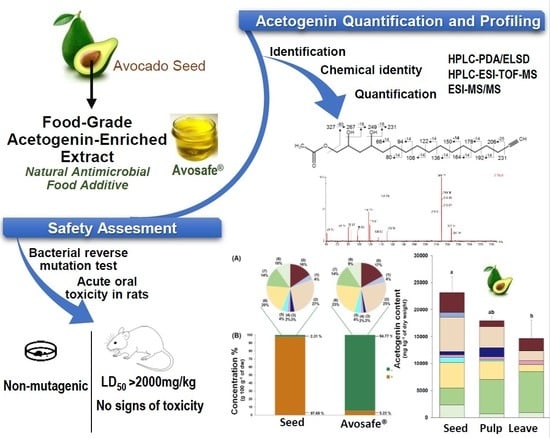
Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed
Abstract
:1. Introduction
2. Results
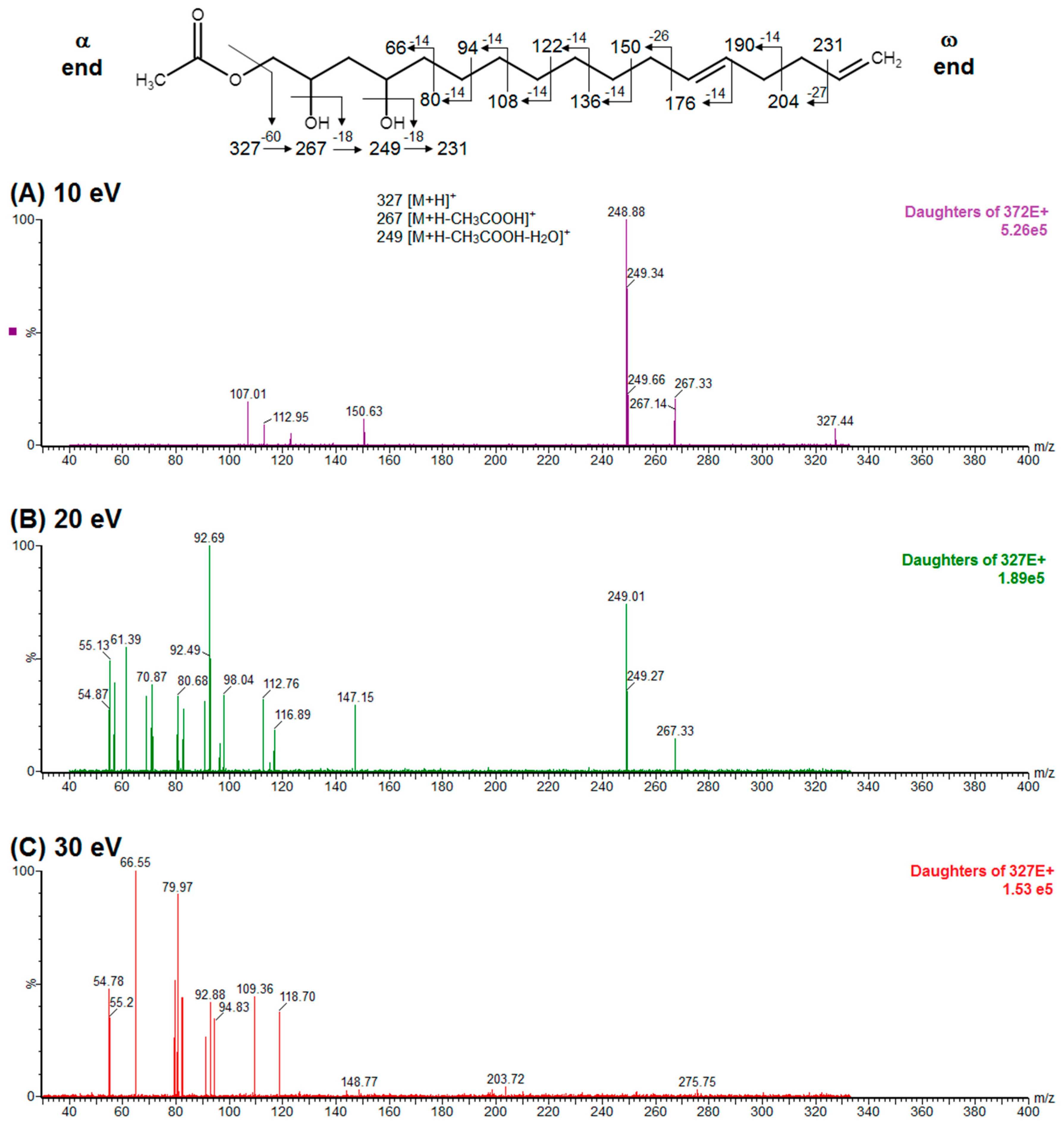
2.1. Confirmation of Chemical Structure of Two Acetogenin Compounds Present in An Acetogenin-Enriched Avocado Seed Extract (Avosafe®)
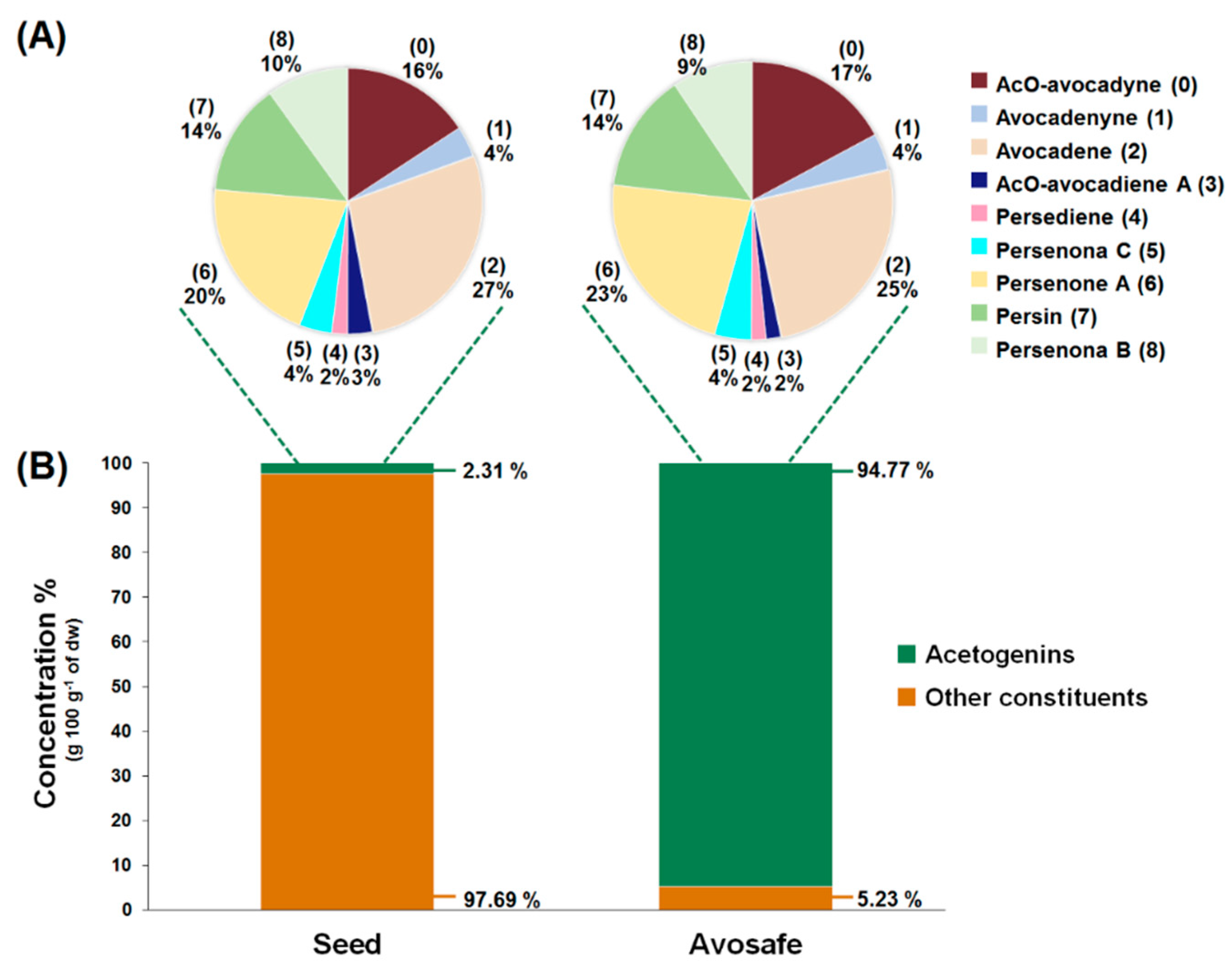
2.2. Quantification of Acetogenins Found in Avosafe®, a Food-Grade Acetogenin-Enriched Extract
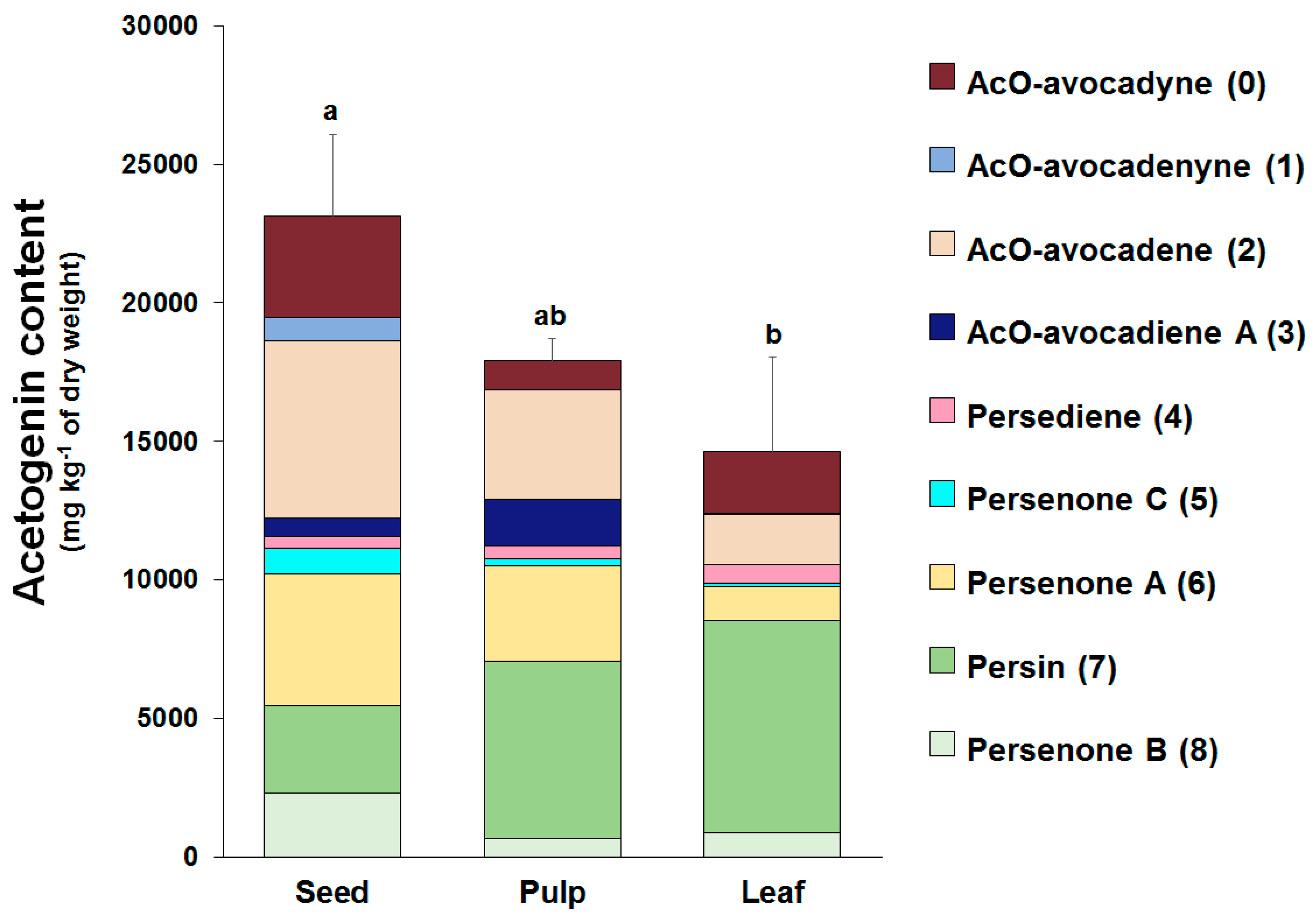
2.3. Individual Profiles and Quantification of Acetogenins Present in Avocado (‘Hass’ cultivar) Seed, Pulp, and Leaves using the Improved Analytical Methodology
2.4. Bacterial Reverse Mutation Test (AMES Test)
2.5. Study I-Acute Oral Toxicity in Rats (Fixed Dose Method): Acute Median Lethal Oral Dose (LD50) and Macroscopic Pathology Analyses
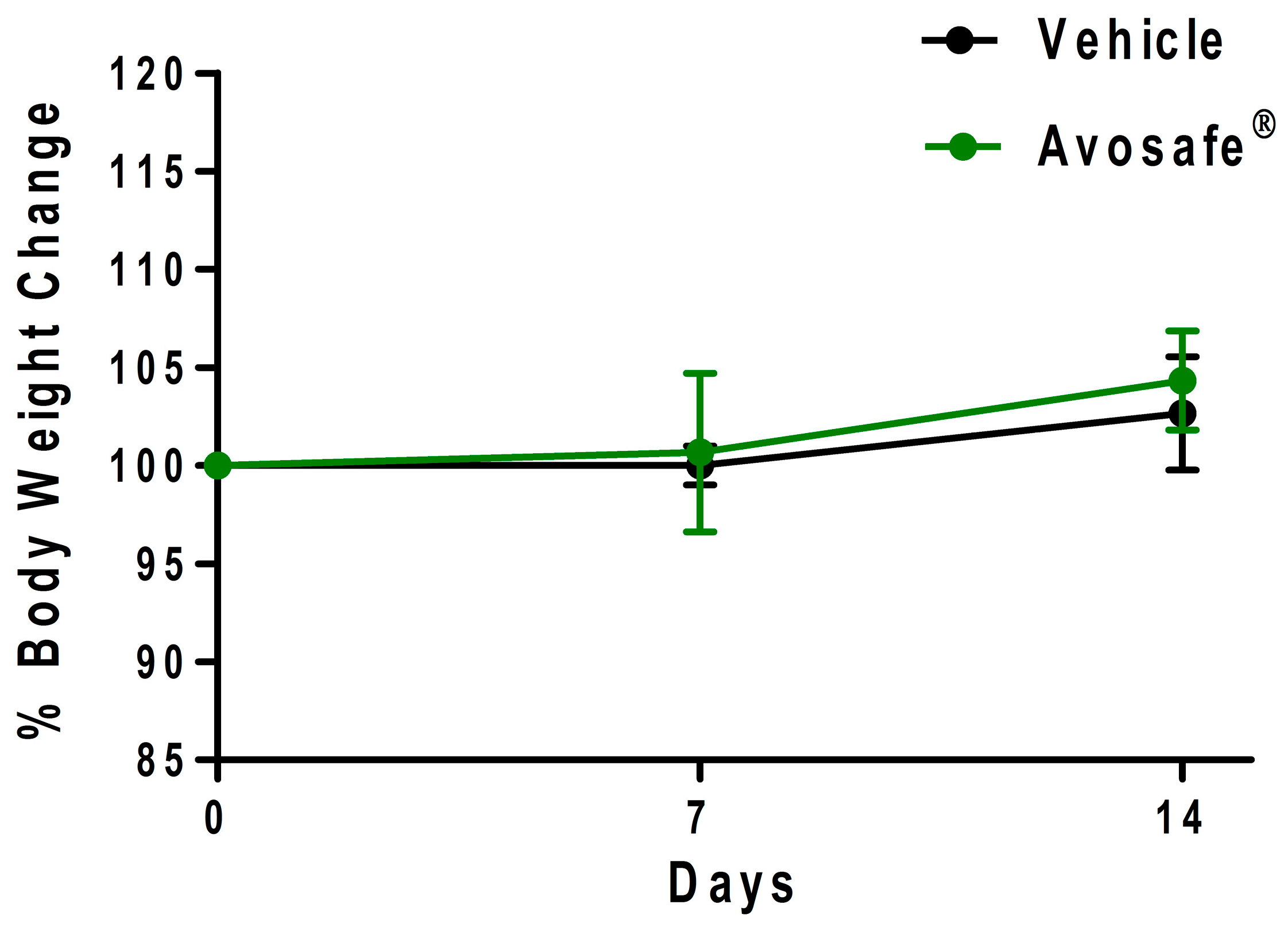
2.6. Study II-Acute Oral Toxicity in Rats (Single Dose of 2000 mg·kg−1): Clinical Observations, Hematology, and Serum Biochemistry
2.6.1. Clinical Observations and Body Weight
2.6.2. Hematology and Serum Biochemistry Analysis
2.6.3. Macroscopic Observations
3. Discussion
3.1. Identification and Quantification of Acetogenins Found in Avocado Seed and in a Food-Grade Acetogenin-Enriched Extract Obtained from Avocado Seed (Avosafe®)
3.2. Improvements in the Quantification of Acetogenins Present in Avocado (‘Hass’ cultivar) Seed, Pulp, and Leaf
3.3. Insights on the Safety of a Food-Grade Avocado Seed Extract Enriched in Acetogenins (94.74% w/w Purity)
4. Materials and Methods
4.1. Materials
4.2. Analyses of Acetogenin Composition
4.2.1. Acetogenin Extraction for HPLC-PDA/ELSD Analysis
4.2.2. Acetogenin Identification and Quantification by HPLC-PDA/ELSD
4.2.3. Elucidation of Chemical identity of Acetogenin Chromatographic Peaks
4.3. Bacterial Reverse Mutation Test (AMES Test)
4.4. Study I-Acute Oral Toxicity in Rats (Fixed Dose Method): Acute Median Lethal Oral Dose (LD50) and Macroscopic Pathology Analyses
4.4.1. Animal Use
4.4.2. Sighting Study and Main Study
4.4.3. Observations and Macroscopic Pathology Analysis
4.5. Study II-Acute Oral Toxicity in Rats (Single Dose of 2000 mg·kg−1): Hematology, Serum Biochemistry, and Histological Examination
4.5.1. Animal Use and Treatment
4.5.2. Clinical Observations and Body Weight
4.5.3. Hematology and Serum Biochemistry Analysis
4.5.4. Necropsy and Histopathology
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rodriguez-Saona, C.; Trumble, J.T. Biologically active aliphatic acetogenins from specialized idioblast oil cells. Curr. Org. Chem. 2000, 4, 1249–1260. [Google Scholar] [CrossRef]
- Rodríguez-López, C.E.; Hernández-Brenes, C.; Diaz de la Garza, R.I. A targeted metabolomics approach to characterize acetogenin profiles in avocado fruit (Persea americana Mill.). RSC Adv. 2015, 5, 106019–106029. [Google Scholar] [CrossRef]
- Carman, R.M.; Handley, P.N. Antifungal diene in leaves of various avocado cultivars. Phytochemistry 1999, 50, 1329–1331. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, D.G.; Pacheco, A.; García-Cruz, M.I.; Gutiérrez-Uribe, J.A.; Benavides-Lozano, J.A.; Hernández-Brenes, C. Isolation and structure elucidation of avocado seed (Persea americana) lipid derivatives that inhibit Clostridium sporogenes endospore germination. J. Agric. Food Chem. 2013, 61, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Rodríguez-Sánchez, D.G.; Villarreal-Lara, R.; Navarro-Silva, J.M.; Senés-Guerrero, C.; Hernández-Brenes, C. Stability of the antimicrobial activity of acetogenins from avocado seed, under common food processing conditions, against Clostridium sporogenes vegetative cell growth and endospore germination. Int. J. Food Sci. Technol. 2017, 52, 2311–2323. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Hernández-Brenes, C.; Rodríguez-Sánchez, D.; Castillo, E.; Navarro-Silva, J.; Pacheco, A. Inhibitory activity of avocado seed fatty acid derivatives (acetogenins) against Listeria monocytogenes. J. Food Sci. 2017, 82, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Lara, R.; Rodríguez-Sánchez, D.G.; Díaz de la Garza, R.; García-Cruz, M.; Castillo, A.; Pacheco, A.; Hernández-Brenes, C. Purified Avocado Seed Acetogenins: Antimicrobial Spectrum and Complete Inhibition of Listeria monocytogenes in a Refrigerated Food Matrix. CyTA-J. Food 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Erickson, M.C.; Doyle, M.P. The challenges of eliminating or substituting antimicrobial preservatives in foods. Annu. Rev. Food Sci. Technol. 2017, 8, 371–390. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Engel, A.; Fattinger, K.; Marbet, U.A.; Criblez, D.; Reichen, J.; Zimmermann, A.; Oneta, C.M. Herbal does not mean innocuous: Ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife® products. J. Hepatol. 2007, 47, 521–526. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Trumble, J. Toxicity, growth, and behavioral effects of an oil extracted from idioblast cells of the avocado fruit on the generalist herbivore beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1996, 89, 1571–1577. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Trumble, J.T. Secretory avocado idioblast oil cells: Evidence of their defensive role against a non-adapted insect herbivore. Entomol. Exp. Appl. 2000, 94, 183–194. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Millar, J.G.; Trumble, J.T. Growth inhibitory, insecticidal, and feeding deterrent effects of (12Z, 15Z)-1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15-diene, a compound from avocado fruit, to Spodoptera exigua. J. Chem. Ecol. 1997, 23, 1819–1831. [Google Scholar] [CrossRef]
- Kobiler, I.; Prusky, D.; Midland, S.; Sims, J.J.; Keen, N.T. Compartmentation of antifungal compounds in oil cells of avocado fruit mesocarp and its effect on susceptibility to Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 1993, 43, 319–328. [Google Scholar] [CrossRef]
- Ramos-Jerz, M.R. Phytochemical Analysis of Avocado Seeds (Persea americana Mill., cv Hass); Cuvillier: Göttingen, Germany, 2007; p. 311. [Google Scholar]
- Domergue, F.; Helms, G.; Prusky, D.; Browse, J. Antifungal compounds from idioblast cells isolated from avocado fruits. Phytochemistry 2000, 54, 183–189. [Google Scholar] [CrossRef]
- Kawagishi, H.; Fukumoto, Y.; Hatakeyama, M.; He, P.; Arimoto, H.; Matsuzawa, T.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Liver injury suppressing compounds from avocado (Persea americana). J. Agric. Food Chem. 2001, 49, 2215–2221. [Google Scholar] [CrossRef]
- Kim, E.J.; Tian, F.; Woo, M.H. Asitrocin, (2,4)-cis- and trans-asitrocinones: Novel bioactive mono-tetrahydrofuran acetogenins from Asimina triloba seeds. J. Nat. Prod. 2000, 63, 1503–1506. [Google Scholar] [CrossRef]
- Gleye, C.; Raynaud, S.; Fourneau, C.; Laurens, A.; Laprévote, O.; Serani, L.; Fournet, A.; Hocquemiller, R. Cohibins C and D, two important metabolites in the biogenesis of acetogenins from Annona muricata and Annona nutans. J. Nat. Prod. 2000, 63, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Millar, J.G.; Maynard, D.F.; Trumble, J.T. Novel antifeedant and insecticidal compounds from avocado idioblast cell oil. J. Chem. Ecol. 1998, 24, 867–889. [Google Scholar] [CrossRef]
- Hsu, F.; Turk, J. Distinction Among Isomeric Unsaturated Fatty Acids as Lithiated Adducts by Electrospray Ionization Mass Spectrometry Using Low Energy Collisionally Activated Dissociation on a Triple Stage Quadrupole Instrument. Am. Soc. Mass Spectrom. 1999, 10, 600–612. [Google Scholar] [CrossRef]
- Yang, K.; Dilthey, B.G.; Gross, R.W. Identification and quantitation of fatty acid double bond positional isomers: A shotgun lipidomics approach using charge-switch derivatization. Anal. Chem. 2013, 85, 9742–9750. [Google Scholar] [CrossRef]
- Haiming, D.; Chin, Y.-W.; Kinghorn, A.D.; D’Ambrosio, S.M. Chemopreventive characteristics of avocado fruit. Semin. Cancer Biol. 2007, 17, 386–394. [Google Scholar] [CrossRef]
- Degenhardt, A.G.; Hofmann, T. Bitter-tasting and kokumi-enhancing molecules in thermally processed avocado (Persea americana Mill.). J. Agric. Food Chem. 2010, 58, 12906–12915. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Tsai, Y.-F.; Huang, T.-T.; Chen, P.-Y.; Liang, W.-L.; Lee, C.-K. Heptadecanols from the leaves of Persea americana var. americana Tzong-Huei. Food Chem. 2012, 132, 921–924. [Google Scholar] [CrossRef]
- Dictionary of Food Compounds. Available online: https://web.archive.org/save/http://dfc.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 19 June 2019).
- OECD (Organisation for Economic Co-operation and Development). Guidelines for the Testing of Chemicals. Genetic Toxicology: Bacterial Reverse Mutation Test, Guideline 471; OECD: Paris, France, 1997. [Google Scholar] [CrossRef]
- Mahon, G.A.T.; Middleton, B.; Robinson, W.D.; Green, M.H.L.; de Mitchell, I.G.; Tweats, D.J. Analysis of data from microbial colony assays. In Statistical Evaluation of Mutagenicity Data; David, J., Kirkland, G.A.T.M., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 26–65. [Google Scholar]
- Oelrichs, P.B.; Ng, J.C.; Seawright, A.A.; Ward, A.; Schaffeler, L.; MacLeod, J.K. Isolation and identification of a compound from avocado (Persea americana) leaves which causes necrosis of the acinar epithelium of the lactating mammary gland and the myocardium. Nat. Toxins 1995, 3, 344–349. [Google Scholar] [CrossRef]
- Freireich, E.J.; Gehan, E.A.; Rail, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative Comparison of Toxicity of Anticancer Agents in Mouse, Rat, Hamster, Dog, Monkey, and Man. Cancer Chemother. Rept 1966, 50, 219–244. [Google Scholar]
- OECD Guideline 420 for testing of chemicals: Acute oral toxicity-Fixed Dose Procedure. 2001. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl420.pdf (accessed on 20 January 2019).
- OECD Harmonised Integrated Hazard Classification for Human Health and Environmental Effects of Chemical Substances and mixtures. Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology in 14 August 2001. Available online: http://www.oecd.org/chemicalsafety/risk-management/37182285.pdf (accessed on 18 January 2019).
- Fajardo, R.J.; Karim, L.; Calley, V.I.; Bouxsein, M.L. A Review of Rodent Models of Type 2 Diabetic Skeletal Fragility. JBMR 2014, 29, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, S.M.; Han, C.; Pan, L.; Kinghorn, A.D.; Ding, H. Aliphatic acetogenin constituents of avocado fruits inhibit human oral cancer cell proliferation by targeting the EGFR/RAS/RAF/MEK/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2011, 409, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Kashman, Y.; Néeman, I.; Lifshitz, A. New compounds from avocado pear. Tetrahedron 1969, 25, 4617–4631. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chang, H.-S.; Peng, C.-F.; Lin, C.-H.; Chen, I.-S. Secondary metabolites from the unripe pulp of Persea americana and their antimycobacterial activities. Food Chem. 2012, 135, 2904–2909. [Google Scholar] [CrossRef]
- Leikin-Frenkel, A.; Prusky, D. Ethylene enhances the antifungal lipid content in idioblasts from avocado mesocarp. Phytochemistry 1998, 49, 2291–2298. [Google Scholar] [CrossRef]
- Carman, R.M.; Duffield, A.R. The isolation of (R)-2-Hydroxy-4-oxohenicosan-1-yl acetate from avocado leaves. Tetrahedron Lett. 1995, 36, 2119–2120. [Google Scholar] [CrossRef]
- Chang, C.F.; Isogai, A.; Kamikado, T.; Murakoshi, S.; Sakurai, A.; Tamura, S. Isolation and structure elucidation of growth inhibitors for silkworm larvae from avocado leaves. Agric. Biol. Chem. 1975, 39, 1167–1168. [Google Scholar] [CrossRef]
- Bowen, J.; Burdon, J.; Billing, D.; Cooney, J.; Connolly, P.; Smith, W. Maturity, storage and ripening effects on anti-fungal compounds in the skin of ‘Hass’ avocado fruit. Postharvest Biol. Technol. 2018, 146, 43–50. [Google Scholar] [CrossRef]
- Brown, B.I. Qualitative and quantitative estimation by thin-layer and gas chromatography of a series of C17 oxygenated aliphatic compounds in the avocado (Persea americana). J. Chromatogr. 1973, 86, 239–245. [Google Scholar] [CrossRef]
- Adnani, N.; Michel, C.R.; Bugni, T.S. Universal quantification of structurally diverse natural products using an evaporative light scattering detector. J. Nat. Prod. 2012, 75, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. (Tokyo). 2014, 67, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Ruiz-Gutierrez, V. Quantification of major lipid classes in human triacylglycerol-rich lipoproteins by high-performance liquid chromatography with evaporative light-scattering detection. J. Sep. Sci. 2004, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Tomer, K.B.; Crow, F.W.; Gross, M.L. Location of Double Bond Position in Unsaturated Fatty Acids by Negative Ion MS/MS. J. Am. Chem. Soc. 1983, 105, 5487–5488. [Google Scholar] [CrossRef]
- Wysocki, V.H.; Ross, M.M. Charge-remote fragmentation of gas-phase ions: Mechanistic and energetic considerations in the dissociation of long-chain functionalized alkanes and alkenes. Int. J. Mass Spectrom. Ion Process. 1991, 104, 179–211. [Google Scholar] [CrossRef]
- Gross, M.L. Charge-remote fragmentation: An account of research on mechanisms and applications. Int. J. Mass Spectrom. 2000, 200, 611–624. [Google Scholar] [CrossRef]
- Mitchell, T.W.; Pham, H.; Thomas, M.C.; Blanksby, S.J. Identification of double bond position in lipids: From GC to OzID. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.M.; Zhou, D.; Wu, J.; Shi, G.; Zeng, L.; McLaughlin, J.L. Screening for annonaceous acetogenins in bioactive plant extracts by liquid chromatography/mass spectrometry. J. Nat. Prod. 1997, 60, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, A.E.; Bummer, P.M. Simple chromatographic method for simultaneous analyses of phosphatidylcholine, lysophosphatidylcholine, and free fatty acids. AAPS PharmSciTech 2010, 11, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.G.; Libong, D.; Rakotomanga, M.; Gaudin, K.; Loiseau, P.M.; Chaminade, P. Comparison between charged aerosol detection and light scattering detection for the analysis of Leishmania membrane phospholipids. J. Chromatogr. A 2008, 1209, 88–94. [Google Scholar] [CrossRef]
- Rodríguez-López, C.E.; Hernández-Brenes, C.; Treviño, V.; Díaz de la Garza, R.I. Avocado fruit maturation and ripening: Dynamics of aliphatic acetogenins and lipidomic profiles from mesocarp, idioblasts and seed. BMC Plant Biol. 2017, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Camberos, E.; Martínez-Velázquez, M.; Flores-Fernández, J.M.; Villanueva-Rodríguez, S. Acute toxicity and genotoxic activity of avocado seed extract (Persea americana Mill., c.v. Hass). Sci. World. J. 2013, 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Grúz, P.; Shimizu, M.; Sugiyama, K.-I.; Honma, M. Mutagenicity of ω-3 fatty acid peroxidation products in the Ames test. Mutat. Res. Toxicol. Environ. Mutagen. 2017, 819, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Liu, J.; Du, S.; Morrow, J.D.; Ames, B.N.; Wander, R.C. Supplementation of postmenopausal women with fish oil rich in eicosapentaenoic acid and docosahexaenoic acid is not associated with greater in vivo lipid peroxidation compared with oils rich in oleate and linoleate as assessed by plasma malondialdehyde an. Am. J. Clin. Nutr. 2000, 72, 714–722. [Google Scholar] [CrossRef]
- OECD Screening Information Data Sets (SIDS). SIDS Initial Assessment Profile on 1,2-Dihydroxypropane (CAS No.: 57-55-6). UNEP Publications, 2001. SIAM 11. Available online: http://www.inchem.org/documents/sids/sids/57-55-6.pdf (accessed on 25 January 2019).
- Butt, A.J.; Roberts, C.G.; Seawright, A.A.; Oelrichs, P.B.; Macleod, J.K.; Liaw, T.Y.E.; Kavallaris, M.; Somers-edgar, T.J.; Lehrbach, G.M.; Watts, C.K.; et al. A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol. Cancer Ther. 2006, 5, 2300–2309. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, D.G.; Flores-García, M.; Silva-Platas, C.; Rizzo, S.; Torre-Amione, G.; De Peña-Diaz, A.; Hernández-Brenes, C.; García-Rivas, G. Isolation and chemical identification of lipid derivatives from avocado (Persea americana) pulp with antiplatelet and antithrombotic activities. Food Funct. 2015, 6, 193–203. [Google Scholar] [CrossRef]
- Craigmill, A.; Seawright, A.; Mattila, T. Pathological changes in the mammary gland and biochemical changes in milk of the goat following oral dosing with leaf of the avocado (Persea americana). Aust. Vet. J. 1989, 66, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanderbali, A.S.; Albert, V.A.; Ashworth, V.E.T.M.; Clegg, M.T.; Litz, R.E.; Soltis, D.E.; Soltis, P.S. Persea americana (avocado): Bringing ancient flowers to fruit in the genomics era. BioEssays 2008, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Seawright, A.; Oelrichs, P.; Ng, J.; MacLeod, J. Method of treatment of cancer as well as method of inhibition of lactation in mammals. US Patent 6,057,366, 2 May 2000. Available online: http://web.archive.org/web/20190619193410/http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=6,057,366.PN.&OS=PN/6,057,366&RS=PN/6,057,366 (accessed on 19 June 2019).
- OECD Screening Information Data Sets (SIDS). SIDS Initial Assessment Profile on sodium nitrite (CAS N°: 7632-00-0). UNEP Publications, 2005. SIAM 20. Available online: http://www.inchem.org/documents/sids/sids/7632000.pdf (accessed on 25 February 2019).
- EC Commission Regulation No. 440/2008. Method, B.13/14: Mutagenicity—Reverse mutation test using bacteria. OJ L 142/248. Available online: http://enfo.agt.bme.hu/drupal/sites/default/files/B1314web2000.pdf (accessed on 25 February 2019).
- US EPA Health Effects Test Guidelines. OPPTS 870.5100 Bacterial Reverse Mutation Rest. EPA 712-C-98-247. Available online: https://web.archive.org/web/20190619194636/https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0156-0022 (accessed on 19 June 2019).
- US Food and Drug Administration. Toxicological Principles for the Safety Assessment of Food. In Redbook: IV.C. 1. a Bacterial Reverse Mutation Test. Available online: https://web.archive.org/web/20190619195038/https://www.fda.gov/media/79074/download (accessed on 19 June 2019).
- FDA. Guidance for industry and other stakeholders toxicological principles for the safety assessment of food ingredients. Available online: http://www.webcitation.org/query?url=https%3A%2F%2Fwww.fda.gov%2Fdownloads%2FFood%2FGuidanceRegulation%2FUCM222779.pdf.&date=2019-01-08 (accessed on 8 January 2019).
- UK Goverment. United Kingdom Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 (The Act); Statutory Instruments, No. 3039; The Stationery Office Limited: London, UK, 2012. Available online: https://web.archive.org/web/20190619195420/http://www.legislation.gov.uk/uksi/2012/3039/made (accessed on 19 June 2019).
- Japanese Ministry of Agriculture, Forestry and Fisheries, Test Data for Registration of Agricultural Chemicals, Acute oral toxicity (2-1-1), 12 Nousan No 8147, Agricultural Production Bureau, November 24. Available online: https://www.mhlw.go.jp/english/topics/foodsafety/residue/dl/01.pdf (accessed on 19 February 2019).
- EPA Health Effects Test Guidelines OPPTS 870.1100 Acute Oral Toxicity EPA 712-C-02-190. Available online: https://web.archive.org/save/https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0156-0003 (accessed on 19 June 2019).
Sample Availability: Samples of Avosafe® are available by request to Tecnologico de Monterrey Technology Transfer Office. http://ott.mty.itesm.mx. |
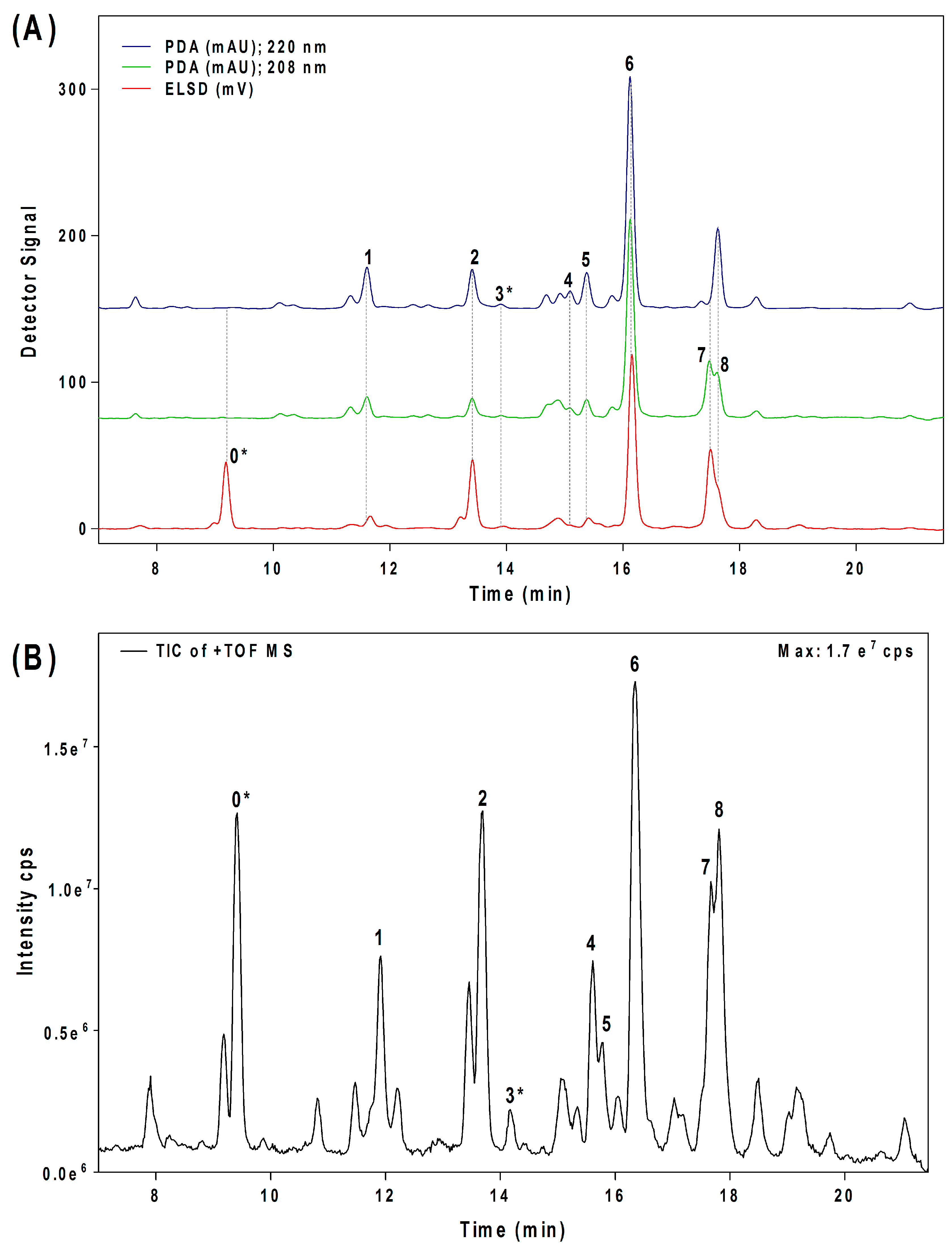

| Compound (Number) a | Elution Time (min) b/Detector | [M + H]+/Ions Pattern (m/z) c | Molecular Formula | Structure | References |
|---|---|---|---|---|---|
| AcO d-avocadyne (0) | 9.19/ELSD | 327/349, 309, 267, 249, 231 | C19H34O4 | 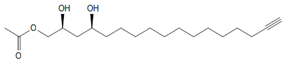 | [14] |
| AcO-avocadenyne (1) | 11.61/PDA @ 220 nm | 325/347, 307, 265 | C19H32O4 | 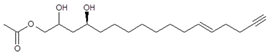 | [2,14] |
| AcO-avocadene (2) | 13.48/PDA @ 220 nm | 329/351, 311, 269, 251 | C19H36O4 |  | [15] |
| AcO-avocadiene B (3) | 13.92/PDA @ 220 nm | 327/349, 309, 267, 249, 231 | C19H34O4 | 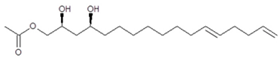 | [2,14] |
| Persediene (4) | 15.11/PDA @ 220 nm | 353/375, 335, 293 | C21H36O4 |  | [4] |
| Persenone C (5) | 15.40/PDA @ 220 nm | 353/375, 335, 293 | C21H36O4 |  | [4] |
| Persenone A (6) | 16.14/PDA @ 220 nm | 379/401, 361, 319, 301 | C23H38O4 | 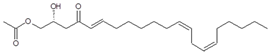 | [15] |
| Persin (7) | 17.50/PDA @ 208 nm | 381/403, 363, 321, 303 | C23H40O4 | 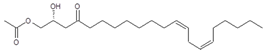 | [16] |
| Persenone B (8) | 17.63/PDA @ 208 nm | 355/377, 337, 295 | C21H38O4 | 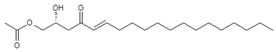 | [17] |
| Test Substance a | Concentration (µg·Plate−1) | Increase in Revertant Bacterial Colony Numbers | |||||
|---|---|---|---|---|---|---|---|
| S. typhimurium | E. coli | ||||||
| TA98 | TA100 | TA1535 | TA1537 | WP2 uvrA | |||
| Without metabolic activation | Avosafe® | 5000.0 | 1.0 | 0.0 | 0.5 | 0.0 | 0.4 |
| 2NF | 2.0 | 3.9 | - | - | - | - | |
| NaN3 | 2.0 | - | 7.2 | - | - | - | |
| NaN3 | 2.0 | - | - | 27.6 | - | - | |
| AAC | 50.0 | - | - | - | 21.5 | - | |
| NQO | 2.0 | - | - | - | - | 14.9 | |
| With metabolic activation | Avosafe® | 5000.0 | 0.5 | 0.1 | 0.3 | 0.0 | 0.5 |
| B[a]P | 5.0 | 4.1 | - | - | - | - | |
| AAN | 5.0 | - | 12.3 | - | - | - | |
| AAN | 5.0 | - | - | 18.3 | - | - | |
| B[a]P | 5.0 | - | - | - | 5.6 | - | |
| AAN | 10.0 | - | - | - | - | 5.4 | |
| Observation | Dosage Groups a | |||
|---|---|---|---|---|
| 0 mg·kg−1 | 2000 mg·kg−1 | |||
| 6 h | 14 Day | 6 h | 14 Day | |
| Fur appearance | Normal | Normal | Normal | Normal |
| Posture | Normal | Normal | Normal | Normal |
| Movement | Diminished | Normal | Diminished | Normal |
| Sleep | Normal | Normal | Normal | Normal |
| Diarrhea | Normal | Normal | Normal b | Normal |
| Parameter (Units) | Dosage Group a | |
|---|---|---|
| 0 mg·kg−1 | 2000 mg·kg−1 | |
| Red blood cell count (×106 μL−1) | 6.5 ± 1.4 | 7.2 ± 0.1 |
| Hemoglobin (g·dL−1) | 14.2 ± 0.7 | 12.8 ± 0.4 * |
| Mean corpuscular hemoglobin (pg) | 22.8 ± 6.6 | 17.7 ± 0.5 |
| Mean corpuscular hemoglobin concentration (%) | 32.9 ± 0.5 | 32.0 ± 0.4 |
| Platelet count (×103 μL−1) | 666.0 ± 381.1 | 847.7 ± 126.5 |
| White blood cell count (×103 μL−1) | 3.7 ± 1.8 | 3.9 ± 1.0 |
| Neutrophils (%) | 25.8 ± 13.3 | 27.1 ± 4.8 |
| Lymphocytes (%) | 67.8 ± 12.5 | 66.7 ± 6.2 |
| Monocytes (%) | 0.9 ± 0.3 | 0.3 ± 0.1 |
| Eosinophils (%) | 2.0 ± 0.4 | 4.2 ± 1.4 |
| Basophils (%) | 3.5 ± 1.2 | 1.7 ± 0.2 |
| Parameter (Units) | Dosage Group a | |
|---|---|---|
| 0 mg·kg−1 | 2000 mg·kg−1 | |
| Aspartate aminotransferase (U·L−1) | 159.3 ± 14.2 | 120.3 ± 9.0 |
| Alanine aminotransferase (U·L−1) | 51.7 ± 5.5 | 45.0 ± 1.0 |
| Alkaline phosphatase (U·L−1) | 142.0 ± 37.2 | 134.7 ± 9.0 |
| Total bilirubin (mg·dL−1) | >0.1 ± 0.0 | >0.1 ± 0.0 |
| Glucose (mg·dL−1) | 118.0 ± 33.9 | 157.3 ± 19.4 * |
| Total cholesterol (mg·dL−1) | 60.3 ± 4.5 | 63.7 ± 3.8 |
| Triglycerides (mg·dL−1) | 49.3 ± 19.6 | 57.0 ± 13.0 |
| Total protein (g·dL−1) | 6.6 ± 0.3 | 6.2 ± 0.5 |
| Albumin (g·dL−1) | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Albumin/globulin ratio | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Blood urea nitrogen (mg·dL−1) | 22.4 ± 4.9 | 20.3 ± 4.8 |
| Creatinine (mg·dL−1) | 0.6 ± 0.0 | 0.6 ± 0.1 |
| Inorganic phosphorus (mg·dL−1) | 8.6 ± 2.0 | 7.6 ± 0.4 |
| Calcium ion (mEq·L−1) | 10.4 ± 0.1 | 10.3 ± 0.3 |
| Sodium ion (mEq·dL−1) | 143.3 ± 1.5 | 141.4 ± 2.4 |
| Potassium ion (mEq·dL−1) | 4.6 ± 1.0 | 4.3 ± 0.2 |
| Chloride ion (mEq·dL−1) | 100.4 ± 0.6 | 97.6 ± 2.1 |
| Organ (% bw) | Dosage Group a | |
|---|---|---|
| 0 mg·kg−1 | 2000 mg·kg−1 | |
| Brain | 0.72 ± 0.09 | 0.76 ± 0.05 |
| Heart | 0.37 ± 0.02 | 0.37 ± 0.01 |
| Liver | 4.21 ± 0.43 | 4.87 ± 0.24 |
| Kidney b | 0.80 ± 0.05 | 0.77 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez, D.G.; Pacheco, A.; Villarreal-Lara, R.; Ramos-González, M.R.; Ramos-Parra, P.A.; Granados-Principal, S.; Díaz de la Garza, R.I.; García-Rivas, G.; Hernández-Brenes, C. Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed. Molecules 2019, 24, 2354. https://doi.org/10.3390/molecules24132354
Rodríguez-Sánchez DG, Pacheco A, Villarreal-Lara R, Ramos-González MR, Ramos-Parra PA, Granados-Principal S, Díaz de la Garza RI, García-Rivas G, Hernández-Brenes C. Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed. Molecules. 2019; 24(13):2354. https://doi.org/10.3390/molecules24132354
Chicago/Turabian StyleRodríguez-Sánchez, Dariana G., Adriana Pacheco, Raúl Villarreal-Lara, Martín R. Ramos-González, Perla A. Ramos-Parra, Sergio Granados-Principal, Rocío I. Díaz de la Garza, Gerardo García-Rivas, and Carmen Hernández-Brenes. 2019. "Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed" Molecules 24, no. 13: 2354. https://doi.org/10.3390/molecules24132354
APA StyleRodríguez-Sánchez, D. G., Pacheco, A., Villarreal-Lara, R., Ramos-González, M. R., Ramos-Parra, P. A., Granados-Principal, S., Díaz de la Garza, R. I., García-Rivas, G., & Hernández-Brenes, C. (2019). Chemical Profile and Safety Assessment of a Food-Grade Acetogenin-Enriched Antimicrobial Extract from Avocado Seed. Molecules, 24(13), 2354. https://doi.org/10.3390/molecules24132354