat | 25 weeks | Decreasing the number of gastric cancers of the glandular stomach | [82] |
| 5 | Gaba from Sigma Company (St. Louis, MO, USA) | Gaba (10 µM), Human liver cancer cells | 24 h | Reducing intrahepatic liver metastasis and inhibiting human liver cancer cell migration and invasion | [83] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. https://doi.org/10.3390/molecules24152678
Ngo D-H, Vo TS. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules. 2019; 24(15):2678. https://doi.org/10.3390/molecules24152678
Chicago/Turabian StyleNgo, Dai-Hung, and Thanh Sang Vo. 2019. "An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid" Molecules 24, no. 15: 2678. https://doi.org/10.3390/molecules24152678
APA StyleNgo, D.-H., & Vo, T. S. (2019). An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules, 24(15), 2678. https://doi.org/10.3390/molecules24152678





