Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization
Abstract
1. Introduction
2. Results
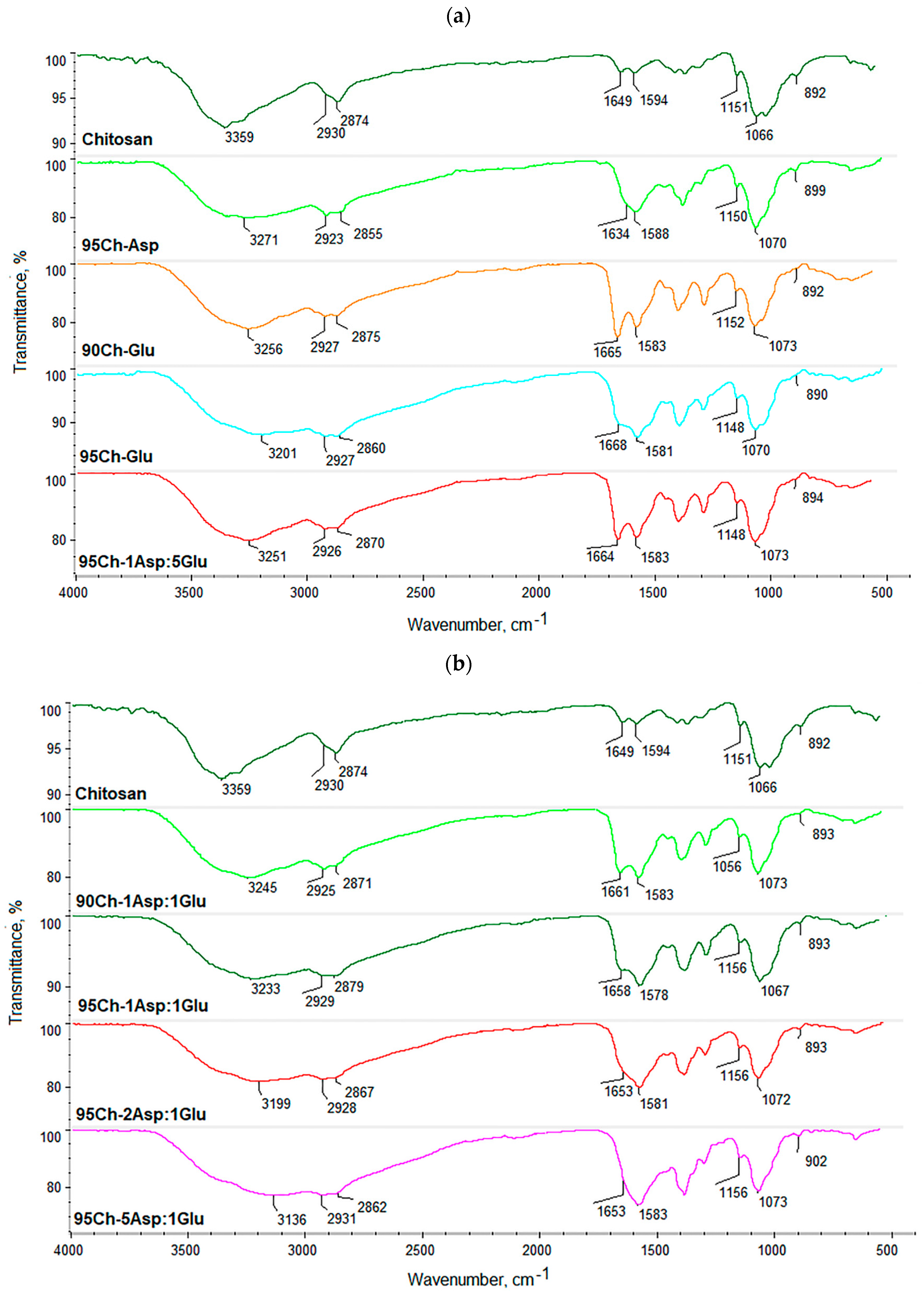
2.1. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
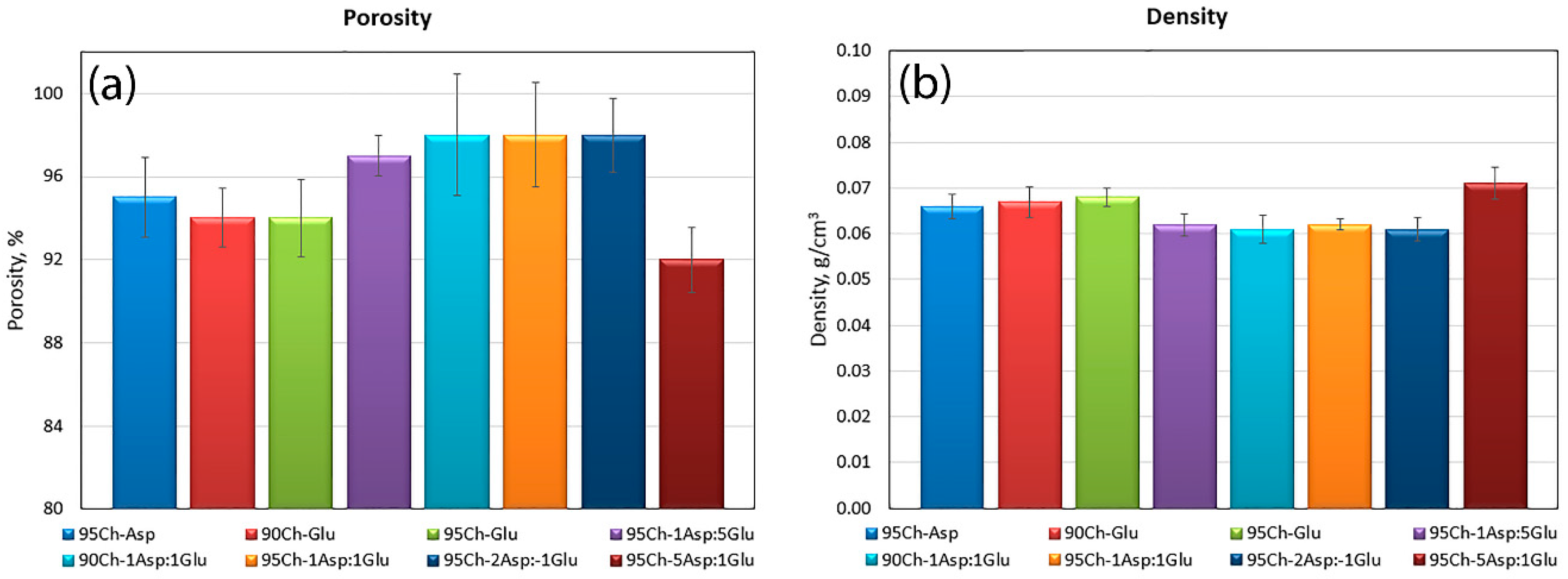
2.2. Porosity and Density
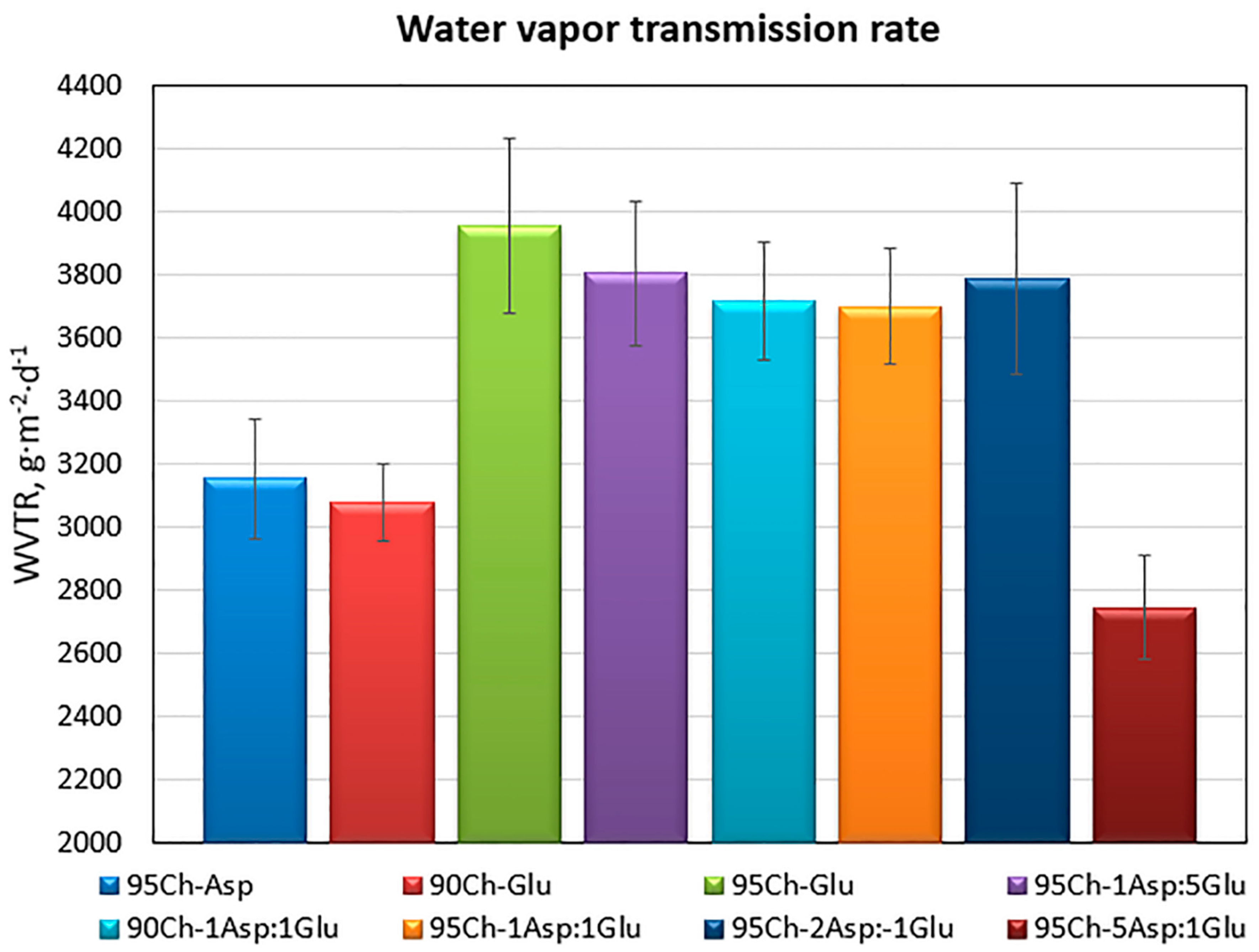
2.3. Water Vapor Transmission Rate of the Chitosan Aerogels
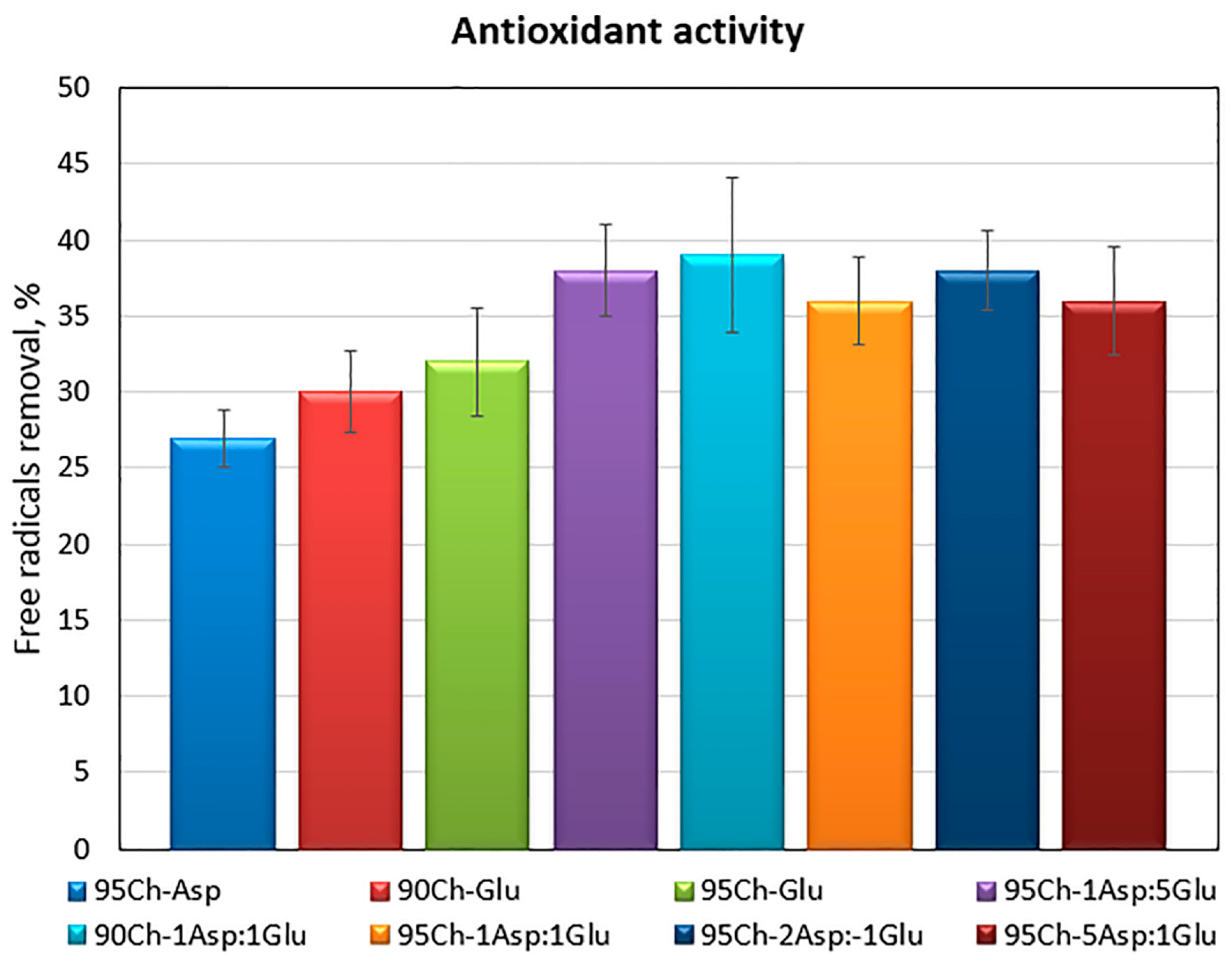
2.4. Antioxidant Activity of the Chitosan Aerogels
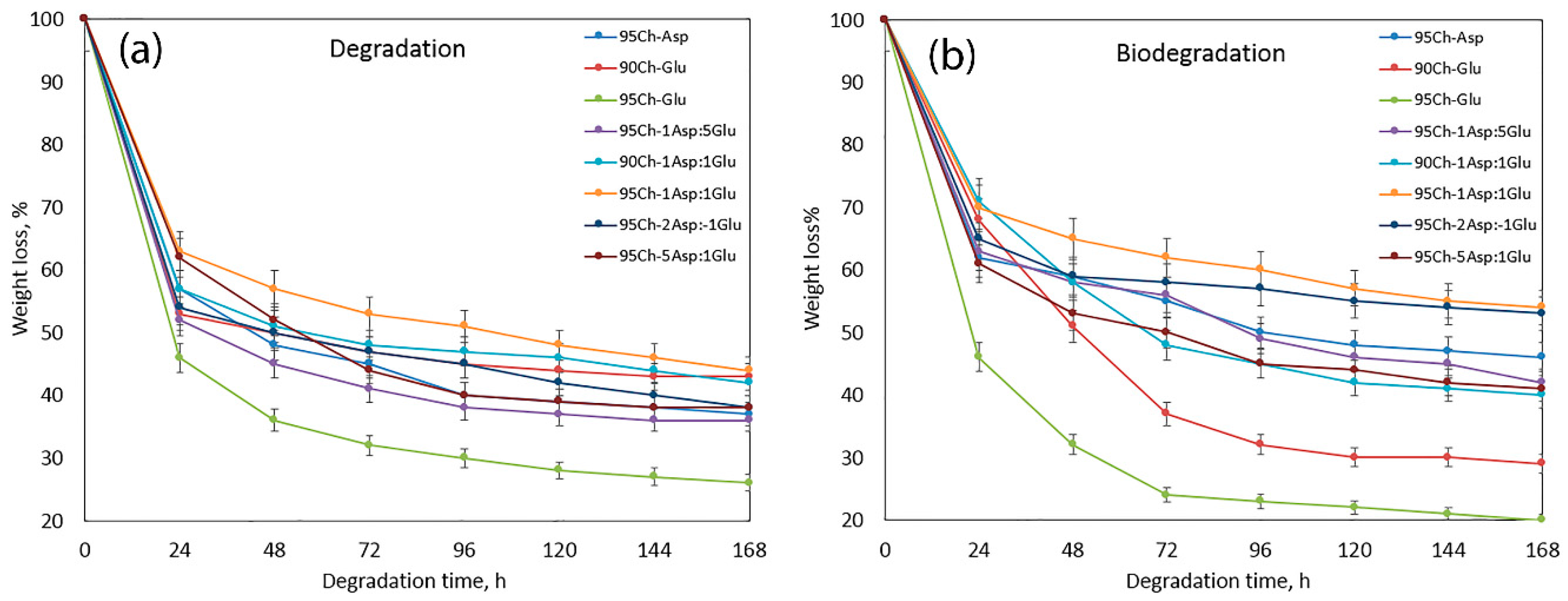
2.5. In Vitro Degradation and Biodegradation Study
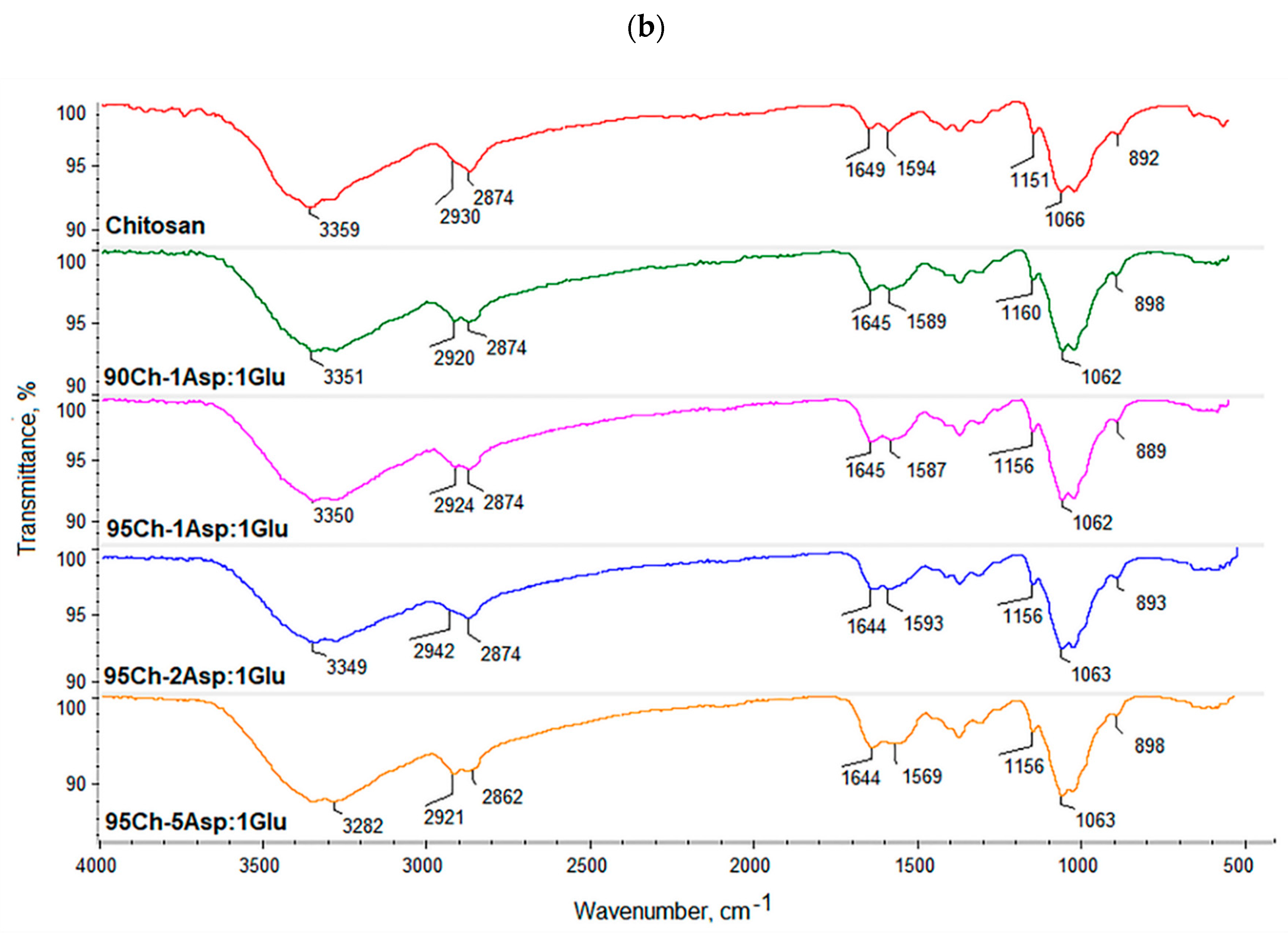
2.6. FT-IR Study
2.7. Blood-Clotting Experiment and Scanning Electron Microscopy (SEM) Analysis
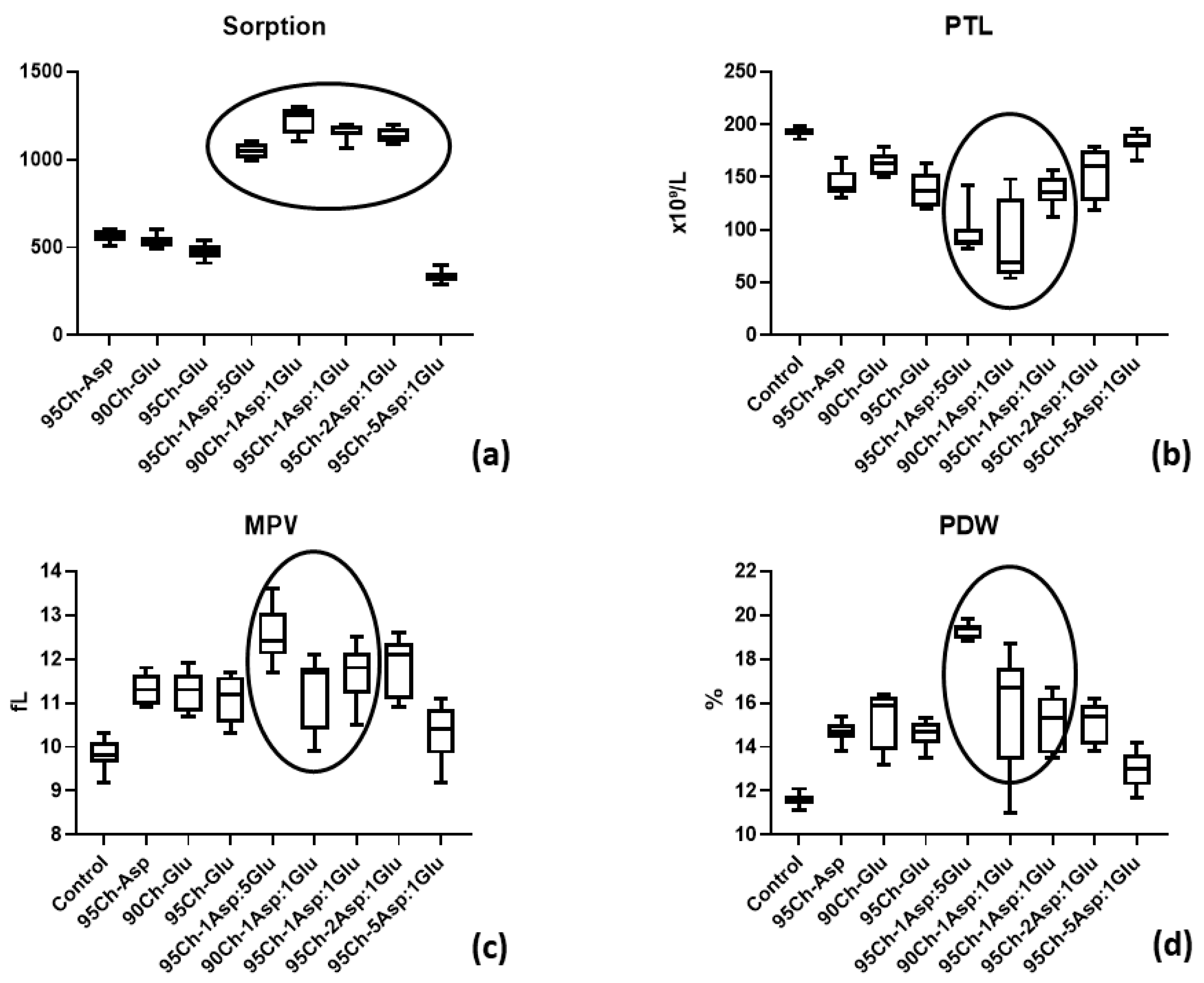
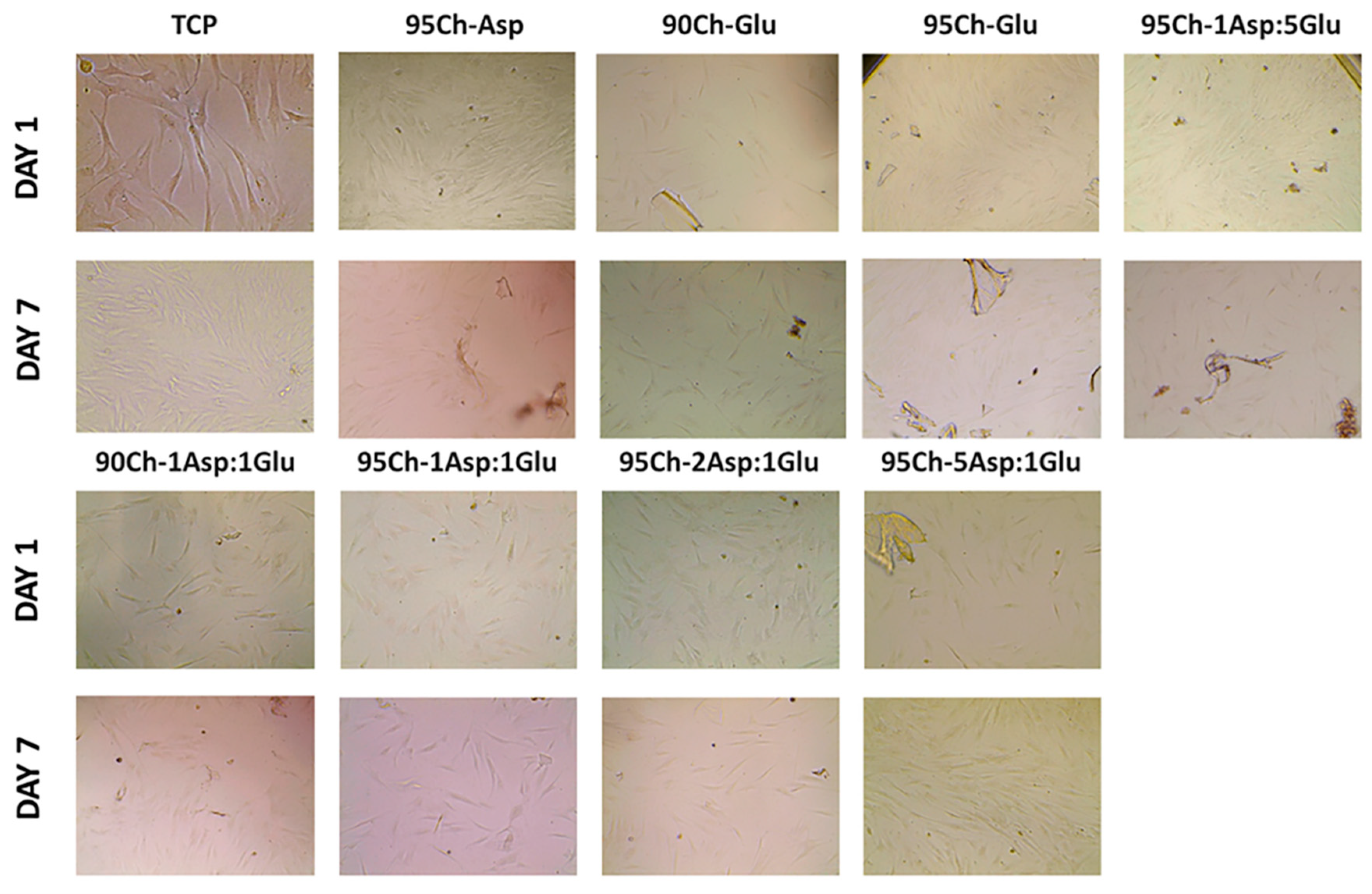
2.8. Cell Toxicity Experiment
2.9. Antibacterial Properties
3. Materials and Methods
3.1. Chitosan Hemostatics Synthesis
3.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.3. Porosity and Density Study
- d—density, g/cm3
- p—porosity, %
- W—weight of the investigated sample, g
- V1—initial volume of isopropanol, cm3
- V2—volume of isopropanol with immersed sample, cm3
- V3—volume of isopropanol after sample removal, cm3
3.4. Water Vapor Transmission Rate
- W0—the initial weight,
- Wt—the weight after time t,
- t—the measuring time
- A—the area of the opening of the polystyrene well
3.5. Antioxidant Activity
- %S—the % of the free radicals which were neutralized
- Ac—the absorbance of the DPPH solution without the sample
- As—the absorbance of the DPPH solution containing the sample
3.6. In Vitro Degradation and Biodegradation Study
- (B)D—(bio)degradation degree, %
- W0—initial weight of the analyzed sample, g
- Wt—sample weight after time = t, min
3.7. Blood Clotting Tests
- W1—initial weight (40 mg)
- W2—weight after the blood clotting test, mg
3.8. Scanning Electron Microscopy (SEM)
3.9. Cell Culture
3.10. Bacteriology Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, 26023. [Google Scholar] [CrossRef] [PubMed]
- Pogorielov, M.; Kalinkevich, O.; Deineka, V.; Garbuzova, V.; Solodovnik, A.; Kalinkevich, A.; Kalinichenko, T.; Gapchenko, A.; Sklyar, A.; Danilchenko, S. Haemostatic chitosan coated gauze: In vitro interaction with human blood and in-vivo effectiveness. Biomater. Res. 2015, 19, 217. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L. Bleeding Control Using Hemostatic Dressings: Lessons Learned. Wilderness Environ. Med. 2017, 28, S39–S49. [Google Scholar] [CrossRef] [PubMed]
- Toledo, S.L.; Guedes, J.V.; Alpoim, P.N.; Rios, D.R.; Pinheiro, M.B. Sickle cell disease: Hemostatic and inflammatory changes, and their interrelation. Clin. Chim. Acta 2019, 493, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, I.; Vasdev, N.; Soomro, N. A Review of Current Hemostatic Agents and Tissue Sealants Used in Laparoscopic Partial Nephrectomy. Rev. Urol. 2011, 13, 131–138. [Google Scholar]
- Ogle, O.E.; Swantek, J.; Kamoh, A. Hemostatic Agents. Dent. Clin. North Am. 2011, 55, 433–439. [Google Scholar] [CrossRef]
- Nakielski, P.; Pierini, F. Blood interactions with nano and microfibers: Recent advances, challenges and applications in nano and microfibrous hemostatic agents. Acta Biomater. 2019, 84, 63–76. [Google Scholar] [CrossRef]
- Lundin, J.G.; McGann, C.L.; Weise, N.K.; Estrella, L.A.; Balow, R.B.; Streifel, B.C.; Wynne, J.H. Iodine binding and release from antimicrobial hemostatic polymer foams. React. Funct. Polym. 2019, 135, 44–51. [Google Scholar] [CrossRef]
- Landsman, T.L.; Touchet, T.; Hasan, S.M.; Smith, C.; Russell, B.; River, J.; Maitland, D.J.; Cosgriff-Hernandez, E. A shape memory foam composite with enhanced fluid uptake and bactericidal properties as a hemostatic agent. Acta Biomater. 2017, 47, 91–99. [Google Scholar] [CrossRef]
- Piatkowski, M.; Janus, Ł.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Biodegradable, pH-sensitive chitosan beads obtained under microwave radiation for advanced cell culture. Coll. Surf. B Biointerfaces 2018, 164, 324–331. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Shaji, J.; Jain, V.; Lodha, S. Chitosan: A novel pharmaceutical excipient. Int. J. Pharm. Appl. Sci. 2010, 1, 11–28. [Google Scholar]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [PubMed]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Logith Kumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, S.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Ma, Y.; Xin, L.; Tan, H.; Fan, M.; Li, J.; Jia, Y.; Ling, Z.; Chen, Y.; Hu, X. Chitosan membrane dressings toughened by glycerol to load antibacterial drugs for wound healing. Mater. Sci. Eng. C 2017, 81, 522–531. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.; Xing, K.; Park, H. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Lim, S.; Song, D.; Oh, S.; Lee-Yoon, D.; Bae, E.; Lee, J. In vitro and in vivo degradation behavior of acetylated chitosan porous beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 453–466. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Yan, T.; Cheng, F.; Wei, X.; Huang, Y.; He, J. Biodegradable collagen sponge reinforced with chitosan/calcium pyrophosphate nanoflowers for rapid hemostasis. Carbohydr. Polym. 2017, 170, 271–280. [Google Scholar] [CrossRef]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Coll. Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.Ö.; Mol, A.; Menteş, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawakami, K.; Miyatake, K.; Morimoto, M.; Shigemasa, Y.; Minami, S. Analgesic effects of chitin and chitosan. Carbohydr. Polym. 2002, 49, 249–252. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar] [CrossRef]
- De Lima, J.M.; Sarmento, R.R.; De Souza, J.R.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.F.B.D.; De Oliveira, J.E.; et al. Evaluation of Hemagglutination Activity of Chitosan Nanoparticles Using Human Erythrocytes. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2002, 22, 261–268. [Google Scholar] [CrossRef]
- Piątkowski, M.; Radwan-Pragłowska, J.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. Microwave-assisted synthesis and characterization of chitosan aerogels doped with Au-NPs for skin regeneration. Polym. Test. 2019, 73, 366–376. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |



| Time of Incubation (hours) | Sample/Isolated Microorganisms in Log (CFU—Colony Forming Unit) | |||||||
|---|---|---|---|---|---|---|---|---|
| 95Ch-Asp | 90Ch-Glu | 95Ch-Glu | 95Ch-1Asp:5Glu | 90Ch-1Asp:1Glu | 95Ch-1Asp:1Glu | 95Ch-2Asp:1Glu | 95Ch-5Asp:1Glu | |
| 0 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 2 | 2 | 3.7 | 0 | 2 | 3.5 | 2 | 0 | 0 |
| 4 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Time of Incubation (hours) | Sample/Isolated Microorganisms in Log (CFU) | |||||||
|---|---|---|---|---|---|---|---|---|
| 95Ch-Asp | 90Ch-Glu | 95Ch-Glu | 95Ch-1Asp:5Glu | 90Ch-1Asp:1Glu | 95Ch-1Asp:1Glu | 95Ch-2Asp:1Glu | 95Ch-5Asp:1Glu | |
| 0 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 |
| 4 | 5 | 3 | 0 | 3 | 4 | 0 | 5 | 3 |
| 6 | 5 | 5 | 0 | 4.7 | 5 | 0 | 6.7 | 5.7 |
| 8 | 5 | 6.7 | 0 | 6.7 | 6.7 | 0 | 6.7 | 0 |
| 10 | 5 | 6.7 | 0 | 6.7 | 6.7 | 0 | 6.7 | 0 |
| 24 | 5 | 6.7 | 0 | 6.7 | 6.7 | 0 | 6.7 | 0 |
| Sample | Crosslinking Agents | Chitosan Deacetylation Degree (DD) | |
|---|---|---|---|
| l-Aspartic (Asp) | l-Glutamic (Glu) | ||
| 95Ch-Asp | 0.84 g | - | 95% |
| 90Ch-Glu | - | 0.84 g | 90% |
| 95Ch-Glu | - | 0.84 g | 95% |
| 95Ch-1Asp:5Glu | 0.74 g | 0.22 g | 95% |
| 90Ch-1Asp:1Glu | 0.50 g | 0.50 g | 90% |
| 95Ch-1Asp:1Glu | 0.50 g | 0.50 g | 95% |
| 95Ch-2Asp:1Glu | 0.70 g | 0.30 g | 95% |
| 95Ch-5Asp:1Glu | 0.17 g | 0.84 g | 95% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules 2019, 24, 2629. https://doi.org/10.3390/molecules24142629
Radwan-Pragłowska J, Piątkowski M, Deineka V, Janus Ł, Korniienko V, Husak E, Holubnycha V, Liubchak I, Zhurba V, Sierakowska A, et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules. 2019; 24(14):2629. https://doi.org/10.3390/molecules24142629
Chicago/Turabian StyleRadwan-Pragłowska, Julia, Marek Piątkowski, Volodymyr Deineka, Łukasz Janus, Viktoriia Korniienko, Evgenia Husak, Viktoria Holubnycha, Iryna Liubchak, Vyacheslav Zhurba, Aleksandra Sierakowska, and et al. 2019. "Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization" Molecules 24, no. 14: 2629. https://doi.org/10.3390/molecules24142629
APA StyleRadwan-Pragłowska, J., Piątkowski, M., Deineka, V., Janus, Ł., Korniienko, V., Husak, E., Holubnycha, V., Liubchak, I., Zhurba, V., Sierakowska, A., Pogorielov, M., & Bogdał, D. (2019). Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules, 24(14), 2629. https://doi.org/10.3390/molecules24142629