Chemical Structures and Pharmacological Profiles of Ginseng Saponins
Abstract
:1. Introduction
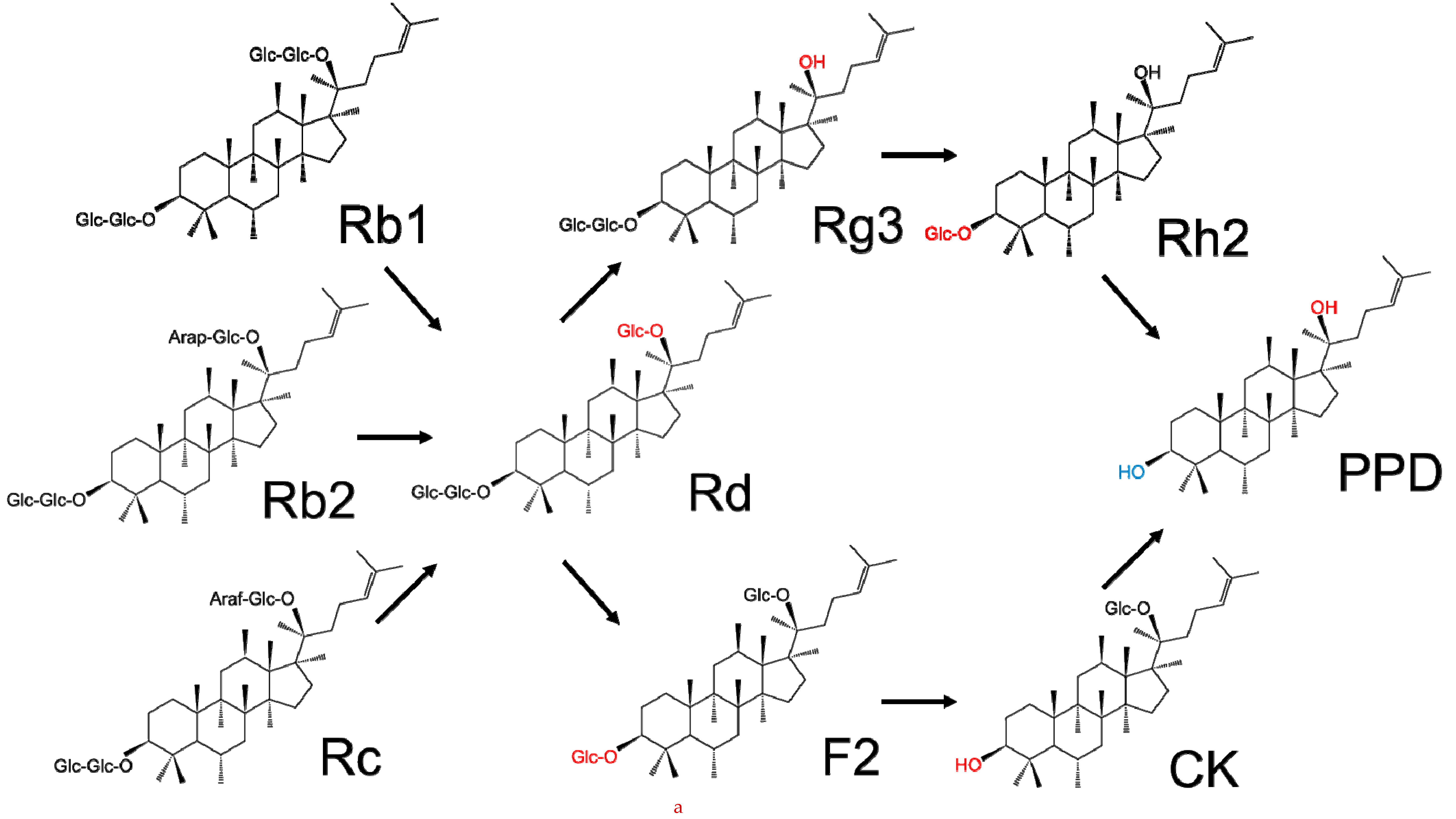
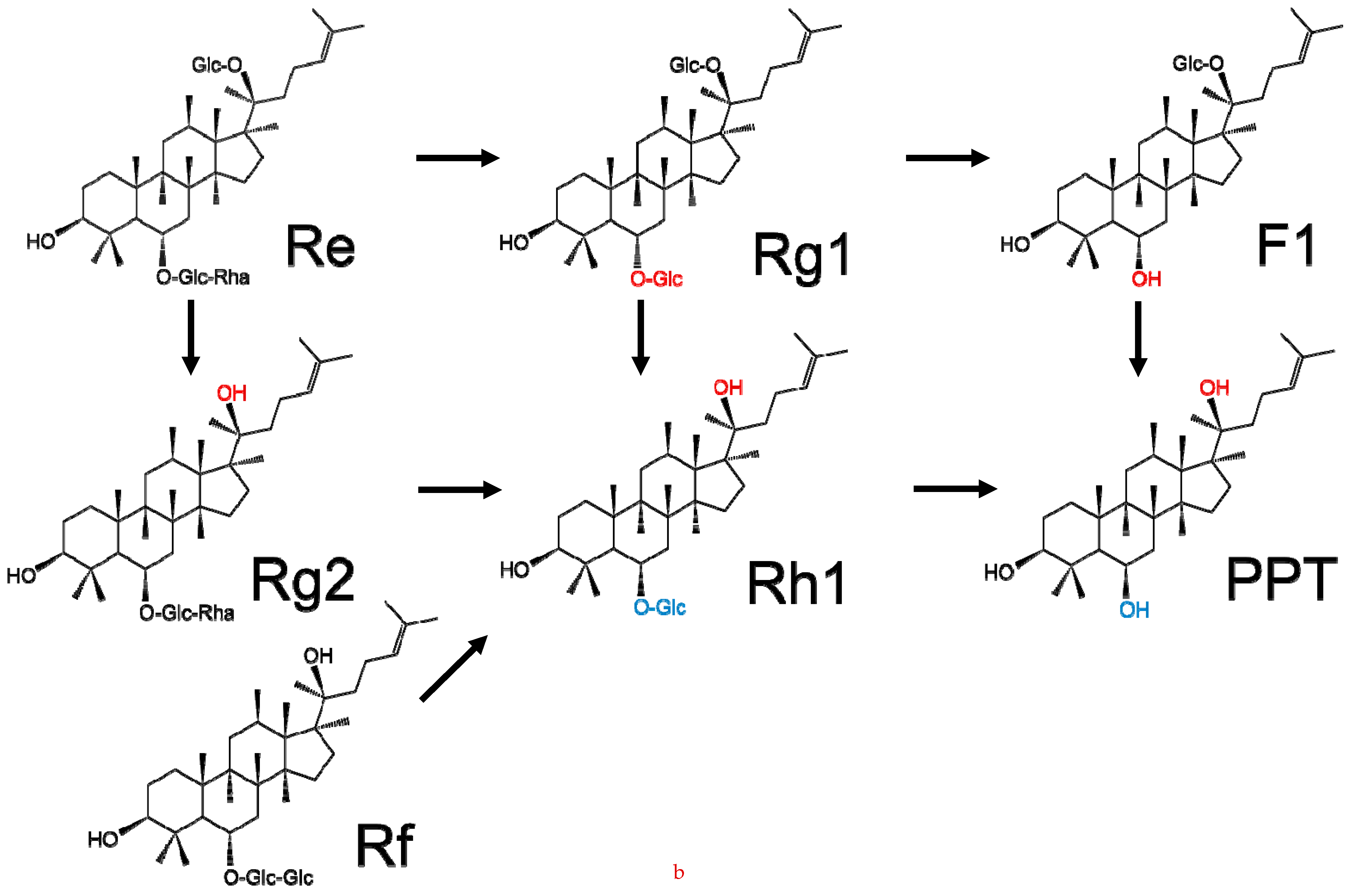
2. Chemical Structure and Classification of Ginseng Saponins
3. Key Saponins Found in Ginseng
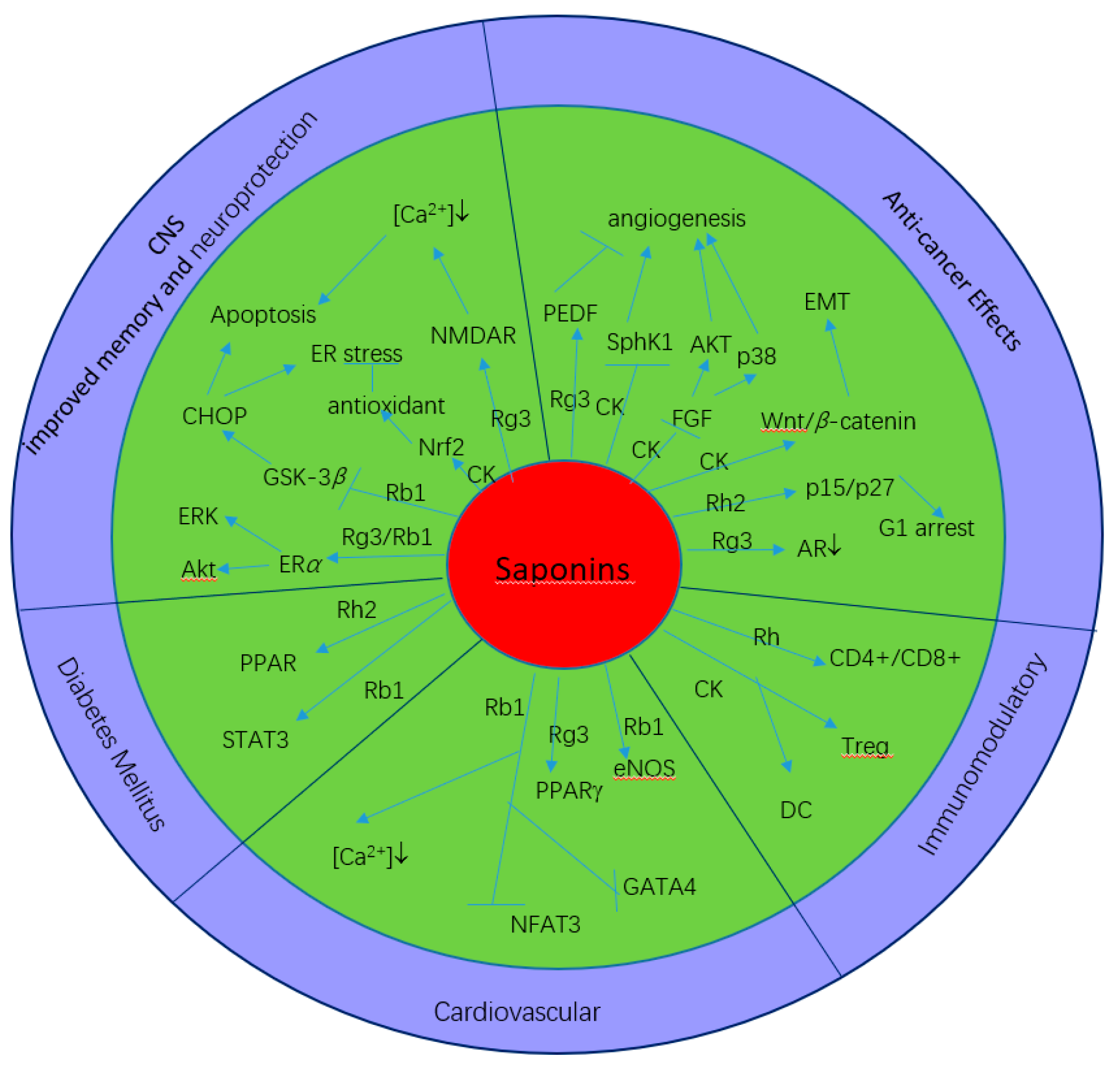
4. Pharmacological Profiles and Mechanisms of Ginseng Saponins
4.1. General Effects of Ginseng Saponins
4.2. Effects on the Central Nervous System (CNS)
4.3. Anticarcinogenic Activities
4.4. Immunomodulatory Effects
4.5. Cardiovascular System
4.6. Diabetes Mellitus
4.7. Clinical Application
5. Other Active Ingredients in Ginseng
6. Conclusion and Future Prospects
Funding
Conflicts of Interest
References
- Yun, T.K. Brief Introduction of Panax Ginseng C.A. Meyer. J. Korean Med. Sci. 2001, 16, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Eom, S.M.; Kim, J.; Kim, S.H.; Huh, E.; Kim, H.; Lee, Y.; Lee, H.; Oh, M.S.A. Comprehensive Review of Recent Studies on Herb-drug Interaction: A focus on Pharmacodynamic Interaction. J. Altern. Complement. Med. 2016, 22, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A. Adaptogenic Herb Ginseng (Panax) as Medical Food: Status Quo and Future Prospects. Biomed. Pharmacother. 2017, 85, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Z.; Shang, H.C.; Gao, X.M.; Zhang, B.L. A Comparison of the Ancient Use of Ginseng in Traditional Chinese Medicine with Modern Pharmacological Experiments and Clinical Trials. Phyther. Res. 2008, 22, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.H.; So, S.H. The World Ginseng Market and the Ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical Diversity of Ginseng Saponins from Panax Ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tanaka, O.; Soma, K.; Iida, Y.; Ando, T.; Nakamura, H. Studies on Saponins and Sapogenins of Ginseng the Structure of Panaxatriol. Tetrahedron Lett. 1965, 42, 207–213. [Google Scholar] [CrossRef]
- Kaku, T.; Miyata, T.; Uruno, T.; Sako, I.; Kinoshita, A. Chemico-pharmacological Studies on Saponins of Panax Ginseng C. A. Meyer; I. Chemical part. Arzneimittelforschung 1975, 25, 343–347. [Google Scholar] [PubMed]
- Lu, J.M.; Jiang, J.; Jamaluddin, M.S.; Liang, Z.; Yao, Q.; Chen, C. Ginsenoside Rb1 Blocks Ritonavir-induced Oxidative Stress and ENOS Downregulation Through Activation of Estrogen Receptor-beta and Upregulation of SOD in Human Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 294. [Google Scholar] [CrossRef]
- Shin, K.C.; Oh, D.K. Classification of Glycosidases that Hydrolyze the Specific Positions and Types of Sugar Moieties in Ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- Wong, A.S.T.; Che, C.M.; Leung, K.W. Recent Advances in Ginseng as Cancer Therapeutics: A Functional and Mechanistic Overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Y.; Wang, C.Z.; Liu, Z.; Zhang, Q.H.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Li, P.; Qi, L.W.; Yuan, C.S. Determination of American Ginseng Saponins and their Metabolites in Human Plasma, Urine and Feces Samples by Liquid Chromatography Coupled with Quadrupole Time-of-flight Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 15, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.K.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.P.; Yeung, H.W.; Wong, R.N.S. Pharmacogenomics and the Yin/Yang Actions of Ginseng: Anti-tumor, Angiomodulating and Steroid-like Activities of Ginsenosides. Chin. Med. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular Signaling of Ginsenosides Rb1, Rg1, and Rg3 and their Mode of Actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Choi, D.K.; Cho, D.Y.; Jakaria, M.; Haque, M.E.; Kim, J. Active Ginseng Components in Cognitive Impairment: Therapeutic Potential and Prospects for Delivery and Clinical Study. Oncotarget 2018, 9, 33601–33620. [Google Scholar] [CrossRef]
- Cho, C.W.; Kim, Y.C.; Kang, J.H.; Rhee, Y.K.; Choi, S.Y.; Kim, K.T.; Lee, Y.C.; Hong, H. Do Characteristic Study on the Chemical Components of Korean Curved Ginseng Products. J. Ginseng Res. 2013, 37, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chu, Y.; Liao, B.; Xiao, S.; Yin, Q.; Bai, R.; Su, H.; Dong, L.; Li, X.; Qian, J.; et al. Panax Ginseng Genome Examination for Ginsenoside Biosynthesis. Gigascience 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Ong, Y.C.; Yong, E.L. Panax (Ginseng)-Panacea or Placebo? Molecular and Cellular Basis of its Pharmacological Activity. Ann. Acad. Med. Singap. 2000, 29, 42–46. [Google Scholar]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng Pharmacology: Multiple Constituents and Multiple Actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Losel, R.; Wehling, M. Nongenomic Actions of Steroid Hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef]
- Oliynyk, S.; Oh, S. Actoprotective Effect of Ginseng: Improving Mental and Physical Performance. J. Ginseng Res. 2013, 37, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Zhang, M.; Xu, M.; Tang, W.; Zhang, Y.; Lu, L.; Gao, J.; Gao, S. Intranasal Delivery of Microspheres Loaded with 20(R)-ginsenoside Rg3 Enhances Anti-fatigue Effect in Mice. Curr. Drug Deliv. 2017, 14, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.A.; Kim, D.E.; Choi, H.J.; Kim, N.J.; Kim, D.H. Anti-fatigue effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatiol in mice. Biol. Pharm. Bull. 2015, 38, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Ju, S.H.; Oh, J.; Lee, S.K.; Kim, J.S. Neuroprotective and Cognition-enhancing Effects of Compound K Isolated from Red Ginseng. J. Agric. Food Chem. 2016, 64, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Rokot, N.T.; Kairupan, T.S.; Cheng, K.C.; Runtuwene, J.; Kapantow, N.H.; Amitani, M.; Morinaga, A.; Amitani, H.; Asakawa, A.; Inui, A. A role of Ginseng and its Constituents in the Treatment of Central Nervous System Disorders. Evid.-Based Complement. Altern. Med. 2016, 2016, 2614742. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.Y.; Park, J.H.; Hong, J.T.; Yoo, H.S.; Song, S.; Hwang, B.Y.; Eun, J.S.; Oh, K.W. Anxiolytic-like Effects of Ginsenosides on the Elevated Plus-maze model in Mice. Biol. Pharm Bull. 2005, 28, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Gu, W.; Liu, Y.; Zhang, M. Ginsenoside Rb1 Protects Hippocampal Neurons from High Glucose-induced Neurotoxicity by Inhibiting GSK3β-mediated CHOP Induction. Mol. Med. Rep. 2014, 9, 1434–1438. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, S.Y.; Lee, J.H.; Jeong, S.M.; Yoon, I.S.; Lee, B.H.; Lee, J.H.; Pyo, M.K.; Lee, S.M.; Chung, J.M.; et al. Neuroprotective Effects of Ginsenoside Rg3 Against Homocysteine-induced Excitotoxicity in Rat Hippocampus. Brain Res. 2007, 1136, 190–199. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, D.D.; Fang, L.; Wu, F.; Kwong, W.H.; Xu, J. Ginsenoside Rg1 Promotes Nonamyloidgenic Cleavage of APP Via Estrogen Receptor Signaling to MAPK/ERK and PI3K/Akt. Biochim. Biophys. Acta. Gen. Subj. 2012, 1820, 453–460. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective Effects of Ginseng on Neurological Disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef]
- Choi, S.; Kim, T.W.; Singh, S.V. Ginsenoside Rh2-mediated G1 Phase Cell Cycle Arrest in Human Breast Cancer Cells is Caused by P15INK4B and P27KIP1-dependent Inhibition of Cyclin-dependent Kinases. Pharm. Res. 2009, 26, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Cho, S.H.; Shin, J.S.; Kim, D.H.; Choi, J.H.; Choi, S.Y.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Ginsenoside Rh2 Induces Cell Cycle Arrest and Differentiation in Human Leukemia Cells by Upregulating TGF-β Expression. Carcinogenesis 2013, 34, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Song, Y.M.; Wang, B.; Zhang, F.R.; Yang, R.; Wang, H.Q.; Zhang, G.J. Ginsenoside Rg3 Sensitizes Human Non-small Cell Lung Cancer Cells to Γ-radiation by Targeting the Nuclear Factor-κB Pathway. Mol. Med. Rep. 2015, 12, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, M.; Wu, X.; Hu, Y.; Cao, Y.; Wang, X.; Xiang, S.; Li, H.; Jiang, L.; Tan, Z.; et al. 20(S)-Ginsenoside Rg3 Promotes Senescence and Apoptosis in Gallbladder Cancer Cells Via the P53 Pathway. Drug Des. Dev. Ther. 2015, 9, 3969–3987. [Google Scholar] [CrossRef]
- Liu, W.K.; Xu, S.X.; Che, C.T. Anti-proliferative Effect of Ginseng Saponins on Human Prostate Cancer Cell Line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef]
- Leung, K.W.; Cheung, L.W.T.; Pon, Y.L.; Wong, R.N.S.; Mak, N.K.; Fan, T.P.P.; Au, S.C.L.; Tombran-Tink, J.; Wong, A.S.T. Ginsenoside Rb1 Inhibits Tube-like Structure Formation of Endothelial Cells by Regulating Pigment Epithelium-derived Factor Through the Oestrogen β receptor. Br. J. Pharmacol. 2007, 152, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yoo, Y.C.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tono-oka, S.; Samukawa, K.; Azuma, I. Inhibitory Effect of Tumor Metastasis in Mice by Saponins, Ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of Red Ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.J.; Shui, Y.M.; Wan, J.B.; Gao, J.L. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-type Ginsenosides and their Metabolites. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Shin, K.O.; Seo, C.H.; Cho, H.H.; Oh, S.; Hong, S.P.; Yoo, H.S.; Hong, J.T.; Oh, K.W.; Lee, Y.M. Ginsenoside Compound K Inhibits Angiogenesis Via Regulation of Sphingosine Kinase-1 in Human Umbilical Vein Endothelial Cells. Arch. Pharm. Res. 2014, 37, 1183–1192. [Google Scholar] [CrossRef]
- Deng, S.; Wong, C.K.C.; Lai, H.-C.; Wong, A.S.T. Ginsenoside-Rb1 Targets Chemotherapy-resistant Ovarian Cancer Stem Cells Via Simultaneous Inhibition of WNT/β-catenin Signaling and Epithelial-to-Mesenchymal Transition. Oncotarget 2016, 8, 25897–25914. [Google Scholar] [CrossRef]
- Kenarova, B.; Neychev, H.; Hadjiivanova, C.; Petkov, V.D. Immunomodulating Activity of Ginsenoside Rg1 from Panax ginseng. Jpn. J. Pharmacol. 1990, 54, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, S.J.; Zhang, H.T.; Li, N.; Liu, T.; Zhang, Y.L.; Li, X.X.; Ma, Q.; Qiu, X.C.; Fan, Q.Y.; et al. Ginsenoside Rh2 Enhances the Antitumor Immunological Response of a Melanoma Mice Model. Oncol. Lett. 2017, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Tachikawa, E.; Umeyama, A. Dendritic Cells Promoted by Ginseng Saponins Drive a Potent Th1 Polarization. Biomark. Insights 2017, 3, 269–286. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Wang, Q.; Chang, Y.; Liu, K.; Song, S.; Yuan, P.; Fu, J.; Sun, W.; Huang, Q.; et al. Ginsenoside Metabolite Compound K Alleviates Adjuvant-induced Arthritis by Suppressing T Cell Activation. Inflammation 2014, 37, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Guo, R.; Wu, S.L.; Sun, M.; Li, M. Protective Effects of Ginsenoside Rb1 on Human Umbilical Vein Endothelial Cells in vitro. J. Cardiovasc. Pharmacol. 2007, 50, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.H.; Guo, G.L.; Lau, J.K.; Cheng, Y.K.; Wang, J.R.; Jiang, Z.H.; Keung, M.H.; Mak, N.K.; Yue, P.Y.; Wong, R.N. Stereoisomers Ginsenosides-20(S)-Rg₃ and -20(R)-Rg₃ Differentially Induce Angiogenesis Through Peroxisome Proliferator-activated Receptor-gamma. Biochem. Pharmacol. 2012, 83, 893–902. [Google Scholar] [CrossRef]
- Yu, J.; Eto, M.; Akishita, M.; Kaneko, A.; Ouchi, Y.; Okabe, T. Signaling Pathway of Nitric Oxide Production Induced by Ginsenoside Rb1 in Human Aortic Endothelial Cells: A Possible Involvement of Androgen Receptor. Biochem. Biophys. Res. Commun. 2007, 353, 764–769. [Google Scholar] [CrossRef]
- Jiang, Q.S.; Huang, X.N.; Dai, Z.K.; Yang, G.Z.; Zhou, Q.X.; Shi, J.S.; Wu, Q. Inhibitory Effect of Ginsenoside Rb1 on Cardiac Hypertrophy Induced by Monocrotaline in Rat. J. Ethnopharmacol. 2007, 111, 567–572. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, L.; Li, C.R.; Wang, X.W.; Ma, Y.J.; Zhong, Z.Y.; Zhao, H.B.; Cui, J.; Xun, S.F.; Huang, X.L.; et al. Ginsenoside Rg1 Protects Rat Cardiomyocyte from Hypoxia/Reoxygenation Oxidative Injury Via Antioxidant and Intracellular Calcium Homeostasis. J. Cell. Biochem. 2009, 108, 117–124. [Google Scholar] [CrossRef]
- Furukawa, T.; Bai, C.X.; Kaihara, A.; Ozaki, E.; Kawano, T.; Nakaya, Y.; Awais, M.; Sato, M.; Umezawa, Y.; Kurokawa, J. Ginsenoside Re, a Main Phytosterol of Panax Ginseng, Activates Cardiac Potassium Channels Via a Nongenomic Pathway of Sex Hormones. Mol. Pharmacol. 2006, 70, 1916–1924. [Google Scholar] [CrossRef]
- Xie, J.T.; Shao, Z.H.; Vanden Hoek, T.L.; Chang, W.T.; Li, J.; Mehendale, S.; Wang, C.Z.; Hsu, C.W.; Becker, L.B.; Yin, J.J.; et al. Antioxidant Effects of Ginsenoside Re in Cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Gong, Q.H.; Wei, L.W.; Wu, Q.; Huang, X.N. Total Ginsenosides Inhibit the Right Ventricular Hypertrophy Induced by Monocrotaline in Rats. Biol. Pharm. Bull. 2008, 31, 1530–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 Protects Against 6-hydroxydopamine-induced Oxidative Stress by Increasing Heme Oxygenase-1 Expression Through an Estrogen Receptor-related PI3K/Akt/Nrf2-dependent Pathway in Human Dopaminergic Cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Haas, M.; Wang, D.Q.H.; May, A.; Lo, C.C.; Obici, S.; Tso, P.; Woods, S.C.; Liu, M. Ginsenoside Rb1 Increases Insulin Sensitivity by Activating AMP-activated Protein Kinase in Male Rats. Physiol. Rep. 2015, 3, 12543. [Google Scholar] [CrossRef]
- Xiong, Y.; Shen, L.; Liu, K.J.; Tso, P.; Xiong, Y.; Wang, G.; Woods, S.C.; Liu, M. Antiobesity and Antihyperglycemic Effects of Ginsenoside Rb1 in Rats. Diabetes 2010, 59, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-diabetic Agent and its Underlying Mechanism Analysis. Cells 2019, 28, 204. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.Z.; Ji, A.W.; Aung, H.H.; Rue, P.A.; Bell, G.I.; et al. Anti-diabetic Effect of Ginsenoside Re in Ob/Ob mice. Biochim. Biophys. Acta-Mol. Basis Dis. 2005, 1740, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, J. Diabetic Cardiomyopathy: Where We are and Where We are Going. Korean J. Intern. Med. 2017, 32, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.; Hsu, C.T.; Niu, H.S.; Niu, C.S.; Cheng, J.T.; Chen, Z.C. Ginsenoside Rh2 Improves Cardiac Fibrosis Via PPARδ-STAT3 Signaling in Type 1-like Diabetic rats. Int. J. Mol. Sci. 2017, 18, 1364. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Y.; Yin, H.; Wang, J.; Chen, K.; Li, Y. Developmental Toxicity Research of Ginsenoside Rb1 Using a Whole Mouse Embryo Culture Model. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2005, 74, 207–209. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, S.W.; Kim, S.Y.; Cho, I.H.; Kim, H.C.; Rhim, H.; Kim, M.; Nah, S.Y. Panax Ginseng as an Adjuvant Treatment for Alzheimer’s Disease. J. Ginseng Res. 2018, 42, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Park, M.H.; Lee, J.H. Effect of Korean Red Ginseng on Cognitive Function and Quantitative EEG in Patients with Alzheimer’s Disease: A Preliminary Study. J. Altern. Complement. Med. 2015, 22, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Gao, Y.T.; Shu, X.O.; Kahkeshani, K.; Ji, B.T.; Yang, G.; Li, H.L.; Rothman, N.; Chow, W.H.; Zheng, W. Ginseng Intake and Gastric Cancer Risk in the Shanghai Women’s Health Study Cohort. Cancer Epidemiol. Biomark. Prev. 2007, 16, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Ovodov, Y.S.; Solov’eva, T.F. Polysaccharides of Panax ginseng. Chem. Nat. Compd. 1966, 2, 242–245. [Google Scholar] [CrossRef]
- Jovanovski, E.; Jenkins, A.; Dias, A.G.; Peeva, V.; Sievenpiper, J.; Arnason, J.T.; Rahelic, D.; Josse, R.G.; Vuksan, V. Effects of Korean Red Ginseng (Panax Ginseng C.A. Mayer) and its Isolated Ginsenosides and Polysaccharides on Arterial Stiffness in Healthy Individuals. Am. J. Hypertens. 2010, 23, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Han, Y.N.; Ryu, S.Y.; Han, B.H.; Woo, L.K. Spinacine From Panax Ginseng. Arch. Pharm. Res. 1987, 10, 258. [Google Scholar] [CrossRef]
- Wright, G.D. Unlocking the Potential of Natural Products in Drug Discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.Y. The Green Tea Polyphenol EGCG Potentiates the Antiproliferative Activity of Sunitinib in Human Cancer Cells. Tumor Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
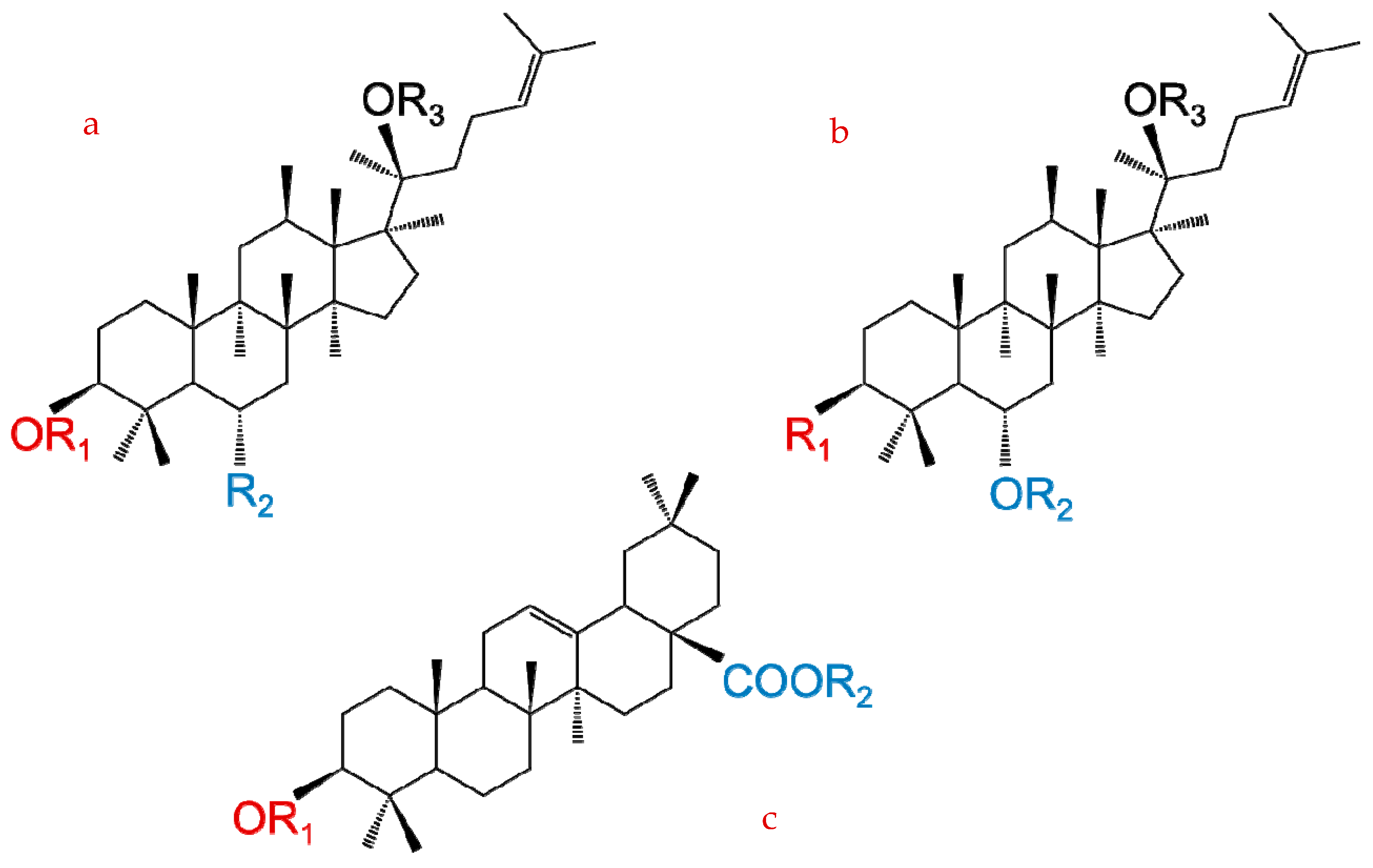
| Formula | Carbohydrate Moieties | |||
|---|---|---|---|---|
| R1 (C3) | R2 (C6) | R3 (C20) | ||
| Protopanaxadiol | C30H52O3 | H | H | H |
| Ra1 | C58H98O26 | Glc-Glc | H | Glc-Ara(p)-Xyl |
| Ra2 | C58H98O26 | Glc-Glc | H | Glc-Ara(f)-Xyl |
| Ra3 | C59H100O27 | Glc-Glc | H | Glc-Glc-Xyl |
| Rb1 | C54H92O23 | Glc-Glc | H | Glc-Glc |
| Rb2 | C54H90O22 | Glc-Glc | H | Glc-Ara(p) |
| Rb3 | C53H90O22 | Glc-Glc | H | Glc-Xly |
| Rc | C53H90O22 | Glc-Glc | H | Glc-Ara(f) |
| Rd | C48H82O18 | Glc-Glc | H | Glc |
| Rg3 | C42H72O13 | Glc-Glc | H | H |
| Rh2 | C36H62O8 | Glc | H | H |
| Rs1 | C36H92O23 | Glc-Glc-Ac | H | Glc-Ara(p) |
| Rs2 | C55H92O23 | Glc-Glc-Ac | H | Glc-Ara(f) |
| Rs3 | C44H74O14 | Glc-Glc-Ac | H | H |
| Compound K | C36H62O8 | H | H | Glc |
| Protopanaxatriol | C30H52O4 | H | H | H |
| Re | C48H82O18 | H | Glc-Rha | Glc |
| Rf | C42H72O14 | H | Glc-Glc | H |
| Rg1 | C42H72O14 | H | Glc | Glc |
| Rg2 | C42H72O13 | H | Glc-Rha | H |
| Rh1 | C36H62O9 | H | Glc | H |
| F1 | C36H62O9 | H | H | Glc |
| F3 | C41H70O13 | H | H | Glc-Ara(p) |
| Oleanolic acid | ||||
| Ro | C48H76O19 | GlcUA-Glc | Glc | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.-Y.; Zeng, J.-Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. https://doi.org/10.3390/molecules24132443
Shi Z-Y, Zeng J-Z, Wong AST. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules. 2019; 24(13):2443. https://doi.org/10.3390/molecules24132443
Chicago/Turabian StyleShi, Ze-Yu, Jin-Zhang Zeng, and Alice Sze Tsai Wong. 2019. "Chemical Structures and Pharmacological Profiles of Ginseng Saponins" Molecules 24, no. 13: 2443. https://doi.org/10.3390/molecules24132443
APA StyleShi, Z.-Y., Zeng, J.-Z., & Wong, A. S. T. (2019). Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules, 24(13), 2443. https://doi.org/10.3390/molecules24132443






