Comparison of Strategies for the Determination of Sterol Sulfates via GC-MS Leading to a Novel Deconjugation-Derivatization Protocol
Abstract
1. Introduction
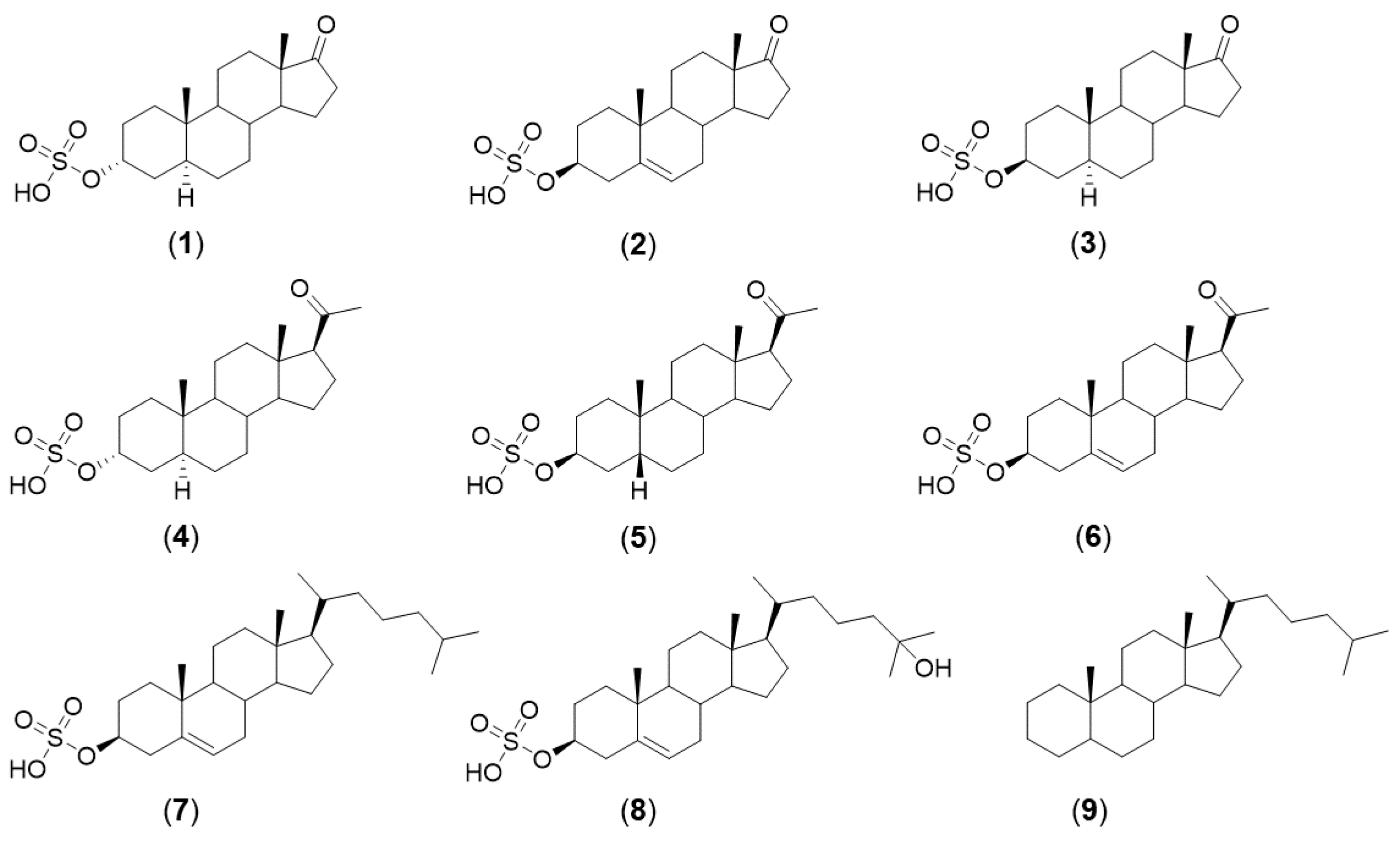
2. Results
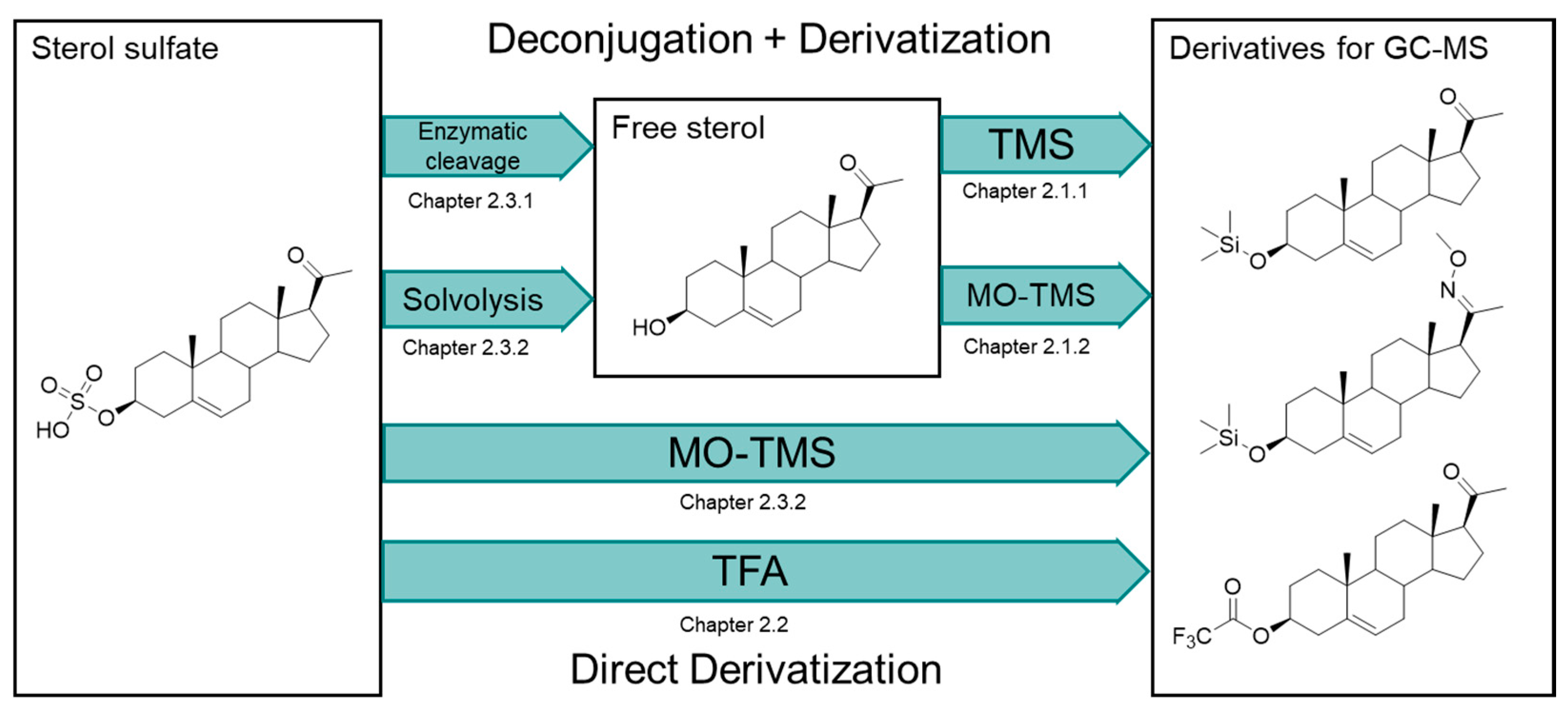
2.1. Derivatization Strategies for Free Sterols (Deconjugated Sterol Sulfates)
2.1.1. Trimethylsilyl (TMS) Derivatives
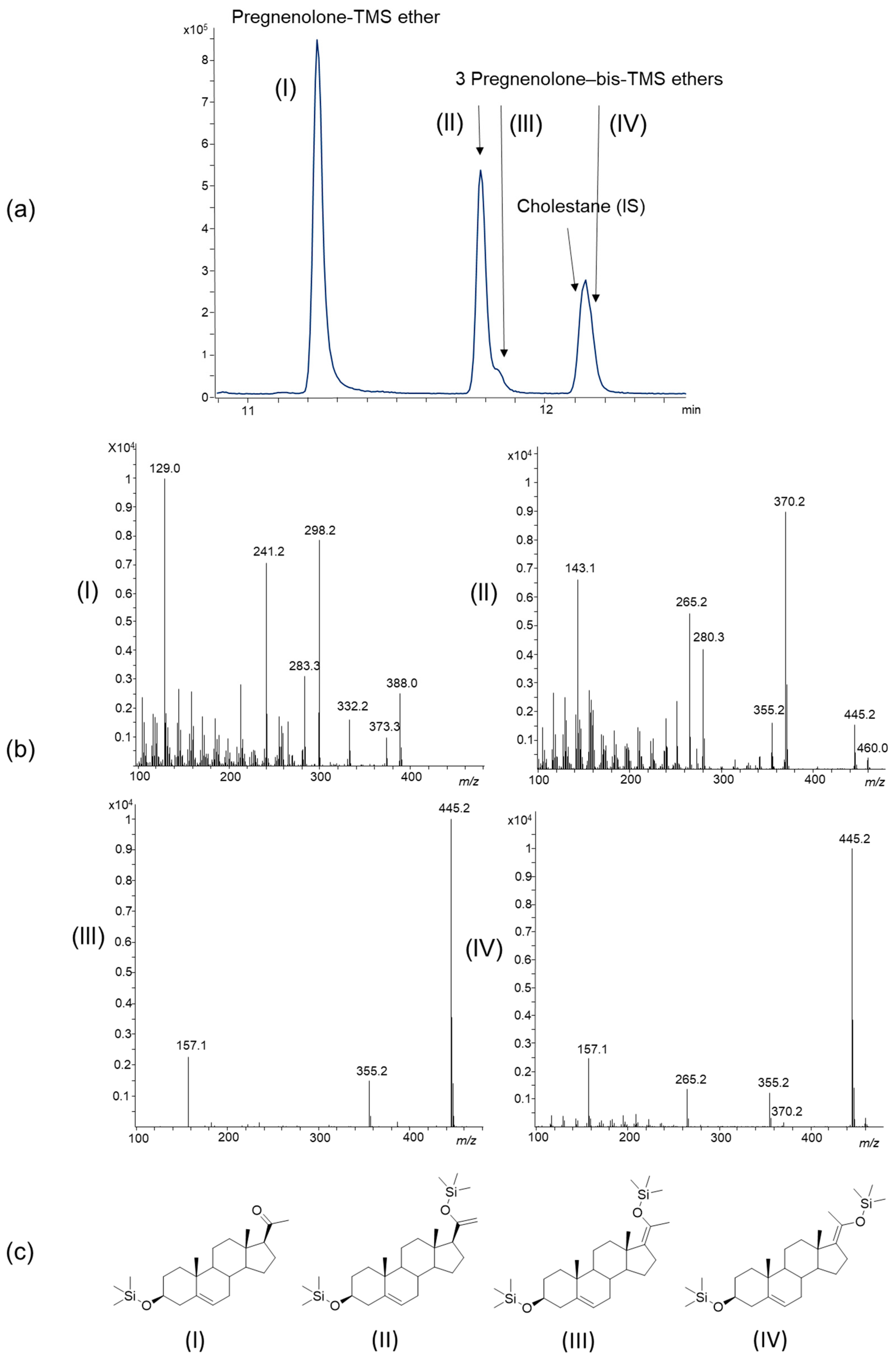
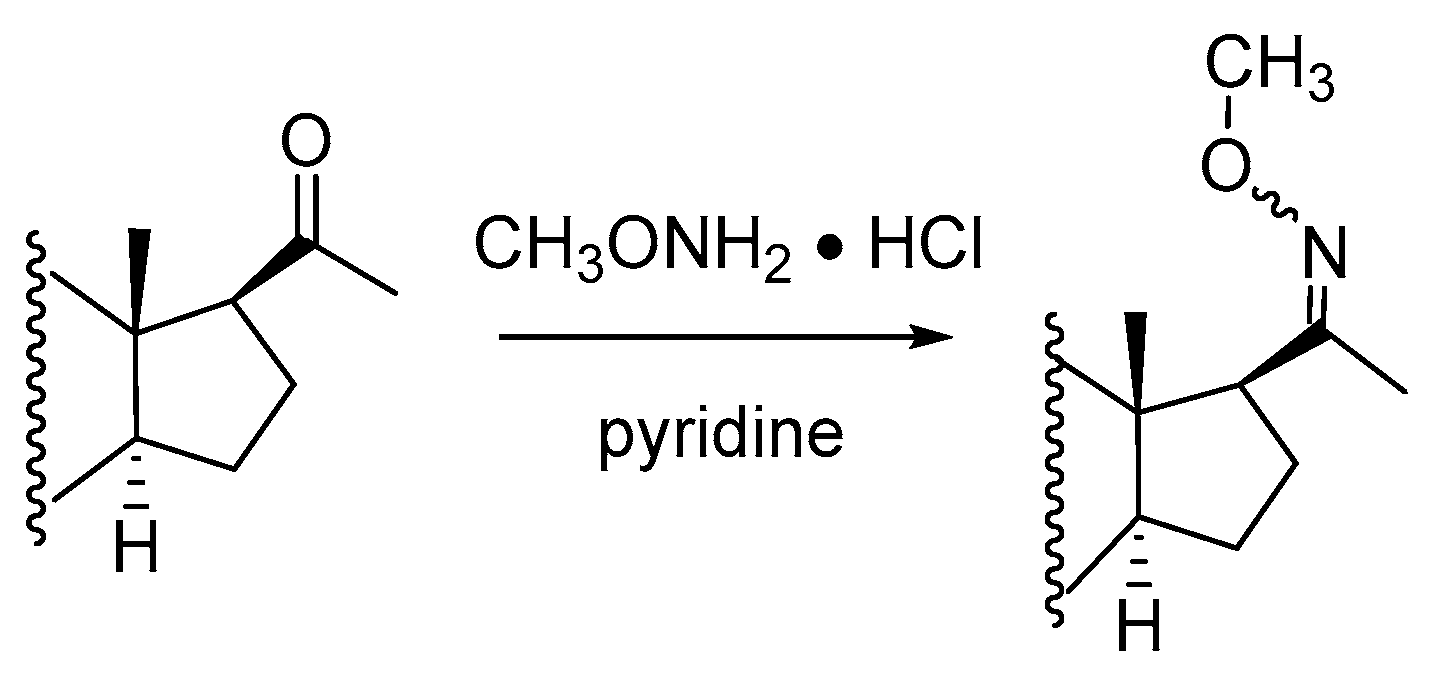
2.1.2. Methyloxime-Trimethylsilyl (MO-TMS) Derivatives
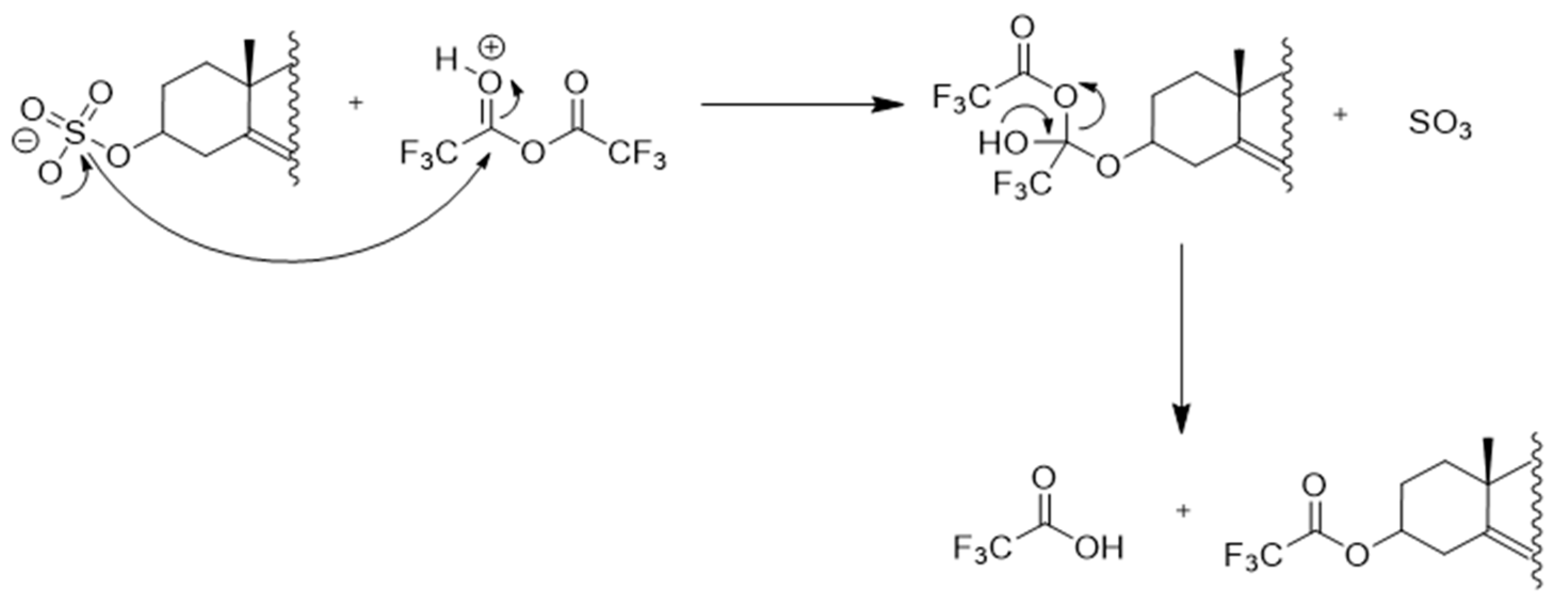
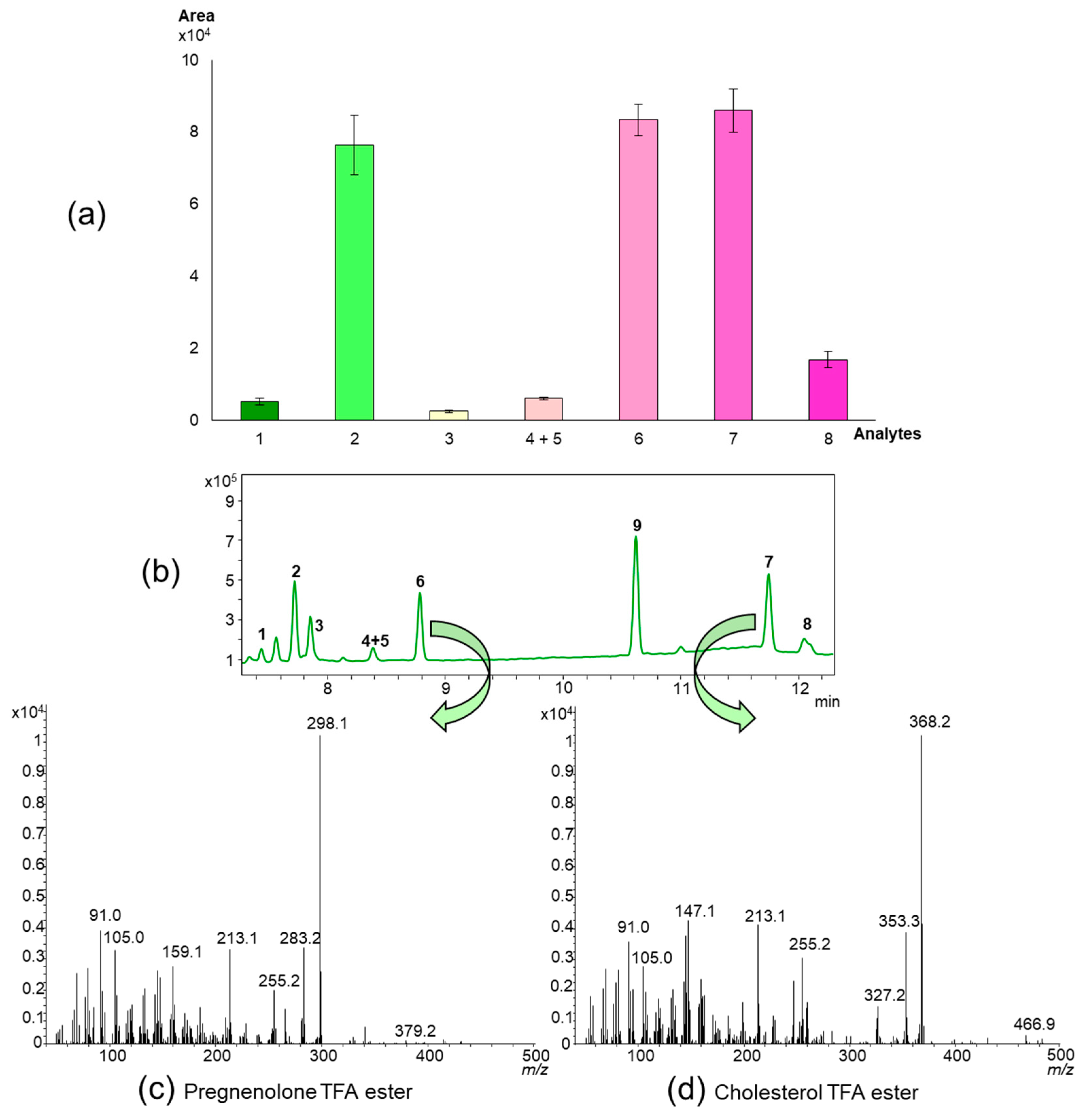
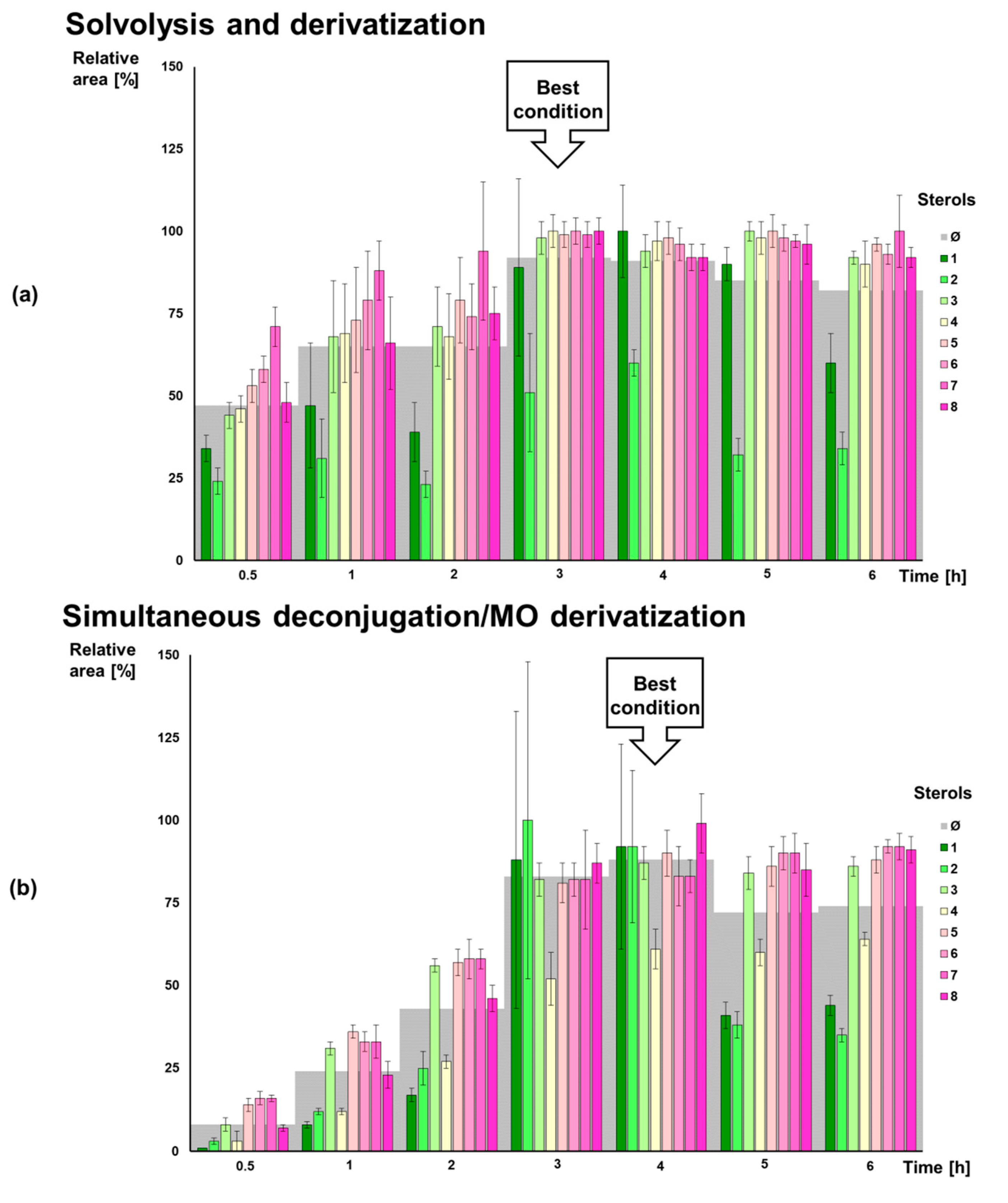
2.2. Direct Deconjugation/Derivatization of Sterol Sulfates to Give Trifluoroacetyl (TFA) Derivatives
2.3. Strategies for Sterol Sulfate Deconjugation
2.3.1. Enzymatic Cleavage of Sterol Sulfates
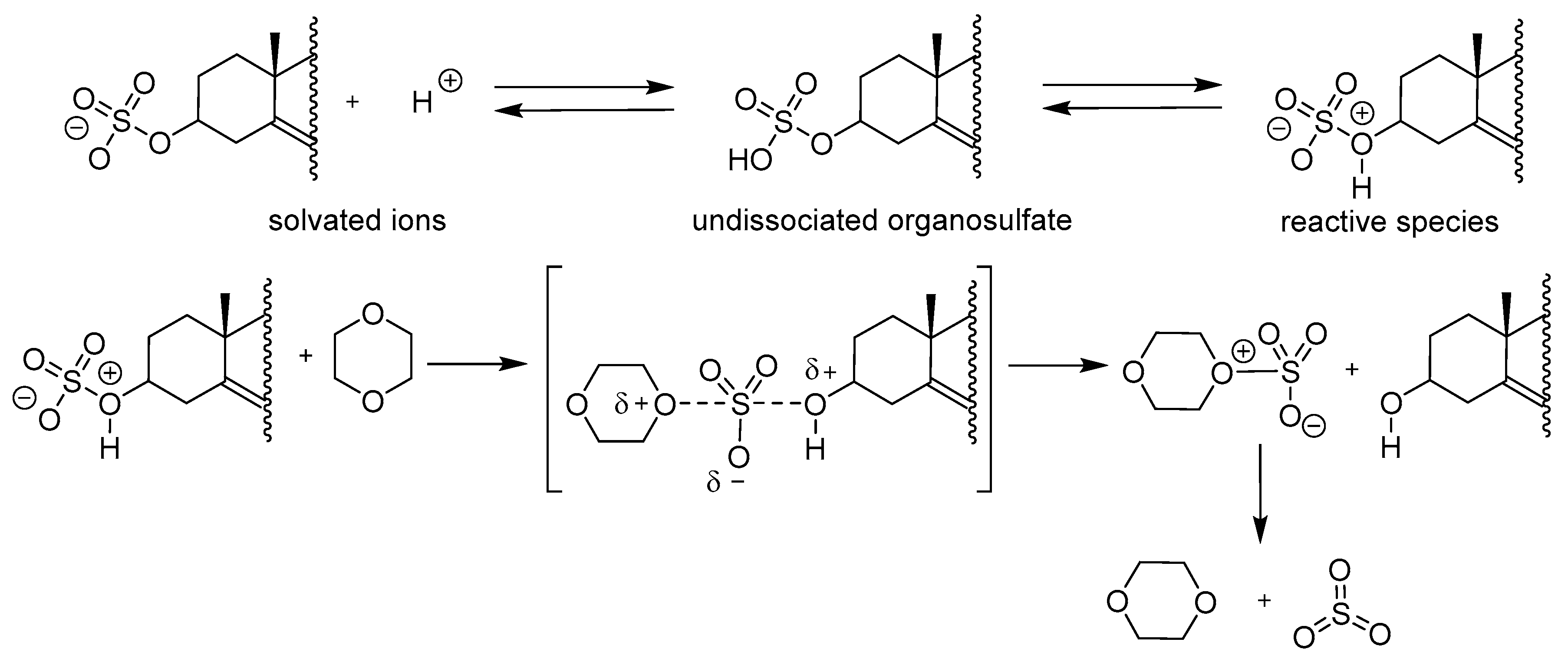
2.3.2. Chemical Cleavage of Sterol Sulfates
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Reagents
5.2. Instruments and Equipment
5.3. Methods
5.3.1. TMS Derivatives by Direct Silylation
5.3.2. Acidic Deconjugation and Formation of MO-TMS Derivatives
5.3.3. TFA Derivatives by Direct Deconjugation/Derivatization
5.3.4. Enzymatic Cleavage of Sulfates and Derivatization
5.3.5. Chemical Cleavage of Sulfates and Derivatization
5.3.5.1. With Acidic Deconjugation (Solvolysis)
5.3.5.2. With Deconjugation/Methoximation with O-Methylhydroxylamine
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Falany, C.N. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997, 11, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Bakhaus, K.; Bernhardt, R.; Blaschka, C.; Dezhkam, Y.; Fietz, D.; Grosser, G.; Hartmann, K.; Hartmann, M.F.; Neunzig, J.; et al. The role of sulfated steroid hormones in reproductive processes. J. Steroid Biochem. Mol. Biol. 2017, 172, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Harteneck, C. Pregnenolone sulfate: From steroid metabolite to TRP channel ligand. Molecules 2013, 18, 12012–12028. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate as a modulator of synaptic plasticity. Psychopharmacology (Berl.) 2014, 231, 3537–3556. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Liere, P.; Akwa, Y.; Rajkowski, K.; Griffiths, W.; Bodin, K.; Sjövall, J.; Baulieu, E.E. Pregnenolone sulfate in the brain: A controversial neurosteroid. Neurochem. Int. 2008, 52, 522–540. [Google Scholar] [CrossRef]
- Fietz, D. Transporter for sulfated steroid hormones in the testis—Expression pattern, biological significance and implications for fertility in men and rodents. J. Steroid Biochem. Mol. Biol. 2018, 179, 8–19. [Google Scholar] [CrossRef]
- Strott, C.A.; Higashi, Y. Cholesterol sulfate in human physiology: What’s it all about? J. Lipid Res. 2003, 44, 1268–1278. [Google Scholar] [CrossRef]
- Iwamori, M.; Iwamori, Y.; Ito, N. Regulation of the activities of thrombin and plasmin by cholesterol sulfate as a physiological inhibitor in human plasma. J. Biochem. 1999, 125, 594–601. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J.; Arlt, W.; Foster, P.A. The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef]
- Luchetti, S.; Huitinga, I.; Swaab, D.F. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience 2011, 191, 6–21. [Google Scholar] [CrossRef]
- Vaňková, M.; Hill, M.; Velíková, M.; Včelák, J.; Vacínová, G.; Dvořáková, K.; Lukášová, P.; Rusina, R.; Holmerová, I.; Jarolímová, E.; et al. Preliminary evidence of altered steroidogenesis in women with Alzheimer’s disease: Have the patients “OLDER” adrenal zona reticularis? J. Steroid Biochem. Mol. Biol. 2016, 158, 157–177. [Google Scholar] [CrossRef]
- Vaňková, M.; Hill, M.; Velíková, M.; Včelák, J.; Vacínová, G.; Lukášová, P.; Vejražková, D.; Dvořáková, K.; Rusina, R.; Holmerová, I.; et al. Reduced sulfotransferase SULT2A1 activity in patients with Alzheimer’s disease. Physiol. Res. 2015, 64 (Suppl. 2), S265–S273. [Google Scholar]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, C. Clinical steroid mass spectrometry: A 45-year history culminating in HPLC–MS/MS becoming an essential tool for patient diagnosis. J. Steroid Biochem. Mol. Biol. 2010, 121, 481–490. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Abdel-Khalik, J.; Yutuc, E.; Morgan, A.H.; Gilmore, I.; Hearn, T.; Wang, Y. Cholesterolomics: An update. Anal. Biochem. 2017, 524, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef]
- Liere, P.; Schumacher, M. Mass spectrometric analysis of steroids: All that glitters is not gold. Expert Rev. Endocrinol. Metab. 2015, 10, 463–465. [Google Scholar]
- Giera, M.; Plössl, F.; Bracher, F. Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post-squalene pathway. Steroids 2007, 72, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Velikanova, L.I.; Strel’nikova, E.G.; Obedkova, E.V.; Krivokhizhina, N.S.; Shafigullina, Z.R.; Grigoryan, K.; Povarov, V.G.; Moskvin, A.L. Generation of urinary steroid profiles in patients with adrenal incidentaloma using gas chromatography–mass spectrometry. J. Anal. Chem. 2016, 71, 748–754. [Google Scholar] [CrossRef]
- Marcos, J.; Pozo, O.J. Derivatization of steroids in biological samples for GC–MS and LC–MS analyses. Bioanalysis 2015, 7, 2515–2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Griffiths, W.J. Chapter 3 steroids, sterols and the nervous system. In Metabolomics, Metabonomics and Metabolite Profiling, 1st ed.; Griffiths, W.J., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2008; Volume 1, pp. 71–115. [Google Scholar]
- Christakoudi, S.; Cowan, D.A.; Taylor, N.F. Steroids excreted in urine by neonates with 21-hydroxylase deficiency. 3. Characterization, using GC–MS and GC–MS/MS, of androstanes and androstenes. Steroids 2012, 77, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Fabregat, A.; Pozo, Ó.J.; Marcos, J.; Segura, J.; Ventura, R. Analytical strategies based on mass spectrometric techniques for the study of steroid metabolism. Trends Anal. Chem. 2014, 53, 106–116. [Google Scholar] [CrossRef]
- Gomes, R.L.; Meredith, W.; Snape, C.E.; Sephton, M.A. Analysis of conjugated steroid androgens: Deconjugation, derivatisation and associated issues. J. Pharm. Biomed. Anal. 2009, 49, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Chung, B.C. Bringing GC–MS profiling of steroids into clinical applications. Mass Spectrom. Rev. 2015, 34, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Giera, M.; Müller, C.; Bracher, F. Analysis and experimental inhibition of distal cholesterol biosynthesis. Chromatographia 2015, 78, 343–358. [Google Scholar] [CrossRef]
- Matysik, S.; Schmitz, G. Determination of steroid hormones in human plasma by GC–triple quadrupole MS. Steroids 2015, 99, 151–154. [Google Scholar] [CrossRef]
- Christakoudi, S.; Cowan, D.A.; Taylor, N.F. Sodium ascorbate improves yield of urinary steroids during hydrolysis with Helix pomatia juice. Steroids 2008, 73, 309–319. [Google Scholar] [CrossRef]
- Ebner, M.J.; Corol, D.I.; Havlíková, H.; Honour, J.W.; Fry, J.P. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology 2006, 147, 179–190. [Google Scholar] [CrossRef]
- Dehennin, L.; Lafarge, P.; Dailly, P.; Bailloux, D.; Lafarge, J.P. Combined profile of androgen glucuro- and sulfoconjugates in post-competition urine of sportsmen: A simple screening procedure using gas chromatography-mass spectrometry. J. Chromatogr. B 1996, 687, 85–91. [Google Scholar] [CrossRef]
- Müller, C.; Binder, U.; Bracher, F.; Giera, M. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography-mass spectrometry. Nat. Protoc. 2017, 12, 947–963. [Google Scholar] [CrossRef]
- Little, J.L. Artifacts in trimethylsilyl derivatization reactions and ways to avoid them. J. Chromatogr. A 1999, 844, 1–22. [Google Scholar] [CrossRef]
- Teubel, J.; Wüst, B.; Schipke, C.G.; Peters, O.; Parr, M.K. Methods in endogenous steroid profiling—A comparison of gas chromatography mass spectrometry (GC–MS) with supercritical fluid chromatography tandem mass spectrometry (SFC-MS/MS). J. Chromatogr. A 2018, 1554, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Alkylsilyl derivatives for gas chromatography. J. Chromatogr. A 2013, 1296, 2–14. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, D.H.; van Ooijen, R.D.; de Boer, D.; Fokkens, R.H.; Nibbering, N.M.; Zwikker, J.W.; Thijssen, J.H.; Maes, R.A. Artifact formation due to ethyl thio-incorporation into silylated steroid structures as determined in doping analysis. J. Chromatogr. A 2002, 954, 199–206. [Google Scholar] [CrossRef]
- Hadef, Y.; Kaloustian, J.; Portugal, H.; Nicolay, A. Multivariate optimization of a derivatisation procedure for the simultaneous determination of nine anabolic steroids by gas chromatography coupled with mass spectrometry. J. Chromatogr. A 2008, 1190, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Pan, X.; Huang, B.; Liu, J.; Wang, Y.; Gao, J. Simultaneous derivatization of hydroxyl and ketone groups for the analysis of steroid hormones by GC–MS. Chromatographia 2010, 72, 949–956. [Google Scholar] [CrossRef]
- Meunier-Solère, V.; Maume, D.; André, F.; Le Bizec, B. Pitfalls in trimethylsilylation of anabolic steroids. New derivatisation approach for residue at ultra-trace level. J. Chromatogr. B 2005, 816, 281–288. [Google Scholar] [CrossRef]
- Shackleton, C.H.L. Profiling steroid hormones and urinary steroids. J. Chromatogr. B Biomed. Sci. Appl. 1986, 379, 91–156. [Google Scholar] [CrossRef]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.P.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef]
- Nicholson, J.D. Derivative formation in the quantitative gas-chromatographic analysis of pharmaceuticals. Part II. A review. The Analyst 1978, 103, 193–222. [Google Scholar] [CrossRef]
- Goad, L.J.; Akihisa, T. Mass spectrometry of sterols. In Analysis of Sterols; Springer: Dordrecht, The Netherlands, 1997; pp. 152–196. [Google Scholar]
- Touchstone, J.C.; Dobbins, M.F. Direct determination of steroidal sulfates. J. Steroid Biochem. 1975, 6, 1389–1392. [Google Scholar] [CrossRef]
- Liere, P.; Pianos, A.; Eychenne, B.; Cambourg, A.; Liu, S.; Griffiths, W.; Schumacher, M.; Sjövall, J.; Baulieu, E.-E. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J. Lipid Res. 2004, 45, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Baillie, T.A. Direct derivatization of sulphate esters for analysis by gas chromatography mass spectrometry. Biol. Mass Spectrom. 1979, 6, 82–89. [Google Scholar] [CrossRef]
- Galli, G.; Maroni, S. Mass spectrometric investigations of some unsaturated sterols biosynthetically related to cholesterol. Steroids 1967, 10, 189–197. [Google Scholar] [CrossRef]
- Knights, B.A. Identification of plant sterols using combined GLC/mass spectrometry. J. Chromatogr. Sci. 1967, 5, 273–282. [Google Scholar] [CrossRef]
- Ferchaud, V.; Courcoux, P.; Le Bizec, B.; Monteau, F.; André, F. Enzymatic hydrolysis of conjugated steroid metabolites: Search for optimum conditions using response surface methodology. The Analyst 2000, 125, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Cawley, L.P.; Faucette, W.; Musser, B.O.; Beckloff, S. Steric hindrance of the sulfatase of Helix pomatia on some 17-ketosteroid sulfate conjugates. Am. J. Clin. Pathol. 1969, 52, 652–655. [Google Scholar] [CrossRef]
- Xu, X.; Keefer, L.K.; Ziegler, R.G.; Veenstra, T.D. A liquid chromatography–mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat. Protoc. 2007, 2, 1350–1355. [Google Scholar] [CrossRef]
- Venturelli, E.; Cavalleri, A.; Secreto, G. Methods for urinary testosterone analysis. J. Chromatogr. B 1995, 671, 363–380. [Google Scholar] [CrossRef]
- Robards, K.; Towers, P. Chromatography as a reference technique for the determination of clinically important steroids. Biomed. Chromatogr. 1990, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Muehlbaecher, C.A.; Smith, E.K. Three hydrolysis methods for 17-ketosteroid sulfates compared by colorimetric and gas—liquid chromatographic analyses. Clin. Chem. 1970, 16, 158–160. [Google Scholar] [PubMed]
- Burstein, S.; Lieberman, S. Hydrolysis of ketosteroid hydrogen sulfates by solvolysis procedures. J. Biol. Chem. 1958, 233, 331–335. [Google Scholar] [PubMed]
- Burstein, S.; Lieberman, S. Kinetics and mechanism of solvolysis of steroid hydrogen sulfates. J. Am. Chem. Soc. 1958, 80, 5235–5239. [Google Scholar] [CrossRef]
- Cohen, S.L.; Oneson, I.B. The conjugated steroids. IV. The hydrolysis of ketosteroid sulfates. J. Biol. Chem. 1953, 204, 245–256. [Google Scholar] [PubMed]
- Hutchins, R.F.N.; Kaplanis, J.N. Sterol sulfates in an insect. Steroids 1969, 13, 605–614. [Google Scholar] [CrossRef]
- Benkovic, S.J.; Benkovic, P.A. Studies on sulfate Esters. I. Nucleophilic reactions of amines with p-nitrophenyl sulfate. J. Am. Chem. Soc. 1966, 88, 5504–5511. [Google Scholar] [CrossRef]
- Kirby, A. Reactions of alpha-nucleophiles with a model phosphate diester. Arkivoc 2008, 2009, 28–38. [Google Scholar]
- Wolfenden, R.; Yuan, Y. Monoalkyl sulfates as alkylating agents in water, alkylsulfatase rate enhancements, and the “energy-rich” nature of sulfate half-esters. PNAS 2007, 104, 83–86. [Google Scholar] [CrossRef]
- Fina, N.J.; Edwards, J.O. The alpha effect. A review. Int. J. Chem. Kinet. 1973, 5, 1–26. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
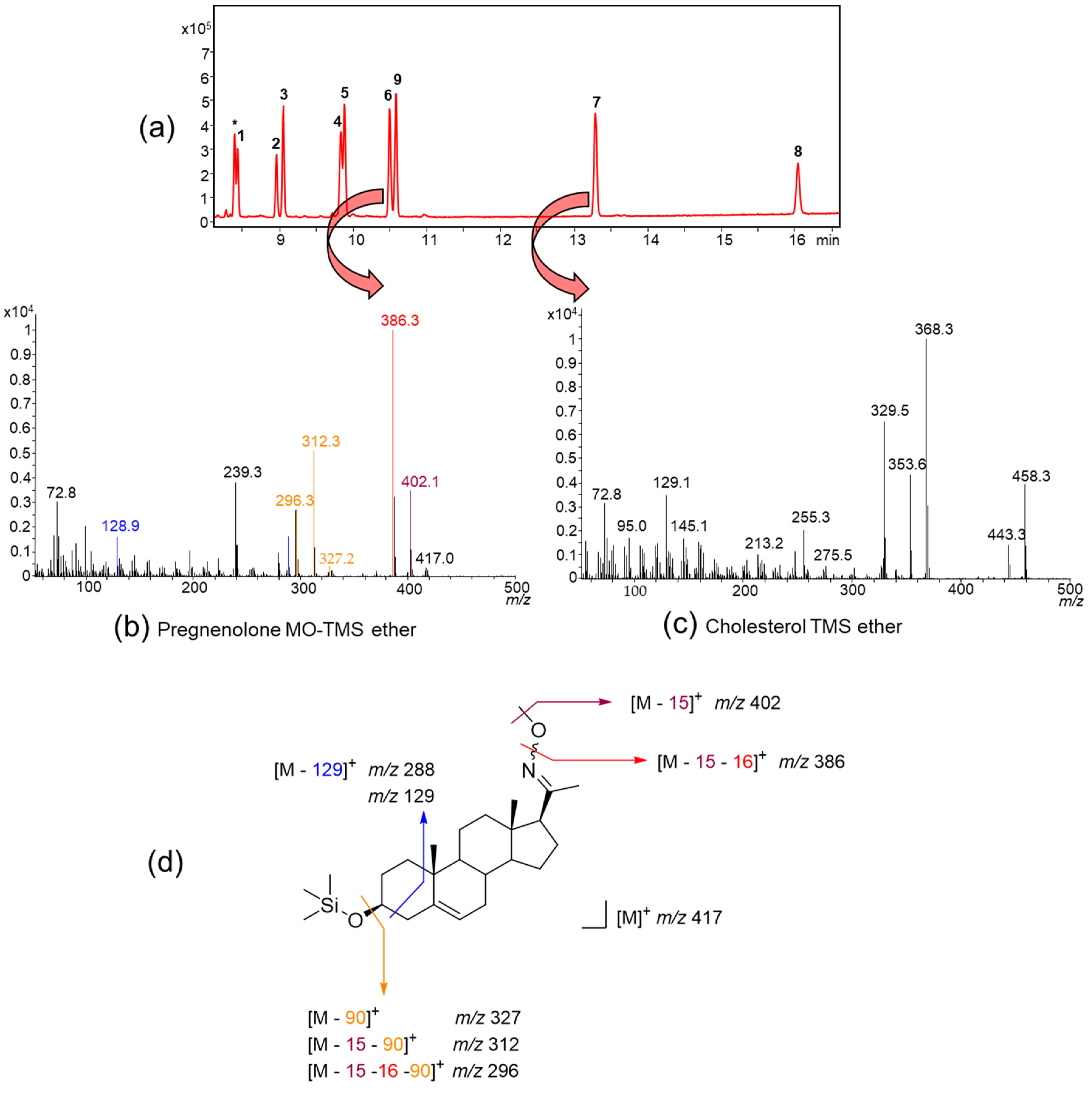
| Analyzed by GC-MS as | Advantages | Disadvantages |
|---|---|---|
| TMS ether (Section 2.1.1 and Section 5.3.1) | Fast and easy workup Mass spectra with molecular ion and characteristic fragmentation pattern | Prior deconjugation step afforded (e.g., solvolysis) Artifact formation with keto groups possible |
| MO-TMS ether (Section 2.1.2, Section 2.3.2, Section 5.3.2 and Section 5.3.5.2) | Mass spectra with molecular ion and characteristic fragmentation pattern Simultaneous deconjugation/MO-derivatization of sterol sulfates possible No artifacts (apart from possible syn- and anti-isomers of the MO group) in presence of keto groups | Time consuming workup with two step derivatization and additional clean up step |
| TFA ester (Section 2.2 and Section 5.3.3) | Fast and easy workup Direct derivatization of sterol sulfates possibleShort GC run times | Direct derivatization is limited to estrogens and ∆5-sterol-3-sulfates Derivatization of additional free hydroxyl groups could be problematic (e.g. 25-hydroxycholesterol sulfate) Residual TFA leads to column bleeding Mass spectra do not show a molecular ion |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junker, J.; Chong, I.; Kamp, F.; Steiner, H.; Giera, M.; Müller, C.; Bracher, F. Comparison of Strategies for the Determination of Sterol Sulfates via GC-MS Leading to a Novel Deconjugation-Derivatization Protocol. Molecules 2019, 24, 2353. https://doi.org/10.3390/molecules24132353
Junker J, Chong I, Kamp F, Steiner H, Giera M, Müller C, Bracher F. Comparison of Strategies for the Determination of Sterol Sulfates via GC-MS Leading to a Novel Deconjugation-Derivatization Protocol. Molecules. 2019; 24(13):2353. https://doi.org/10.3390/molecules24132353
Chicago/Turabian StyleJunker, Julia, Isabelle Chong, Frits Kamp, Harald Steiner, Martin Giera, Christoph Müller, and Franz Bracher. 2019. "Comparison of Strategies for the Determination of Sterol Sulfates via GC-MS Leading to a Novel Deconjugation-Derivatization Protocol" Molecules 24, no. 13: 2353. https://doi.org/10.3390/molecules24132353
APA StyleJunker, J., Chong, I., Kamp, F., Steiner, H., Giera, M., Müller, C., & Bracher, F. (2019). Comparison of Strategies for the Determination of Sterol Sulfates via GC-MS Leading to a Novel Deconjugation-Derivatization Protocol. Molecules, 24(13), 2353. https://doi.org/10.3390/molecules24132353










