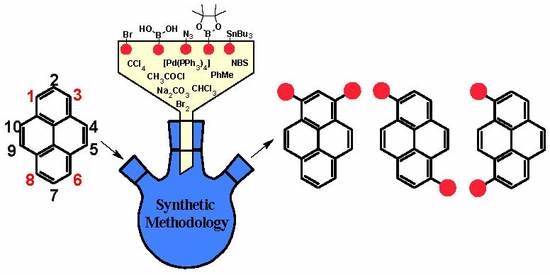
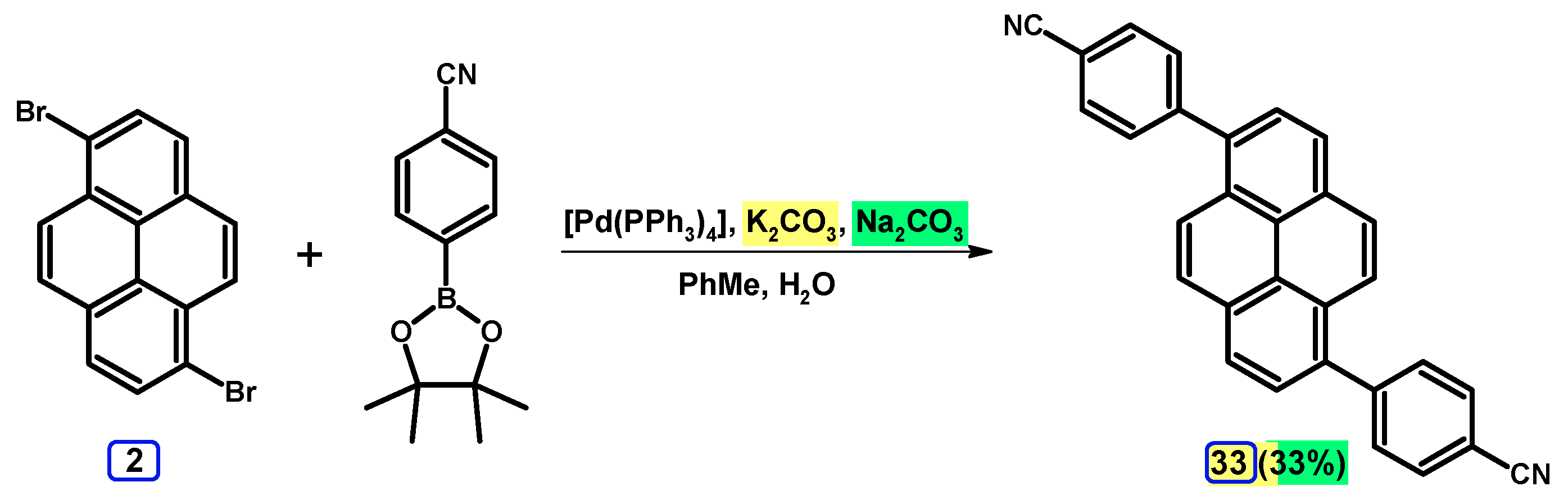Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review
Abstract
:1. Introduction
2. Dibromopyrenes
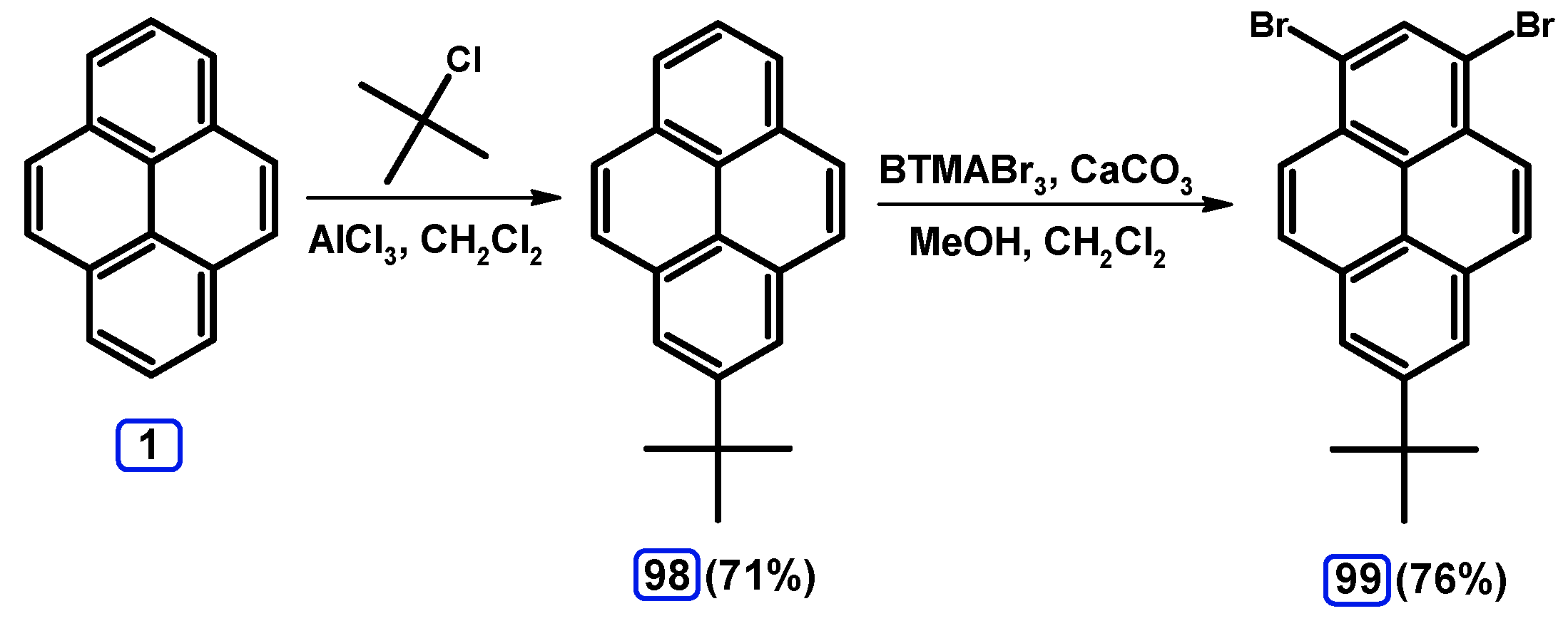
2.1. 1,6- And 1,8-dibromopyrene
2.2. 1,3-Dibromopyrene
2.3. Suzuki-Miyaura Coupling
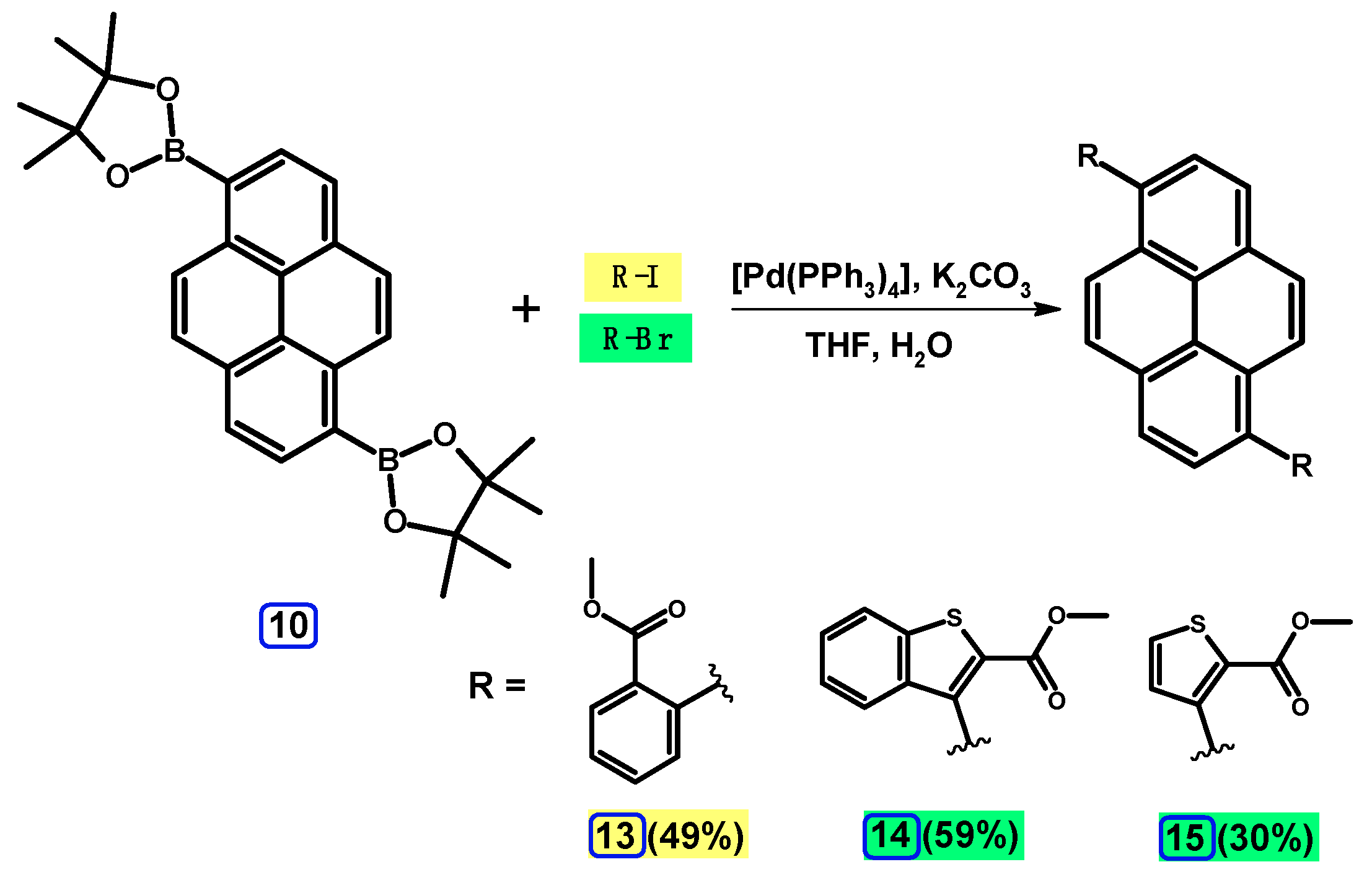
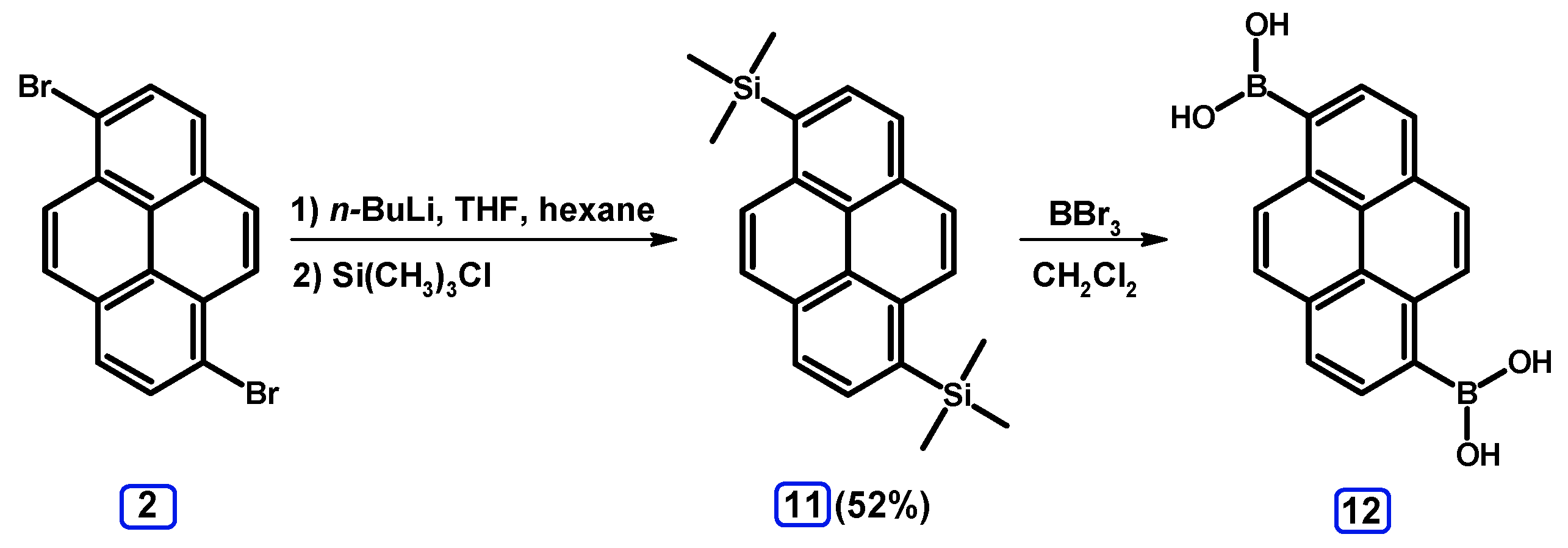
2.3.1. Pyrene Derivative Acting as a Boroorganic Compound
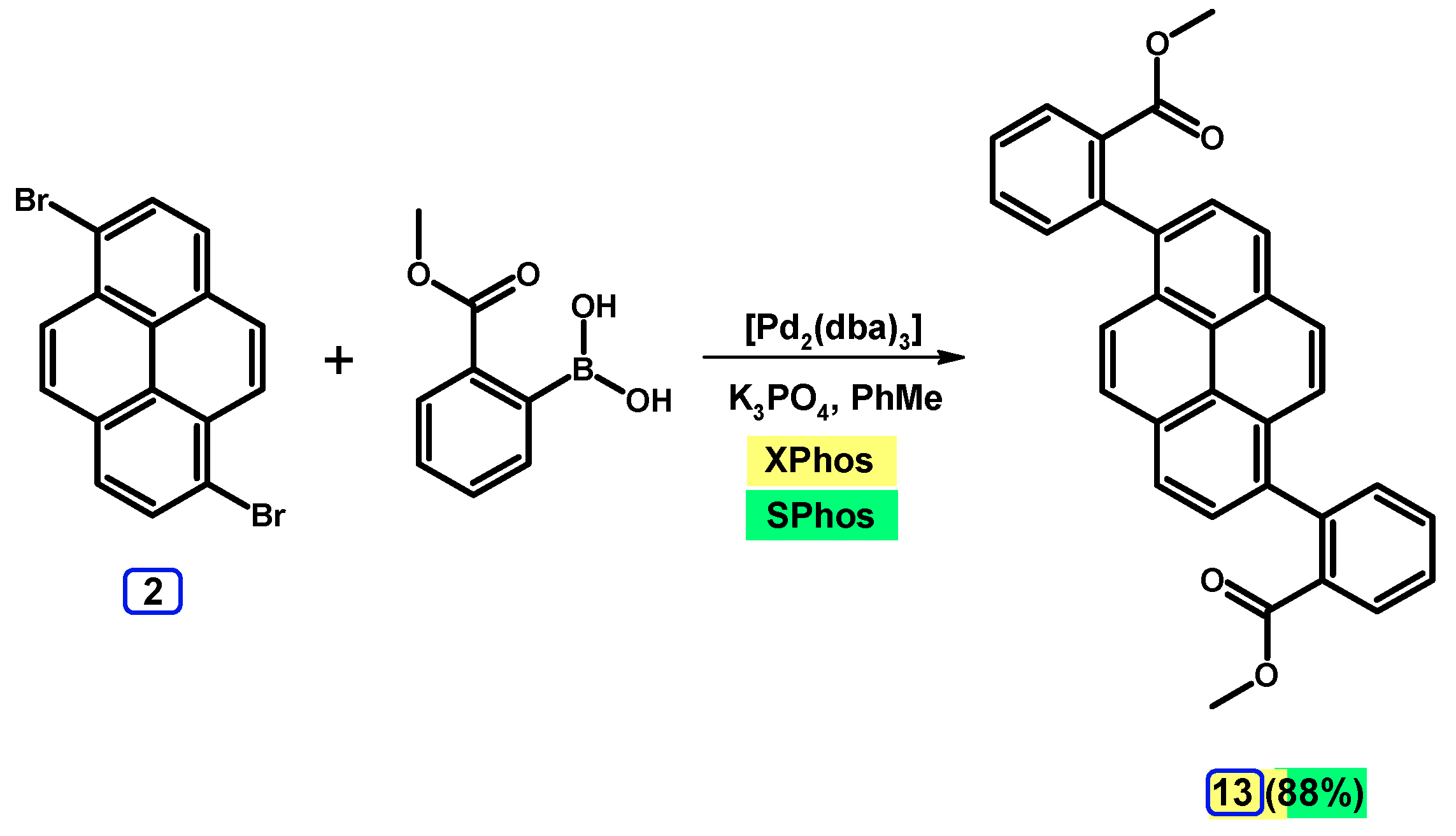
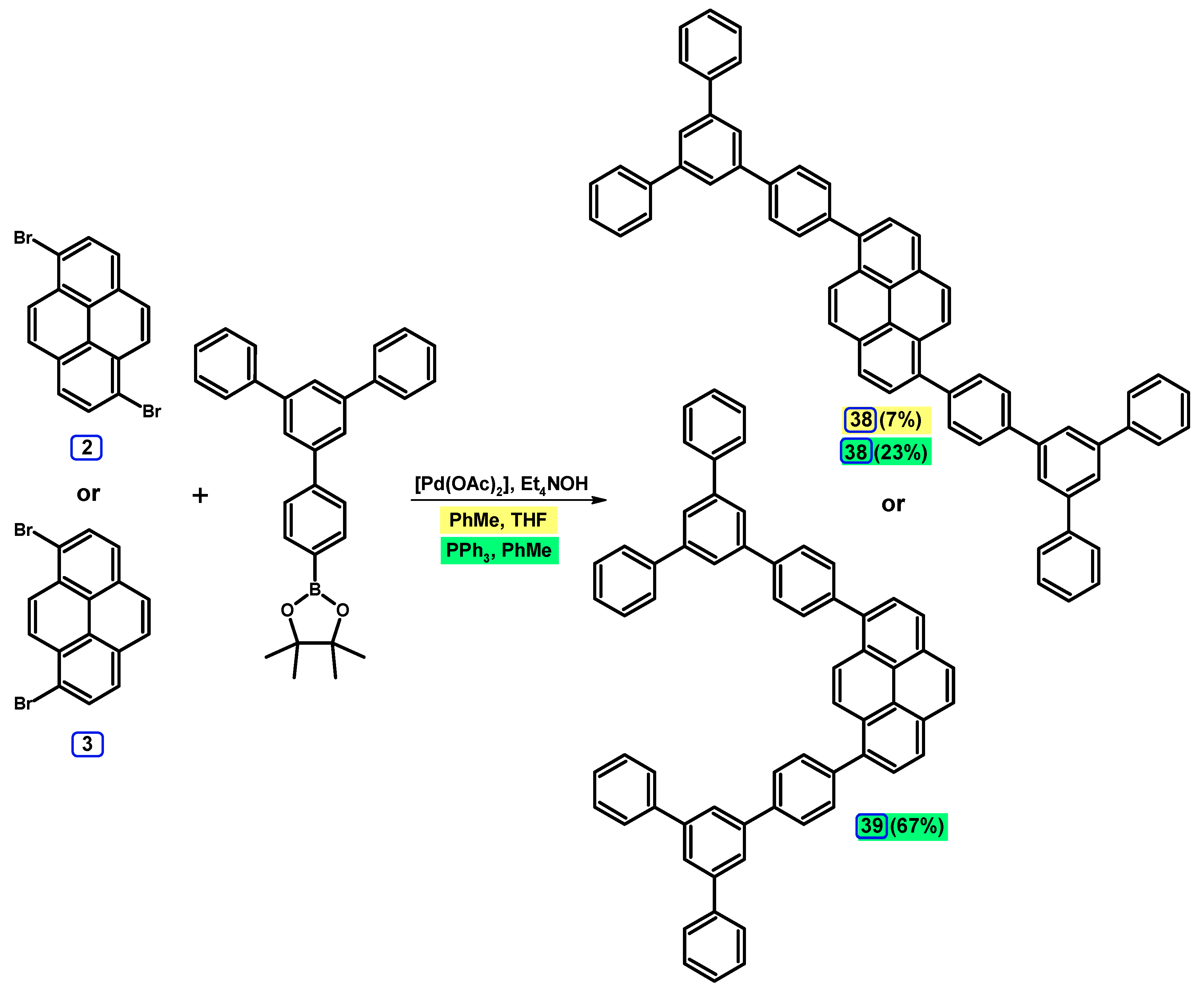
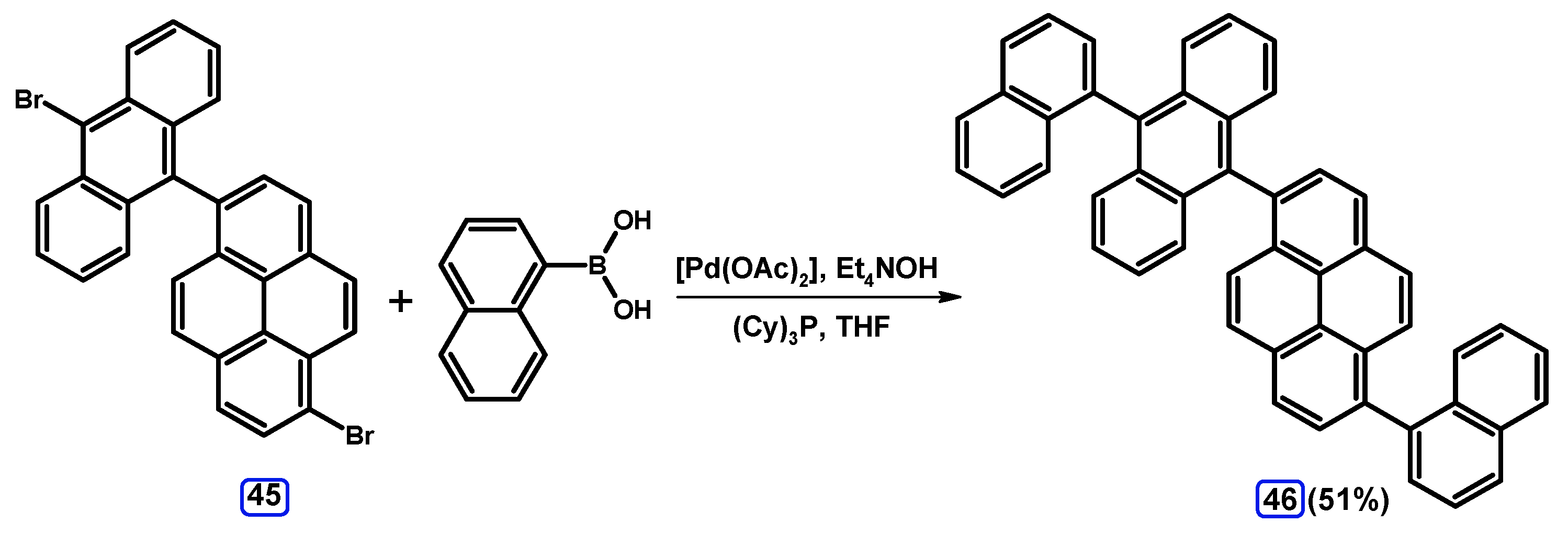
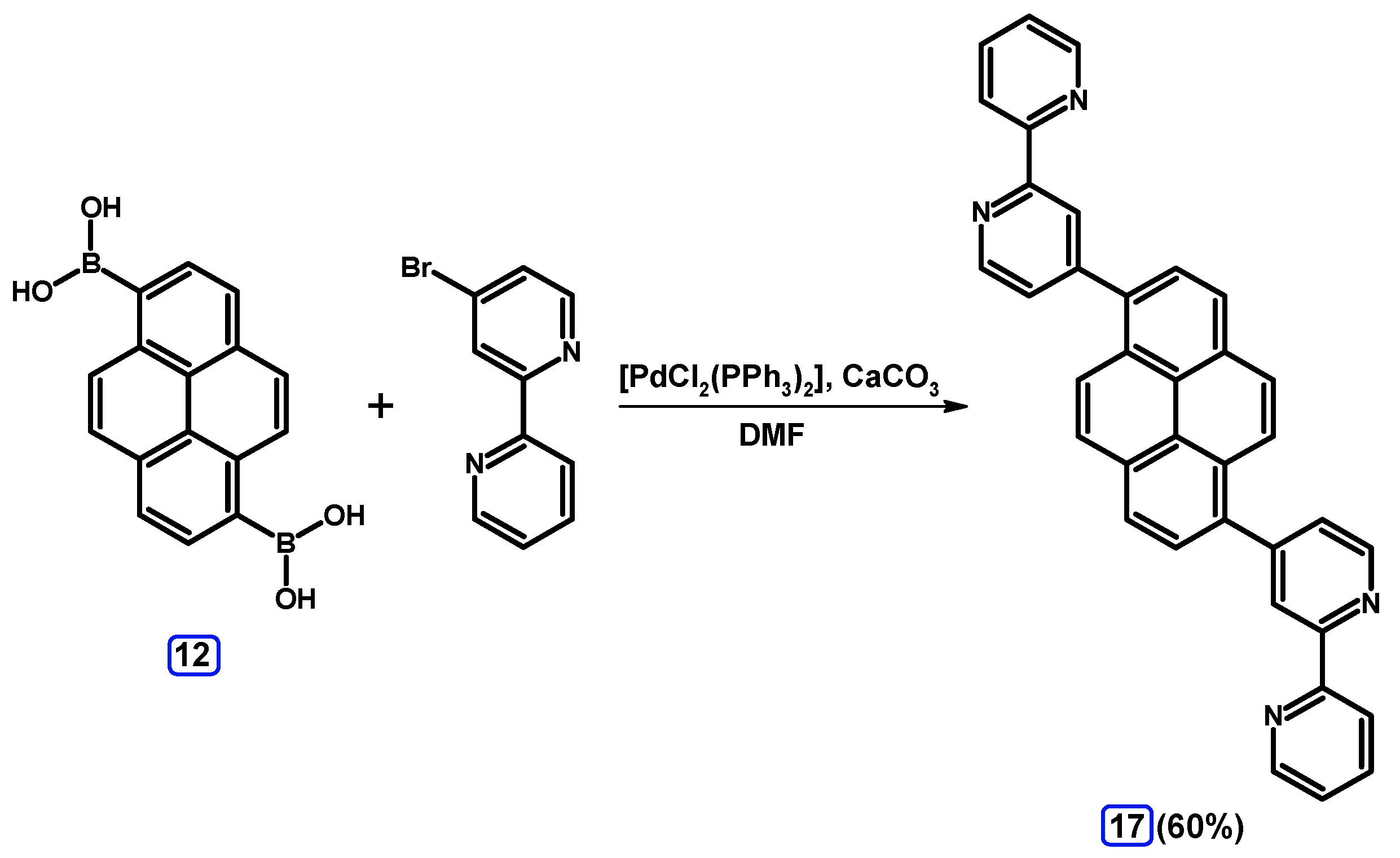
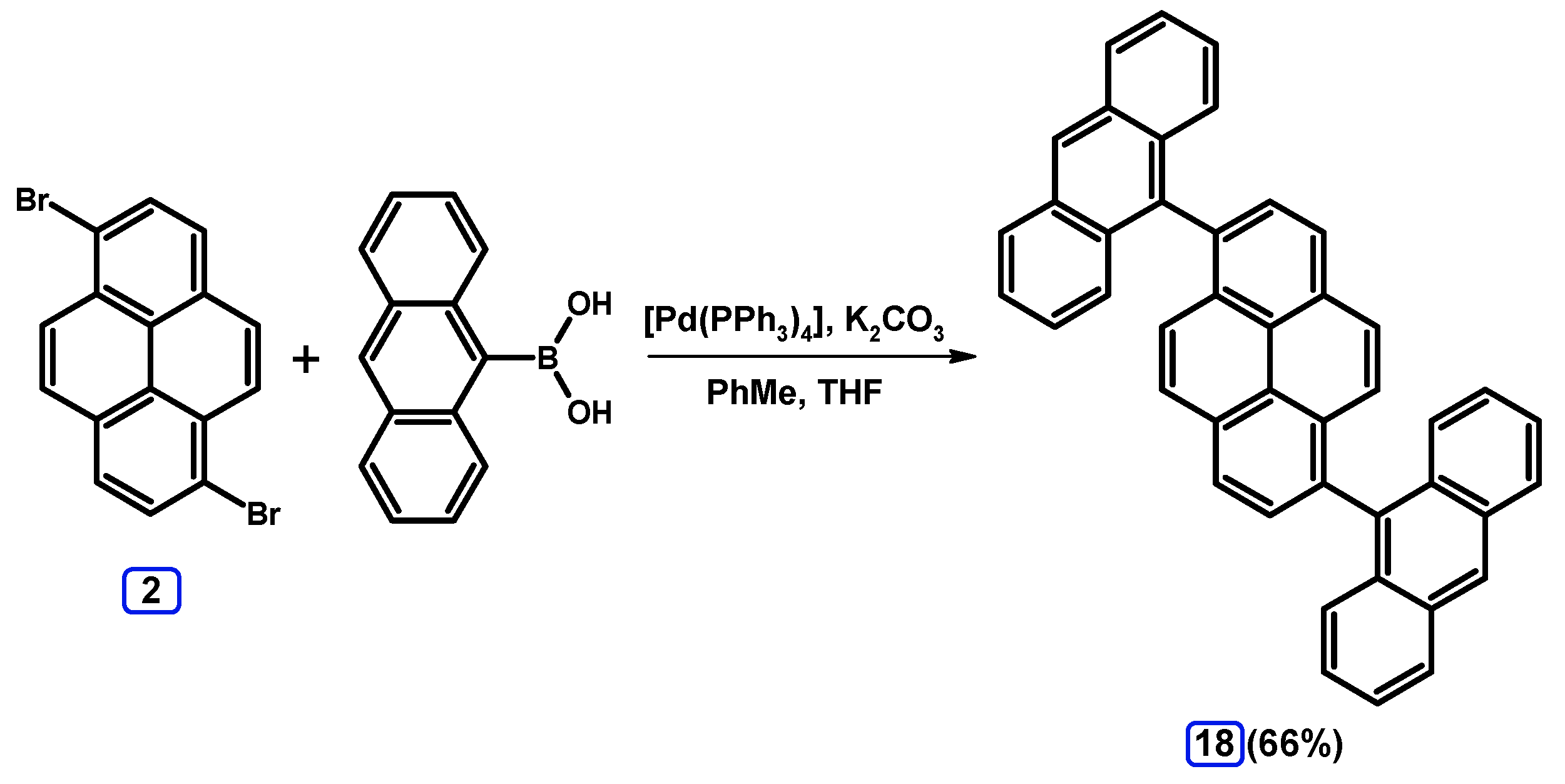
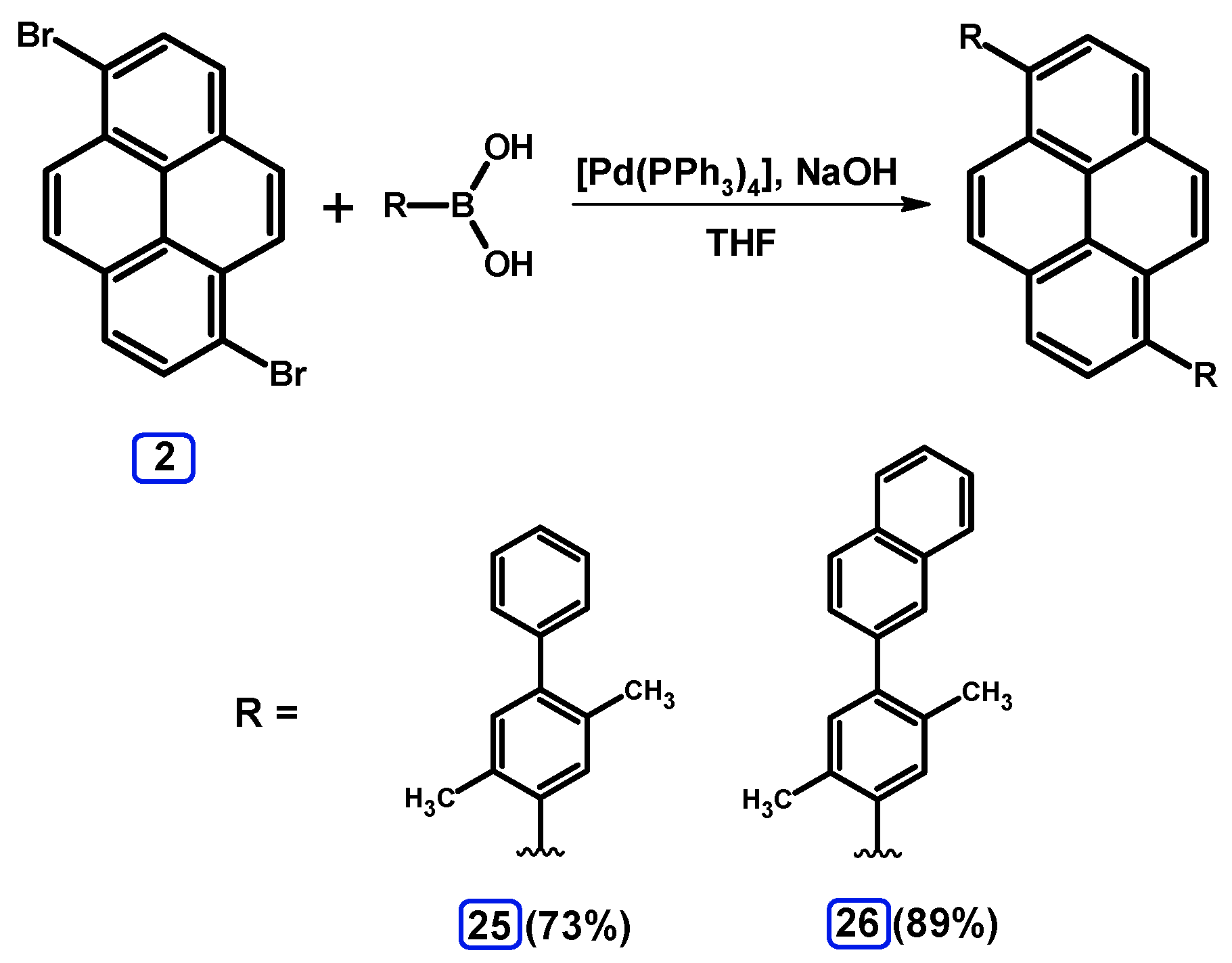
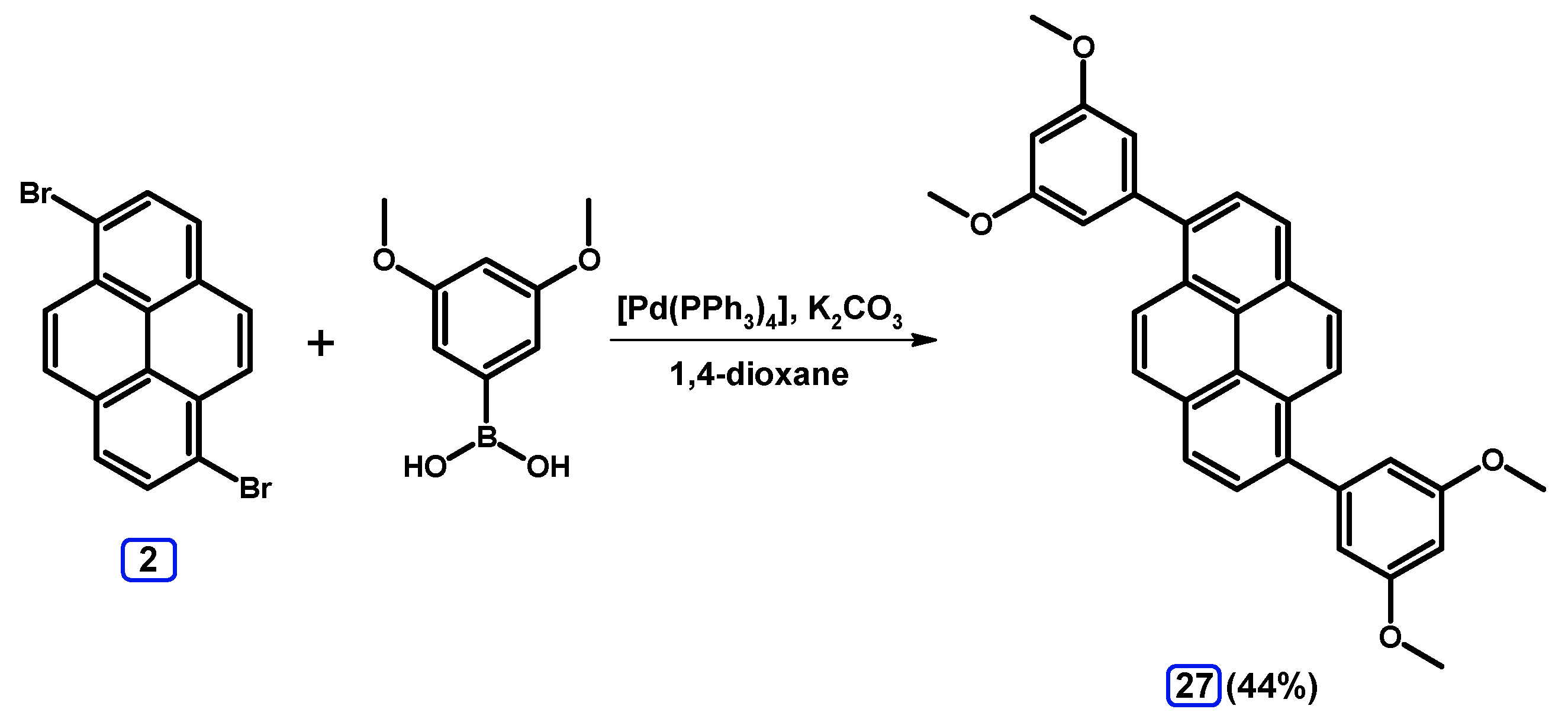
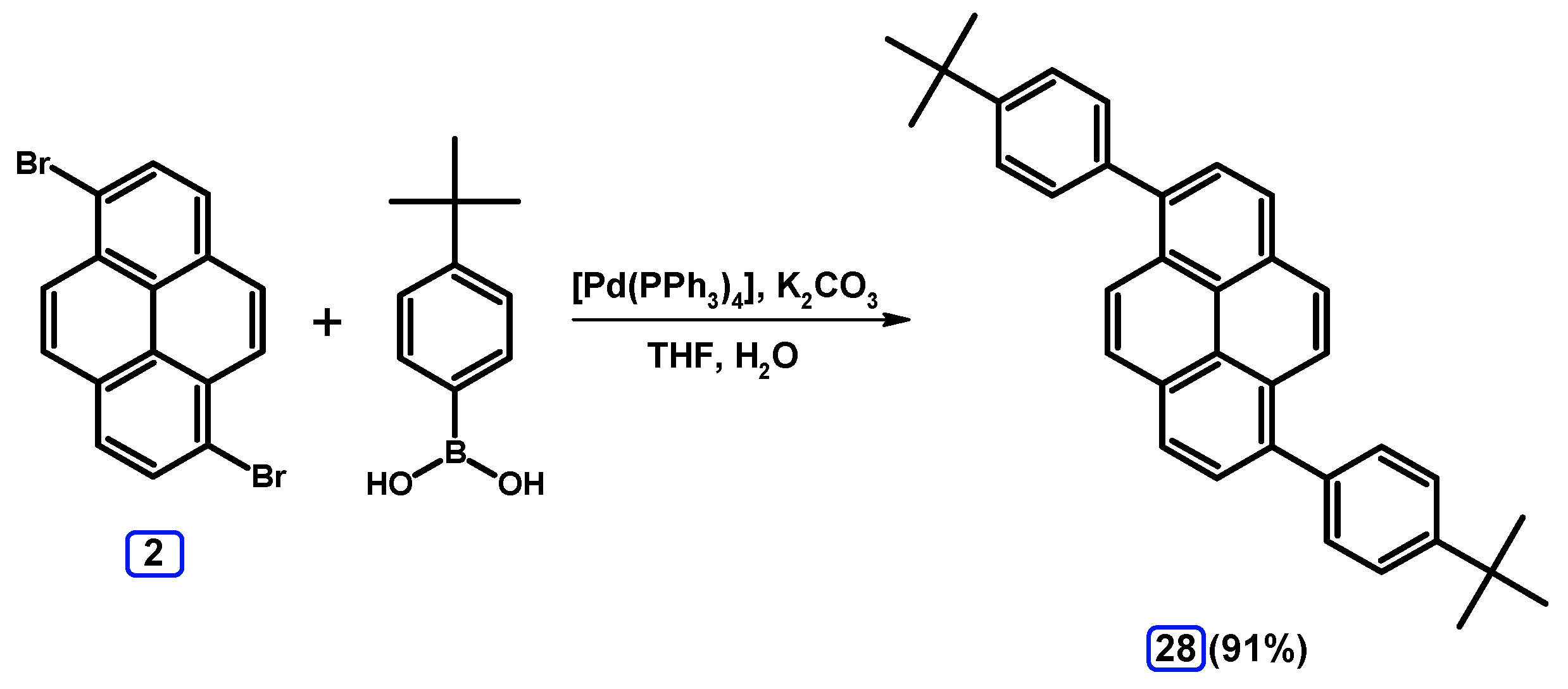
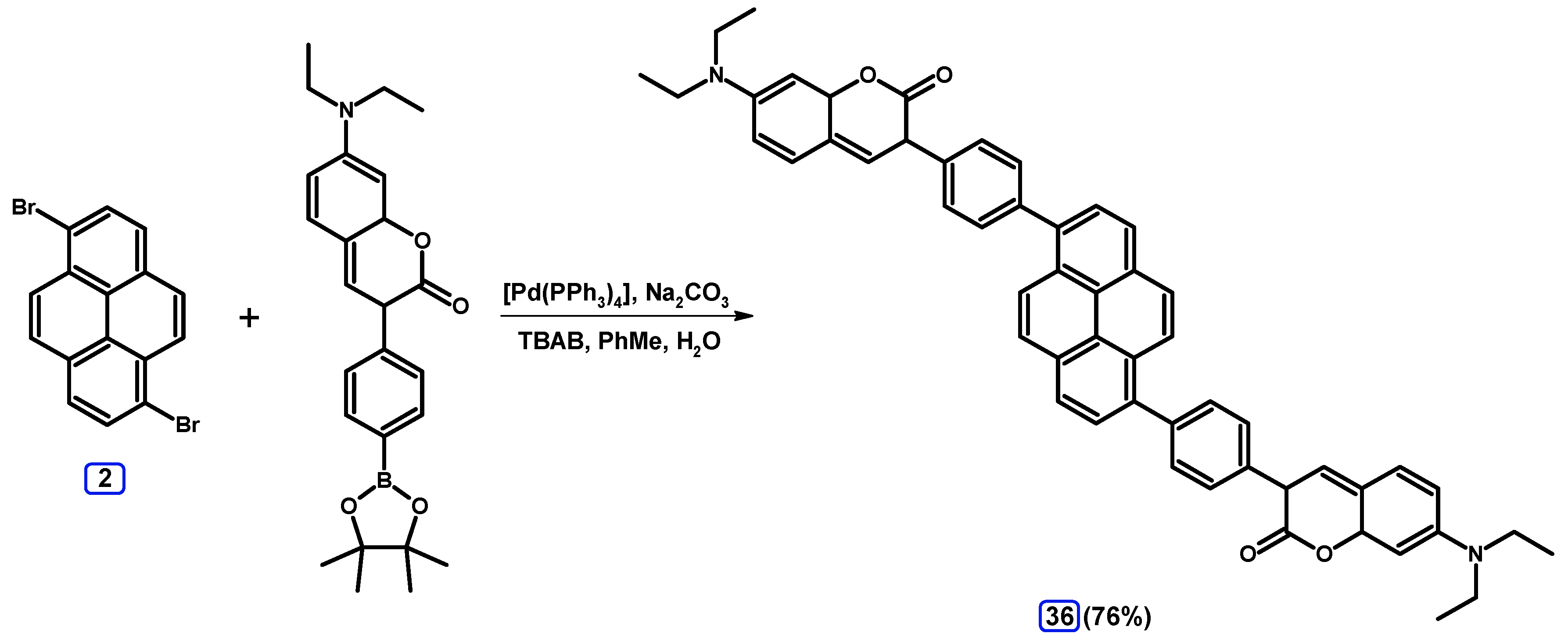
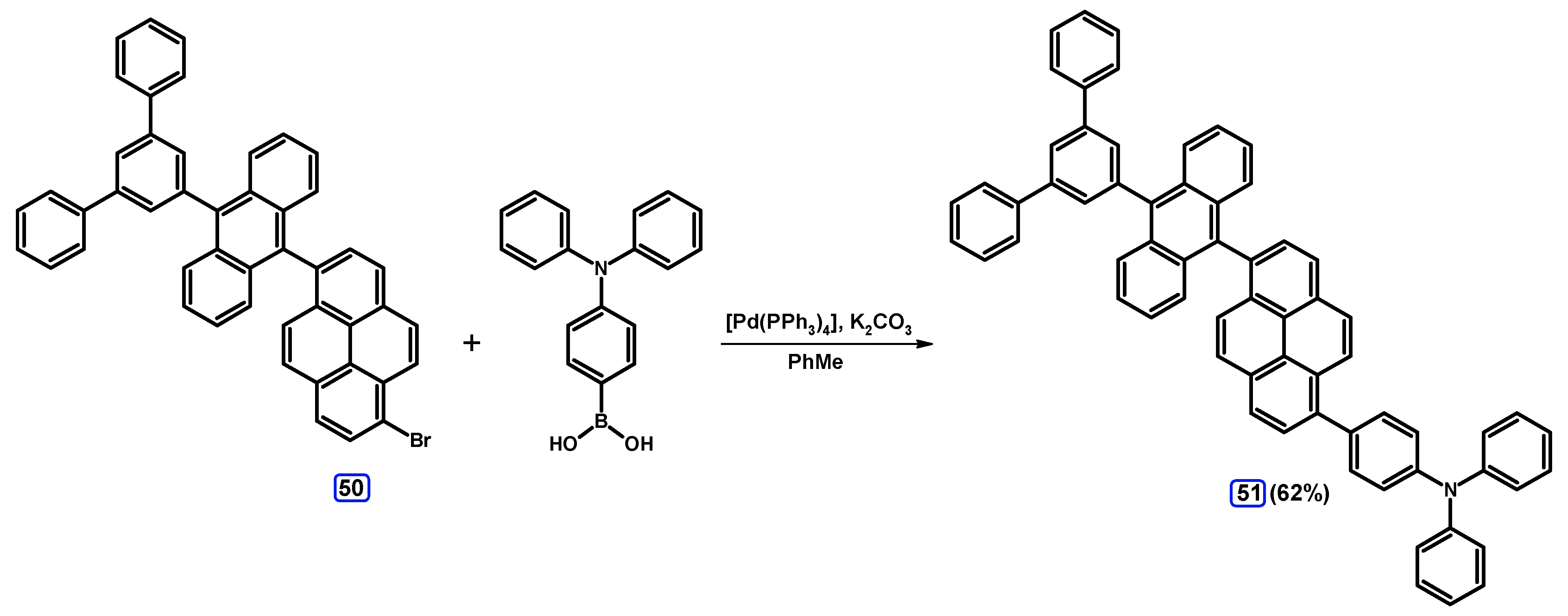
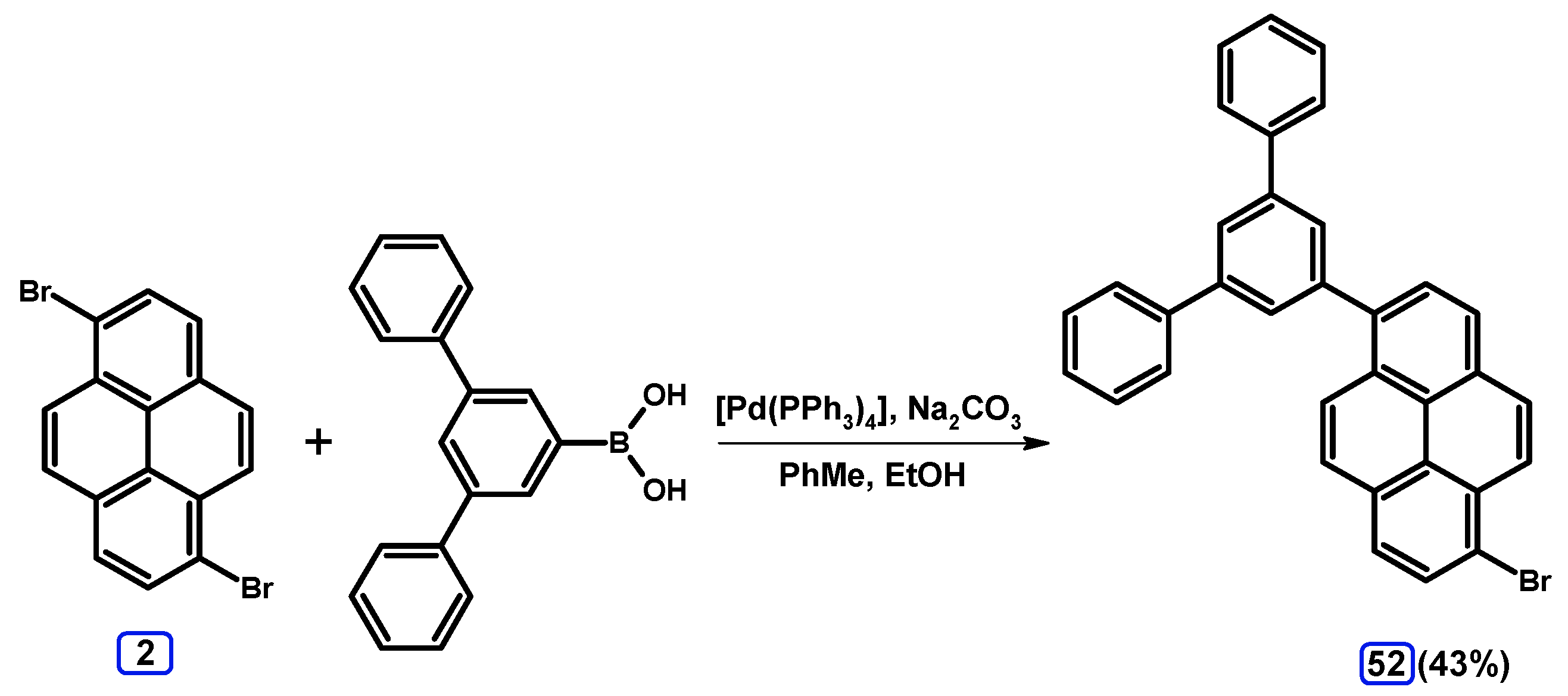
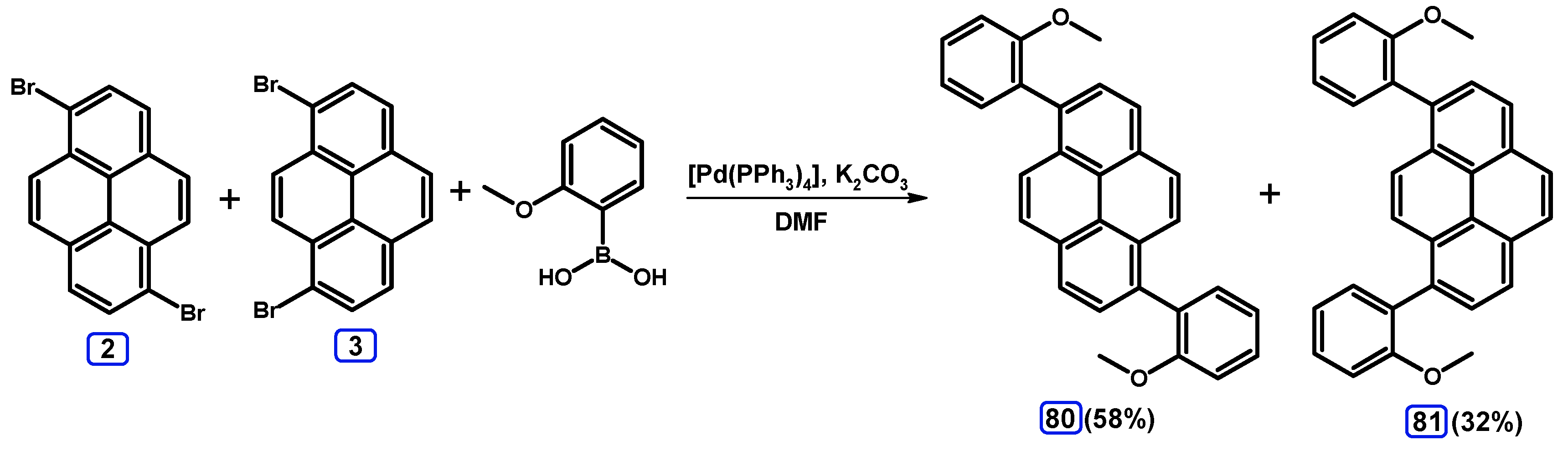
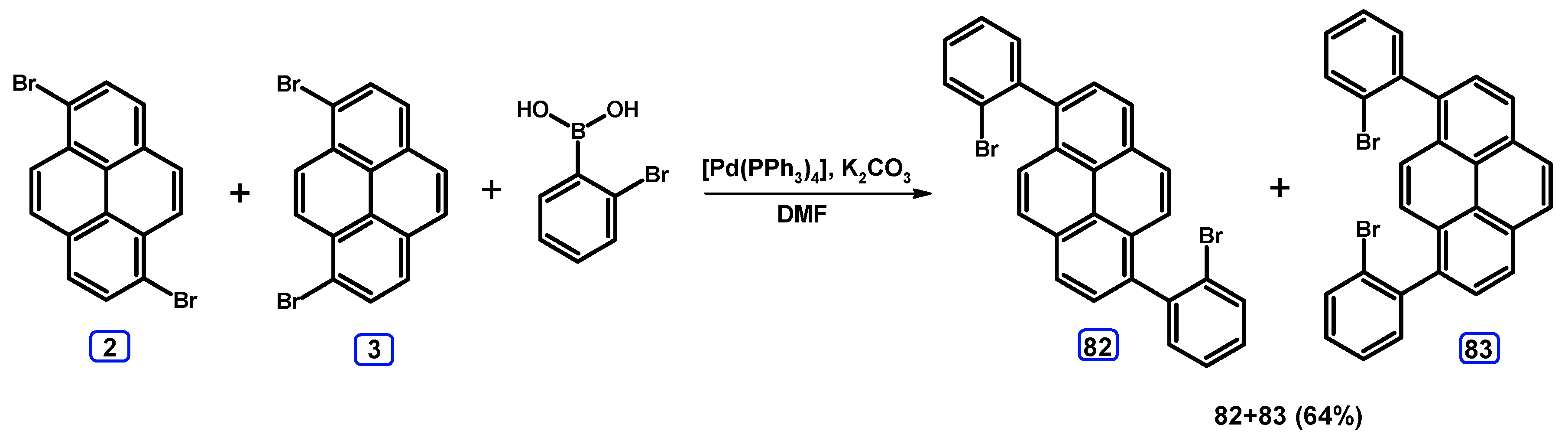
2.3.2. Suzuki-Miyaura Coupling of Dibromopyrenes with (Hetero)Arylboronic Acids
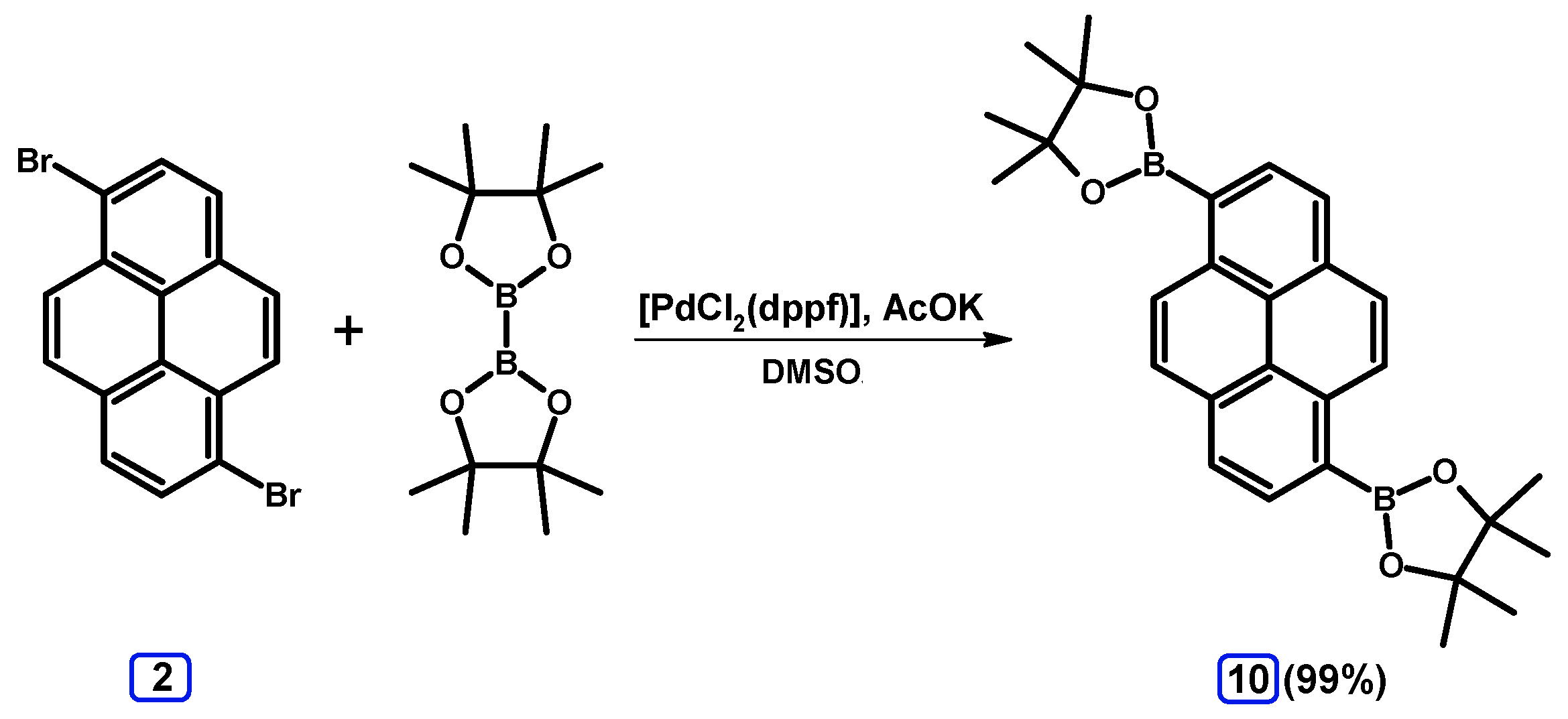
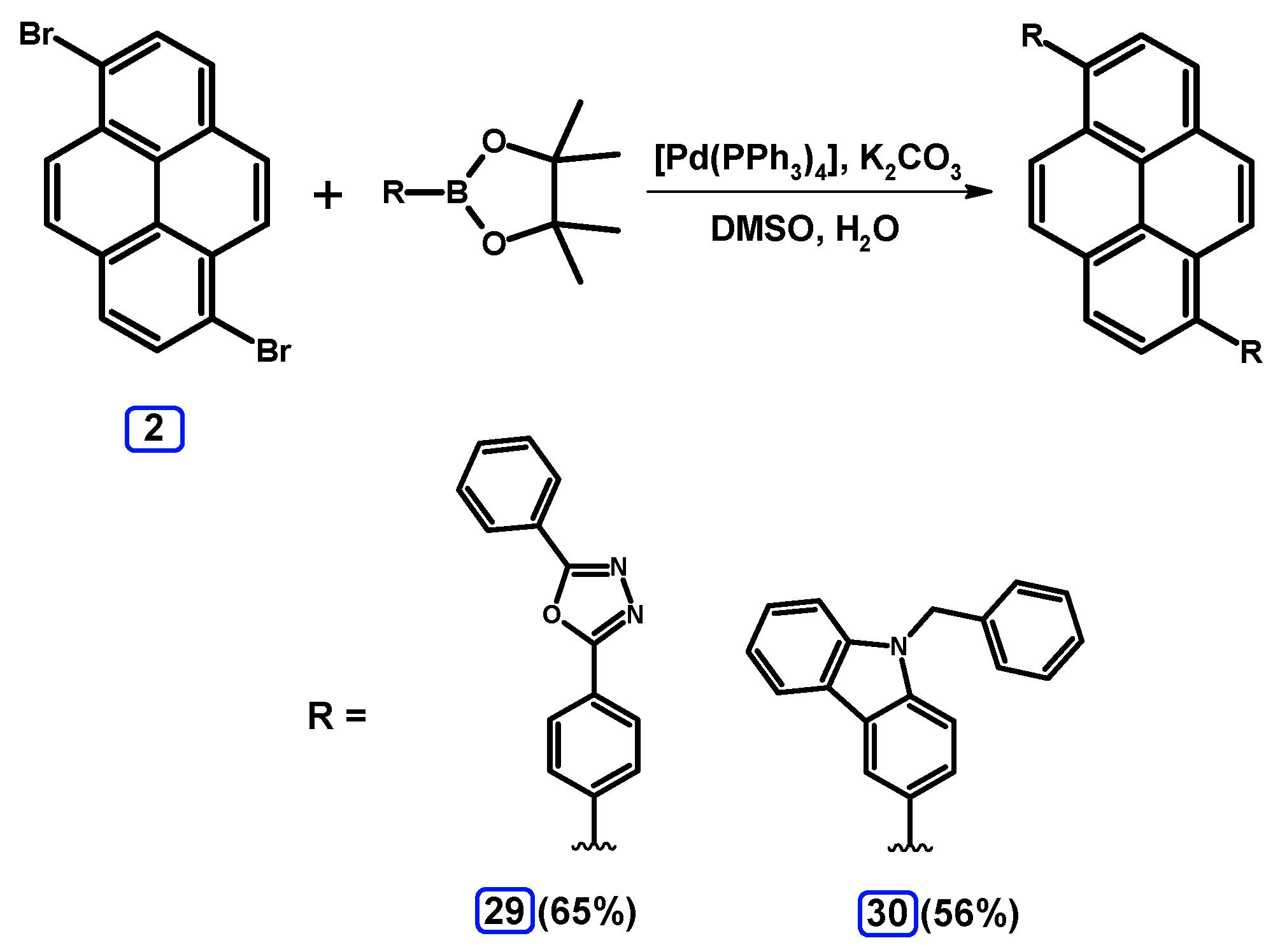
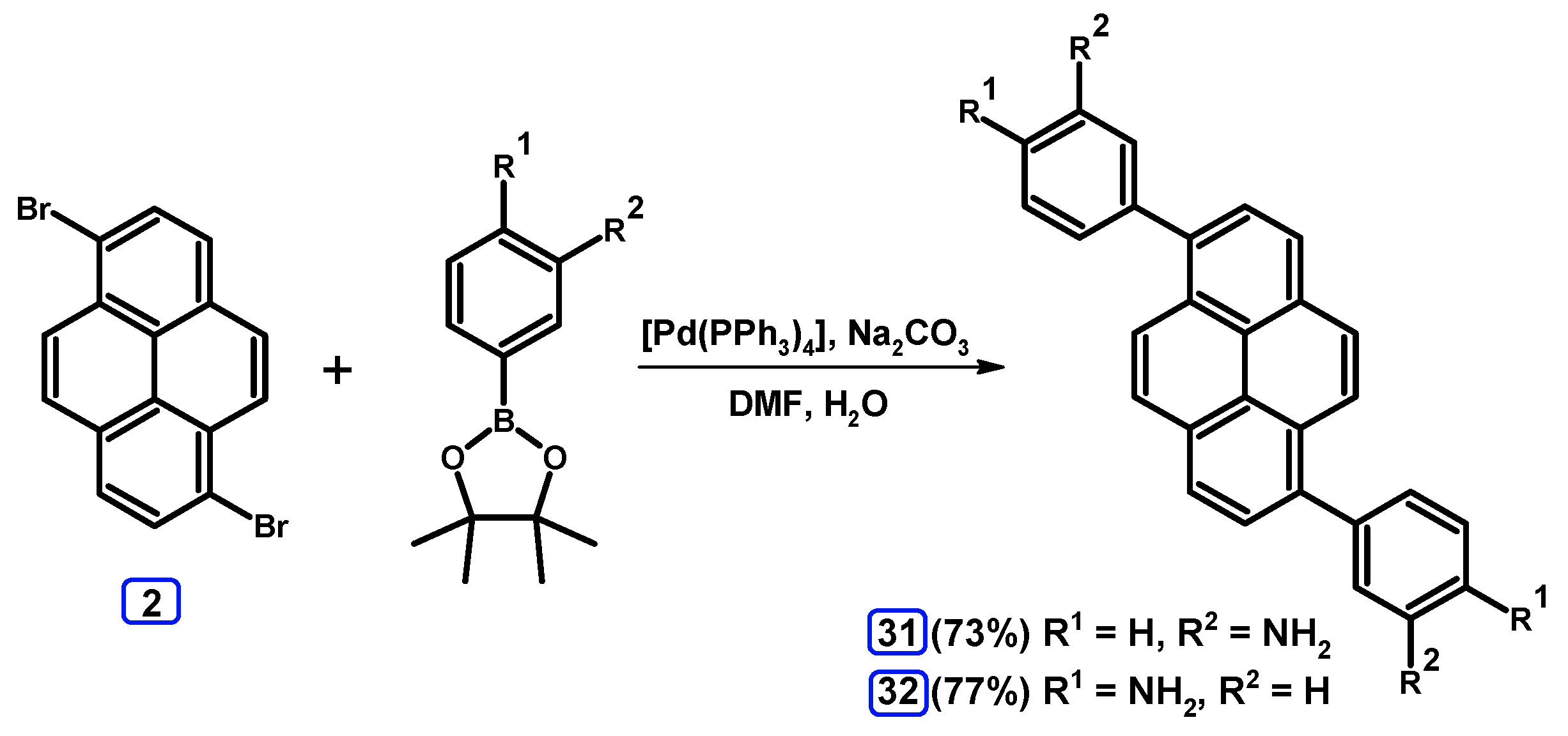
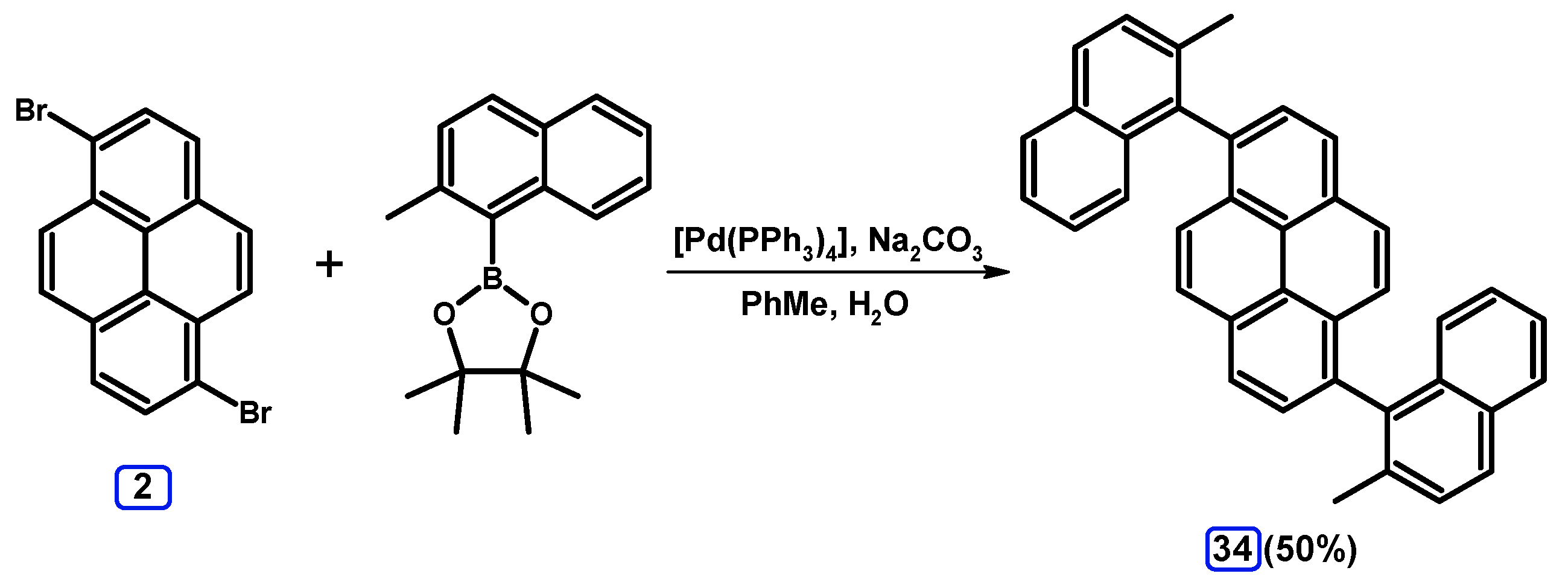
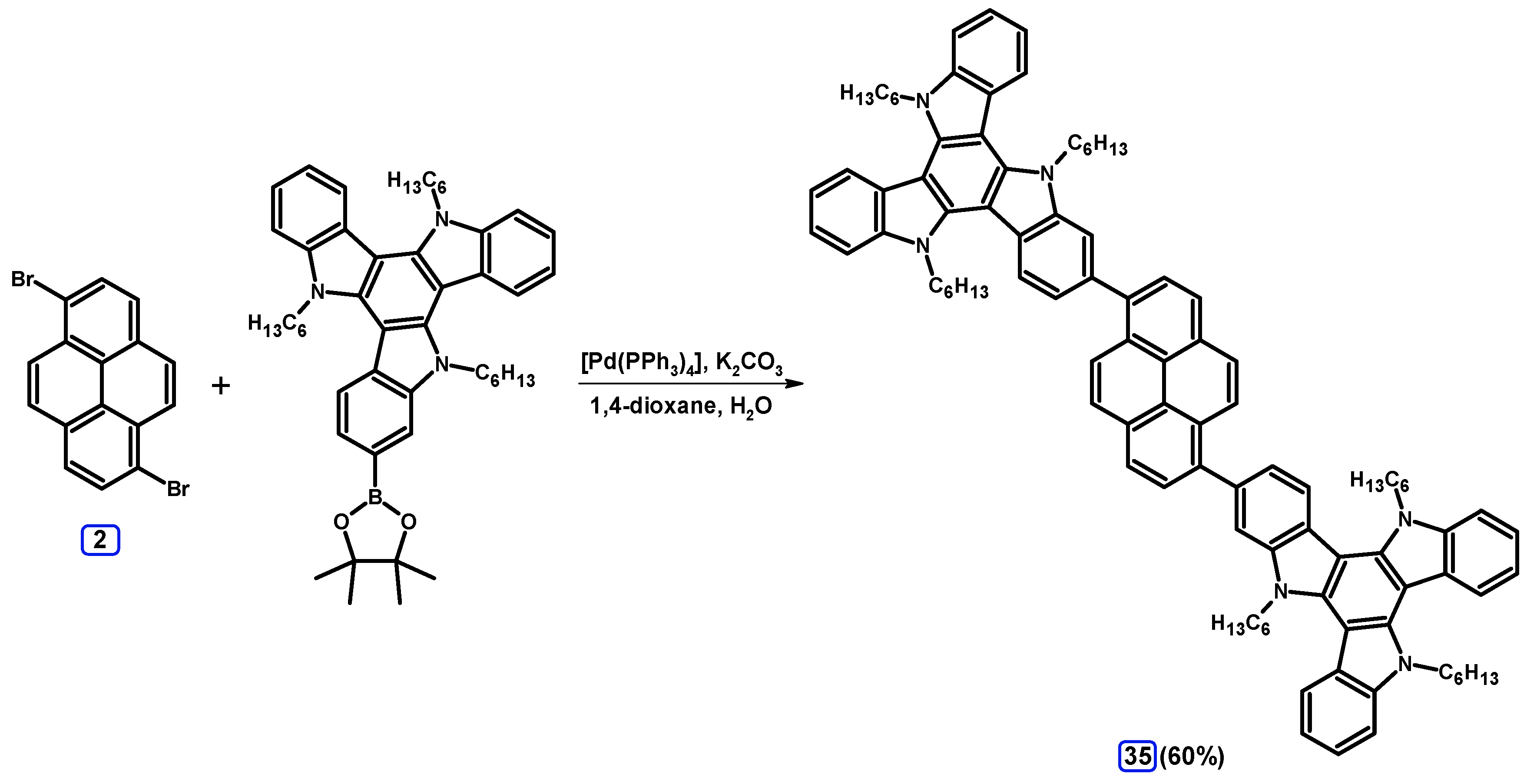
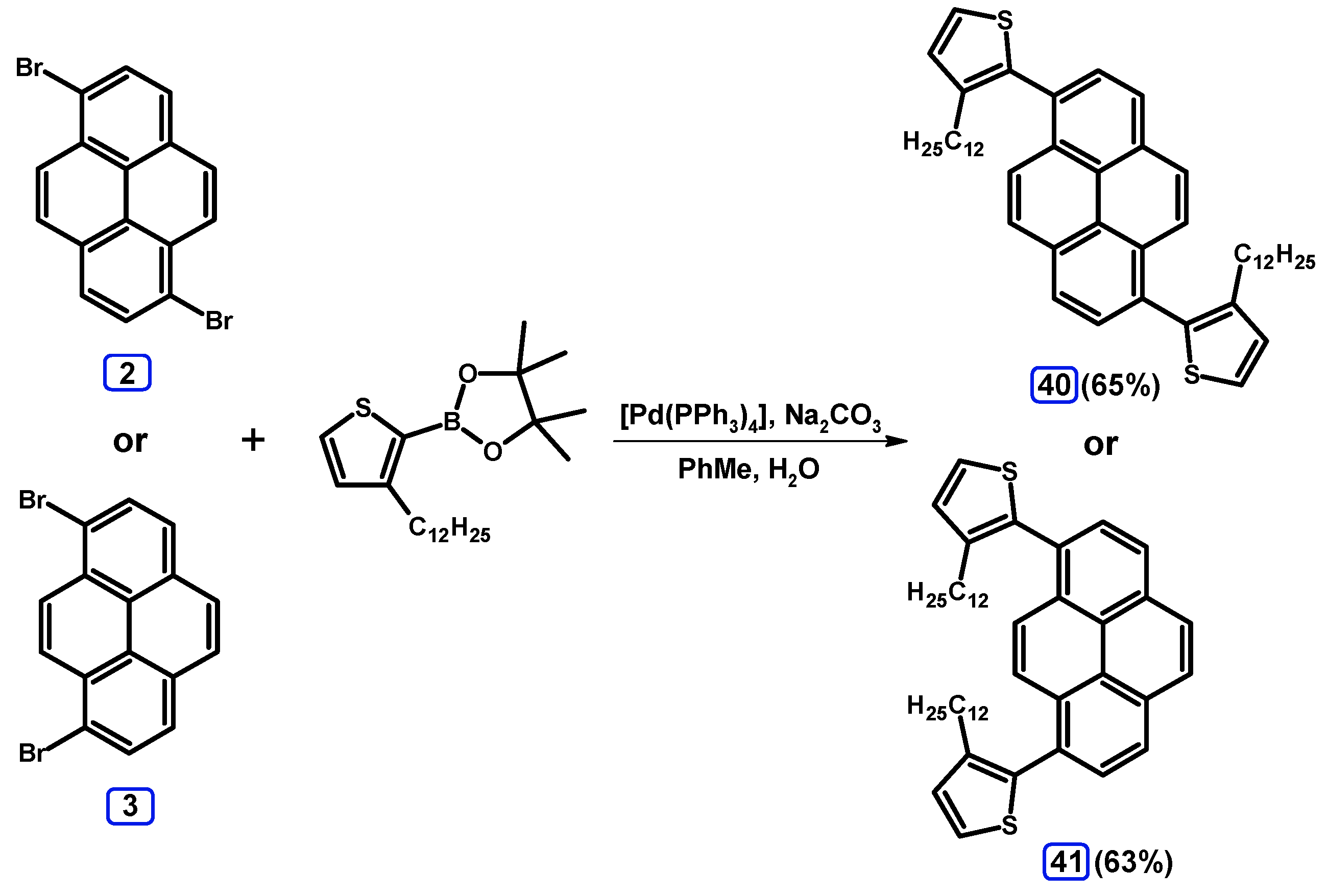
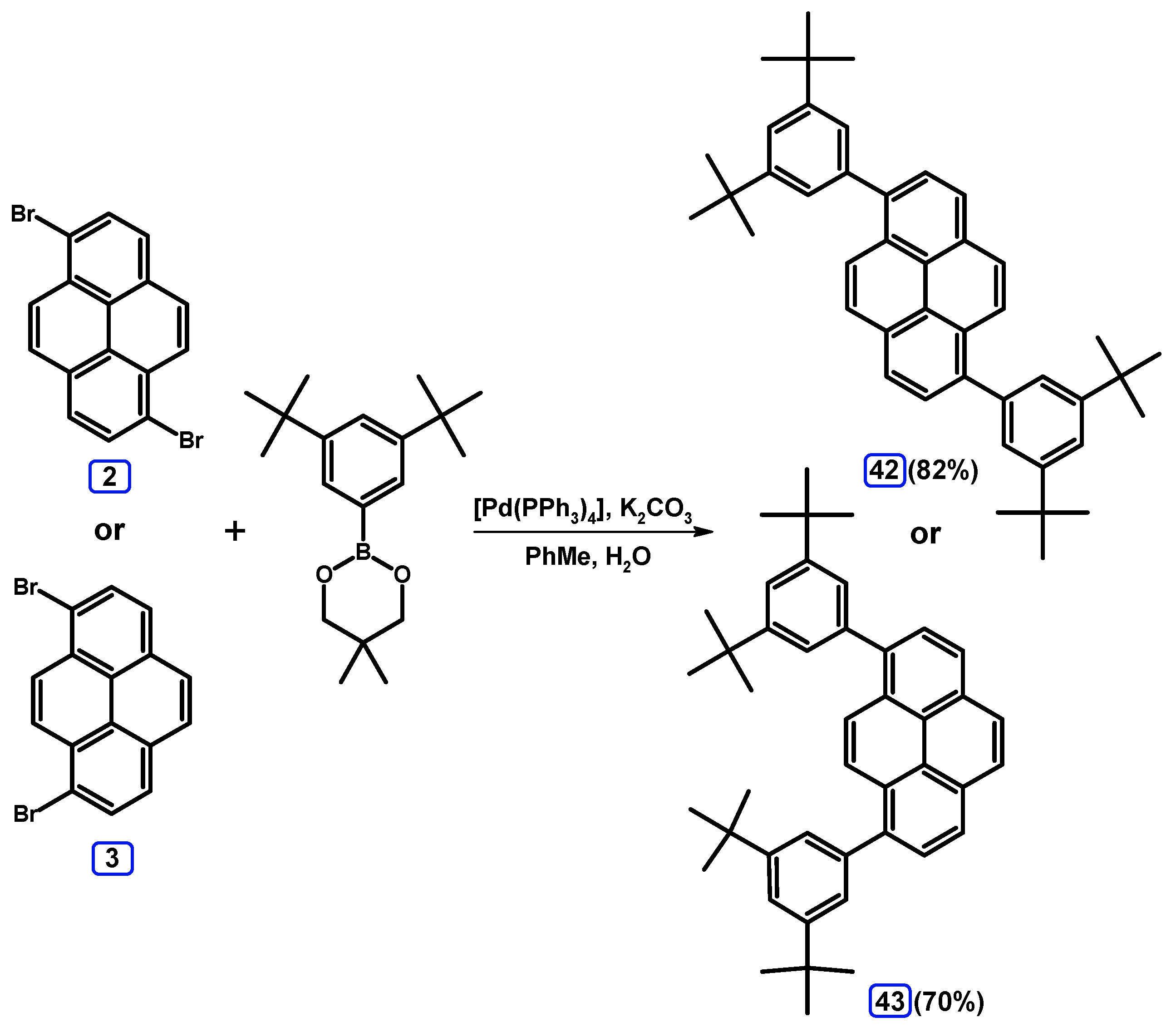
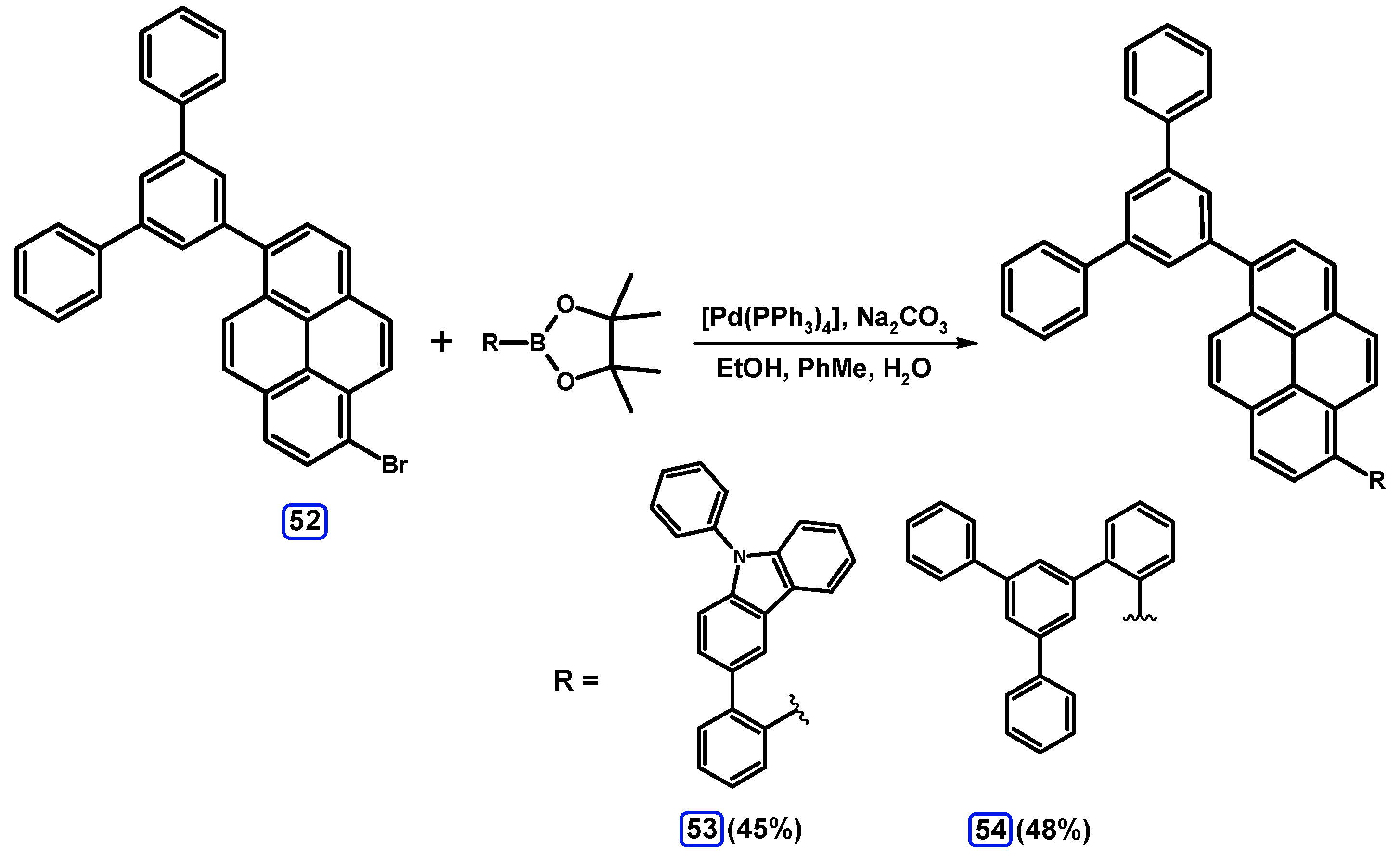
2.3.3. Suzuki-Miyaura Coupling of Dibromopyrenes with (Hetero)Arylboronates
2.3.4. Mono-Suzuki-Miyaura Coupling
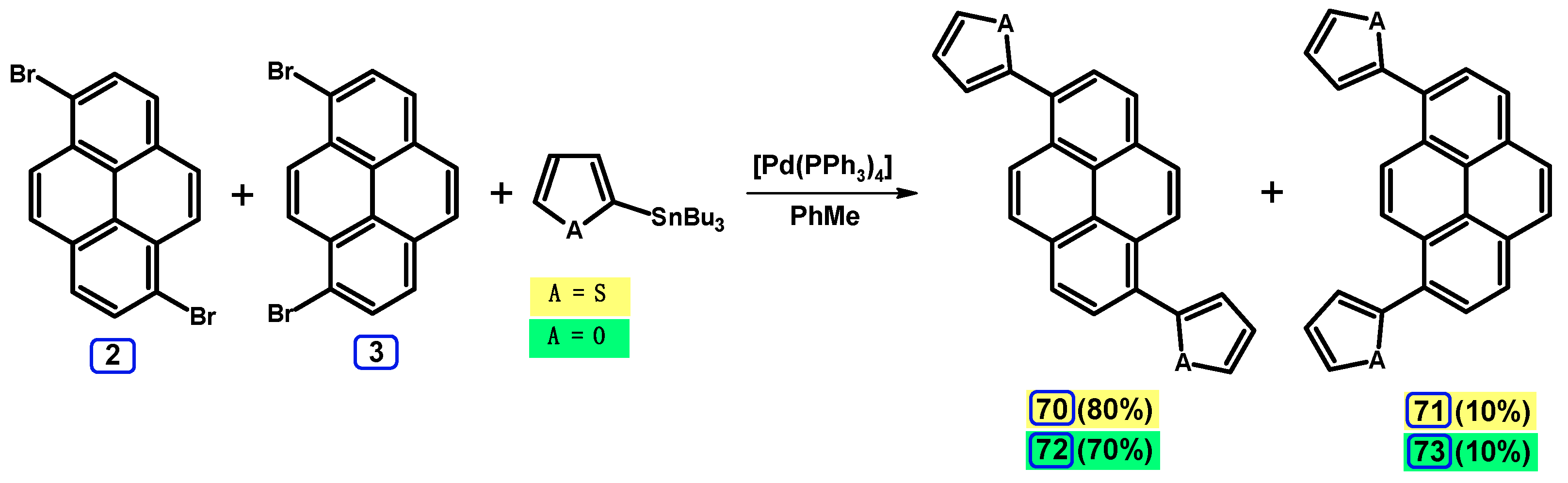
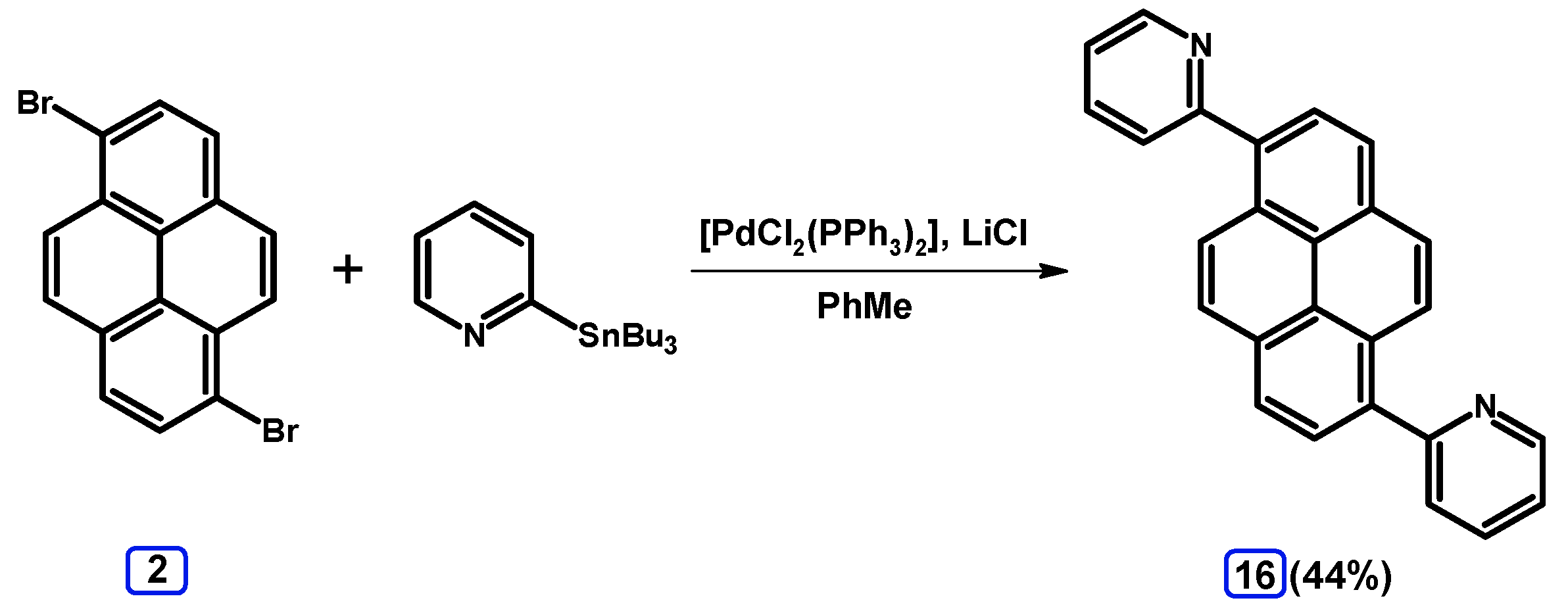
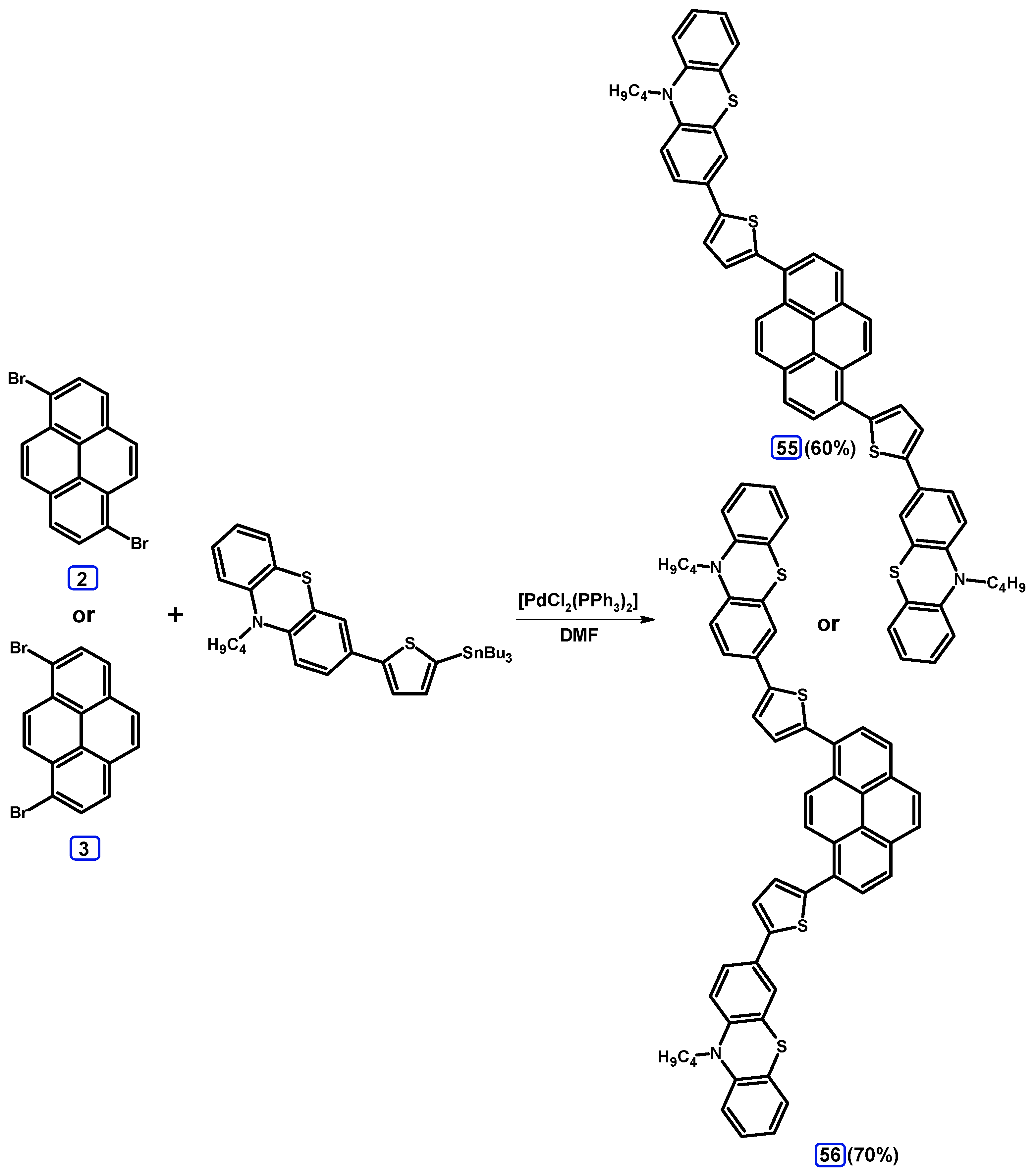
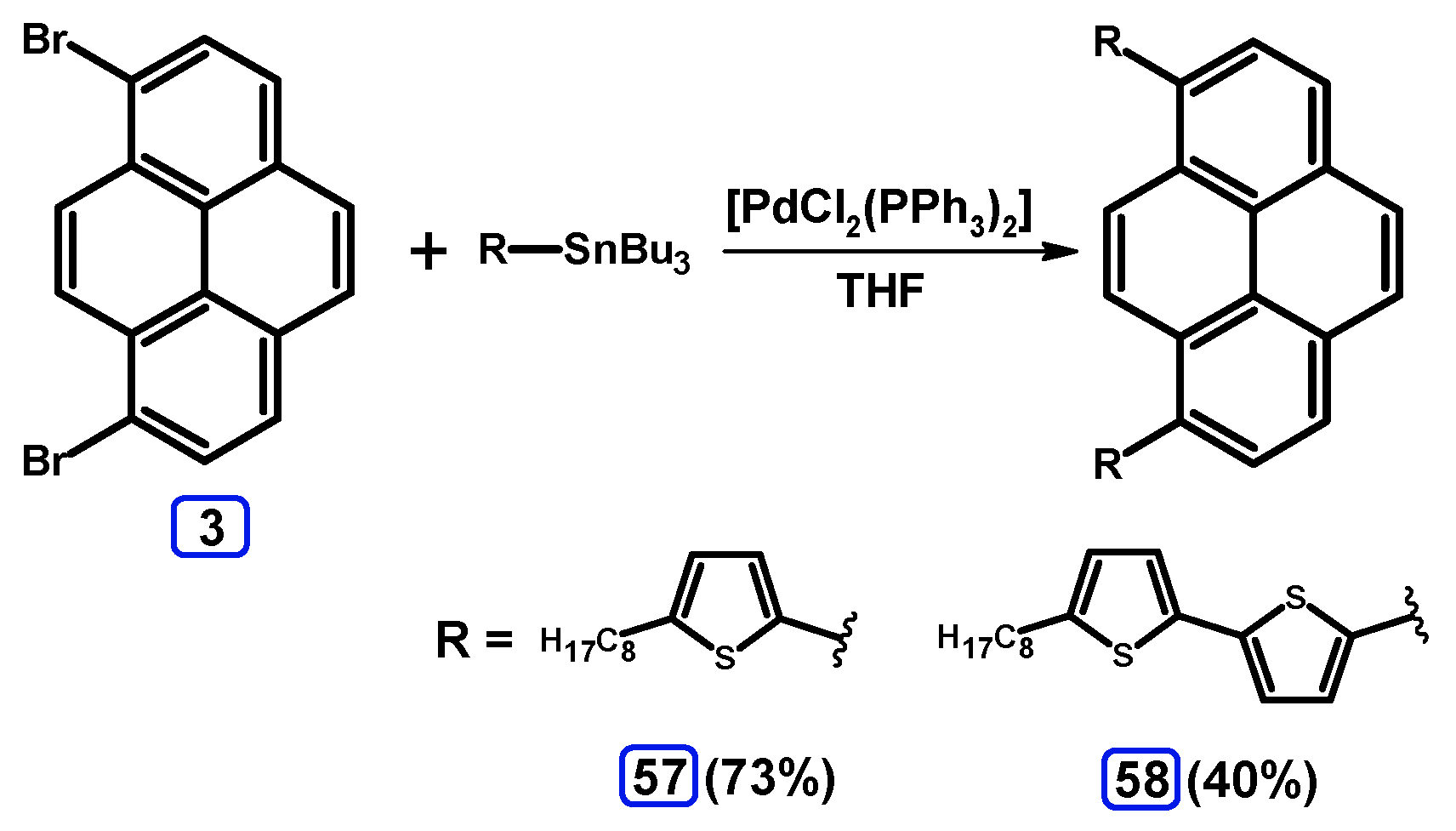
2.4. Stille Coupling
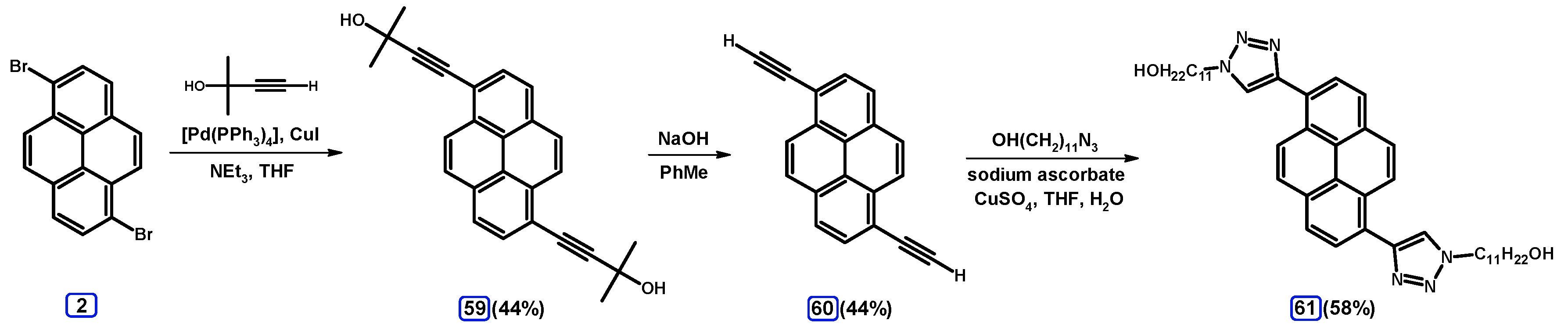
2.5. Sonogashira Coupling
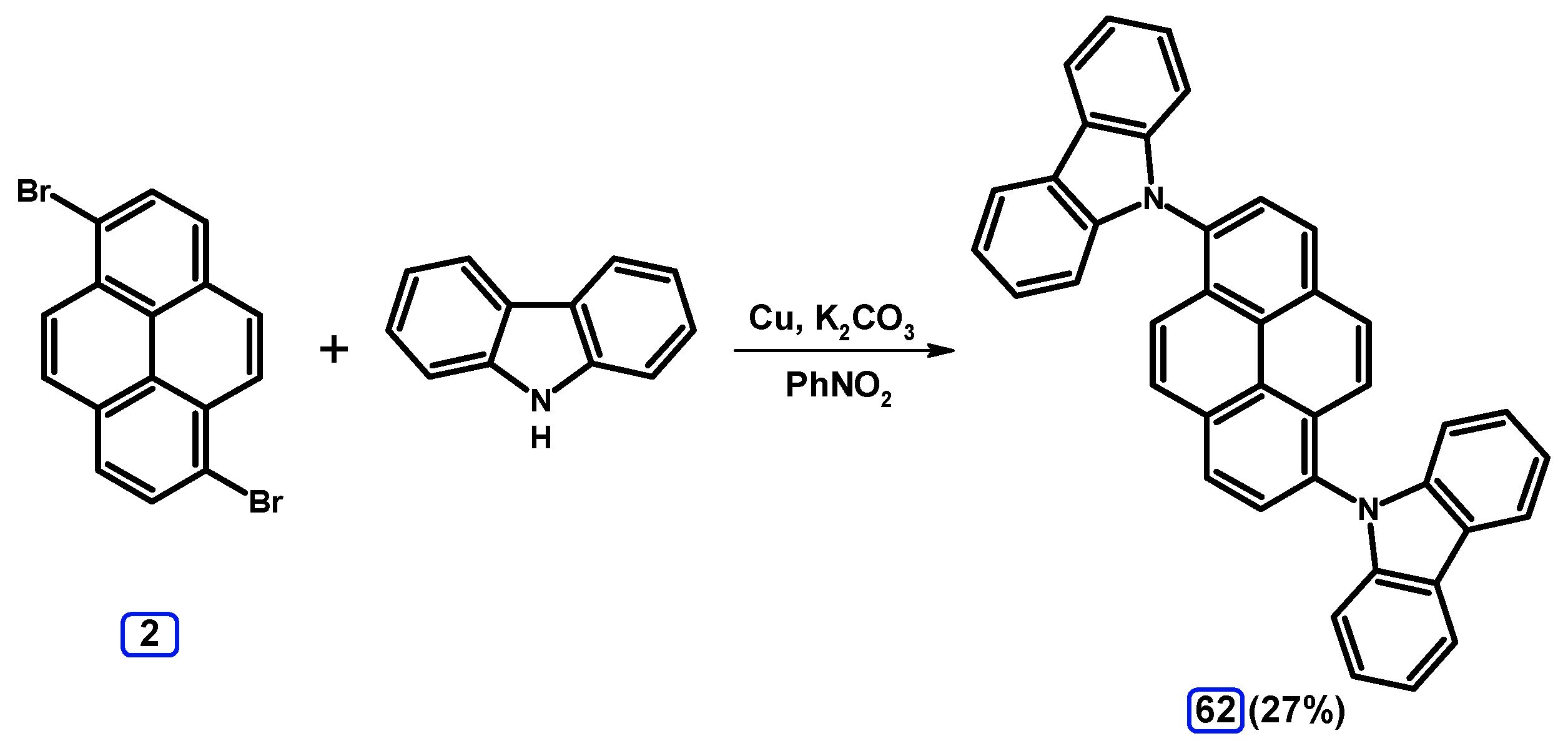
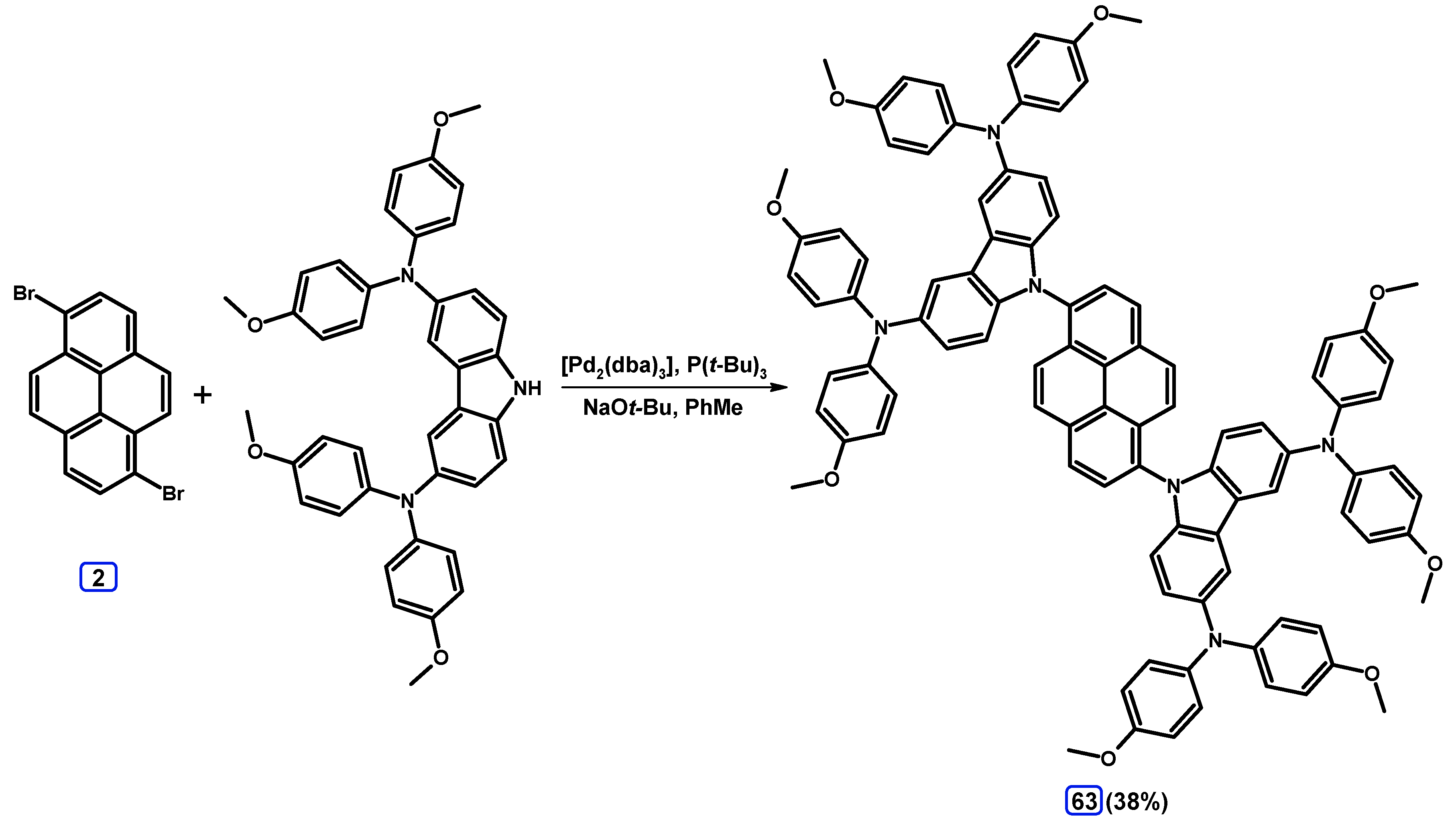
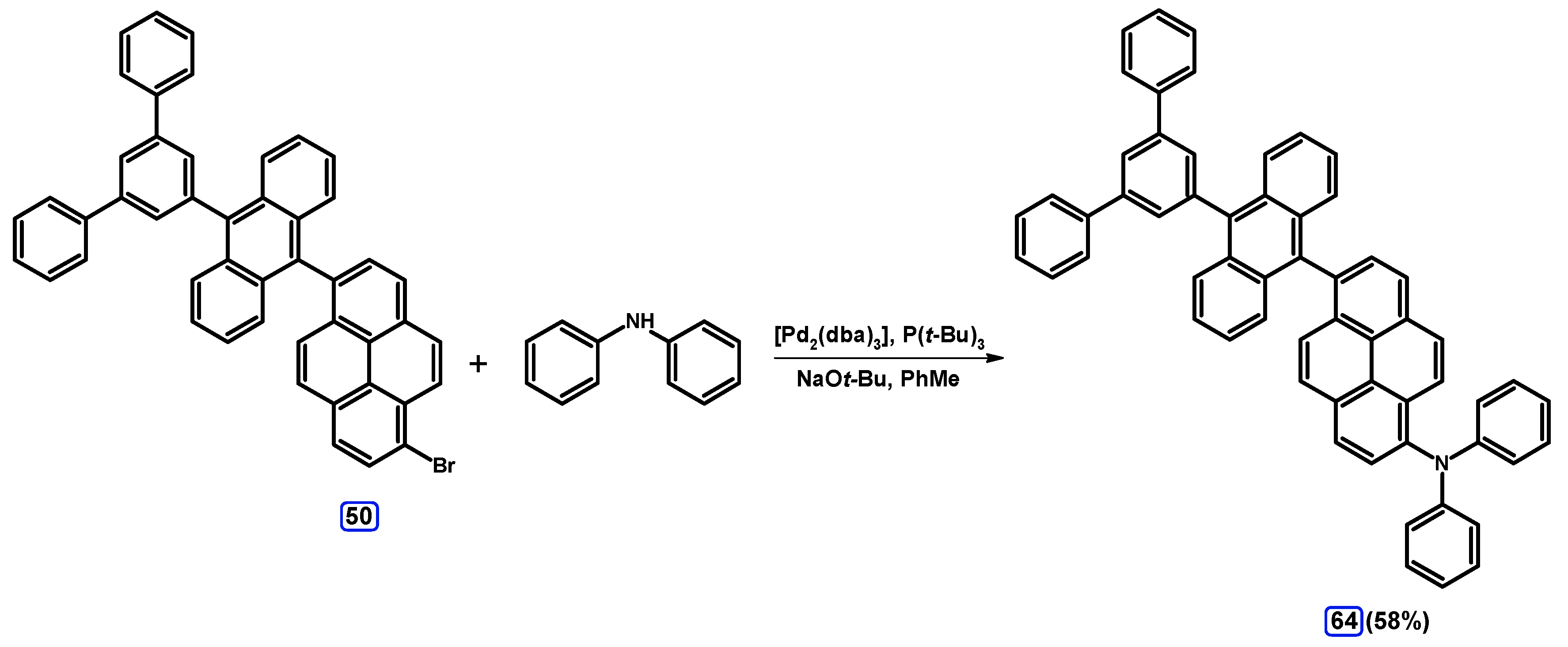
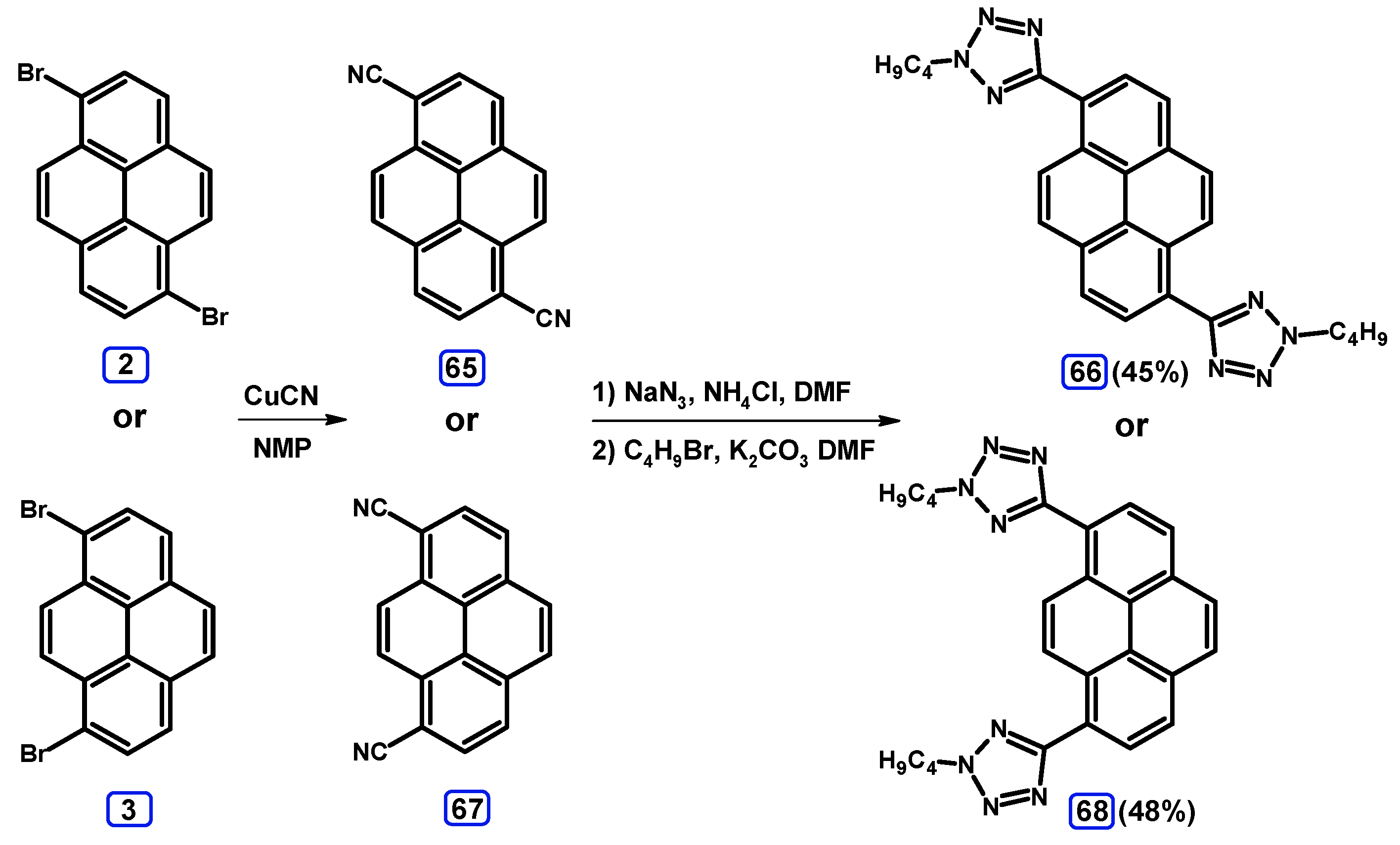
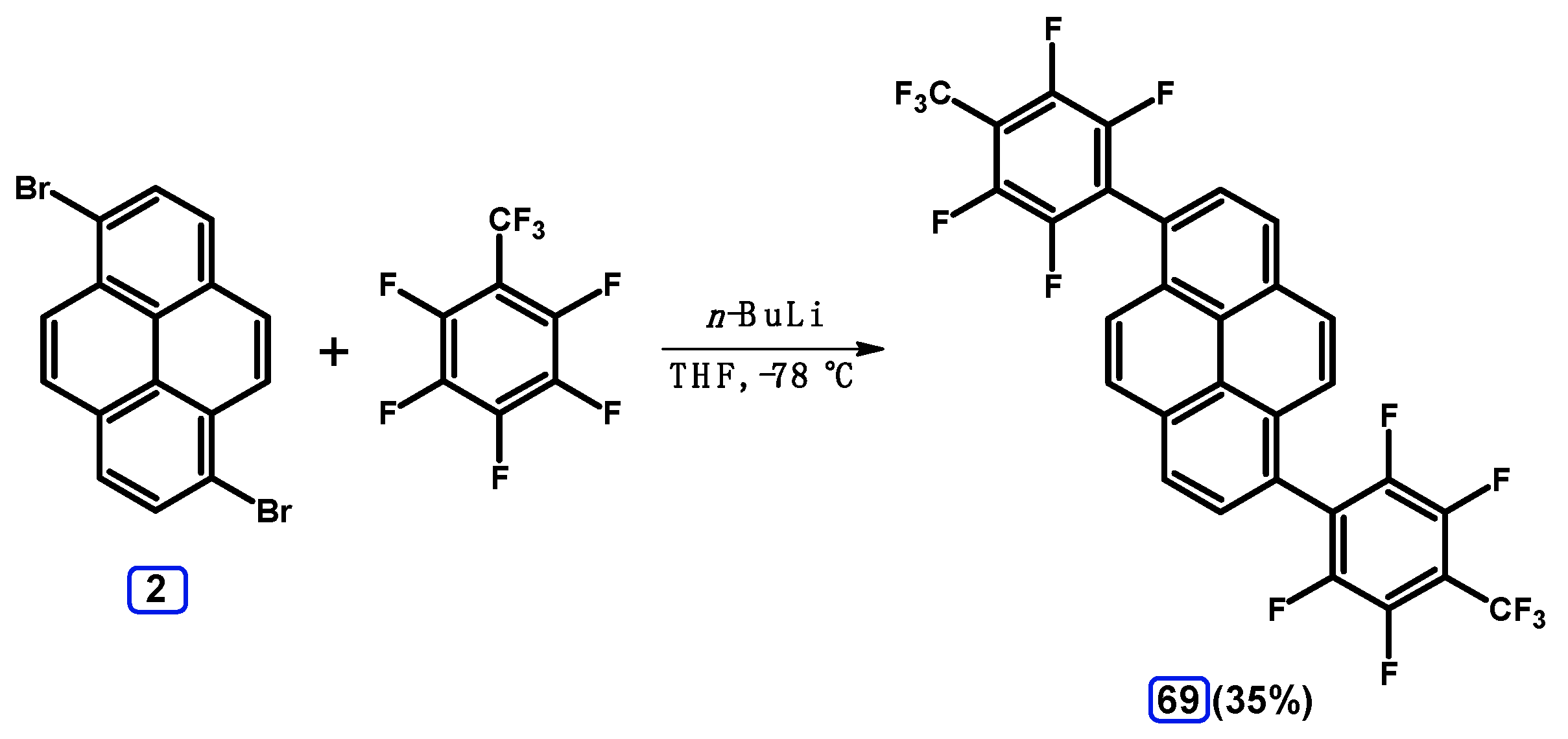
2.6. Ullmann, Buchwald-Hartwig, Rosenmund-von Braun, and Substitution Reactions
2.7. Reaction with a Mixture of 1,6- and 1,8-dibromo Isomers
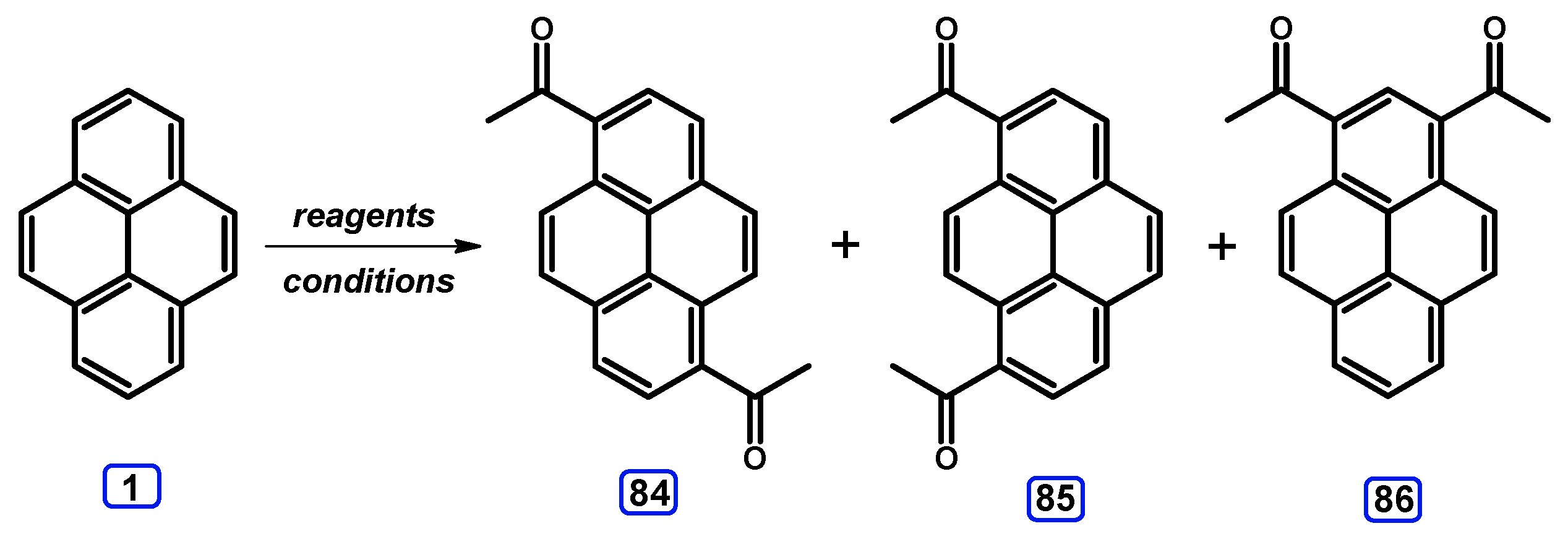
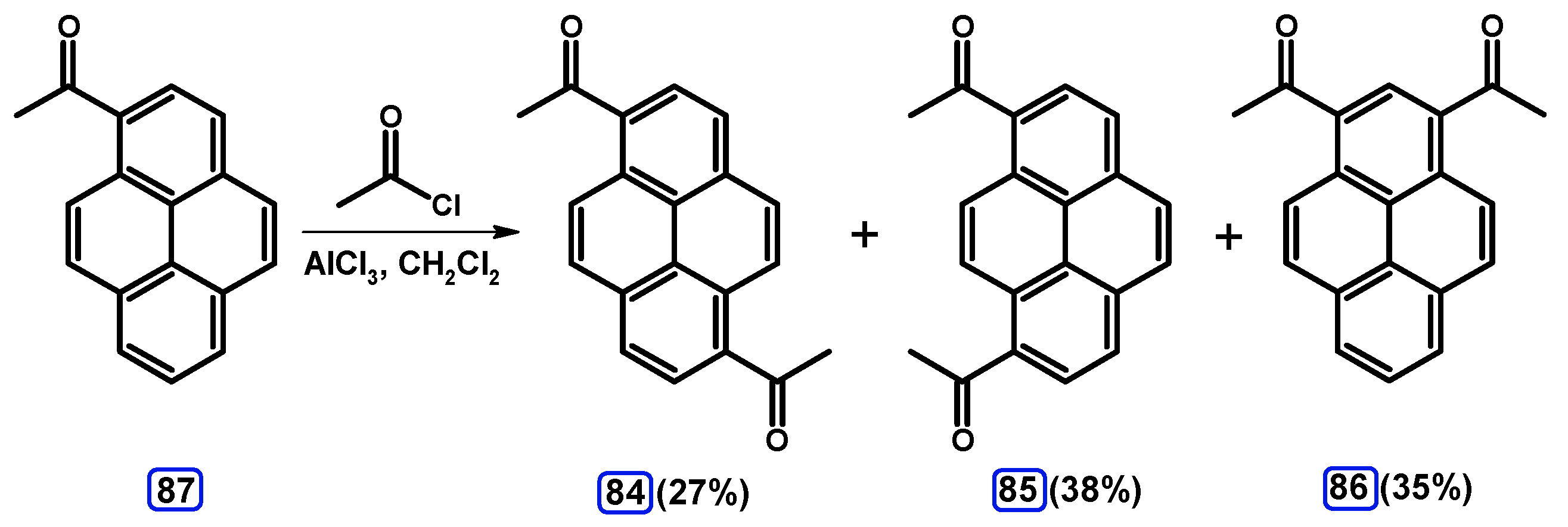
3. Acetylpyrenes
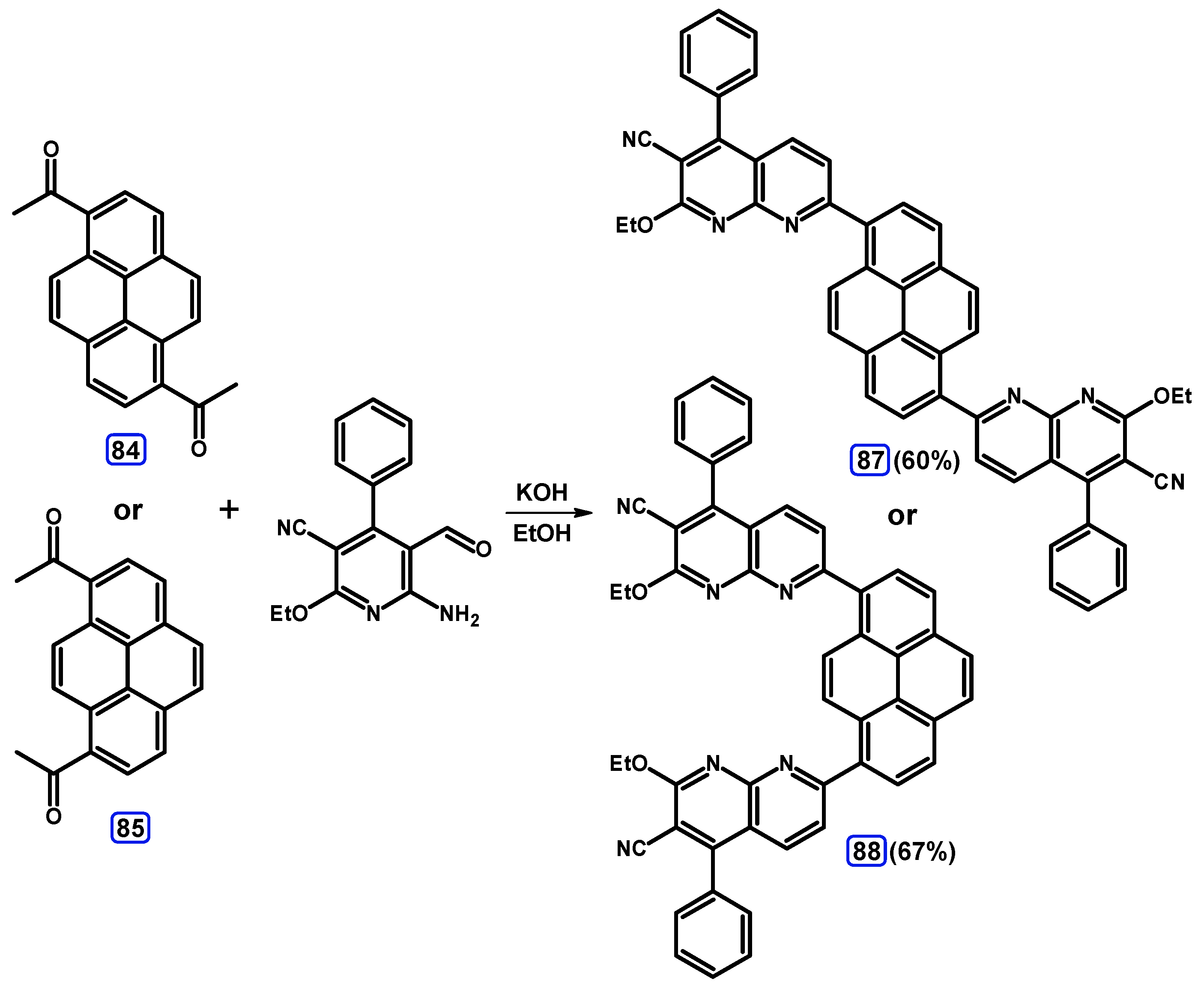
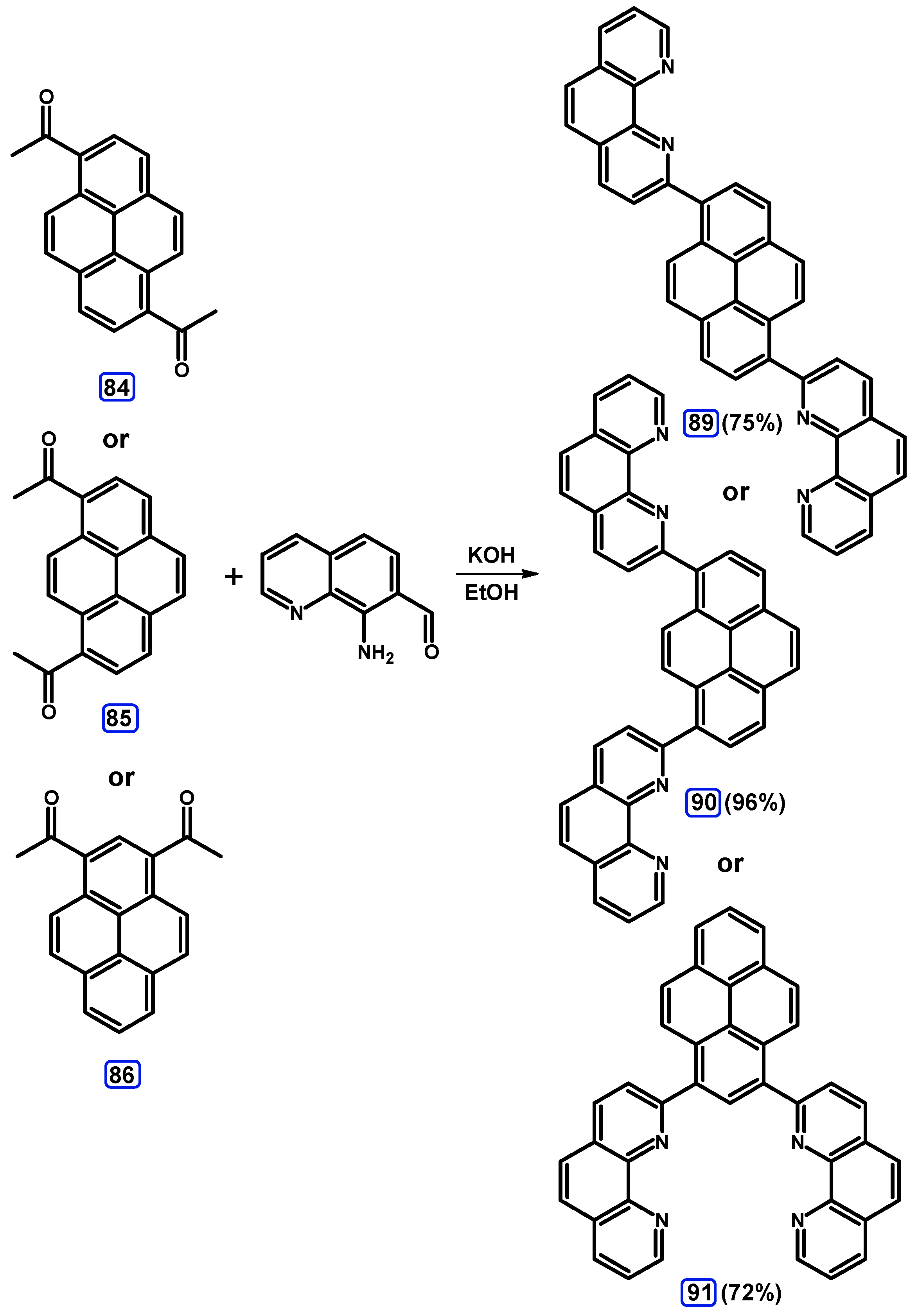
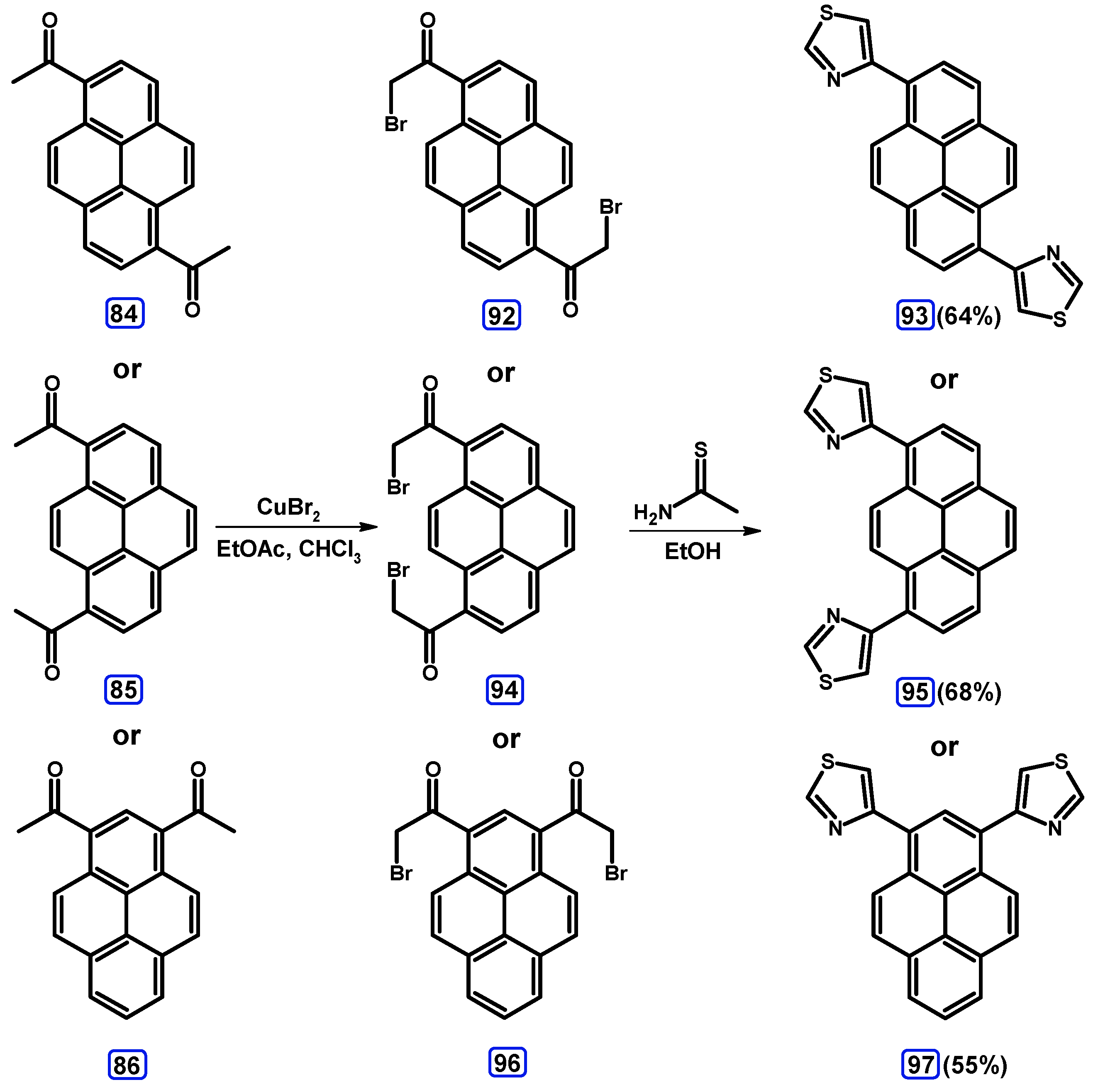
Condensation Reactions with Acetylpyrenes
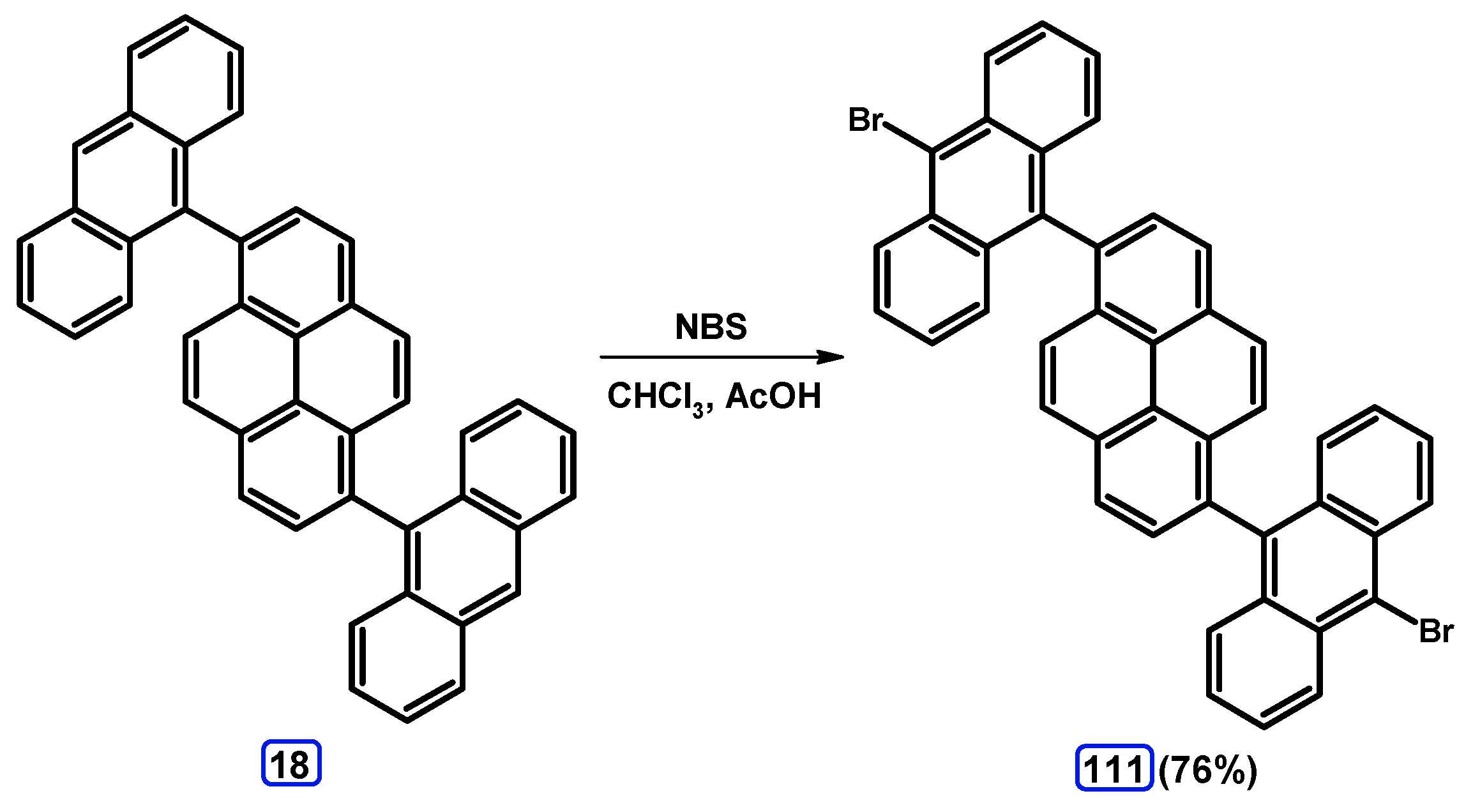
4. 1,3-Disubstituted Pyrene
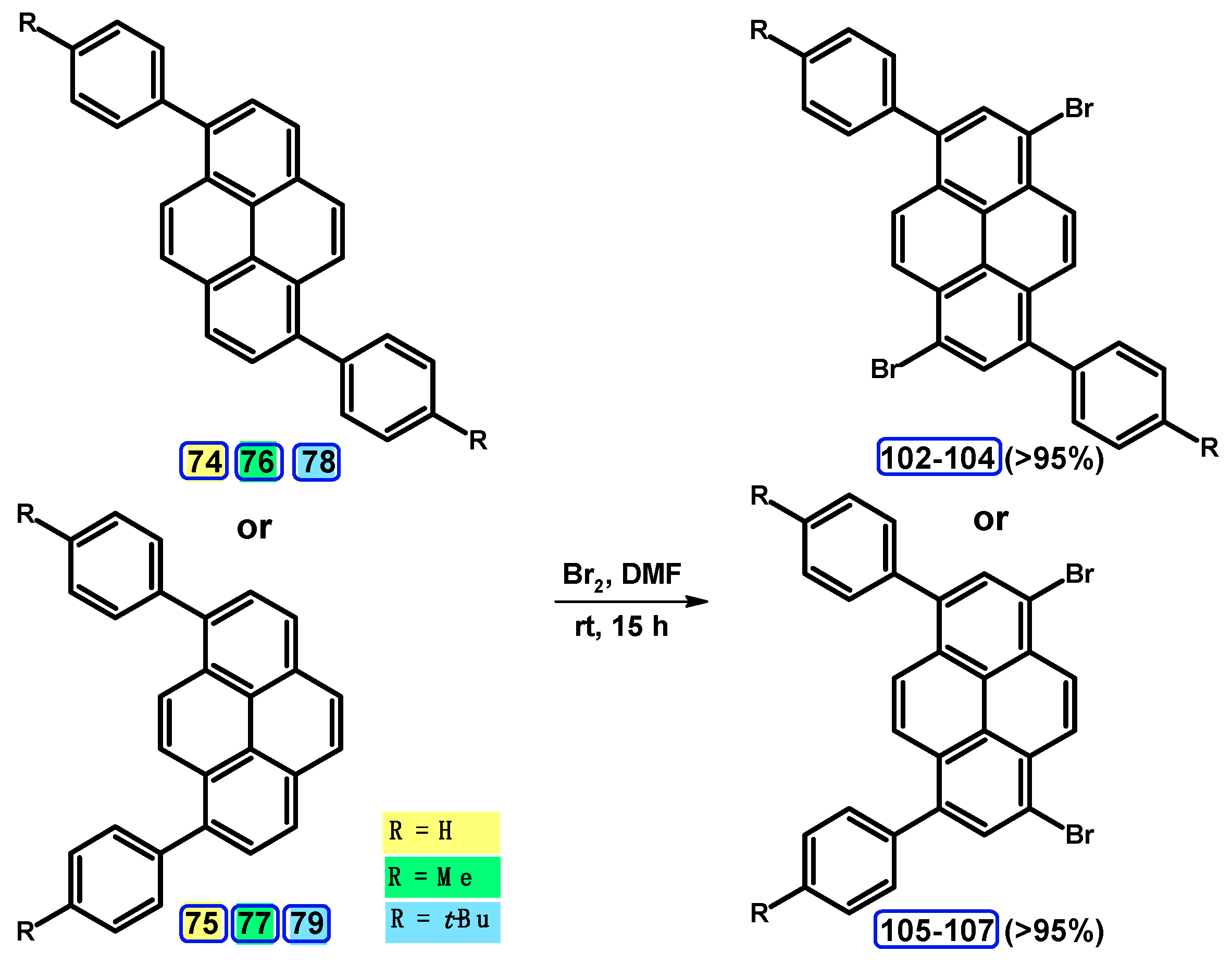
5. Synthesis of 1,3,6,8-tetrasubstituted Starting from Disubstituted Pyrenes
6. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, J.-Y.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials-Typical Examples of Synthetic Methodology. Chem.—A Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Kurpanik, A.; Slodek, A.; Maroń, A.; Pajak, M.; Szafraniec-Gorol, G.; Matussek, M.; Krompiec, S.; Schab-Balcerzak, E.; Kotowicz, S.; et al. NCN-Coordinating Ligands based on Pyrene Structure with Potential Application in Organic Electronics. Chem.—A Eur. J. 2017, 23, 15746–15758. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yoon, J.-Y.; Lee, S.J.; Lee, H.W.; Kim, Y.K.; Yoon, S.S. Various Blue Emitting Materials Based on Pyrene Derivatives for Organic Light-Emitting Diodes. J. Nanosci. Nanotechnol. 2015, 15, 5246–5249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, J.; Wakamiya, A.; Park, J. New blue emitting materials based on triple-core chromophores for organic light-emitting diodes. Mol. Cryst. Liq. Cryst. 2017, 654, 40–46. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.; Zhang, T.; Xu, L.; Ni, Z. A series of short axially symmetrically 1,3,6,8-tetrasubstituted pyrene-based green and blue emitters with 4-tert-butylphenyl and arylamine attachments. Dyes Pigments 2016, 130, 106–115. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Kwon, J.E.; Kim, J.; Yokoyama, D.; Suzuki, K.; Nishimura, H.; Wakamiya, A.; Park, S.Y.; Park, J. Excimer formation in organic emitter films associated with a molecular orientation promoted by steric hindrance. Chem. Commun. 2014, 50, 14145–14148. [Google Scholar] [CrossRef] [PubMed]
- Mizoshita, N.; Inagaki, S. Enhanced Photoluminescence of Mesostructured Organosilica Films with a High Density of Fluorescent Chromophores. Macromol. Chem. Phys. 2018, 219, 1700596. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, T.; Xu, L.; Han, F.; Zhao, Y.; Ni, Z. A new series of short axially symmetrically and asymmetrically 1,3,6,8-tetrasubstituted pyrenes with two types of substituents: Syntheses, structures, photophysical properties and electroluminescence. J. Mol. Struct. 2017, 1127, 237–246. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Chen, Y.; Tan, Y.-Z.; Tu, Z.; Gao, X.J.; Dong, H.; Yi, Y.; Zhang, Y.; Hu, W.; et al. A novel angularly fused bistetracene: facile synthesis, crystal packing and single-crystal field effect transistors. J. Mater. Chem. C 2017, 5, 1308–1312. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Zheng, C.; Feng, X.; Huan, Y.; Li, J.; Yi, M.; Fu, Z.; Huang, W.; Gao, D. 1,8-Substituted Pyrene Derivatives for High-Performance Organic Field-Effect Transistors. Chem.—An Asian J. 2018, 13, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, J.-Y.; Li, Y.; Li, Y.; Deng, L.-Y.; Zhong, Y.-W.; Meng, Q. New hole transporting materials for planar perovskite solar cells. Chem. Commun. 2018, 54, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Lungerich, D.; Papaianina, O.; Feofanov, M.; Liu, J.; Devarajulu, M.; Troyanov, S.I.; Maier, S.; Amsharov, K. Dehydrative π-extension to nanographenes with zig-zag edges. Nat. Commun. 2018, 9, 4756. [Google Scholar] [CrossRef] [PubMed]
- Ronson, T.K.; Roberts, D.A.; Black, S.P.; Nitschke, J.R. Stacking Interactions Drive Selective Self-Assembly and Self-Sorting of Pyrene-Based M II 4 L 6 Architectures. J. Am. Chem. Soc. 2015, 137, 14502–14512. [Google Scholar] [CrossRef]
- Ronson, T.K.; League, A.B.; Gagliardi, L.; Cramer, C.J.; Nitschke, J.R. Pyrene-Edged Fe II 4 L 6 Cages Adaptively Reconfigure During Guest Binding. J. Am. Chem. Soc. 2014, 136, 15615–15624. [Google Scholar] [CrossRef] [PubMed]
- Casas-Solvas, J.M.; Howgego, J.D.; Davis, A.P. Synthesis of substituted pyrenes by indirect methods. Org. Biomol. Chem. 2014, 12, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Ehrenhauser, F.S. PAH and IUPAC Nomenclature. Polycycl. Aromat. Compd. 2015, 35, 161–176. [Google Scholar] [CrossRef]
- Grimshaw, J.; Trocha-Grimshaw, J. Characterisation of 1,6- and 1,8-dibromopyrenes. J. Chem. Soc. Perkin Trans. 1 1972, 0, 1622. [Google Scholar] [CrossRef]
- Bheemireddy, S.R.; Ubaldo, P.C.; Finke, A.D.; Wang, L.; Plunkett, K.N. Contorted aromatics via a palladium-catalyzed cyclopentannulation strategy. J. Mater. Chem. C 2016, 4, 3963–3969. [Google Scholar] [CrossRef]
- Gong, X.; Xie, X.; Chen, N.; Zheng, C.; Zhu, J.; Chen, R.; Huang, W.; Gao, D. Two Symmetrically Bis-substituted Pyrene Derivatives: Synthesis, Photoluminescence, and Electroluminescence. Chin. J. Chem. 2015, 33, 967–973. [Google Scholar] [CrossRef]
- Liu, M.; Gong, X.; Zheng, C.; Gao, D. Development of Pyrene Derivatives as Promising n-Type Semiconductors: Synthesis, Structural and Spectral Properties. Asian J. Org. Chem. 2017, 6, 1903–1913. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Kim, S.; Kim, J.; Lee, J.; Shin, H.; Lee, J.-H.; Park, J. Synthesis and electroluminescence properties of highly efficient dual core chromophores with side groups for blue emission. J. Mater. Chem. C 2014, 2, 4737–4747. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Lee, J.; Yokoyama, D.; Lee, J.-H.; Kido, J.; Park, J. Synthesis and electroluminescence properties of highly efficient blue fluorescence emitters using dual core chromophores. J. Mater. Chem. C 2013, 1, 432–440. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.; Jung, H.; Park, J. Synthesis and Physical Properties of New Pyrene Derivative with Bulky Side Groups for Blue Emission. J. Nanosci. Nanotechnol. 2016, 16, 8796–8799. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.; Jung, H.; Kang, S.; Wakamiya, A.; Park, J. Highly efficient pyrene blue emitters for OLEDs based on substitution position effect. Dye. Pigment. 2018, 158, 42–49. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.; Kang, I.-N.; Park, M.-J.; Hwang, D.-H. Photovoltaic devices using semiconducting polymers containing head-to-tail-structured bithiophene, pyrene, and benzothiadiazole derivatives. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3415–3424. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.U.; Kang, I.-N.; Lee, S.K.; Moon, S.-J.; Shin, W.S.; Hwang, D.-H. Incorporation of Pyrene Units to Improve Hole Mobility in Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 8628–8638. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Sharma, G.D.; Godovskii, D.Y.; Belomoina, N.M.; Geng, Y.; Zou, Y.; Kochurov, V.S.; Stakhanov, A.I.; Khokhlov, A.R. Novel electron-withdrawing π-conjugated pyrene-containing poly(phenylquinoxaline)s. Dokl. Chem. 2014, 456, 65–71. [Google Scholar] [CrossRef]
- Connor, D.M.; Kriegel, R.M.; Collard, D.M.; Liotta, C.L.; Schiraldi, D.A. Pyrene and anthracene dicarboxylic acids as fluorescent brightening comonomers for polyester. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1291–1301. [Google Scholar] [CrossRef]
- Suenaga, H.; Nakashima, K.; Mizuno, T.; Takeuchi, M.; Hamachi, I.; Shinkai, S. Pyrenylboronic acids as a novel entry for photochemical DNA cleavage: diradical-forming pyrene-1,6-diyldiboronic acid mimics the cleavage mechanism of enediyne antitumor antibiotics. J. Chem. Soc. Perkin Trans. 1 1998, 0, 1263–1268. [Google Scholar] [CrossRef]
- Kaplunov, M.G.; Yakushchenko, I.K.; Krasnikova, S.S.; Echmaev, S.B. Novel 1,8-bis(diarylamino)pyrenes as OLED materials. Mendeleev Commun. 2016, 26, 437–439. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Hiyoshi, H.; Do, J.-H.; Yamato, T. Synthesis and fluorescence emission properties of 1,3,6,8-tetrakis(9H-fluoren-2-yl)pyrene derivative. J. Chem. Res. 2010, 2010, 278–282. [Google Scholar] [CrossRef]
- Arai, R.; Uemura, S.; Irie, M.; Matsuda, K. Reversible Photoinduced Change in Molecular Ordering of Diarylethene Derivatives at a Solution−HOPG Interface. J. Am. Chem. Soc. 2008, 130, 9371–9379. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, Q.; Chen, Q.; Shi, L.; Cao, H.; Cheng, J.; Deng, C.; Lin, T. Highly fluorescent intramolecular dimmers of two pyrenyl-substituted fluorenes bridged by 1,6-hexanyl: synthesis, spectroscopic, and self-organized properties. Tetrahedron Lett. 2010, 51, 1317–1321. [Google Scholar] [CrossRef]
- Nakamura, H.; Tomonaga, Y.; Miyata, K.; Uchida, M.; Terao, Y. Reaction of Polycyclic Aromatic Hydrocarbons Adsorbed on Silica in Aqueous Chlorine. Environ. Sci. Technol. 2007, 41, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Minabe, M.; Takeshige, S.; Soeda, Y.; Kimura, T.; Tsubota, M. Electrophilic Substitution of Monosubstituted Pyrenes. Bull. Chem. Soc. Jpn. 1994, 67, 172–179. [Google Scholar] [CrossRef]
- Cabral, L.I.L.; Henriques, M.S.C.; Paixão, J.A.; Cristiano, M.L.S. Synthesis and structure of 2-substituted pyrene-derived scaffolds. Tetrahedron Lett. 2017, 58, 4547–4550. [Google Scholar] [CrossRef]
- Casas-Solvas, J.; Mooibroek, T.; Sandramurthy, S.; Howgego, J.; Davis, A. A Practical, Large-Scale Synthesis of Pyrene-2-Carboxylic Acid. Synlett 2014, 25, 2591–2594. [Google Scholar]
- Gerasimenko, Y.E.P. No Title. J. Org. Chem. USSR (Engl. Transl.) 1972, 8, 1084. [Google Scholar]
- Nielsen, T.; Siigur, K.; Helweg, C.; Jørgensen, O.; Hansen, P.E.; Kirso, U. Sorption of Polycyclic Aromatic Compounds to Humic Acid As Studied by High-Performance Liquid Chromatography. Environ. Sci. Technol. 1997, 31, 1102–1108. [Google Scholar] [CrossRef]
- Xu, K.; Fu, Y.; Zhou, Y.; Hennersdorf, F.; Machata, P.; Vincon, I.; Weigand, J.J.; Popov, A.A.; Berger, R.; Feng, X. Cationic Nitrogen-Doped Helical Nanographenes. Angew. Chemie Int. Ed. 2017, 56, 15876–15881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Zhang, S.; Wang, Q.; Dai, G.; Liu, B.; Zhu, X.; Li, Z.; Kolodziej, C.; McCleese, C.; et al. Stable 2D Bisthienoacenes: Synthesis, Crystal Packing, and Photophysical Properties. Chem.—A Eur. J. 2018, 24, 14442–14447. [Google Scholar] [CrossRef]
- Soujanya, T.; Philippon, A.; Leroy, S.; Vallier, M.; Fages, F. Tunable Photophysical Properties of Two 2,2‘-Bipyridine-Substituted Pyrene Derivatives. J. Phys. Chem. A 2000, 104, 9408–9414. [Google Scholar] [CrossRef]
- Sbargoud, K.; Mamada, M.; Jousselin-Oba, T.; Takeda, Y.; Tokito, S.; Yassar, A.; Marrot, J.; Frigoli, M. Low Bandgap Bistetracene-Based Organic Semiconductors Exhibiting Air Stability, High Aromaticity and Mobility. Chem.—A Eur. J. 2017, 23, 5076–5080. [Google Scholar] [CrossRef]
- Jousselin-Oba, T.; Sbargoud, K.; Vaccaro, G.; Meinardi, F.; Yassar, A.; Frigoli, M. Novel Fluorophores based on Regioselective Intramolecular Friedel-Crafts Acylation of the Pyrene Ring Using Triflic Acid. Chem.—A Eur. J. 2017, 23, 16184–16188. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Wei, C.; Chen, S.; Yin, X.; Xiao, J.; Su, W. Synthesis and red electroluminescence of a dimesityl-functionalized bistetracene. Chinese Chem. Lett. 2018, 29, 293–296. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Wang, W.; Dong, X.; Zhao, B.; Ji, B. Novel pyrene derivatives: Synthesis, properties and highly efficient non-doped deep-blue electroluminescent device. Dyes Pigments 2012, 92, 732–736. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Liu, C.-L.; Zheng, C.-J.; Wang, W.-Z.; Xu, C.; Zhu, M.; Ji, B.-M.; Li, F.; Zhang, X.-H. Efficient violet non-doped organic light-emitting device based on a pyrene derivative with novel molecular structure. Org. Electron. 2015, 23, 179–185. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, K.H.; Joo, C.W.; Lee, J.-I.; Lee, J.; Kim, Y.-H. Highly twisted pyrene derivatives for non-doped blue OLEDs. Dyes Pigments 2016, 128, 19–25. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Han, Y.; Song, T.; Zhao, X.; Xiao, J. Synthesis, physical properties and electroluminescence of functionalized pyrene derivative. Dyes Pigments 2019, 167, 22–28. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, N.; Feng, L. Two non-doped blue emitters for electroluminescent devices: Preparation, photophysics and electroluminescence. Dyes Pigments 2016, 132, 121–127. [Google Scholar] [CrossRef]
- Gong, X.; Heeran, D.; Zhao, Q.; Zheng, C.; Yufit, D.S.; Sandford, G.; Gao, D. Synthesis of Fluoro and Cyanoaryl-Containing Pyrene Derivatives and their Optical and Electrochemical Properties. Asian J. Org. Chem. 2019, 8, 722–730. [Google Scholar] [CrossRef]
- Gong, X.; Pan, Y.; Xie, X.; Tong, T.; Chen, R.; Gao, D. Synthesis, characterization and electroluminescence of two highly-twisted non-doped blue light-emitting materials. Opt. Mater. (Amst). 2018, 78, 94–101. [Google Scholar] [CrossRef]
- Sang, M.; Cao, S.; Yi, J.; Huang, J.; Lai, W.-Y.; Huang, W. Multi-substituted triazatruxene-functionalized pyrene derivatives as efficient organic laser gain media. RSC Adv. 2016, 6, 6266–6275. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Luo, Q.; Zhao, Y.; Yu, T. Synthesis, Characterization and Luminescent Properties of Anthracen- or Pyrene-Based Coumarin Derivatives. J. Fluoresc. 2018, 28, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Mikami, S.; Iwasaki, K.; Hino, S.; Asato, E.; Sakata, Y. Synthesis, properties, molecular structure and electron transfer salts of 13,13,14,14-tetracyano-1,6- and -1,8-pyrenoquinodimethanes (1,6-TCNP and 1,8-TCNP). J. Mater. Chem. 2000, 10, 315–319. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, S.; Lee, H.; Shin, H.; Jung, H.; Park, J.; Koo, K.-K. Efficient White Organic Light Emitting Diodes Using New Blue Fluorescence Emitter Based on Vacuum and Solution Process. J. Nanosci. Nanotechnol. 2017, 17, 4339–4342. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.; Jung, H.; Shin, H.; Lee, H.; Lee, J.; Park, J. Synthesis and electroluminescence properties of new blue dual-core OLED emitters using bulky side chromophores. Dye. Pigment. 2017, 136, 255–261. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Park, J. Excimer Formation Promoted by Steric Hindrance in Dual Core Chromophore for Organic Light-Emitting Diodes Emitters. J. Nanosci. Nanotechnol. 2016, 16, 8854–8857. [Google Scholar] [CrossRef]
- Shin, H.; Kim, B.; Jung, H.; Lee, J.; Lee, H.; Kang, S.; Moon, J.; Kim, J.; Park, J. Achieving a high-efficiency dual-core chromophore for emission of blue light by testing different side groups and substitution positions. RSC Adv. 2017, 7, 55582–55593. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, C.; Fu, W.; Xu, C.; Wu, J.; Ji, B. Efficient non-doped deep-blue electroluminescence devices based on unsymmetrical and highly twisted pyrene derivatives. New J. Chem. 2017, 41, 14152–14160. [Google Scholar] [CrossRef]
- He, Y.-Q.; Zhong, Y.-W. The synthesis of 2- and 2,7-functionalized pyrene derivatives through Ru(II)-catalyzed C–H activation. Chem. Commun. 2015, 51, 3411–3414. [Google Scholar] [CrossRef] [PubMed]
- Konidena, R.K.; Justin Thomas, K.R.; Singh, M.; Jou, J.-H. Thienylphenothiazine integrated pyrenes: an account on the influence of substitution patterns on their optical and electroluminescence properties. J. Mater. Chem. C 2016, 4, 4246–4258. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Wu, G.; Yan, L.; Dong, L.; Lai, X.; Yin, S.; Song, B. Mono-molecule-layer nano-ribbons formed by self-assembly of bolaamphiphiles. Soft Matter 2014, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Werder, S.; Malinovskii, V.L.; Häner, R. Triazolylpyrenes: Synthesis, Fluorescence Properties, and Incorporation into DNA. Org. Lett. 2008, 10, 2011–2014. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Wu, Y.; Liao, Q. Luminescence emission-modulated based on specific two-photon compound of triazole-conjugated pyrene derivative. RSC Adv. 2017, 7, 19002–19006. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Park, S.H.; Kim, K.-S.; Kwon, Y.; Ha, K.-R.; Choi, B.-D.; Han, Y.S. Synthesis and Electro-Optical Properties of Carbazole-Substituted Pyrene Derivatives. J. Nanosci. Nanotechnol. 2011, 11, 4351–4356. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A.; Frankowska, A. Is it worthwhile to deal with 1,3-disubstituted pyrene derivatives? – Photophysical, optical and theoretical study of substitution position effect of pyrenes containing tetrazole groups. Comput. Mater. Sci. 2019, 165, 101–113. [Google Scholar] [CrossRef]
- Idzik, K.R.; Licha, T.; Lukeš, V.; Rapta, P.; Frydel, J.; Schaffer, M.; Taeuscher, E.; Beckert, R.; Dunsch, L. Synthesis and Optical Properties of Various Thienyl Derivatives of Pyrene. J. Fluoresc. 2014, 24, 153–160. [Google Scholar] [CrossRef]
- Idzik, K.R.; Ledwon, P.; Licha, T.; Kuznik, W.; Lapkowski, M.; Frydel, J. Furyl derivatives of pyrene: Efficient synthesis and relevant optical properties. Dye. Pigment. 2014, 103, 55–61. [Google Scholar] [CrossRef]
- Wegner, H.A.; Reisch, H.; Rauch, K.; Demeter, A.; Zachariasse, K.A.; Meijere, A.; Scott, L.T. Oligoindenopyrenes: A New Class of Polycyclic Aromatics. J. Org. Chem. 2006, 71, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Gomha, S.M. The Chemistry of Acetylpyrazoles and Its Utility in Heterocyclic Synthesis. J. Heterocycl. Chem. 2019, 56, 726–758. [Google Scholar] [CrossRef]
- Rajagopal, S.K.; Philip, A.M.; Nagarajan, K.; Hariharan, M. Progressive acylation of pyrene engineers solid state packing and colour via C–H⋯H–C, C–H⋯O and π–π interactions. Chem. Commun. 2014, 50, 8644–8647. [Google Scholar] [CrossRef] [PubMed]
- Chouai, L.; Wu, F.; Jang, Y.; Thummel, R.P. Pyrene-Bridged Bis(phenanthroline) Ligands and Their Dinuclear Ruthenium(II) Complexes. Eur. J. Inorg. Chem. 2003, 2774–2782. [Google Scholar] [CrossRef]
- Harvey, R.G.; Pataki, J.; Lee, H. The Friedel-Crafts acylation and benzoylation of pyrene. Org. Prep. Proced. Int. 1984, 16, 144–148. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R.; Adams, C.J.; Roberts, G. Friedel–Crafts reactions in room temperature ionic liquids. Chem. Commun. 1998, 0, 2097–2098. [Google Scholar] [CrossRef]
- Fernández-Mato, A.; Blanco, G.; Quintela, J.M.; Peinador, C. Synthesis of new bis(2-[1,8]naphthyridinyl) bridging ligands with multidentate binding sites. Tetrahedron 2008, 64, 3446–3456. [Google Scholar] [CrossRef]
- Rajagopal, S.K.; Salini, P.S.; Hariharan, M. S···π, π–π, and C–H···π Contacts Regulate Solid State Fluorescence in Regioisomeric Bisthiazolylpyrenes. Cryst. Growth Des. 2016, 16, 4567–4573. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.-Y.; Yi, L.; Seto, N.; Tao, Z.; Redshaw, C.; Elsegood, M.R.J.; Yamato, T. Pyrene-Based Y-shaped Solid-State Blue Emitters: Synthesis, Characterization, and Photoluminescence. Chem.—An Asian J. 2012, 7, 2854–2863. [Google Scholar] [CrossRef]
- Feng, X.; Tomiyasu, H.; Hu, J.-Y.; Wei, X.; Redshaw, C.; Elsegood, M.R.J.; Horsburgh, L.; Teat, S.J.; Yamato, T. Regioselective Substitution at the 1,3- and 6,8-Positions of Pyrene for the Construction of Small Dipolar Molecules. J. Org. Chem. 2015, 80, 10973–10978. [Google Scholar] [CrossRef] [Green Version]
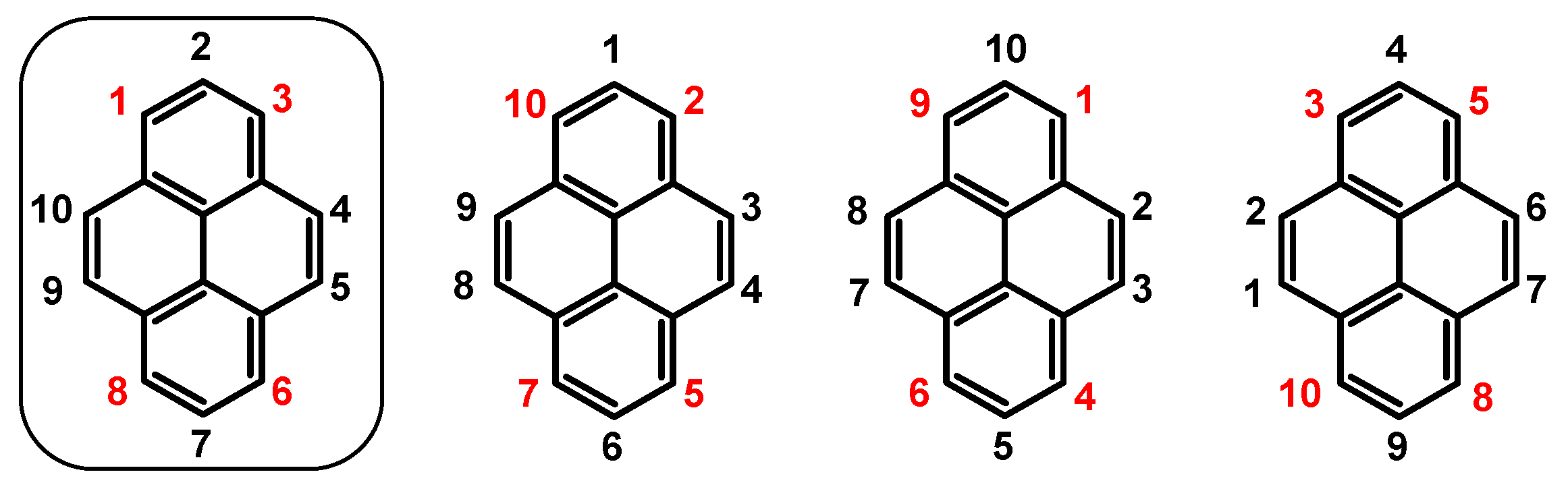
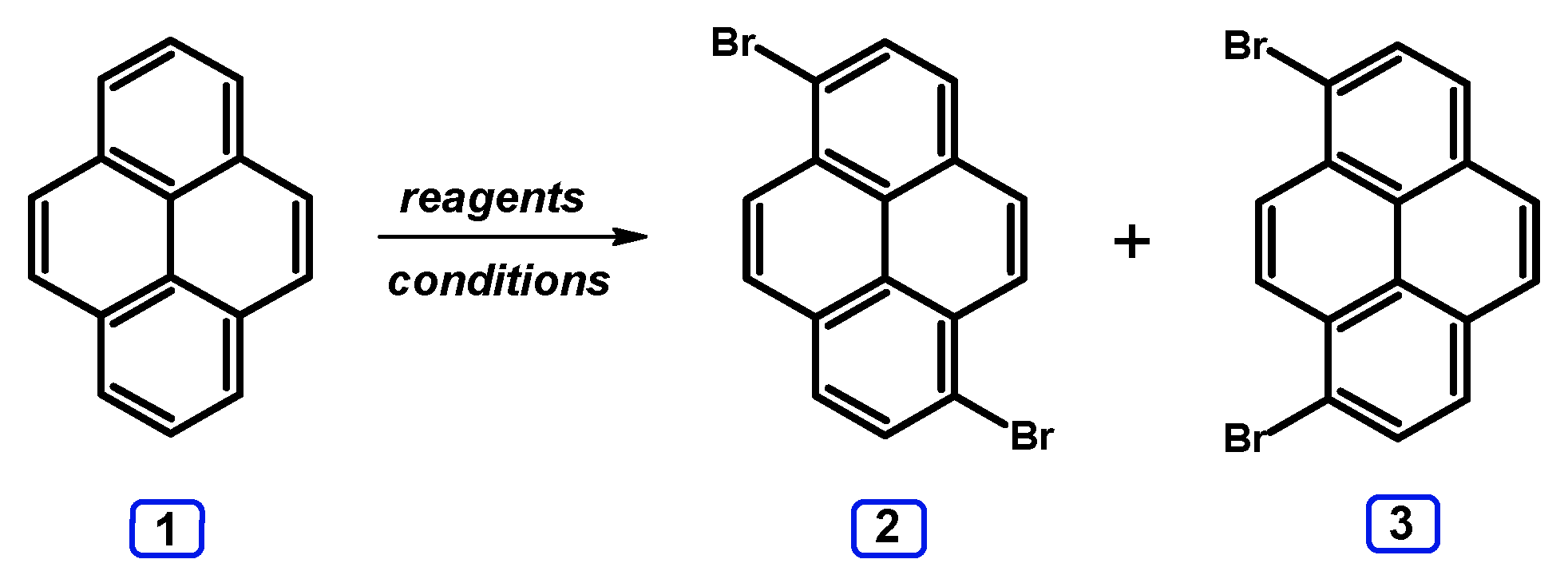
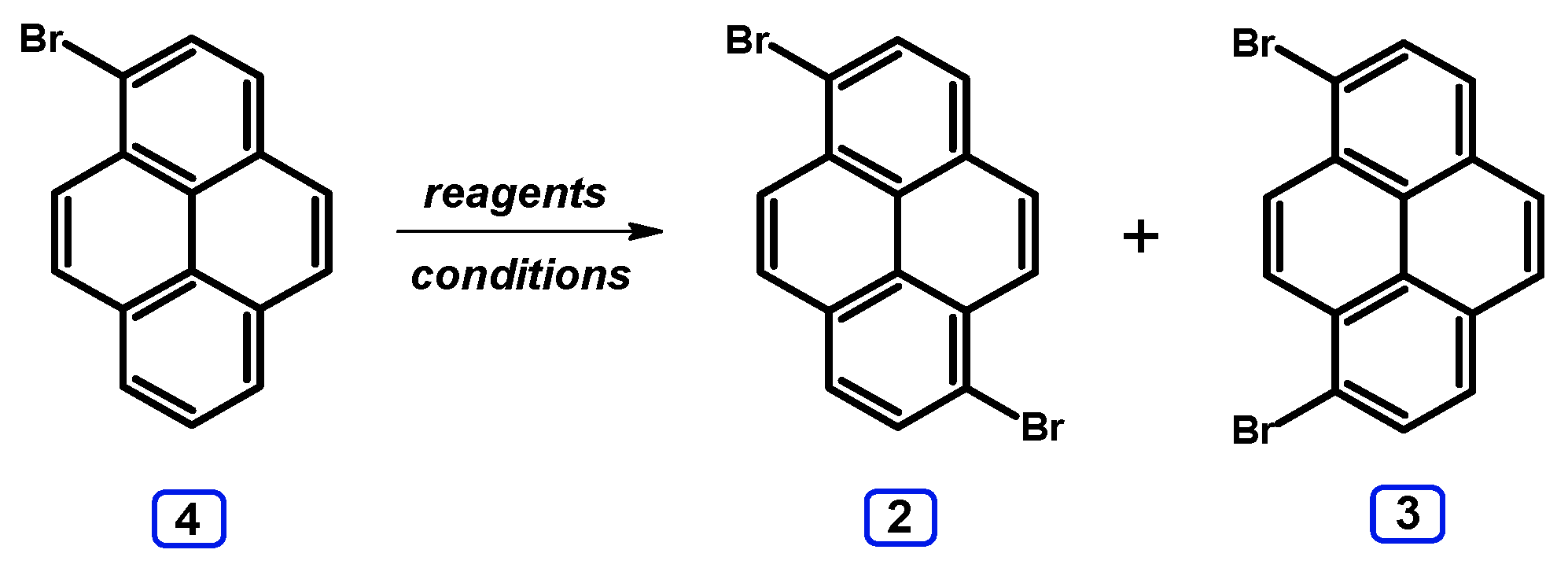
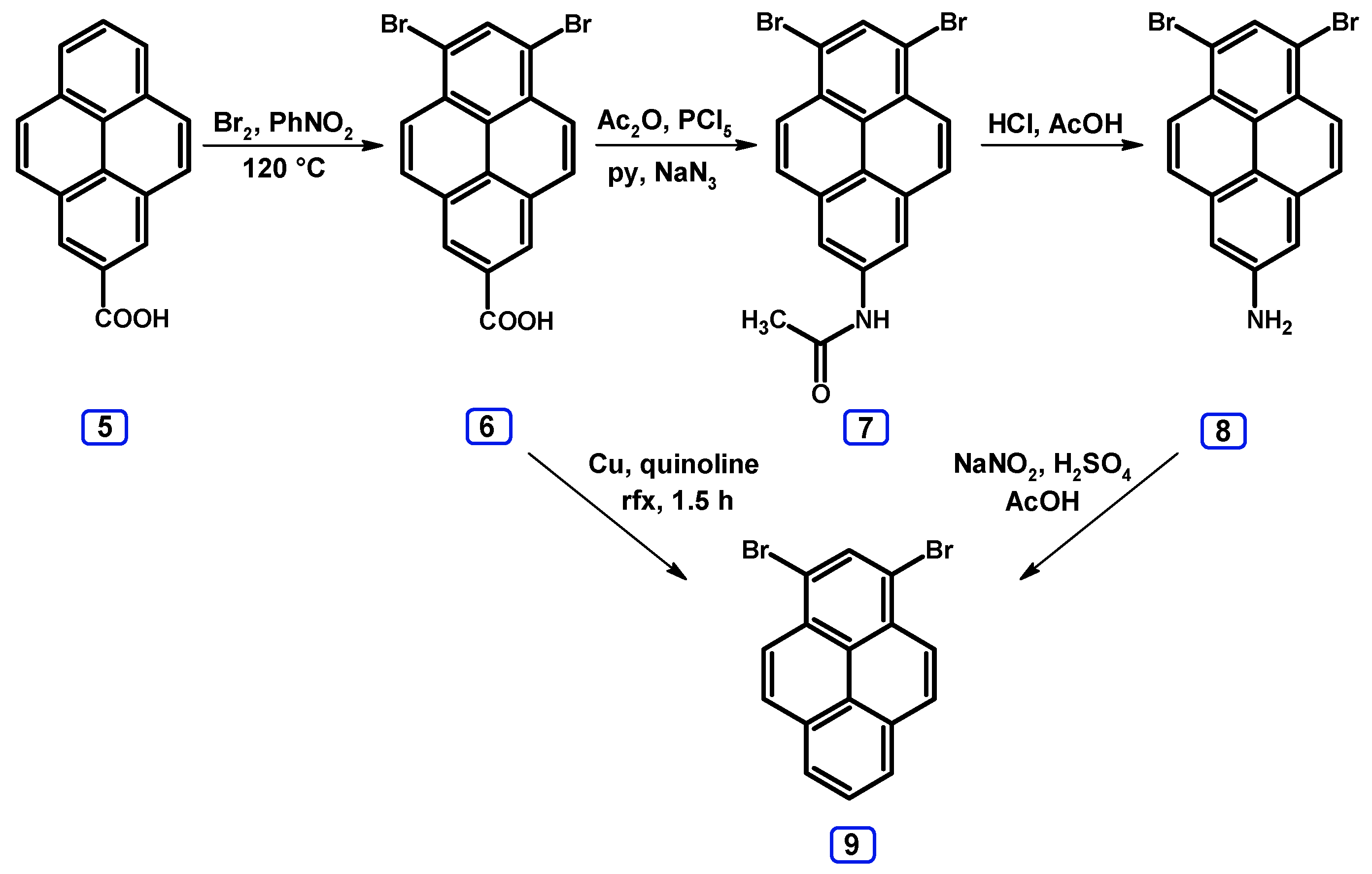
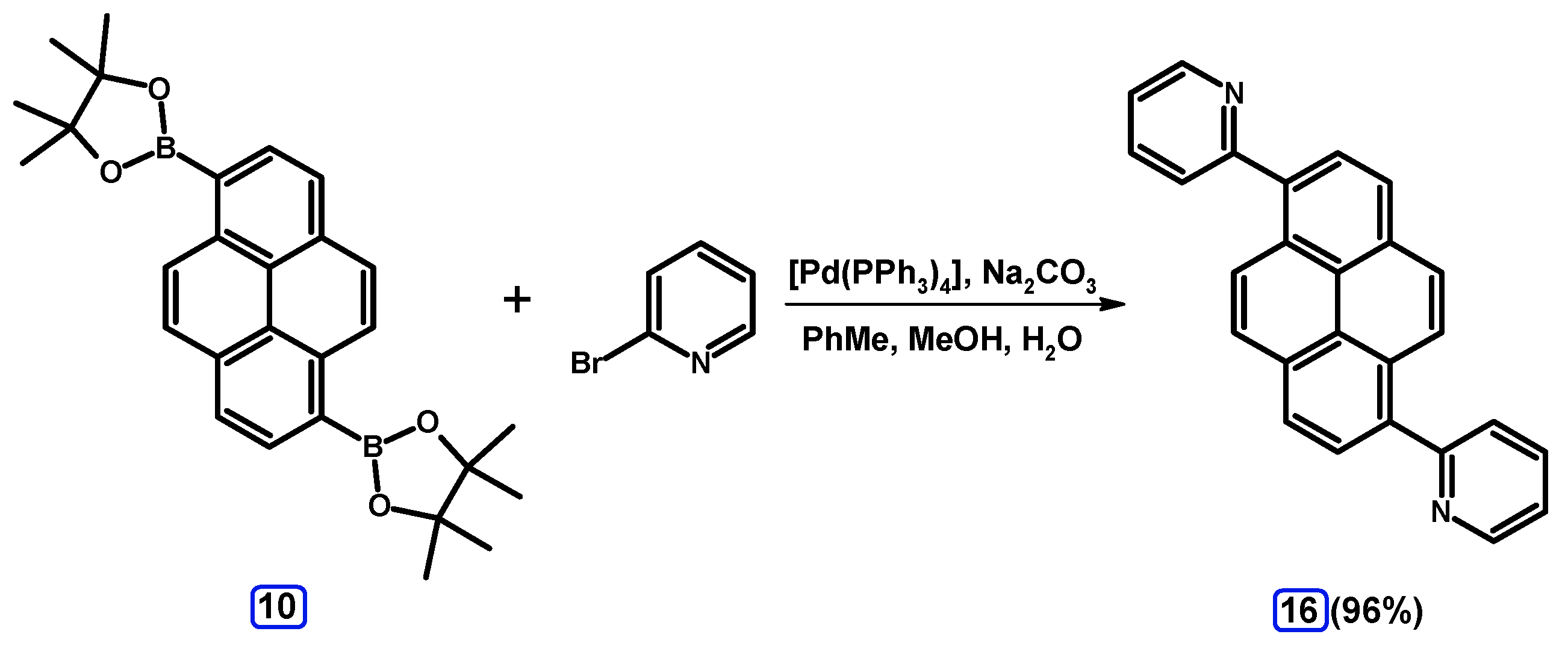
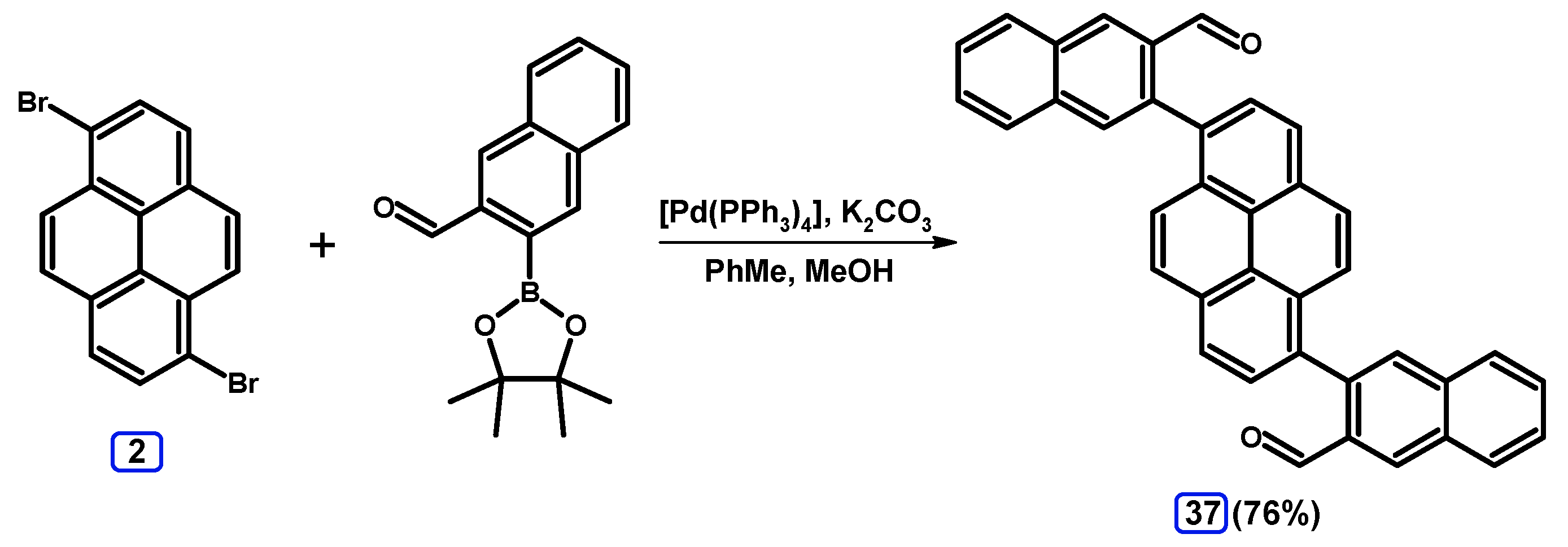
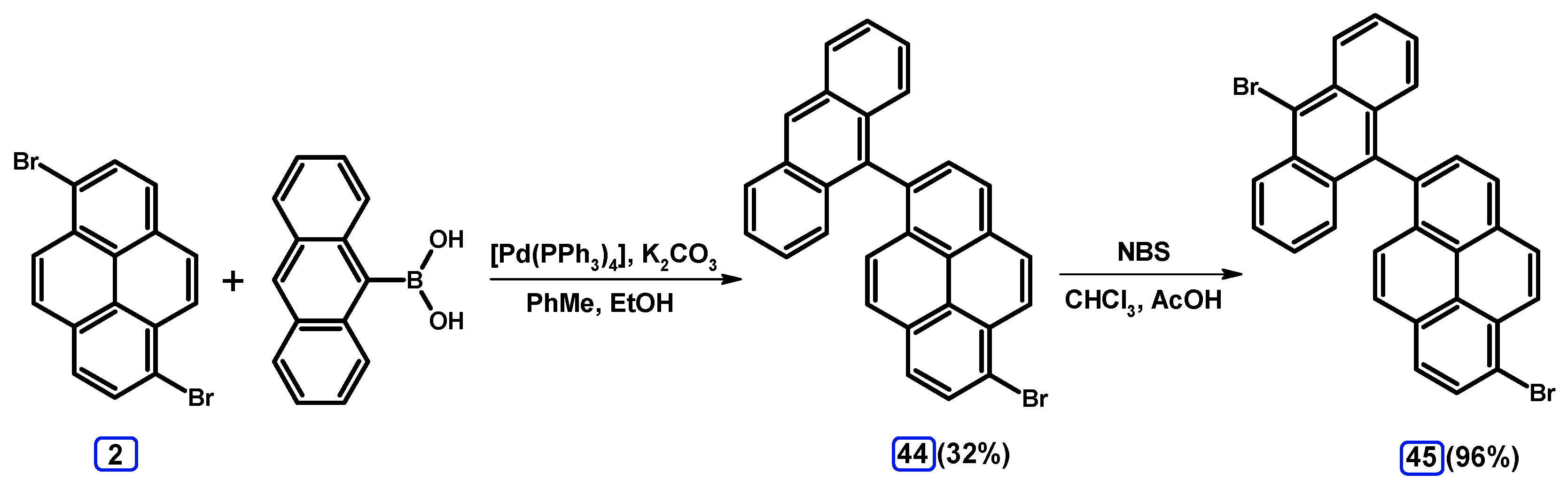
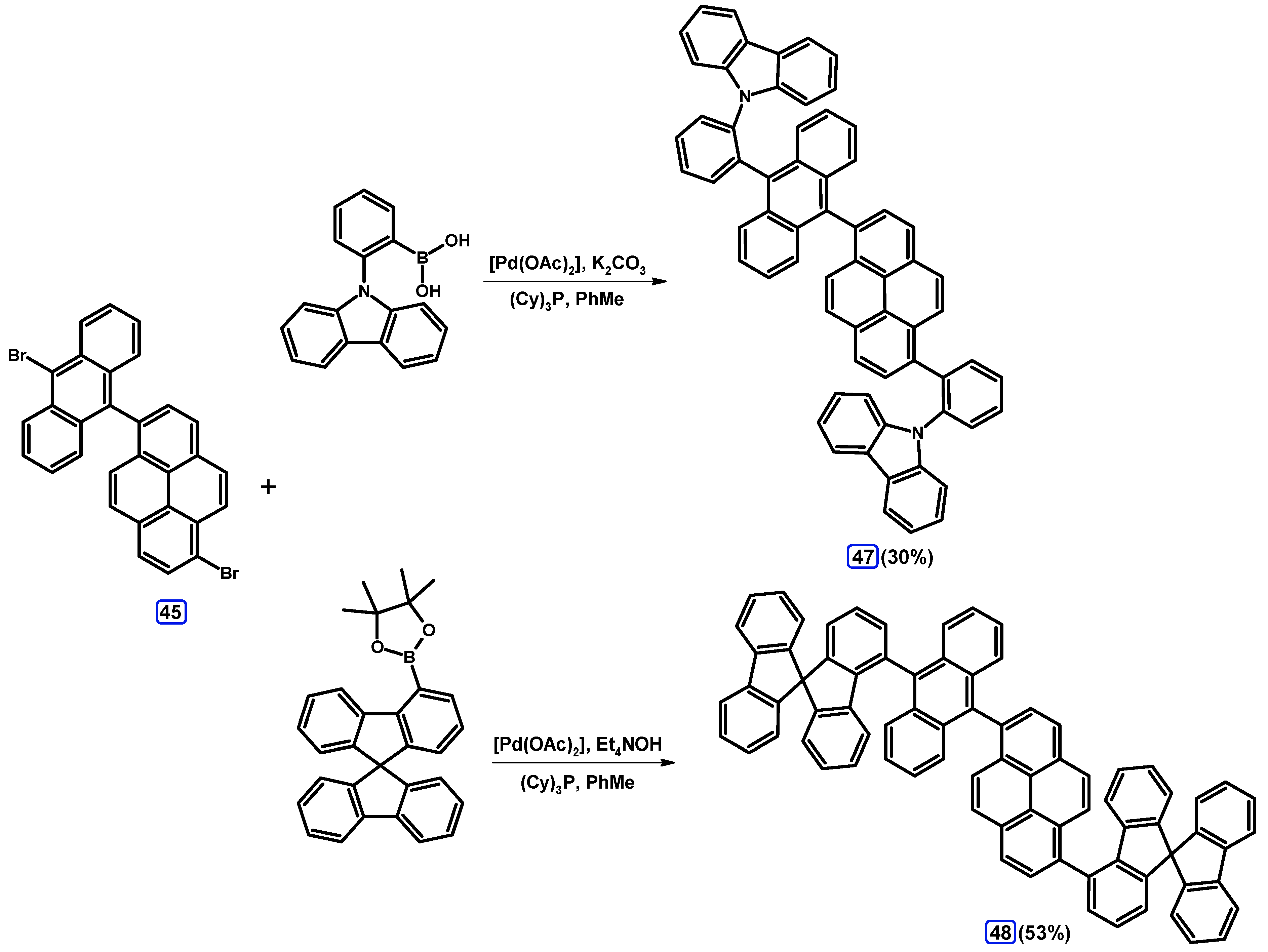
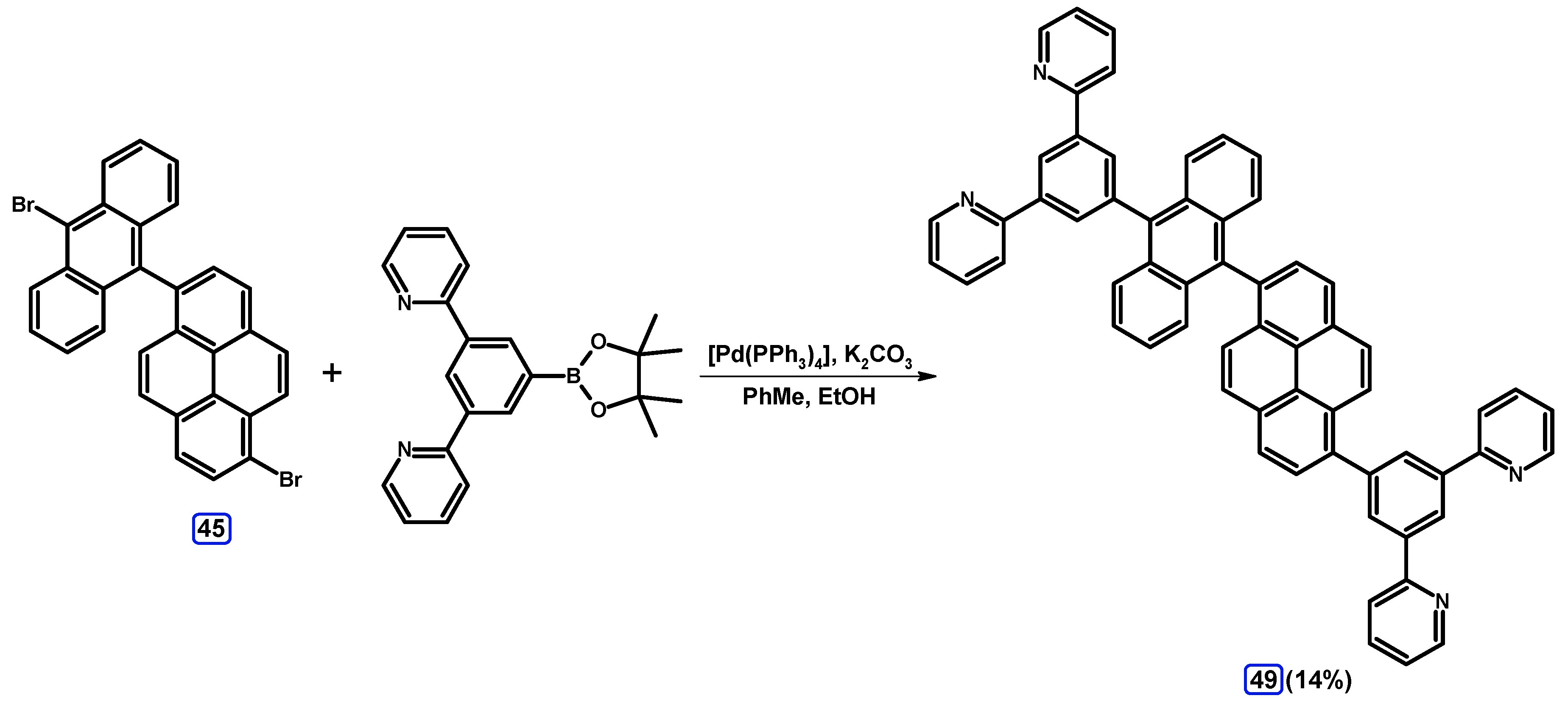
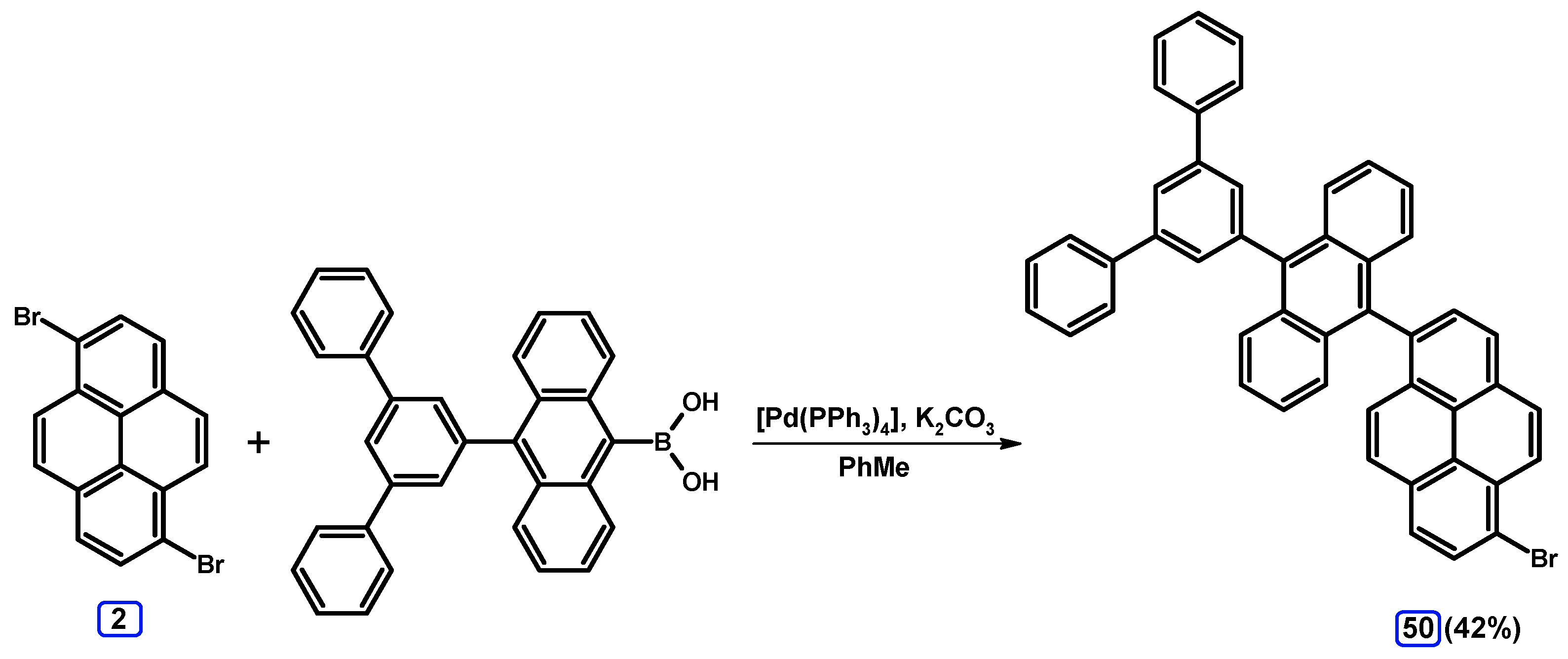
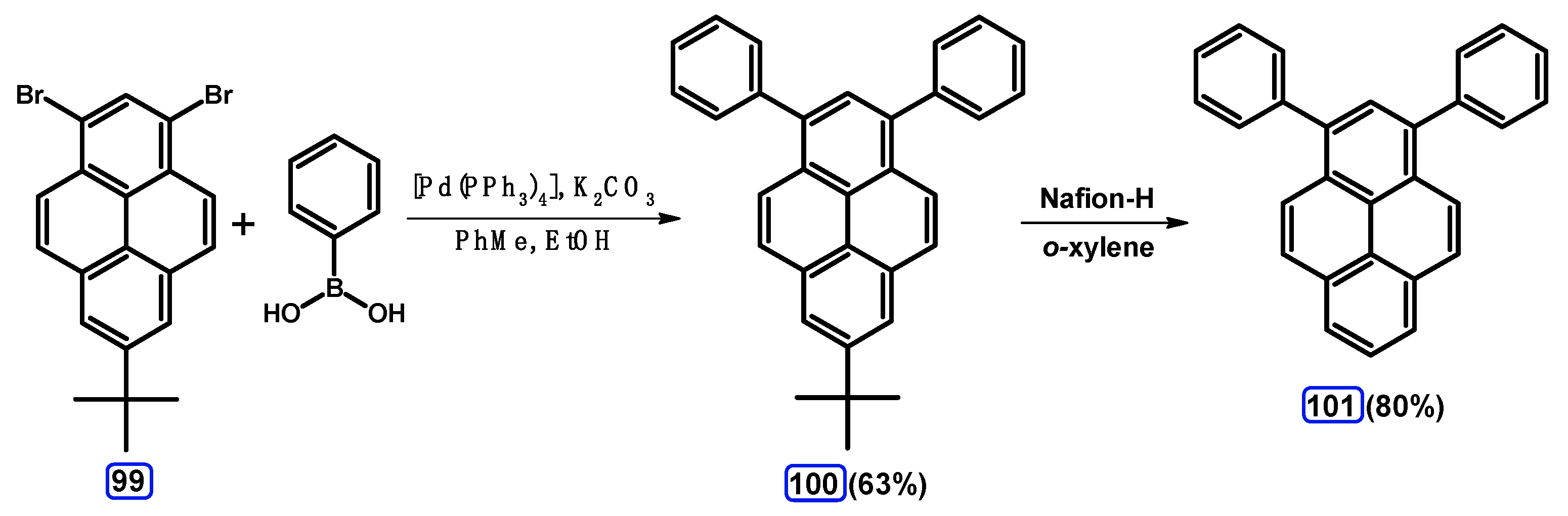
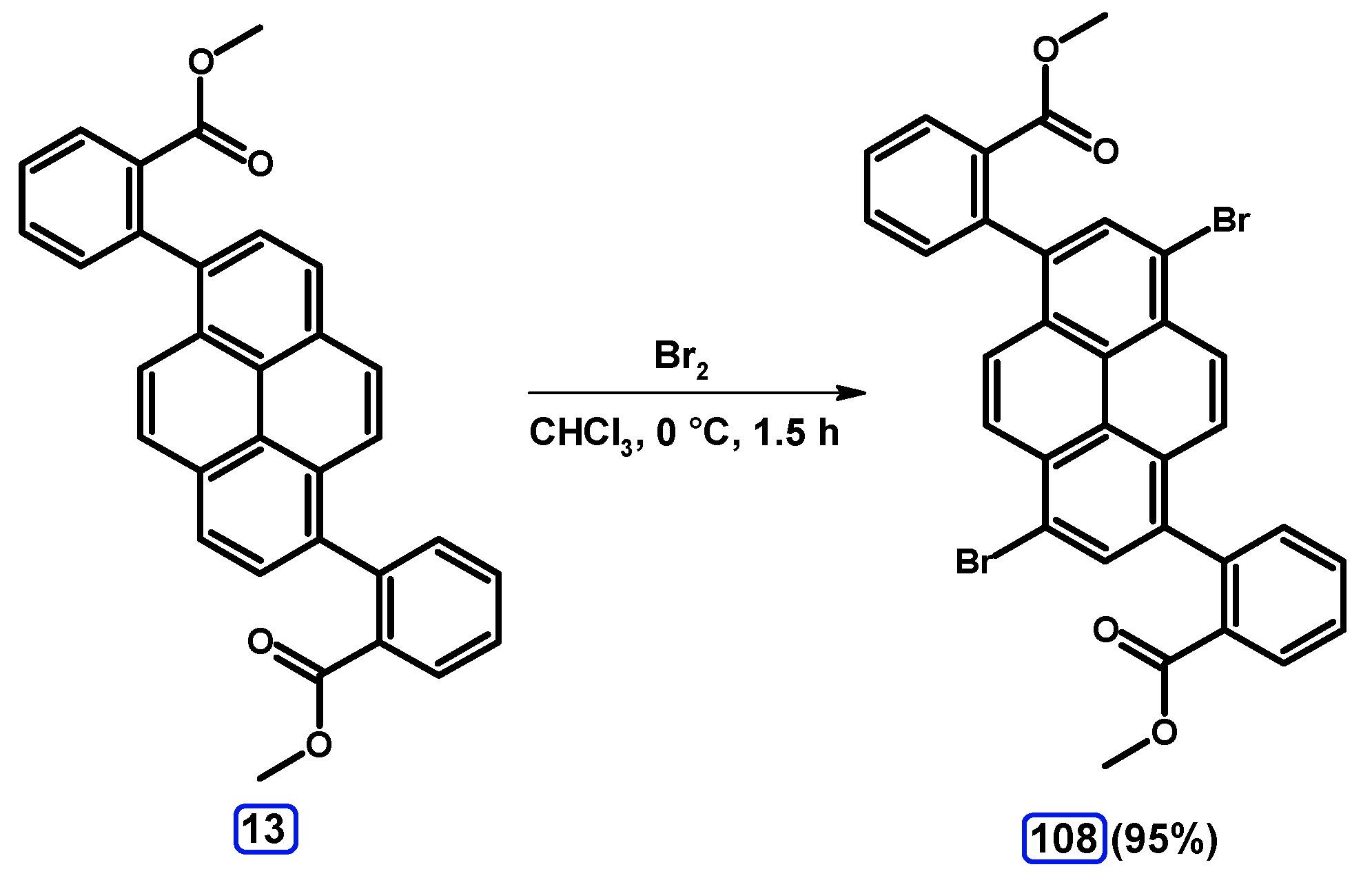
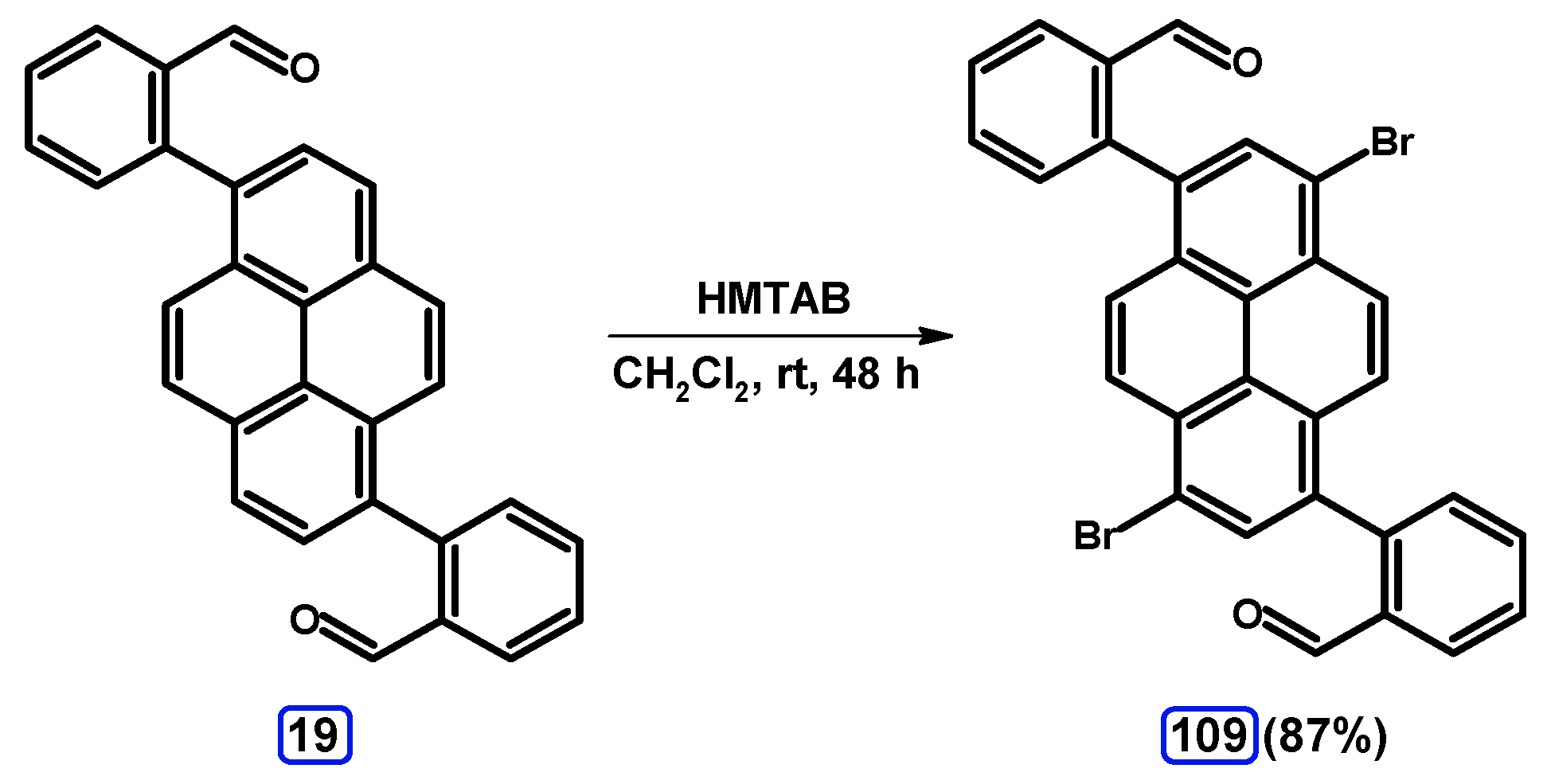
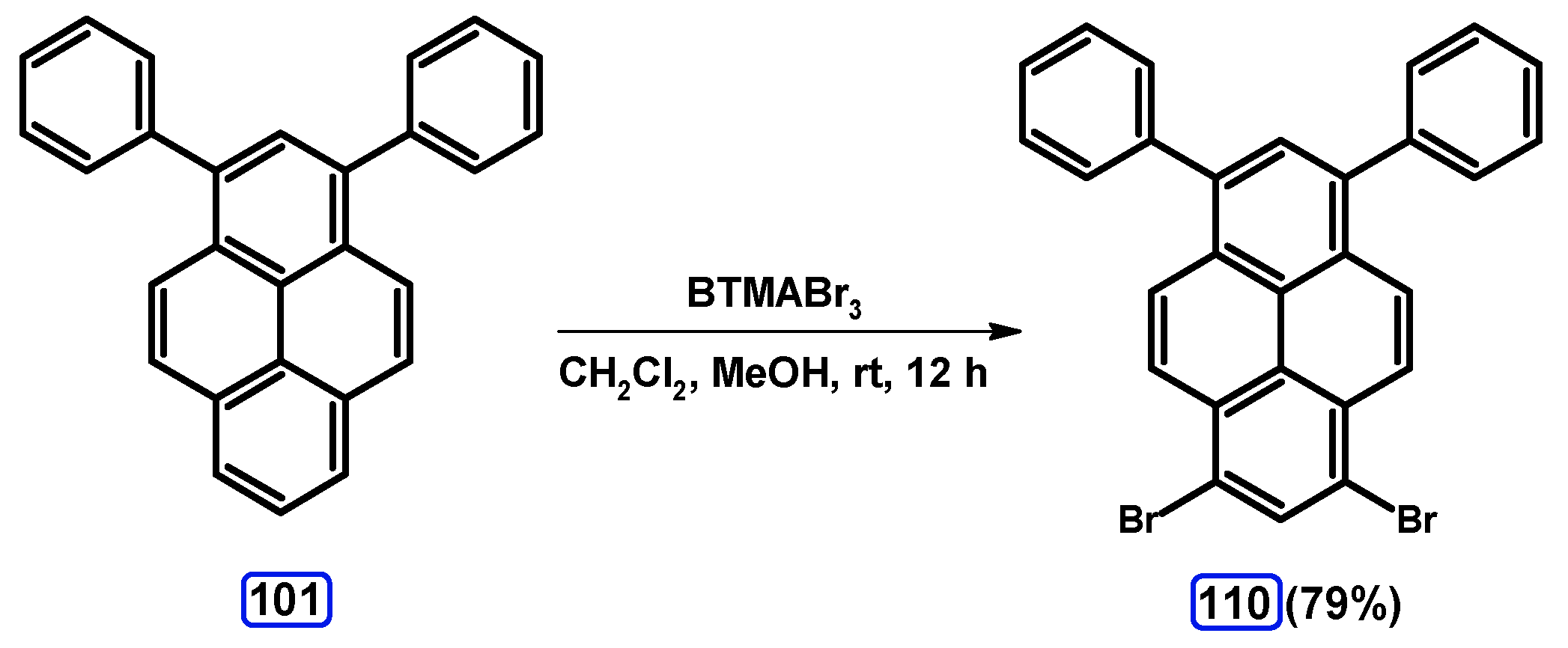
| Entry | Brominating Agent | Solvent | Reaction Conditions | Yield [%] | |
|---|---|---|---|---|---|
| 1,6- | 1,8- | ||||
| 1[19] | Br2 | CH2Cl2 | rt, 24 h | 15 | - |
| 2[20] | Br2 | CH2Cl2 | rt, 2 h | 50 | - |
| 4[21] | Br2 | CH2Cl2 | rt, 20 h | 25 | 9 |
| 4[22,23] | Br2 | CHCl3 | rt,17 h | 33 | - |
| 5 [13] | Br2 | CHCl3 | rt, 24 h | 36 | - |
| 6[24] | Br2 | CHCl3 | rt, 5 h | 14 | - |
| 7[25] | Br2 | CHCl3 | rt, 17 h | 14 | 6 |
| 8[26] | Br2 | CCl4 | 110 °C, 12 h, darkness | 63 | - |
| 9[27] | Br2 | CCl4 | rt, 16 h | 21 | - |
| 10[18] | Br2 | CCl4 | rt, 17 h | 44 | 45 |
| 11[28] | Br2 | CCl4 | rt, 17 h | 61 | - |
| 12[29] | Br2 | CCl4 | rt, 24 h | 28 | 13 |
| 13[30] | Br2 | CCl4 | rt, 48 h | 38 | - |
| 14[31] | Br2 | CCl4 | rt, 54 h | 25 | 50 |
| 15[6,9] | Br2 | CS2 | rt, 17 h | 15 | 85 |
| 16[32] | DBMH | CH2Cl2 | rt, 1 h | 97 | |
| 17[33] | BTMABr3 + ZnCl2 | CH2Cl2, MeOH | rt, 16 h | quant. | |
| DBMH–1,3-dibromo-5,5-dimethylhydantoin BTMABr3–benzyltrimethylammonium tribromide | |||||
| Entry | Brominating Agent | Solvent | Reaction Conditions | Yield [%] | |
|---|---|---|---|---|---|
| 1,6- | 1,8- | ||||
| 1[35] | KBr + NaClO | HCl, MeOH | rt, 24 h | 43 | |
| 2[36] | Br2 | CH2Cl2 | rt, 6 h | 35 | 36 |
| Entry | Reaction Conditions | Yield [%] | ||
|---|---|---|---|---|
| 1,6- | 1,8- | 1,3- | ||
| 1[73] | AcCl, AlCl3, CS2, rt, 3 h | 9.6 | 37.5 | 9.4 |
| 2[74] | AcCl, AlCl3, CS2, rt, 2 h | 25.0 | 46.0 | 11.0 |
| 3[75] | AcCl, AlCl3, CS2, rt, 2 h | 14.8 | 40.2 | 12.3 |
| 4[76] | AcCl, [emim]Cl–AlCl3, rt, 2 h | 55.0 | - | |
| [emim]Cl–1-methyl-3-ethylimidazolium chloride | ||||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, D. Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules 2019, 24, 2551. https://doi.org/10.3390/molecules24142551
Zych D. Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules. 2019; 24(14):2551. https://doi.org/10.3390/molecules24142551
Chicago/Turabian StyleZych, Dawid. 2019. "Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review" Molecules 24, no. 14: 2551. https://doi.org/10.3390/molecules24142551
APA StyleZych, D. (2019). Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules, 24(14), 2551. https://doi.org/10.3390/molecules24142551