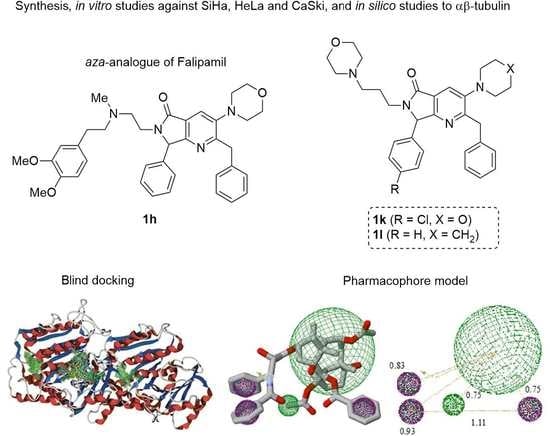
Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines
Abstract
1. Introduction
2. Results and Discussion
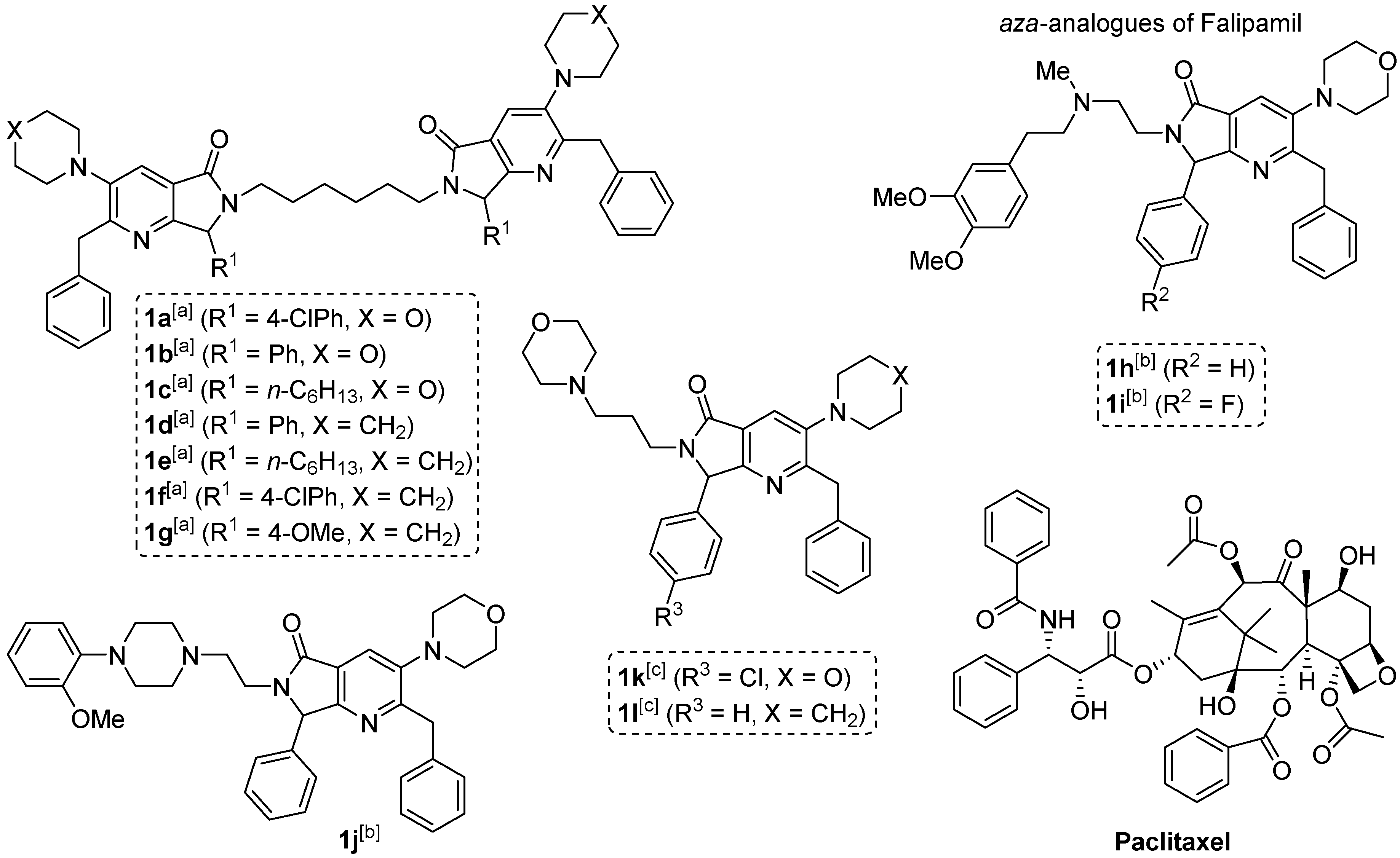
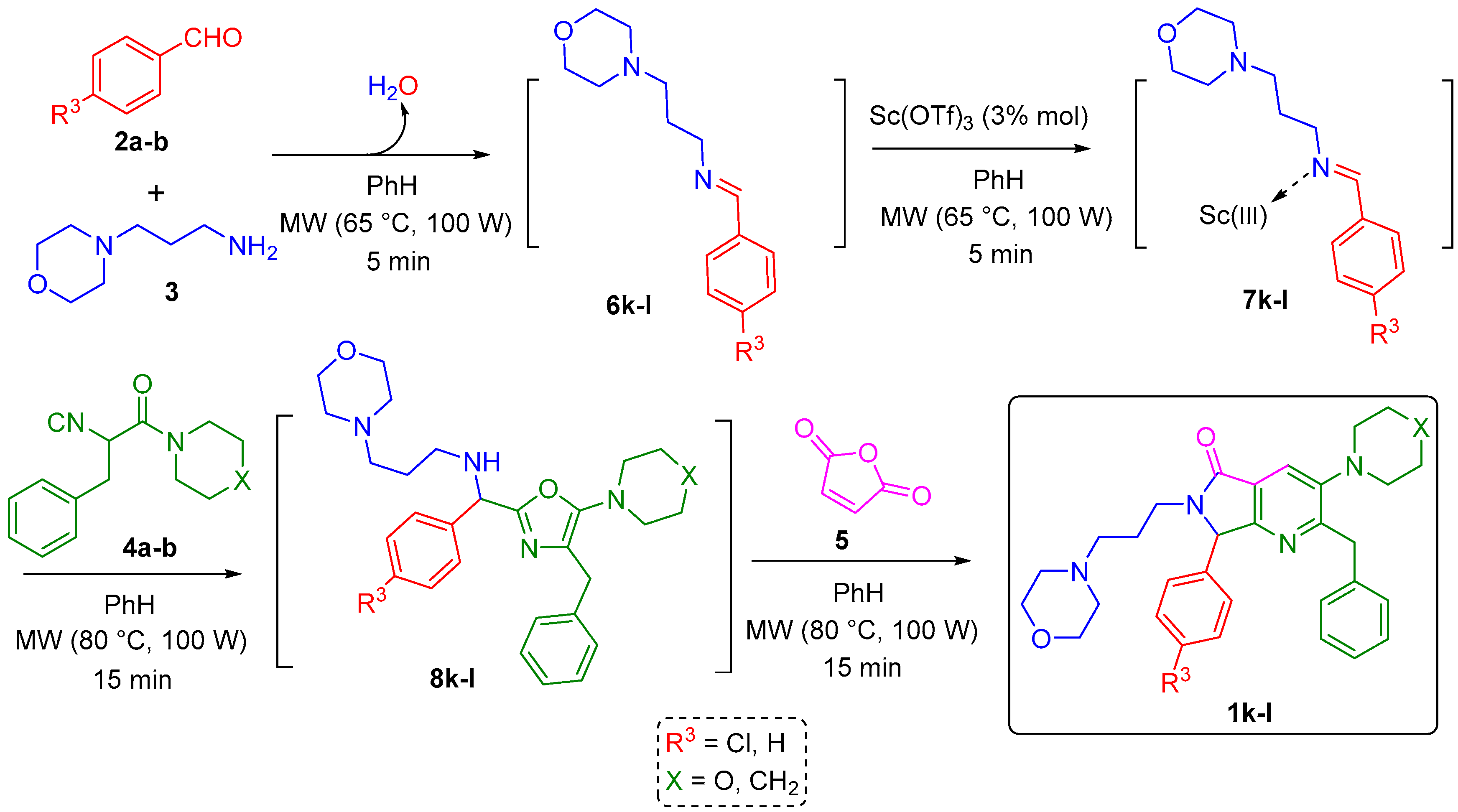
2.1. Synthesis
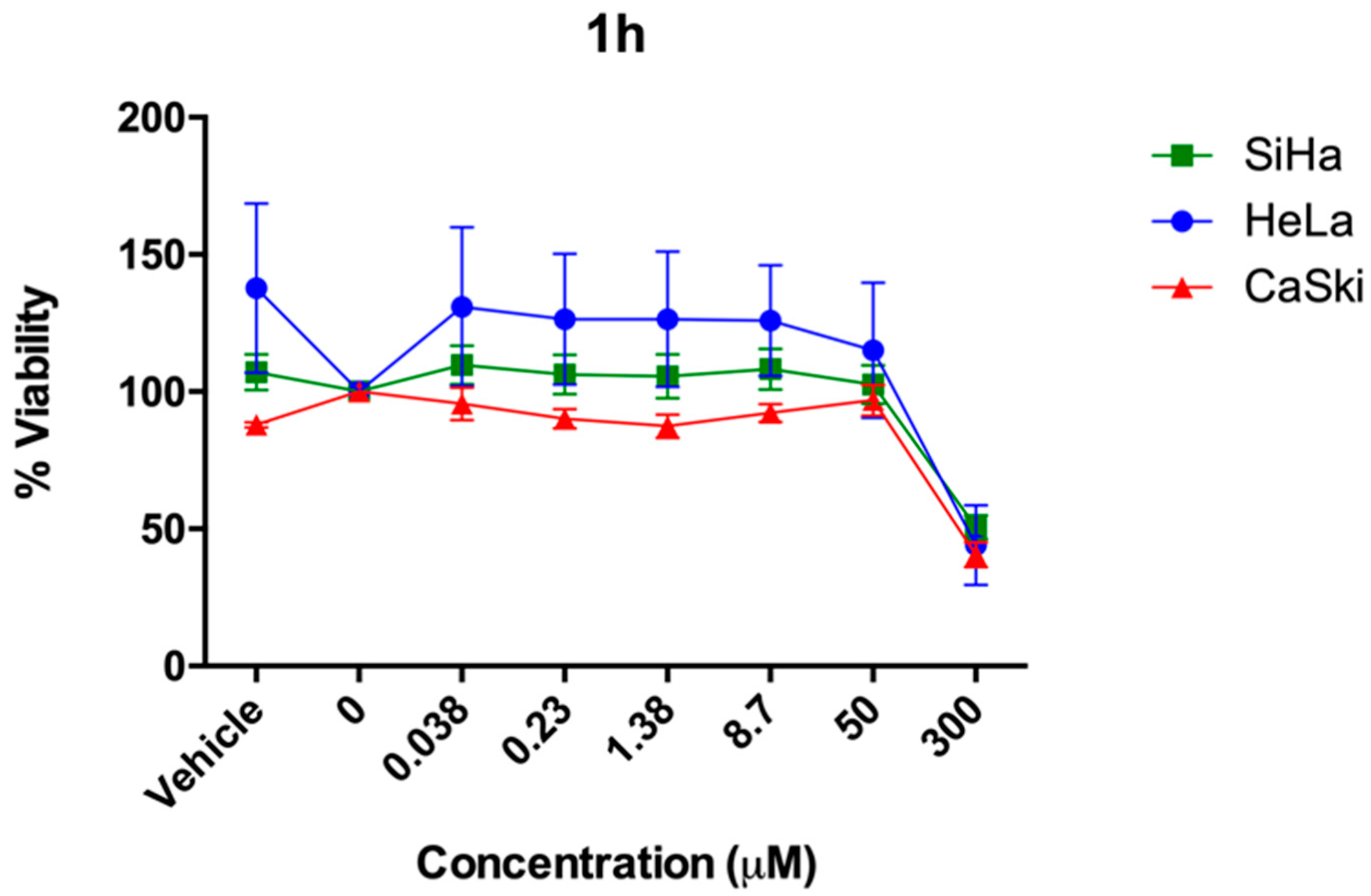
2.2. In Vitro Studies
2.3. In Silico Studies
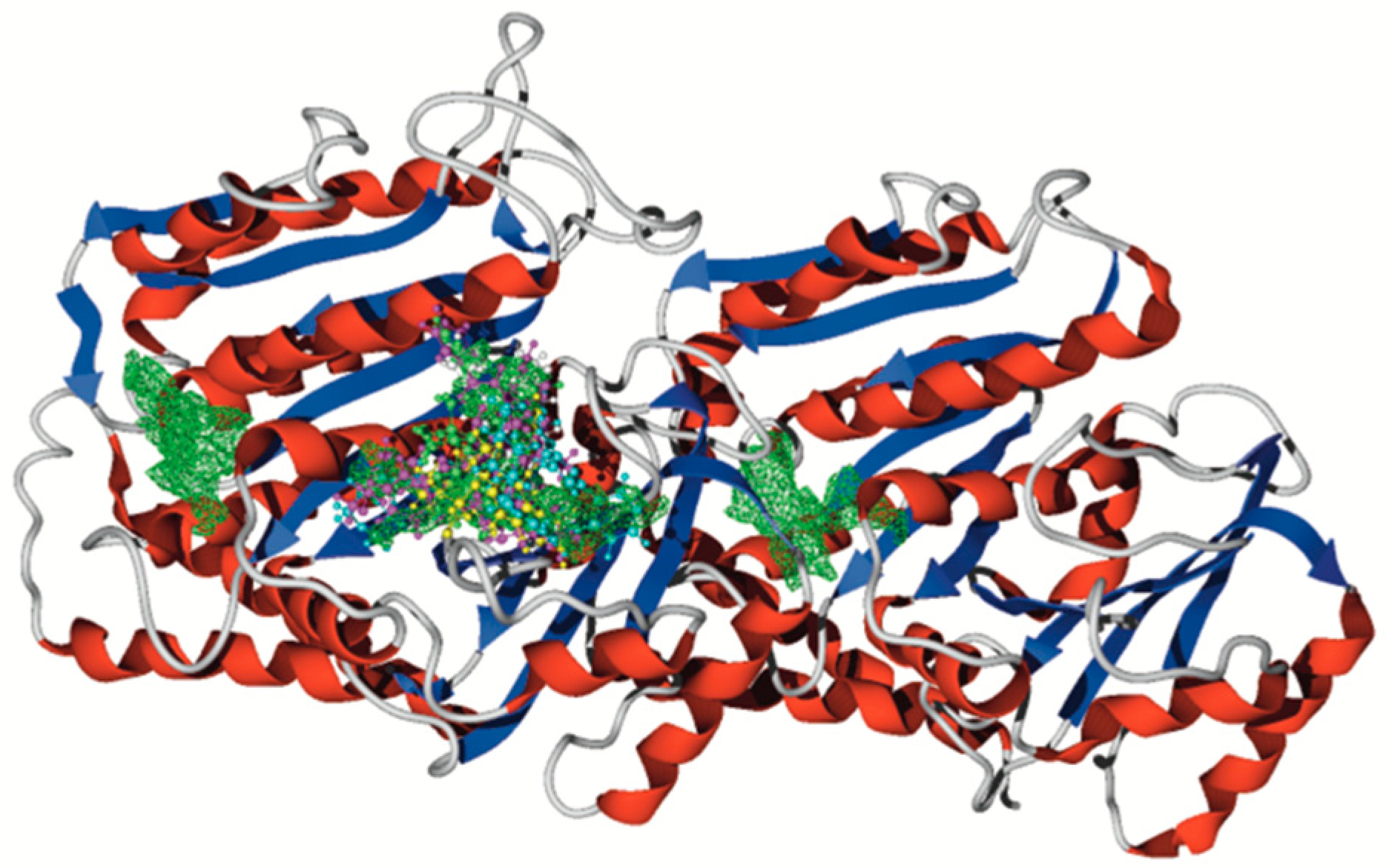
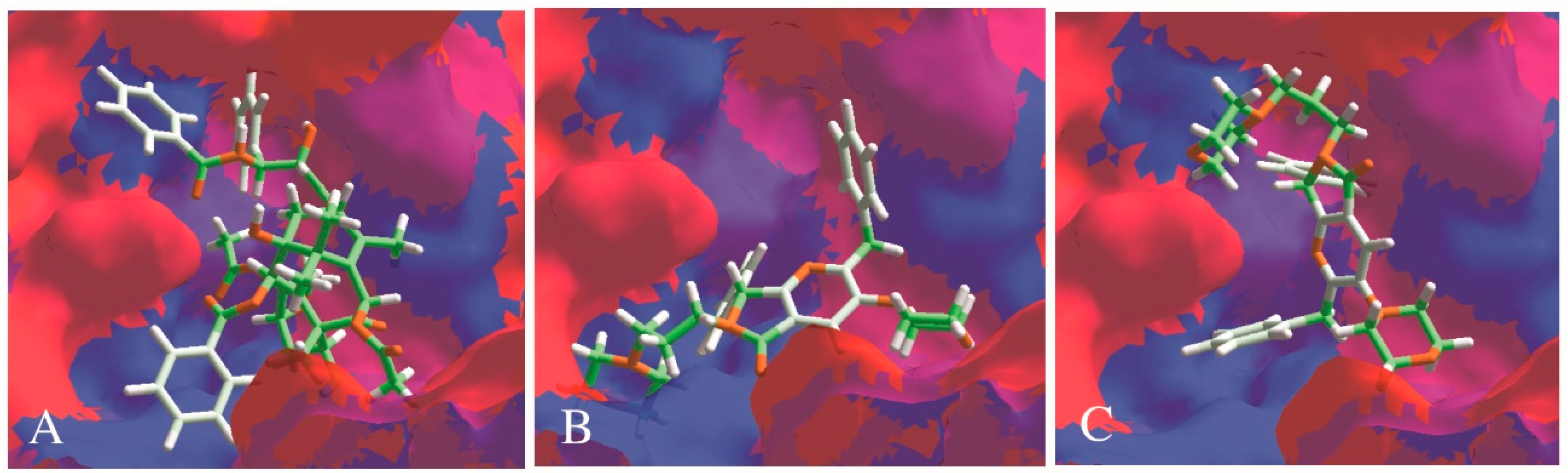
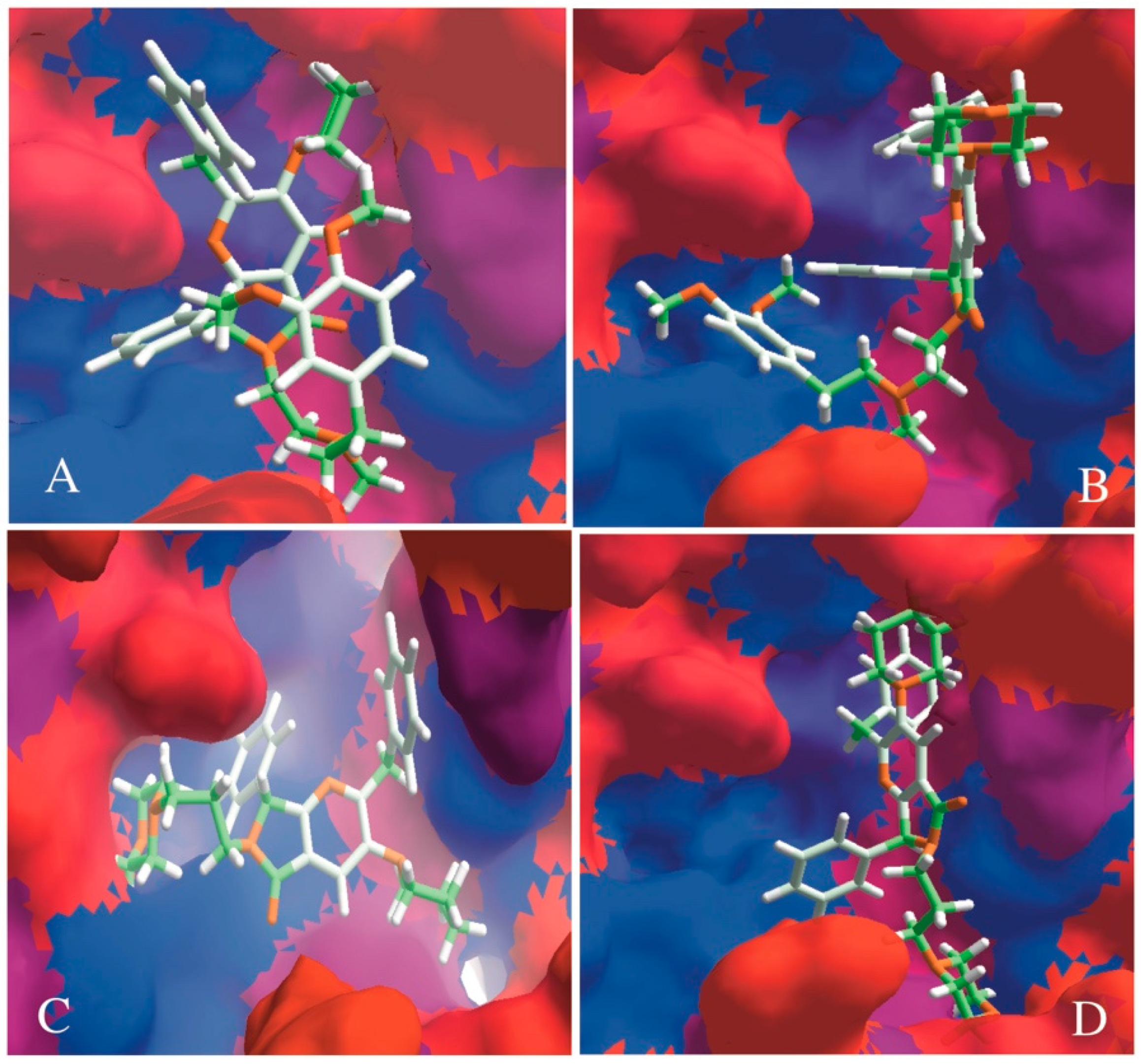
2.3.1. Docking Assays
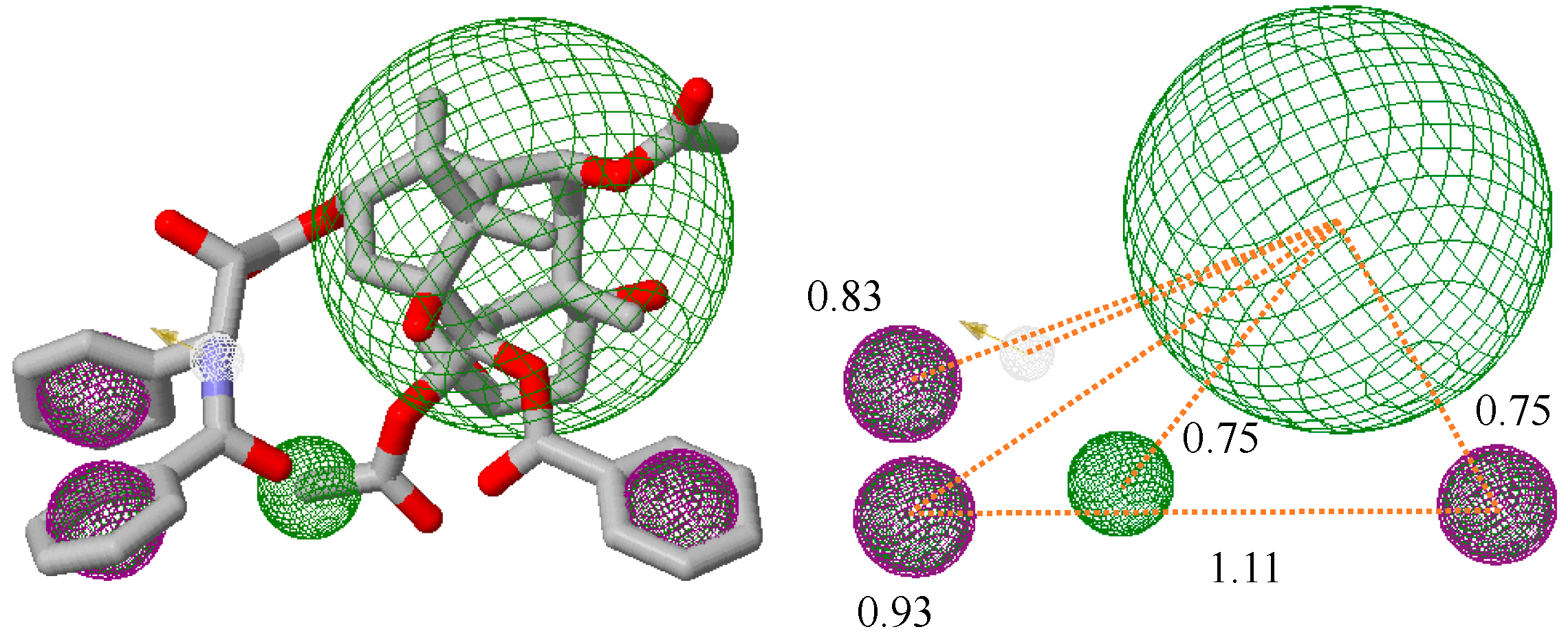
2.3.2. QSAR and Pharmacophoric Modeling
Q2loop = 95.99; R2 = 0.991
Q2loop = 97.88; R2 = 0.996
Q2loop = 71.41; R2 = 0.983
3. Experimental Part
3.1. Synthesis
3.1.1. General Information, Instrumentation, Software, and Chemicals
3.1.2. Synthesis and Characterization of the Pyrrolo[3,4-b]pyridin-5-ones 1k–l
General Procedure (GP)
2-Benzyl-7-(4-chlorophenyl)-3-morpholino-6-(3-morpholinopropyl)-6,7-dihydro-5H-pyrrolo [3,4-b]pyridin-5-one (1k)
2-Benzyl-6-(3-morpholinopropyl)-7-phenyl-3-(piperidin-1-yl)-6,7-dihydro-5H-pyrrolo[3,4-b] pyridin-5-one (1l)
3.2. In Vitro Studies
3.3. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sikora, K.; Timbs, O. Cancer 2025: Introduction. Expert Rev. Anticancer Ther. 2004, 4, 11–12. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Canc. Res. 2011, 30, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Naresh, R.; Nazeer, Y.; Palani, S. In silico evaluation of modes of action of anticancer compounds on molecular targets for cancer. Med. Chem. Res. 2013, 22, 1938–1947. [Google Scholar] [CrossRef]
- Zarchi, M.K.; Behtash, N.; Chiti, Z.; Kargar, S. Cervical Cancer and HPV Vaccines in Developing Countries. Asian Pac. J. Cancer Prev. 2009, 10, 969–974. [Google Scholar]
- Andrei, G.; Snoeck, R.; Piette, J.; Delvenne, P.; De Clercq, E. Inhibiting Effects of Cidofovir (HPMPC) on the Growth of the Human Cervical Carcinoma (SiHa) Xenografts in Athymic Nude Mice. Oncol. Res. 1998, 10, 533–539. [Google Scholar] [PubMed]
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef]
- Cui, L.; Song, J.; Wu, L.; Cheng, L.; Chen, A.; Wang, Y.; Huang, Y.; Huang, L. Role of Annexin A2 in the EGF-induced epithelial-mesenchymal transition in human CaSki cells. Oncol. Lett. 2017, 13, 377–383. [Google Scholar] [CrossRef]
- Rupavani, B.; Peesapati, V.; Naidu, V.G.M.; Ramakrishna, S. Synthesis of some new physiologically active polyheterocycles derived from benzocycloheptene-5-ones. Indian J. Chem. 2016, 55B, 88–93. [Google Scholar]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discovery Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Keating, T.A.; Armstrong, R.W. Molecular Diversity via a Convertible Isocyanide in the Ugi Four-Component Condensation. J. Am. Chem. Soc. 1995, 117, 7842–7843. [Google Scholar] [CrossRef]
- Botes, M.G.; Pelly, S.C.; Blackie, M.A.L.; Kornienko, A.; Otterlo, W.A.L. Synthesis of 4-azapodophyllotoxins with Anticancer Activity by Multicomponent Reactions (Review). Chem. Heterocycl. Com. 2014, 50, 119–138. [Google Scholar] [CrossRef]
- Saquib, M.; Khan, M.F.; Ansari, M.I.; Khan, I.; Hussain, M.K.; Singh, J. Multicomponent Reactions for Generation of Molecular Libraries in Anticancer Drug Discovery. In Multicomponent Reactions: Synthesis of Bioactive Heterocycles; Ameta, K.L., Dandia, A., Eds.; CRS Press: Boca Raton, FL, USA, 2017; pp. 117–137. [Google Scholar]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. α-Tubulin Acetylation Elevated in Metastatic and Basal-like Breast Cancer Cells Promotes Microtentacle Formation, Adhesion and Invasive Migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Alushin, G.M.; Lander, G.C.; Kellogg, E.H.; Zhang, R.; Baker, D.; Nogales, E. High-Resolution Microtubule Structures Reveal the Structural Transitions in α β-Tubulin upon GTP Hydrolysis. Cell 2014, 157, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The Novel Microtubule-Destabilizing Drug BAL27862 Binds to the Colchicine Site of Tubulin with Distinct Effects on Microtubule Organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; García-Revilla, M.A.; Robles, J.; Aguilera-Granja, F. Solubility of functionalized single-wall carbon nanotubes in water: A theoretical study. Theor. Chem. Acc. 2017, 136, 127. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; Islas-Jácome, A.; Rentería-Gómez, A.; Robles, J.; Gámez-Montaño, R. In vitro and in silico evaluation of twelve newly-synthesized 1-acetamide-5-methoxy-2-oxindoles as 5-Ht7 receptor ligands. Bioorg. Med. Chem. Lett. 2015, 25, 1580–1585. [Google Scholar]
- Basavanag, U.M.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Conejo, A.S.; Kurva, M.; Jiménez-Halla, J.O.C.; Velusamy, J.; Ramos-Ortiz, G.; Gámez-Montaño, R. Synthesis of 2-julolidin-imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé reaction and studies of optical properties. New J. Chem. 2017, 41, 3450–3459. [Google Scholar] [CrossRef]
- Zamudio-Medina, A.; García-González, A.N.; Herrera-Carrillo, G.K.; Zárate-Zárate, D.; Benavides-Macías, A.; Tamariz, J.; Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of Polyheterocyclic Pyrrolo[3,4-b]pyridin-5-ones via a One-Pot (Ugi-3CR/aza Diels-Alder/N-acylation/aromatization/SN2) Process. A Suitable Alternative towards Novel Aza-Analogues of Falipamil. Molecules 2018, 23, 763. [Google Scholar] [CrossRef] [PubMed]
- Islas-Jácome, A.; Rentería-Gómez, A.; Rentería-Gómez, M.A.; González-Zamora, E.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Selective reaction route in the construction of the pyrrolo[3,4-b] pyridin-5-one core from a variety of 5-aminooxazoles and maleic anhydride. A DFT study. Tetrahedron Lett. 2016, 57, 3496–3500. [Google Scholar] [CrossRef]
- Speck, K.; Magauer, T. The chemistry of isoindole natural products. Beilstein J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, M.; Salahinejad, M.; Zarezadeh, N.; Ghandi, M.; Baghery, M.K. Antitumor evaluation and 3D-QSAR studies of a new series of the spiropyrroloquinoline isoindolinone/aza-isoindolinone derivatives by comparative molecular field analysis (CoMFA). Mol. Divers. 2017, 21, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Devasthale, P.; Wang, Y.; Wang, W.; Fevig, J.; Feng, J.X.; Wang, A.; Harrity, T.; Egan, D.; Morgan, N.; Cap, M.; et al. Optimization of Activity, Selectivity, and Liability Profiles in 5-Oxopyrrolopyridine DPP4 Inhibitors Leading to Clinical Candidate (Sa)-2-(3-(Aminomethyl)-4-(2,4-dichlorophenyl)-2-methyl-5-oxo-5H-pyrrolo[3,4-b]pyridin-6(7H)-yl) N,N-dimethylacetamide (BMS-767778). Bioorg. Med. Chem. 2013, 56, 7343–7357. [Google Scholar]
- Sun, X.; Janvier, P.; Zhao, G.; Bienaymé, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and Its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef]
- Zamudio-Medina, A.; García-González, M.C.; Padilla, J.; González-Zamora, E. Synthesis of a tetracyclic lactam system of Nuevamine by four-component reaction and free radical cyclization. Tetrahedron Lett. 2010, 51, 4837–4839. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; González-Zamora, E.; Gámez-Montaño, R. A short microwave-assisted synthesis of tetrahydroisoquinolinpyrrolopyridinones by a triple process: Ugi-3CR–aza Diels–Alder/S-oxidation/Pummerer. Tetrahedron Lett. 2011, 52, 5245–5248. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Cárdenas-Galindo, L.E.; Jerezano, A.V.; Tamariz, J.; González-Zamora, E.; Gámez-Montaño, R. Synthesis of Nuevamine Aza-Analogues by a Sequence: I-MCR – Aza-Diels – Alder – Pictet–Spengler. Synlett 2012, 23, 2951–2956. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Gutierrez-Carrillo, A.; García-Garibay, M.A.; González-Zamora, E. One-Pot Synthesis of Nuevamine Aza-Analogues by Combined Use of an Oxidative Ugi Type Reaction and Aza-Diels–Alder Cycloaddition. Synlett 2014, 25, 403–406. [Google Scholar] [CrossRef]
- Zamudio-Medina, A.; García-González, M.C.; Gutierrez-Carrillo, A.; González-Zamora, E. Synthesis of cyclic analogues of hexamethylenebis(3-pyridine)amide (HMBPA) in a one-pot process. Tetrahedron Lett. 2015, 56, 627–629. [Google Scholar] [CrossRef]
- Vázquez-Vera, O.; Sánchez-Badillo, J.S.; Islas-Jácome, A.; Rentería-Gómez, M.A.; Pharande, S.G.; Cortes-García, C.J.; Rincón-Guevara, M.A.; Ibarra, I.A.; Gámez-Montaño, R.; González-Zamora, E. An efficient Ugi-3CR/aza Diels–Alder/Pomeranz–Fritsch protocol towards novel aza-analogues of (±)-nuevamine, (±)-lennoxamine and magallanesine: A diversity oriented synthesis approach. Org. Biomol. Chem. 2017, 15, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, M.A.; Pharande, S.G.; Islas-Jácome, A.; González-Zamora, E.; Gámez-Montaño, R. MW-Assisted Synthesis of Eight New 6-Nitrilmethyl-Pyrrolo[3,4-b]pyridin-5-Ones via a Domino Process: aza Diels–Alder/N-Acylation/Aromatization. Proceedings 2019, 9, 5. [Google Scholar] [CrossRef]
- Schwock, J.; Ho, J.C.; Luther, E.; Hedley, D.W.; Geddie, W.R. Measurement of signaling pathway activities in solid tumor fine-needle biopsies by slide-based cytometry. Diagn. Mol. Pathol. 2007, 16, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Yashar, C.; Taylor, D.D.; Gercel-Taylor, C. Cellular response to chemotherapy and radiation in cervical cancer. Am. J. Obstet. Gynecol. 2005, 192, 1399–1403. [Google Scholar] [CrossRef]
- Filippova, M.; Filippov, V.; Kagoda, A.M.; Garnett, T.; Fodor, N.; Duerksen-Hughes, P.J. Complexes of human papillomavirus type 16 E6 proteins form pseudo-death-inducing signaling complex structures during tumor necrosis factormediated apoptosis. J. Virol. 2009, 83, 210–227. [Google Scholar] [CrossRef]
- Meissner, J.D. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J. Gen. Virol. 1999, 80, 1725–1733. [Google Scholar] [CrossRef]
- Filippova, M.; Filippov, V.; Williams, V.M.; Zhang, K.; Kokoza, A.; Bashkirova, S.; Duerksen-Hughes, P. Cellular Levels of Oxidative Stress Affect the Response of Cervical Cancer Cells to Chemotherapeutic Agents. BioMed. Res. Int. 2014, 574659, 1–14. [Google Scholar] [CrossRef]
- Rowinsky, E.K. Paclitaxel pharmacology and other tumor types. Semin. Oncol. 1997, 24, 1–12. [Google Scholar]
- Cheng, H.Y.; Zhang, T.; Qu, Y.; Shi, W.J.; Lou, G.; Liu, Y.X.; Cheng, L. Synergism between RIZ1 gene therapy and Paclitaxel in SiHa cervical cancer cells. Cancer Gene Therapy 2016, 23, 392–395. [Google Scholar] [CrossRef]
- La Regina, G.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New Pyrrole Derivatives with Potent Tubulin Polymerization Inhibiting Activity As Anticancer Agents Including Hedgehog-Dependent Cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef] [PubMed]
- Da, C.; Telang, N.; Barelli, P.; Jia, X.; Gupton, J.T.; Mooberry, S.L.; Kellogg, G.E. Pyrrole-Based Antitubulin Agents: Two Distinct Binding Modalities Are Predicted for C-2 Analogues in the Colchicine Site. ACS Med. Chem. Lett. 2012, 3, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Carta, D.; Bortolozzi, R.; Hamel, E.; Basso, G.; Moro, S.; Viola, G.; Ferlin, M.G. Novel 3-Substituted 7-Phenylpyrrolo[3,2-f]quinolin-9(6H)-ones as Single Entities with Multitarget Antiproliferative Activity. J. Med. Chem. 2015, 58, 7991–8010. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, R.; Mattiuzzo, E.; Dal-Para, M.; Sturlese, M.; Moro, S.; Hamel, E.; Carta, D.; Viola, G.; Ferlin, M.G. Targeting tubulin polymerization by novel 7-aryl-pyrroloquinolinones: Synthesis, biological activity and SARs. Eur. J. Med. Chem. 2018, 143, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Fornabaio, M.; Kellogg, G.E.; Gupton, J.T.; Gewirtz, D.A.; Yeudall, W.A.; Vegae, N.E.; Mooberry, S.L. Docking and hydropathic scoring of polysubstituted pyrrole compounds with antitubulin activity. Bioorg. Med. Chem. 2008, 16, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Chen, C.-C. GEMDOCK: A generic evolutionary method for molecular docking. Proteins 2004, 55, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Löwe, J.; Li, H.; Downing, K.H.; Nogales, E. Refined Structure of αβ-Tubulin at 3.5 Å Resolution. J. Mol. Biol. 2001, 313, 1045–1057. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009; Volume 41, pp. 1–1257. [Google Scholar]
- Thiemann, T.; Vill, V. Preliminary communication: Development of an incremental system for the prediction of the nematic-isotropic phase transition temperature of liquid crystals with two aromatic rings. Liq. Cryst. 1997, 22, 519–523. [Google Scholar] [CrossRef]
- Todeschini, R.; Ballabio, D.; Consonni, V.; Mauri, A.; Pavan, M. Talete srl Home Page. Available online: http://www.talete.mi.it (accessed on 10 June 2019).
- Koes, D.R.; Camacho, C.J. ZINC Pharmer: Pharmacophore search of the ZINC database. Nucleic Acids Res. 2012, 40, W409–W414. [Google Scholar] [CrossRef]
- López-Méndez, L.J.; González-Méndez, I.; Aguayo-Ortiz, R.; Domínguez, L.; Alcaraz-Estrada, S.L.; Rojas-Aguirre, Y.; Guadarrama, P. Synthesis of a poly(ester) dendritic β-cyclodextrin derivative by “click” chemistry: Combining the best of two worlds for complexation enhancement. Carbohyd. Polym. 2018, 184, 20–29. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest Graph™ ED50 Calculator. Available online: https://www.aatbio.com/tools/ed50-calculator (accessed on 31 May 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revisions C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; III, W.A.G.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–l in racemic form are available from the authors. |

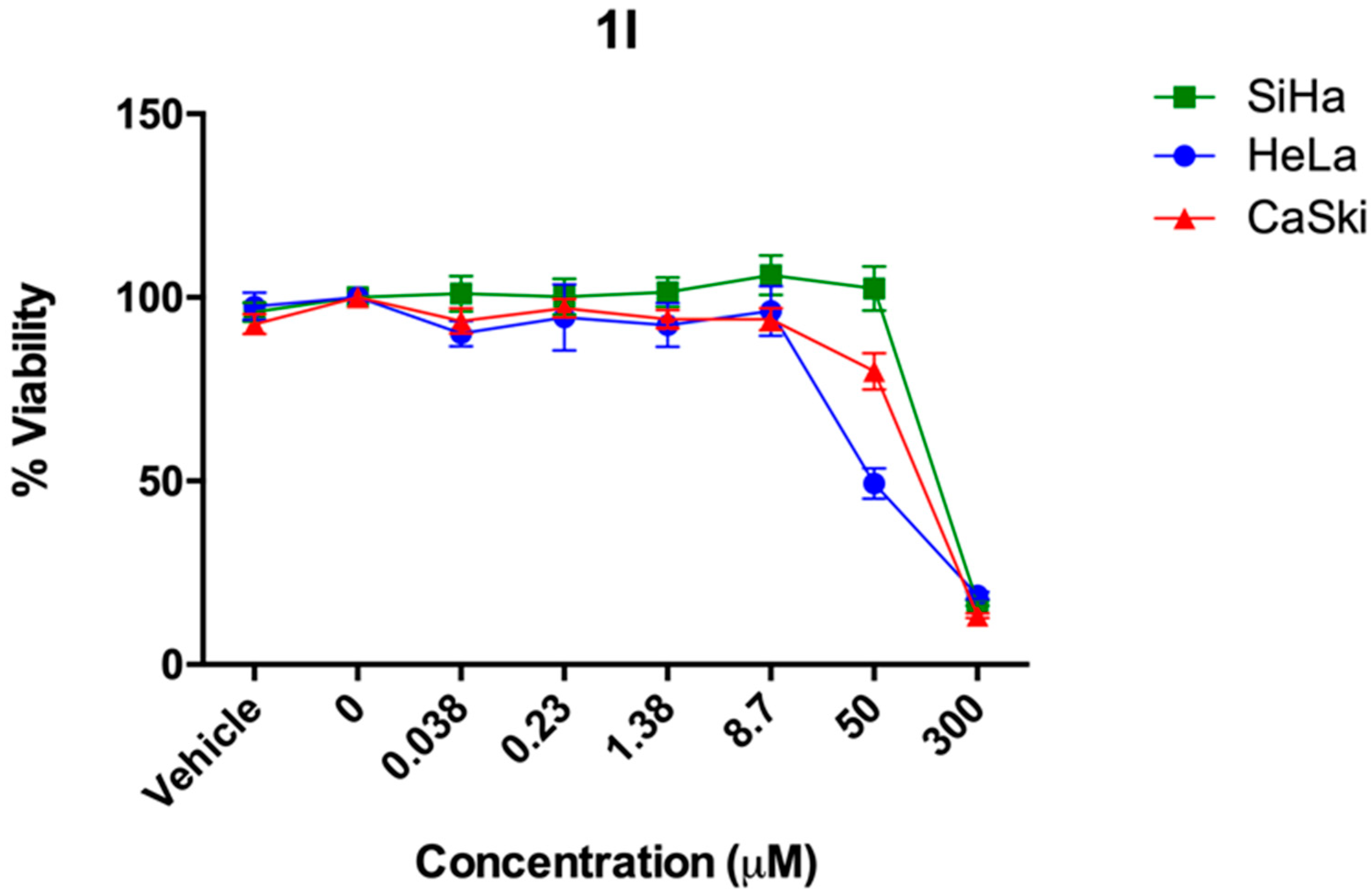
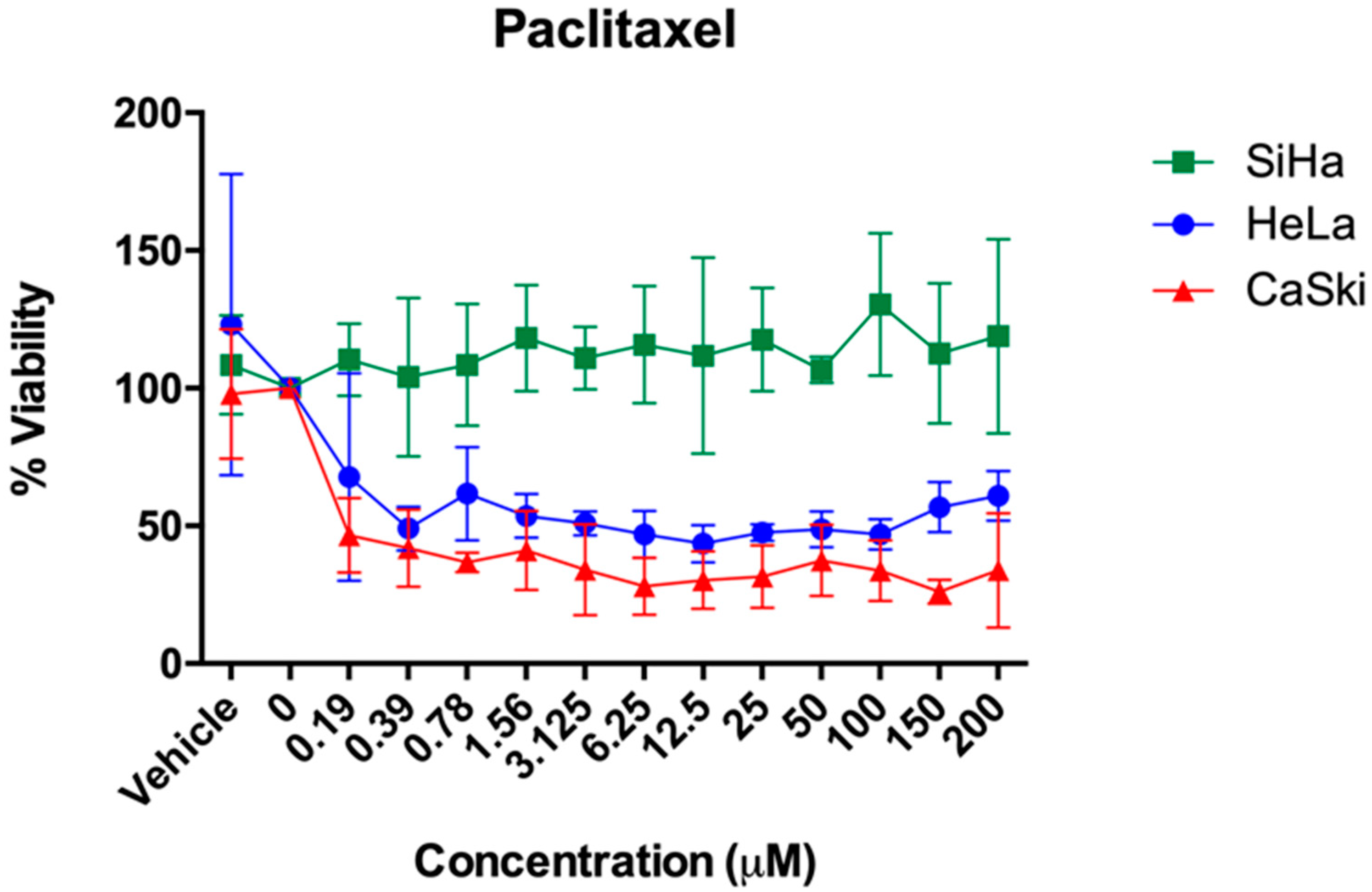
| % Cell Viability ± SD (ED50 Values) | |||
|---|---|---|---|
| Compound | SiHa | HeLa | CaSki |
| 1h[a] | 50.58 ± 4.33 (281.09) | 44.04 ± 14.45 (180.66) | 4.57 ± 4.54 (288.76) |
| 1k[a] | 22.7 ± 2.6 (212.92) | 24.9 ± 3.38 (206.02) | 16.2 ± 1.7 (237.04) |
| 1l[a] | 16.8 ± 0.67 (212.64) | 18.8 ± 1.03 (64.44) | 13.3 ± 0.66 (119.27) |
| [a] n = 8 at 300 μM | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura-Olvera, D.; García-González, A.N.; Morales-Salazar, I.; Islas-Jácome, A.; Rojas-Aguirre, Y.; Ibarra, I.A.; Díaz-Cervantes, E.; Alcaraz-Estrada, S.L.; González-Zamora, E. Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines. Molecules 2019, 24, 2648. https://doi.org/10.3390/molecules24142648
Segura-Olvera D, García-González AN, Morales-Salazar I, Islas-Jácome A, Rojas-Aguirre Y, Ibarra IA, Díaz-Cervantes E, Alcaraz-Estrada SL, González-Zamora E. Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines. Molecules. 2019; 24(14):2648. https://doi.org/10.3390/molecules24142648
Chicago/Turabian StyleSegura-Olvera, Daniel, Ailyn N. García-González, Ivette Morales-Salazar, Alejandro Islas-Jácome, Yareli Rojas-Aguirre, Ilich A. Ibarra, Erik Díaz-Cervantes, Sofía Lizeth Alcaraz-Estrada, and Eduardo González-Zamora. 2019. "Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines" Molecules 24, no. 14: 2648. https://doi.org/10.3390/molecules24142648
APA StyleSegura-Olvera, D., García-González, A. N., Morales-Salazar, I., Islas-Jácome, A., Rojas-Aguirre, Y., Ibarra, I. A., Díaz-Cervantes, E., Alcaraz-Estrada, S. L., & González-Zamora, E. (2019). Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines. Molecules, 24(14), 2648. https://doi.org/10.3390/molecules24142648