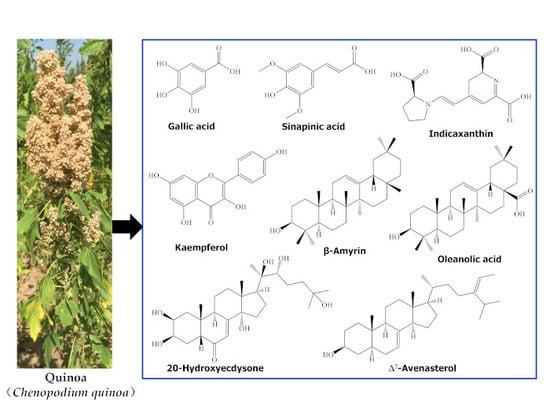
Quinoa Secondary Metabolites and Their Biological Activities or Functions
Abstract
1. Introduction
2. Phenolic Acids and Their Biological Activities or Functions
2.1. Benzoic Acid Analogues and Their Biological Activities or Functions
2.2. Cinnamic Acid Analogues and Their Biological Activities or Functions
3. Flavonoids and Their Biological Activities or Functions
3.1. Flavones and Their Biological Activities or Functions
3.2. Flavonols and Their Biological Activities or Functions
3.3. Flavanones and Their Biological Activities or Functions
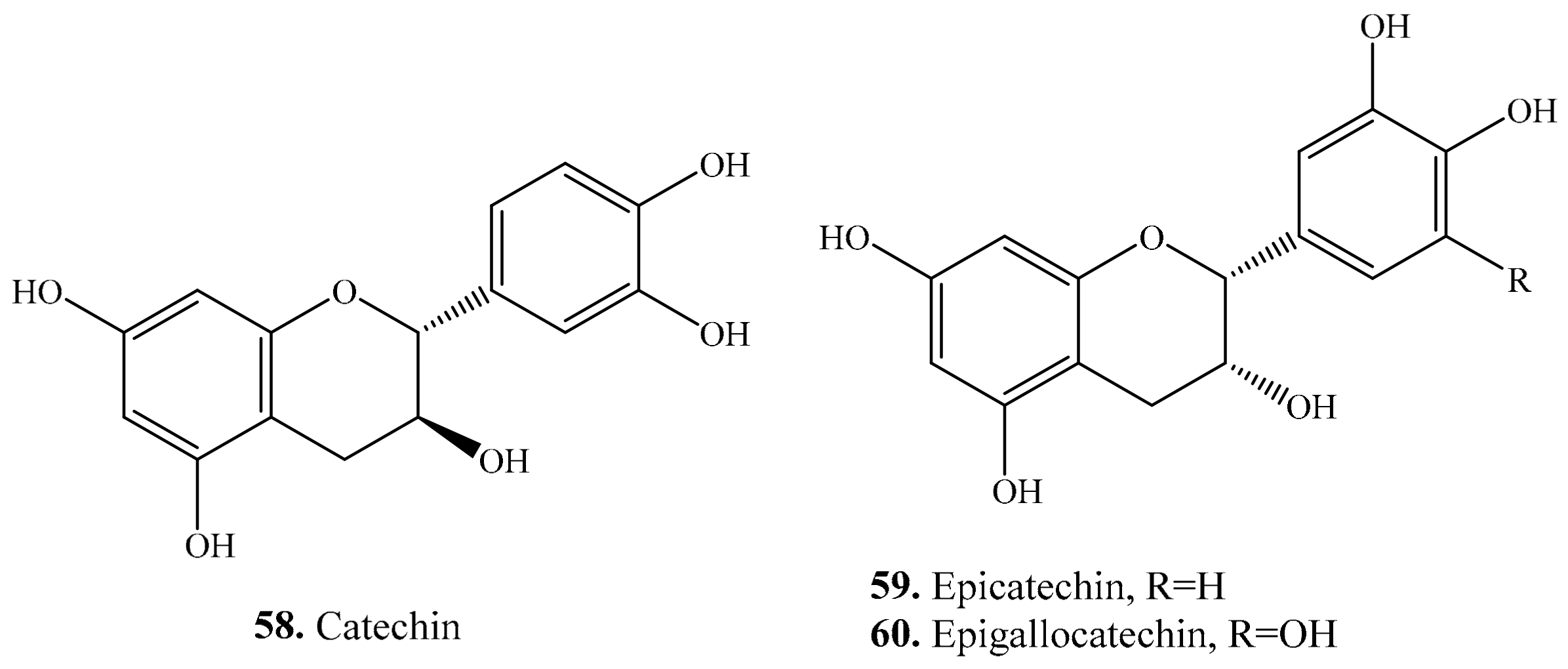
3.4. Flavanols and Their Biological Activities or Functions
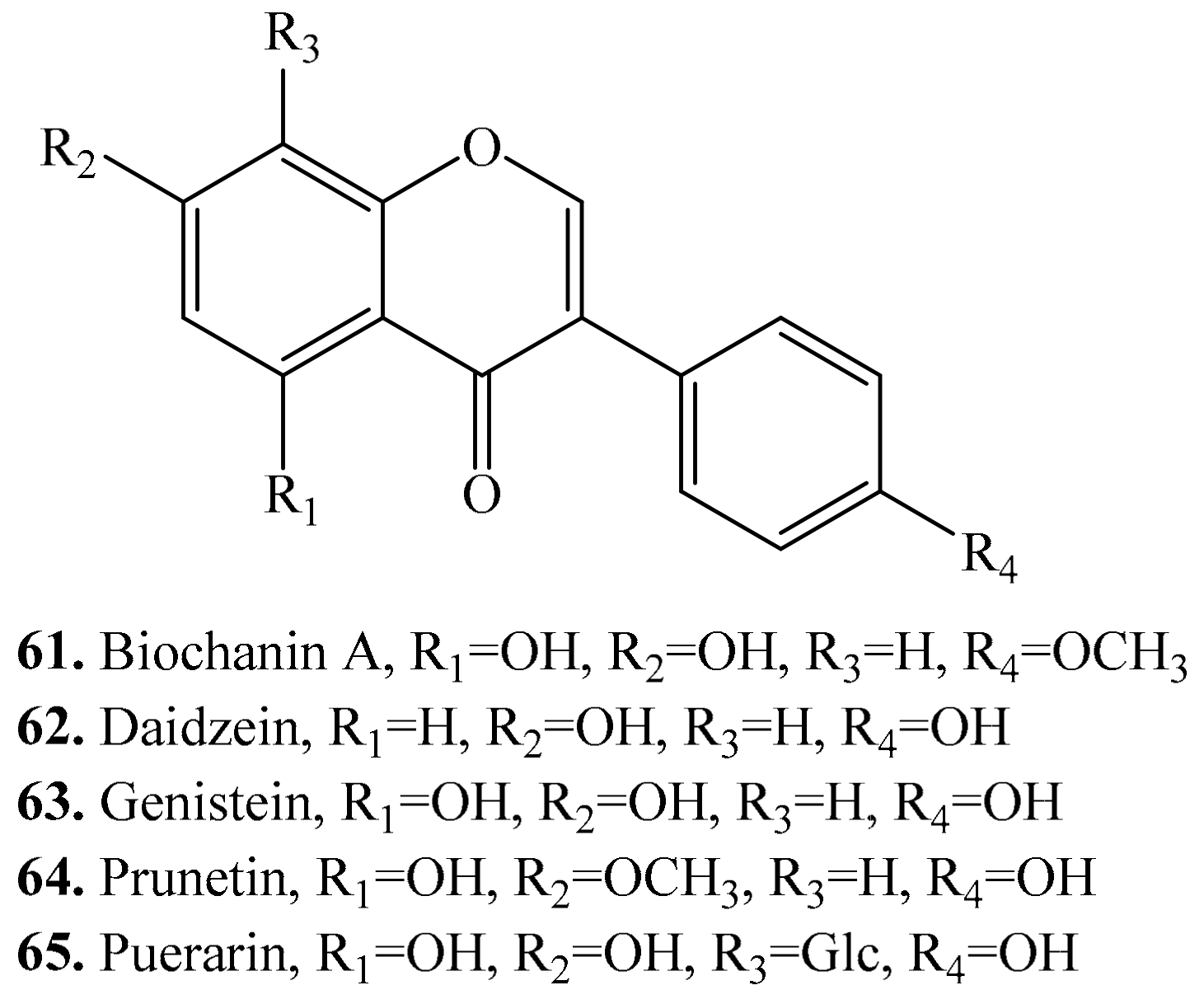
3.5. Isoflavones and Their Biological Activities or Functions
4. Terpenoids and Their Biological Activities or Functions
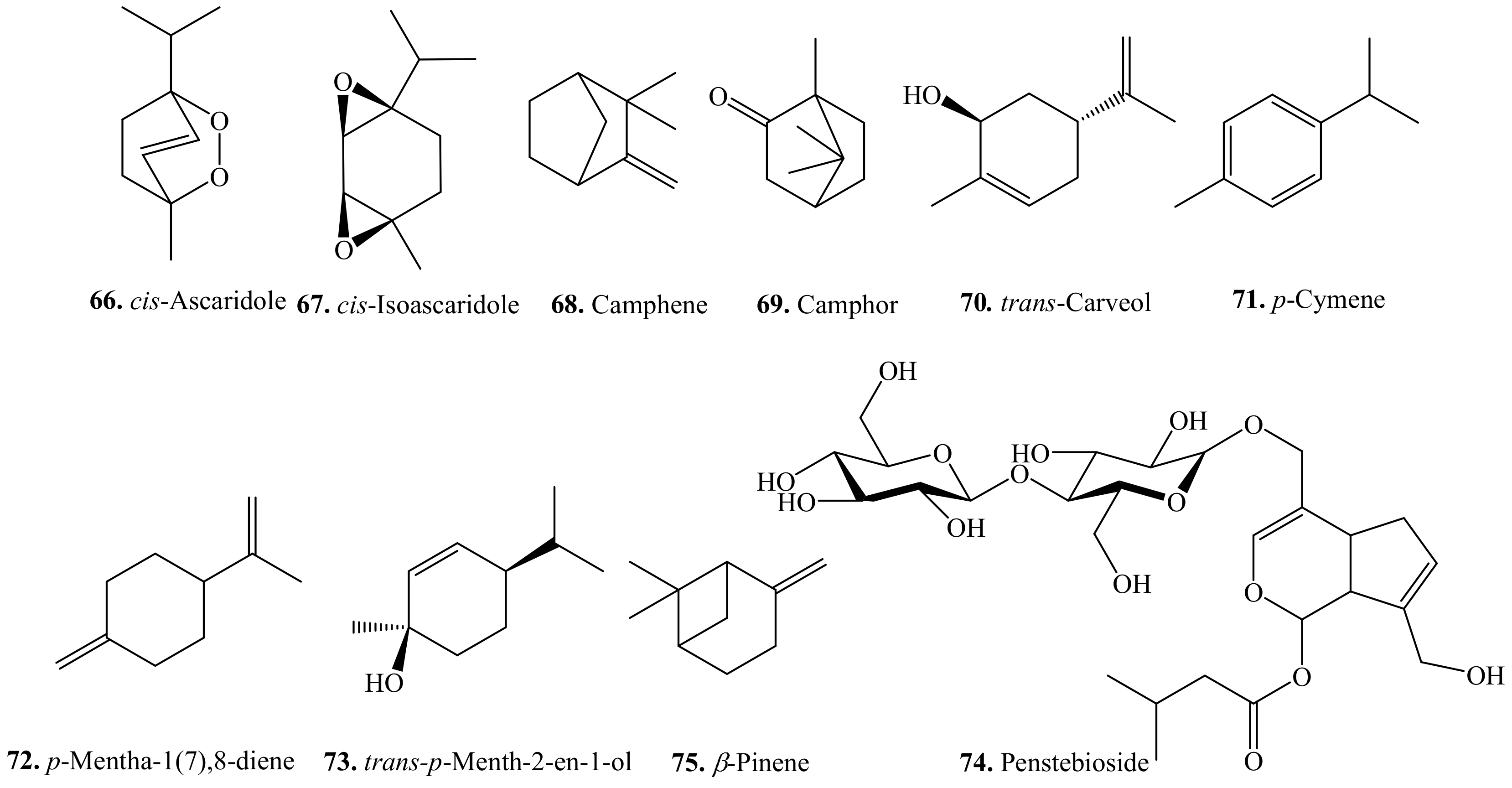
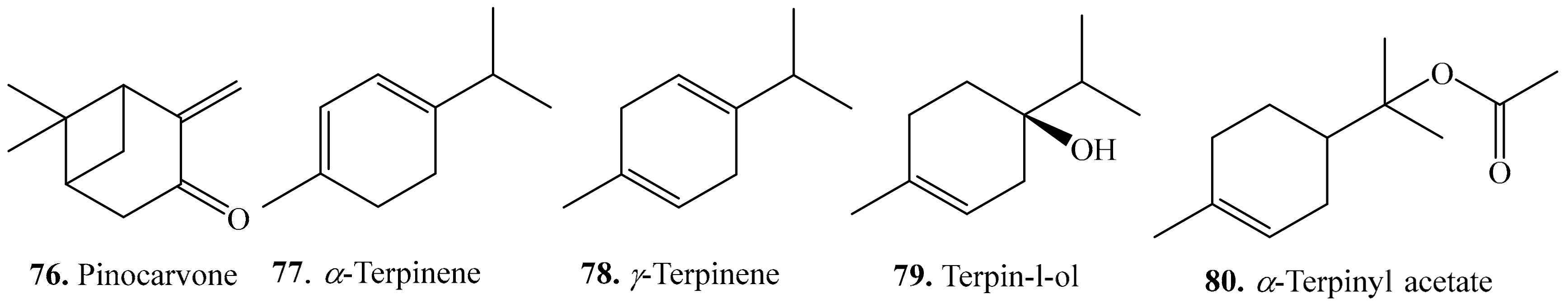
4.1. Monoterpenoids and Their Biological Activities or Functions
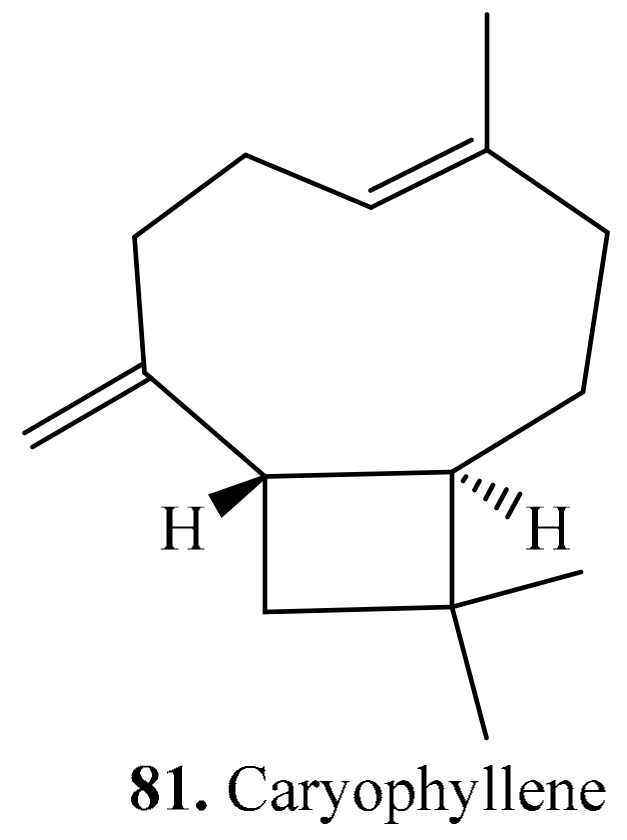
4.2. Sesquiterpenoids and Their Biological Activities or Functions
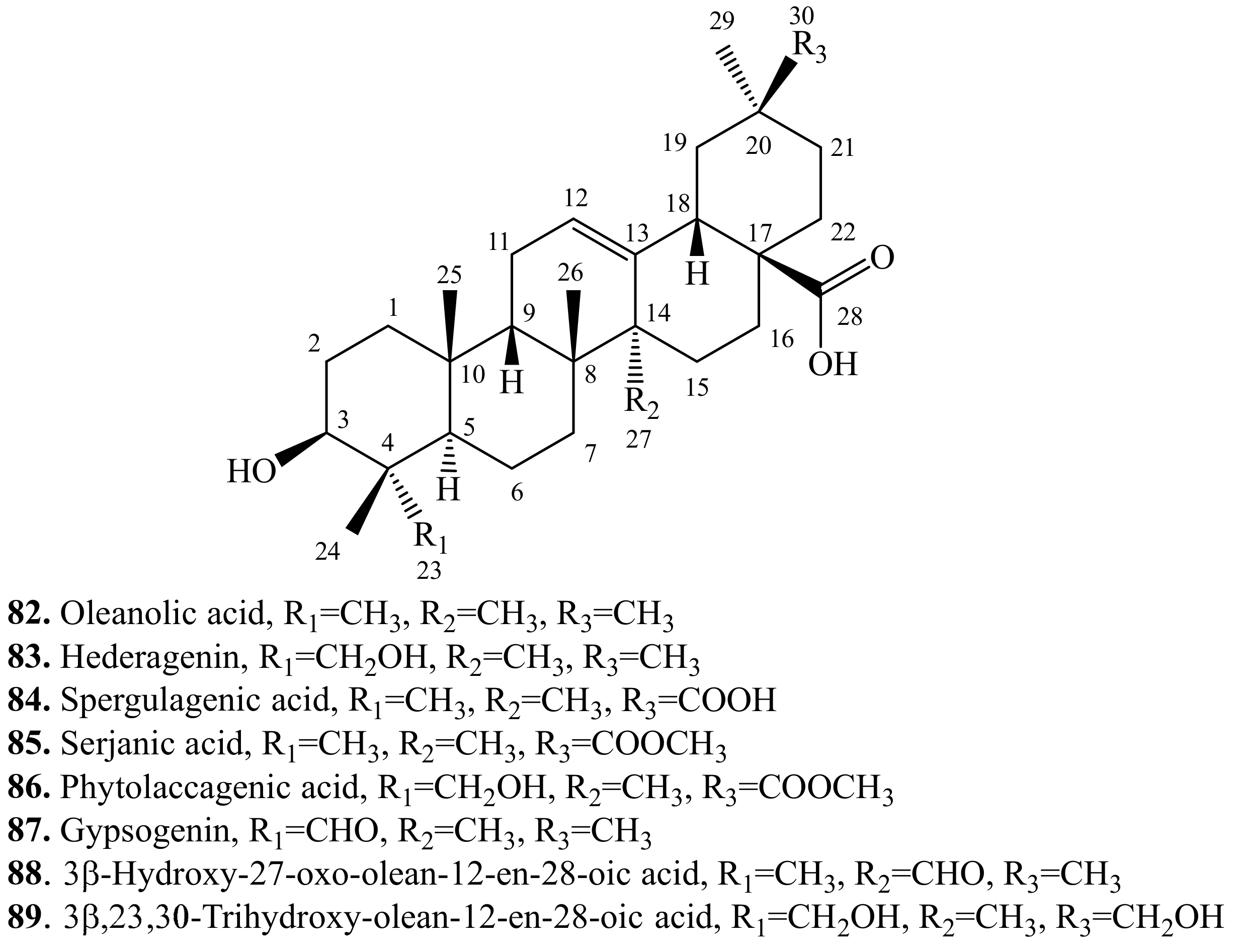
4.3. Triterpenoids and Their Biological Activities or Functions
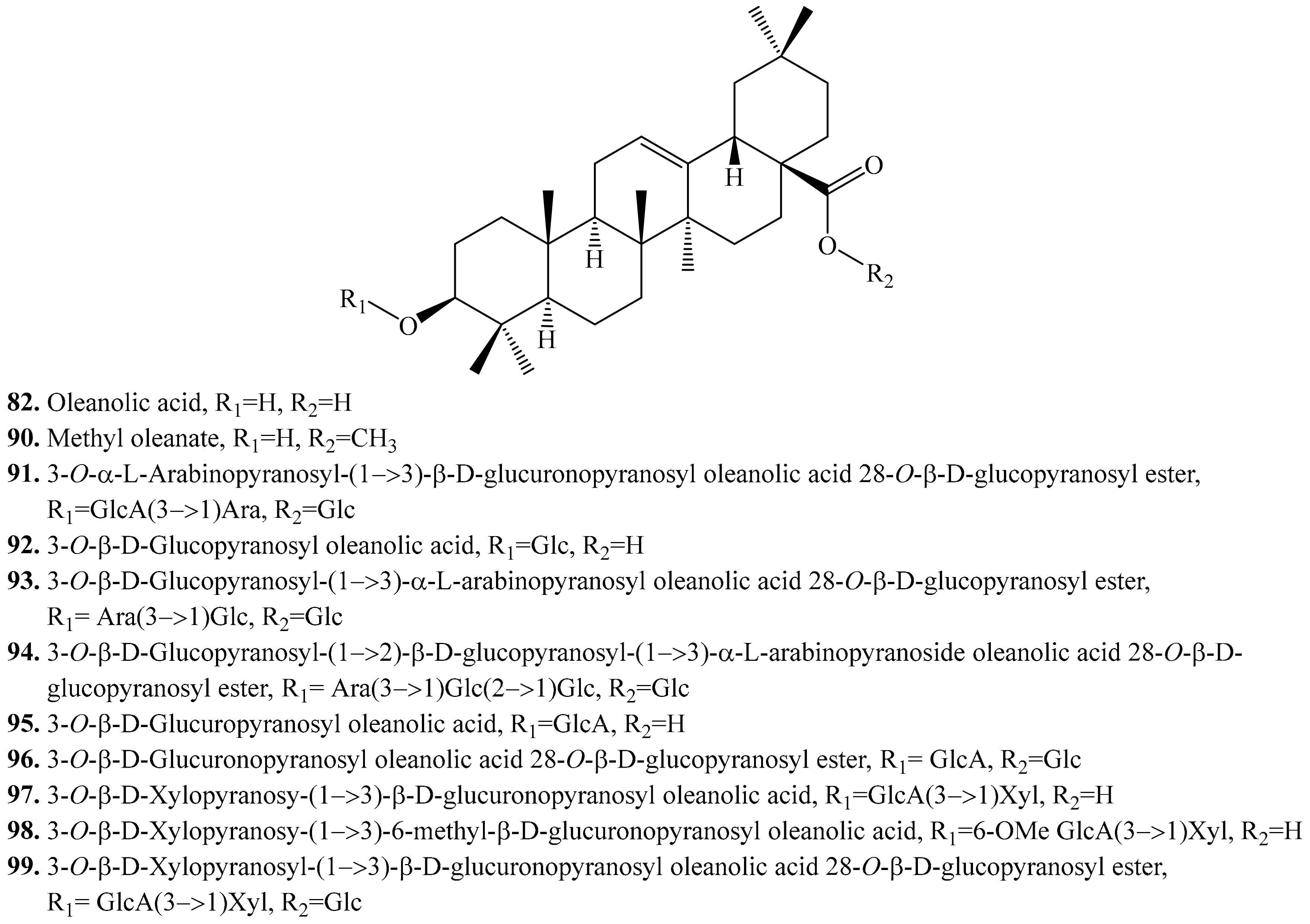
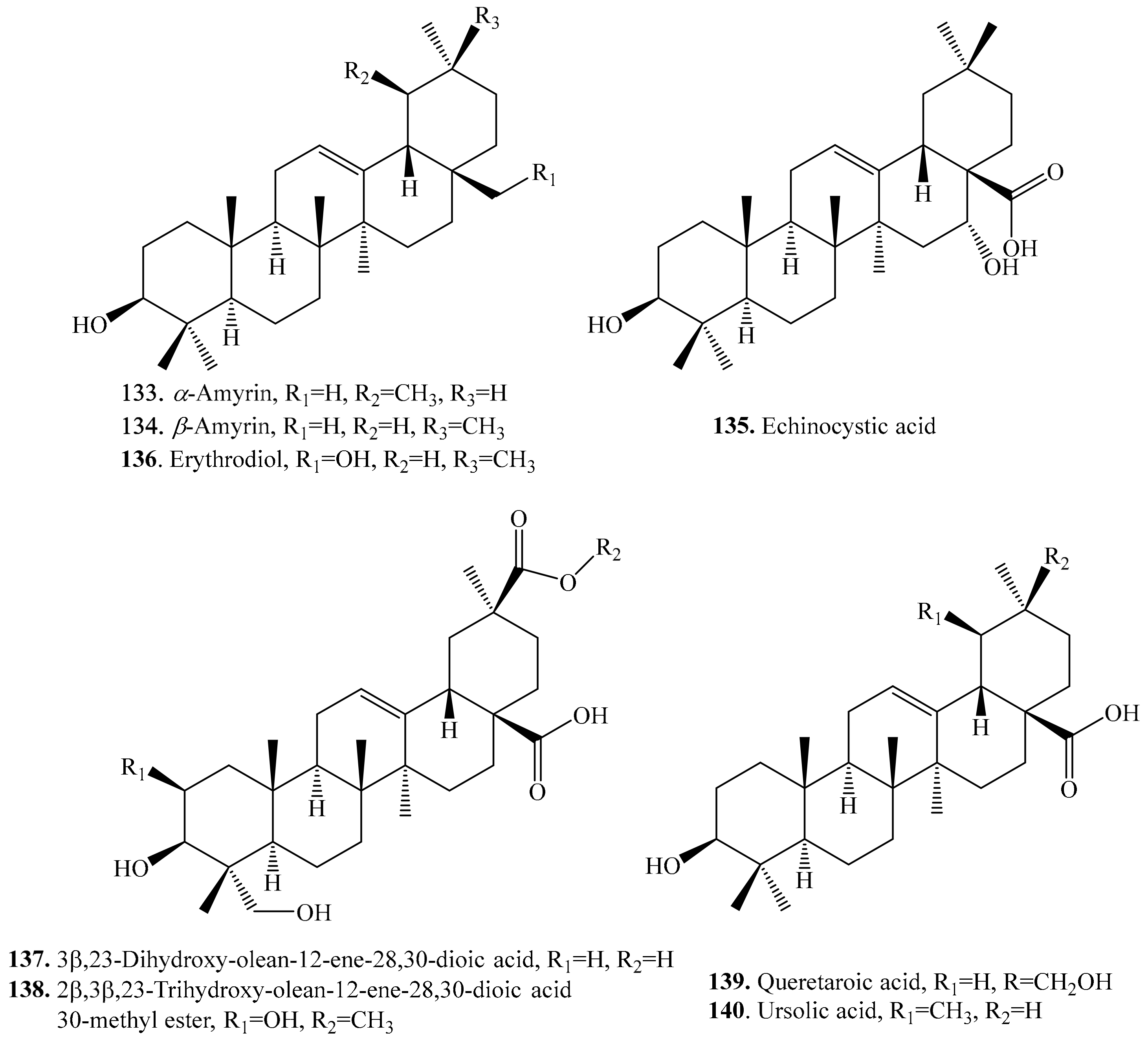
4.3.1. Oleanolic Acid Derivatives and Their Biological Activities or Functions
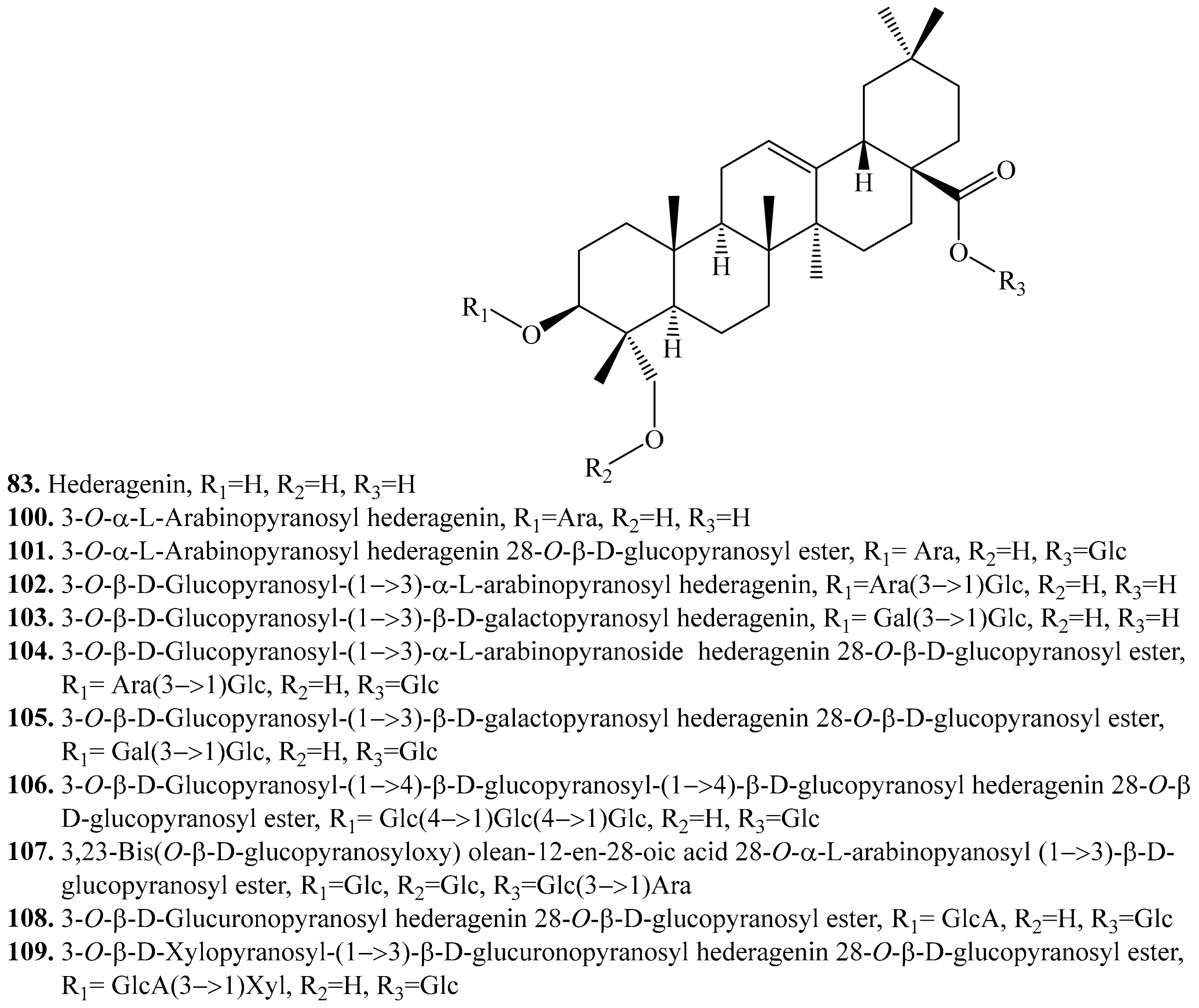
4.3.2. Hederagenin Derivatives and Their Biological Activities or functions
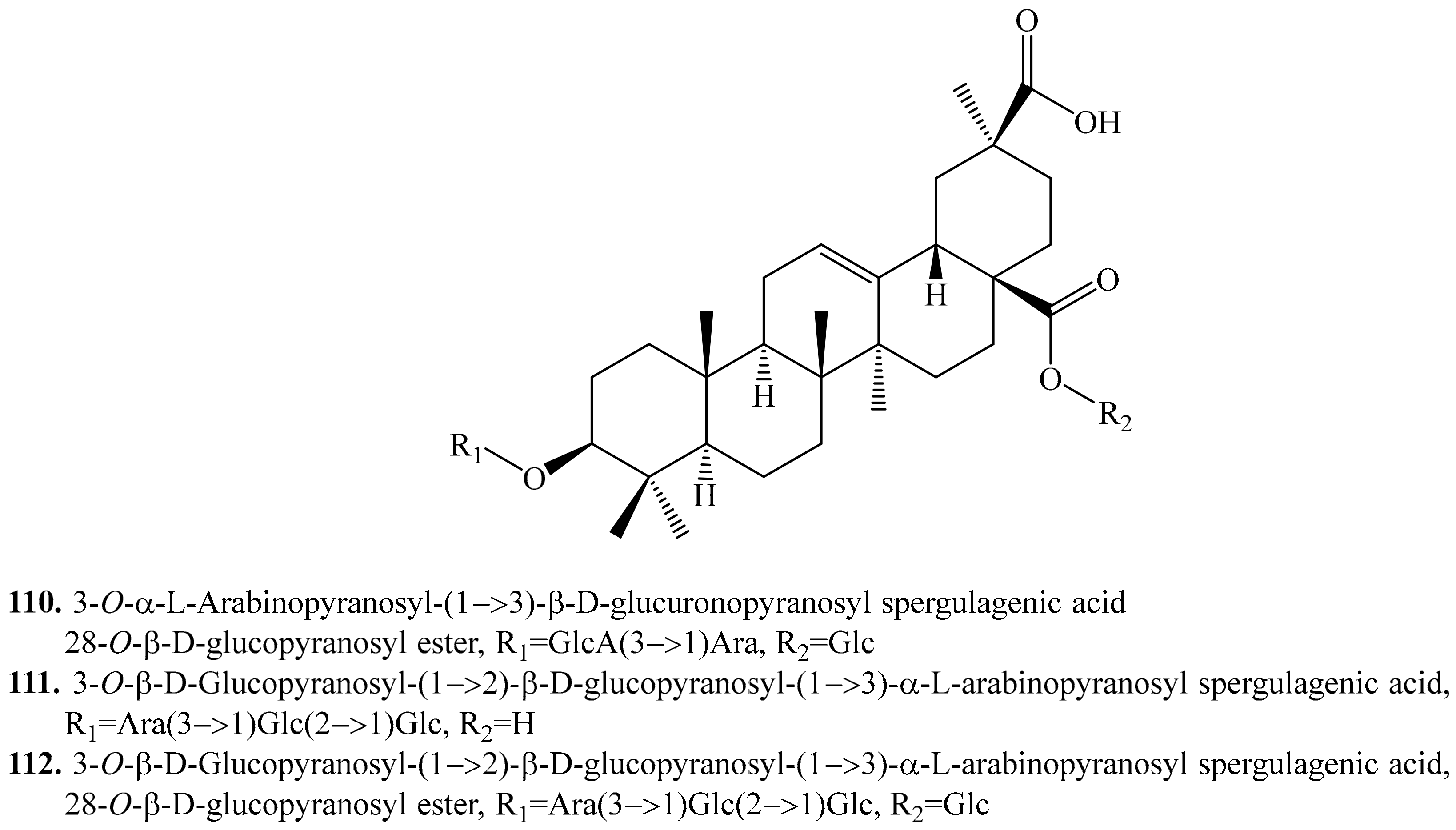
4.3.3. Spergulagenic Acid Derivatives and Their Biological Activities or Functions
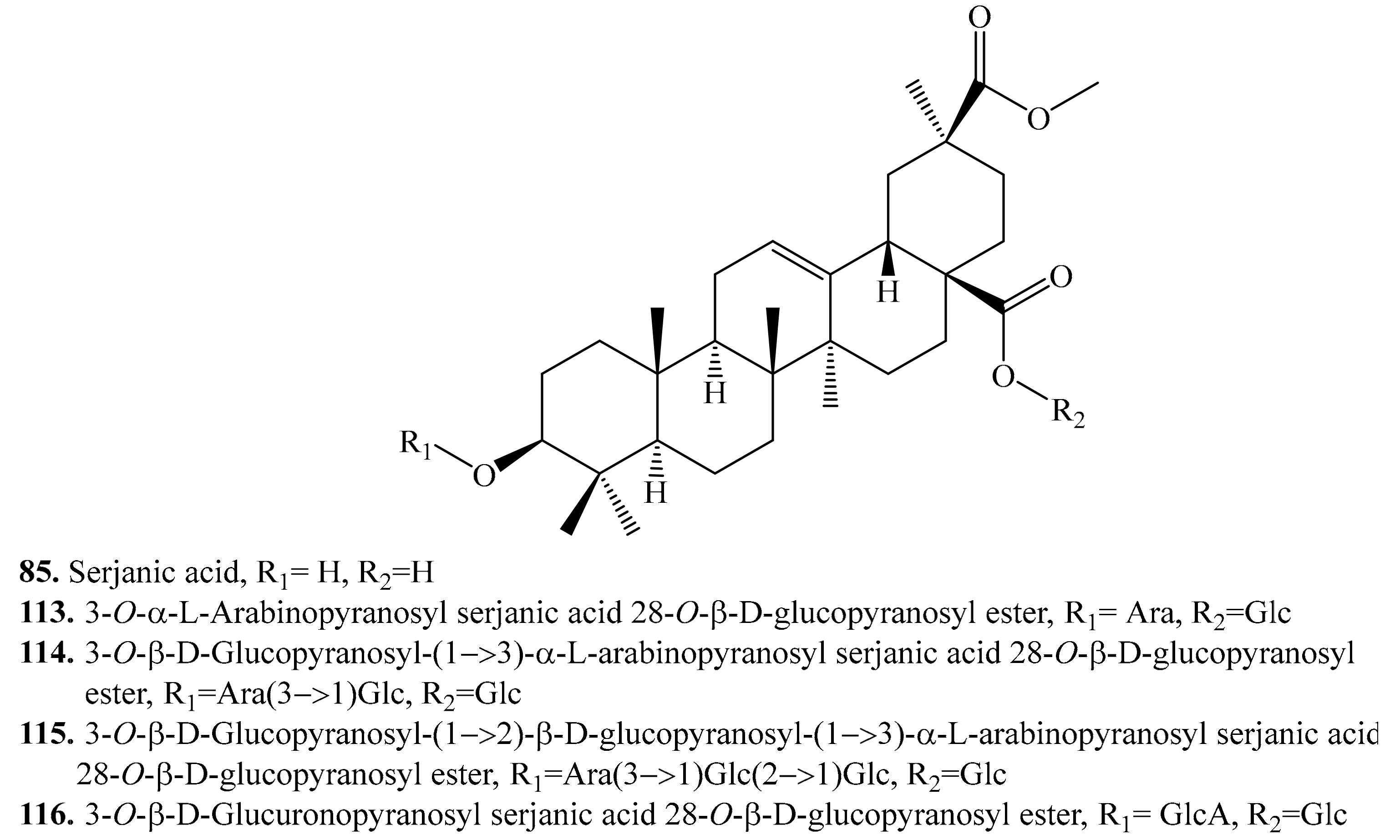
4.3.4. Serjanic Acid Derivatives and Their Biological Activities or Functions
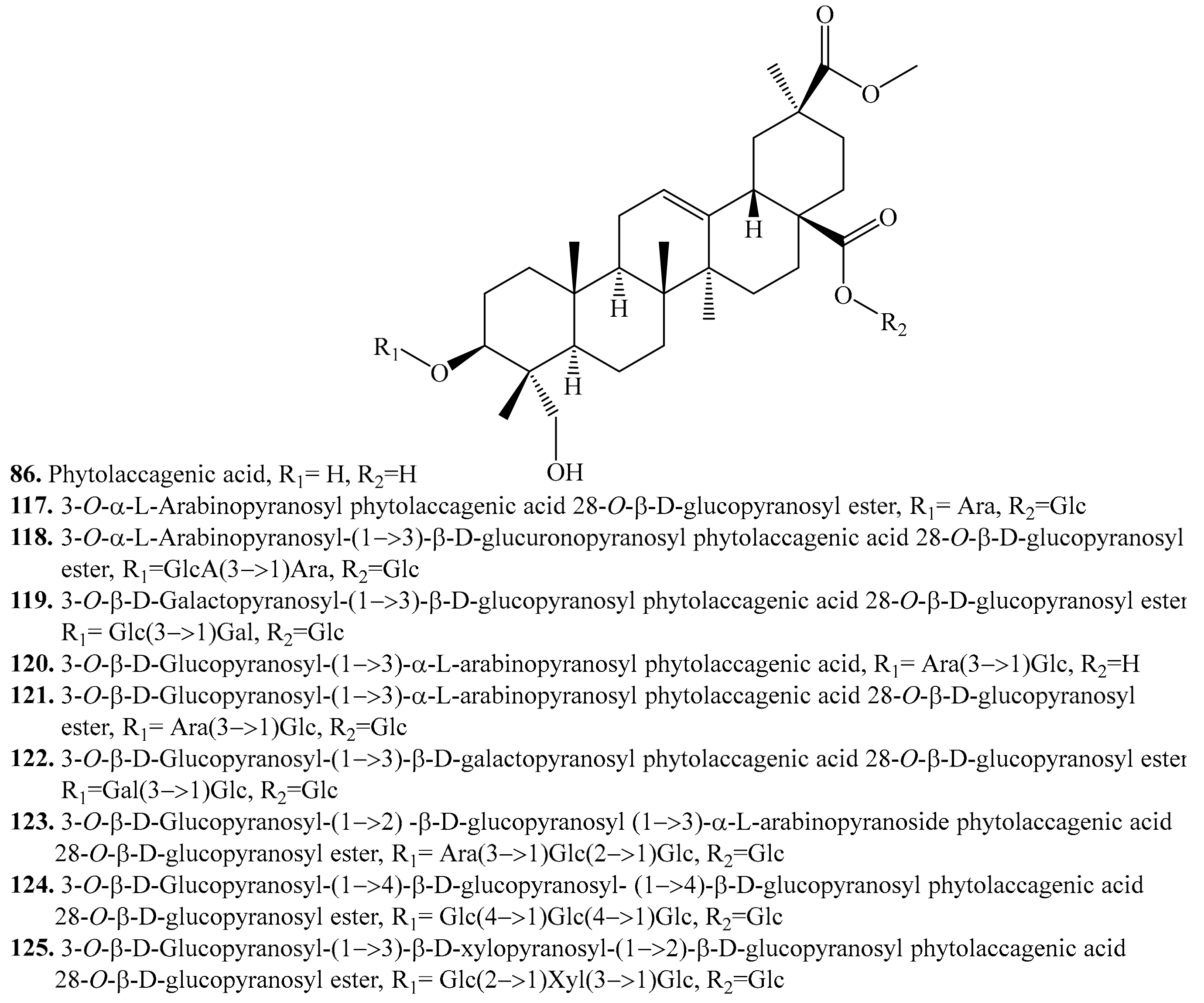
4.3.5. Phytolaccagenic Acid Derivatives and Their Biological Activities or Functions
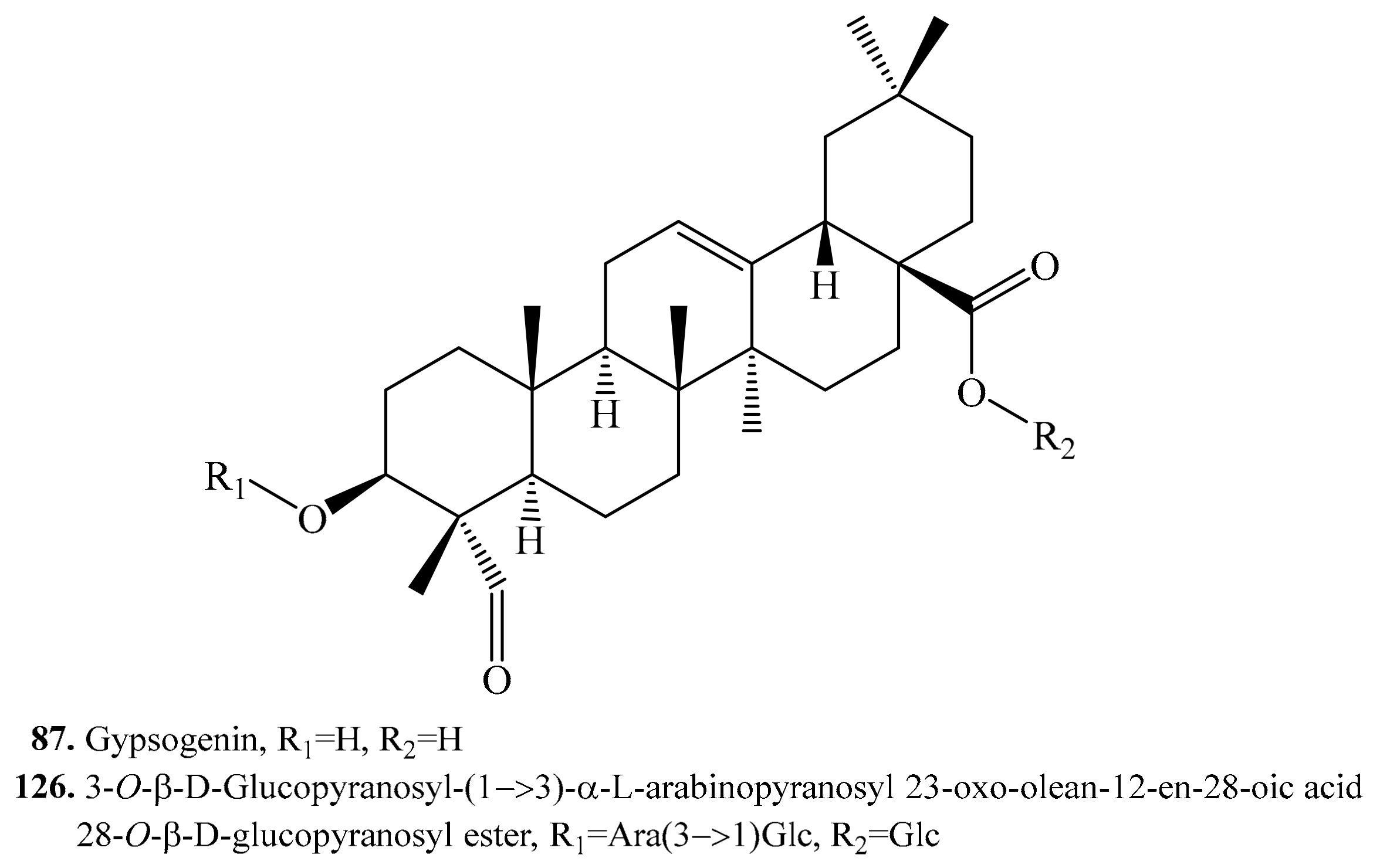
4.3.6. Gypsogenin Derivatives and Their Biological Activities or Functions
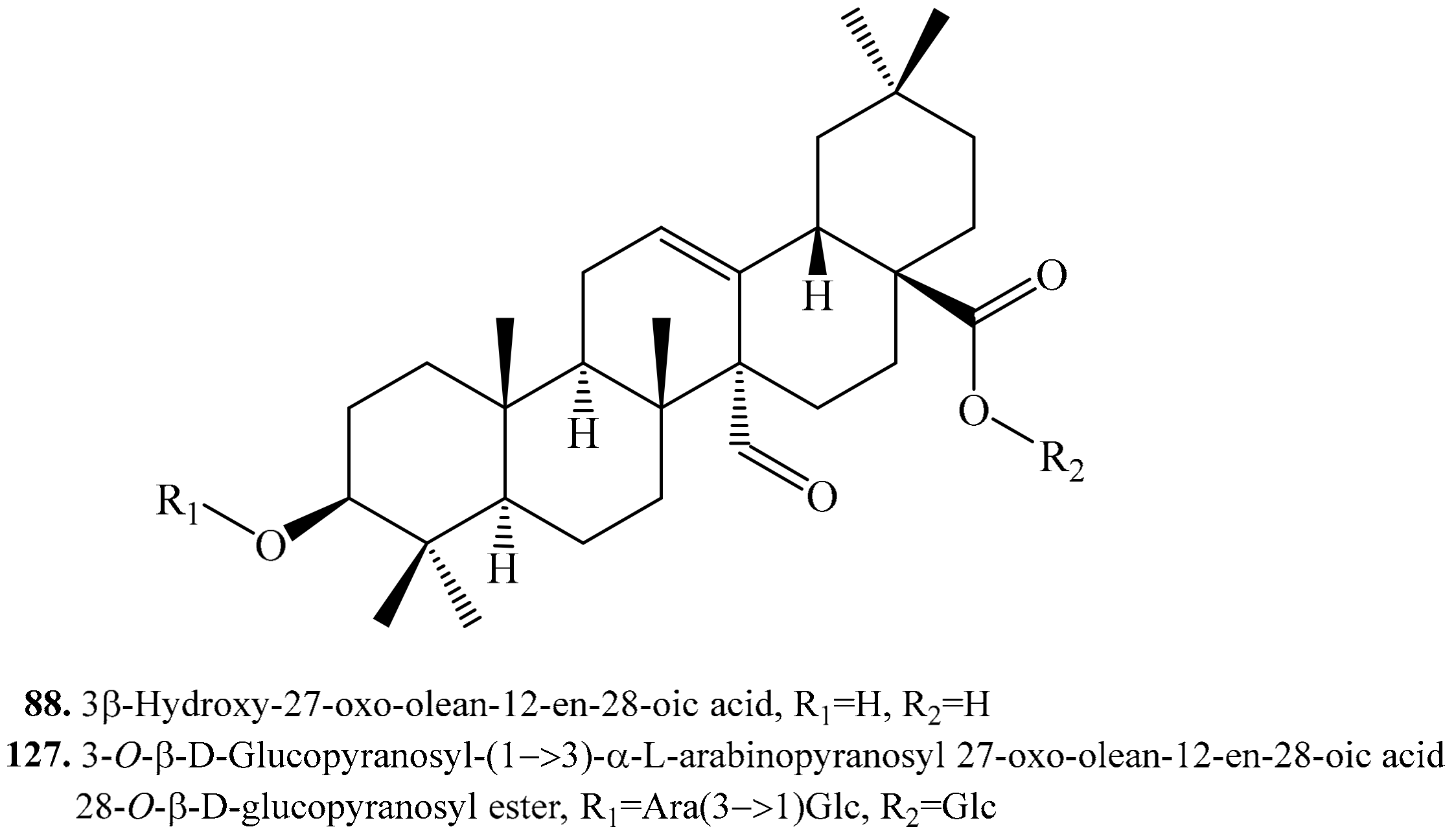
4.3.7. 3β-Hydroxy-27-oxo-olean-12-en-28-oic Acid Derivatives and Their Biological Activities or Functions
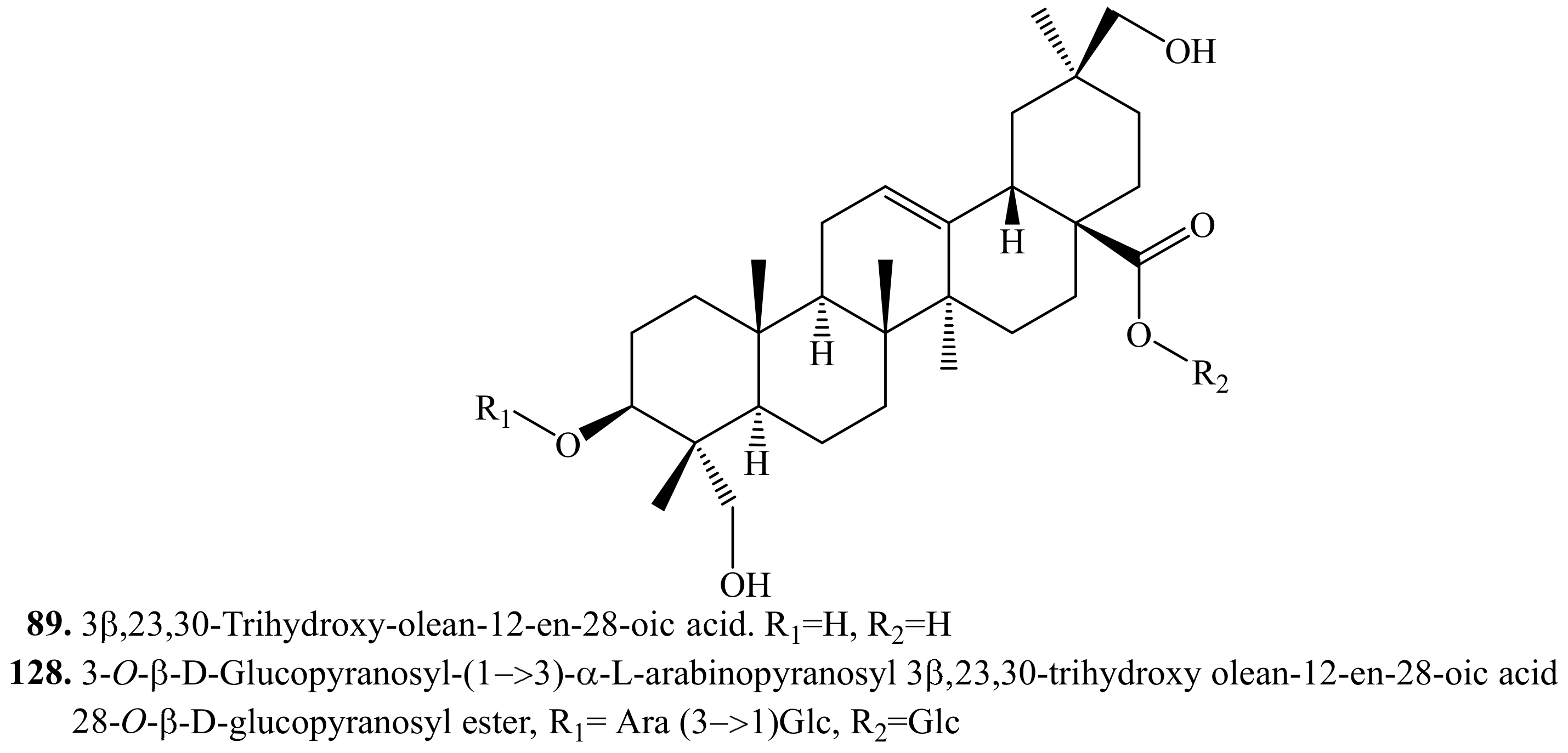
4.3.8. 3β,23,30-Trihydroxy-olean-12-en-28-oic acid Triterpenoids and Their Biological Activities or Functions
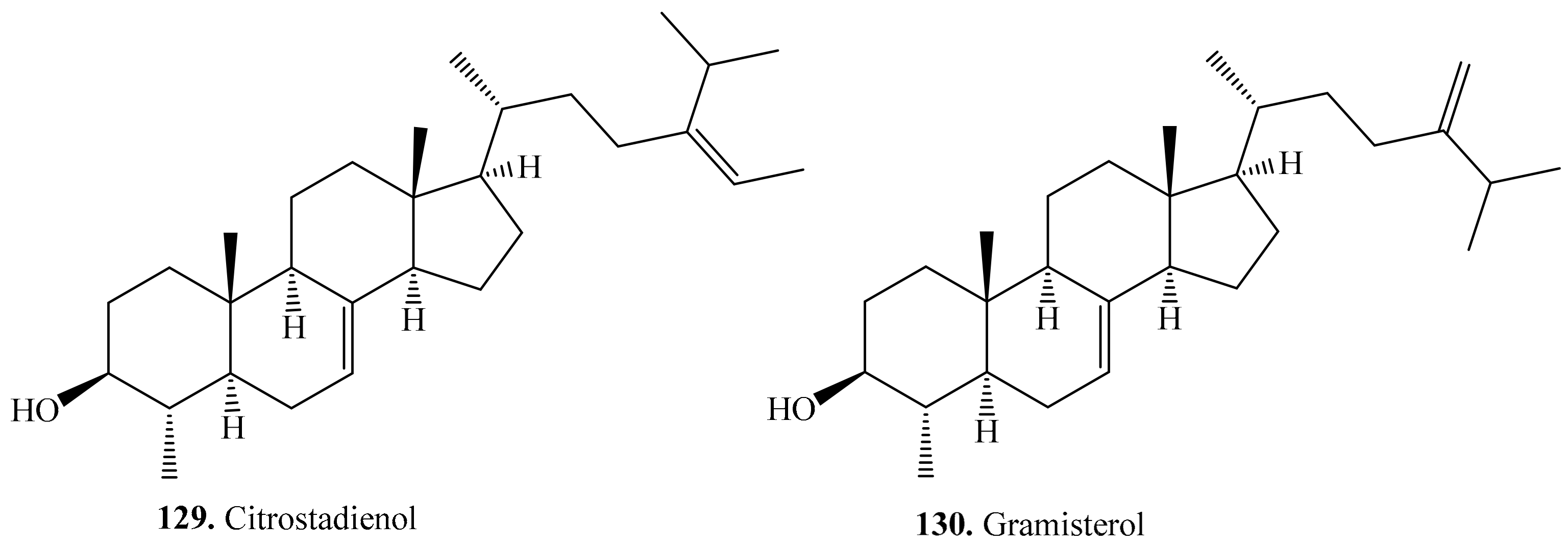
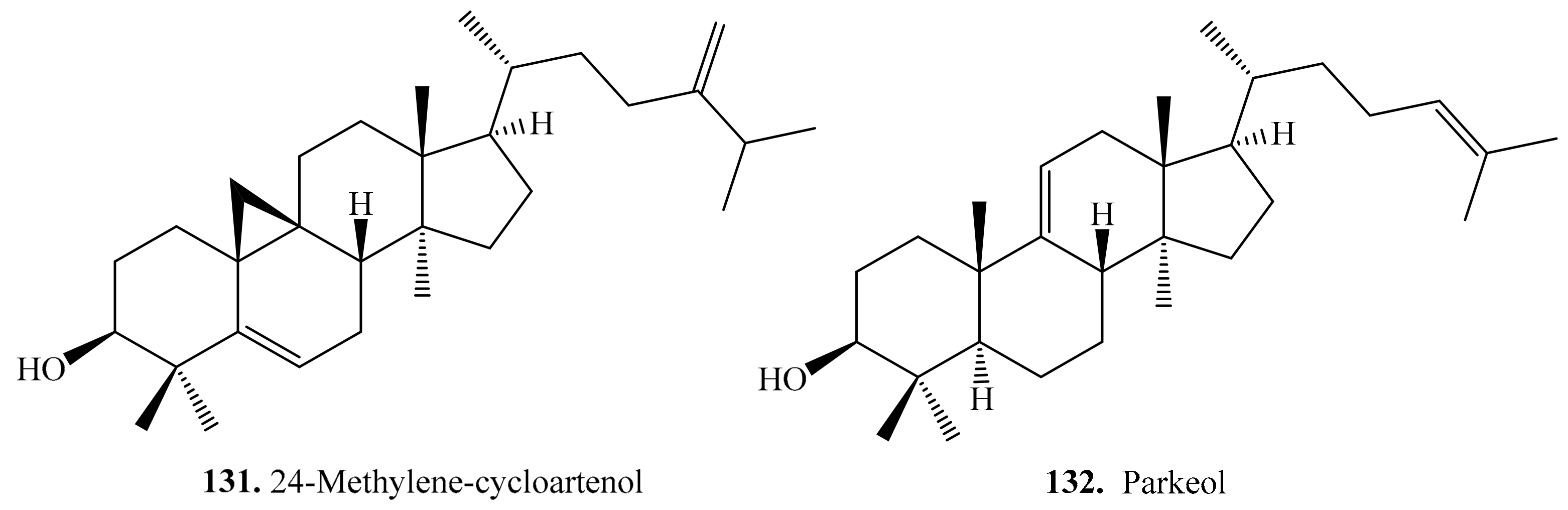
4.3.9. Other Triterpenoids and Their Biological Activities or Functions
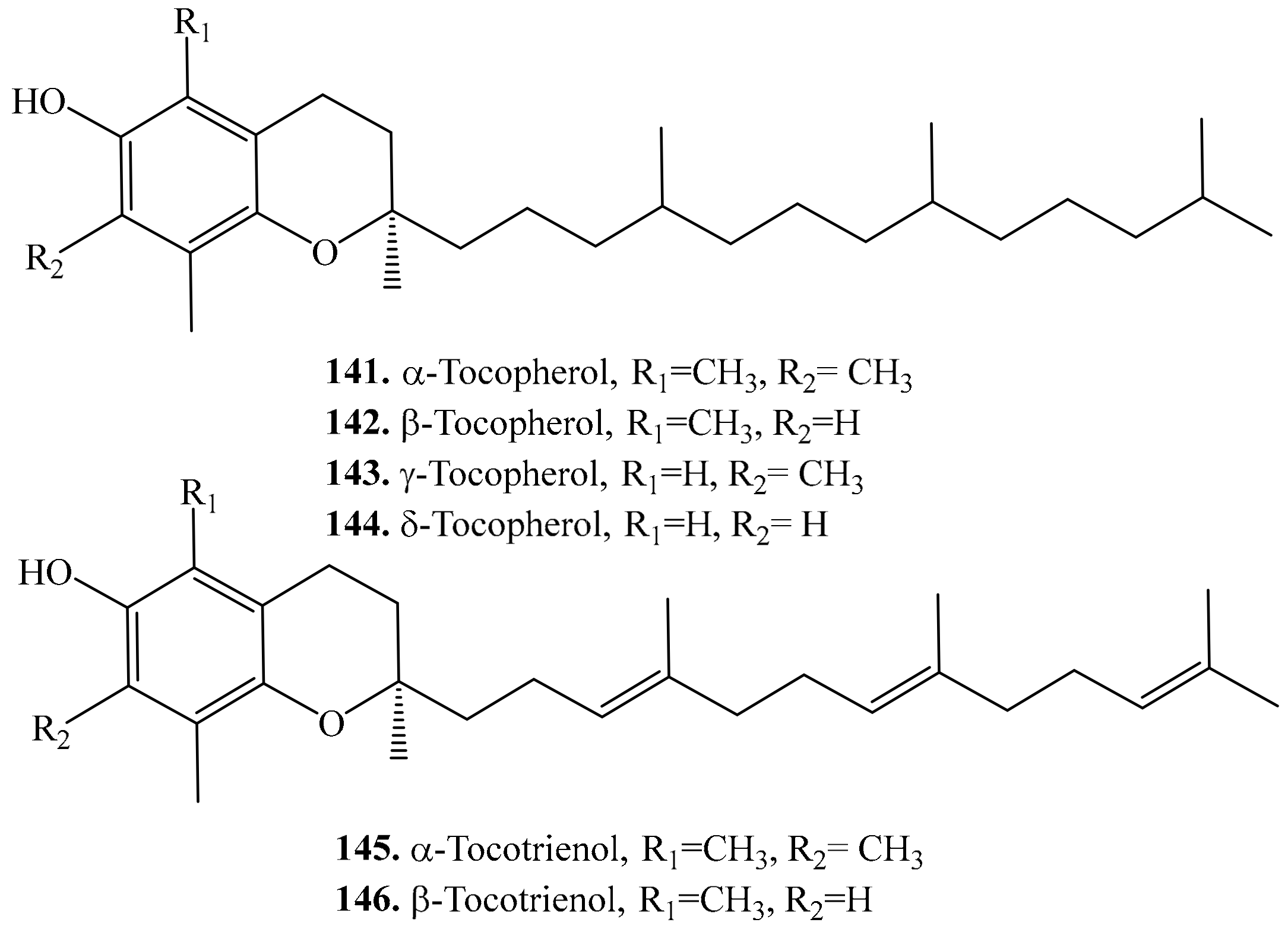
4.4. Meroterpenoids and Their Biological Activities or Functions
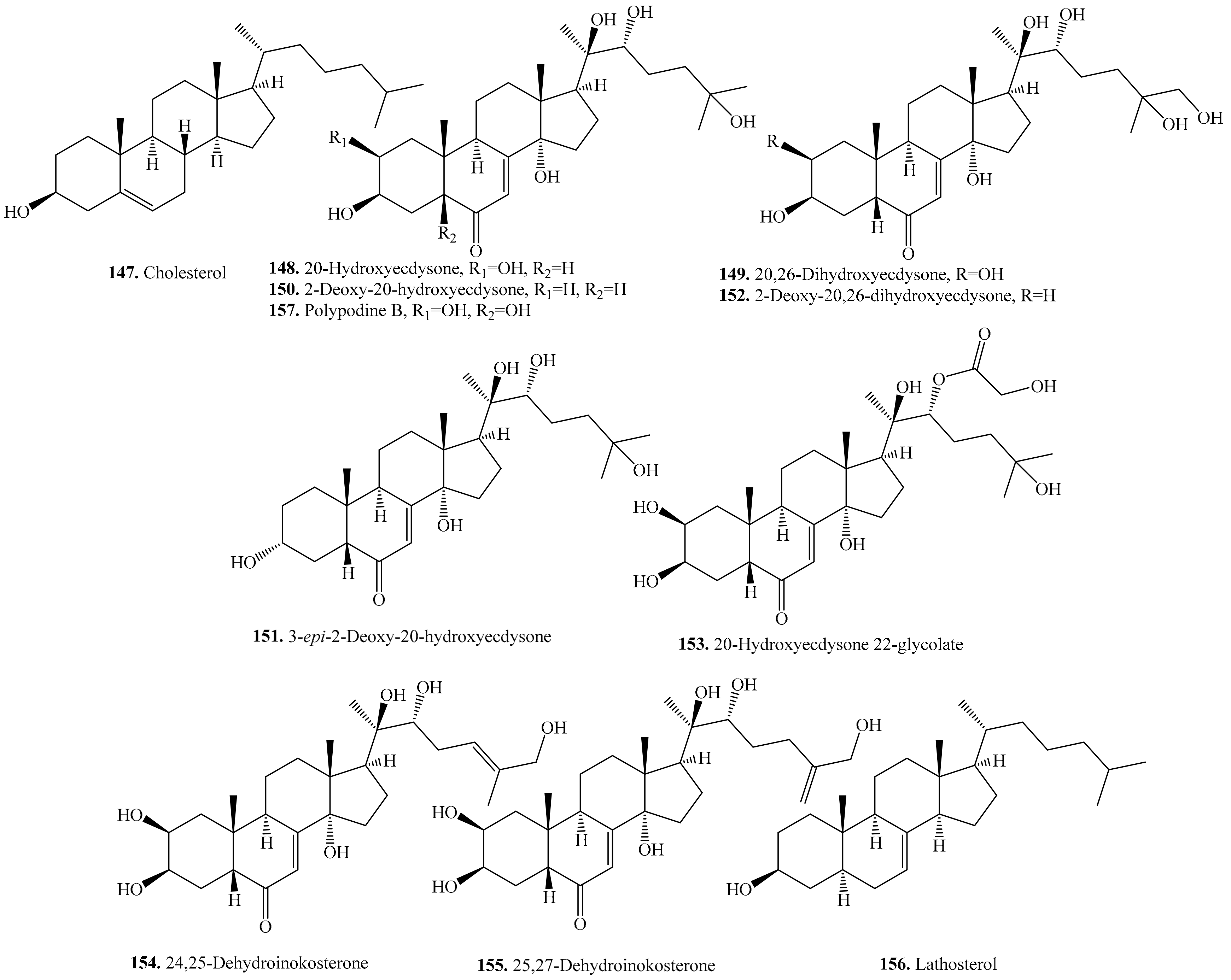
5. Steroids and Their Biological Activities or Functions
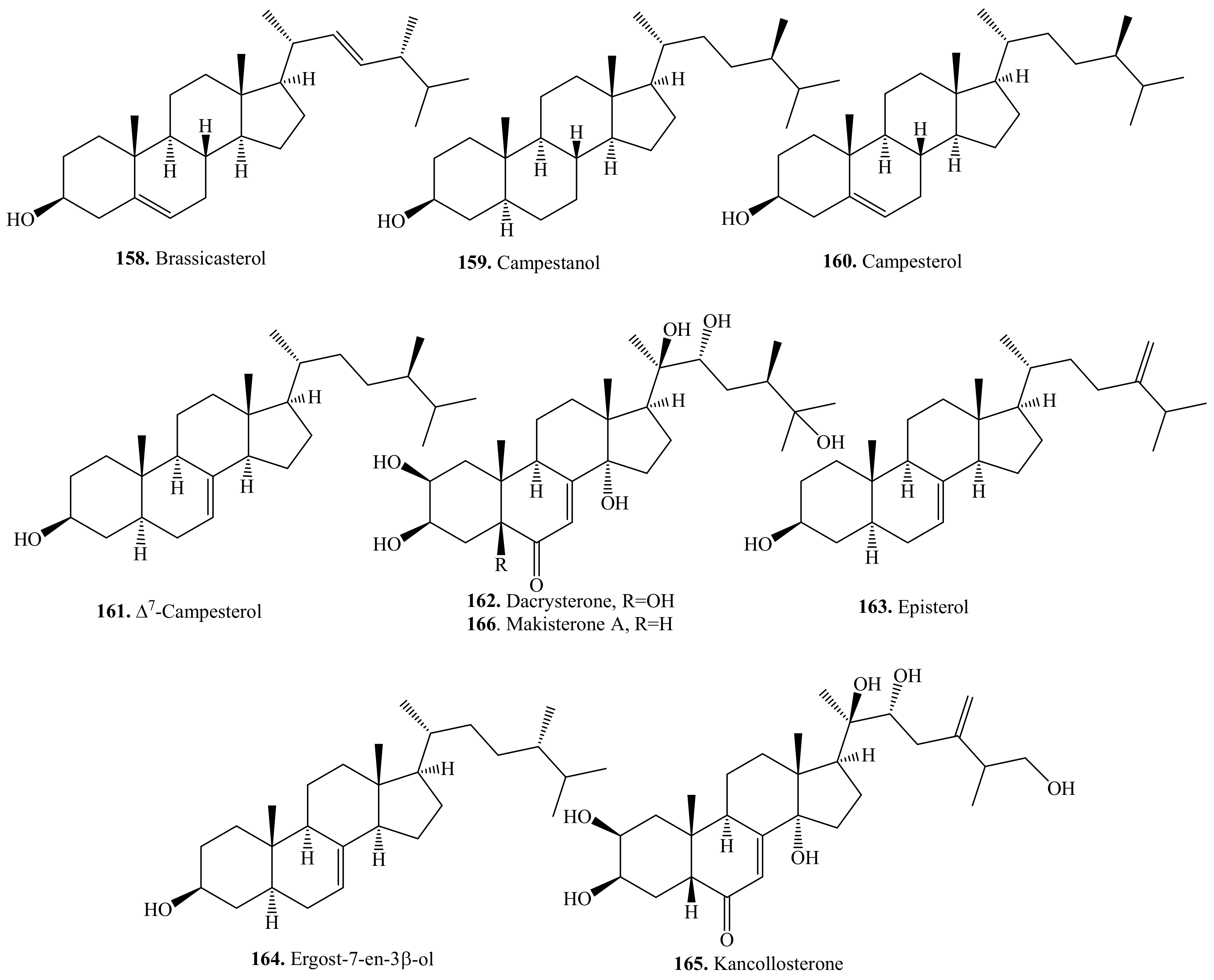
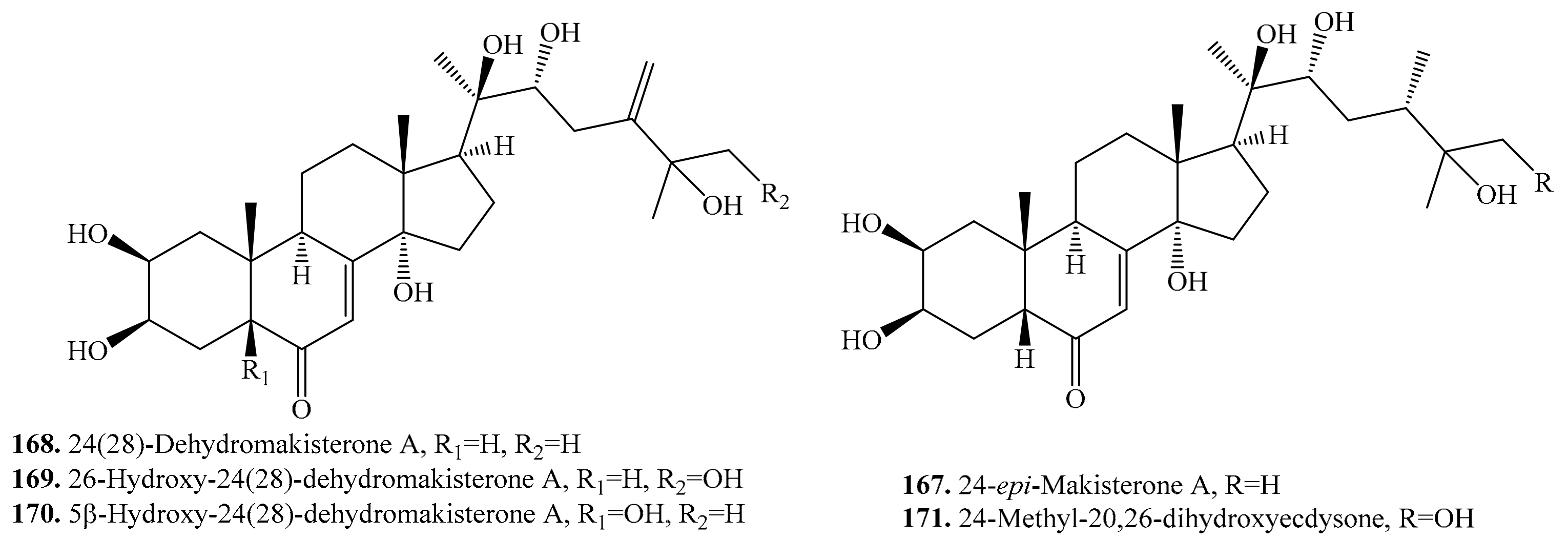
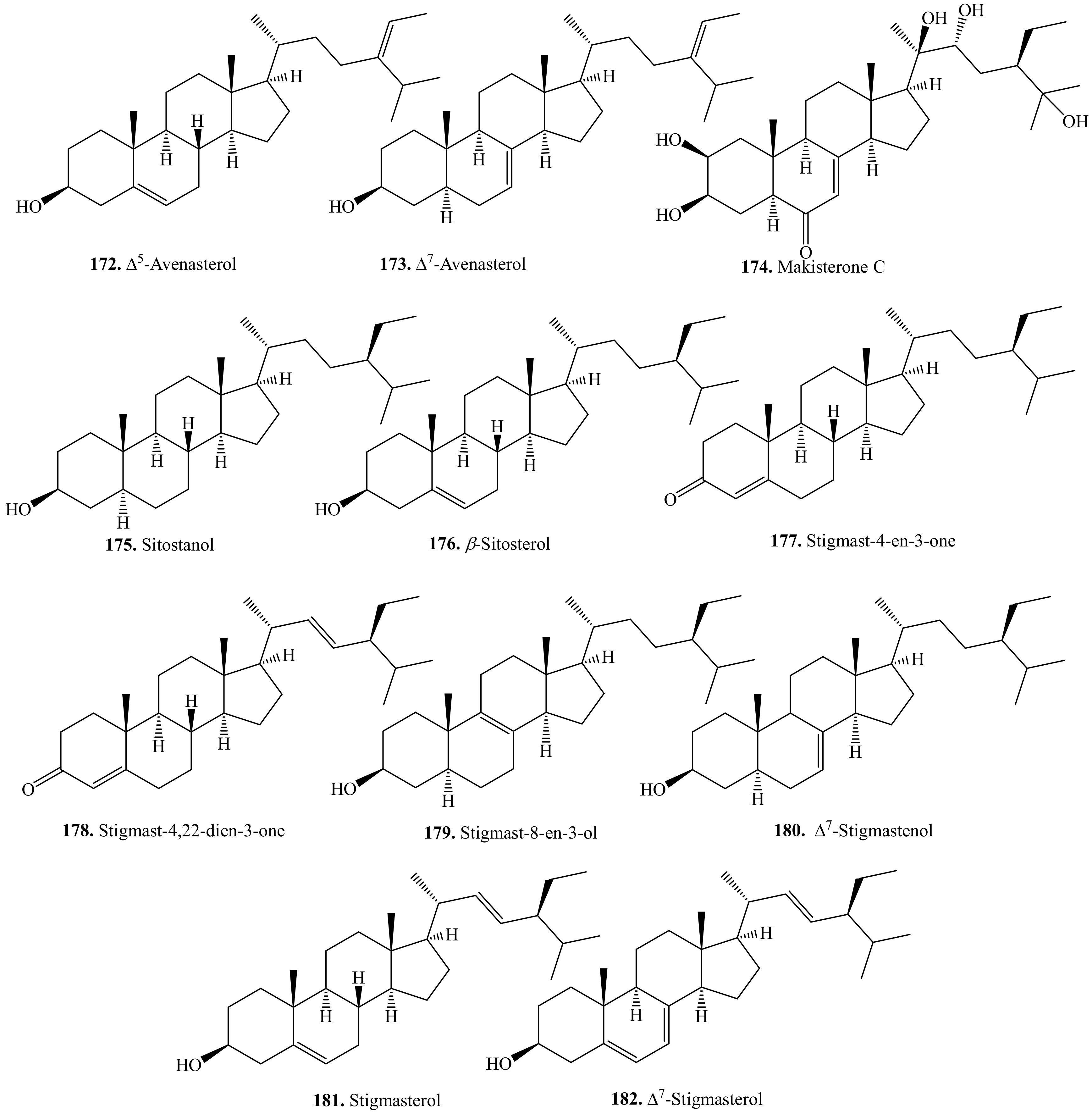
5.1. C27-Steroids and Their Biological Activities or Functions
5.2. C28-Steroids and Their Biological Activities or Functions
5.3. C29-Steroids and Their Biological Activities or Functions
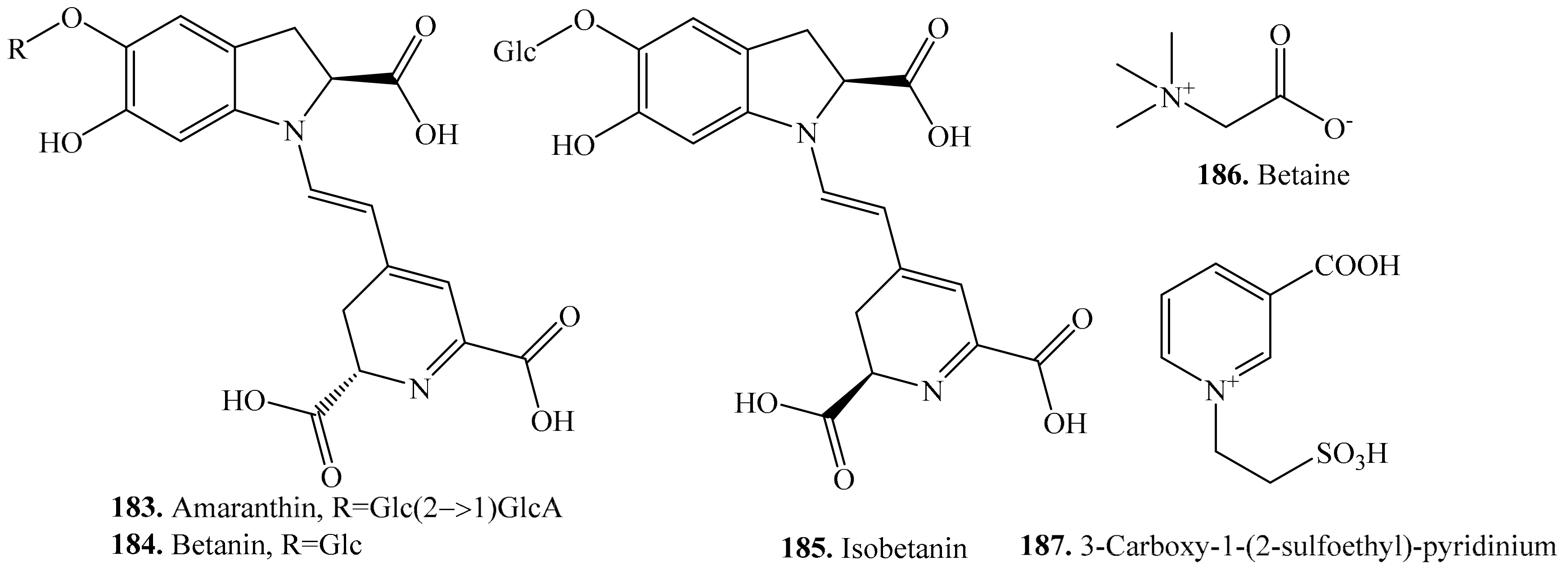
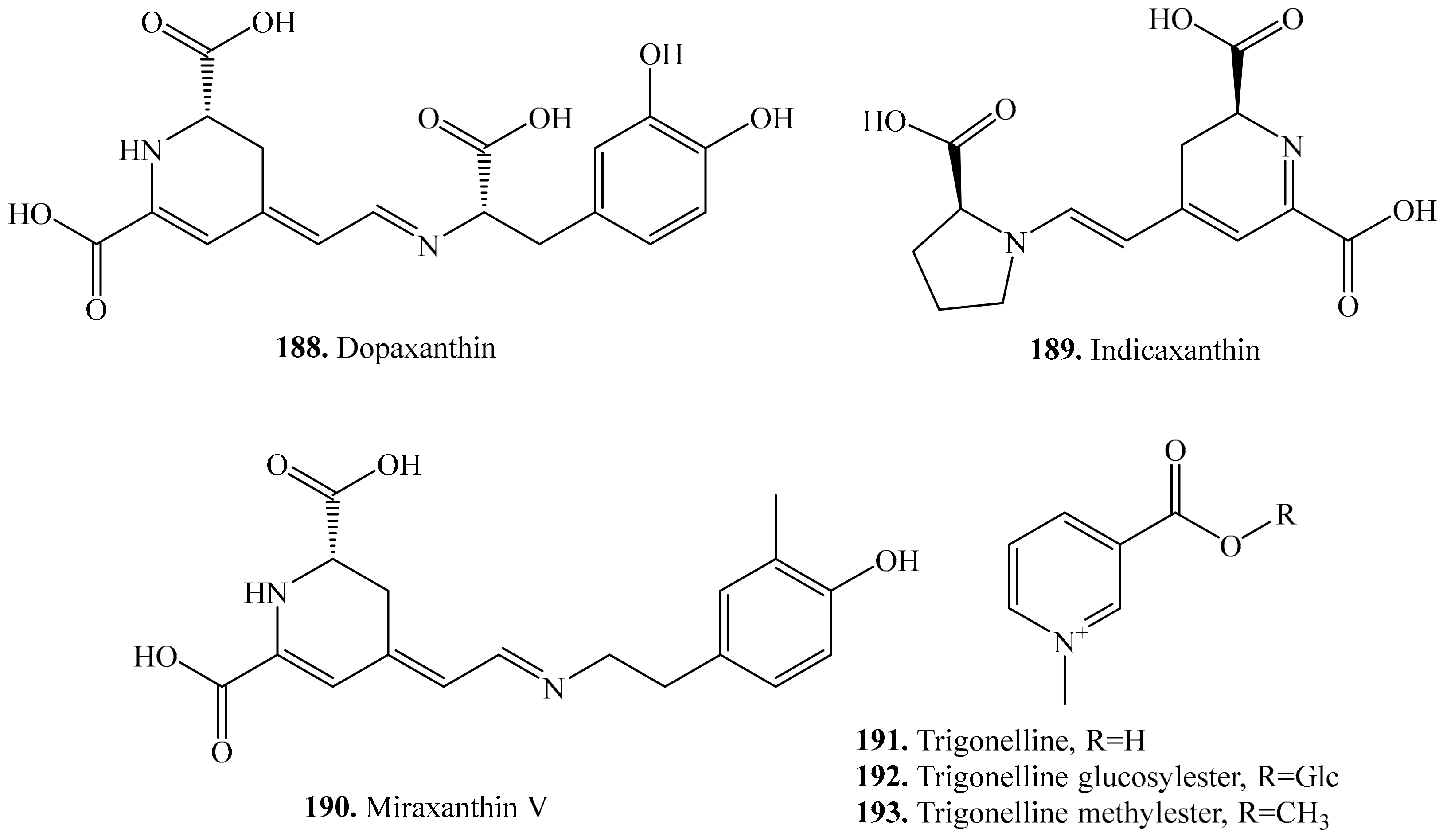
6. Nitrogen-Containing Metabolites and Their Biological Activities or Functions
7. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jacobsen, S.E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar]
- Ng, S.C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of lipid oxidation products in quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar]
- Abugoch, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Jancurova, M.; Minarovicova, L.; Dandar, A. Quinoa—A review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar]
- Kim, S.J.; Pham, T.H.; Bak, Y.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamokkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar]
- Miranda, M.; Delatorre-Herrera, J.; Vega-Galvez, A.; Jorquera, E.; Quispe-Fuentes, I.; Martinez, E.A. Antimicrobial potential and phytochemical content of six diverse sources of quinoa seeds (Chenopodium quinoa Willd. Agric. Sci. 2014, 5, 1015–1024. [Google Scholar]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar]
- Yao, Y.; Shi, Z.; Ren, G. Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.). Int. J. Mol. Sci. 2014, 15, 19307–19318. [Google Scholar]
- Estrada, A.; Li, B.; Laarveld, B. Adjuvant action of Chenopodium quinoa saponins on the induction of antibody responses to intragastric and intranasal administered antigens in mice. Comp. Immunol. Microbiol. Infect. Dis. 1998, 21, 225–236. [Google Scholar]
- Filho, A.M.M.; Pirozi, M.R.; Borge, J.T.D.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar]
- Suarez-Estrella, D.; Torri, L.; Pagani, M.A.; Marti, A. Quinoa bitterness: Causes and solutions for improving product acceptability. J. Sci. Food Agric. 2018, 98, 4033–4041. [Google Scholar]
- Hinojosa, L.; Gonzalez, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuna-Rodriguez, I.S.; Antognoni, F.; Martinez-Mosquieira, E.A.; Coulidaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phychemistry 2001, 57, 325–353. [Google Scholar]
- Kumpun, S.; Maria, A.; Crouzet, S.; Evrard-Todeschi, N.; Girault, J.P.; Lafont, R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011, 125, 1226–1234. [Google Scholar]
- Kuljanabhagavad, T.; Wink, M. Biological activities and chemisty of saponins from Chenopodium quioa Willd. Phytochem. Rev. 2009, 8, 473–490. [Google Scholar]
- Abd El-Mawla, A.M.A.; Beerhues, L. Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta 2002, 214, 727–733. [Google Scholar]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar]
- Gawlik-Dziki, U.; Swieca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotech. Biochem. 1998, 62, 2273–2276. [Google Scholar]
- Tsou, M.F.; Hung, C.F.; Lu, H.F.; Wu, L.T.; Chang, S.H.; Chang, H.L.; Chen, G.W.; Chung, J.G. Effects of caffeic acid, chlorogenic acid and ferulic acid on growth and arylamine N-acetyltransferase activity in Shigella sonnei (group D). Microbios 2000, 101, 37–46. [Google Scholar]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in Southern China. Food Chem. 2014, 159, 166–174. [Google Scholar]
- Abou-Zaid, M.M.; Helson, B.V.; Nozzolillo, C.; Arnason, J.T. Ethyl m-digallate from red maple, Acer rubrum L., as the major resistance factor to forest tent caterpillar, Malacosoma disstria Hbn. J. Chem. Ecol. 2001, 27, 2517–2527. [Google Scholar]
- Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar]
- Pasko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar]
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar]
- Liu, K.S.; Tsao, S.M.; Yin, M.C. In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytother. Res. 2005, 19, 942–945. [Google Scholar]
- Kore, K.J.; Bramhakule, P.P.; Rachhadiya, R.M.; Shete, R.V. Evaluation of antiulcer activity of protocatechuic acid ethyl ester in rats. Int. J. Pharm. Life Sci. 2011, 2, 909–915. [Google Scholar]
- Shi, G.F.; An, L.J.; Jiang, B.; Guan, S.; Bao, Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar]
- Zhou, Z.; Zhang, Y.; Ding, X.R.; Chen, S.H.; Yang, J.; Wang, X.J.; Wang, S.Q. Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antivir. Res. 2007, 74, 59–64. [Google Scholar]
- Galano, A.; Francisco-Márquez, M.; Alvarez-Idaboy, J.R. Mechanism and kinetics studies on the antioxidant activity of sinapinic acid. Phys. Chem. Chem. Phys. 2011, 13, 11199–11205. [Google Scholar]
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009, 1, 15. [Google Scholar]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar]
- Fernandez, M.A.; Saenz, M.T.; Garcia, M.D. Natural Products: Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 1998, 50, 1183–1186. [Google Scholar]
- El-Hawary, S.; Mohammed, R.; AbouZid, S.; Ali, Z.Y.; El-Gendy, A.O.; Elwekeel, A. In-vitro cyclooxygenase inhibitory, antioxidant and antimicrobial activities of phytochemicals isolated from Crassula arborescens (Mill.) Willd. Int. J. Appl. Res. Nat. Prod. 2016, 9, 8–14. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Phenolic constituents of Kancolla seeds. Food Chem. 2004, 84, 163–168. [Google Scholar]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. BBA Gen. Subjects 2011, 1810, 170–177. [Google Scholar]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7 in milk. Food Bioprocess Technol. 2012, 5, 2120–2131. [Google Scholar]
- Shoeb, A.; Chowta, M.; Pallempati, G.; Rai, A.; Singh, A. Evaluation of antidepressant activity of vanillin in mice. Indian J. Pharmacol. 2013, 45, 141. [Google Scholar]
- Lim, E.J.; Kang, H.J.; Jung, H.J.; Song, Y.S.; Lim, C.J.; Park, E.H. Anti-angiogenic, anti-inflammatory and anti-nociceptive activities of vanillin in ICR mice. Biomol. Ther. 2008, 16, 132–136. [Google Scholar]
- Gerig, T.M.; Blum, U. Effects of mixtures of four phenolic acids on leaf area expansion of cucumber seedlings grown in Portsmouth B 1 soil materials. J. Chem. Ecol. 1991, 17, 29–40. [Google Scholar]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. BBA Gen. Subjects 2006, 1760, 283–289. [Google Scholar]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar]
- Chang, W.S.; Chang, Y.H.; Lu, F.J.; Chiang, H.C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res. 1994, 14, 501–506. [Google Scholar]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Toda, S.; Shirataki, Y.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Jang, C.G. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar]
- Kapil, A.; Koul, I.B.; Suri, O.P. Antihepatotoxic effects of chlorogenic acid from Anthocephalus cadamba. Phytother. Res 1995, 9, 189–193. [Google Scholar]
- Karunanidhi, A.; Thomas, R.; Van Belkum, A.; Neela, V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed. Res. Int. 2013, 2013, 392058. [Google Scholar]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar]
- Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial activity of selected phenolic acids. Food Microbiol. 2003, 20, 305–311. [Google Scholar]
- Graf, E. Antioxidant potential of ferulic acid. Free Radical Biol. Med. 1992, 13, 435–448. [Google Scholar]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar]
- Ou, S.; Bao, H.; Lan, Z. Advances on pharmacological study of ferulic acid and its derivatives. J. Chin. Med. Mater. 2001, 24, 220–221. [Google Scholar]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar]
- Sakai, S.; Kawamata, H.; Kogure, T.; Mantani, N.; Terasawa, K.; Umatake, M.; Ochiai, H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264. 7 cells. Mediat. Inflamm. 1999, 8, 173–175. [Google Scholar]
- Mori, H.; Kawabata, K.; Yoshimi, N.; Tanaka, T.; Murakami, T.; Okada, T.; Murai, H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999, 19, 3775–3778. [Google Scholar]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar]
- Englberger, W.; Hadding, U.; Etschenberg, E.; Graf, E.; Leyck, S.; Winkelmann, J.; Parnham, M.J. Rosmarinic acid: A new inhibitor of complement C3-convertase with anti-inflammatory activity. Int. J. Immunopharmacol. 1988, 10, 729–737. [Google Scholar]
- Cao, H.; Cheng, W.X.; Li, C.; Pan, X.L.; Xie, X.G.; Li, T.H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. 2005, 719, 177–183. [Google Scholar]
- Furtado, M.A.; de Almeida, L.C.F.; Furtado, R.A.; Cunha, W.R.; Tavares, D.C. Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2008, 657, 150–154. [Google Scholar]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar]
- Yoon, B.H.; Jung, J.W.; Lee, J.J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar]
- Karakida, F.; Ikeya, Y.; Tsunakawa, M.; Yamaguchi, T.; Ikarashi, Y.; Takeda, S.; Aburada, M. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol. Pharm. Bull. 2007, 30, 514–519. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar]
- Wuyts, N.; Swennen, R.; De Waele, D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 2006, 8, 89–101. [Google Scholar]
- Kang, G.-H.; Chang, E.-J.; Choi, S.-W. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorius L.) seeds. J. Food Sci. Nutr. 1999, 4, 221–225. [Google Scholar]
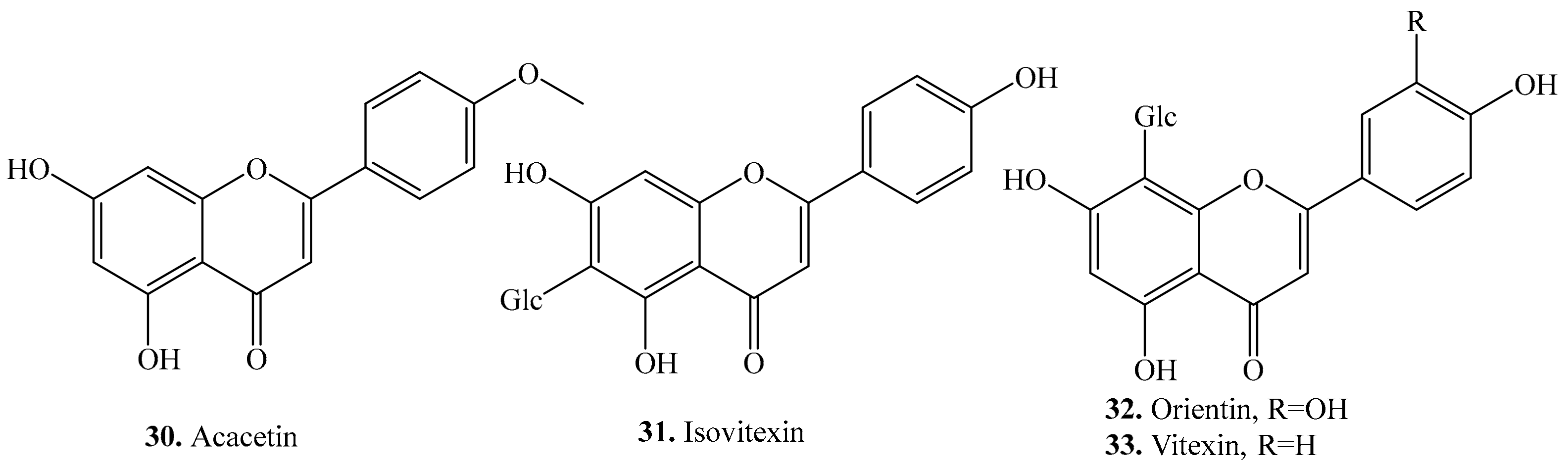
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016, 12, 72. [Google Scholar]
- González-Trujano, M.E.; Ventura-Martínez, R.; Chávez, M.; Díaz-Reval, I.; Pellicer, F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 2012, 78, 793–796. [Google Scholar]
- Hsu, Y.L.; Kuo, P.L.; Liu, C.F.; Lin, C.C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004, 212, 53–60. [Google Scholar]
- Hayashi, K.; Hayashi, T.; Arisawa, M.; Morita, N. Antiviral agents of plant origin. Antiherpetic activity of acacetin. Antivir. Chem. Chemoth. 1993, 4, 49–53. [Google Scholar]
- Liu, L.Z.; Jing, Y.; Jiang, L.L.; Jiang, X.E.; Jiang, Y.; Rojanasakul, Y.; Jiang, B.H. Acacetin inhibits VEGF expression, tumor angiogenesis and growth through AKT/HIF-1α pathway. Biochem. Bioph. Res. Commun. 2011, 413, 299–305. [Google Scholar]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Lopez-Munoz, F.J. Evidence of mechanism of action of anti-inflammatory/antinociceptive activities of acacetin. Eur. J. Pain. 2014, 18, 396–405. [Google Scholar]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Shi, L.; Zaidi, S.F.H.; Ueda, J.Y.; Kadota, S. Hypouricemic effects of acacetin and 4,5-O-dicaffeoylquinic acid methyl ester on serum uric acid levels in potassium oxonate-pretreated rats. Biol. Pharm. Bull. 2005, 28, 2231–2234. [Google Scholar]
- Shochet, G.E.; Drucker, L.; Pasmanik-Chor, M.; Pomeranz, M.; Fishman, A.; Matalon, S.T.; Lishner, M. First trimester human placental factors induce breast cancer cell autophagy. Breast Cancer Res. Treat. 2015, 149, 645–654. [Google Scholar]
- Lee, C.Y.; Chien, Y.S.; Chiu, T.H.; Huang, W.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012, 28, 1883–1888. [Google Scholar]
- De Oliveira, D.R.; Zamberlam, C.R.; Gaiardo, R.B.; Rêgo, G.M.; Cerutti, J.M.; Cavalheiro, A.J.; Cerutti, S.M. Flavones from Erythrina falcata are modulators of fear memory. BMC Complement. Altern. Med. 2014, 14, 288. [Google Scholar]
- Soulimani, R.; Younos, C.; Jarmouni, S.; Bousta, D.; Misslin, R.; Mortier, F. Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J. Ethnopharmacol. 1997, 57, 11–20. [Google Scholar]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar]
- Peng, X.; Zheng, Z.; Cheng, K.W.; Shan, F.; Ren, G.X.; Chen, F.; Wang, M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar]
- Shibano, M.; Kakutani, K.; Taniguchi, M.; Yasuda, M.; Baba, K. Antioxidant constituents in the dayflower (Commelina communis L.) and their α-glucosidase-inhibitory activity. J. Nat. Med. 2008, 62, 349. [Google Scholar]
- Li, H.M.; Hwang, S.H.; Kang, B.G.; Hong, J.S.; Lim, S.S. Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules 2014, 19, 13212–13224. [Google Scholar]
- Yoo, H.; Ku, S.-K.; Lee, T.; Bae, J.-S. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation 2014, 37, 1705–1717. [Google Scholar]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565. [Google Scholar]
- Li, F.; Zong, J.; Zhang, H.; Zhang, P.; Xu, L.; Liang, K.; Qian, W. Orientin reduces myocardial infarction size via eNOS/NO signaling and thus mitigates adverse cardiac remodeling. Front. Pharm. 2017, 8, 926. [Google Scholar]
- Lee, W.; Bae, J.S. Antithrombotic and antiplatelet activities of orientin in vitro and in vivo. J. Funct. Foods 2015, 17, 388–398. [Google Scholar]
- Thangaraj, K.; Natesan, K.; Palani, M.; Vaiyapuri, M. Orientin, a flavanoid, mitigates 1,2-dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet. Toxicol. Rep. 2018, 5, 977–987. [Google Scholar]
- Chen, J.; Zhong, J.; Liu, Y.; Huang, Y.; Luo, F.; Zhou, Y.; Wang, J. Purified vitexin compound 1, a new neolignan isolated compound, promotes PUMA-dependent apoptosis in colorectal cancer. Cancer Med. 2018, 7, 6158–6169. [Google Scholar]
- Je, H.G.; Hong, S.M.; Je, H.D.; Sohn, U.D.; Choi, Y.S.; Seo, S.Y.; Park, E.S. The inhibitory effect of vitexin on the agonist-induced regulation of vascular contractility. J. Pharm. Sci. 2014, 69, 224–228. [Google Scholar]
- Praveena, R.; Sadasivam, K.; Kumaresan, R.; Deepha, V.; Sivakumar, R. Experimental and DFT studies on the antioxidant activity of a C-glycoside from Rhynchosia capitata. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013, 103, 442–452. [Google Scholar]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar]
- Abbasi, E.; Nassiri-Asl, M.; Sheikhi, M.; Shafiee, M. Effects of vitexin on scopolamine-induced memory impairment in rats. Chin. J. Physiol. 2013, 56, 184–189. [Google Scholar]
- Can, O.D.; Ozkay, U.D.; Ucel, U.I. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharm. 2013, 699, 250–257. [Google Scholar]
- Abbasi, E.; Nassiri-Asl, M.; Shafeei, M.; Sheikhi, M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012, 80, 274–278. [Google Scholar]
- Aseervatham, G.S.B.; Suryakala, U.; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharm. 2016, 82, 54–64. [Google Scholar]
- Ozkay, U.D.; Can, O.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharm. Biochem. Behav. 2013, 109, 23–30. [Google Scholar]
- Min, J.-W.; Hu, J.-J.; He, M.; Sanchez, R.M.; Huang, W.-X.; Liu, Y.-Q.; Bsoul, N.B.; Han, S.; Yin, J.; Liu, W.-H.; et al. Vitexin reduces hypoxia–ischemia neonatal brain injury by the inhibition of HIF-1alpha in a rat pup model. Neuropharmacology 2015, 99, 38–50. [Google Scholar]
- Wang, Y.; Zhen, Y.; Wu, X.; Jiang, Q.; Li, X.; Chen, Z.; Dong, L. Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen-activated protein kinase and apoptosis signaling in mice. Phytomedicine 2015, 22, 379–384. [Google Scholar]
- Brahmbhatt, S.; Brahmbhatt, R.M.; Boyages, S.C. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: A study in rural/tribal schoolchildren from Gujarat (Western India). Eur. J. Endocrinol. 2000, 143, 37–46. [Google Scholar]
- Basile, A.; Giordano, S.; Lopez-Saez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar]
- Knipping, K.; Garssen, J.; van’t Land, B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol. J. 2012, 9, 137. [Google Scholar]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biol. Med. 1998, 24, 1355–1363. [Google Scholar]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res. 1995, 22, 375–383. [Google Scholar]
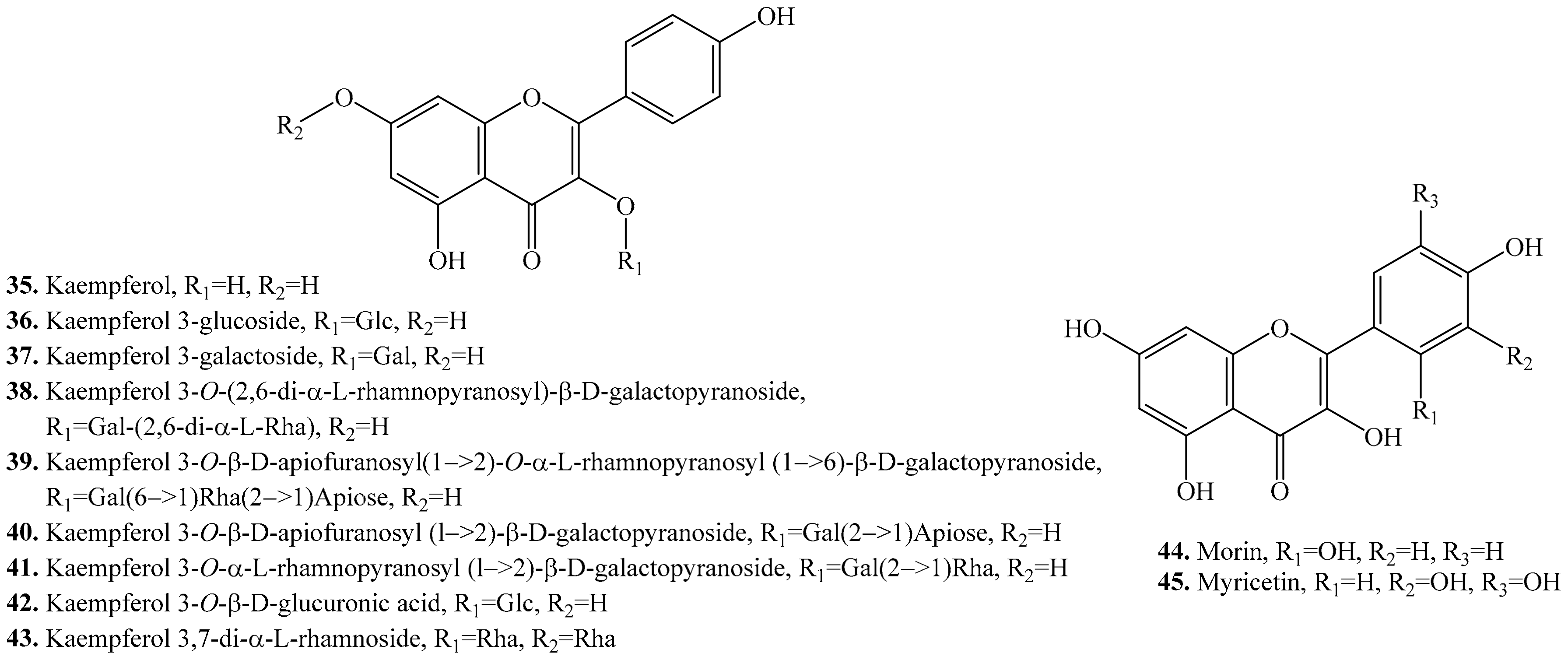
- Zhu, N.; Sheng, S.; Li, D.; LaVoie, E.J.; Karwe, M.V.; Rosen, R.T.; HO, C.T. Antioxidative flavonoid glycosides from quinoa seeds (Chenopodium quinoa Willd). J. Food Lipids 2001, 8, 37–44. [Google Scholar]
- Bloor, S.J. An antimicrobial kaempferol-diacyl-rhamnoside from Pentachondra pumila. Phytochemistry 1995, 38, 1033–1035. [Google Scholar]
- Martini, N.D.; Katerere, D.R.P.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar]
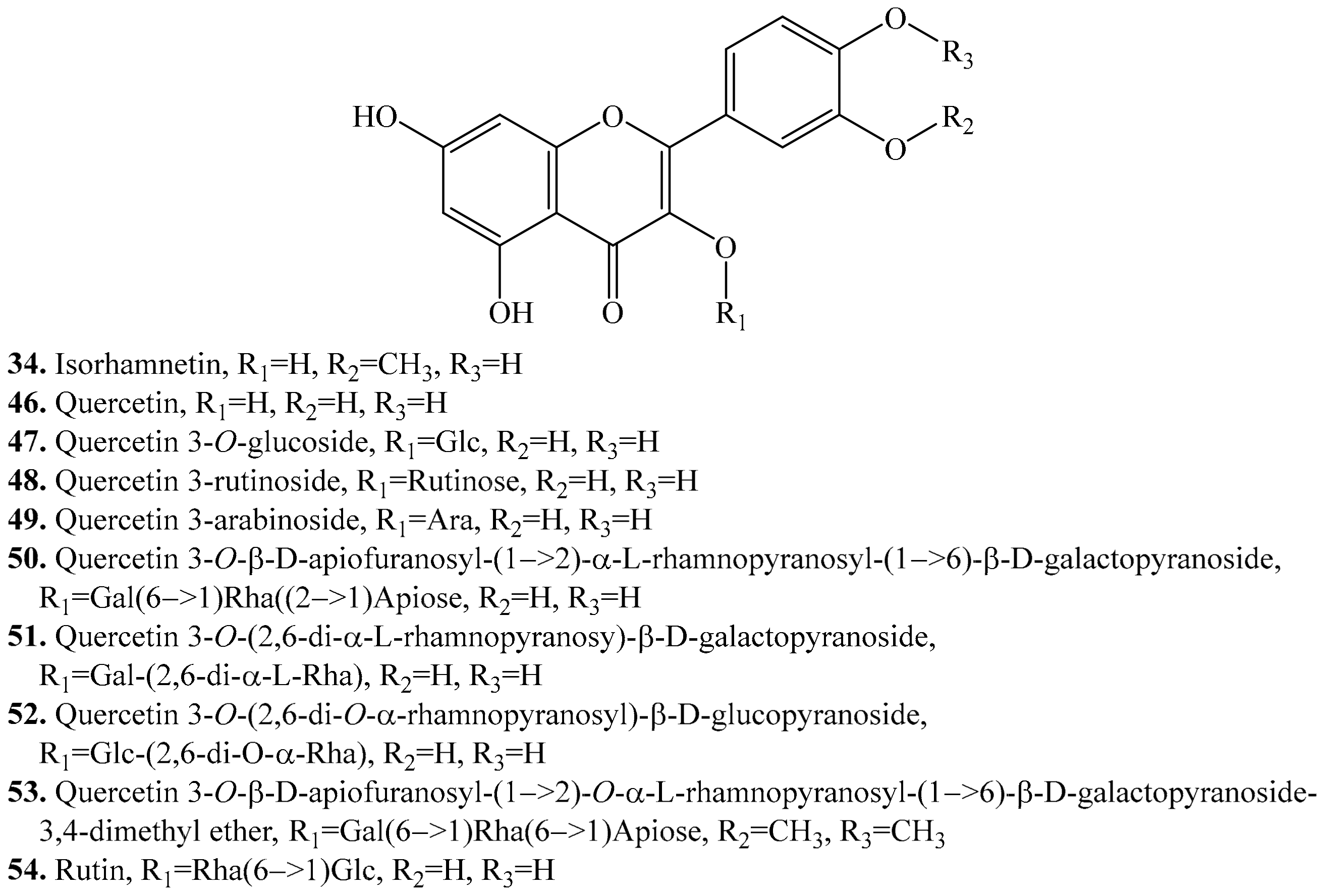
- Parvez, M.M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar]
- Bahrman, N.; Jay, M.; Gorenflot, R. Contribution to the chemosystematic knowledge of some species of the genus Chenopodium, L. Lett. Bot. 1985, 2, 107–113. [Google Scholar]
- Saud, S.M.; Young, M.R.; Jones-Hall, Y.L.; Ileva, L.; Evbuomwan, M.O.; Wise, J.; Bobe, G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013, 73, 5473–5484. [Google Scholar]
- Jnawali, H.N.; Jeon, D.; Jeong, M.-C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.M.; et al. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar]
- Torres, R.; Faini, F.; Modak, B.; Urbina, F.; Labbé, C.; Guerrero, J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry 2006, 67, 984–987. [Google Scholar]
- Teng, B.S.; Lu, Y.H.; Wang, Z.T.; Tao, X.Y.; Wei, D.Z. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharm. Res. 2006, 54, 186–194. [Google Scholar]
- Li, C.; Yang, D.; Zhao, Y.; Qiu, Y.; Cao, X.; Yu, Y.; Yin, X. Inhibitory effects of isorhamnetin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Nutr. Cancer 2015, 67, 1191–1200. [Google Scholar]
- Oh, H.M.; Kwon, B.M.; Baek, N.I.; Kim, S.H.; Chung, I.S.; Park, M.H.; Kim, D.K. Inhibitory activity of isorhamnetin from Persicaria thunbergii on farnesyl protein transferase. Arch. Pharm. Res. 2005, 28, 169–171. [Google Scholar]
- Chirumbolo, S. Anti-inflammatory action of isorhamnetin. Inflammation 2014, 37, 1200. [Google Scholar]
- Ku, S.K.; Kim, T.H.; Bae, J.S. Anticoagulant activities of persicarin and isorhamnetin. Vasc. Pharm. 2013, 58, 272–279. [Google Scholar]
- Kim, S.K.; Kim, H.J.; Choi, S.E.; Park, K.H.; Choi, H.K.; Lee, M.W. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E 2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch. Pharm. Res. 2008, 3, 424. [Google Scholar]
- Lee, K.M.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Heo, Y.S.; Bode, A.M.; Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharm. 2010, 80, 2042–2049. [Google Scholar]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar]
- Prouillet, C.; Mazière, J.C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharm. 2004, 67, 1307–1313. [Google Scholar]
- De Simone, F.; Dini, A.; Pizza, C.; Saturnino, P.; Schettino, O. Two flavonol glycosides from Chenopodium quinoa. Phytochemistry 1990, 29, 3690–3692. [Google Scholar]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar]
- Chemmugil, P.; Lakshmi, P.T.V.; Annamalai, A. Exploring Morin as an anti-quorum sensing agent (anti-QSA) against resistant strains of Staphylococcus aureus. Microb. Pathog. 2019, 127, 304–315. [Google Scholar]
- Qu, Y.; Wang, C.; Liu, N.; Gao, C.; Liu, F. Exhibits anti-inflammatory effects on IL-1β-stimulated human osteoarthritis chondrocytes by activating the Nrf2 signaling pathway. Cell. Physiol. Biochem. 2018, 51, 1830–1838. [Google Scholar]
- Ji, Y.; Jia, L.; Zhang, Y.; Xing, Y.; Wu, X.; Zhao, B.; Qiao, X. Antitumor activity of the plant extract morin in tongue squamous cell carcinoma cells. Oncol. Rep. 2018, 40, 3024–3032. [Google Scholar]
- Yuan, W.; Ahmad, S.; Najar, A. Morin, a plant derived flavonoid, modulates the expression of peroxisome proliferator-activated receptor-γ coactivator-1α mediated by AMPK pathway in hepatic stellate cells. Am. J. Transl. Res. 2017, 9, 5662. [Google Scholar]
- Chen, X.; Deng, Z.; Zhang, C.; Zheng, S.; Pan, Y.; Wang, H.; Li, H. Is antioxidant activity of flavonoids mainly through the hydrogen-atom transfer mechanism? Food Res. Int. 2019. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar]
- Wang, N.; Zhang, J.; Qin, M.; Yi, W.; Yu, S.; Chen, Y.; Zhang, R. Amelioration of streptozotocin-induced pancreatic β cell damage by morin: Involvement of the AMPK-FOXO3-catalase signaling pathway. Int. J. Mol. Med. 2018, 41, 1409–1418. [Google Scholar]
- Carmona, V.; Martín-Aragon, S.; Goldberg, J.; Schubert, D.; Bermejo-Bescós, P. Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutr. Neurosci. 2019. [Google Scholar] [CrossRef]
- Bhakuni, G.S.; Bedi, O.; Bariwal, J.; Kumar, P. Hepatoprotective activity of morin and its semi-synthetic derivatives against alcohol induced hepatotoxicity in rats. Indian J. Physiol. Pharmacol. 2017, 61, 175–190. [Google Scholar]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar]
- Wang, S.J.; Tong, Y.; Lu, S.; Yang, R.; Liao, X.; Xu, Y.F.; Li, X. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010, 76, 1492–1496. [Google Scholar]
- Tong, Y.; Zhou, X.M.; Wang, S.J.; Yang, Y.; Cao, Y.L. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch. Pharm. Res. 2009, 32, 527–533. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; Galvez, J.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar]
- Silva, T.B.; Costa, C.O.; Galvão, A.F.; Bomfim, L.M.; Rodrigues, A.C.; Mota, M.C.; Dantas, A.A.; Santos, T.R.; Soares, M.B.; Bezerra, D.P. Cytotoxic potential of selected medicinal plants in northeast Brazil. BMC Complement. Altern. Med. 2016, 16, 199. [Google Scholar]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar]
- Shimoyama, A.T.; Santin, J.R.; Machado, I.D.; de Silva, A.M.D.O.; De Melo, I.L.P.; Mancini-Filho, J.; Farsky, S.H. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. N-S. Arch. Pharmacol. 2013, 386, 5–14. [Google Scholar]
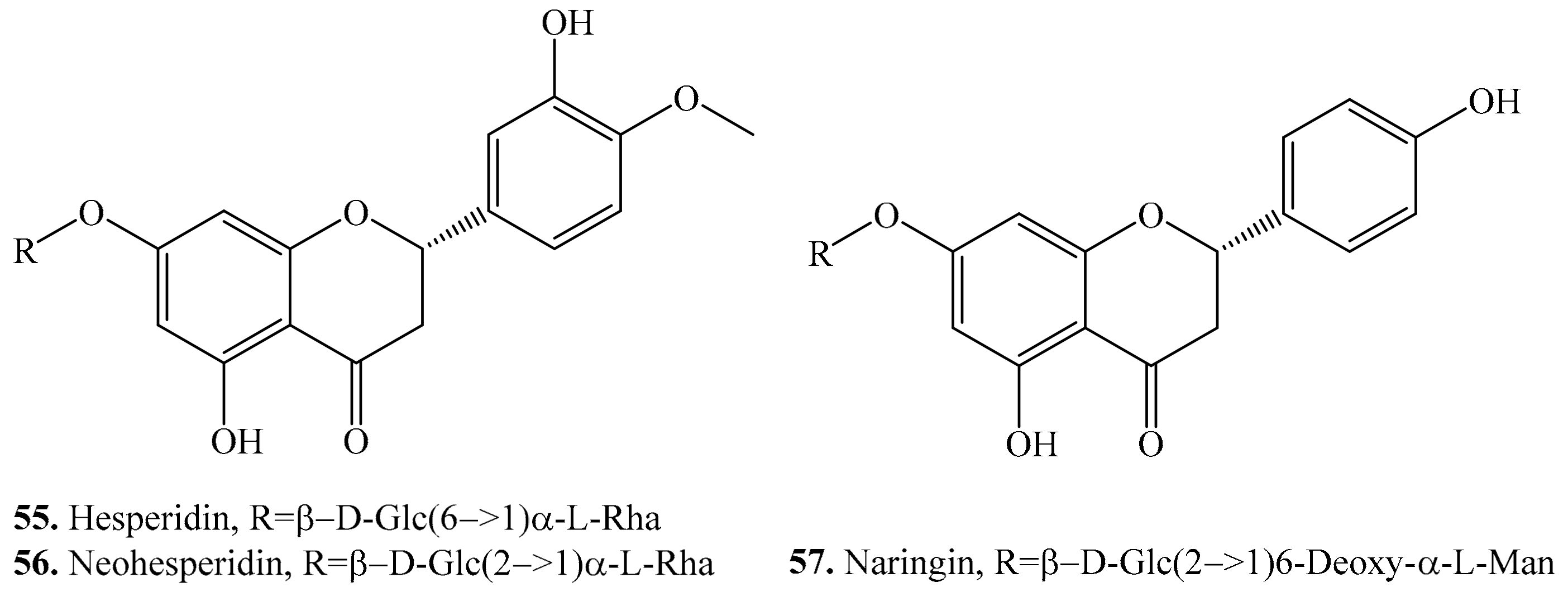
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 2015, 38, 195–3204. [Google Scholar]
- Mahmoud, A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharm. 2014, 92, 717–724. [Google Scholar]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212. [Google Scholar]
- Lee, J.H.; Lee, S.H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res. 2009, 23, 1748–1753. [Google Scholar]
- Xu, F.; Zang, J.; Chen, D.; Zhang, T.; Zhan, H.; Lu, M.; Zhuge, H. Neohesperidin induces cellular apoptosis in human breast denocarcinoma MDA-MB-231 cells via activating the Bcl-2/Bax-mediated signaling pathway. Nat. Prod. Commun. 2012, 7, 1475–1478. [Google Scholar]
- Jeon, S.M.; Bok, S.H.; Jang, M.K.; Lee, M.K.; Nam, K.T.; Park, Y.B.; Choi, M.S. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001, 69, 2855–2866. [Google Scholar]
- Wei, M.; Yang, Z.; Li, P.; Zhang, Y.; Sse, W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007, 35, 663–667. [Google Scholar]
- Amaro, M.I.; Rocha, J.; Vila-Real, H.; Eduardo-Figueira, M.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar]
- Huang, S.W.; Frankel, E.N. Antioxidant activity of tea catechins in different lipid systems. J. Agric. Food Chem. 1997, 45, 3033–3038. [Google Scholar]
- Geetha, T.; Garg, A.; Chopra, K.; Kaur, I.P. Delineation of antimutagenic activity of catechin, epicatechin and green tea extract. Mutat. Res.-Fund. Mol. Mech. Mutagen. 2004, 556, 65–74. [Google Scholar]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Anti-metastatic activity of curcumin and catechin. Cancer Lett. 1999, 141, 159–165. [Google Scholar]
- Hirasawa, M.; Takada, K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicro. Chemoth. 2004, 53, 225–229. [Google Scholar]
- Saeki, K.; Hayakawa, S.; Isemura, M.; Miyase, T. Importance of a pyrogallol-type structure in catechin compounds for apoptosis-inducing activity. Phytochemistry 2000, 53, 391–394. [Google Scholar]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar]
- Kinjo, J.; Nagao, T.; Tanaka, T.; Nonaka, G.I.; Okawa, M.; Nohara, T.; Okabe, H. Activity-guided fractionation of green tea extract with antiproliferative activity against human stomach cancer cells. Biol. Pharm. Bull. 2002, 2, 1238–1240. [Google Scholar]
- Han, Z.; Wang, G.; Yao, W.; Zhu, W. Isoflavonic phytoestrogens-new prebiotics for farm animals: A review on research in China. Curr. Issues Intest. Microbiol. 2006, 7, 53–60. [Google Scholar]
- Lutz, M.; Martínez, A.; Martínez, E.A. Daidzein and genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Ind. Crop. Prod. 2013, 49, 117–121. [Google Scholar]
- Foti, P.; Erba, D.; Riso, P.; Spadafranca, A.; Criscuoli, F.; Testolin, G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch. Biochem. Biophys. 2005, 433, 421–427. [Google Scholar]
- Cho, K.W.; Lee, O.H.; Banz, W.J.; Moustaid-Moussa, N.; Shay, N.F.; Kim, Y.C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J. Nutr. Biochem. 2010, 21, 841–847. [Google Scholar]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar]
- Picherit, C.; Dalle, M.; Neliat, G.; Lebecque, P.; Davicco, M.J.; Barlet, J.P.; Coxam, V. Genistein and daidzein modulate in vitro rat uterine contractile activity. J. Steroid Biochem. Mol. Biol. 2000, 75, 201–208. [Google Scholar]
- Zeng, J.; Huang, Z.; Qiu, F.; Yao, X.; Ye, H. The anti-hypoxia activity of daidzein. Chin. J. Mod. Appl. Pharm. 2004, 21, 454–456. [Google Scholar]
- Choo, M.K.; Park, E.K.; Yoon, H.K.; Kim, D.H. Antithrombotic and antiallergic activities of daidzein, a metabolite of puerarin and daidzin produced by human intestinal microflora. Biol. Pharm. Bull. 2002, 25, 1328–1332. [Google Scholar]
- Lepri, S.R.; Luiz, R.C.; Zanelatto, L.C.; Da Silva, P.B.G.; Sartori, D.; Ribeiro, L.R.; Mantovani, M.S. Chemoprotective activity of the isoflavones, genistein and daidzein on mutagenicity induced by direct and indirect mutagens in cultured HTC cells. Cytotechnology 2013, 65, 213–222. [Google Scholar]
- Fujioka, M.; Uehara, M.; Wu, J.; Adlercreutz, H.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr. 2004, 134, 2623–2627. [Google Scholar]
- Choi, E.J.; Kim, G.H. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013, 7, 781–784. [Google Scholar]
- Finking, G.; Wohlfrom, M.; Lenz, C.; Wolkenhauer, M.; Eberle, C.; Hanke, H. The phytoestrogens genistein and daidzein, and 17 beta-estradiol inhibit development of neointima in aortas from male and female rabbits in vitro after injury. Coron. Artery Dis. 1999, 10, 607–615. [Google Scholar]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. The antioxidant activity of genistein in vitro. J. Nutr. Biochem. 1995, 6, 481–485. [Google Scholar]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.I.; Itoh, N.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar]
- Banerjee, S.; Zhang, Y.; Ali, S.; Bhuiyan, M.; Wang, Z.; Chiao, P.J.; Sarkar, F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005, 65, 9064–9072. [Google Scholar]
- Uckun, F.M.; Narla, R.K.; Jun, X.; Zeren, T.; Venkatachalam, T.; Waddick, K.G.; Rostostev, A.; Myers, D.E. Cytotoxic activity of epidermal growth factor-genistein against breast cancer cells. Clin. Cancer Res. 1998, 4, 901–912. [Google Scholar]
- Büchler, P.; Reber, H.A.; Büchler, M.W.; Friess, H.; Lavey, R.S.; Hines, O.J. Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer 2004, 10, 201–210. [Google Scholar]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar]
- Ye, F.; Wu, J.; Dunn, T.; Yi, J.; Tong, X.; Zhang, D. Inhibition of cyclooxygenase-2 activity in head and neck cancer cells by genistein. Cancer Lett. 2004, 211, 39–46. [Google Scholar]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.H.; Ko, S. Curcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar]
- Raynal, N.J.M.; Momparler, L.; Charbonneau, M.; Momparler, R.L. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2007, 71, 3–7. [Google Scholar]
- Kim, J.S.; Nam, Y.J.; Kwon, T.W. Induction of quinone reductase activity by genistein, soybean isoflavone. Food Sci. Biotechnol. 1996, 5, 70–75. [Google Scholar]
- Khaw, A.K.; Yong, J.W.Y.; Kalthur, G.; Hande, M.P. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Gene. Chromosome Canc. 2012, 51, 961–974. [Google Scholar]
- Yang, G.; Ham, I.; Choi, H.Y. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem. Toxicol. 2013, 58, 124–132. [Google Scholar]
- Xu, X.; Zhang, S.; Zhang, L.; Yan, W.; Zheng, X. The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005, 71, 585–591. [Google Scholar]
- Cherdshewasart, W.; Sutjit, W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine 2008, 15, 38–43. [Google Scholar]
- Hsu, F.L.; Liu, I.M.; Kuo, D.H.; Chen, W.C.; Su, H.C.; Cheng, J.T. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J. Nat. Prod. 2003, 66, 788–792. [Google Scholar]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar]
- Dembitsky, V.; Shkrob, I.; Hanus, L.O. Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Palacky. Olomouc. Czech. Repub. 2008, 152, 209–215. [Google Scholar]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (PsTPS24) encodes a jamonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar]
- Ruiz, K.B.; Khakimov, B.; Engelsen, S.B.; Bak, S.; Biondi, S.; Jacobsen, S.-E. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: An investigation of genotypic diversity in saponin profiles. Ind. Crop. Prod. 2017, 104, 156–163. [Google Scholar]
- Mastebroek, H.D.; Limburg, H.; Gilles, T.; Marvin, H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 2000, 80, 152–156. [Google Scholar]
- Woldemichael, G.M.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar]
- Sun, X.; Yang, X.; Xue, P.; Zhang, Z.; Ren, G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complem. Altern. Med. 2019, 19, 46. [Google Scholar]
- Stuardo, M.; San Martín, R. Antifungal properties of quinoa (Chenopodium quinoa Willd) alkali treated saponins against Botrytis cinerea. Ind. Crop. Prod. 2008, 27, 296–302. [Google Scholar]
- San Martín, R.; Ndjoko, K.; Hostettmann, K. Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponins. Crop Prot. 2008, 27, 310–319. [Google Scholar]
- Castillo-Ruiz, M.; Cañon-Jones, H.; Schlotterbeck, T.; Lopez, M.A.; Tomas, A.; San Martin, R. Safety and efficacy of quinoa (Chenopodium quinoa) saponins derived molluscicide to control of Pomacea maculata in rice fields in the Ebro Delta, Spain. Crop Prot. 2018, 111, 42–49. [Google Scholar]
- Joshi, R.C.; San Martin, R.; Saez-Navarrete, C.; Alarcon, J.; Sainz, J.; Antolin, M.M.; Martin, R.; Sebastian, L.S. Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions. Crop Prot. 2008, 27, 553–557. [Google Scholar]
- Madl, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass. Spectr. 2006, 17, 795–806. [Google Scholar]
- Burnouf-Radosevich, M.; Delfel, N.E.; England, R. Gas chromatography-mass spectrometry of oleanane-and ursane-type triterpenes–application to Chenopodium quinoa triterpenes. Phytochemistry 1985, 24, 2063–2066. [Google Scholar]
- Mizui, F.; Kasai, R.; Ohtani, K.; Tanaka, O. Saponins from brans of quinoa, Chenopodium quinoa Willd. I. Chem. Pharm. Bull. 1988, 36, 1415–1418. [Google Scholar]
- Mizui, F.; Kasai, R.; Ohtani, K.; Tanaka, O. Saponins from brans of quinoa, Chenopodium quinoa Willd. II. Chem. Pharm. Bull. 1990, 38, 375–377. [Google Scholar]
- Burnouf-Radosevich, M.; Delfel, N.E. High-performance liquid chromatography of oleanane-type triterpenes. J. Chromatogr. A 1984, 292, 403–409. [Google Scholar]
- Quispe-Fuentes, I.; Vega-Galvez, A.; Miranda, M.; Lemus-Mondaca, R.; Lozano, M.; Ah-Hen, K. A kinetic approach to saponin extraction during washing of quinoa (Chenopodium quinoa Willd.) seeds. J. Food Process Eng. 2013, 36, 202–210. [Google Scholar]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar]
- Kashiwada, Y.; Wang, H.K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Ikeshiro, Y. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar]
- Singh, G.B.; Singh, S.; Bani, S.; Gupta, B.D.; Banerjee, S.K. Anti–inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar]
- Ghosh, D.; Thejomoorthy, P. Anti-inflammatory and analgesic activities of oleanolic acid 3-/3-glucoside (RDG-1) from Randia dumetorum (Rubiaceae). Indian J. Pharmacol. 1983, 15, 331. [Google Scholar]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem.-Biol. Interact. 2010, 184, 328–337. [Google Scholar]
- Rajasekaran, M.; Bapna, J.S.; Lakshmanan, S.; Nair, A.R.; Veliath, A.J.; Panchanadam, M. Antifertility effect in male rats of oleanolic acid, a triterpene from Eugenia jambolana flowers. J. Ethnopharmacol. 1988, 24, 115–121. [Google Scholar]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drug. 2009, 20, 880–892. [Google Scholar]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar]
- Yoshikawa, M.; Matsuda, H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. BioFactors 2000, 13, 231–237. [Google Scholar]
- Park, S.H.; Oh, S.R.; Jung, K.Y.; Ahn, K.S.; Kim, J.G.; Lee, J.J.; Lee, H.K. Anticomplement activities of oleanolic acid monodesmosides and bisdesmosides isolated from Tiarella polyphylla. Arch. Pharm. Res. 1999, 22, 428–431. [Google Scholar]
- Facino, R.M.; Carini, M.; Stefani, R.; Aldini, G.; Saibene, L. Anti–elastase and anti–hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors contributing to their efficacy in the treatment of venous insufficiency. Archiv. Der. Pharm. 1995, 328, 720–724. [Google Scholar]
- Ruiz, W.A.; Farfan, J.A. Determination of oleanolic acid in quinoa by gas-liquid chromatography (Chenopodium quinoa, Willd cv Kcancolla). Bol. Soc. Quim. Peru. 1979, 45, 266–276. [Google Scholar]
- Lozano, M.; Gonzales, E.; Flores, Y.; Almanza, G.R. Effect in acute inflammation of sapogenin extract and isolated sapogenins from quinoa waste (Chenopodium quinoa Willd). Rev. Boliv. Quim. 2013, 30, 115–121. [Google Scholar]
- Dini, I.; Schettino, O.; Simioli, T.; Dini, A. Studies on the constituents of Chenopodium quinoa seeds: Isolation and characterization of new triterpene saponins. J. Agric. Food Chem. 2001, 49, 741–746. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Oleanane saponins in “Kancolla”, a sweet variety of Chenopodium quinoa. J. Nat. Prod. 2002, 65, 1023–1026. [Google Scholar]
- Ma, W.W.; Heinstein, P.F.; Mclaughlin, J.L. Additional toxic, bitter saponins from the seeds of Chenopodium quinoa. J. Nat. Prod. 1989, 52, 1132–1135. [Google Scholar]
- Zhu, N.; Sheng, S.; Sang, S.; Jhoo, J.-W.; Bai, N.; Karwe, M.V.; Rosen, R.T.; Ho, C.-T. Triterpene saponins from debittered quinoa (Chenopodium quinoa) seeds. J. Agric. Food Chem. 2002, 50, 865–867. [Google Scholar]
- Chauhan, G.S.; Eskin, N.A.M.; Tkachuk, R. Nutrients and antinutrients in quinoa seed. Cereal Chem 1992, 69, 85–88. [Google Scholar]
- Hostettmann, K. Saponins with molluscicidal activity from Hedera helix L. Helv. Chim. Acta 1980, 63, 606–609. [Google Scholar]
- Barthomeuf, C.; Debiton, E.; Mshvildadze, V.; Kemertelidze, E.; Balansard, G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002, 68, 672–675. [Google Scholar]
- Favel, A.; Steinmetz, M.D.; Regli, P.; Vidal-Ollivier, E.; Elias, R.; Balansard, G. In vitro antifungal activity of triterpenoid saponins. Planta Med. 1994, 60, 50–53. [Google Scholar]
- Majester-Savornin, B.; Elias, R.; Diaz-Lanza, A.M.; Balansard, G.; Gasquet, M.; Delmas, F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991, 57, 260–262. [Google Scholar]
- Lee, S.J.; Shin, E.J.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the major constituents of Lonicera japonica. Arch. Pharm. Res. 1995, 18, 133. [Google Scholar]
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar]
- Khalil, A.H.; El-Adawy, T.A. Isolation, identification and toxicity of saponin from different legumes. Food Chem. 1994, 50, 197–201. [Google Scholar]
- Liu, B.-X.-Z.; Zhou, J.-Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.-F.; Yuan, J.-R.; Zhao, B.-J.; Feng, L.; Jia, X.-B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412. [Google Scholar]
- Lee, K.-T.; Sohn, I.-C.; Park, H.-J.; Kim, D.-W.; Jung, G.-O.; Park, K.-Y. Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus. Planta Med. 2000, 66, 329–332. [Google Scholar]
- Park, H.J.; Kwon, S.H.; Lee, J.H.; Lee, K.H.; Miyamoto, K.I.; Lee, K.T. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. 2001, 67, 118–121. [Google Scholar]
- Voutquenne, L.; Lavaud, C.; Massiot, G.; Men-Olivier, L.L. Structure-activity relationships of haemolytic saponins. Pharm. Biol. 2002, 40, 253–262. [Google Scholar]
- Houghton, P.; Patel, N.; Jurzysta, M.; Biely, Z.; Cheung, C. Antidermatophyte activity of medicago extracts and contained saponins and their structure–activity relationships. Phytother. Res. 2006, 20, 1061–1066. [Google Scholar]
- He, W.; Van Puyvelde, L.; Maes, L.; Bosselaers, J.; De Kimpe, N. Antitrichomonas in vitro activity of Cussonia holstii Engl. Nat. Prod. Res. 2003, 17, 127–133. [Google Scholar]
- Gopalsamy, N.; Gueho, J.; Julien, H.R.; Owadally, A.W.; Hostettmann, K. Molluscicidal saponins of Polyscias dichroostachya. Phytochemistry 1990, 29, 793–795. [Google Scholar]
- Oh, S.R.; Jung, K.Y.; Son, K.H.; Park, S.H.; Ahn, K.S.; Lee, H.K. In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacus asper. Arch. Pharm. Res. 1999, 22, 317–319. [Google Scholar]
- Jung, H.-J.; Lee, C.O.; Lee, K.-T.; Choi, J.; Park, H.-J. Structure–activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004, 27, 744–747. [Google Scholar]
- Dini, I.; Tenore, G.C.; Schettino, O.; Dini, A. New oleanane saponins in Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 3976–3981. [Google Scholar]
- Meyer, B.N.; Heinstein, P.F.; Burnoufradosevich, M.; Delfel, N.E.; Mclaughlin, J.L. Bioactivity-directed isolation and characterization of quinoside A: One of the toxic/bitter principles of quinoa seeds (Chenopodium quinoa Willd.). J. Agric. Food Chem. 1990, 38, 205–208. [Google Scholar]
- Montoya, G.; Gutierrez, G.; D’vries, R.; Ellena, J.; Panay, A.J. Spergulagenic acid A: Isolation and single crystal structure elucidation. J. Mol. Struct. 2018, 1173, 937–941. [Google Scholar]
- Lazo-Vélez, M.A.; Guajardo-Flores, D.; Mata-Ramírez, D.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Characterization and quantitation of triterpenoid saponins in raw and sprouted Chenopodium berlandieri spp. (Huauzontle) grains subjected to germination with or without selenium stress conditions. J. Food Sci. 2016, 81, C19–C26. [Google Scholar]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar]
- Fanali, C.; Beccaria, M.; Salivo, S.; Tranchida, P.; Tripodo, G.; Farnetti, S.; Dugo, L.; Dugo, P.; Mondello, L. Non-polar lipids characterization of quinoa (Chenopodium quinoa) seed by comprehensive two-dimensional gas chromatography with flame ionization/mass spectrometry detection and non-aqueous reversed-phase liquid chromatography with atmospheric pressure chemical ionization mass spectrometry detection. J. Sep. Sci. 2015, 38, 3151–3160. [Google Scholar]
- Lee, I.S.; Oh, S.R.; Jung, K.Y.; Kim, D.S.; Kim, J.H.; Lee, H.K. Anticomplementary activity and complete 13C NMR assignment of citrostadienol from Schizandra chinensis. Int. J. Pharmacogn. 1997, 35, 358–363. [Google Scholar]
- Saeed, M.A.; Sabir, A.W. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia 2001, 72, 807–809. [Google Scholar]
- Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Zamilpa, A.; Hidalgo-Figueroa, S.; Navarrete-Vazquez, G.; Garcia-Macedo, R.; Almanza-Perez, J.C. Triterpenoids from Hibiscus sabdariffa L. with PPAR δ/γ dual agonist action: In vivo, in vitro and in silico studies. Planta Med. 2019, 85, 412–423. [Google Scholar]
- Cardoso, B.K.; de Oliveira, H.L.M.; Melo, U.Z.; Fernandez, C.M.M.; Campo, C.F.D.A.A.; Goncalves, J.E.; Gazim, Z.C. Antioxidant activity of α- and β-amyrin isolated from Myrcianthes pungens leaves. Nat. Prod. Res. 2019, 33. [Google Scholar] [CrossRef]
- Chen, D.; Xu, F.; Zhang, P.; Deng, J.; Sun, H.; Wen, X.; Liu, J. Practical synthesis of α-amyrin, β-amyrin, and lupeol: The potential natural inhibitors of human oxidosqualene cyclase. Arch. Pharm. 2017, 350, 1700178. [Google Scholar]
- Kannan, S.; Vijayakumar, B.; Sureshkumar, C.; Mohankumar, R.; Narasimhan, S. Insect antifeedant and growth regulating activities of β-amyrin from Sarcostemma acidum. Asian J. Chem. 2013, 25, 1167–1168. [Google Scholar]
- Mhalla, D.; Ben Farhat-Touzri, D.; Tounsi, S.; Trigui, M. Combinational effect of Rumex tingitanus (Polygonaceae) hexane extract and Bacillus thuringiensis δ-endotoxin against Spodoptera littoralis (Lepidoptera: Noctuidae). BioMed. Res. Int. 2018, 2018, 3895834. [Google Scholar]
- Kemboi, D. Phytochemistry and antimicrobial activity of extracts from medicinal plant Olea africana and Olea europea. Int. J. Biochem. Res. Rev. 2016, 12, 25863. [Google Scholar]
- Zhang, J.; Yamada, S.; Ogihara, E.; Kurita, M.; Banno, N.; Qu, W.; Akihisa, T. Biological activities of triterpenoids and phenolic compounds from Myrica cerifera bark. Chem. Biodivers. 2016, 13, 1601–1609. [Google Scholar]
- Ntchapda, F.; Talla, E.; Sakava, P.; Tanzi, F.; Fohouo, F.N.T.; Tanyi, J.M.; Dimo, T. Nitric oxide-dependent vasodilation and Ca2+ signalling induced by erythrodiol in rat aorta. Asian Pac. J. Trop. Dis. 2015, 5, S214–S223. [Google Scholar]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells. Mol. Nutr. Food. Res. 2008, 52, 595–599. [Google Scholar]
- Zheng, Q.; Li, P.; Jin, F.; Yao, C.; Zhang, G.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell. Signal. 2013, 25, 206–213. [Google Scholar]
- Wang, C.M.; Tsai, S.J.; Jhan, Y.L.; Yeh, K.L.; Chou, C.H. Anti-proliferative activity of triterpenoids and sterols isolated from Alstonia scholaris against non-small-cell lung carcinoma cells. Molecules 2017, 22, 2119. [Google Scholar]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar]
- Alvarez-Jubete, L.; Holse, M.; Hansen, A.; Arendt, E.K.; Gallagher, E. Impact of baking on vitamin E content of pseudocereals amaranth, quinoa, and buckwheat. Cereal Chem. 2009, 86, 511–515. [Google Scholar]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2009, 31, 533–542. [Google Scholar]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar]
- Zingg, J.-M. Vitamin E: An overview of major research directions. Mol. Asp. Med. 2007, 28, 400–422. [Google Scholar]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar]
- Kozioł, M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar]
- Sookwong, P.; Murata, K.; Nakagawa, K.; Shibata, A.; Kimura, T.; Yamaguchi, M.; Kojima, Y.; Miyazawa, T. Cross-fertilization for enhancing tocotrienol biosynthesis in rice plants and QTL analysis of their F2 progenies. J. Agric. Food Chem. 2009, 57, 4620–4625. [Google Scholar]
- Ahamed, N.T.; Singhal, R.S.; Kulkarni, P.R.; Pal, M. A lesser-known grain, Chenopodium quinoa: Review of the chemical composition of its edible parts. Food Nutr. Bull. 1998, 19, 61–70. [Google Scholar]
- Foucault, A.S.; Mathé, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tomé, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulangé, A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity 2012, 20, 270–277. [Google Scholar]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Ecdysteroids act as inhibitors of calf skin collagenase and oxidative stress. J. Biochem. Mol. Toxic. 2008, 22, 240–250. [Google Scholar]
- Dini, A.; Rastrelli, L.; Saturnino, P.; Schettino, O. A compositional study of Chenopodium quinoa seeds. Nahrung 1992, 36, 400–404. [Google Scholar]
- Zhu, N.; Kikuzaki, H.; Vastano, B.C.; Nakatani, N.; Karwe, M.V.; Rosen, R.T.; Ho, C.T. Ecdysteroids of quinoa seeds (Chenopodium quinoa Willd.). J. Agric. Food Chem. 2001, 49, 2576–2578. [Google Scholar]
- Xu, D.; Ali, S.; Huang, Z. Insecticidal activity influence of 20-hydroxyecdysone on the pathogenicity of Isaria fumosorosea against Plutella xylostella. Biol. Control 2011, 56, 239–244. [Google Scholar]
- Choi, J.M.; Lee, E.O.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Shim, B.S.; Kim, N.I.; Song, M.C.; Baek, N.I.; Kim, S.H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar]
- Villacrés, E.; Pástor, G.; Quelal, M.B.; Zambrano, I.; Morales, S.H. Effect of processing on the content of fatty acids, tocopherols and sterols in the oils of quinoa (Chenopodium quinoa Willd), lupine (Lupinus mutabilis Sweet), amaranth (Amaranthus caudatus L.) and sangorache (Amaranthus quitensis L.). Glob. Adv. Res. J. Food Sci. Technol. 2013, 2, 44–53. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Nutritional and antinutritional composition of Kancolla seeds: An interesting and underexploited andine food plant. Food Chem. 2005, 92, 125–132. [Google Scholar]
- Prieto, J.M.; Recio, M.C.; Giner, R.M. Anti-inflammatory activity of β-sitosterol in a model of oxazoloneinduced contact-delayed-type hypersensitivity. Bol. Lat. Am. Caribb. Bull. Med. Plants 2006, 5, 57–62. [Google Scholar]
- Vivancos, M.; Moreno, J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Bio. Med. 2005, 39, 91–97. [Google Scholar]
- Radika, M.K.; Viswanathan, P.; Anuradha, C.V. Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide Biol. Chem. 2013, 32, 43–53. [Google Scholar]
- Garcia, M.D.; Saenz, M.T.; Gomez, M.A.; Fernandez, M.A. Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother. Res. 1999, 13, 78–80. [Google Scholar]
- Ghosh, T.; Maity, T.K.; Singh, J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011, 11, 41–49. [Google Scholar]
- Mbambo, B.; Odhav, B.; Mohanlall, V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker. (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar]
- Batta, A.K.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar]
- Huang, J.G.; Zhou, L.J.; Xu, H.H.; Li, W.O. Insecticidal and cytotoxic activities of extracts of Cacalia tangutica and its two active ingredients against Musca domestica and Aedes albopictus. J. Econ. Entomol. 2009, 102, 1444–1447. [Google Scholar]
- Chai, J.W.; Kuppusamy, U.R.; Kanthimathi, M.S. Beta-sitosterol induces apoptosis in MCF-7 cells. Malay. J. Biochem. Mol. Biol. 2008, 16, 28–30. [Google Scholar]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590. [Google Scholar]
- Sugano, M.; Morioka, H.; Ikeda, I. A comparison of hypocholesterolemic activity of β-sitosterol and β-sitostanol in rats. J. Nutr. 1977, 107, 2011–2019. [Google Scholar]
- Moon, E.J.; Lee, Y.M.; Lee, O.H.; Lee, M.J.; Lee, S.K.; Chung, M.H.; Kim, K.W. A ncovel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis 1999, 3, 117–123. [Google Scholar]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Molina-Jasso, D.; Gallaga, J.P.; Silva-Miranda, A.; Chamorro, G. Genotoxic and cytotoxic studies of beta-sitosterol and pteropodine in mouse. Biomed. Res. Int. 2005, 2005, 242–247. [Google Scholar]
- Villasenor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar]
- Bouic, P.J.D.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; Van Jaarsveld, P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar]
- Shi, C.; Wu, F.; Zhu, X.; Xu, J. Incorporation of β-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3β signaling. BBA Gen. Subj. 2013, 1830, 2538–2544. [Google Scholar]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar]
- Imamura, T.; Takagi, H.; Miyazato, A.; Ohki, S.; Mizukoshi, H.; Mori, M. Isolation and characterization of the betalain biosynthesis gene involved in hypocotyl pigmentation of the allotetraploid Chenopodium quinoa. Biochem. Bioph. Res. Commun. 2018, 496, 280–286. [Google Scholar]
- Kobayashi, N.; Schmidt, J.; Wray, V.; Schliemann, W. Formation and occurrence of dopamine-derived betacyanins. Phytochemistry 2001, 56, 429–436. [Google Scholar]
- Dini, I.; Tenore, G.C.; Trimarco, E.; Dini, A. Two novel betaine derivatives from Kancolla seeds (Chenopodiaceae). Food Chem. 2006, 98, 209–213. [Google Scholar]
- Tramontano, W.A.; Jouve, D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar]
- Jones, G.P.; Paleg, L.G. In vitro thermal and salt stability of pyruvate kinase are increased by proline analogues and trigonelline. Funct. Plant. Biol. 1991, 18, 279–286. [Google Scholar]
- Escribano, J.; Cabanes, J.; Jimenez-Atienzar, M.; Ibañez-Tremolada, M.; Gomez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar]
- Hirakawa, N.; Okauchi, R.; Miura, Y.; Yagasaki, K. Anti-invasive activity of niacin and trigonelline against cancer cells. Biosci. Biotech. Biochem. 2005, 69, 653–658. [Google Scholar]
- Shah, S.N.; Bodhankar, S.L.; Bhonde, R.; Mohan, V. Hypoglycemic activity of the combination of active ingredients isolated from Trigonella foenumgraecum in alloxan induced diabetic mice. Pharmacologyonline 2006, 1, 65–82. [Google Scholar]
- Letelier, M.E.; Rodríguez-Rojas, C.; Sánchez-Jofré, S.; Aracena-Parks, P. Surfactant and antioxidant properties of an extract from Chenopodium quinoa Willd seed coats. J. Cereal Sci. 2011, 53, 239–243. [Google Scholar]
- Johnson, I.T.; Gee, J.M.; Price, K.; Curl, C.; Fenwick, G.R. Influence of saponins on gut permeability and active nutrient transport in vitro. J. Nutr. 1986, 116, 2270–2277. [Google Scholar]
- Gee, J.M.; Price, K.R.; Ridout, C.L.; Wortley, G.M.; Hurrell, R.F.; Johnson, I.T. Saponins of quinoa (Chenopodium quinoa): Effects of processing on their abundance in quinoa products and their biological effects on intestinal mucosal tissue. J. Sci. Food Agric. 1993, 63, 201–209. [Google Scholar]
- Sharma, V.; Chandra, S.; Dwivedi, P.; Parturkar, M. Quinoa (Chenopodium quinoa Willd.): A nutritional healthy grain. Int. J. Adv. Res. 2015, 3, 725–736. [Google Scholar]
- Risi, J.C. The Chenopodium grains of the Andes: Inca crops for modern agriculture. Adv. Appl. Biol. 1984, 10, 145–216. [Google Scholar]
- Jiang, X.; Hansen, H.C.B.; Strobel, B.W.; Cedergreen, N. What is the aquatic toxicity of saponin-rich plant extracts used as biopesticides? Environ. Pollut. 2018, 236, 416–424. [Google Scholar]
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel natural food antimicrobials. Annu. Rev. Food Sci. Technol. 2012, 3, 381–403. [Google Scholar]
- Fiallos-Jurado, J.; Pollier, J.; Moses, T.; Arendt, P.; Barriga-Medina, N.; Morillo, E.; Arahana, V.; de Lourdes Torres, M.; Goossens, A.; Leon-Reyes, A. Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves. Plant Sci. 2016, 250, 188–197. [Google Scholar]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmockel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar]
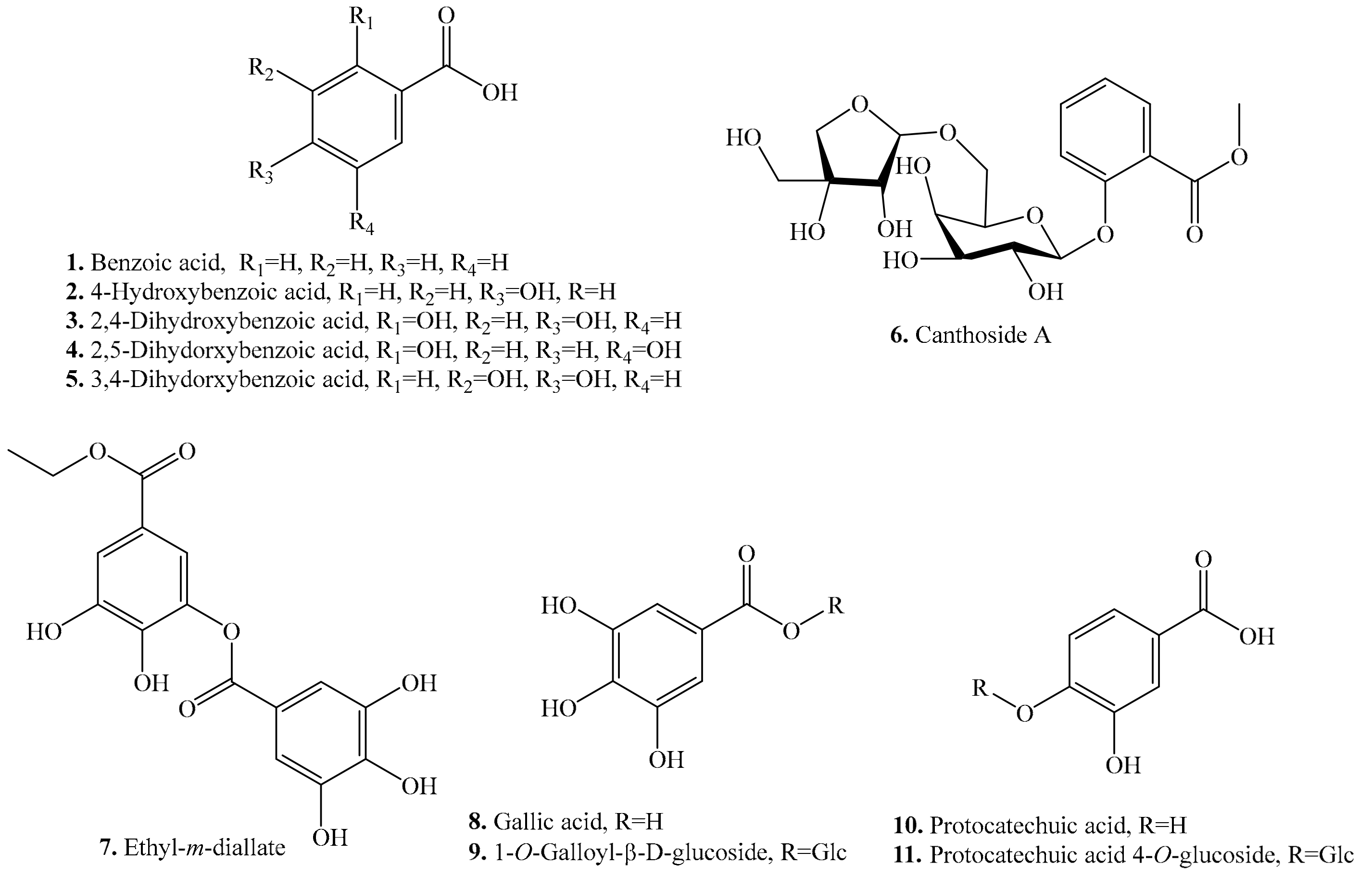
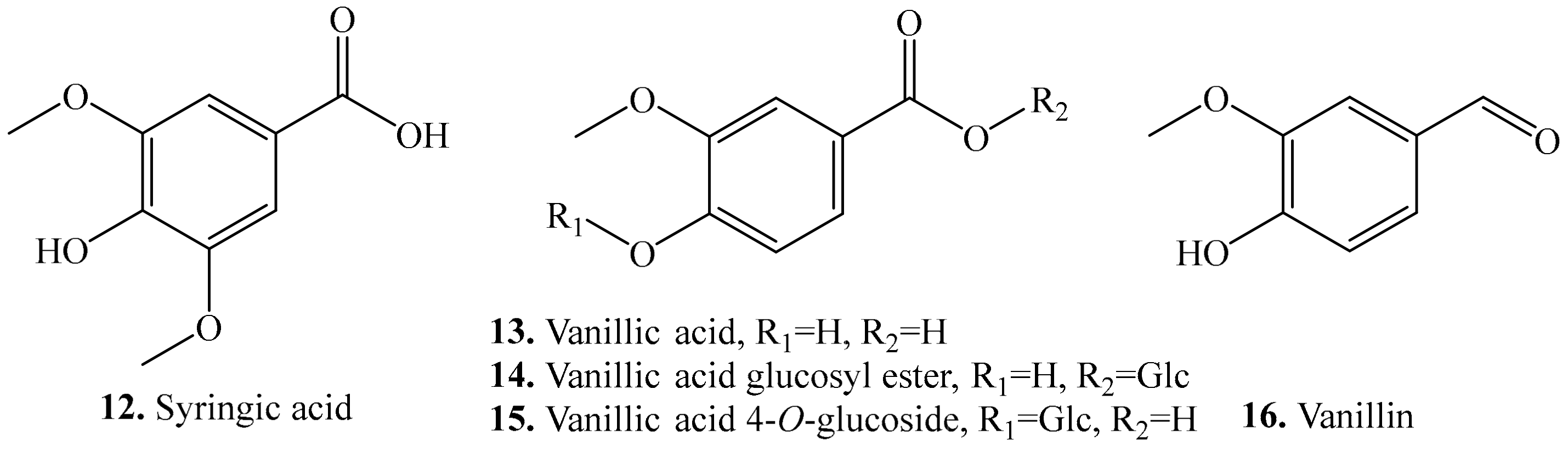
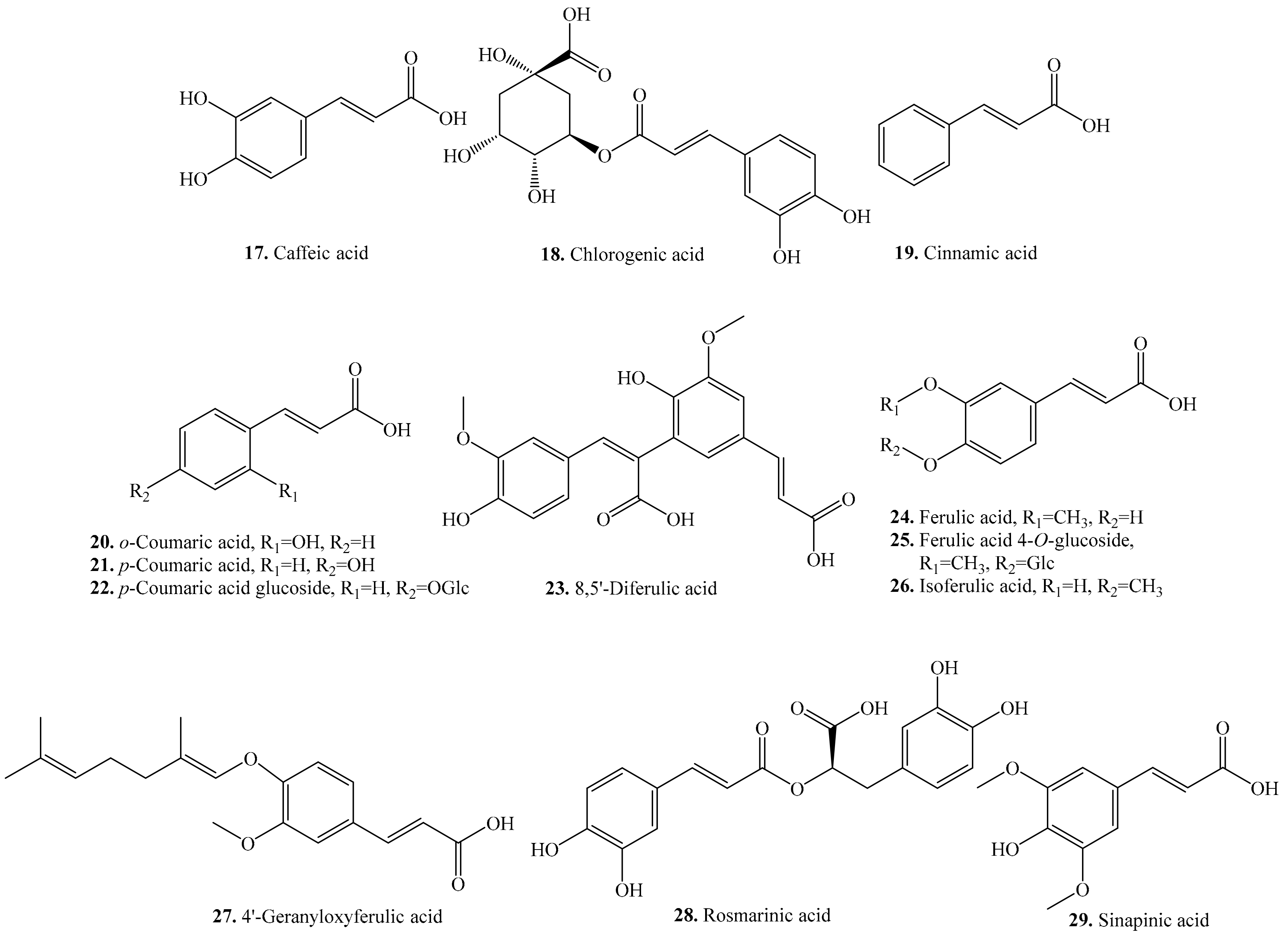
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Benzoic acid (1) | Leaves and flour | - | [27,33] |
| 4-Hydroxybenzoic acid = p-Hydroxybenzoic acid (2) | Seeds | - | [25] |
| Leaves and seeds | - | [27,34] | |
| Antimicrobial activity | [28] | ||
| Allelopathic effect | [30] | ||
| 2,4-Dihydroxybenzoic acid (3) | Seeds | - | [25,35] |
| 2,5-Dihydroxybenzoic acid (4) | Seeds | - | [35] |
| 3,4-Dihydroxybenzoic acid (5) | Seeds | - | [35] |
| Canthoside A (6) | Flour | - | [33] |
| Ethyl-m-digallate (7) | Flour | - | [33] |
| Antifeedant activity | [32] | ||
| Gallic acid (8) | Leaves, sprouts and seeds | - | [27,34] |
| Antioxidant activity | [31] | ||
| Antibacterial activity | [36] | ||
| 1-O-Galloyl-β-d-glucoside (9) | Seeds and flour | - | [33] |
| Protocatechuic acid (10) | Sprouts and seeds | - | [25,37] |
| Antioxidant activity | [31] | ||
| Anticancer activity | [38] | ||
| Antibacterial activity | [39] | ||
| Antiulcer activity | [40] | ||
| Antiageing activity | [41] | ||
| Anti-inflammatory, antiibrotic, antiatherosclerotic, hyperlipidemic, analgesic, hepatoprotective and nephroprotective activities | [42] | ||
| Antiviral activity | [43] | ||
| Protocatechuic acid 4-O-glucoside (11) | Flour | - | [33] |
| Antioxidant activity | [44] | ||
| Syringic acid (12) | Leaves and seeds | - | [25,26] |
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| Antimicrobial activity | [45] | ||
| Hepatoprotective effect | [46] | ||
| Anti-inflammatory activity | [47] | ||
| Vanillic acid (13) | Leaves and seeds | - | [25,34] |
| Allelopathic effect | [30] | ||
| Hepatoprotective effect | [46] | ||
| Antioxidant and antimicrobial activities, and inhibitory activity on COX-I and COX-II | [48] | ||
| Vanillic acid glucosyl ester (14) | Seeds | - | [49] |
| Vanillic acid 4-O-glucoside (15) | Seeds | - | [35] |
| Vanillin (16) | Seeds and flour | - | [25,33,35] |
| Antioxidant activity | [50] | ||
| Antimicrobial activity | [51] | ||
| Antidepressant activity | [52] | ||
| Anti-angiogenic, anti-inflammatory and anti-nociceptive activities | [53] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Caffeic acid (17) | Seeds | - | [25,34] |
| Antimicrobial activity | [29] | ||
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| Anti-apoptotic activity | [55] | ||
| Inhibitory activity on xanthine oxidase | [57] | ||
| Chlorogenic acid (18) | Leaves and seeds | - | [25,26] |
| Antimicrobial activity | [29] | ||
| Antioxidant activity | [31] | ||
| Anti-diabetic activity | [56] | ||
| Hemolytic activity | [58] | ||
| Neuroprotective effects | [59] | ||
| Anti-obesity activity | [60] | ||
| Antihepatotoxic effect | [61] | ||
| Antibiofilm activity | [62] | ||
| Cinnamic acid (19) | Sprouts and seeds | - | [34] |
| o-Coumaric acid (20) | Leaves and seeds | - | [25,26] |
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| p-Coumaric acid (21) | Leaves and seeds | - | [27,63] |
| Antilisterial activity | [64] | ||
| p-Coumaric acid glucoside (22) | Seeds | - | [35] |
| 8,5′-Diferulic acid (23) | Seeds | - | [25] |
| Ferulic acid (24) | Leaves, sprouts and seeds | - | [27,34,35] |
| Antimicrobial activity | [29] | ||
| Anti-apoptotic activity | [55] | ||
| Antioxidant activity | [65] | ||
| Cholesterol-lowering activity | [66] | ||
| Anti-thrombosis and anti-atherosclerosis effects | [67,68] | ||
| Anti-inflammatory activity | [69] | ||
| Anti-cancer activity | [70] | ||
| Ferulic acid 4-O-glucoside (25) | Flour | - | [33] |
| Isoferulic acid (26) | Seeds | - | [35] |
| Antioxidant activity | [71] | ||
| 4’-Geranyloxyferulic acid (27) | Seeds | - | [72] |
| Rosmarinic acid (28) | Seeds | - | [25] |
| Antimicrobial activity | [73] | ||
| Anti-inflammatory activity | [74] | ||
| Antioxidant activity | [75] | ||
| Antimutagenicity activity | [76] | ||
| Antiviral and anti-inflammatory effects | [77] | ||
| Sinapinic acid = trans-Sinapic acid (29) | Leaves | - | [27] |
| Seeds | - | [25] | |
| Antioxidant activity | [44] | ||
| Anxiolytic-like effects | [78] | ||
| Cerebral protective and cognition-improving effects | [79] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Acacetin (30) | Flour | - | [33] |
| Antioxidant activity | [83] | ||
| Spasmolytic and antinociceptive activities | [85] | ||
| Antiproliferative activity | [86] | ||
| Antiherpetic activity | [87] | ||
| Anticancer activity | [88] | ||
| Anti-inflammatory and antinociceptive activities | [89] | ||
| Hypouricemic effect | [90] | ||
| Isovitexin (31) | Sprouts | - | [34] |
| Anti-inflammatory and antioxidant activities | [84] | ||
| Anti-neoplastic effect | [91] | ||
| Anti-tumour activity | [92] | ||
| Neuroprotective effect | [93] | ||
| Anxiolytic property | [94] | ||
| Anti-Alzheimer‘s disease | [95] | ||
| Reduced postprandial blood glucose | [96] | ||
| Inhibitory effect on α-glucosidase | [97] | ||
| Inhibitory activity on rat lens aldose reductase | [98] | ||
| Orientin (32) | Seeds | - | [34] |
| Anticancer activity | [7] | ||
| Anti-inflammatory activity | [99] | ||
| Antioxidant activity | [100] | ||
| Antiapoptosis activity | [101] | ||
| Antithrombotic and antiplatelet activities | [102] | ||
| Antiproliferative activity | [103] | ||
| Vitexin (33) | Sprouts and seeds | - | [34] |
| Anti-carcinogenic effect | [91] | ||
| Anxiolytic property | [94] | ||
| Anti-Alzheimer’s disease property | [95] | ||
| Reduced postprandial blood glucose | [96] | ||
| Inhibitory effect on α-glucosidase | [97] | ||
| Induced apoptosis property | [104] | ||
| Agonist-induced regulation of vascular contractility | [105] | ||
| Antioxidant activity | [106] | ||
| Anti-inflammatory activity | [107] | ||
| Neuroprotective effect | [108] | ||
| Anti-depressant effect | [109] | ||
| Anti-convulsant effect | [110] | ||
| Antiepileptic effect | [111] | ||
| Anti-nociceptive effect | [112] | ||
| Anti-hypoxia/ischemia injury | [113] | ||
| Anti-ischemia/reperfusion injury | [114] | ||
| Anti-thyroid effect | [115] | ||
| Antimicrobial activity | [116] | ||
| Anti-viral effect | [117] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Isorhamnetin (34) | Leaves | - | [27,125] |
| Chemopreventive activity | [126] | ||
| Antituberculosis activity | [127] | ||
| Antioxidant activity | [128] | ||
| Anti-tumor activity | [129,130] | ||
| Inhibitory activity on farnesyl protein transferase | [131] | ||
| Anti-inflammatory activity | [132] | ||
| Anticoagulant activity | [133] | ||
| Kaempferol (35) | Leaves and seeds | - | [25,26,35] |
| Antibacterial activity | [36] | ||
| Antioxidant activitiy | [134] | ||
| Inhibit UVB-induced COX-2 expression | [135] | ||
| Anti-inflammatory activitiy | [136] | ||
| Stimulate osteoblastic activity | [137] | ||
| Kaempferol 3-glucoside (36) | Seeds | - | [35] |
| Kaempferol 3-galactoside (37) | Seeds | - | [35] |
| Kaempferol 3-O-(2,6-di-α-l-rhamnopyranosyl)-β-d-galactopyranoside (38) | Seeds | Antioxidant activity | [49,120,138,139] |
| Kaempferol 3-O-β-d-apiofuranosyl-(1→2)-O-α-l-rhamnopyranosyl(1→6)-β-d-galactopyranoside (39) | Seeds | Antioxidant activity | [49,120,138] |
| Kaempferol 3-O-β-d-apiofuranosyl-(l→2)-β-d-galactopyranoside (40) | Seeds | Antioxidant activity | [120,138] |
| Kaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside (41) | Seeds | Antioxidant activity | [120] |
| Kaempferol 3-O-β-d-glucuronic acid (42) | Seeds | Antioxidant activity | [49] |
| Kaempferol 3,7-dirhamnoside (43) | Seeds | - | [35] |
| Morin (44) | Sprouts and seeds | - | [34] |
| Anti-biofilm activity | [140] | ||
| Anti-inflammatory activity | [141] | ||
| Antitumor activity | [142] | ||
| Inhibitory effect on the expression of α1 (I) collagen | [143] | ||
| Antioxidant activity | [144] | ||
| Anticancer activity | [145] | ||
| Inhibited the increase of ROS and reduced the apoptotic cell | [146] | ||
| Neuroprotective effect | [147] | ||
| Hepatoprotective activity | [148] | ||
| Myricetin (45) | Seeds | - | [63] |
| Antibacterial activity | [36] | ||
| Antioxidant and prooxidant activities | [149] | ||
| Anticancer activity | [150] | ||
| Anti-inflammatory activity | [151] | ||
| Analgesic activity | [152] | ||
| Quercetin (46) | Leaves and seeds | - | [27,35,125,139] |
| COX-I and COX-II inhibition activity | [48] | ||
| Stimulate osteoblastic activity | [137] | ||
| Antioxidant and prooxidant activities | [149] | ||
| Anti-inflammatory activity | [153] | ||
| Cytotoxic activity | [154] | ||
| Quercetin 3-O-glucoside (47) | Flour | - | [33] |
| Quercetin-3-rutinoside (48) | Seeds | - | [35] |
| Quercetin 3-arabinoside (49) | Seeds | - | [35] |
| Quercetin 3-O-β-d-apiofuranosyl-(1→2)-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside (50) | Seeds | Antioxidant activity | [120,139] |
| Quercetin 3-O-(2,6-di-α-l-rhamnopyranosy)-β-d-galactopyranoside (51) | Seeds | Antioxidant activity | [49,120] |
| Quercetin 3-O-(2,6-di-O-α-rhamnopyranosyl)-β-glucopyranoside (52) | Seeds | - | [139] |
| Quercetin 3-O-β-d-apiofuranosyl-(1→2)-O-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside-3,4-dimethyl ether (53) | Seeds | Antioxidant activity | [49] |
| Rutin (54) | Leaves, sprouts and seeds | - | [27,34] |
| Anti-diabetic activity | [56] | ||
| Antioxidant activity | [155] | ||
| Antiulcerogenic activity | [156] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Hesperidin (55) | Seeds | - | [34] |
| Neuroprotective effect | [147] | ||
| Antioxidant and cytotoxic activities | [157] | ||
| Anti-inflammatory activity | [158] | ||
| Antifungal activity | [159] | ||
| Anti-proliferative and apoptotic activities | [160] | ||
| Protects the liver against drug-induced injury | [161] | ||
| Cardioprotective activity | [162] | ||
| Neohesperidin (56) | Seeds | - | [34] |
| Neuroprotective effect | [147] | ||
| Antifungal activity | [159] | ||
| Antioxidant activity | [163] | ||
| Induces cell apoptosis | [164] | ||
| Naringin (57) | Seeds | - | [35] |
| Antifungal activity | [159] | ||
| Antioxidative activity | [165] | ||
| Anti-osteoporosis activity | [166] | ||
| Anti-inflammatory activity | [167] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Catechin (58) | Seeds | - | [25] |
| Antioxidant activity | [149] | ||
| Antimutagenic activity | [169] | ||
| Anti-metastatic activity | [170] | ||
| Antifungal activity | [171] | ||
| Apoptosis-inducing activity | [172] | ||
| Epicatechin (59) | Seeds | - | [35] |
| Antimutagenic activity | [169] | ||
| Antioxidant activity | [173] | ||
| Antiproliferative activity | [174] | ||
| Epigallocatechin (60) | Seeds | - | [33,35] |
| Antioxidant activity | [168] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Biochanin A (61) | Seeds | - | [35] |
| Daidzein (62) | Seeds | - | [176] |
| Antioxidant activity | [177] | ||
| Enhance adipocyte differentiation and PPARγ transcriptional activities | [178] | ||
| Affected human nonhormone-dependent cervical cancer cells | [179] | ||
| Modulate in vitro rat uterine contractile activity | [180] | ||
| Anti-hypoxia activity | [181] | ||
| Antithrombotic and antiallergic activities | [182] | ||
| Chemoprotective activity | [183] | ||
| Inhibits bone loss in ovariectomized mice | [184] | ||
| Antiproliferative activity | [185,186] | ||
| Genistein (63) | Seeds | - | [176] |
| Antiproliferative activity on human breat cancer cells | [185,186] | ||
| Modulate in vitro rat uterine contractile activity | [180] | ||
| Antioxidant activity | [187] | ||
| Inhibitory activity on tyrosine-specific protein kinases | [188] | ||
| Antitumor activity | [189] | ||
| Cytotoxic activity and anticancer activities | [190] | ||
| Antitumor and antiangiogenic activities | [191,192] | ||
| Antibacterial activity | [193] | ||
| Inhibition of cyclooxygenase-2 activity | [194] | ||
| Antiprostate cancer activity | [195] | ||
| Antileukemic activity | [196] | ||
| Induction of quinone reductase activity | [197] | ||
| Induces growth arrest and suppresses telomerase activities | [198] | ||
| Prunetin (64) | Seeds | - | [25] |
| Anti-inflammatory activity | [199] | ||
| Puerarin (65) | Seeds | - | [35] |
| Antithrombotic and antiallergic activities | [182] | ||
| Anti-apoptosis activity | [200] | ||
| Antioxidant activity | [201] | ||
| Antihyperglycemic effect | [202] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| cis-Ascaridole (66) | Leaves | - | [204] |
| cis-Isoascaridole (67) | Leaves | - | [204] |
| Camphene (68) | Leaves | - | [204] |
| Camphor (69) | Leaves | - | [204] |
| trans-Carveol (70) | Leaves | - | [204] |
| p-Cymene (71) | Leaves | - | [204] |
| p-Mentha-1(7),8-diene (72) | Leaves | - | [204] |
| trans-p-Menth-2-en-1-ol (73) | Leaves | - | [204] |
| Penstebioside (74) | Flour | - | [33] |
| β-Pinene (75) | Leaves | - | [204] |
| Pinocarvone (76) | Leaves | - | [204] |
| α-Terpinene (77) | Leaves | - | [204] |
| γ-Terpinene (78) | Leaves | - | [204] |
| Antibacterial activity | [205] | ||
| Terpin-1-ol (79) | Leaves | - | [204] |
| α-Terpinyl acetate (80) | Leaves | - | [204] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Oleanolic acid (82) | Seeds and bran | - | [217,233] |
| Antimicrobial activity | [220,221] | ||
| Anti-HIV activity | [222] | ||
| Anti-inflammatory activity | [223] | ||
| Antioxidant activity | [225] | ||
| Antifertility activity | [226] | ||
| Antitumor activity | [227,228] | ||
| Inhibitory activities on serin protease and porcine pancreatic elastase | [232] | ||
| Methyl oleanate (90) | Bran | Anti-inflammatory activity | [234] |
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (91) | Seeds | - | [235,236] |
| 3-O-β-d-Glucopyranosyl oleanolic acid (92) | Seeds | - | [237] |
| Antidiabetogenic activity | [230] | ||
| Anti-inflammatory activity | [224] | ||
| Hemolytic activity | [231] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (93) | Flowers, fruits, seeds and bran | - | [11,235,236] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (94) | Flowers, fruits, seeds and bran | - | [11,217,238] |
| 3-O-β-d-Glucuropyranosyl oleanolic acid (95) | Seeds | - | [208,238] |
| 3-O-β-d-Glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (96) | Flowers, fruits, seeds and bran | Hemolytic activity | [11,208,217,236] |
| 3-O-β-d-Xylopyranosy-(1→3)-β-d-glucuronopyranosyl oleanolic acid (97) | Seeds | - | [237] |
| 3-O-β-d-Xylopyranosy(1→3)-6-methyl-β-d-glucuronopyranosyl oleanolic acid (98) | Seeds | - | [237] |
| 3-O-β-d-Xylopyranosyl-(1→3)-β-d-glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (99) | Flowers, fruits, seeds and bran | - | [11,217,237] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Hederagenin (83) | Seeds and bran | - | [215,216,217] |
| Inhibitory activity on serin protease, and porcine pancreatic elastase | [232] | ||
| Cytotoxic activity on P-388 mouse lymphoma, L-1210 mouse lymphomatic leukemia, HL-60 human promyelocytic leukemia and SNU-5 human stomach cancer cells | [248,249] | ||
| Haemolytic activity | [250] | ||
| Anti-inflammatory activity | [244] | ||
| Antidermatophytic activity | [251] | ||
| Antitrichomonas activity | [252] | ||
| Inducing apoptosis in human LoVo colon cells | [247] | ||
| 3-O-α-l-Arabinopyranosyl hederagenin (100) | Seeds | - | [208] |
| Molluscicidal activity | [140,253] | ||
| Cytotoxic activity on human carcinoma and melanoma cell lines DLD-1, PA1, A549, MCF7, PC3, and M4 | [241] | ||
| Antifungal activity | [242] | ||
| Leishmanicidic activity | [243] | ||
| Antidermatophytic activity | [251] | ||
| 3-O-α-l-Arabinopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (101) | Flowers, fruits, seeds and bran | - | [11,208,216,217] |
| Antidermatophytic activity | [251] | ||
| Anticomplementary activity | [254] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl hederagenin (102) | Seeds and bran | - | [208,216,238] |
| Cytotoxic activity on A549, SK-OV-3, SK-MEL-2, XF498 and HCT15 | [255] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranosyl hederagenin (103) | Bran | - | [216] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranoside hederagenin 28-O-β-d-glucopyranosyl ester (104) | Flowers, fruits, seeds and bran | - | [11,208,216,217,236,238,256] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (105) | Flowers, fruits, seeds and bran | - | [11,216,238] |
| 3-O-β-d-Glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (106) | Seeds | - | [256] |
| 3,23-Bis(O-β-d-glucopyranosyloxy) olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl-(1→3)-α-l-arabinopyanosyl ester (107) | Seeds | - | [257] |
| 3-O-β-d-Glucuronopyranosyl hederagenin 28-O-β-glucopyranosyl ester (108) | Flowers, fruits, seeds and bran | - | [11,217,238] |
| 3-O-β-d-Xylopyranosyl-(1→3)-β-d-glucuronopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (109) | Bran | - | [217] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl spergulagenic acid 28-O-β-d-glucopyranosyl ester (110) | Seeds | - | [256] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl spergulagenic acid (111) | Bran | - | [217] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl spergulagenic acid 28-O-β-d-glucopyranosyl ester (112) | Seeds | - | [256] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Serjanic acid (85) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11,217] |
| 3-O-α-l-Arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (113) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester = 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl-30-O-methyl spergulagenate 28-O-β-d-glucopyranosyl ester (114) | Flowers, fruits, seeds and bran | - | [11,236,238] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (?) = 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl-30-O-methyl spergulagenate 28-O-β-d-glucopyranosyl ester (115) | Flowers, fruits, seeds and bran | - | [11,217,238,256] |
| 3-O-β-d-Glucuronopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (116) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Phytolaccagenic acid (86) | Bran | - | [216,217] |
| Bran | Anti-inflammatory activity | [234] | |
| 3-O-α-l-Arabinopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (117) | Flowers, fruits, seeds and bran | - | [11,208,216,217,238] |
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (118) | Seeds | - | [235,236,256] |
| 3-O-β-d-Galactopyranosyl-(1→3)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (119) | Seeds | - | [238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl phytolaccagenic acid (120) | Seeds | Antifungal activity | [208,238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (121) | Flowers, fruits, seeds and bran | - | [11,208,216,217,235,236,238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranoside phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (122) | Flowers, fruits, seeds and bran | - | [11,216,217,238] |
| 3-O-β-d-Glucopyranosyl(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranoside phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (123) | Flowers, fruits, seeds and bran | - | [11,217,235,238] |
| 3-O-β-d-Glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (124) | Flowers, fruits, seeds and bran | - | [11,256] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→2)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (125) | Seeds | - | [235] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Gypsogenin = 3β-Hydroxy-23-oxo-olean-12-en-28-oic acid (87) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester = 3β-[(O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl)oxy]-23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (126) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3β-Hydroxy-27-oxo-olean-12-en-28-oic acid (88) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 27-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester = 3β-[(O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl)oxy]-27-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranoside (127) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3β,23,30-Trihydroxy-olean-12-en-28-oic acid (89) | Flowers, fruits, seeds and bran | - | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 3β,23,30-trihydroxyolean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (128) | Flowers, fruits, seeds and bran | - | [11,214] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Tetracyclic triterpenoids | |||
| Citrostadienol (129) | Seeds | - | [261] |
| Anticomplementary activity | [262] | ||
| Gramisterol (130) | Seeds | - | [261] |
| Anti-cancer activity on mouse leukemic cell line WEHI-3 | [8] | ||
| 24-Methylene-cycloartenol (131) | Seeds | - | [261] |
| Parkeol (132) | Seeds | - | [261] |
| Pentacyclic triterpenoids | |||
| α-Amyrin (133) | Seeds | - | [215] |
| Antibacterial activity | [263] | ||
| Antidiabetic effect | [264] | ||
| Antioxidant activity | [265] | ||
| Inhibitory activity against human oxidosqualene cyclase | [266] | ||
| β-Amyrin (134) | Seeds | - | [215] |
| Antibacterial activity | [263] | ||
| Antioxidant activity | [265] | ||
| Inhibitory activity against human oxidosqualene cyclase | [266] | ||
| Antifeedant and growth regulating activities | [267] | ||
| Insecticidal activity | [268] | ||
| Echinocystic acid (135) | Seeds | - | [215] |
| Erythrodiol (136) | Seeds | - | [215,261] |
| Antibacterial activity | [269] | ||
| Melanogenesis-inhibitory activity | [270] | ||
| Protecting the cardiovascular system | [271] | ||
| Antiproliferative and apoptotic activity | [272] | ||
| 3β,23-Dihydroxy-olean-12-ene-28,30-dioic acid (137) | Seeds | - | [208] |
| 2β,3β,23-Trihydroxy-olean-12-ene-28,30-dioic acid 30-methyl ester (138) | Bran | - | [216] |
| Queretaroic acid (139) | Seeds | - | [215] |
| Ursolic acid (140) | Seeds | - | [215] |
| Spasmolytic and antinociceptive activities | [85] | ||
| Cytotoxic activity | [270] | ||
| Anticancer activity | [273,274] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| α-Tocopherol (141) | Seeds | - | [283] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| β-Tocopherol (142) | Seeds | - | [37] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| γ-Tocopherol (143) | Seeds | - | [283] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| δ-Tocopherol (144) | Seeds | - | [37] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| α-Tocotrienol (145) | Seeds | - | [275] |
| Antioxidant and anti-inflammatory activities | [282] | ||
| β-Tocotrienol (146) | Seeds | - | [275] |
| Antioxidant and anti-inflammatory activities | [282] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Cholesterol (147) | Seeds | - | [285,288] |
| 20-Hydroxyecdysone (148) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| Insecticidal activity | [290] | ||
| 20,26-Dihydroxyecdysone (149) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 2-Deoxy-20-hydroxyecdysone (150) | Seeds | - | [22] |
| 3-epi-2-Deoxy-20-hydroxyecdysone (151) | Seeds | - | [22] |
| 2-Deoxy-20,26-dihydroxyecdysone (152) | Seeds | - | [22] |
| 20-Hydroxyecdysone 22-glycolate (153) | Seeds | Antioxidant activity and inhibitory activity on collagenase | [287] |
| 24,25-Dehydroinokosterone (154) | Seeds | - | [22] |
| 25,27-Dehydroinokosterone (155) | Seeds | - | [22] |
| Lathosterol (156) | Seeds | - | [261] |
| Polypodine B (157) | Seeds | - | [22] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Brassicasterol (158) | Seeds | - | [292] |
| Campestanol (159) | Seeds | - | [261] |
| Campesterol = Δ5-Campesterol (160) | Seeds | - | [261,285,288] |
| Antiangiogenic activity | [291] | ||
| Δ7-Campesterol (161) | Seeds | - | [285,288] |
| Dacrysterone (162) | Seeds | - | [22] |
| Episterol (163) | Seeds | - | [261] |
| Ergost-7-en-3β-ol = Δ7-Ergostenol (164) | Seeds | - | [261] |
| Kancollosterone (165) | Seeds | - | [293] |
| Makisterone A (166) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 24-epi-Makisterone A (167) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 24(28)-Dehydromakisterone A (168) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 26-Hydroxy-24(28)-dehydromakisterone A (169) | Seeds | Antioxidant activity, inhibitory activity on collagenase | [287] |
| 5β-Hydroxy-24(28)-dehydromakisterone A (170) | Seeds | - | [22] |
| 24-Methyl-20,26-dihydroxyecdysone (171) | Seeds | - | [22] |
| Seeds | Antioxidant activity | [287] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Δ5-Avenasterol = Δ5,24(28)-Avenasterol (172) | Seeds | - | [261,285,288] |
| Δ7-Avenasterol = Δ7,24(28)-Avenasterol (173) | Seeds | - | [261] |
| Makisterone C (174) | Seeds | - | [22] |
| Sitostanol (175) | Seeds | - | [261] |
| β-Sitosterol (176) | Seeds | - | [285,288] |
| Insecticidal activity | [268] | ||
| Anti-inflammatory activity | [294] | ||
| Anti-oxidant activity | [295] | ||
| Antidiabetic activity | [296] | ||
| Inducing apoptosis | [302] | ||
| Hypocholesterolemic activity | [303,304] | ||
| Angiogenic effect | [305] | ||
| Genotoxicity effect | [306] | ||
| Anthelminthic and Anti-mutagenic activity | [307] | ||
| Immunomodulatory activity | [308] | ||
| Neuroprotection effect | [309] | ||
| Stigmast-4-en-3-one (177) | Seeds | [292] | |
| Stigmast-4,22-dien-3-one (178) | Seeds | [292] | |
| Stigmast-8-en-3-ol (179) | Seeds | - | [261] |
| Δ7-stigmastenol (180) | Seeds | - | [261] |
| Stigmasterol = Δ5-Stigmasterol (181) | Seeds | - | [261] |
| Anti-inflammatory activity | [297] | ||
| Anti-tumor activity | [298] | ||
| Antifungal activity | [299] | ||
| Anti-hypercholestrolemic activity | [300] | ||
| Cytotoxicity activity | [301] | ||
| Anti-osteoarthritic activity | [310] | ||
| Δ7-Stigmasterol (182) | Seeds | - | [285,288] |
| Seeds | - | [261] |
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Amaranthin (183) | Seeds | - | [316] |
| Betanin (184) | Seeds | - | [35] |
| Antioxidant activity | [317] | ||
| Isobetanin (185) | Seeds | - | [35] |
| Betaine (186) | Seeds | - | [35] |
| 3-Carboxy-1-(2-sulfoethyl)-pyridinium (187) | Seeds | - | [313] |
| Dopaxanthin (188) | Seeds | - | [316] |
| Antioxidant activity | [318] | ||
| Indicaxanthin (189) | Seeds | - | [316] |
| Miraxanthin V (190) | Seeds | - | [316] |
| Trigonelline (191) | Seeds | - | [313] |
| Anti-invasive activity | [319] | ||
| Hypoglycemic effect | [320] | ||
| Trigonelline glucosylester (192) | Seeds | - | [313] |
| Trigonelline methylester (193) | Seeds | - | [313] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules 2019, 24, 2512. https://doi.org/10.3390/molecules24132512
Lin M, Han P, Li Y, Wang W, Lai D, Zhou L. Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules. 2019; 24(13):2512. https://doi.org/10.3390/molecules24132512
Chicago/Turabian StyleLin, Minyi, Peipei Han, Yuying Li, Weixuan Wang, Daowan Lai, and Ligang Zhou. 2019. "Quinoa Secondary Metabolites and Their Biological Activities or Functions" Molecules 24, no. 13: 2512. https://doi.org/10.3390/molecules24132512
APA StyleLin, M., Han, P., Li, Y., Wang, W., Lai, D., & Zhou, L. (2019). Quinoa Secondary Metabolites and Their Biological Activities or Functions. Molecules, 24(13), 2512. https://doi.org/10.3390/molecules24132512