Abstract
4-(4-Aminophenyl)-1-thia-4-azaspiro[4.5]decan-3-one 1 was prepared and allowed to react with nitrogen nucleophiles to give the corresponding hydrazones 2–4. Further, compound 1 underwent diazotization and afforded the parallel hydrazono derivative 5; moreover, compound 1 refluxed with active methylene derivatives yielded the corresponding aminospirothiazolo pyridine–carbonitrile derivative 6 and spirothiazolopyridinone–carbonitrile derivative 7. Condensation of spirothiazolidine 1 with 4-chlorobenzaldehyde gave the corresponding spiro arylidiene derivative 8, which was utilized as a component of Micheal addition to react with excess of nitrogen nucleophiles to yield novel ring frameworks 4-(3′-(4-chlorophenyl)–spiro [cyclohexane-1,5′-pyrazolo[3,4-d]thiazol]-6′(1′H)-yl)aniline (9) and 4-(3′-(4-chlorophenyl)-6′H- spiro[cyclohexane-1,5′-thiazolo[5,4-d]isoxazol]-6′-yl)aniline (10). Finally, when spirothiazolo pyridinone–carbonitrile derivative 7 sodium salt generated in situ was reacted with different alkyl halides, it produced the corresponding N-derivatives 12–16. Three compounds, 6, 14, and 16, showed high significantly anticancer activities compared with Doxorubicin® (positive control) against human breast carcinoma (MCF-7) and human liver carcinoma (HepG-2) cell lines. On the other hand, compounds 6 and 9 showed higher therapeutic indices for both of alpha-amylase inhibitor and alpha-glucosidase inhibitor than the other tested compounds compared with the antidiabetic Acarbose (positive control).
1. Introduction
Cancer is a horrible disease, which concerns the medical community all over the world and is likely to become the principal cause of death in most developed countries by 2030 [1,2,3,4,5,6]. Development of resistance is a key related issue commonly observed in drug therapy [7,8,9,10]. Despite the great efforts in cancer research, resistance is currently insufficient [11,12,13,14,15,16]. Therefore, there is a crucial need to logically design novel, molecularly targeted antineoplastic treatments, which are more selective, preferably less toxic, and eventually more effective than conventional therapies [17].
Diabetes mellitus is a metabolic unrest primarily characterized by high blood glucose level [18]. Diabetes mellitus was initially counted as a disease of slight significance to world health, but now it is considered as an epidemic and one of the major principal threats to human health in the 21st century. The World Health Organization postulates that the Middle East area will possess the highest prevalence rate of diabetes, growing at 163% by the year 2030 [19]. The dramatic increase in the number of diabetic patients is due to changes in lifestyle and behavioral factors, such as sedentary lifestyle, excessive feeding, and obesity. After 1995, a number of new classes of pharmacological agents [20,21,22] were introduced in the market. The classes currently available are insulin and insulin analogues for type-1/type-2 diabetes [23], sulfonylureas [24,25], glinides [22], biguinides [26,27], glitazones (thiazolidine diones) [28], and α–glycosidase inhibitors for type-2 diabetes (T2D) [29]. Scientific researchers have suggested there is a common link between cancer and diabetes that is related to sugar. Research suggests people who have diabetes have a higher risk of developing various forms of cancers [30]. Sugar plays a vital role in how our bodies process nutrients needed for energy and how our cells are fed [31]. Research has found cancer cells thrive on sugar, especially processed sugars like high fructose corn syrup and white sugar [32].
In search of bioactive entities, we found that compounds comprising thiazole moiety which is easily metabolized inside the body are a base structure in many synthetic drugs that have been applied in the therapy of some diseases [33,34,35,36,37,38,39]. The chemistry of heterocyclic candidates incorporating the thiazole nucleus was particularly interesting due to their potential application in medicinal chemistry as antidiabetic [40,41], anti-inflammatory [42,43], antibacterial [42,44,45,46], anticonvulsant [47,48,49], anticancer [50,51,52,53,54], and anti-HIV [55,56,57,58,59]. Otherwise, heterocyclic analogs containing a Spiro-thiazolidines skeleton have a significant place in chemotherapy due to them exhibiting a broad spectrum of potent pharmacological activities [60,61,62,63,64,65].
As a continuation to our program directed towards the synthesis of useful chemotherapeutic agency [66,67,68,69,70,71,72,73,74,75,76,77,78], we decided to explore other spirothiazolidinone derivatives for multiple potential biological activities. The current study was designed to locate novel important scaffold spiro (cyclohexane-thiazolidine) derivatives that can inhibit human cancer targets MCF-7 and HepG-2. Further, we studied the antidiabetic activity intends to screen alpha-amylase and alpha-glucoidase inhibition activity. Hopefully, the newly synthesized spiro derivatives will have the capability to target the disease without harming healthy tissue.
2. Results
2.1. Chemistry
In continuation of our earlier interest on the synthesis of a wide range of applicable heterocyclic compounds [11,12,13,14,15,16,64,65,66,67,68,69,70,71,72,73,74,75,76,77], the starting compound 4-(4-aminophenyl)-1-thia-4-azaspiro [4.5]decan-3-one (1) was prepared by condensation between cyclohexanone, p-phenyllenediamine, and thioglycolic acid in dry toluene. Compound 1 was reacted with nitrogen nucleophiles such as hydrazine hydrate, phenylhydrazine, and/or thiosemicarbaxzide to give the hydrazones 2–4, respectively. The IR and 13C-NMR spectra of compounds 2–4 displayed the absence of the C=O group and the presence of a new signal discriminatory for NH2 and NH groups in both IR and 1H-NMR spectra, in addition to the presence of the C=S signal in 13C-NMR for compound 4 at δ 179.69 ppm (Scheme 1; c.f. experimental). Further, compound 1 underwent diazotization condition in the presence of 4-nitroaniline, which afforded the hydrazono derivative 4-(4-aminophenyl)-2-(2-(4-nitrophenyl) hydrazono)-1-thia-4-azaspiro[4.5]decan-3-one (5) in good yield. The 1H-NMR spectrum for hydrazono derivative 5 exhibited the absence of two signals specified to active thiazolomethylene protons and the presence of new signals distinguished for the NH group at δ 7.33 ppm (s, 1H; D2O exchangeable); (Scheme 1; c.f. experimental). Furthermore, compound 1 was refluxed with active methylene derivatives, namely, malononitrile and/or ethyl cyanoacetate in the presence of both 4-chlorobenzaldehyde and ammonium acetate, which yielded the corresponding aminospirothiazolo pyridine–carbonitrile derivative 6 and spirothiazolo pyridinone–carbonitrile derivative 7, respectively. The IR spectrum of compound 6 showed absorption bands characteristic for new C≡N and NH2 groups at 3122 and 2210 cm−1, respectively, in addition to the disappearance of the signal characteristic for the C=O group. The 1H-NMR spectrum for derivative 6 showed absence of signals specified to active thiazolomethylene protons in addition to the appearance of new signals for the new NH2 group at δ 8.61 ppm, exchangeable with D2O. Furturmore, 13C-NMR for compound 6 showed a specified signal for the C≡N group at δ 114.69 ppm with the absence of the signal for the C=O group (Scheme 1; c.f. experimental).
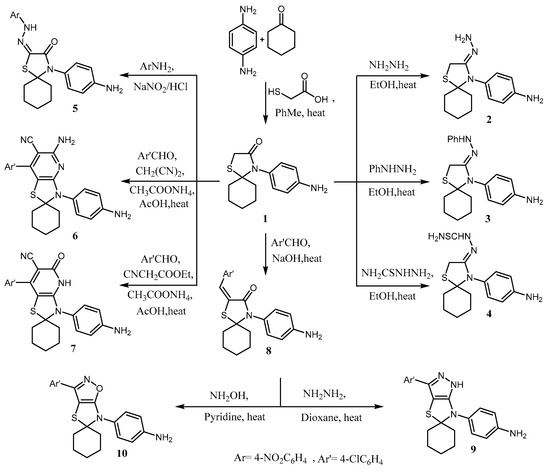
Scheme 1.
Synthesis of compounds 1–10.
The structure of derivative 7 was confirmed on the basis of its spectral data. The IR spectrum for 7 showed the absorption band characteristic for (C=O; amide), (C≡N), and (NH; amide) at 1658, 2214, and 3119 cm−1, respectively. The 1H-NMR of compound 7 revealed a new D2O exchangeable signal at δ 7.01 due to the NH group (Scheme 1; c.f. experimental).
Condensation of spirothiazolidine 1 with 4-chlorobenzaldehyde gave the corresponding arylidiene derivative 8 in quantitative yields. Arylidiene derivative 8 was confirmed by the presence of a new singlet signal at δ 8.01 ppm in the 1H-NMR spectrum corresponding to excocyclic vinylic proton (Scheme 1; c.f. experimental). The arylidiene of spiro thiazolidine 8 contained the α,β-unsaturated function system which has been used as a component of Micheal addition to react with excess of nitrogen nucleophiles, namely, hydrazine hydrate and/or hydroxyl amine hydrochloride to yield novel ring frameworks 4-(3′-(4-chlorophenyl)-spiro[cyclohexane-1,5′- pyrazolo[3,4-d]thiazol]-6′ (1′H)-yl)aniline 9 and 4-(3′-(4-chlorophenyl)-6′H-spiro[cyclohexane-1, 5′-thiazolo [5,4-d]isoxazol]-6′-yl)aniline 10 (Scheme 1; c.f. experimental). The 1H-NMR spectra for compounds 9 and 10 were devoid of the excocyclic vinylic proton signal, which was present in its precursor, a confirmed heterocyclization reaction. Additionally, IR and 13C-NMR spectra free from the C=O group signal and agree with the proposed structures (Scheme 1; c.f. experimental).
On the other hand, the spirothiazolopyridinone–carbonitrile derivative 7 was utilized as a key starting material for the synthesis of novel heterocyclic ring systems. The pyridinone derivative 7 was reacted with a mixture of PCl5/POCl3 on water bath afforded the corresponding chloro derivative 11. Compound 11 was elucidated by the absence of the (C=O) group in both the IR and 13C-NMR spectra. In addition to the absence of the NH signal in both the IR and 1H-NMR spectra, its mass spectrum afforded the molecular ion peak M+ at m/z 466 as well as the presence of an isotopic pattern of 2 chlorine atoms in agreement with its molecular formula (Scheme 2; c.f. experimental).
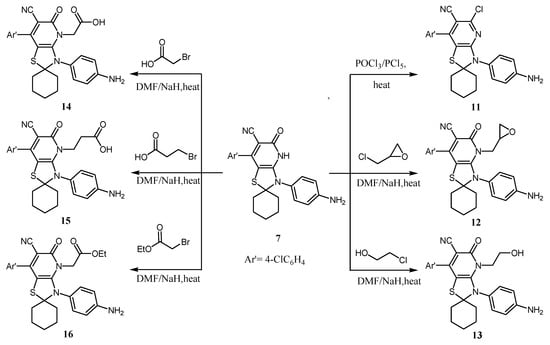
Scheme 2.
Synthesis of compounds 11–16.
Finally, spirothiazolopyridinone–carbonitrile derivative 7 sodium salt generated in situ was reacted with epichlorohydrine, chloroethanol, bromoacetic acid, bromopropionic acid, and/or ethyl bromoacetate and produced the corresponding N-derivatives, 12–16, respectively (Scheme 2; c.f. experimental). The 13C-NMR and IR spectra for the abovementioned N-derivatives 12–16 detected the attack position which was on the nitrogen and not oxygen atom. Further, NMR spectra exhibited signals for oxiran-2-ylmethyl, hydroxyethyl, acetic acid, propionic acid, and ethyl acetate groups (Scheme 2; c.f. experimental).
2.2. Anticancer
Anticancer bioassays were carried out to test compounds 4, 6, 8, 12, 14, and 16 towards the MCF-7 and HepG-2 cell lines. The HepG-2 and MCF-7 cell lines’ inhibition activities are given in Figure 1 and Figure 2. The concentration required for 50% inhibition of cell viability (IC50) was calculated from the graph shown the relation between concentrations of tested compound (µg/mL) and cell viability %, and the results are given in Table 1 (for details see Supplementary Materials). It was obvious that at different concentrations (3.90, 7.8, 15.6, 31.25, 62.5, 125, 250, and 500 µg/mL), compounds 6, 14, and 16 showed higher therapeutic indices for both breast cell line MCF-7 and liver cell line HepG-2 than the other tested compounds, and the results were compared with the anticancer drug Doxorubicin® (positive control).
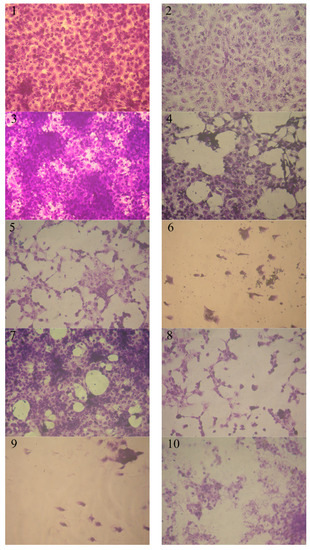
Figure 1.
Effect of various concentrations of tested compounds on HepG-2 cell inhibition activity. (1) Control HepG-2 (2) compound 6 (7.8 µg/mL), (3) compound 14 (7.8 µg/mL), (4) compound 14 (125 µg/mL), (5) compound 6 (125 µg/mL), (6) compound 6 (500 µg/mL), (7) compound 12 (7.8 µg/mL), (8) compound 12 ( 125 µg/mL), (9) compound 16 (500 µg/mL), and (10) compound 14 (500 µg/mL).
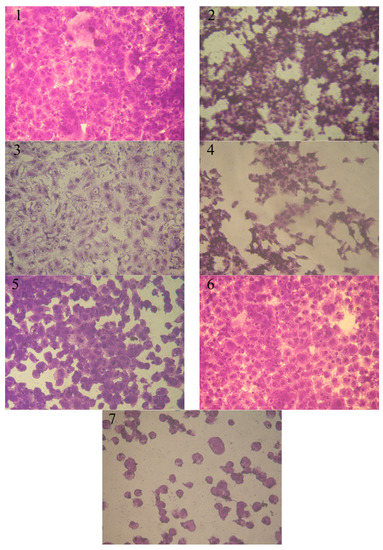
Figure 2.
Effect of various concentrations of tested compounds on MCF-7 cell Inhibition activity. (1) Control MCF-7 (2) compound 14 (500 µg/mL), (3) compound 6 (7.8 µg/mL), (4) compound 6 (500 µg/mL), (5) compound 12 (7.8 µg/mL), (6) compound 12 (31.25 µg/mL), and (7) compound 12 (500 µg/mL).

Table 1.
Effect of treatment at various concentrations of prepared compounds on MCF-7 and HepG-2 cell cytotoxicity.
2.3. Antidiabetic
Antidiabetic bioassays were carried out to test compounds 1, 2, 6, 7, 8, 9, 13, and 16 as alpha-amylase inhibitor and alpha-glucosidase inhibitor. The concentrations of the tested compounds, which exhibited 50% of their activities at the same condition (IC50), were determined, and the results are given in Table 2. It was obvious that by comparison with the antidiabetic Acarbose (positive control) at different concentrations (7.81, 15.63, 31.25, 62.5, 125, 250, 500, and 1000 µg/ml), compounds 6 and 9 showed a higher therapeutic effect for both alpha–amylase inhibitor and alpha–glucosidase inhibitor than the other tested compounds. Further, both compounds 6 and 9 showed higher inhibition against alpha-glucosidase than alpha-amylase.

Table 2.
Effect of treatment at various concentrations of prepared compounds on alpha-amylase inhibitor and alpha-glucosidase inhibitor.
3. Materials and Methods
3.1. General Information
Melting points were measured using an Electro-Thermal IA 9100 digital melting point apparatus (Büchi, Flawil, Switzerland) and are uncorrected. Infrared spectra were recorded on a Perkin-Elmer 1600 FTIR (Perkin-Elmer, Waltham, MA, USA) discs. NMR spectra were determined on a Jeol-Ex-500 NMR spectrometer (JEOL, Tokyo, Japan), and chemical shifts were expressed as part per million; (δ values, ppm) against TMS as internal reference, National Research Center, Cairo, Egypt. The mass spectra were run at 70 eV with a Finnigan SSQ 7000 spectrometer (Thermo Electron Corporation, Madison, WI, USA) using EI, and the values of m/z are indicated in Dalton. Elemental analyses were performed on a Perkin-Elmer 2400 analyzer (Perkin-Elmer) and were found within the accepted range (±0.30) of the calculated values. Reaction monitoring and verification of the purity of the compounds was done by TLC on silica gel precoated aluminum sheets (type 60 F254, Merck, Darmstadt, Germany). All solvents and chemical reagents were purchased from Aldrich (Munich, Germany).
3.2. Chemistry
3.2.1. 4-(4-aminophenyl)-1-thia-4-azaspiro[4.5]decan-3-one (1)
A mixture of cyclohexanone (0.98 mL, 0.01 mol), p-phenyllenediamine (0.01 mol), and thioglycolic acid (0.92 mL, 0.01 mol) in dry toluene (50 mL) was refluxed for 10 h. The solution was concentrated and the formed solid was filtered off, dried, and crystallized from dioxane/methanol to give compound 1. Pale yellow powder, Yield 92%; m.p.170–172 °C; IR (KBr, υ, cm−1): 3230 (NH2), 1666 (C=O); 1H-NMR (DMSO): δ (ppm) 1.52–2.00 (m, 10H, 5CH2), 3.41 (d, J = 8.06 Hz, 1H, CH2), 3.48 (d, J = 8.05 Hz, 1H, CH2), 3.87 (s, 2H, NH2; D2O exchangeable), 7.11–7.67 (m, 4H, Ar-H); 13C-NMR spectrum (CDCl3, δ ppm): 22.41, 25.64, 35.28, 35.61, 80.49, 117.08, 126.95, 131.38, 140.04, 169.98; MS, m/z (%): 262 (M+, 100); Analysis calc. for C14H18N2OS (262.37):C, 64.09; H, 6.92; N, 10.68; S, 12.22. Found: C, 63.84; H, 6.66; N, 10.42; S, 11.97.
3.2.2. General procedure for the synthesis of compounds 2 and 3
A mixture of compound 1 (0.01 mol) and hydrazine hydrate or phenylhydrazine (0.02 mol) was refluxed in absolute ethanol (30 mL) for 6 h. The obtained solid was filtered off, dried, and crystallized from the proper solvent to give compounds 2 and 3.
4-(3-hydrazono-1-thia-4-azaspiro[4.5]decan-4-yl)aniline (2)
From dioxane. Pale yellow fine crystals, Yield 83%; m.p. 263–265 °C; IR (KBr, υ, cm−1): 3343, 3232 (2NH2); 1H-NMR (DMSO): δ (ppm) 1.54–1.90 (m, 10H, 5CH2), 3.57 (d, J = 6.60 Hz, 1H, CH2), 3.58 (d, J = 6.61 Hz, 1H, CH2), 4.24 (s, 2H, NH2; D2O exchangeable), 5.24 (s, 2H, NH2; D2O exchangeable), 6.55–6.77 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.46, 22.71, 31.96, 35.28, 78.82, 116.09, 125.43, 134.93, 142.48, 151.30; MS, m/z (%): 276 (M+, 34); Analysis calc. for C14H20N4S (276.14): C, 60.84; H, 7.29; N, 20.27; S, 11.60. Found: C, 60.58; H, 7.02; N, 20.02; S, 11.36.
4-(3-(2-phenylhydrazono)-1-thia-4-azaspiro[4.5]decan-4-yl)aniline (3)
From methanol. Brown needle fine crystals, Yield 79%; m.p. 280–282 °C; IR (KBr, υ, cm−1): 3342 (NH2), 3226 (NH); 1H-NMR (DMSO): δ (ppm) 1.53‒1.91 (m, 10H, 5CH2), 3.58 (d, J = 6.62 Hz, 1H, CH2), 3.63 (d, J = 6.61 Hz, 1H, CH2), 5.28 (s, 2H, NH2; D2O exchangeable), 6.27(s, 1H, NH; D2O exchangeable), 6.57‒6.78(m, 4H, Ar-H), 7.28‒7.59 (m, 5H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.42, 25.67, 33.66, 35.24, 78.78, 115.19, 116.05, 124.00, 125.39, 128.82, 134.89, 142.44, 143.59, 151.33; MS, m/z (%): 352 (M+, 47). Analysis calc. for C20H24N4S (352.17): C, 68.15; H, 6.86; N, 15.89; S, 9.10. Found: C, 67.89; H, 6.61; N, 15.63; S, 8.82.
3.2.3. 2-(4-(4-aminophenyl)-1-thia-4-azaspiro[4.5]decan-3-ylidene)hydrazine-1-carbothioamide (4)
Two milliliters of concentrated HCl was added to a solution of compound 1 (2.6 g, 0.01 mol), thiosemicarbazide (0.01 mol) in absolute ethanol (30 mL). The reaction mixture was refluxed for 3 h, then left to cool and the formed solid was filtered off, washed with water, and crystallized from dioxane to give compound 4. Pale yellow needle fine crystals, Yield 67%; m.p. 255–257 °C; IR (KBr, υ, cm−1): 3332, 3226 (2NH2), 3121 (NH); 1H-NMR (DMSO): δ (ppm) 1.54‒1.90 (m, 10H, 5CH2), 3.59 (d, J = 6.61 Hz, 1H, CH2), 3.62 (d, J = 6.61 Hz, 1H, CH2), 5.27(s, 2H, NH2; D2O exchangeable), 6.64‒6.86 (m, 4H, Ar-H), 7.11 (s, 2H, NH2; D2O exchangeable), 8.21 (s, 1H, NH; D2O exchangeable); 13C-NMR spectrum (DMSO, δ ppm): 22.48, 25.73, 33.71, 35.29, 78.81, 116.11, 125.44, 134.95, 142.49, 152.85, 179.69; MS, m/z (%): 335 (M+, 22). Analysis calc. for C15H21N5S2 (335.12): C, 53.70; H, 6.31; N, 20.88; S, 19.11. Found: C, 53.44; H, 6.07; N, 20.63; S, 18.85.
3.2.4. 4-(4-aminophenyl)-2-(2-(4-nitrophenyl)hydrazono)-1-thia-4-azaspiro[4.5]decan-3-one (5)
A solution of hydrochloric acid (6 mL); 4-nitroaniline (0.01 mol) and an aqueous solution (3 mL) of sodium nitrite (0.72 g, 0.015 mol) was stirred at 0 °C for 1 h, followed by addition of (0.01 mol) of compound 1 in 10 mL pyridine, and stirring was continued at 0 °C for 2 h. The resulting product was filtered off, washed with water, dried, and crystallized from dioxane to give compound 5. White powder, m.p. over 300 °C.; IR (KBr, υ, cm−1): 3211 (NH2), 3111 (NH), 1651 (C=O); 1H-NMR (DMSO): δ (ppm) 1.52‒1.92 (m, 10H, 5CH2), 5.30 (s, 2H, NH2; D2O exchangeable), 6.56‒6.81 (m, 4H, Ar-H), 7.33 (s, 1H, NH; D2O exchangeable),7.47‒7.79 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.41, 25.65, 34.68, 82.18, 115.95, 116.45, 126.33, 126.94, 128.12, 129.96, 143.64, 143.82, 150.33, 172.58; MS, m/z (%): 411 (M+, 28). Analysis calc. for C20H21N5O3S (411.14): C, 58.38; H, 5.14; N, 17.02; S, 7.79. Found: C, 58.14; H, 4.90; N, 16.78; S, 7.54.
3.2.5. General procedure for the synthesis of compounds 6 and 7
A mixture of compound 1 (2.6 g, 0.01 mol), 4-chlorobenzaldehyde (1.4 g, 0.01 mol), ammonium acetate (0.02 mol), and malononitrile (0.06 g, 0.01 mol) or ethylcyanoacetate (1.1 g, 0.01 mol) in glacial acetic acid (40 mL) was refluxed for 24 h. The reaction mixture was cooled and poured into water. The formed solid was filtered off, dried, and crystallized from proper solvent to give compounds 6 and 7.
5′-amino-3′-(4-aminophenyl)-7′-(4-chlorophenyl)-3′H-spiro[cyclohexane-1,2′-thiazolo[4,5-b] pyridine]-6′-carbonitrile (6)
From methanol. Brown powder, Yield 81%; m.p. 233–235 °C; IR (KBr, υ, cm−1): 3295, 3122 (2NH2), 2210 (C≡N); 1H-NMR (DMSO): δ (ppm) 1.54‒1.93 (m, 10H, 5CH2), 5.31 (s, 2H, NH2; D2O exchangeable), 6.57‒6.83 (m, 4H, Ar-H), 7.11‒7.37 (m, 4H, Ar-H), 8.61 (s, 2H, NH2; D2O exchangeable); 13C-NMR spectrum (DMSO, δ ppm): 22.40, 25.63, 34.28, 81.48, 91.25, 114.69, 116.45, 124.49, 128.63, 129.57, 129.81, 133.24, 133.92, 135.16, 145.64, 154.02, 154.53, 159.58; MS, m/z (%): 477 (M+, 67), 479 (M+ + 2, 21). Analysis calc. for C24H22ClN5S (447.13): C, 64.35; H, 4.95; Cl, 7.91; N, 15.63; S, 7.16. Found: C, 64.08; H, 4.71; Cl, 7.66; N, 15.38; S, 6.87.
3′-(4-aminophenyl)-7′-(4-chlorophenyl)-5′-oxo-4′,5′-dihydro-3′H-spiro[cyclohexane-1,2′- thiazolo[4,5-b]pyridine]-6′-carbonitrile (7)
From dioxane. Deep yellow powder, Yield 77%; m.p. 225–227 °C; IR (KBr, υ, cm−1): 3294 (NH2), 3119 (NH), 2214 (C≡N), 1658 (C=O); 1H-NMR (DMSO): δ (ppm) 1.53‒1.91 (m, 10H, 5CH2), 5.29 (s, 2H, NH2; D2O exchangeable), 6.61‒6.84 (m, 4H, Ar-H), 7.01 (s, 1H, NH2; D2O exchangeable), 7.14‒7.40 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.41, 25.62, 34.33, 79.01, 101.16, 114.45, 116.67, 125.09, 129.13, 130.45, 132.31, 136.04, 136.52, 136.66, 143.99, 147.29, 149.75, 162.47; MS, m/z (%): 448 (M+, 57), 450 (M+ + 2, 18). Analysis calc. for C24H21ClN4OS (448.11): C, 64.21; H, 4.71; Cl, 7.90; N, 12.48; S, 7.14. Found: C, 63.93; H, 4.42; Cl, 7.66; N, 12.18; S, 6.87.
3.2.6. 4-(4-aminophenyl)-2-(4-chlorobenzylidene)-1-thia-4-azaspiro[4.5]decan-3-one (8)
A mixture of compound 1 (0.01 mol) and 4-chlorobenzaldehyde (0.01 mol) was refluxed in ethanolic NaOH for 2 h. The product was poured onto ice and neutralized with dilute HCl, and then the formed solid was filtered off and crystallized from dioxane to give 8. Pink powder, Yield 66%; m.p. 293–295 °C; IR (KBr, υ, cm−1): 3226 (NH2), 1670 (C=O); 1H-NMR (DMSO): δ (ppm) 1.52‒1.91 (m, 10H, 5CH2), 5.28 (s, 2H, NH2; D2O exchangeable), 6.62‒6.86 (m, 4H, Ar-H), 7.12‒7.39 (m, 4H, Ar-H), 8.01 (s, 1H, exocyclic vinylic-H); 13C-NMR spectrum (DMSO, δ ppm): 22.43, 25.62, 34.43, 82.67, 116.47, 126.93, 126.96, 128.93, 129.97, 130.85, 131.77, 133.74, 137.53, 143.67, 165.61; MS, m/z (%): 384 (M+, 53), 386 (M+ + 2, 16). Analysis calc. for C21H21ClN2OS (384.11): C, 65.53; H, 5.50; Cl, 9.21; N, 7.28; S, 8.33. Found: C, 65.24; H, 5.22; Cl, 8.93; N, 6.99; S, 8.03.
3.2.7. 4-(3′-(4-chlorophenyl)-spiro[cyclohexane-1,5′-pyrazolo[3,4-d]thiazol]-6′ (1′H)-yl)aniline (9)
A mixture of compound 8 (0.01 mol) and hydrazine hydrate (0.02 mol) was refluxed in 30 mL dioxane for 10 h. The reaction mixture was concentrated under reduced pressure; the formed solid was filtered off, dried, and crystallized from dioxane to give compound 9. White powder, Yield 69%; m.p. 278–280 °C; IR (KBr, υ, cm−1): 3231 (NH2), 3131 (NH); 1H-NMR (DMSO): δ (ppm) 1.51‒1.92 (m, 10H, 5CH2), 5.33 (s, 2H, NH2; D2O exchangeable), 6.61‒6.87 (m, 4H, Ar-H), 7.35 (s, 1H, NH; D2O exchangeable), 7.53‒7.64 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.42, 25.63, 34.38, 78.97, 116.87, 118.48, 125.42, 128.30, 129.46, 129.95, 132.78, 136.37, 139.38, 147.39, 152.60; MS, m/z (%): 396 (M+, 36), 398 (M+ + 2, 11). Analysis calc. for C21H21ClN4S (396.12): C, 63.54; H, 5.33; Cl, 8.93; N, 14.12; S, 8.08. Found: C, 63.26; H, 5.04; Cl, 8.67; N, 13.84; S, 7.81.
3.2.8. 4-(3′-(4-chlorophenyl)-6′H-spiro[cyclohexane-1,5′-thiazolo[5,4-d]isoxazol]-6′-yl)aniline (10)
A mixture of compound 8 (0.01 mol) and hydroxyl amine hydrochloride (0.5 g, 0.01 mol) was refluxed in pyridine (20 mL) for 10 h. The reaction mixture was cooled, poured into 100 mL water, and neutralized with dilute HCl. The product was filtered off, dried, and crystallized to give compound 10. Brown powder, Yield 57%; m.p. 290–292 °C; IR (KBr, υ, cm−1): 3222 (NH2); 1H-NMR (DMSO): δ (ppm) 1.51‒1.92 (m, 10H, 5CH2), 5.32 (s, 2H, NH2; D2O exchangeable), 6.60‒6.86 (m, 4H, Ar-H), 7.51‒7.62 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.41, 25.62, 34.41, 81.06, 107.55, 117.67, 125.61, 127.39, 127.91, 129.49, 132.18, 133.47, 145.49, 156.64; MS, m/z (%): 397 (M+, 44), 399 (M+ + 2, 14). Analysis calc. for C21H20ClN3S (397.10): C, 63.39; H, 5.07; Cl, 8.91; N, 10.56; S, 8.06. Found: C, 63.10; H, 4.78; Cl, 8.67; N, 10.27; S, 7.78.
3.2.9. 3′-(4-aminophenyl)-5′-chloro-7′-(4-chlorophenyl)-3′H-spiro[cyclohexane-1,2′-thiazolo[4,5-b] pyridine]-6′-carbonitrile (11)
A suspension of compound 7 (0.01 mol), POCl3 (3 mL), and PCl5 (0.5 gm) was heated in a steam bath for 2 h. The reaction mixture was poured gradually onto crushed ice. The separated solid was filtered off and recrystallized from dioxane to give compound 11. Black oil. Yield 52%; IR (KBr, υ,cm−1): 3219 (NH2), 2214 (C≡N); 1H-NMR (DMSO): δ (ppm) 1.50‒1.91 (m, 10H, 5CH2), 5.30 (s, 2H, NH2; D2O exchangeable), 6.59‒6.84 (m, 4H, Ar-H), 7.49‒7.60 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.42, 25.64, 34.19, 87.42, 96.01, 115.91, 116.42, 124.34, 128.27, 129.57, 133.72, 139.69, 135.21, 135.58, 141.79, 146.67, 153.26, 156.62; MS, m/z (%): 466 (M+, 44), 468 (M+ + 2, 29), 470 (M+ + 4, 4). Analysis calc. for C24H20Cl2N4S (466.08): C, 61.67; H, 4.31; Cl, 15.17; N, 11.99; S, 6.86. Found: C, 61.39; H, 4.03; Cl, 14.88; N, 11.69; S, 6.58.
3.2.10. General procedure for the synthesis of compounds 12‒16
To a solution of compound 7 (0.01 mol) in dry DMF (20 mL), sodium hydride (0.24g, 0.01 mol) was added, then the reaction mixture was stirred at 70 °C for 3 h; the reaction mixture was cooled and then epichlorohydrine, chloroethanol, bromoacetic acid, bromopropionic acid, and/or ethyl bromo acetate (0.01 mol) was added, and stirring at room temperature was continued for 5 h. The reaction mixture was evaporated under reduced pressure; the residue was washed with distilled water, filtered off, dried, and recrystallized from dioxane to give compounds 12‒16.
3′-(4-aminophenyl)-7′-(4-chlorophenyl)-4′-(oxiran-2-ylmethyl)-5′-oxo-4′,5′-dihydro-3′H-spiro[cyclo hexane-1,2′-thiazolo[4,5-b]pyridine]-6′-carbonitrile (12)
Deep brown powder, Yield 76%; m.p. 240–242 °C; IR (KBr, υ, cm−1): 3228 (NH2), 2217(C≡N), 1673 (C=O); 1H-NMR (DMSO): δ (ppm) 1.52‒1.92 (m, 10H, 5CH2), 2.94 (dd, 1H, J = 1.49 Hz; J = 4.51 Hz, OCH2), 2.98 (dd, 1H, J = 4.21 Hz; J =4.54 Hz, OCH2), 4.30–4.42 (m, 1H, OCH), 4.79 (dd, 1H, J = 2.18 Hz; J = 5.22 Hz, NCH2), 4.82 (dd, 1H, J = 2.26 Hz; J = 4.98 Hz, NCH2), 5.28 (s, 2H, NH2; D2O exchangeable), 6.60‒6.83 (m, 4H, Ar-H), 7.47‒7.59 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.41, 25.63, 34.33, 45.73, 46.60, 47.94, 79.21, 107.63, 114.57, 116.07, 124.69, 126.68, 129.30, 130.24, 132.92, 135.97, 136.25, 143.18, 143.90, 147.44, 158.22. MS, m/z (%): 447 (M+- 57, 100), 449 ([M+ + 2]-57, 32). Analysis calc. for C27H25ClN4O2S (504.14): C, 64.21; H, 4.99; Cl, 7.02; N, 11.09; S, 6.35. Found: C, 63.93; H, 4.72; Cl, 6.74; N, 10.82; S, 6.06.
3′-(4-aminophenyl)-7′-(4-chlorophenyl)-4′-(2-hydroxyethyl)-5′-oxo-4′,5′-dihydro-3′H-spiro[cyclo hexane-1,2′-thiazolo[4,5-b]pyridine]-6′-carbonitrile (13)
Pale yellow powder, Yield 86%; m.p. 208–210 °C; IR (KBr, υ, cm−1): 3400 (OH), 3294 (NH2), 2211 (C≡N), 1678 (C=O); 1H-NMR (DMSO): δ (ppm) 1.50‒1.89 (m, 10H, 5CH2), 3.79 (t, J = 4.51 Hz, 2H, NCH2), 4.58 (t, J = 4.53 Hz, 2H, CH2O), 5.28 (s, 2H, NH2; D2O exchangeable), 5.54 (bs, 1H, OH; D2O exchangeable), 6.61‒6.84 (m, 4H, Ar-H), 7.46‒7.58 (m, 4H, Ar-H); 13C-NMR spectrum (DMSO, δ ppm): 22.42, 25.62, 34.41, 46.76, 59.29, 79.49, 107.72, 114.89, 116.67, 124.72, 126.77, 129.34, 130.28, 135.99, 136.55, 142.48, 143.19, 147.38, 157.87. MS, m/z (%): 447 (M+- 45, 100), 449 ([M+ + 2]-45, 31). Analysis calc. for C26H25ClN4O2S (492.14): C, 63.34; H, 5.11; Cl, 7.19; N, 11.36; S, 6.50. Found: C, 63.05; H, 4.85; Cl, 6.91; N, 11.07; S, 6.21.
2-(3′-(4-aminophenyl)-7′-(4-chlorophenyl)-6′-cyano-5′-oxo-3′,5′-dihydro-4′H-spiro[cyclohexane -1, 2′-thiazolo[4,5-b]pyridin]-4′-yl)acetic acid (14)
Brown powder, Yield 69%; m.p. 280–282 °C; IR (KBr, υ, cm−1): 3410 (OH), 3288 (NH2), 2216 (C≡N), 1740 (C=O), 1672 (C=O); 1H-NMR (DMSO): δ (ppm) 1.51‒1.90 (m, 10H, 5CH2), 4.87 (s, 2H, NCH2), 5.29 (s, 2H, NH2; D2O exchangeable), 6.65‒6.89 (m, 4H, Ar-H), 7.51‒7.64 (m, 4H, Ar-H), 10.45 (bs, 1H, OH; D2O exchangeable); 13C-NMR spectrum (DMSO, δ ppm): 22.41, 25.63, 34.43, 43.16, 79.87, 104.69, 114.56, 116.77, 124.69, 126.68, 129.30, 130.33, 132.92, 135.97, 136.25, 142.28, 143.23, 147.49, 161.38, 172.79. MS, m/z (%): 447 (M+-59, 100), 449 ([M+ + 2]-59, 31). Analysis calc. for C26H23ClN4O3S (506.12): C, 61.59; H, 4.57; Cl, 6.99; N, 11.05; O, 9.47; S, 6.32. Found: C, 61.31; H, 4.28; Cl, 6.72; N, 10.76; S, 5.93.
3-(3′-(4-aminophenyl)-7′-(4-chlorophenyl)-6′-cyano-5′-oxo-3′,5′-dihydro-4′H-spiro[cyclo hexane-1,2′-thiazolo[4,5-b]pyridin]-4′-yl)propanoic acid (15)
Brown powder, Yield 72%; m.p. 200–202 °C; IR (KBr, υ, cm−1): 3411 (OH), 3261 (NH2), 2214 (C≡N), 1733 (C=O), 1668 (C=O); 1H-NMR (DMSO): δ (ppm) 1.50‒1.91 (m, 10H, 5CH2), 3. 51 (t, J = 4.47 Hz, 2H, CH2CO), 4.35 (t, J = 4.49 Hz, 2H, NCH2), 5.28 (s, 2H, NH2; D2O exchangeable), 6.66‒6.90 (m, 4H, Ar-H), 7.53‒7.65 (m, 4H, Ar-H), 10.67 (bs, 1H, OH; D2O exchangeable); 13C-NMR spectrum (DMSO, δ ppm): 22.40, 25.64, 34.36, 35.60, 44.43, 79.93, 107.72, 114.81, 116.70, 124.69, 126.65, 129.32, 130.41, 132.32, 135.97, 136.27, 142.21, 143.11, 147.51, 159.98, 173.47. MS, m/z (%): 447 (M+-73, 100), 449 ([M+ + 2]-73, 30). Analysis calc. for C27H25ClN4O3S (520.13): C, 62.24; H, 4.84; Cl, 6.80; N, 10.75; S, 6.15. Found: C, 61.98; H, 4.56; Cl, 6.53; N, 10.46; S, 5.87.
Ethyl 2-(3′-(4-aminophenyl)-7′-(4-chlorophenyl)-6′-cyano-5′-oxo-3′,5′-dihydro-4′H-spiro [cyclohexane-1,2′-thiazolo[4,5-b]pyridin]-4′-yl)acetate (16)
Brown powder, Yield 91%; m.p. 190–192 °C; IR (KBr, υ, cm−1): 3257 (NH2), 2217 (C≡N), 1718 (C=O), 1663 (C=O); 1H-NMR (DMSO): δ (ppm) 1.17 (t, J = 7.94 Hz, 3H, CH3CH2O), 1.51‒1.92 (m, 10H, 5CH2), 4.17 (q, J = 7.96 Hz, 2H, CH3CH2O), 5.11 (s, 2H, NCH2), 5.30 (s, 2H, NH2; D2O exchangeable), 6.67‒6.92 (m, 4H, Ar-H), 7.54‒7.67 (m, 4H, Ar-H), 10.67 (bs, 1H, OH; D2O exchangeable); 13C-NMR spectrum (DMSO, δ ppm): 14.73, 22.63, 25.16, 34.47, 45.85, 61.21, 79.77, 103.99, 114.11, 116.72, 124.64, 126.69, 129.55, 130.33, 132.17, 135.60, 136.38, 142.31, 143.18, 147.61, 161.00, 172.53. MS, m/z (%): 447 (M+-87, 100), 449 ([M+ + 2]-87, 32). Analysis calc. for C28H27ClN4O3S (534.15):C, 62.85; H, 5.09; Cl, 6.63; N, 10.47; S, 5.99. Found: C, 62.56; H, 4.80; Cl, 6.34; N, 10.18; S, 5.71.
3.3. Anticancer Activity
Carried out at Al-Azhar University, The Regional Center for Mycology and Biotechnology, Cairo, Egypt.
3.3.1. Chemicals
Dimethyl sulfoxide (DMSO), crystal violet, and trypan blue dye were purchased from Sigma (St. Louis, MO, USA). Fetal Bovine serum, DMEM, RPMI-1640, HEPES buffer solution, L-glutamine, gentamycin, and 0.25% Trypsin-EDTA were purchased from Lonza (Basel, Switzerland). Crystal violet stain (1%): It was composed of 0.5% (w/v) crystal violet and 50% methanol then made up to volume with ddH2O and filtered through a Whatmann No.1 filter paper purchased from Sigma-Aldrich, Saint Louis, MO, USA.
3.3.2. Mammalian Cell Lines
MCF-7 (human breast cancer cell line) and HepG-2 (human liver cancer cell line) were obtained from the American Type Culture Collection (Rockville, MD, USA)
3.3.3. Cell Line Propagation
The cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, HEPES buffer, and 50 µg/mL gentamycin. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were subcultured two times a week. Cytotoxicity evaluation using viability assay: For cytotoxicity assay, the cells were seeded in a 96-well plate at a cell concentration of 1 × 104 cells per well in 100 µL of growth medium. Fresh medium containing different concentrations of the test sample was added after 24 h of seeding. Serial two-fold dilutions of the tested chemical compound were added to confluent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, NJ, USA) using a multichannel pipette. The microtiter plates were incubated at 37 °C in a humidified incubator with 5% CO2 for a period of 48 h. Three wells were used for each concentration of the test sample. Control cells were incubated without a test sample and with or without DMSO. The little percentage of DMSO present in the wells (maximal 0.1%) was found not to affect the experiment. After incubation of the cells for at 37 °C, various concentrations of the sample were added, and the incubation was continued for 24 h and viable cells yield was determined through a colorimetric method [79].
In brief, after the end of the incubation period, media were aspirated, and the crystal violet solution (1%) was added to each well for at least 30 minutes. The stain was removed, and the plates were rinsed using tap water until all excess stain was removed. Glacial acetic acid (30%) was then added to all wells and mixed thoroughly, and then the absorbance of the plates was measured after they were gently shaken on Microplate reader (TECAN, Inc.), using a test wavelength of 490 nm. All results were corrected for background absorbance detected in wells without added stain. Treated samples were compared with the cell control in the absence of the tested compounds. All experiments were carried out in triplicate. The cell cytotoxic effect of each tested compound was calculated. The optical density was measured with the microplate reader (SunRise, TECAN, Inc, Zanker Road, San Jose, CA, USA) to determine the number of viable cells, and the percentage of viability was calculated as [1 − (ODt/ODc)] × 100%, where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The relation between surviving cells and drug concentration was plotted to get the survival curve of each tumor cell line after treatment with the specified compound.
IC50 calculations. To assess the compounds’ anticancer potency, the IC50 values (the concentration that inhibited cell viability to 50% of the control) were determined. The IC50 values were calculated from the best-fit (R2 > 0.95) of the Hill slope curve to experimental data using nonlinear regression analysis in Graph Pad Prism (Graph Pad Software Version 7, San Diego, CA. USA), according to the formula:
where X = log of dose, Y = growth inhibition value normalized to control, and HillSlope = unitless slope factor or Hill slope [80].
Y = 100/1 +10((LogIC50 − X) × HillSlope),
3.4. In Vitro Antidiabetic Assay
Carried out at Al-Azhar University, The Regional Center for Mycology and Biotechnology, Cairo, Egypt.
3.4.1. Alpha-Amylase Inhibitory Activity
Chemicals
Alpha-amylase and 3,5-dinitro salicylic acid (DNS) were purchased from Sigma-Aldrich, Saint Louis, MO, USA, while starch, sodium dihydrogen phosphate, and di-sodium hydrogen phosphate were purchased from Hi-Media, PA, USA.
Alpha-Amylase Inhibition Method
In the alpha–amylase inhibition method, the enzyme solution was prepared by dissolving α–amylase in 20 mM phosphate buffer (6.9) at a concentration of 0.5 mg/mL. One milliliter of the extract of various concentrations (1000–7.81 μg/mL) and 1 mL of enzyme solution were mixed together and incubated at 25 °C for 10 min. After incubation, 1 mL of starch (0.5%) solution was added to the mixture and further incubated at 25 °C for 10 min. The reaction was then stopped by adding 2 mL of 3,5-dinitro salicylic acid (DNS, color reagent), heating the reaction mixture in a boiling water bath (5 min). After cooling, the absorbance was measured colorimetrically at 565 nm [81]. The inhibition percentage was calculated using the given formula:
where As is the absorbance in the presence of test substance and Ac is the absorbance of the control. Acarbose was used as a standard drug. The IC50 value was defined as the concentration of alpha–amylase inhibitor to inhibit 50% of its activity under the assay conditions.
Inhibitory activity (%) = (1−As/Ac) × 100,
3.4.2. Alpha-Glucoidase Inhibitory Activity
Chemicals
Alpha-glucosidase (Saccharomyces cerevisiae) and 3,5-dinitro salicylic acid (DNS) were purchased from Sigma-Aldrich, Saint Louis, Missouri, USA, while P-nitrophenyl-α–D-glucopyranoside (p-NPG), sodium carbonate (Na2CO3), sodium dihydrogen phosphate, and di-sodium hydrogen phosphate were purchased from Hi-Media, Pennsylvania, USA.
Alpha-Glucoidase Inhibition Method
The alpha–glucosidase inhibitory activity of B. vulgaris subspecies cicla L. var. flavescens leaves different extracts and fractions was carried out according to the standard method with minor modification [82]. In a 96-well plate, reaction mixture containing 50 μL phosphate buffer (100 mM, pH = 6.8), 10 μL alpha–glucosidase (1 U/mL), and 20 μL of varying concentrations of extracts and fractions (1000 to 7.81 μg/mL) was preincubated at 37 ° C for 15 min. Then, 20 μL P-NPG (5 mM) was added as a substrate and incubated further at 37 °C for 20 min. The reaction was stopped by adding 50 μL Na2CO3 (0.1 M). The absorbance of the released p-nitrophenol was measured at 405 nm using Multiplate Reader. Acarbose at various concentrations (1000 to 7.81 μg/mL) was included as a standard. Without a test, the substance was set up in parallel as a control, and each experiment was performed in triplicates. The results were expressed as percentage inhibition, which was calculated using the formula:
where As is the absorbance in the presence of test substance and Ac is the absorbance of control. The IC50 value was defined as the concentration of alpha-glucosidase inhibitor to inhibit 50% of its activity under the assay conditions.
Inhibitory activity (%) = (1 – As/Ac) × 100,
4. Conclusion
Our study concerned the preparation of new spirothiazolidene derivatives and its fused analogs, which were prepared and elucidated using spectral and elemental analysis. Generally, the type of heterocyclic framework of these derivatives had a notable effect on the anticancer and antidiabetic activity. The prepared compounds were screened for anticancer activity towards the breast and liver human cell line. Compounds 6, 14, and 16, containing spirothiazolopyridine–carbonitrile derivatives with amino or acetic acid or propanoic acid groups showed excellent anticancer activity at all concentrations. Furthermore, it is apparent that compounds 6 and 9 containing amino spirothiazolopyridine–carbonitrile and pyrazolo spirothiazolidine groups display high activity against both of alpha-amylase and alpha-glucosidase enzyme in all concentrations.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/13/2511/s1, evaluation of cytotoxicity against HepG-2 and MCF-7cell lines.
Author Contributions
M.E.-S. conceived and designed the experiment; M.E.-S. and W.I.E.-S. performed the experiments and analyzed the data; M.E.-S. wrote the paper; E.M.F. and R.A.K.A.-H. modified the manuscript, supervised the project and checked the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Cancer Facts & Figures. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf (accessed on 11 December 2017).
- Cancer Facts & Figures. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf (accessed on 9 November 2018).
- Common Cancer Types. Available online: https://www.cancer.gov/types/common-cancers (accessed on 26 February 2018).
- The Genetics of Cancer. Available online: https://www.cancer.gov/about-cancer/causes-prevention/genetics (accessed on 12 October 2007).
- What is Cancer? Available online: https://www.cancer.org/cancer/cancer-basics/what-is-cancer.html (accessed on 8 December 2015).
- What is Cancer? Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 9 February 2015).
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Lonati, V.; Barni, S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: A literature-based meta-analysis of 24 trials. Lung Cancer 2012, 78, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, H.; Huang, F.; Zhu, D.; Bi, J.; Kel, Y.; Zhang, T. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: A systematic review and meta-analysis. World J. Surg. Oncol. 2013, 11, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Gore, L.; De Gregori, J.; Porter, C.C. Targeting developmental pathways in children with cancer: What price success? Lancet Oncol. 2013, 4, 70–78. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sayed, W.A.; Mohamed, A.M.; El-Sofany, W.I.; Awad, H.M. Synthesis and Anticancer Activity of New 1-Thia-4-azaspiro[4.5]decane, Their Derived Thiazolopyrimidine and 1,3,4-Thiadiazole Thioglycosides. Molecules 2017, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Khaireldin, N.Y.; El-Shahat, M.; El-Hefny, E.A.; El-Saidi, M.M.T.; Ali, M.M.; Mahmoud, A.E. Antiproliferative Activity For Newly Heterofunctionalized Pyridine Analogues. Ponte 2016, 72, 106–118. [Google Scholar]
- Shamroukh, A.H.; El-Shahat, M.; Drabowicz, J.; Ali, M.M.; Rashad, A.E. Anticancer evaluation of some newly synthesized N-nicotinonitrile derivative. Eur. J. Med. Chem. 2013, 69, 521–526. [Google Scholar] [CrossRef]
- Rashad, A.E.; Shamroukh, A.H.; Yousif, N.M.; Salama, M.A.; Ali, M.A.; Mahmoud, A.E.; El-Shahat, M. New Pyrimidinone and Fused Pyrimidinone Derivatives as Potential Anticancer Chemotherapeutics. Arch. Pharm. Chem. Life Sci. 2012, 345, 729–738. [Google Scholar] [CrossRef]
- Flefel, E.E.; Salama, M.A.; El-Shahat, M.; El-Hashash, M.A.; El-Farargy, A.F. A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1739–1756. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; El-Sofany, W.I.; Hussein, H.A.; Fathi, N.M. Synthesis and anticancer activity of new[(indolyl)pyrazolyl]-1,3,4-oxadiazole thioglycosides and acyclic nucleoside analogs. Nucleosides Nucleotides Nucleic Acids. 2017, 36, 474–495. [Google Scholar] [CrossRef]
- Johnson, K.A.; Brown, P.H. Drug development for cancer chemoprevention: Focus on molecular targets. Semin. Oncol. 2010, 37, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Diabetes, M.; Alvin, C. Powers in Harrison’s Principles of Internal Medicine, 18th ed.; Chapter 345; McGraw Hill Education Books: New York, NY, USA, 2004; ISBN 978-0071748896. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Initial management of glycemia in type 2 diabetes mellitus. N. Engl. J. Med. 2002, 347, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. J. Am. Med. Assoc. 2002, 287, 360–372. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, D.E.; Hirsch, I.B. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. J. Am. Med. Assoc. 2003, 289, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drug 2004, 64, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin secretagogues: Old and new. Diabetes Rev. 1999, 7, 139–153. [Google Scholar]
- Cusi, K.; DeFronzo, R.A. Metformin: A review of its metabolic effects. Diabetes Rev. 1998, 6, 98–131. [Google Scholar]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar]
- Diamant, M.; Heine, R.J. Thiazolidinediones in type 2 diabetes mellitus. Drug 2003, 63, 1373–1406. [Google Scholar] [CrossRef]
- Lebovitz, H.E. α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998, 6, 132–145. [Google Scholar]
- Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2890380/ (accessed on 15 July 2010).
- Available online: http://scienceblog.cancerresearchuk.org/2017/05/15/sugar-and-cancer-what-you-need-to-know/ (accessed on 15 May 2017).
- Available online: https://beatcancer.org/blog-posts/5-reasons-cancer-and-sugar-are-best-friends/ (accessed on 02 April 2000).
- Gressier, B.; Lebegue, N.; Brunet, C.; Luyckx, M.; Dine, T.; Cazin, M.; Cazin, J.C. Scavenging of reactive oxygen species by letosteine, a molecule with two blocked-SH groups. Pharm. World Sci. 1995, 17, 76–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, H.; Zhai, S.; Yan, B. Natural product-inspired synthesis of thiazolidine and thiazolidinone compounds and their anticancer activities. Curr. Pharm. Des. 2010, 16, 1826–1842. [Google Scholar] [CrossRef] [PubMed]
- Tomasić, T.; Masic, L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Sharma, N. Epalrestat, an aldose reductase inhibitor, in diabetic neuropathy: An Indian perspective. Ann. Indian Acad. Neurol. 2008, 11, 231–235. [Google Scholar] [CrossRef]
- Rizzo, S. Clinical trial with arginine tidiacicate in symptomatic chronic persistent hepatitis. Int. J. Clin. Pharmacol. Res. 1986, 6, 225–230. [Google Scholar]
- Missbach, M.; Jagher, B.; Sigg, I.; Nayeri, S.; Carlberg, C.; Wiesenberg, I. Thiazolidine diones, specific ligands of the nuclear receptor retinoid Z receptor/retinoid acid receptor-related orphan receptor α with potent antiarthritic activity. J. Biol. Chem. 1996, 271, 13515–13522. [Google Scholar] [CrossRef]
- Gonzalez-Estrada, A.; Radojicic, C. Penicillin allergy: A practical guide for clinicians. Clev. Clin. J. Med. 2015, 82, 295–300. [Google Scholar] [CrossRef]
- Norisada, N.; Masuzaki, H.; Fujimoto, M.; Inoue, G.; Hosoda, K.; Hayashi, T.; Watanabe, M.; Muraoka, S.; Yoneda, F.; Nakao, K. Antidiabetic and adipogenic properties in a newly synthesized thiazolidine derivative, FPFS-410. Metabolism 2004, 53, 1532–1537. [Google Scholar] [CrossRef]
- Ottanà, R.; Maccari, R.; Giglio, M.; Del Corso, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2, 4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agent. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Taranalli, A.D.; Thimmaiah, N.V.; Srinivas, S.; Saravanan, E.; Bhat, A.R. Anti-inflammatory, analgesic and anti ulcer activity of certain thiazolidinones. Asian J. Pharm. Clin. Res. 2009, 2, 209–211. [Google Scholar]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClerq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4’-chlorophenyl) thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Shiradkar, M.R.; Murahari, K.K.; Gangadasu, H.R.; Suresh, T.; Kalyan, C.A.; Panchal, D.; Kaur, R.; Burange, P.; Ghogare, J.; Mokale, V.; et al. Synthesis of new S-derivatives of clubbed triazole as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. 2007, 15, 3997–4008. [Google Scholar] [CrossRef]
- Upadhyay, A. Conventional and microwave assisted synthesis of some new N-[(4-oxo-2-substituted aryl-1, 3-thiazolidine)-acetamidyl]-5-nitroindazoles and its antimicrobial activity. Eur. J. Med. Chem. 2010, 45, 3541–3548. [Google Scholar] [CrossRef]
- Amin, K.M.; Rahman, A.D.E.; Al-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef]
- Agarwal, A.; Lata, S.; Saxena, K.K.; Srivastava, V.K.; Kumar, A. Chemistry and Biological Activities of 1, 3-Thiazolidin-4-ones. Eur. J. Med. Chem. 2006, 41, 1223–1229. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Kumar, A. Synthesis of newer thiadiazolyl and thiazolidinonyl quinazolin-4 (3H)-ones as potential anticonvulsant agents. Eur. J. Med. Chem. 2002, 37, 873–882. [Google Scholar]
- Ban, J.O.; Kwak, D.H.; Oh, J.H.; Park, E.J.; Cho, M.C.; Song, H.S.; Song, M.J.; Han, S.B.; Moon, D.C.; Kang, K.W.; et al. Suppression of NF-κB and GSK-3β is involved in colon cancer cell growth inhibition by the PPAR agonist troglitazone. Chem. Biol. Interact. 2010, 188, 75–85. [Google Scholar] [CrossRef]
- Beharry, Z.; Zemskova, M.; Mahajan, S.; Zhang, F.; Ma, J.; Xia, Z.; Lilly, M.; Smith, C.D.; Kraft, A.S. Novel benzylidene-thiazolidine-2, 4-diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Chandrappa, S.; Benaka, P.S.B.; Vinaya, K.; Ananda, K.C.S.; Thimmegowda, N.R.; Rangappa, K.S. Synthesis and in vitro antiproliferative activity against human cancer cell lines of novel 5-(4-methyl-benzylidene)-thiazolidine-2, 4-diones. Investig. New Drugs 2008, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gududuru, V.; Hurh, E.; Dalton, J.T.; Miller, D.D. Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorg. Med. Chem. Lett. 2004, 14, 5289–5293. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of novel nonfused bicyclic thiazolidinone derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 638–650. [Google Scholar] [CrossRef]
- Balzarini, J.; Orzeszko, B.; Maurin, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Balzarini, J.; Carbone, A.; Chimirri, A.; De Clercq, E.; Monforte, A.M.; Monforte, P.; Pannecouque, C.; Zappalà, M. Synthesis of new 2, 3-diaryl-1, 3-thiazolidin-4-ones as anti-HIV agents. II Farmaco 2004, 59, 33–39. [Google Scholar] [CrossRef]
- Rao, A.; Carbone, A.; Chimirri, A.; De Clercq, E.; Monforte, A.M.; Monforte, P.; Pannecouque, C.; Zappalà, M. Synthesis and anti-HIV activity of 2, 3-diaryl-1, 3-thiazolidin-4-(thi) one derivatives. II Farmaco 2002, 57, 747–751. [Google Scholar] [CrossRef]
- Barreca, M.L.; Chimirri, A.; De Luca, L.; Monforte, A.M.; Monforte, P.; Rao, A.; Zappalà, M.; Balzarini, J.; De Clercq, E.; Pannecouque, C.; et al. Discovery of 2, 3-diaryl-1, 3-thiazolidin-4-ones as potent anti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2001, 11, 1793–1796. [Google Scholar] [CrossRef]
- Kamila, S.; Ankati, H.; Biehl, E.R. An efficient microwave assisted synthesis of novel class of Rhodanine derivatives as potential HIV-1 and JSP-1 inhibitors. Tetrahedron Lett. 2011, 52, 4375–4377. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.; Brazeau, P.; Hariton, L.B. Spiro (Steroid) Thiazolidines1. J. Am. Chem. Soc. 1948, 70, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Kovach, N.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Synthesis and Anticancer Activity of Isatin-Based Pyrazolines and Thiazolidines Conjugates. Arch. Pharm. Chem. Life Sci. 2011, 344, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ramshid, P.K.; Jagadeeshan, S.; Krishnan, A.; Mathew, M.; Nair, S.A.; Pillai, M.R. Synthesis and in vitro evaluation of some isatin-thiazolidinone hybrid analogues as anti-proliferative agents. Med. Chem. 2010, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Zhang, G.; Lv, Y.; Zhang, N.; Gong, P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011, 46, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; Sayed, H.H.; Hashem, A.I.; Saleh, D.O.; El-Sofany, W.; Abdel-Megeid, F.M.E. Hyperglycedemia and hypertriglyceridemia activities of newly synthesized compounds derived from 3’-(4-halophenyl)-5’-arylidene spiro(cyclohexane-(1,2’)-thiazolidin)-4’-one. Der Pharma Chem. 2015, 7, 142–157. [Google Scholar]
- Flefel, E.M.; Sayed, H.H.; Hashem, A.I.; Shalaby, E.A.; El-Sofany, W.; Abdel-Megeid, F.M.E. Pharmacological evaluation of some novel synthesized compounds derived from spiro(cyclohexane-1,2’-thiazolidines). Med. Chem. Res. 2014, 23, 2515–2527. [Google Scholar] [CrossRef]
- Soliman, H.A.; El-Shahat, M.; Soliman, A. Silica-supported Zinc Chloride (ZnCl2/SiO2)-induced Efficient Protocol for the Synthesis of N-sulfonyl imines and 2-Arylbenzothiazole. Lett. Org. Chem. 2019, 16, 584–591. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-El-Shahat, M. Fabrication of ZIF-67@ MIL-125-NH2 nanocomposite with enhanced visible light photoreduction activity. J. Environ. Chem. Eng. 2019, 7, 103194. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Sayed, H.A.; El-Shahat, M.; El-Sayed, A.A.; Darwesh, O.M. Novel triazolothiadiazole and triazolothiadiazine derivatives containing pyridine moiety: Design, synthesis, bactericidal and fungicidal activities. Curr. Bioact. Compd. 2018, 14, 169–179. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, Molecular Docking and In Vitro Screeningof Some Newly Synthesized Triazolopyridine, Pyridotriazine and Pyridine–Pyrazole Hybrid Derivatives. Molecules 2018, 23, 2548. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; Tantawy, W.A.; El-Sofany, W.; El-Shahat, M.; El-Sayed, A.A.; Abd-Elshafy, D.N. Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation. Molecules 2017, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; El-Shahat, M.; Ullah, B.; Bao, Z.; Xing, H.; Xiao, L.; Ren, Q.; Zhang, Z. Cyclopentadiene-based Brønsted acid as a new generation of organocatalyst for transfer hydrogenation of 2-substituted quinoline derivatives. Tetrahedron Lett. 2017, 58, 2050–2053. [Google Scholar] [CrossRef]
- Rashad, A.E.; Shamroukh, A.H.; El-Hashash, M.A.; El-Farargy, A.F.; Yousif, N.M.; Salama, M.A.; Mostafa, A.; El-Shahat, M. Synthesis and Anti-Avian Influenza Virus (H5N1) Evaluation of Some Novel Nicotinonitriles and Their N-Acylic Nucleosides. J. Heterocycl. Chem. 2012, 49, 1130–1135. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Khalil, A.M.; El-Shahat, M.; Khaireldin, N.Y.; Rabie, S.T. Antimicrobial activity of PVC-pyrazolone-silver nanocomposites. J. Macromol. Sci. Part A Pure Appl.Chem. 2016, 53, 346–353. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; El-Shahat, M.; Rabie, S.T.; Flefel, E.M.; Abd-Elshafy, D.N. New pyrimidine and fused pyrimidine derivatives: Synthesis and anti hepatitis a virus (HAV) evaluation. Int. J. Pharm. 2015, 5, 69–79. [Google Scholar]
- Salama, M.A.M.; El-Shahat, M.; Elhefny, E.A.; El-Sayed, A.A. A Novel Fused Pyridopyrimidine Derivatives: Synthesis And Characterization. Int. J. Pharm 2015, 5, 53–58. [Google Scholar]
- Flefel, E.M.; El-Hashash, M.A.; El-Farargy, A.F.; Salama, M.A.; El-Shahat, M. Utility of 1-(3,4-dimethyl-phenyl)-3-thien-2-yl-propenone as ring transformer in preparing heterocyclic compounds and their antimicrobial and analgesic activities. Egypt. J. Pharm. Sci. 2013, 54–69. [Google Scholar]
- Rashad, A.E.; Shamroukh, A.H.; El-Hashash, M.A.; El-Farargy, A.F.; Yousif, N.M.; Salama, M.A.; Abdelwahed, N.M.A.; El-Shahat, M. 1,3-Bis(4-chlorophenyl)-2,3-epoxypropanone as Synthons in Synthesis of Some Interesting Potential Antimicrobial Agents. Org. Chem. Indian J. 2013, 9, 287–294. [Google Scholar]
- Sayed, H.H.; Flefel, E.M.; Abd El-Fatah, A.M.; El-Sofany, W.I. Focus on the Synthesis and Reactions of Some New Pyridine Carbonitrile Derivatives as Antimicrobial and Antioxidant Agents. Egypt. J. Chem. 2010, 53, 17–35. [Google Scholar]
- Saotome, K.; Morita, H.; Umeda, M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol. In Vitro 1989, 3, 317–321. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(vinylpyridine) as Basic Catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Narkhede, M.B.; Ajimire1, P.V.; Wagh1, A.E.; Mohan, M.; Shivashanmugam, A.T. In vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. Asian J. Plant Sci. Res. 2011, 1, 101–106. [Google Scholar]
- Shai, L.J.; Magano, S.R.; Lebelo, S.L.; Mogale, A.M. Inhibitory effects of five medicinal plants on alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase. J. Med. Plant Res. 2011, 5, 2863–2867. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).