Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens
Abstract
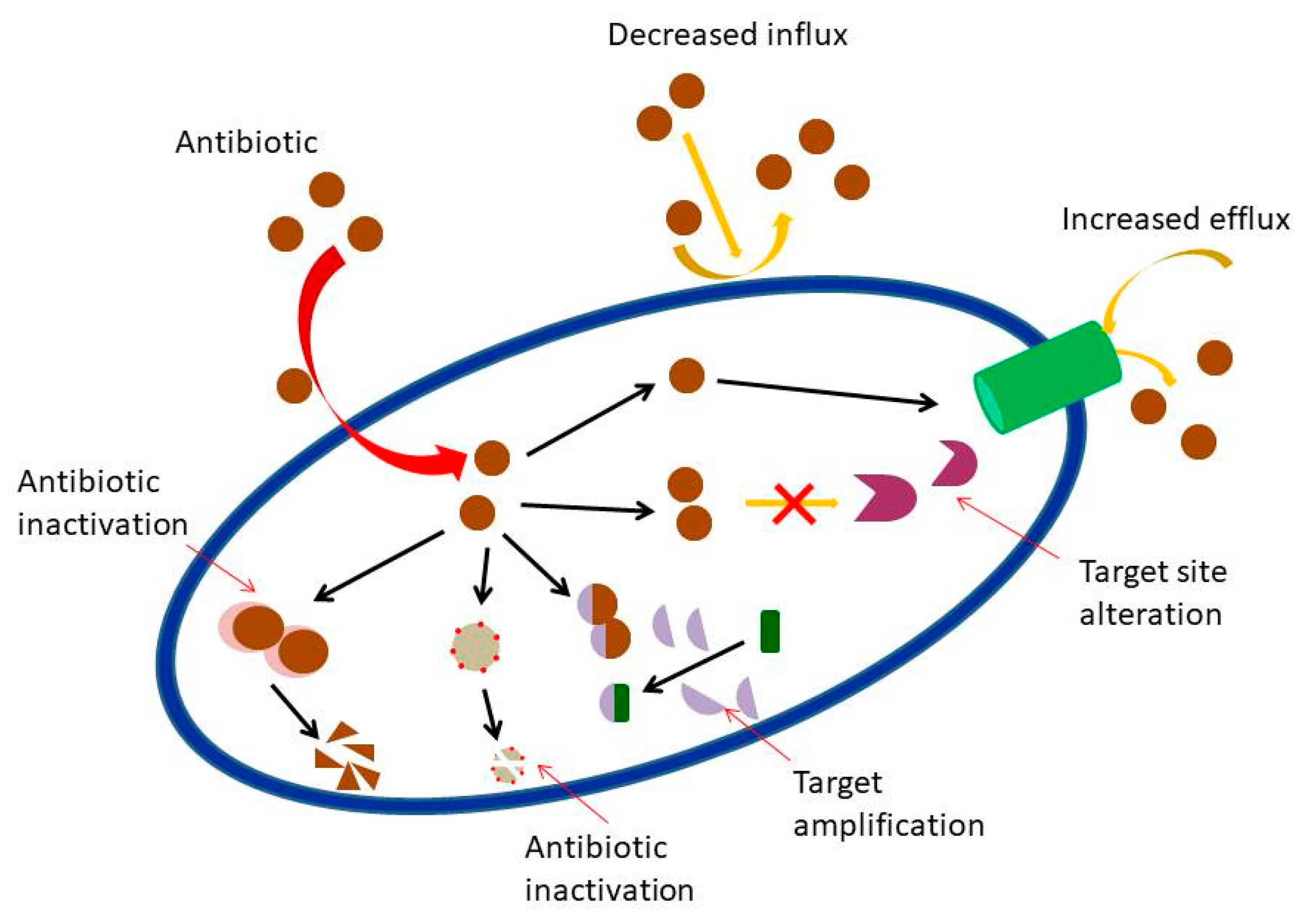
1. Introduction
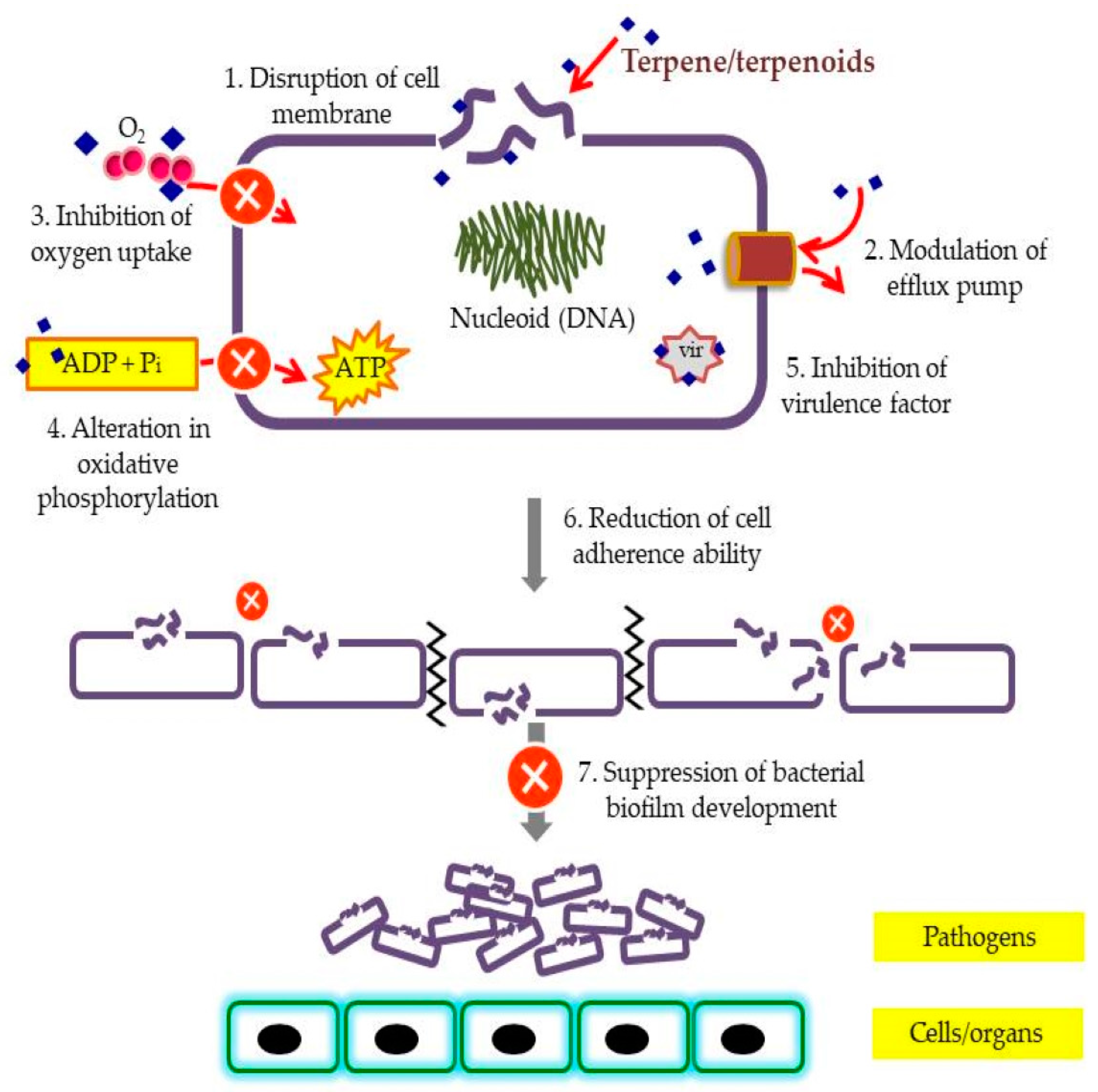
2. Terpenes and Their Derivatives
2.1. Bioactive Terpenes and Terpenoids
2.1.1. Monoterpenes and Monoterpenoids
2.1.2. Sesquiterpenes and Sesquiterpenoids
2.1.3. Diterpenes and Diterpenoids
2.1.4. Triterpenes and Triterpenoids
2.2. Therapeutic Implementation
2.2.1. Drugs and Antibiotics
2.2.2. Terpenes Bioavailability
2.2.3. Evaluation of Compounds Interaction in Combination Therapies
2.2.4. Methods for Antimicrobial Evaluation
3. Perspectives
3.1. Ongoing Research
3.2. Application of Terpenoids in Clinical Settings: Challenges
3.3. Future Prospects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandal, S.M.; Roy, A.; Ghosh, A.K.; Hazra, T.K.; Basak, A.; Franco, O.L. Challenges and future prospects of antibiotic therapy: From peptides to phages utilization. Front. Pharmacol. 2014, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Zacchino, S.A.; Butassi, E.; Cordisco, E.; Svetaz, L.A. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine 2017, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.; Martin, M.J.; Santiago, M.; Lee, W.; Kos, V.N.; Meredith, T.; Gilmore, M.S.; Walker, S. Multidrug intrinsic resistance factors in Staphylococcus aureus identified by profiling fitness within high-diversity transposon libraries. MBio 2016, 7, 4. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Touani, F.K.; Seukep, A.J.; Djeussi, D.E.; Fankam, A.G.; Noumedem, J.A.K.; Kuete, V. Antibiotic-potentiation activities of four Cameroonian dietary plants against multidrug-resistant Gram-negative bacteria expressing efflux pumps. BMC Complement. Altern. Med. 2014, 1, 258. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 3, 417–433. [Google Scholar] [CrossRef]
- Zaidi, A.K.M.; Huskins, W.C.; Thaver, D.; Bhutta, Z.A.; Abbas, Z.; Goldmann, D.A. Hospital-acquired neonatal infections in developing countries. Lancet 2005, 365, 1175–1188. [Google Scholar] [CrossRef]
- Saleem, A.F.; Ahmed, I.; Mir, F.; Ali, S.R.; Zaidi, A.K.M. Pan-resistant Acinetobacter infection in neonates in Karachi, Pakistan. J. Infect. Dev. Ctries. 2010, 4, 30–37. [Google Scholar] [CrossRef]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Shen, J.; Davis, L.E.; Wallace, J.M.; Cai, Y.; Lawson, L.D. Enhanced diallyl trisulfide has in vitro synergy with amphotericin B against Cryptococcus neoformans. Planta Med. 1996, 62, 415–418. [Google Scholar] [CrossRef]
- Yang, S.K.; Low, L.Y.; Yap, P.S.X.; Yusoff, K.; Mai, C.W.; Lai, K.S.; Lim, S.H. Plant-derived antimicrobials: Insights into mitigation of antimicrobial resistance. Rec. Nat. Prod. 2018, 12, 295–316. [Google Scholar] [CrossRef]
- Spratt, B.G. Resistance to antibiotics mediated by target alterations. Science 1994, 264, 388–393. [Google Scholar] [CrossRef]
- Baroud, M.; Dandache, I.; Araj, G.F.; Wakim, R.; Kanj, S.; Kanafani, Z.; Khairallah, M.; Sabra, A.; Shehab, M.; Dbaibo, G.; et al. Underlying mechanisms of carbapenem resistance in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates at a tertiary care centre in Lebanon: Role of OXA-48 and NDM-1 carbapenemases. Int. J. Antimicrob. Agents 2013, 41, 75–79. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Insights into antibiotic resistance through metagenomic approaches. Future Microbiol. 2012, 7, 73–89. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005, 5, 209–218. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2019, 16. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Li, L.; Zheng, C.; Guo, L.; Li, W.; Sun, P.; Qin, L. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 2010, 165, 437–449. [Google Scholar] [CrossRef]
- Thapa, D.; Louis, P.; Losa, R.; Zweifel, B.; Wallace, R.J. Essential oils have different effects on human pathogenic and commensal bacteria in mixed faecal fermentations compared with pure cultures. Microbiology 2015, 161, 441–449. [Google Scholar] [CrossRef]
- Lewis, R.E.; Kontoyiannis, D.P. Rationale for combination antifungal therapy. Pharmacotherapy 2001, 21, 149S–164S. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 7. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. Agric. Food Chem. 1998, 46, 590–595. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 2015, 60, 21–30. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yang, S.K.; Lai, K.S.; Lim, S.H. Essential oils: The ultimate solution to antimicrobial resistance in Escherichia coli? In Escherichia coli-Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Samie, A., Ed.; Intech Open: Rijeka, Croatia, 2017; pp. 299–313. [Google Scholar]
- Yang, S.K.; Yap, P.S.X.; Krishnan, T.; Yusoff, K.; Chan, K.G.; Yap, W.S.; Lai, K.S.; . Lim, S.H. Mode of action: Synergistic interaction of peppermint (Mentha x piperita L. Carl) essential oil and meropenem against plasmid-mediated resistant E. coli. Rec. Nat. Prod. 2018, 12, 582–594. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.E.; Lai, K.S. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS ONE 2019, 1–20. [Google Scholar] [CrossRef]
- Maida, I.; Lo Nostro, A.; Pesavento, G.; Barnabei, M.; Calonico, C.; Perrin, E.; Chiellini, C.; Fondi, M.; Mengoni, A.; Maggini, V.; et al. Exploring the anti- Burkholderia cepacia complex activity of essential oils: A preliminary analysis. Evidence-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Rasooli, I.; Shayegh, S.; Astaneh, S.D.A. The effect of Mentha spicata and Eucalyptus camaldulensis essential oils on dental biofilm. Int. J. Dent. Hyg. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef]
- Shrigod, N.M.; Swami Hulle, N.R.; Prasad, R.V. Supercritical fluid extraction of essential oil from mint leaves (Mentha spicata): Process optimization and its quality evaluation. J. Food Process Eng. 2017, 40, 12488. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- De Andrade, F.B.; Midena, R.Z.; Koga-Ito, C.Y.; Duarte, M.A. Conventional and natural products against oral infections. Microbial pathogens and strategies for combating them: Science, technology and education. In Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; FORMATEX Research Center: Badajoz, Spain, 2013; pp. 1574–1583. [Google Scholar]
- Vasireddy, L.; Bingle, L.E.H.; Davies, M.S. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Grzesiak, B.; Głowacka, A.; Krukowski, H.; Lisowski, A.; Lassa, H.; Sienkiewicz, M. The in vitro efficacy of essential oils and antifungal drugs against Prototheca zopfii. Mycopathologia 2016, 181, 609–615. [Google Scholar] [CrossRef]
- Rafiq, R.; Hayek, S.; Anyanwu, U.; Hardy, B.; Giddings, V.; Ibrahim, S.; Tahergorabi, R.; Kang, H. Antibacterial and antioxidant activities of essential oils from Artemisia herba-alba Asso., Pelargonium capitatum radens and Laurus nobilis L. Foods 2016, 5, 28. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.M.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef]
- De Rapper, S.; Viljoen, A.; Van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evidence-Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medical importance of Anthemis nobilis—A review. As. J. Pharm. Sci. Technol. 2016, 6, 89–95. [Google Scholar]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Garzoli, S.; Božović, M.; Baldisserotto, A.; Sabatino, M.; Cesa, S.; Pepi, F.; Vicentini, C.B.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-Candida activity of Foeniculum vulgare Miller–new approaches. Nat. Prod. Res. 2018, 32, 1254–1259. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef]
- Zrira, S.; Ghanmi, M. Chemical composition and antibacterial activity of the essential of Cedrus atlantica (Cedarwood oil). J. Essent. Oil-Bearing Plants. 2016, 19, 1267–1272. [Google Scholar] [CrossRef]
- El Omari, K.; Hamze, M.; Alwan, S.; Jama, C.; Chihib, N.E. Antifungal activity of the essential oil of Micromeria barbata an endemic lebanese micromeria species collected at North Lebanon. J. Mater. Environ. Sci. 2016, 7, 4158–4167. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Roberts, S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007, 3, 387–395. [Google Scholar] [CrossRef]
- Shaw, M.K.; Ingraham, J.L. Synthesis of macromolecules by Escherichia coli near the minimal temperature for growth. J. Bacteriol. 1967, 1, 157–164. [Google Scholar]
- Zengin, H.; Baysal, A. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 11, 17773–17798. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 12, 1709. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.L. Geraniol restores antibiotic activities against multidrug-resistant isolates from Gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- De Inés, C.; Argandoña, V.H.; Rovirosa, J.; San-Martín, A.; Díaz-Marrero, A.R.; Cueto, M.; González-Coloma, A. Cytotoxic activity of halogenated monoterpenes from Plocamium cartilagineum. Zeitschrift fur Naturforsch-Sect. C J. Biosci. 2004, 59, 339–344. [Google Scholar] [CrossRef]
- Kurita, N.; Miyaji, M.; Kuraney, R.; Takahara, Y.; Ichimura, K. Antifungal activity and molecular orbital energies of aldehyde compounds from oils of higher plants. Agric. Biol. Chem. 1979, 43, 2365–2371. [Google Scholar]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Zhou, J. Antimicrobial activity of an endophytic Xylaria sp. YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin. Appl. Microbiol. Biotechnol. 2008, 78, 241–247. [Google Scholar] [CrossRef]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Khan, S.S.; Khan, A.; Rani, M.; Ahmad, V.U.; Choudhary, M.I. Biotransformation of monoterpenoids and their antimicrobial activities. Phytomedicine. 2014, 21, 1597–1626. [Google Scholar] [CrossRef]
- Smid, E.J.; de Witte, Y.; Gorris, L.G.M. Secondary plant metabolites as control agents of postharvest Penicillium rot on tulip bulbs. Postharvest Biol. Technol. 1995, 6, 303–312. [Google Scholar] [CrossRef]
- Dunkic, V.; Bezic, N.; Vuko, E.; Cukrov, D. Antiphytoviral activity of Satureja montana L. ssp. variegata (host) P. W. Ball essential oil and phenol compounds on CMV and TMV. Molecules 2010, 15, 6713–6721. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Torres-Romero, D.; Jiménez, I.A.; Rojas, R.; Gilman, R.H.; López, M.; Bazzocchi, I.L. Dihydro-β-agarofuran sesquiterpenes isolated from Celastrus vulcanicola as potential anti-Mycobacterium tuberculosis multidrug-resistant agents. Bioorganic Med. Chem. 2011, 19, 2182–2189. [Google Scholar] [CrossRef]
- Koo, H.; Pearson, S.K.; Scott-Anne, K.; Abranches, J.; Cury, J.A.; Rosalen, P.L.; Park, Y.; Marquis, R.E.; Bowen, W.H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 2002, 17, 337–343. [Google Scholar] [CrossRef]
- Gomes, F.I.A.; Teixeira, P.; Azeredo, J.; Oliveira, R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr. Microbiol. 2009, 59, 118–122. [Google Scholar] [CrossRef]
- Masako, K.; Yusuke, K.; Hideyuki, I.; Atsuko, M.; Yoshiki, M.; Kayoko, M.; Makoto, K. Corrigendum to “A novel method to control the balance of skin microflora. Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin”. J. Dermatol. Sci. 2005, 39, 197. [Google Scholar] [CrossRef]
- Castelo-Branco, D.S.C.M.; Riello, G.B.; Vasconcelos, D.C.; Guedes, G.M.M.; Serpa, R.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Rocha, M.F.; Sidrim, J.J.; et al. Farnesol increases the susceptibility of Burkholderia pseudomallei biofilm to antimicrobials used to treat melioidosis. J. Appl. Microbiol. 2016, 120, 600–606. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Hwang, J.K. Effect of coating the wells of a polystyrene microtiter plate with xanthorrhizol on the biofilm formation of Streptococcus mutans. J. Basic Microbiol. 2006, 46, 410–415. [Google Scholar] [CrossRef]
- Jin, J.; Guo, N.; Zhang, J.; Ding, Y.; Tang, X.; Liang, J.; Li, L.; Deng, X.; Yu, L. The synergy of honokiol and fluconazole against clinical isolates of azole-resistant Candida albicans. Lett. Appl. Microbiol. 2010, 51, 351–357. [Google Scholar] [CrossRef]
- Gonçalves, O.; Pereira, R.; Gonçalves, F.; Mendo, S.; Coimbra, M.A.; Rocha, S.M. Evaluation of the mutagenicity of sesquiterpenic compounds and their influence on the susceptibility towards antibiotics of two clinically relevant bacterial strains. Mutat. Res. -Genet. Toxicol. Environ. Mutagen. 2011, 723, 18–25. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Tirapelli, C.R.; da Costa, F.B.; de Oliveira, A.M. Kaurane and pimarane-type diterpenes from the Viguiera species inhibit vascular smooth muscle contractility. Life Sci. 2006, 79, 925–933. [Google Scholar] [CrossRef]
- Souza, A.B.; Martins, C.H.G.; Souza, M.G.M.; Furtado, N.A.J.C.; Heleno, V.C.G.; De Sousa, J.P.B.; Rocha, E.M.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.; et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phythother. Res. 2011, 25, 215–220. [Google Scholar] [CrossRef]
- Różalski, M.; Walencka, E.; Różalska, B.; Wysokińska, H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: Salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine. 2007, 14, 31–35. [Google Scholar]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine. 2016, 23, 654–661. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from Salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef]
- Cunha, W.R.; De Matos, G.X.; Souza, M.G.M.; Tozatti, M.G.; Andrade, E.; Silva, M.L.; Martins, C.H.G.; Silva, R.D.; Da Silva Filho, A.A. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, Y.; Chen, W.; Zhang, P.; Chen, Y.; Lv, X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis. 2013, 19, 494–500. [Google Scholar] [CrossRef]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia. 2014, 94, 48–54. [Google Scholar] [CrossRef]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time – kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Rodriguez, M.V.; Sortino, M.A.; Ivancovich, J.J.; Pellegrino, J.M.; Favier, L.S.; Raimondi, M.P.; Gattuso, M.A.; Zacchino, S.A. Detection of synergistic combinations of Baccharis extracts with Terbinafine against Trichophyton rubrum with high throughput screening synergy assay (HTSS) followed by 3D graphs. Behavior of some of their components. Phytomedicine 2013, 13, 1230–1239. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Di Liberto, M.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from forests and human health. Toxicol. Res. 2017, 2, 97. [Google Scholar] [CrossRef]
- Schwab, W.; Fuchs, C.; Huang, F.C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Tech. 2013, 1, 3–8. [Google Scholar] [CrossRef]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and-resistant human pathogenic Candida species. BMC infectious diseases 2006, 1, 158. [Google Scholar] [CrossRef]
- Tona, L.; Mesia, K.; Ngimbi, N.P.; Chrimwami, B.; Okond’Ahoka; Cimanga, K.; Bruyne, T.D.; Apers, S.; Hermans, N.; Totte, J.; et al. In-vivo antimalarial activity of Cassia Occidentalism, Morinda morindoides and Phyllanthus niruri. Ann. Trop. Med. Parasitol. 2001, 1, 47–57. [Google Scholar] [CrossRef]
- Donald, P.R.; Lamprecht, J.H.; Freestone, M.; Albrecht, C.F.; Bouic, P.J.; Kotze, D.; Van Jaarsveld, P.P. A randomised placebo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 1997, 6, 518–522. [Google Scholar]
- Kohlert, C.; Van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 6, 495–505. [Google Scholar] [CrossRef]
- Papada, E.; Gioxari, A.; Brieudes, V.; Amerikanou, C.; Halabalaki, M.; Skaltsounis, A.L.; Smyrnioudis, I.; Kaliora, A.C. Bioavailability of terpenes and postprandial effect on human antioxidant potential. An open-label study in healthy subjects. Mol. Nutr. Food Res. 2018, 3, 1700751. [Google Scholar] [CrossRef]
- Amin, T.; Bhat, S.V. A review on phytosome technology as a novel approach to improve the bioavailability of nutraceuticals. Int. J. Adv. Res. Technol. 2012, 3, 1–5. [Google Scholar]
- Yang, S.K.; Yusoff, K.; Mai, C.W.; Lim, W.M.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef]
- Bodede, O.; Shaik, S.; Chenia, H.; Singh, P.; Moodley, R. Quorum sensing inhibitory potential and in silico molecular docking of flavonoids and novel terpenoids from Senegalia nigrescens. J. Ethnopharmacol. 2018, 216, 134–146. [Google Scholar] [CrossRef]
- Avalos, M.; van Wezel, G.P.; Raaijmakers, J.M.; Garbeva, P. Healthy scents: Microbial volatiles as new frontier in antibiotic research? Curr. Opin. Microbiol. 2018, 45, 84–91. [Google Scholar] [CrossRef]
- Chudasama, R.G.; Dhanani, N.J.; Amrutiya, R.M.; Chandni, R.; Jayanthi, G.; Karthikeyan, K. Screening of selected plants from semi-arid region for its phytochemical constituents and antimicrobial activity. J. Pharmacog. Phytochem. 2018, 7, 2983–2988. [Google Scholar]
- Gupta, S.; Bhagat, M.; Sudan, R.; Rajput, S.; Rajput, K. Analysis of chemical composition of Cupressus torulosa (D.Don) essential oil and bioautography guided evaluation of its antimicrobial fraction. Indian J. Exp. Biol. 2018, 56, 252–257. [Google Scholar]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma Lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Bin Sayeed, M.; Karim, S.; Sharmin, T.; Morshed, M. Critical analysis on characterization, systemic effect, and therapeutic potential of beta-sitosterol: A plant-derived orphan phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Medicine 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- De Moraes, M.M.; da Camara, C.A.G.; Da Silva, M.M.C. Comparative toxicity of essential oil and blends of selected terpenes of Ocotea species from Pernambuco, Brazil, against Tetranychus urticae Koch. An. Acad. Bras. Cienc. 2017, 89, 1417–1429. [Google Scholar] [CrossRef]
- Sparagano, O.; Khallaayoune, K.; Duvallet, G.; Nayak, S.; George, D. Comparing terpenes from plant essential oils as pesticides for the poultry red mite (Dermanyssus gallinae). Transbound. Emerg. Dis. 2013, 60, 150–153. [Google Scholar] [CrossRef]
- Van der Werf, M.J.; de Bont, J.A.; Leak, D.J. Opportunities in microbial biotransformation of monoterpenes. In Biotechnology of Aroma Compounds; Springer: Berlin/Heidelberg, Germany, 1997; pp. 147–177. [Google Scholar]
| Plants spp. | Common Name | Pathogens Tested | MIC/Sensitivity/Inhibition Zone | Citation |
|---|---|---|---|---|
| Eugenia caryophyllata | Clove | Burkholderia cepacia complex | ES | [35] |
| Origanum vulgare | Oregano | B. cepacia complex | ES | [35] |
| Thymus vulgaris | Thyme | B. cepacia complex | ES | [35] |
| Eucalyptus camadulensis | Eucalyptus | Streptococcus pyogenes | 1 mg mL−1 | [36] |
| Fusarium oxysporum f. sp. lycopersici | 15.93% to 72.5% | [37] | ||
| Mentha spicata | Spearmint | S. pyogenes | 2 mg mL−1 | [36] |
| E. coli | 21 mm at 150 µL | [38] | ||
| Salmonella thyphi | 13 mm at 150 µL | |||
| S. aureus | 12 mm at 150 µL | |||
| Cymbopogon citratus | Lemongrass | Acinetobacter baumannii | 0.65% (v/v) | [39] |
| F. oxysporum f. sp. lycopersici | 250 ppm | [37] | ||
| Syzygium aromaticum | Clove | Candida albicans | 360 µg mL−1 | [40] |
| F. oxysporum f. sp. lycopersici | 125 ppm | [37] | ||
| Pelargonium graveolens | Geranium | B. cepacia complex | 0.4% (v/v) | [41] |
| Prototheca zopfii | 3.5 to 4.0 µL mL−1 | [42] | ||
| Laurus nobilis | Bay laurel | S. typhimurium | 3 % (v/v) | [43] |
| E. coli | 1 % (v/v) | [44] | ||
| Candida spp. | 250 to 500 µg mL−1 | |||
| Melaleuca alternifolia | Tea tree | Campylobacter spp. | 0.00% | [45] |
| Leptospermum petersonii | Manuka | Campylobacter spp. | 0.01% | [45] |
| Backhousia citriodora | Lemon myrtle | Campylobacter spp. | 0.01% | [45] |
| Lavandula angustifolia | Lavender | S. aureus | 2 mg mL−1 | [46] |
| Pseudomonas aeruginosa | 2 mg mL−1 | |||
| C. albicans | 3 mg mL−1 | |||
| Mentha x piperita | Peppermint | Clostridium perfringens Fusarium oxysporum f. sp. lycopersici | 10 mg mL−1 | [47] |
| 500 ppm | [37] | |||
| Chamaemelum nobile | Roman chamomile | Porphyromonas gingivalis | 20.5 ± 0.5 mm | [48] |
| Origanum majorana | Marjoram | Micrococcus luteus | 0.097 mg mL−1 | [49] |
| Vibrio alginolyticus | 0.39 mg mL−1 | |||
| Foeniculum vulgare | Fennel | Candida spp. | 1.56 to 12.48 mg mL−1 | [50] |
| Pinus sylvestris | Pine | Pseudomonas spp. | 4.33 ± 0.58 mm | [51] |
| Cedrus atlantica | Cedarwoood | E. coli | 0.4 µL mL−1 | [52] |
| Bacillus subtilis | 0.2 µL mL−1 | |||
| Bacillus cereus | 0.4 µL mL−1 | |||
| Aniba rosaeodora | Rosewood | Trichophyton mentagrophytes | 0.002 M | [53] |
| Terpenoids Class | Chemical Compounds | Tested Microorganism | Antimicrobial Effect | Reference |
|---|---|---|---|---|
| Monoterpenes and monoterpenoids | Carvacrol Thymol Geraniol | Resistant Enterobacter aerogenes | Efflux pump inhibition | [60] |
| Linalyl acetate (+)- Menthol Thymol | S. aureus E. coli | Growth inhibition | [61] | |
| Carvacrol Trans-cinnamaldehyde (+)-Carvone | E. coli S. typhimurium | Growth inhibition | [30] | |
| (+)-Terpinen-4-ol γ-Terpinene α-Terpinene Terpinolene α-Pinene 1,8-Cineole ρ-Cymene (+)-Limonene β-Myrcene (+)-β-Pinene (±)-Linalool α-Phellandrene α-Terpinoel | T. mentagrophytes Trichophyton violaceum Microsporium gypseum Histoplasma capsulatum Blastomyces dermatitidis…etc. | Growth inhibition | [63] | |
| Carvacrol Eugenol Thymol | Acinetobacter calcoacetica Aeromonas hydrophila B. subtilis | Growth inhibition | [64] | |
| Borneol d-3-Carene Carvacrol Carvacrol methyl ester cis/trans Citral Eugenol Geraniol Geranyl acetate cis-hex-3-en-1-ol R(+)Limonene (2)-Linalool Menthone Nerol α-Pinene β-Pinene (+)sabinene α-Terpinene Terpinen-4-ol α-terpineole (−)-Thujone Thymol | S. aureus E. coli Salmonella typhia S. typhimurium Salmonella enteritidis A. hydrophila Yersinia sp. Vibrio anguillarum Shigella sp. Vibrio parahaemolyticus C. albicans Penicillium expansum Aspergillus niger…etc. | Growth inhibition | [65] | |
| Carvacrol | S.aureus S. typhimurium | Biofilm inhibition | [66] | |
| Linalool | Resistant K. pneumoniae carbapenemase (KPC) | Cell membrane disruption | [34] | |
| Terpinen-4-ol α-Terpineol 1, 8-Cineole Linalool | A. niger Botrytis cinerea | Growth inhibition | [67] | |
| α-Terpinene γ-Terpinene α-Pinene ρ-Cymene Terpinen-4-ol α-Terpineol Thymol Citral 1, 8-Cineole Borneol Bornyl acetate Isoborneol 1, 8-Cineole Thujone Camphor | Herpes simplex virus type 1 (HSV-1) | Growth inhibition | [69] | |
| Thymol Carvacrol | Tobacco mosaic virus (TMV) Cucumber mosaic virus (CMV) | Growth inhibition | [70] | |
| Sesquiterpenes and Sesquiterpenoids | 1α-Acetoxy-6β, 9β-dibenzoyloxy-dihydro-β-agarofuran | Bacillus spp. | Growth inhibition | [71] |
| Farnesol | Streptococcus mutans Streptococcus sobrinus | Biofilm formation inhibition | [72] | |
| S. aureus S. epidermidis | [73] [74] | |||
| B. pseudomallei | Potentiation effect—combination therapy | [75] | ||
| Xanthorrhizol | Staphylococcus mutans | Reduction of cell adherence ability | [76] | |
| Mycobacterium smegmatis | Growth inhibition | [77] | ||
| Diterpenes and diterpenoids | (-)-Carvone Thymol Dihydrocarveol (-)-Perilla alcohol Carvacrol (-)-Carveol…etc. | P. aeruginosa E. coli S. aureus C. albicans | Growth inhibition | [55] |
| Ent-kaurane Ent-pimarane | Dental carries pathogens | Growth inhibition | [80] | |
| Salvipisone | S. aureus S. epidermidis | Bacterial cell adherence prevention Biofilm development inhibition | [81] | |
| 16αHydroxycleroda-3, 13 (14)-Z-dien-15, 16-olide (CD) | MRSA | Antibiotic potentiation Efflux pump modulation | [82] | |
| Salvipisone Aethiopinone | S. aureus Enterococcus faecalis S. epidermidis | Biofilm production inhibition | [81] | |
| MRSA MRSE | Synergistic activity alongside antibiotic | [83] | ||
| Triterpenes and triterpenoids | 24, 24-Dimethyl-5β-tirucall-9 | Tubercular strains | Growth inhibition | [11] |
| 25-Dien-3-one Oleanic acid (OA) Bonianic acid A Bonianic acid B | Mycobacterium tuberculosis | Synergistic activity alongside antibiotic | [2] | |
| OA Ergosterol peroxide Ursolic acid (UA) | Synergistic activity—combination therapy | |||
| OA UA | B. cereus Vibrio cholerae S. choleraesuis K. pneumoniae S. pneumoniae | Growth inhibition | [85] | |
| Planktonic cariogenic microorganism S. mutans S. sobrinus | Biofilm inhibition | [86] [31] | ||
| Listeria monocytogenes | [45] | |||
| OA | A. baumanii | Antibiotic potentiation | [4] | |
| Amyrin Betulinic acid Betulinaldehyde | MRSA MMSA | Growth inhibition | [87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahizan, N.A.; Yang, S.-K.; Moo, C.-L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. https://doi.org/10.3390/molecules24142631
Mahizan NA, Yang S-K, Moo C-L, Song AA-L, Chong C-M, Chong C-W, Abushelaibi A, Lim S-HE, Lai K-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules. 2019; 24(14):2631. https://doi.org/10.3390/molecules24142631
Chicago/Turabian StyleMahizan, Nik Amirah, Shun-Kai Yang, Chew-Li Moo, Adelene Ai-Lian Song, Chou-Min Chong, Chun-Wie Chong, Aisha Abushelaibi, Swee-Hua Erin Lim, and Kok-Song Lai. 2019. "Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens" Molecules 24, no. 14: 2631. https://doi.org/10.3390/molecules24142631
APA StyleMahizan, N. A., Yang, S.-K., Moo, C.-L., Song, A. A.-L., Chong, C.-M., Chong, C.-W., Abushelaibi, A., Lim, S.-H. E., & Lai, K.-S. (2019). Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules, 24(14), 2631. https://doi.org/10.3390/molecules24142631






