Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of SJ Extract
2.2. Total Phenolic Content and Evaluation of Antioxidant Activity In Vitro
2.3. In Vivo Antioxidant Activity Using the C. elegans Model
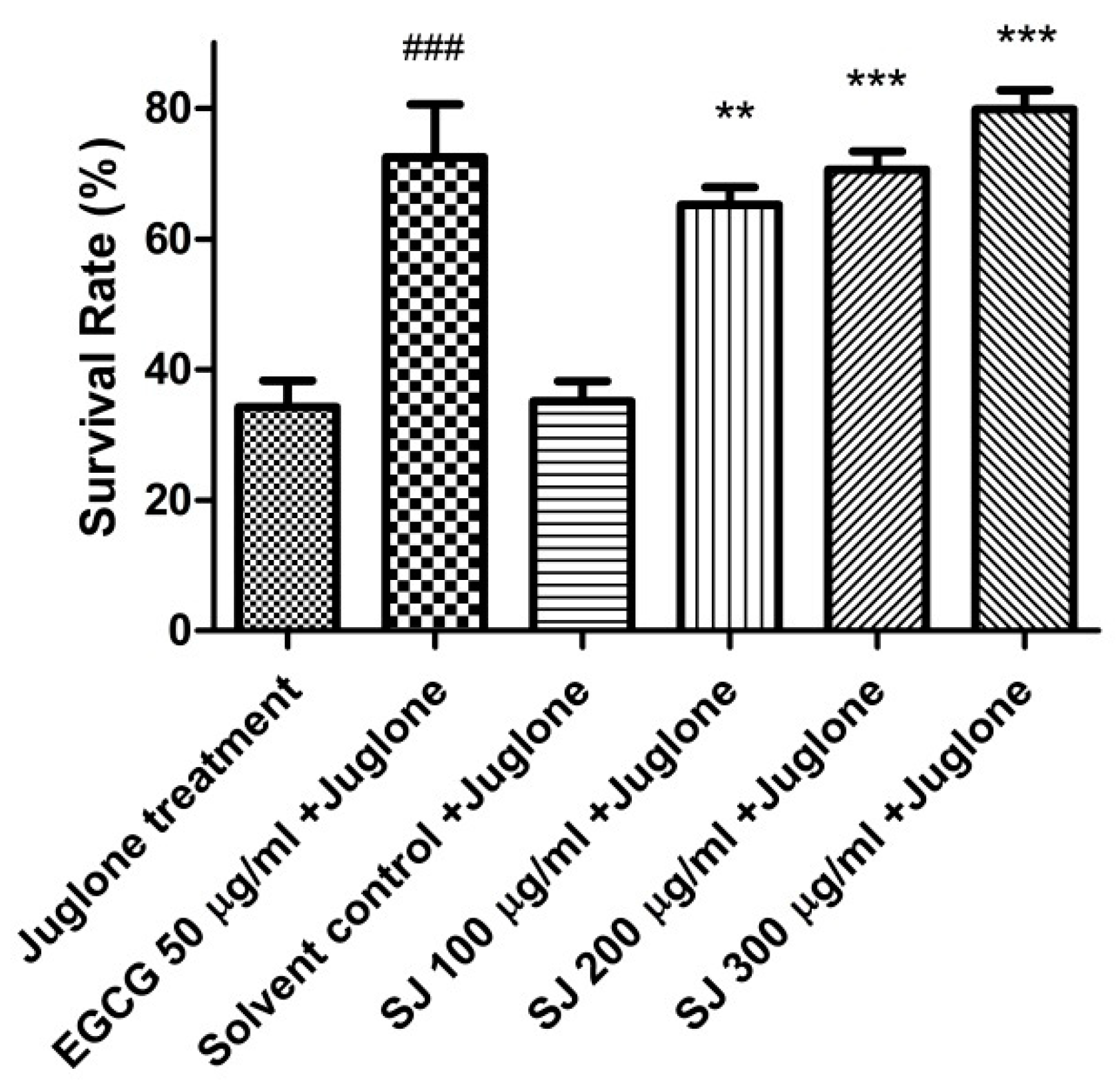
2.3.1. Survival Assay
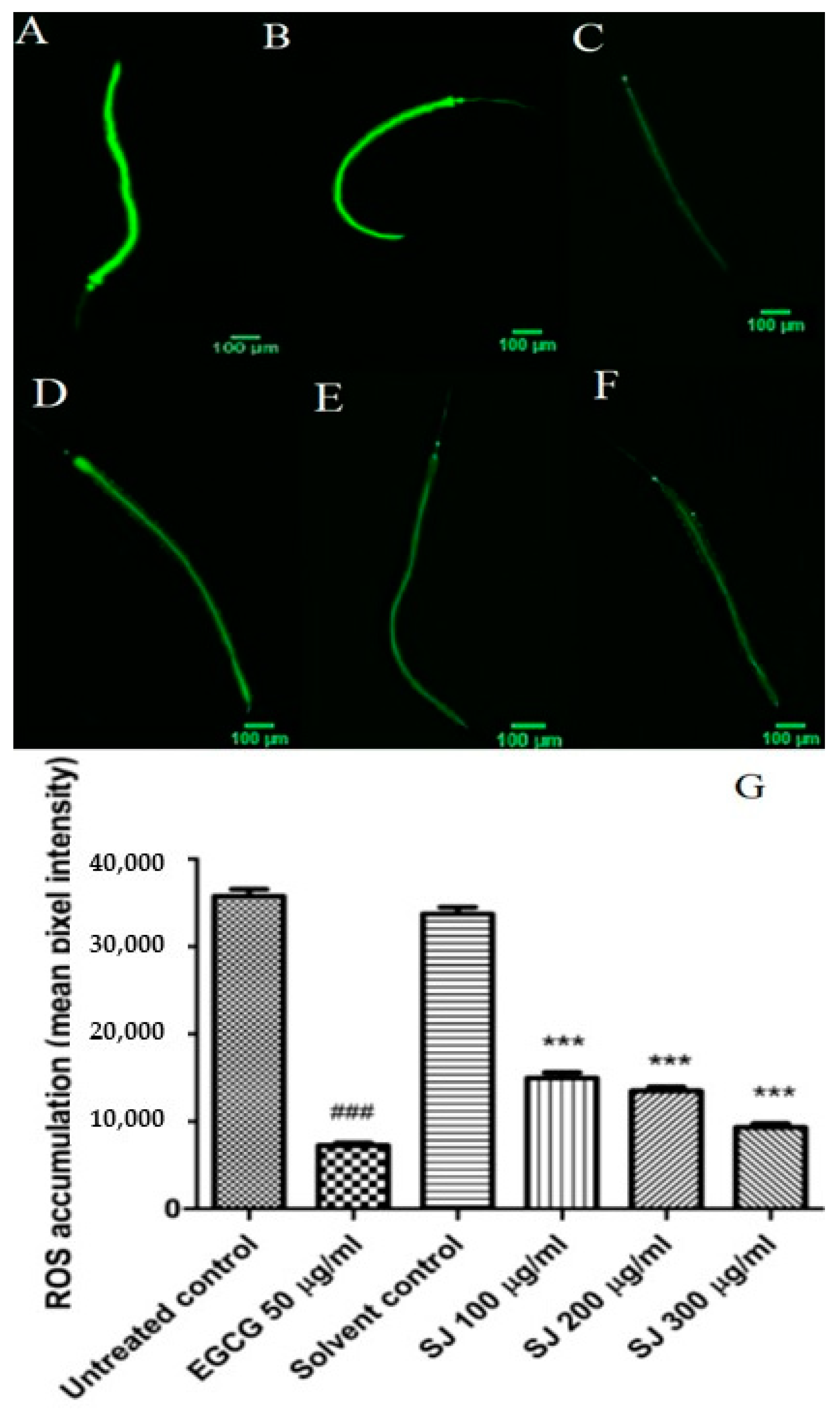
2.3.2. ROS Intracellular Accumulation
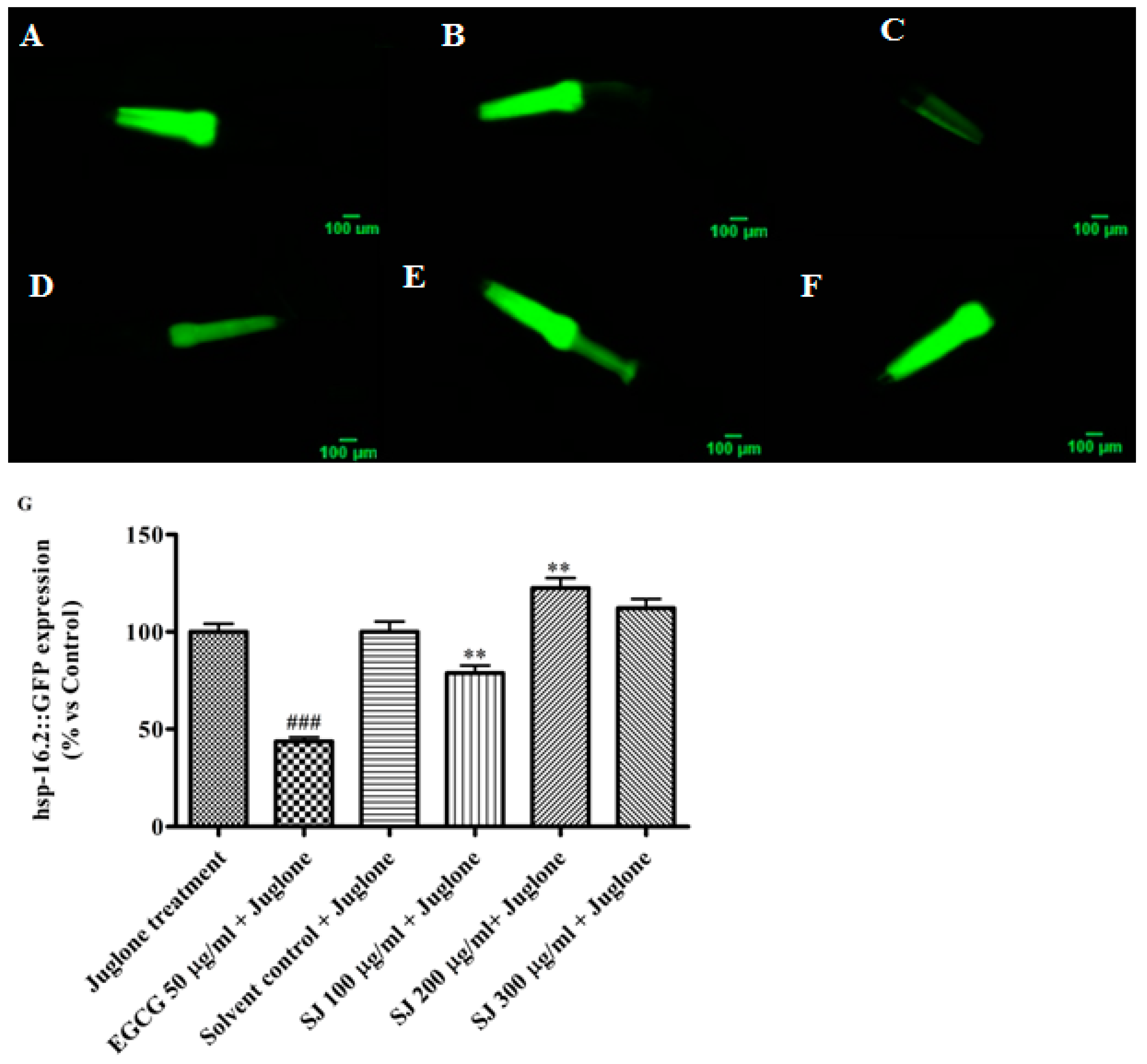
2.3.3. Expression of Hsp-16.2 Gene
2.3.4. Expression of Sod-3 Gene
2.4. In Vivo Antioxidant Activity Using TMT Mouse Model
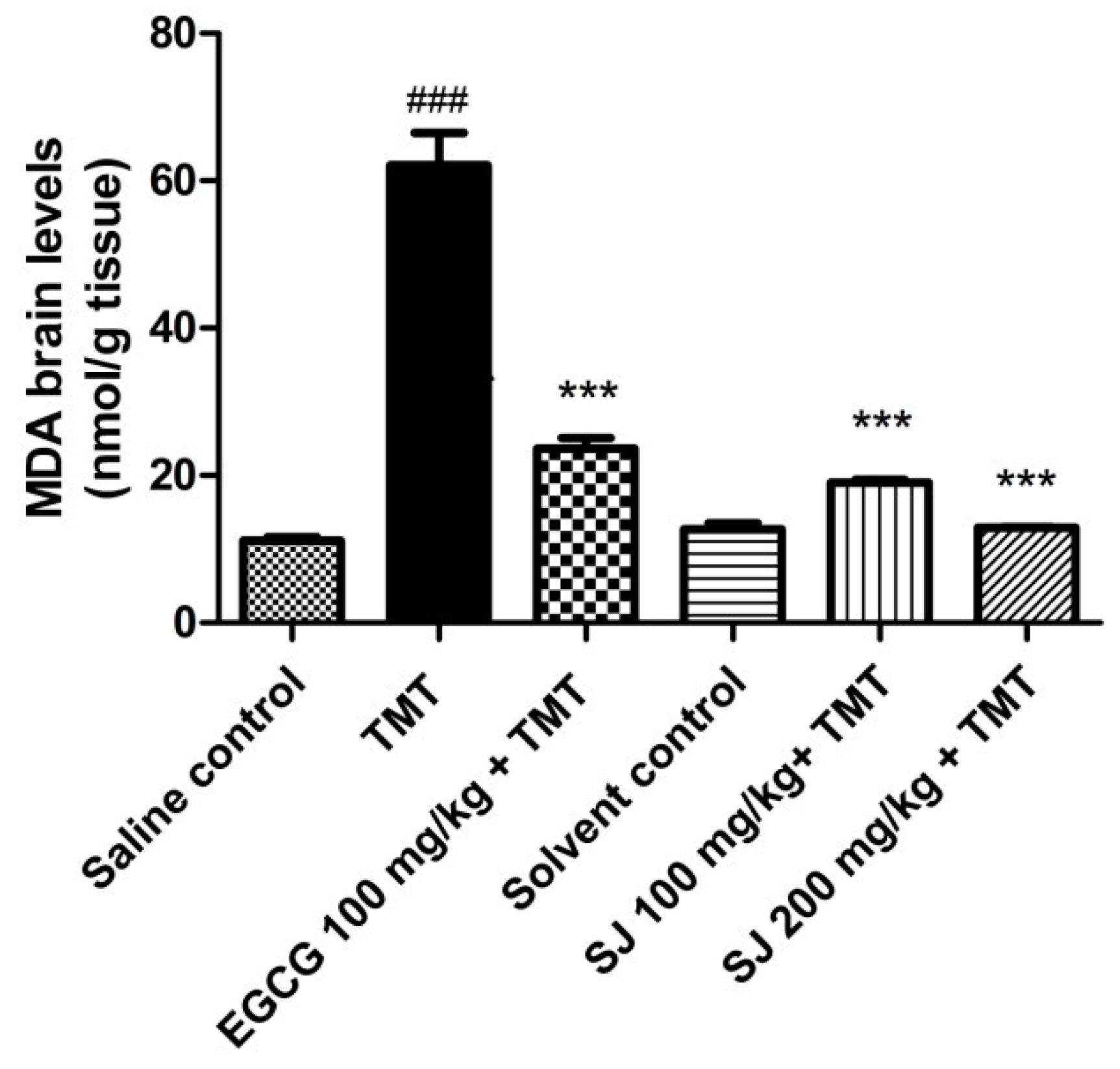
2.4.1. Levels of the Oxidative Stress Marker MDA
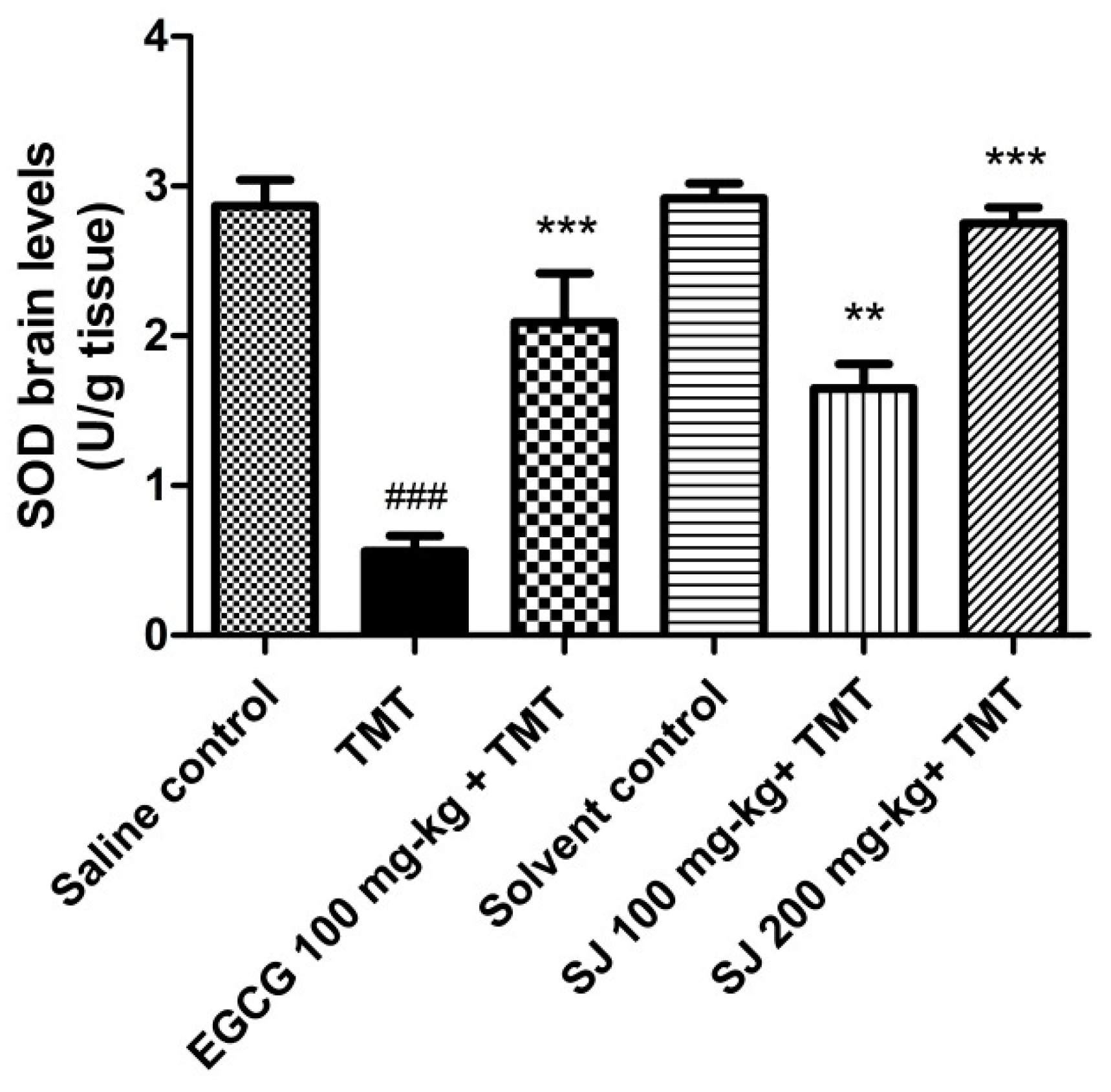
2.4.2. Brain SOD Levels
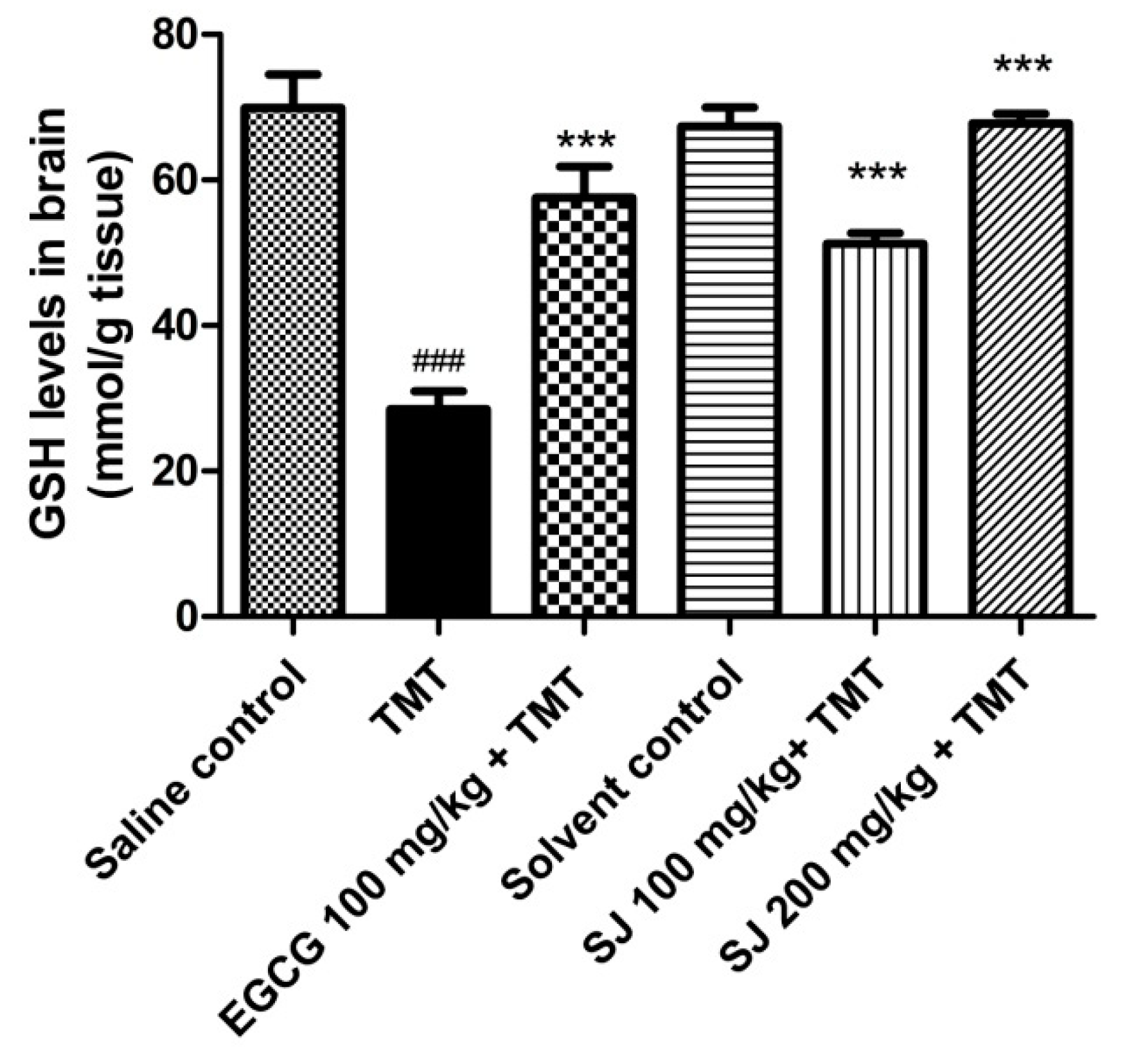
2.4.3. GSH Brain Levels
2.4.4. HO-1 Levels in Brain
3. Materials and Methods
3.1. Plant Material
3.2. Chemical Profiling of Phenolic Constituents with HPLC-PDA-ESI-MS/MS
3.3. Antioxidant Activity In Vitro
3.3.1. Measurement of Total Phenolic Content
3.3.2. DPPH• Assay
3.4. In Vivo Antioxidant Activity
3.4.1. Strains and Culture Conditions of Caenorhabditis Elegans
3.4.2. Survival Assay
3.4.3. ROS Assay
3.4.4. Expression of Hsp-16.2::GFP
3.4.5. Expression of Sod-3::GFP
3.4.6. Animals
3.4.7. Experimental Design
3.4.8. Biochemical Analysis
Estimation of MDA Brain Levels
Estimation of SOD Brain Levels
Estimation of GSH Brain Levels
Estimation of HO-1 Brain Levels
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference Publications Inc.: Algonac, MI, USA, 1985; Volume 2, pp. 363–705. [Google Scholar]
- Kite, G.C.; Veitch, N.C.; Boalch, M.E.; Lewis, G.P.; Leon, C.J.; Simmonds, M.S. Flavonol tetraglycosides from fruits of Styphnolobium japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry 2009, 70, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Beretz, A.; Cazenave, J.-P. The effect of flavonoids on blood-vessel wall interactions. Prog. Clin. Biol. Res. 1988, 280, 187–200. [Google Scholar] [PubMed]
- Bown, D. Encyclopedia of Herbs & Their Uses; Dorling Kindersley Publishing: New York, NY, USA, 1995; pp. 143–153. [Google Scholar]
- Nguyẽn, V.D.; Nguyẽn, G.C. Selected Medicinal Plants in Vietnam; Science and Technology Publishing House: Hanoi, Vietnam, 1999. [Google Scholar]
- Stuart, G. Chinese Materia Medica; Vegetable Kingdom; Southern Materials Center Inc.: Taipei, Taiwan, 1979; pp. 15–18. [Google Scholar]
- Vo, V. Dictionary of Medicinal Plants in Vietnam; Y Hoc: Ho Chi Minh City, Vietnam, 1997; Volume 435, p. 1249. [Google Scholar]
- WHO. Medicinal Plants in the Republic of Korea: Information on 150 Commonly Used Medicinal Plants; WHO Regional Office for the Western Pacific: Manila, Philippines, 1998. [Google Scholar]
- Yoon, H.-J.; Seo, C.-R.; Kim, M.; Kim, Y.-J.; Song, N.-J.; Jang, W.-S.; Kim, B.-J.; Lee, J.; Hong, J.-W.; Nho, C.W. Dichloromethane extracts of Sophora japonica L. stimulate osteoblast differentiation in mesenchymal stem cells. Nutr. Res. 2013, 33, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Al-Abd, A.M.; Asaad, G.F.; Abdel-Naim, A.B.; El-Halawany, A.M. Isolation of antiosteoporotic compounds from seeds of Sophora japonica. PLoS ONE 2014, 9, e98559. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-T.; Wong, M.; Lam, C. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-S.; Won, T.-J.; Kang, H.-C.; Lee, D.-I. Isoflavones extracted from sophorae fructus upregulate IGF-I and TGF-β and inhibit osteoclastogenesis in rat born marrow cells. Arch. Pharmacal Res. 2004, 27, 99–105. [Google Scholar] [CrossRef]
- Lao, C.-J.; Lin, J.-G.; Kuo, J.-S.; Chao, P.-D.L.; Cheng, C.-Y.; Tang, N.-Y.; Hsieh, C.-L. Microglia, apoptosis and interleukin-1β expression in the effect of Sophora Japonica L. on cerebral infarct induced by ischemia-reperfusion in rats. Am. J. Chin. Med. 2005, 33, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Yamada, H. Interaction in the antibacterial activity of flavonoids from Sophora japonica L. to Propionibacterium. Yakugaku Zasshi J. Pharma. Soc. Jpn. 1984, 104, 340. [Google Scholar] [CrossRef]
- Park, K.W.; Lee, J.-E.; Park, K.-M. Diets containing Sophora japonica L. prevent weight gain in high-fat diet-induced obese mice. Nutr. Res. 2009, 29, 819–824. [Google Scholar] [CrossRef]
- Tang, Y.-P.; Li, Y.-F.; Hu, J.; Lou, F.-C. Isolation and identification of antioxidants from Sophora japonica. J. Asian Nat. Prod. Res. 2002, 4, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Thabit, S.; Handoussa, H.; Roxo, M.; El Sayed, N.S.; de Azevedo, B.C.; Wink, M. Evaluation of antioxidant and neuroprotective activities of Cassia fistula (L.) using the Caenorhabditis elegans model. PeerJ 2018, 6, e5159. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.; Roxo, M.; Silva, E.; Valente, K.; Braun, M.; Wang, X.; Wink, M. Bark Extract of the Amazonian Tree Endopleura uchi (Humiriaceae) Extends Lifespan and Enhances Stress Resistance in Caenorhabditis elegans. Molecules 2019, 24, 915. [Google Scholar] [CrossRef] [PubMed]
- Braungart, E.; Gerlach, M.; Riederer, P.; Baumeister, R.; Hoener, M.C. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener. Dis. 2004, 1, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, N. Bioaccessibility and HPLC-MS/MS chemical characterization of phenolic antioxidants in Red Chicory (Cichorium intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC–DAD–ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Wan, C.; Yu, Y.; Zhou, S.; Tian, S.; Cao, S. Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacogn. Mag. 2011, 7, 101. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of ultra performance liquid chromatography-photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) method for the characterization of phenolic compounds of Lepidium sativum L. sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Schatz, P.; Lappierr, C.; Ralph, J. Synthesis of hydroxycinnamoyl-L-malic acids and identification of 5-hydroxyferuloyl-L-malic acid in COMT-downregulated Arabidopsis. In Proceedings of the International Symposium on Wood and Pulping Chemistry, Madison, WI, USA, 9–12 June 2003; Volume 3, pp. 159–162. [Google Scholar]
- Ferreres, F.; Sousa, C.; Justin, M.; Valentão, P.; Andrade, P.B.; Llorach, R.; Rodrigues, A.; Seabra, R.M.; Leitão, A. Characterisation of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem. Anal. 2005, 16, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.E.P.; De Vos, R.C.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a potential chemotaxonomical tool: Application in the genus Vernonia Schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Ferreira, I.C.; Carvalho, A.M.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Bamawa, C.M.; Ndjele, L.M.; Foma, F.M. Characterization of leaf phenolic compounds of Hymenocardia ulmoides (Wild plant tea) by HPLC-MS. Int. J. Pharm. Biol. Res. 2013, 4, 170–174. [Google Scholar]
- Beelders, T.; De Beer, D.; Stander, M.; Joubert, E. Comprehensive phenolic profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and-MS/MS reveals novel xanthone and benzophenone constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; Van Chuyen, N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef]
- Strayer, A.; Wu, Z.; Christen, Y.; Link, C.D.; Luo, Y. Expression of the small heat-shock protein Hsp16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB J. 2003, 17, 2305–2307. [Google Scholar] [CrossRef]
- Rattan, S.I. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid-and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Balaban, C.; Callaghan, J.; Billingsle, M. Trimethyltin-induced neuronal damage in the rat brain: Comparative studies using silver degeneration stains, immunocytochemistry and immunoassay for neuronotypic and gliotypic proteins. Neuroscience 1988, 26, 337–361. [Google Scholar] [CrossRef]
- Feldman, R.G.; White, R.F.; Eriator, I.I. Trimethyltin encephalopathy. Arch. Neurol. 1993, 50, 1320–1324. [Google Scholar] [CrossRef]
- Fortemps, E.; Amand, G.; Bomboir, A.; Lauwerys, R.; Laterre, E.-C. Trimethyltin poisoning·report of two cases. Int. Arch. Occup. Environ. Health 1978, 41, 1–6. [Google Scholar] [CrossRef]
- Kreyberg, S.; Torvik, A.; Bjørneboe, A.; Wiik-Larsen, W.; Jacobsen, D. Trimethyltin poisoning: Report of a case with postmortem examination. Clin. Neuropathol. 1992, 11, 256–259. [Google Scholar]
- O’Connell, A.W.; Strada, O.; Earley, B.; Leonard, B.E. Altered expression of amyloid protein precursor mRNA in the rat hippocampus following trimethyltin intoxication: An in situ hybridization study. Neurochem. Int. 1997, 30, 313–320. [Google Scholar] [CrossRef]
- Ali, S.; LeBel, C.; Bondy, S. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Naalsund, L.; Fonnum, F. The effect of trimethyltin on three glutamergic and gabaergic transmitter parameters in vitro: High affinity uptake, release and receptor binding. Neurotoxicology 1986, 7, 53–62. [Google Scholar] [PubMed]
- Ha, J.S.; Jin, D.E.; Park, S.K.; Park, C.H.; Seung, T.W.; Bae, D.-W.; Kim, D.-O.; Heo, H.J. Antiamnesic effect of Actinidia arguta extract intake in a mouse model of TMT-induced learning and memory dysfunction. EVID-Based Complem. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Kim, C.-W.; Lee, U.; Kim, S.-H.; Heo, H.J. Ethyl Acetate Fraction from Persimmon (Diospyros kaki) Ameliorates Cerebral Neuronal Loss and Cognitive Deficit via the JNK/Akt Pathway in TMT-Induced Mice. Int. J. Mol. Sci. 2018, 19, 1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Choi, G.N.; Kim, J.H.; Kwak, J.H.; Jeong, C.-H.; Jeong, H.R.; Lee, U.; Heo, H.J. Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure. Food Chem. 2012, 132, 1019–1024. [Google Scholar] [CrossRef]
- Shin, E.-J.; Suh, S.; Lim, Y.; Jhoo, W.-K.; Hjelle, O.; Ottersen, O.; Shin, C.; Ko, K.; Kim, W.-K.; Kim, D. Ascorbate attenuates trimethyltin-induced oxidative burden and neuronal degeneration in the rat hippocampus by maintaining glutathione homeostasis. Neuroscience 2005, 133, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S. Magnolol protects against trimethyltin-induced neuronal damage and glial activation in vitro and in vivo. Neurotoxicology 2016, 53, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Marnett, L.J. Lipid peroxidation—DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Cini, M.; Fariello, R.; Bianchetti, A.; Moretti, A. Studies on lipid peroxidation in the rat brain. Neurochem. Res. 1994, 19, 283–288. [Google Scholar] [CrossRef]
- Kaur, S.; Chhabra, R.; Nehru, B. Ginkgo biloba extract attenuates hippocampal neuronal loss and cognitive dysfunction resulting from trimethyltin in mice. Phytomedicine 2013, 20, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of quercetin in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. EVID-Based Complem. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Terpstra, M.; McCarten, J.R.; Hodges, J.; Hemmy, L.S.; Grant, A.; Deelchand, D.K.; Marjanska, M. Noninvasively Measured Human Brain Glutathione and Ascorbate Concentrations in Healthy Aging and in Alzheimer’s Disease. Free Radic. Biol. Med. 2016, 100, S89. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Hajizadeh Moghaddam, A. The effect of olive leaf methanolic extract on hippocampal antioxidant biomarkers in an animal model of Parkinson’s disease. J. Basic. Clin. Pathophysiol. 2017, 5, 9–14. [Google Scholar]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr. Pharm. Des. 2008, 14, 429–442. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Motterlini, R.; Green, C.J.; Foresti, R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid. Redox Signal. 2002, 4, 615–624. [Google Scholar] [CrossRef]
- Shibahara, S.; Han, F.; Li, B.; Takeda, K. Hypoxia and heme oxygenases: Oxygen sensing and regulation of expression. Antioxid. Redox Signal. 2007, 9, 2209–2226. [Google Scholar] [CrossRef]
- Chen, K.; Gunter, K.; Maines, M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J. Neurochem. 2000, 75, 304–313. [Google Scholar] [CrossRef]
- Huang, S.-W.; Wang, W.; Zhang, M.-Y.; Liu, Q.-B.; Luo, S.-Y.; Peng, Y.; Sun, B.; Wu, D.-L.; Song, S.-J. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine 2016, 23, 694–704. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. C. Elegans 1999, 2, 51–67. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Koolen, H.; da Silva, F.; Silva, E.; Braun, M.; Wang, X.; Wink, M. Calycophyllum spruceanum (Benth.), the amazonian “tree of youth” prolongs longevity and enhances stress resistance in Caenorhabditis elegans. Molecules 2018, 23, 534. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Krstin, S.; Röhrig, T.; Richling, E.; Wink, M. An anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Beutler, E.; Kelly, B.M. The effect of sodium nitrite on red cell GSH. Experientia 1963, 19, 96–97. [Google Scholar] [CrossRef]
- Abraham, N.; Lutton, J.; Levere, R. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838–843. [Google Scholar]
Sample Availability: Samples of the plant material are available from the authors. |
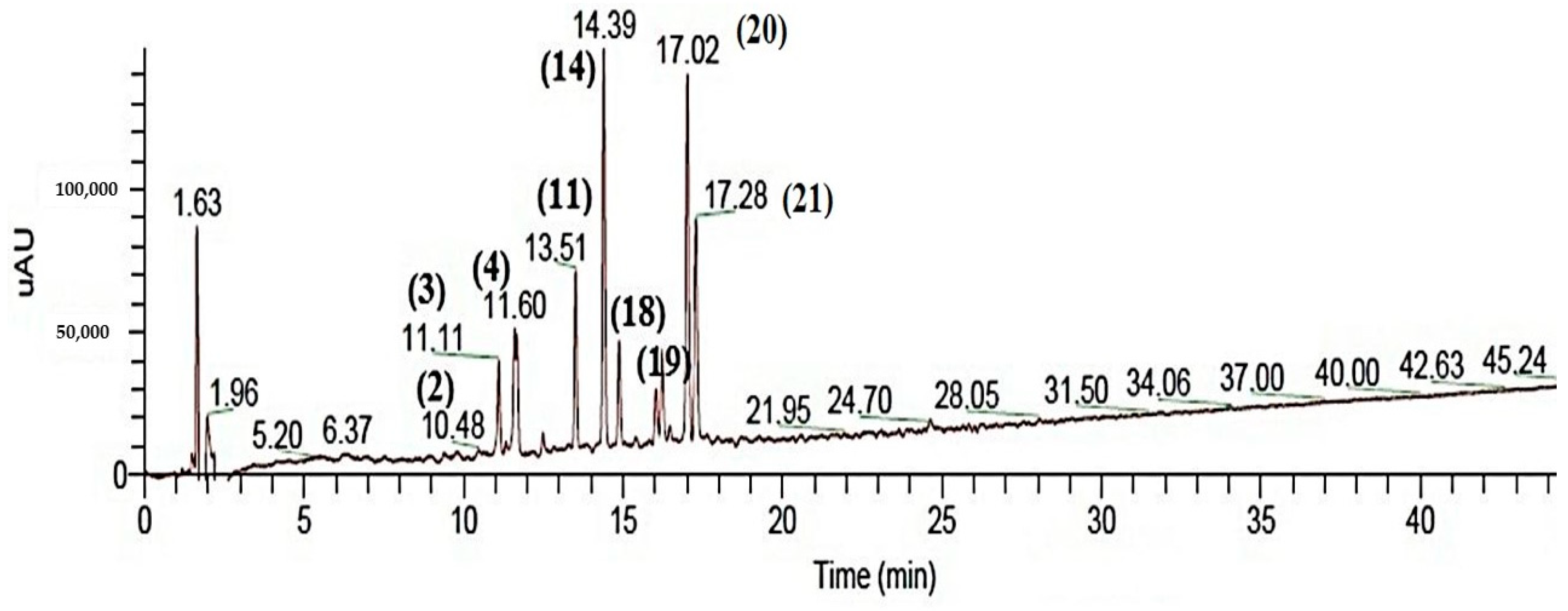


| Peak no. | Tentative Identification | Rt (min) | UV-Vis (λ max) | Peak Area (%) | [M − H]− | Fragment Ions (Ms/Ms) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Apigenin acetyl dihexoside * | 9.45 | 272, 334 | 0.3 | 639 | 638, 592, 431 | Tentative |
| 2 | Kaempferol-3-O-rutinoside-7-O-β-D hexopyranoside * | 10.48 | 290, 348 | 0.5 | 755 | 754, 609, 593, 285 | [25] |
| 3 | Digalloyl-HHDP-Hexose * | 11.11 | 240, 272 | 1.8 | 784 | 633, 631, 301 | [27] |
| 4 | Kaempferol 3-O-[6″-O-(hexosyl) hexoside] 7-O-deoxyhexoside | 11.60 | 290, 348 | 2.8 | 755 | 609, 431, 284 | [28] |
| 5 | Kaempferol dihexoside | 12.30 | 278, 349 | 0.7 | 609 | 429, 284 | Tentative |
| 6 | Quercetin 7-O-hexoside-3-O-Rutinoside * | 12.53 | 255, 354 | 0.25 | 771 | 463, 300 | [24] |
| 7 | Trihydroxy-dimethoxy-flavone hexosyl-hexoside | 12.65 | 271, 342 | 0.3 | 654 | 473 | [29] |
| 8 | Kaempferol-feruloyl-di-hexose-pentose * | 12.98 | 266, 333 | 0.18 | 931 | 785 | [26] |
| 9 | Kaempferol 3-O-[6″-O-(hexosyl) hexoside] 7-O-deoxyhexoside | 13.38 | 290, 348 | 0.15 | 755 | 284 | [28] |
| 10 | Apigenin hexoside * | 13.4 | 270, 338 | 0.19 | 431 | 269 | [23] |
| 11 | Kaempferol rutinoside | 13.51 | 254, 367 | 2.3 | 593 | 285 | [24] |
| 12 | Kaempferol-3-O-hexose-O-caffeoyl-Odeoxyhexoside * | 13.62 | 254, 330 | 0.2 | 755 | 284 | [30] |
| 13 | Apigenin 7-O-rutinoside * | 14.19 | 268, 334 | 0.1 | 577 | 269 | [24] |
| 14 | Apigenin 7-O-(2’’-dihydrogalloyl)-deoxyhexosyl-6-C-(2″′-pentosyl)-hexoside * | 14.39 | 232, 336 | 3.5 | 862 | 700 | [22] |
| 15 | Kaempferol derivative | 14.5 | 278, 330 | 0.08 | 654 | 284 | Tentative |
| 16 | Kaempferol di-O-deoxyhexoside | 14.55 | 246, 344 | 0.07 | 577 | 431 | [31] |
| 17 | Kaempferol-O-deoxyhexoside | 14.94 | 245, 344 | 0.05 | 431 | 284 | [31] |
| 18 | Galloyl-bis-HHDP hexose * | 14.98 | 256 | 1.8 | 935 | 633 | [32] |
| 19 | Coumaroyl quinic acid * | 16.35 | 264,325 | 1.72 | 337 | 191 | [33] |
| 20 | Naringenin-O-hexose-O-deoxyhexose | 17.02 | 281 | 1.85 | 578 | 433, 271 | [34] |
| 21 | Naringenin-O-hexose | 17.28 | 281 | 0.82 | 432 | 271 | [34] |
| 22 | Apigenin 7-O-(2″-dihydrogalloyl)-rhamonsyl-6-C-(2″′-pentosyl)-hexoside * | 18.2 | 232, 336 | 0.9 | 863 | 269 | [22] |
| 23 | Proanthocyanidin tetramer * | 40.59 | 278 | 0.1 | 1121 | 849, 831 | [32] |
| 24 | Apigenin 6-C-hexosyl 8-C- (6″-O-methoxygalloyl)- hexoside * | 43.07 | 272, 340 | 0.4 | 758 | 269 | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thabit, S.; Handoussa, H.; Roxo, M.; Cestari de Azevedo, B.; S.E. El Sayed, N.; Wink, M. Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models. Molecules 2019, 24, 2633. https://doi.org/10.3390/molecules24142633
Thabit S, Handoussa H, Roxo M, Cestari de Azevedo B, S.E. El Sayed N, Wink M. Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models. Molecules. 2019; 24(14):2633. https://doi.org/10.3390/molecules24142633
Chicago/Turabian StyleThabit, Sara, Heba Handoussa, Mariana Roxo, Bruna Cestari de Azevedo, Nesrine S.E. El Sayed, and Michael Wink. 2019. "Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models" Molecules 24, no. 14: 2633. https://doi.org/10.3390/molecules24142633
APA StyleThabit, S., Handoussa, H., Roxo, M., Cestari de Azevedo, B., S.E. El Sayed, N., & Wink, M. (2019). Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models. Molecules, 24(14), 2633. https://doi.org/10.3390/molecules24142633






