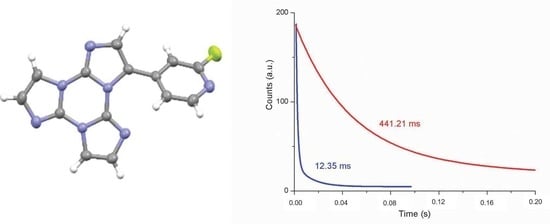
Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-yl)triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]triazine
Abstract
1. Introduction
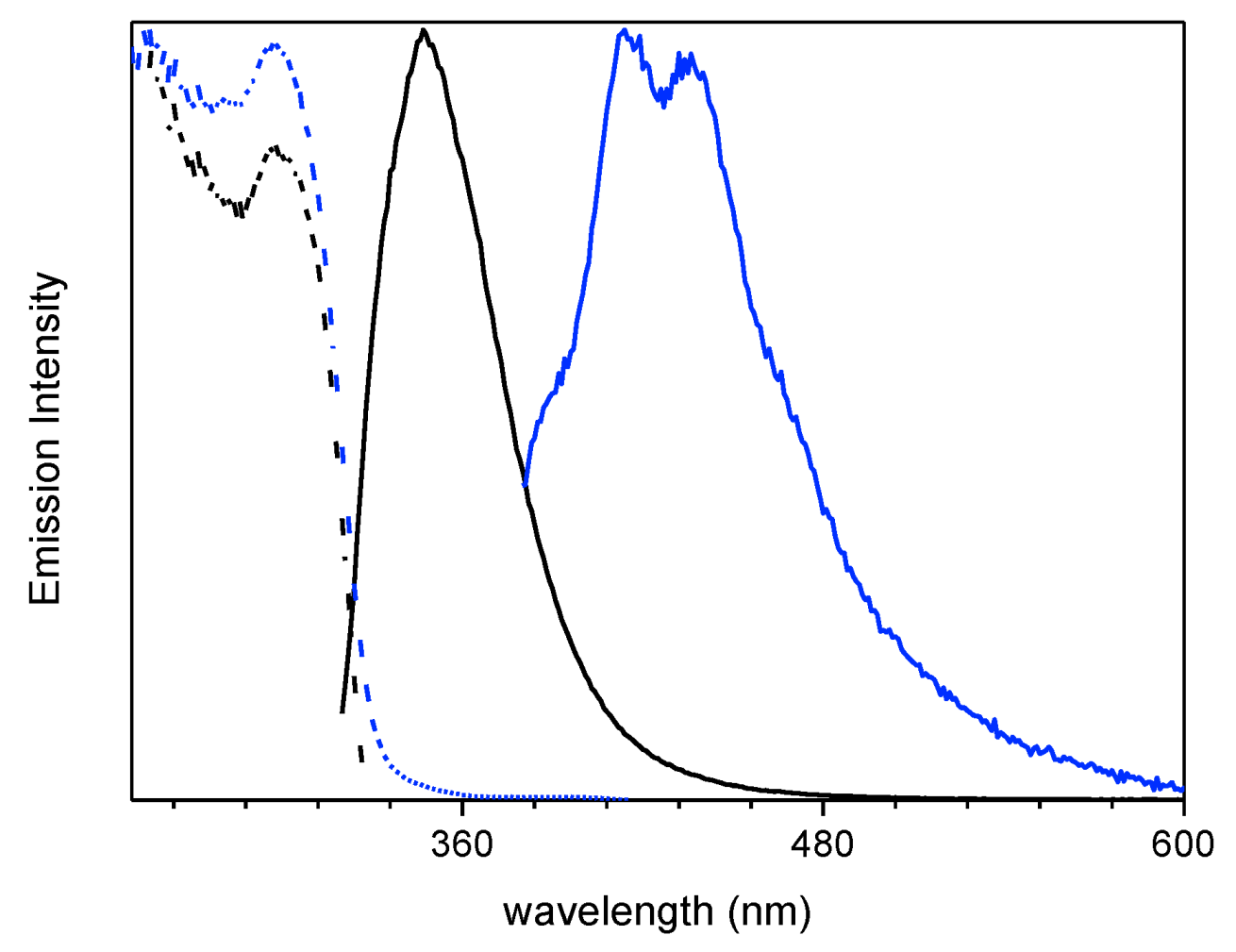
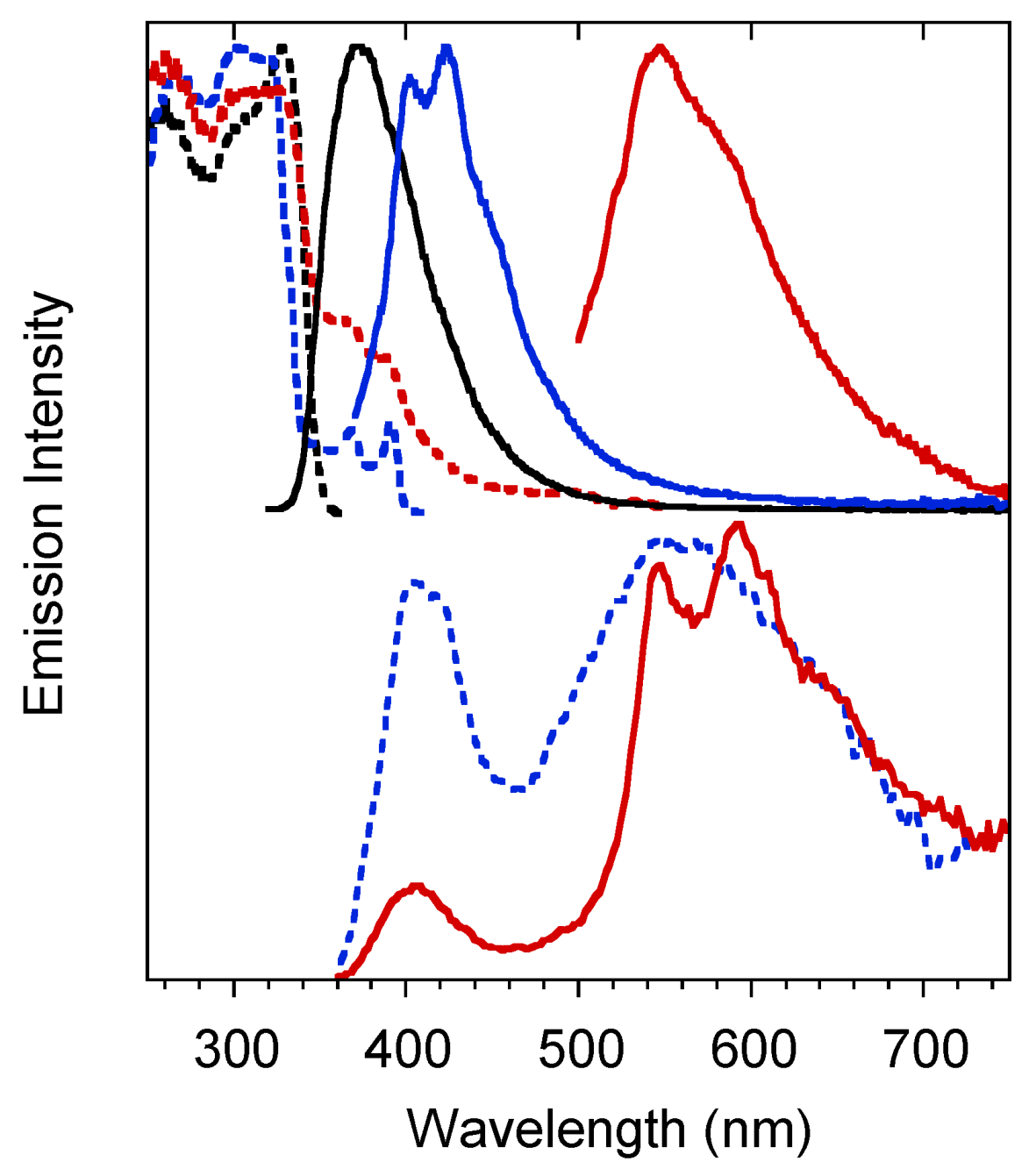
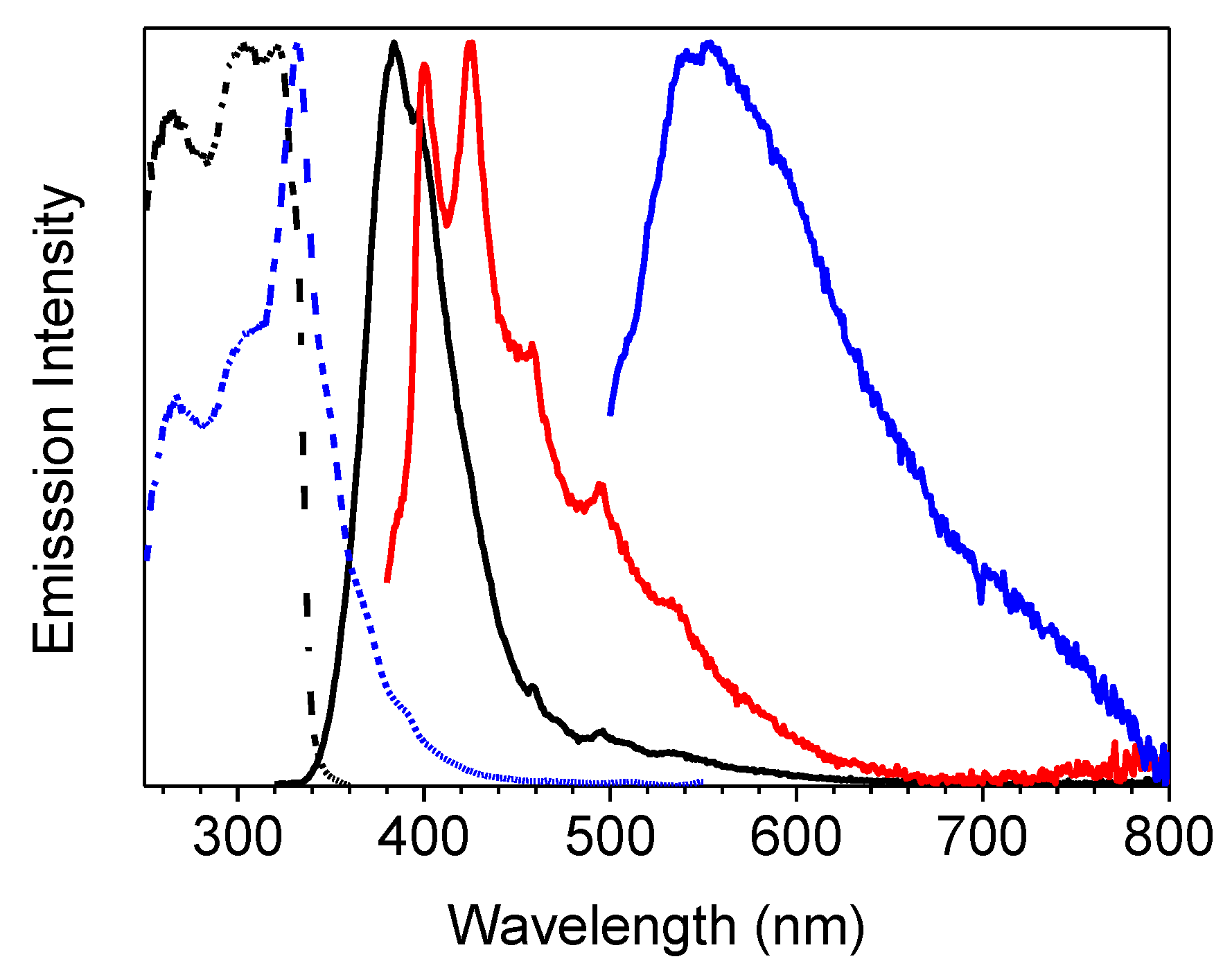
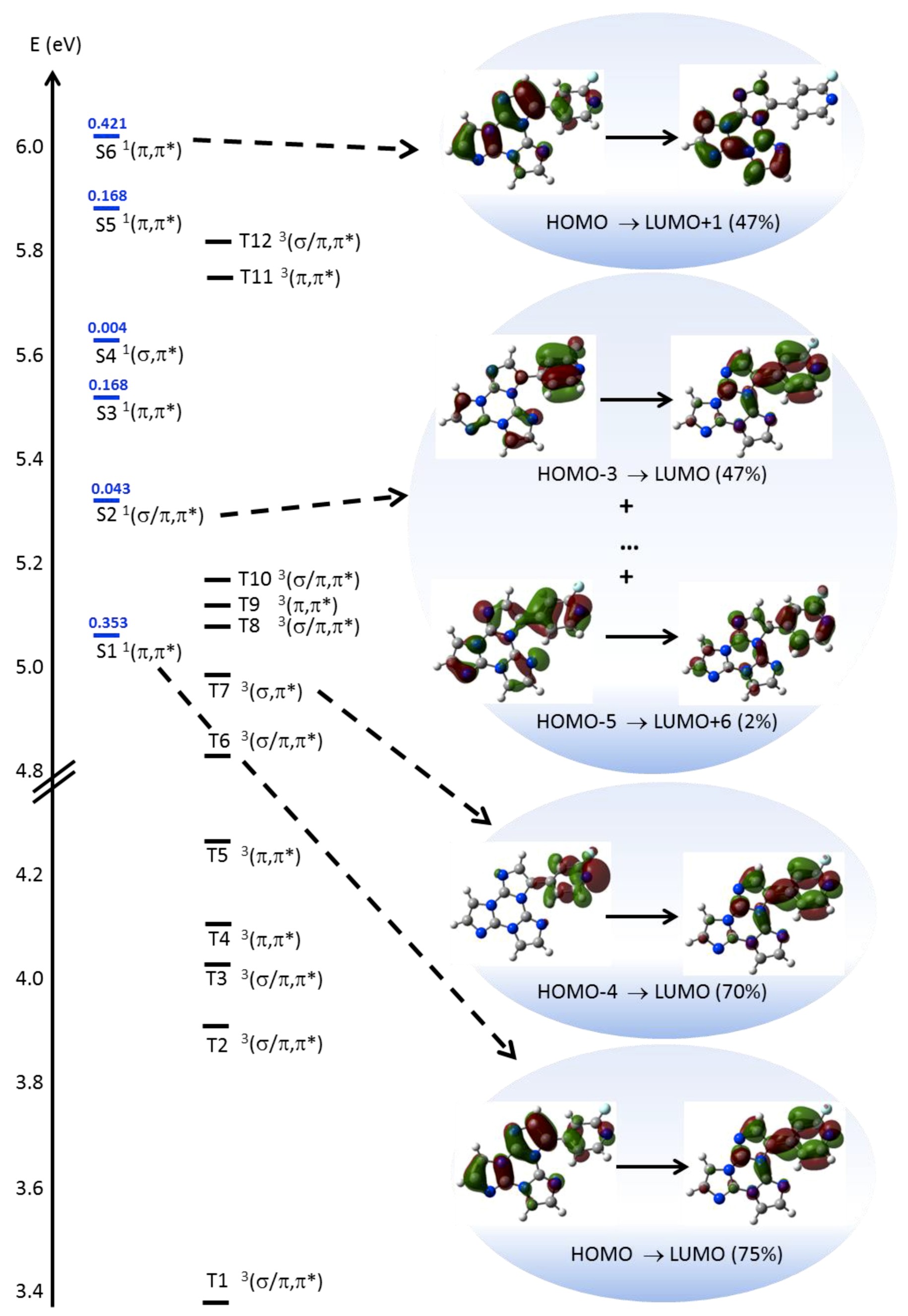
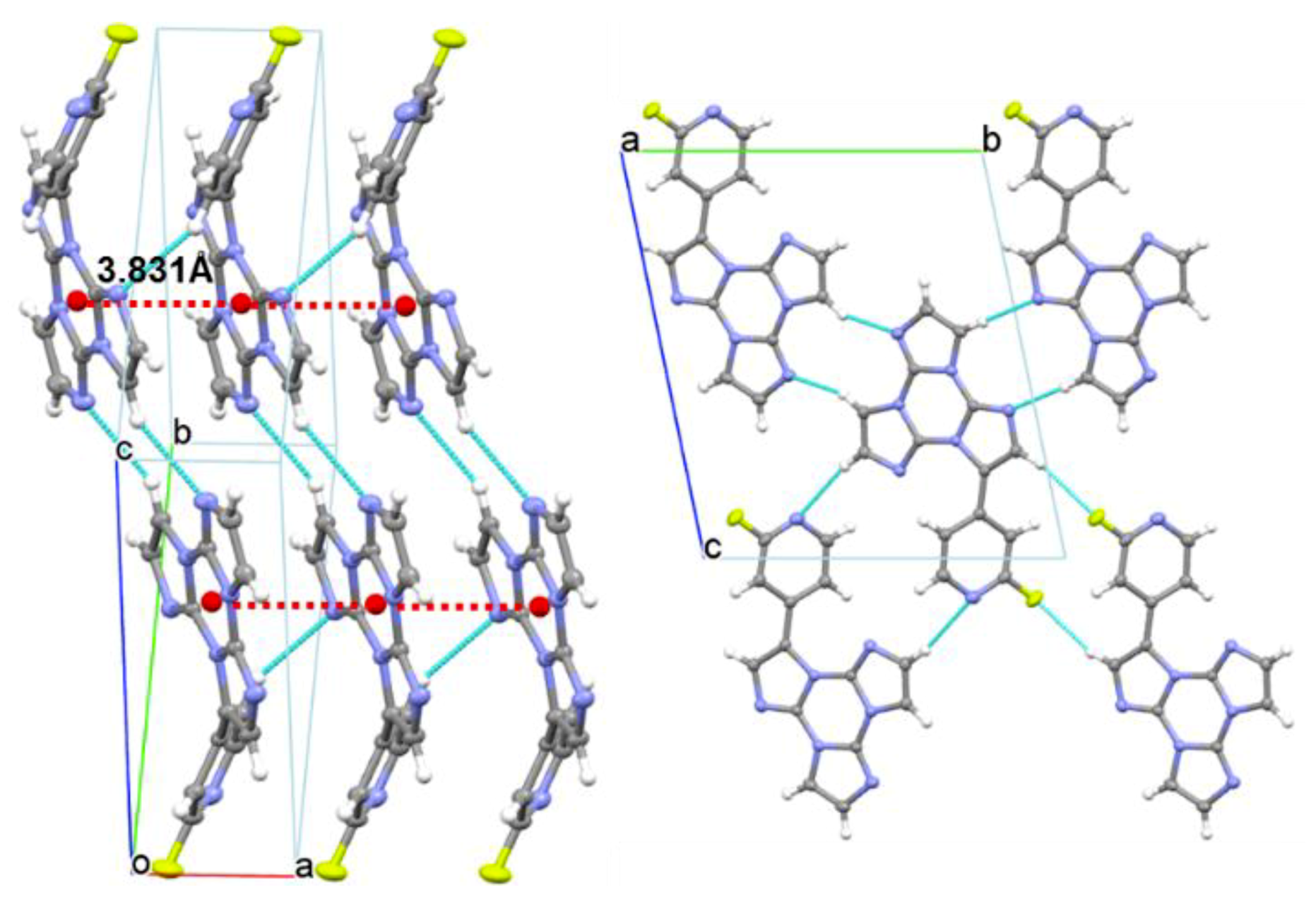
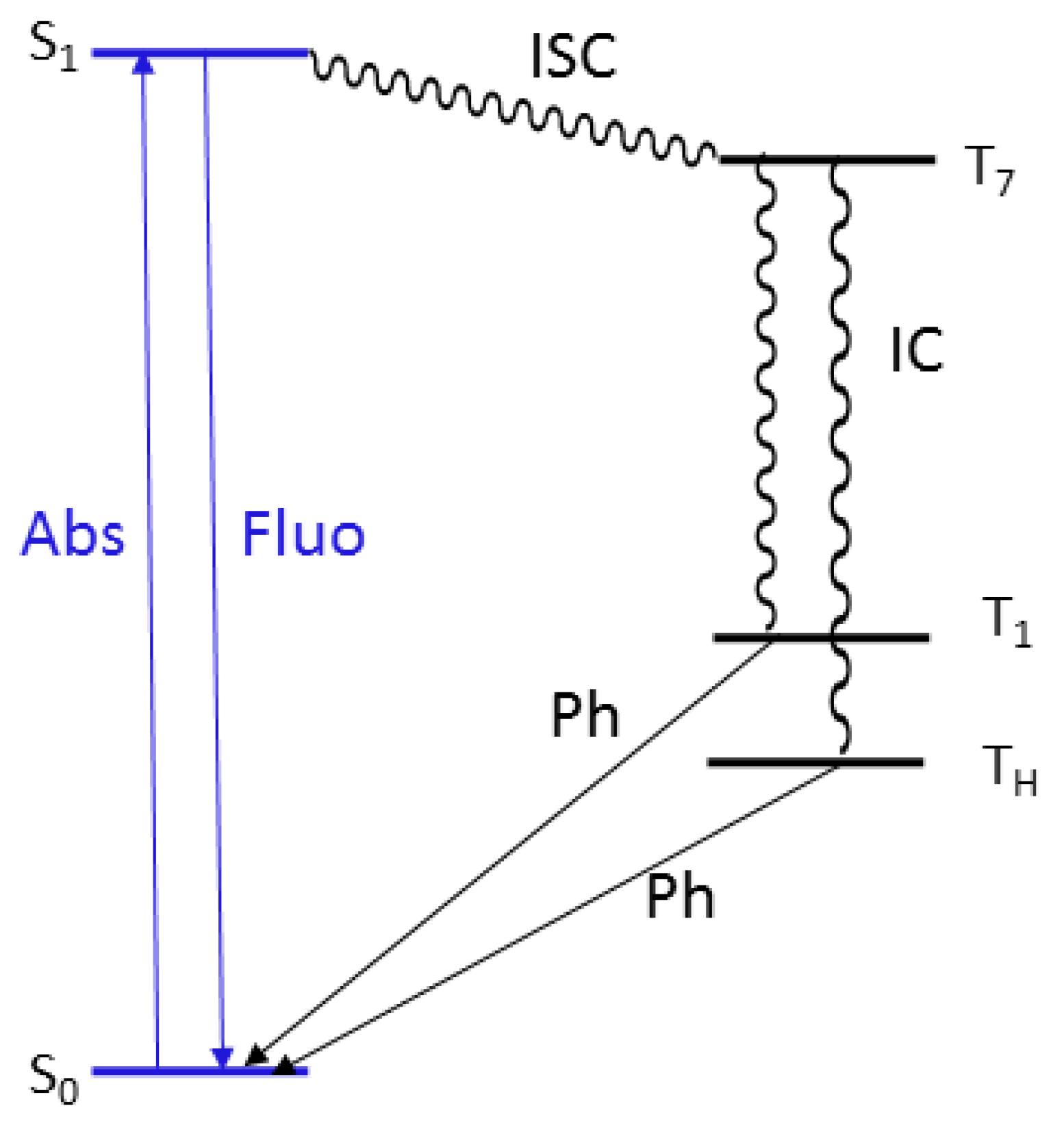
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Single Crystal X-Ray Studies
4.3. Computational Details
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Zhao, W.; Cheung, T.S.; Jiang, N.; Huang, W.; Lam, J.W.Y.; Zhang, X.; He, Z.; Tang, B.Z. Boosting the efficiency of organic persistent room-temperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 2019, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of Carbon Dots from Fluorescence to Ultralong Room-Temperature Phosphorescence by Heating for Security Applications. Adv. Mater. 2018, 30, 1800783. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, S.; Lin, W.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Xiao, H.; Chen, P.Z.; Yang, Q.Z.; Chen, B.; Tung, C.H.; Chen, Y.Z.; Wu, L.Z. Pure Organic Room Temperature Phosphorescence from Excited Dimers in Self-Assembled Nanoparticles under Visible and Near-Infrared Irradiation in Water. J. Am. Chem. Soc. 2019, 141, 5045–5050. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Xie, C.; Pu, K. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Kabe, R.; Notsuka, N.; Yoshida, K.; Adachi, C. Afterglow Organic Light-Emitting Diode. Adv. Mater. 2016, 28, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Fu, H. Enhanced Room-Temperature Phosphorescence through Intermolecular Halogen/Hydrogen Bonding. Chem. Eur. J. 2019, 25, 714–723. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. H-Aggregates Granting Crystallization-Induced Emissive Behavior and Ultralong Phosphorescence from a Pure Organic Molecule. J. Phys. Chem. Lett. 2017, 8, 1894–1898. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef]
- Pan, S.; Chen, Z.; Zheng, X.; Wu, D.; Chen, G.; Xu, J.; Feng, H.; Qian, Z. Ultralong Room-Temperature Phosphorescence from Supramolecular Behavior via Intermolecular Electronic Coupling in Pure Organic Crystals. J. Phys. Chem. Lett. 2018, 9, 3939–3945. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Wang, Q.; Shao, P.; Ma, D.; Yuan, S.; Lei, P.; Li, P.; Feng, X.; Wang, B. Covalent organic frameworks: A platform for the experimental establishment of the influence of intermolecular distance on phosphorescence. J. Mater. Chem. C 2018, 6, 5369–5374. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Ji, Y.; Chen, R.; Zhu, Q.; Wang, Y.; Zheng, C.; Tao, Y.; Fan, Q.; Huang, W. Invoking ultralong room temperature phosphorescence of purely organic compounds through H-aggregation engineering. Mater. Horiz. 2019. [Google Scholar] [CrossRef]
- Mao, Z.; Yang, Z.; Xu, C.; Xie, Z.; Jiang, L.; Gu, F.L.; Zhao, J.; Zhang, Y.; Aldred, M.P.; Chi, Z. Two-photon-excited ultralong organic room temperature phosphorescence by dual-channel triplet harvesting. Chem. Sci. 2019. [Google Scholar] [CrossRef]
- Ma, X.; Wang, J.; Tian, H. Assembling-Induced Emission: An Efficient Approach for Amorphous Metal-Free Organic Emitting Materials with Room-Temperature Phosphorescence. Acc. Chem. Res. 2019, 52, 738–748. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Colombo, A.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. The Effect of Bromo Substituents on the Multifaceted Emissive and Crystal-Packing Features of Cyclic Triimidazole Derivatives. ChemPhotoChem 2018, 2, 801–805. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Pavanello, A.; Previtali, A.; Righetto, S.; Cariati, E. Cyclic Triimidazole Derivatives: Intriguing Examples of Multiple Emissions and Ultralong Phosphorescence at Room Temperature. Angew. Chem. Int. Ed. 2017, 56, 16302–16307. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. Intrinsic and Extrinsic Heavy-Atom Effects on the Multifaceted Emissive Behavior of Cyclic Triimidazole. Chem. Eur. J. 2019, 25, 2452–2456. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, R.; Tang, X.; Tao, Y.; Xu, S.; Jin, L.; Chen, C.; Zhou, X.; Zheng, C.; Huang, W. Direct population of triplet excited states through singlet–triplet transition for visible-light excitable organic afterglow. Chem. Sci. 2019, 10, 5031–5038. [Google Scholar] [CrossRef]
- Majoube, M.; Henry, M.; Chinsky, L.; Turpin, P.Y. Preresonance Raman spectra for imidazole and imidazolium ion: Interpretation of the intensity enhancement from a precise assignment of normal modes. Chem. Phys. 1993, 169, 231–241. [Google Scholar] [CrossRef]
- Schubert, D.M.; Natan, D.T.; Wilson, D.C.; Hardcastle, K.I. Facile Synthesis and Structures of Cyclic Triimidazole and Its Boric Acid Adduct. Cryst. Growth Des. 2011, 11, 843–850. [Google Scholar] [CrossRef]
- Bruker. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 16, Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
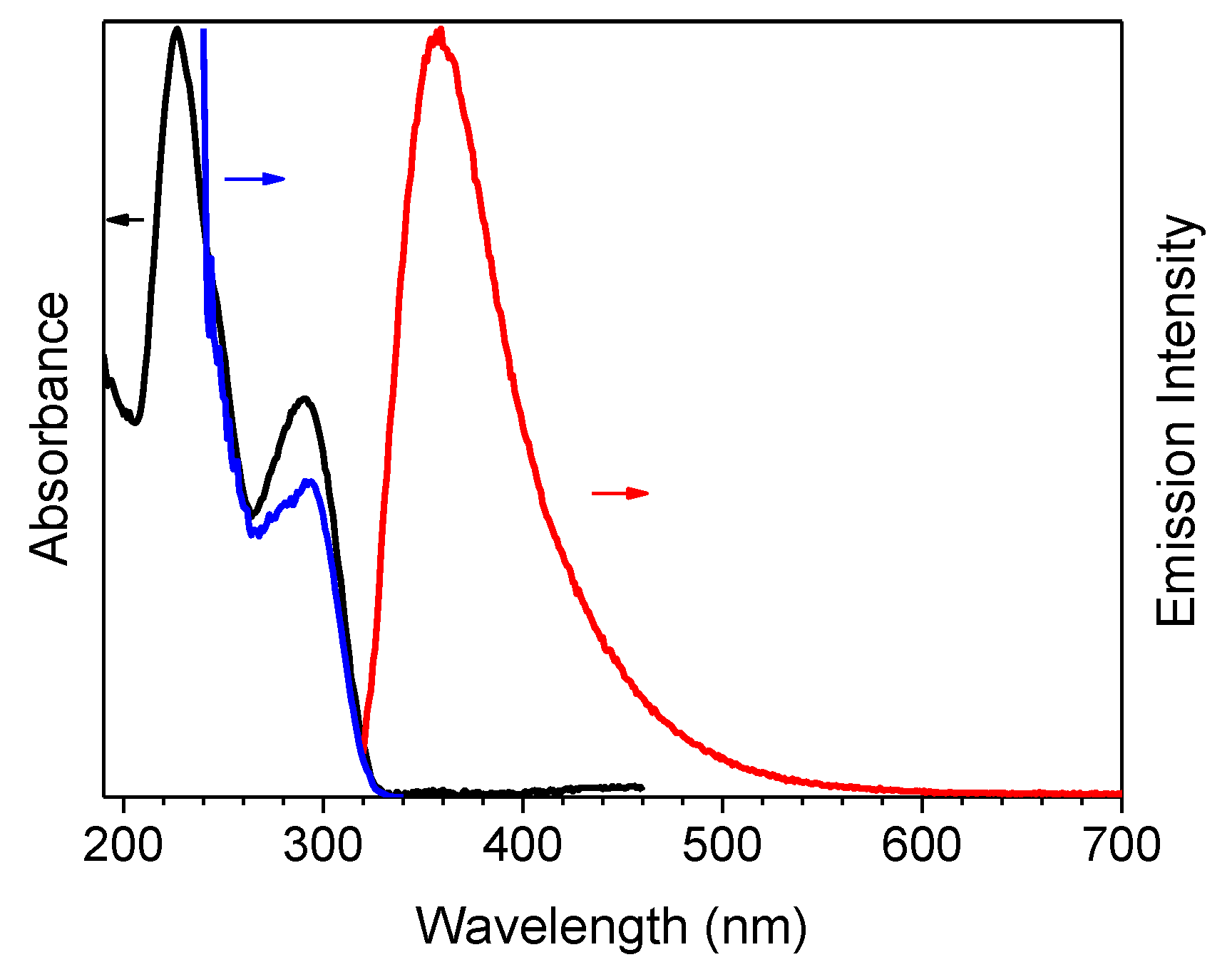
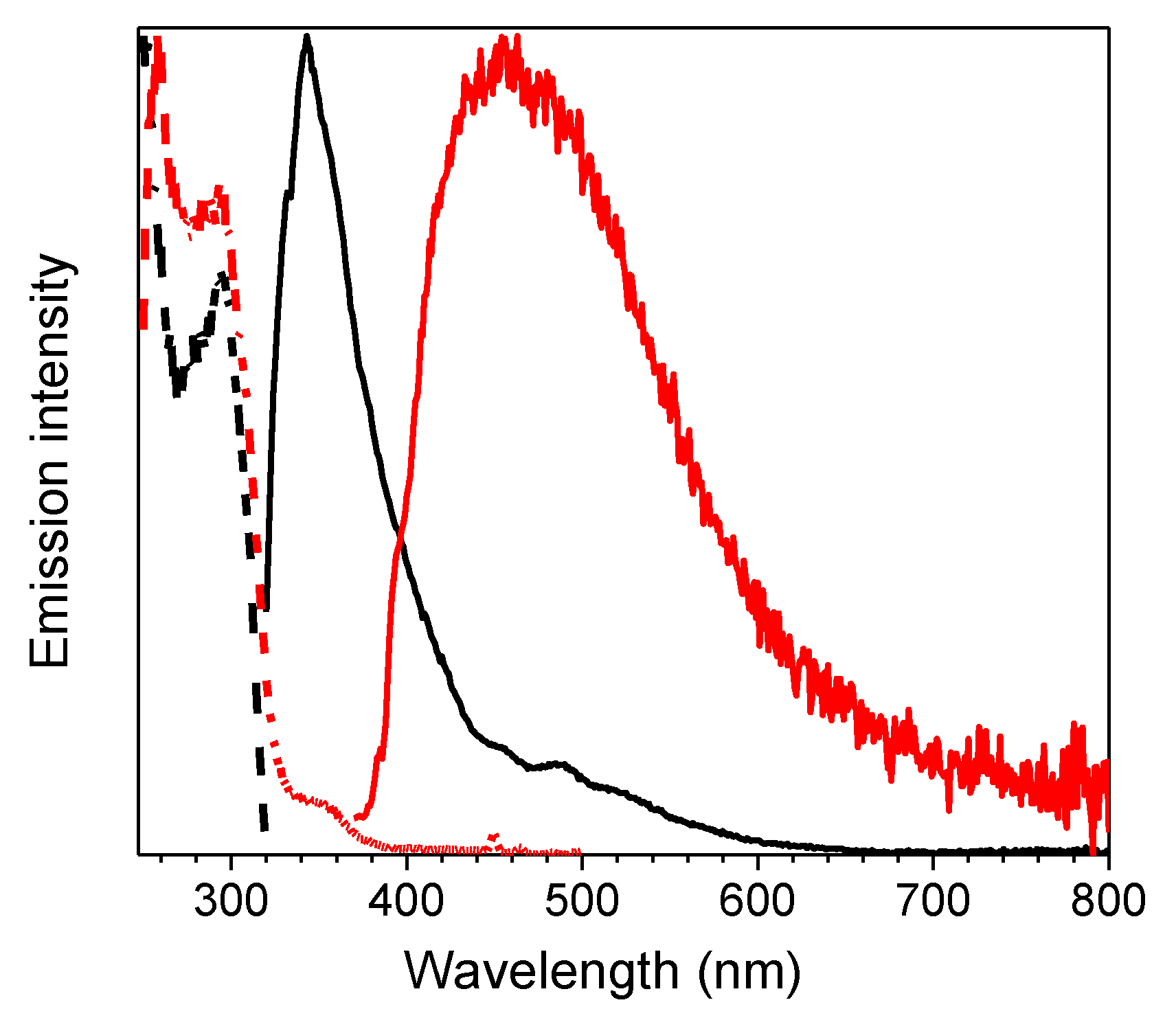
| Sample | 298 K | 77 K | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Φ (%) | λabs 1 (nm) | λem (nm) | τ (λexc 300nm) | Origin | λabs 1 (nm) | λem (nm) | τ (λexc 300nm) | Origin | |
| 1 (CH3CN) | 50 | 290 | 358 | 4.26ns 2 | S1-S0 | 295 | 344 | 0.44 ns (0.52), 1.74 ns (0.40) 7.9 ns (0.08) 3 | S1-S0 |
| 350 | 454 | 127 ms (0.07), 1640 ms (0.93) 4 | T1-S0 | ||||||
| 1 (crystals) | 25 | 301 | 373 | 1.78 ns (0.19), 5.02 ns (0.81) 5 | S1-S0 | 306 | 385 | 7.56 ns (0.10), 16.87 ns (0.90) 5 | S1-S0 |
| 368, 392 | 403, 424, 446 | 1.26 ms (0.40), 12.35 ms (0.60) 6 | T1-S0 | 369, 390 | 401, 425, 457 | 1.52 ms (0.45), 13.97 ms (0.55) 6 | T1-S0 | ||
| 500, 533 | 546 592 | 42.27 ms (0.38), 441.21 ms (0.62) 7 | -S0 | 513 | 549 | 175.95 ms (0.03), 1190.09 ms (0.49)2320.45 ms (0.48)7 | -S0 | ||
| 1(film) | 298 | 348 | 0.84 ns (0.28), 2.06 ns (0.72) 8 | S1-S0 | |||||
| 415, 436 | 0.57 ms (0.50), 3.50 ms (0.50) 9 | T1-S0 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Previtali, A.; Lucenti, E.; Forni, A.; Mauri, L.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Righetto, S.; Cariati, E. Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-yl)triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]triazine. Molecules 2019, 24, 2552. https://doi.org/10.3390/molecules24142552
Previtali A, Lucenti E, Forni A, Mauri L, Botta C, Giannini C, Malpicci D, Marinotto D, Righetto S, Cariati E. Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-yl)triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]triazine. Molecules. 2019; 24(14):2552. https://doi.org/10.3390/molecules24142552
Chicago/Turabian StylePrevitali, Andrea, Elena Lucenti, Alessandra Forni, Luca Mauri, Chiara Botta, Clelia Giannini, Daniele Malpicci, Daniele Marinotto, Stefania Righetto, and Elena Cariati. 2019. "Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-yl)triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]triazine" Molecules 24, no. 14: 2552. https://doi.org/10.3390/molecules24142552
APA StylePrevitali, A., Lucenti, E., Forni, A., Mauri, L., Botta, C., Giannini, C., Malpicci, D., Marinotto, D., Righetto, S., & Cariati, E. (2019). Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-yl)triimidazo[1,2-a:1′,2′-c:1″,2″-e][1,3,5]triazine. Molecules, 24(14), 2552. https://doi.org/10.3390/molecules24142552