Abstract
Macrocyclic diterpenoids produced by plants of the Euphorbiaceae family are of considerable interest due to their high structural diversity; and their therapeutically relevant biological properties. Over the last decade many studies have reported the ability of macrocyclic diterpenoids to inhibit in cellulo the cytopathic effect induced by the chikungunya virus. This review; which covers the years 2011 to 2019; lists all macrocyclic diterpenoids that have been evaluated for their ability to inhibit viral replication. The structure–activity relationships and the probable involvement of protein kinase C in their mechanism of action are also detailed.
Keywords:
chikungunya; Euphorbiaceae; phorbol; tigliane; daphnane; ingenane; jatrophane; pre-myrsinane; flexibilane; PKC 1. Introduction
Chikungunya virus (CHIKV) is an arthropod-borne virus causing an infectious disease characterized by fever, arthralgia and, sometimes, a maculopapular rash [1]. Despite high morbidity rate, there is currently no approved vaccine or antiviral treatment. Different classes of compounds that target either a viral or a host factor have been reported to inhibit CHIKV replication in vitro [2], but none have progressed to further development. The development of potent antiviral drugs against CHIKV is, therefore, urgently needed.
Plant species of the Euphorbiaceae family are known to produce a vast array of macrocyclic diterpenoids. They possess various types of carbon skeletons (e.g., jatrophane, lathyrane, myrsinane, ingenane, tigliane, daphnane etc.), many of which are unique to this large plant family [3,4]. The genus Euphorbia, which includes more than 2000 species, alone provided several hundred macrocyclic diterpenoids, representative of more than 20 skeletal types [5,6]. With regard to their biogenesis, these compounds also called lower diterpenoids are formed by a head-to-tail cyclization of a tetraprenylphosphate precursor through the agency of a type I cyclase, leading to the formation in a step-wise fashion of the monocyclic cembranes, the bicyclic casbanes and the tricyclic lathyrane derivatives, the latter may lead to jatrophane after cyclopropane ring opening [7]. From a structural standpoint phorboids, i.e., tiglianes, daphnanes, ingenanes and rhamnopholanes, are polycyclic diterpenoids but are biogenetically derived from macrocyclic precursors. However, Appendino pointed out that the relationship between macrocyclic diterpenoids and phorboids has remained a mechanistic black box, despite obvious structural similarities between the lathyrane and the tigliane skeletons [7].
Macrocyclic diterpenoids are of considerable interest due to their therapeutically relevant biological properties, one of the most widely described being their ability to modulate protein kinase C (PKCs) activities [4,5]. PKCs are involved in many physiological functions [8]. In addition, it has been shown that biologically active diterpenes containing a gem-dimethylcyclopropane subunit such as lathyrane-, casbane-, premyrsinane-, ingenane- or tigliane-type diterpenoids are an intriguing source of PKC modulators [9]. PKC activation is responsible for a wide variety of biological activities such as platelet aggregation, tumor-promotion and anti-HIV activity.
Since the discovery of their anti-HIV properties in the early 1990s, macrocyclic diterpenoids have attracted the interest of the scientific community [10,11,12,13,14] and a significant number of studies have focused on their anti-HIV potential through PKC activation [15,16,17,18]. Some non-tumor-promoting tiglianes such as prostratin and DDP (12-deoxyphorbol-13-phenylacetate) exhibit potent in vitro activity toward the induction of HIV expression in latently infected cell lines and primary cells [19] and are considered to be promising anti-HIV agents [20].
In contrast, the discovery of antiviral properties of macrocyclic diterpenes against CHIKV is much more recent [20,21,22,23,24] as it followed the chikungunya African outbreak in the 2000s and its subsequent emergence in the Indian Ocean [24]. The main vectors are Aedes mosquitoes from the Culicidae family such as A. furcifer, A. africanus, A. luteocephalus, A. taylori and A. aegypti. Between 2005 and 2006, a new vector (A. albopictus) spread in most of the tropical and subtropical areas and led to massive outbreak in the Indian Ocean region.
As CHIKV reached epidemic level, the quest for novel and selective antiviral compounds was launched on a large scale. A project entitled ‘Biodiversity and emerging viruses in the Indian Ocean: selection of drug candidates targeting the Chikungunya virus′, was selected by the Centre for Research and Monitoring of Emerging Diseases in the Indian Ocean (CRVOI) for financial support and was developed between 2009 and 2011 [24]. The main objective was to discover and characterize new selective antiviral compounds from the plant biodiversity of the Indian Ocean (Madagascar, Reunion and Mauritius), which was then extended to that of New Caledonian and Mediterranean flora. The research program, led by a tight network of virologists and natural products chemists, quickly revealed the Euphorbiaceae as the most promising plant family in the fight against CHIKV.
This review focuses on the anti-CHIKV activity of about 80 naturally occurring macrocyclic diterpenoids isolated from plant species belonging to the Euphorbiaceae family from 2011 to 2019, along with about 30 commercially available natural diterpenoids. These compounds, which have been classified according to their chemical features into 14 skeletal types have all been evaluated using the methodology described by Bourjot et al. [21]. The discussion focuses on the structure–activity relationships that are detailed when a sufficient number of compounds have been tested in each series. The mechanism of action of the most promising compounds that involve PKCs is also discussed, highlighting the close analogy with their anti-human immunodeficiency virus (HIV) activities.
2. Tiglianes and Ingenanes
Tigliane diterpenoids form the largest group of phorboids. They possess a 5/7/6/3-tetracyclic ring system, in which rings A and B, and B and C, are trans-fused while rings C and D are cis-fused. A carbonyl is located at C-3, a double-bond at C-1, and most of the tiglianes are hydroxylated in positions 4, 9, 12, 13, and 20 [5]. Also called phorbol esters (PE), most tigliane derivatives exist in the form of 12,13 or 13,20-diesters, and a few also exist as 12- or 13-monoesters and 12,13,20-triesters. They are classified as (i) phorbol esters including 12- and 13-monoesters, 12,13- and 13,20-diesters, and 12,13,20-triesters, (ii) 4-deoxyphorbol esters, (iii) 12-deoxyphorbol esters, (iv) 4,12- dideoxyphorbol esters and (v) 4,20-dideoxyphorbol esters [4]. Ingenane diterpenoids, which are a biogenetically advanced group of phorboids [7], possesses a scaffold composed of a 5/7/7/3-tetracyclic ring system including a ketone bridge between C-8 and C-10 and are β-hydroxylated at C-4. Rings A and B are trans-fused and double bonds can be found between C-1 and C-2 in ring A, and between C-6 and C-7 in ring B. The C-3, C-5, C-13, C-17, and C-20 positions can be oxygenated and/or esterified [5].
A total of 51 tiglianes (1–51) and three ingenanes (52–54) has been evaluated in a virus-cell-based assay against CHIKV (Figure 1). These compounds were either commercially available.
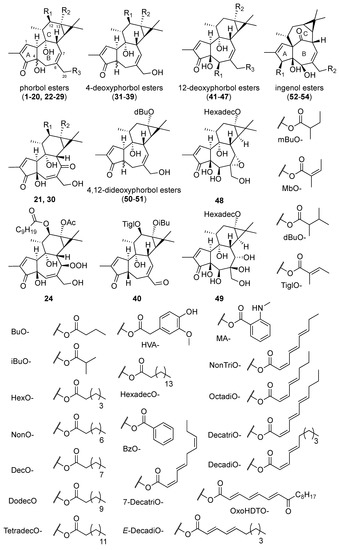
Figure 1.
Structures of tiglianes 1–51 and ingenanes 52–54.
(1–20,25,26,41–43,52–54) [16] or isolated from various Euphorbiaceae species i.e., Trigonostemon howii (21) [21], Croton mauritianus (23,24) [25], Euphorbia semiperfoliata (33–35,40) [26], Stillingia lineata (22,31,32,44,45) [27], Bocquillonia nervosa (46–49) [28], Euphorbia pithyusa (42,43,50,51) [29], Euphorbia dendroides (36–39) [30], Euphorbia cupanii (27–30) [31]. Additionally, phorbols have also been identified in Sandwithia guyanensis [32] and Sagotia racemosa bark extracts as potent putative anti-CHIKV agents.
First, the cytotoxicity of all compounds was evaluated against African green monkey kidney epithelial cell line (Vero cells). The CC50 (50% antimetabolic concentration) values ranged from 4.1 to > 343 µM, phorbol (1) being the less cytotoxic compound. Among compounds with a selective index > 20 (see below), the highest cytotoxicity was obtained for compounds with a long acyl chain either at C-12 or C-13 position (11, 15 and 48).
Most diterpenes have shown significant CHIKV inhibitory activities but the level of activity seems to be highly dependent on the structural type and its decoration (Table 1). Phorbol-12,13-didecanoate (11), 12-O-tetradecanoylphorbol-13-acetate (TPA, 15) and to a lesser extent 12-deoxyphorbol-13-hexadecanoate (46) were found to be the most potent inhibitors yet reported as evidenced by their lower EC50 (effective concentration or concentration which is calculated to inhibit virus induced cell death by 50%) and higher selectivity indices values (EC50 = 6.0, 2.9 and 20 nM, and SI = 686, 1965 and 1500, respectively). Interestingly TPA did not show any significant antiviral activities against Sindbis virus (SINV) and Semliki Forest virus (SFV), two other members of the genus Alphavirus [21]. Thirty-two other tiglianes and one ingenane have shown significant anti-CHIKV activities with EC50 values between 20 nM and 5 µM. They belong to all structural sub-classes defined previously. Among these, phorbol esters 22, and 27–29, 4-deoxyphorbol esters 33, 35, 37 and 38, and 12-deoxyphorbol esters 41 (prostratin), 44, and 47–49 exhibited selective indices >20.

Table 1.
Anti-chikungunya virus (CHIKV) activities of tiglianes 1–51 and ingenanes 52–54.
Although it is difficult to draw clear structure–activity relationships within this family of compounds, general rules can be highlighted. As was previously reported [16,26], the anti-CHIKV activity of some phorbols can be modulated by the length and location at C-12 and/or C-13 positions of the acyl chain(s) on the phorbol backbone, the relative configuration at C-4 and the presence of additional carbonyl function at C-7 and/or C-20 (Figure 2). Generally, a stronger anti-CHIKV activity was reported for phorbol monoesters (3,6,7) and phorbol di- and triesters (9,11,15,17, 19, and 26–30) possessing long aliphatic side chains at C-12 and/or C-13. Conversely, those having short side chains at C-12 and C-13 are less active and less selective, with the exception of compound 22 possessing an acetyl side chain and a 2-methylbutyryl side chain at C-12 and C-13, respectively (EC50 = 3.3 µM, and SI = 41). Comparison of the anti-CHIKV activities of compounds 11 and 12, and 15 (TPA) and 16 indicated that 4β-phorbol derivatives are much more potent than their 4α-counterparts. A similar observation can be noted by comparing the antiviral activities of the 4α-deoxyphorbols 31 and 34, much weaker than those of 4β-deoxyphorbols 35 and 33, all possessing short side chains in C-12 and C-13. The 4α-deoxyphorbols 31 and 34 showed lower activities. In contrast, 4α-deoxyphorbol 32, which possess a nona-2-enoyl side chain at C-13, showed a significant anti-CHIKV activity (EC50 = 1.4 µM, and SI = 5.1). It should be noted that compound 32 as well as compound 45 also showed a significant antiviral activity on the replication of SINV [27]. All 4β-deoxyphorbols but compound 39, exhibited potent anti-CHIKV activity, among which 4β-deoxyphorbol 12-acetate-13-isobutyrate (35) has the highest selective index (EC50 = 0.44 µM, and SI = 390). Finally, most of the 12-deoxyphorbols have shown strong anti-CHIKV activities. In particular, compounds 46–48, which all have an hexadecanoyl side chain at C-13, exhibited the best indices of selectivity. Among the latter, the presence of a 6,7-epoxy function instead of a 6,7-dihydroxy moiety has a favorable effect on the anti-CHIKV activity (48 vs. 49). [28] In general, oxidation of phorbol esters [33] leading to an α,β-unsaturated carbonyl function at C-20 or C-7 appears to be responsible for a significant decrease in antiviral activity (25 vs. 9, 26 vs. 15, 40, 21). Finally, it should be noted that ingenol-3,20-dibenzoate 54, was the first ingenane-type diterpenoid showing a significant anti-CHIKV activity [16].
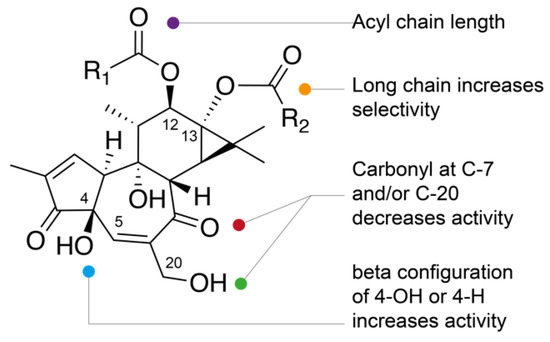
Figure 2.
Structure–activity relationships of tiglianes.
Most of tigliane and ingenane derivatives mentioned in Table 1 have also been evaluated against HIV-1 and HIV-2 replications [16,27,28]. Overall, the structure–activity relationships that were established for the anti-CHIKV activities of the tested phorbol derivatives were found to be similar to those observed for anti-HIV-1 and anti-HIV-2. This concerns the role of the length and the position of the acyl chains at C-12 and C-13, the requirement of a C-4β configuration for a strong antiviral effect, and the deleterious effect of an oxidation at C-20. In particular the close antiviral profiles of the tested compounds against CHIKV on one hand and HIV-1 and HIV-2 on the other hand have been confirmed by the calculation of Pearson correlation coefficients between the EC50 values for each virus pair. The results showed that EC50s against CHIKV and HIVs were positively correlated (CHIKV/HIV-1, r = 0.81 ± 0.09; CHIKV/HIV-2, r = 0.84 ± 0.07) [16]. The authors concluded that, similarly to the mechanism of action proposed for HIV inhibitors, phorbol ester derivatives could operate according to a CHIKV-specific mechanism possibly associated with the activation of PKCs [34,35].
3. Daphnanes
Daphnane diterpenoids, which are believed to be derived from a tigliane precursor [36] are based on a 5/7/6-tricyclic skeleton, rings A and B and rings B and C being trans-fused. A double bond or an epoxy group may be present between C-6 and C-7 carbons. A large number of daphnane diterpenoids possess an orthoester moiety (Daphnane Diterpenoid Orthoesters, DDO), which can be attached on ring C at various positions i.e., C-9, C-11, C-12, C-13, and C-14 [36,37].
In several recent studies, the anti-CHIKV activities of the commercially available resiniferatoxin (55) [16], as well as DDOs isolated from Trigonostemon cherrieri (56-63) [22,23,38], Neoguillauminia Cleopatra (64) [28], and Codiaeum peltatum (65,66) [37], have been reported (Figure 3). Most of them have shown significant anti-CHIKV activities with EC50 values ranging from 0.6 to 18 µM (Table 2). From this chemical series, trigocherrierin A (56) possessing a 2-methyl-decanoyl side chain at C-12, and a 9,13,14-orthoester moiety exhibited the strongest antiviral activity with the highest selective index (EC50 = 0.6 µM, and SI = 72). Finally, it has been shown that anti-HIV activities of trigocherriolides are 100 to 1000 times higher than those of trigocherrins, suggesting a different mechanism of action [39]. Interestingly, compounds 59–62, and to a lesser extent compounds 57 and 58, showed significant antiviral activities on the replication of SINV and SFV viruses. [23] Finally, compounds 57, 60 and 61 also showed significant inhibitory activity against NS5 RNA-dependent RNA polymerase of dengue virus (DENV) [23].
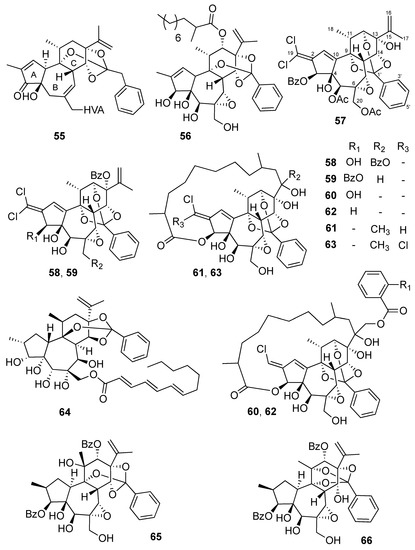
Figure 3.
Structures of daphanes 55–66.

Table 2.
Anti-CHIKV activities of daphnanes 55–66.
4. Jatrophanes
Jatrophane diterpenes are based on a 5/12-bicyclic ring system. The number of substitutable positions on the bicyclic core, provides jatrophanes with a great chemical diversity [5].
In 2014 and 2016, 25 jatrophanes (Figure 4) were isolated from Euphorbia amygdaloides ssp. semiperfoliata [40], and Euphorbia dendroides [41]. Their anti-CHIKV activities are reported in Table 3. Within the 9,14-dioxojatropha-dienes (67–73), an acetyl group at position 2 proved to be deleterious for anti-CHIKV activity (69 vs. 72, and 70 vs. 73). Regarding compounds 67–70 and 71–75, the authors ranged the influence of the C-8 substitution on the activity as follows: tiglyloxy > benzoyloxy > acetyloxy ≈ isobutyryloxy. In the 9-oxojatropha-dienes series, the 2-methylbutyryl group of 76 seemed to be deleterious for the antiviral activity (76 vs. 74 and 75). It should be noted that compound 69 exhibited moderate anti-SINV activity, while compounds 74–76 exhibited significant, albeit weak, antiviral activities on the replication of SINV and SFV viruses [40].
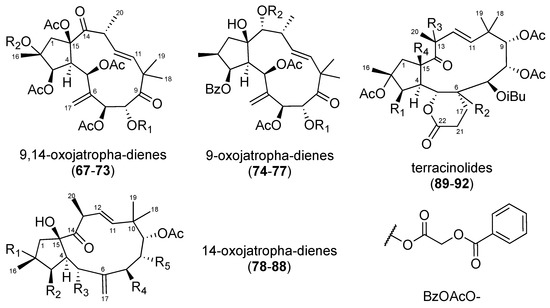
Figure 4.
Structures of jatrophanes 67–92.

Table 3.
Anti-CHIKV activities of jatrophanes 67–92.
5. Myrsinanes and Premyrsinanes
Myrsinane and premyrsinane diterpenes possess a 5/7/6-fused tricyclic or a 5/7/6/3-fused tetracyclic skeleton, respectively. Rings A and B and rings B and C are trans-fused in both series, and an additional cyclopropane ring is present in premyrsinanes. Myrsinanes generally possess ester groups at positions C-3, C-5, C-7, and C-15, and a double bond between C-8 and C-9. In premyrsinanes, an hemiacetal ring or a 13/17-epoxy function can be present [5]. Seven premyrsinols (93–99) and one myrsinol (100) (Figure 5) were evaluated on the CHIKV-cell-based assay (Table 4). None of the compounds tested except compound 98 (EC50 = 11 µM, SI = 5.8) showed significant anti-viral activity. In the premyrsinol series, all compounds but 98 possess an ester group at C-7. It was suggested that this ester group could have a deleterious effect on anti-CHIKV activity [29].
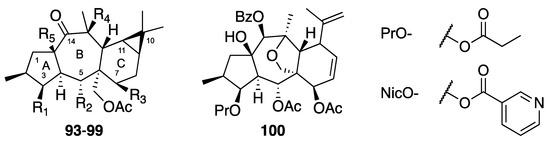
Figure 5.
Structures of premysinanes 93–99 and myrsinane 100.

Table 4.
Anti-CHIKV activities of premysinanes 93–99 and myrsinane 100.
6. Flexibilanes
Flexibilanes are rare 15-membered macrocyclic diterpenes that possess an intramolecular furan, a hydroxy group in position 10 and a side chain in position 8. In general, a pyranol ring complement their structural features. The structure of flexibilanes is rigid due to strong internal hydrogen bonds between the hydroxy group at C-10 and the ester oxygens of the side chain. The presence of five methyl groups on the macrocycle increases the rigidity of the flexibilanes [42,43]. Among the 10 flexibilanes evaluated (101–110) (Figure 6), tonantzitlolones B, C and F (102, 103 and 106) showed moderate anti-CHIKV activities with EC50 values of 12, 24 and 19 µM, and SI = 10.2, >9 and 3, respectively (Table 5) [27]. Compound 106 also showed moderate anti-SINV and anti-SFV activities.
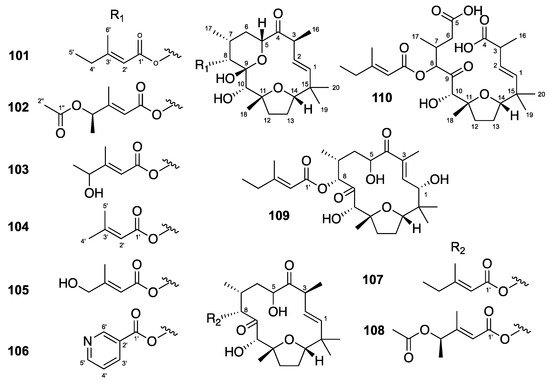
Figure 6.
Structures of flexibilanes 101–110.

Table 5.
Anti-CHIKV activities of flexibilanes 101–110.
7. Protein Kinase C (PKCs) as Targets of Phorbol Esters for Inhibition of Chikungunya Virus (CHIKV)
Recently, it has been shown that HIV-1 and HIV-2 inhibitory effects of phorbols esters were strongly correlated with those occurring on CHIKV [16]. These results were quite surprising given the fact that CHIKV and HIV belong to two different virus genera, Alphavirus and Lentivirus, respectively, but most probably can be explained through a common PKC-based mechanism of action. Although the mechanism remains poorly defined, this provides evidences that inhibition of CHIKV-induced cell death of phorbol esters might result from an activation of PKCs [44], and that PKC is an important target in CHIKV replication.
Protein kinase C (PKC) is a family of related serine/threonine kinases that regulate many cellular processes such as proliferation, differentiation and apoptosis. They have been classified into several distinct subfamilies depending on their specific requirements for activation. Classical isoforms (α, βI, βII, and γ) require calcium and diacylglycerol (DAG); novel isoforms (PKC-δ, -ε, -η, and -θ) require DAG but not calcium for activation, while activation of the atypical isoforms (Mζ- ι/λ isoforms) is independent of calcium and DAG. Following activation, PKCs undergo translocation from the cytoplasm to the plasma membrane and act trough phosphorylation of downstream signaling factors [45,46,47]. Due to their structural similarity with DAG, phorbol esters are powerful ligands of the regulatory domain of all classical and novel PKC isoforms.
The interaction of phorbols with PKC is dependent on their substitution pattern and requires a combination of optimal hydrogen bonding and hydrophobic contacts for high potency. Phorbols bind to a cysteine-rich site replacing a molecule of water and establishing hydrogen bond interactions through the oxygen atoms bound to carbons C-3, C-4, and C-20 [48,49,50]. The hydrophobic acyl chains of phorbol esters allow complex formation with PKCs and their anchoring to the membrane [50]. Changes on the C-3 oxygen atom led to lower PKC activation due to the loss of inductive and steric effects exerted on the C-4 hydroxy group [49,51]. Since the cis-configuration of the A/B rings junction might create a spatial arrangement incompatible with PKCs binding, the most potent PEs that modulate PKCs activity belong to the β-series (trans-fused A/B rings) [52]. By using computer-assisted modeling, it has been shown that the pharmacophore model for PEs required a hydrophobic region consisting of acyl substituents on C-12 and/or C-13 and a cyclohexane- cyclopropane-annellated ring system, and a hydrophilic domain spanning the C-3 to C-9 region including four groups able to form hydrogen bonds. An adequate distance and orientation of the ring system relative to membrane lipid bilayer are also necessary [51,53].
Tigliane diterpenoids, are one of the most important classes of diterpenoids from the Euphorbiaceae family [4,5]. Among tiglianes, prostratin is a 12-deoxyphorbol ester that has been demonstrated to be a potent activator of PKCs [47]. This compound, which has no pro-tumoral effect [10], was reported to inhibit the entry of HIV and to compromise latent HIV viral reservoirs through PKC-dependent mechanisms [54,55]. During the past 20 years many studies showed that phorbol derivatives stimulate HIV replication while inhibiting virion formation thus suppressing viral latent reservoirs through the same “kick and kill” strategy [13,34,35,56,57,58]. Recently, prostratin was shown to be a potent and selective inhibitor of CHIKV [21,44]. In particular, Neyts and colleagues demonstrated that its antiviral activity was dependent on the multiplicity of infection of the virus, and proved to be strongly dependent on the cell type [44]. A potent antiviral activity was observed in human skin fibroblast cells, the primary target cells of CHIKV infection. Prostratin mainly inhibits CHIKV replication at the post-entry stage hence exhibit antiviral activity when added to cells several hours post-infection. When tested in association with PKC inhibitors of known spectrum, the effect of prostratin appeared to be mediated mainly by the activation of classical PKC isoforms [44].
Considering the anti-CHIKV activity of PEs related to the present work, the structure–activity relationships have suggested the importance of the C-4 configuration, the influence of carbonyl at C-20 or C-7 and 6,7-epoxy function (which is spatially close to C-20) and the key role of acyl chains (See Section 1). These assumptions are in complete agreement with the pharmacophore model developed for phorbol-PKCs interactions [44,45,46,47,48].
A major concern about phorbols is their pro-tumoral effect. It has been attributed to hydrophobic acyl chains that exposed outward from the PKC/phorbol complex and likely retain complexes at the plasma membrane. This results in a sustained PKC activation which ultimately lead to the loss of its regulatory activity. Among PEs, a long side chain in position 13 associated with the absence of hydroxyl in position 12 (12-deoxyphorbol esters) seemed to be responsible for a strong tumor-promoting activity. Accordingly, PEs bearing short or medium acyl chain(s) may activate classical PKCs and should be devoid of tumor promoter activity. This sub-class of compounds might ideally be considered for anti-CHIKV compounds development [15,35,59,60].
8. Conclusions
Macrocyclic diterpenoids are an important source of lead compounds for drug development [61,62,63]. The non-tumor-promoting tiglianes, prostratin and DDP, are considered to be promising anti-HIV agents [20]. Ingenol mebutate a natural product identified from Euphorbia peplus is used as a topical gel (Picato®) for treatment of keratose actinic [61,62,63]. Others are currently in preclinical or clinical studies. Tigilanol tiglate (EBC-46®) has completed safety and efficacy studies for the treatment of solid tumors in dogs [64] and is currently in clinical study for the treatment of head and neck tumors in human adults [65].
In this review, we have shown than macrocyclic diterpenoids can also provide compounds with powerful antiviral activities. In particular, phorbol esters, 4-deoxy and 12-deoxyphorbol esters proved to be among the most promising anti-CHIKV agents yet reported. Considering that PKCs are potential host targets for the inhibition of CHIKV replication and that PEs most likely act though a PKC-dependent pathway, these compounds and their analogs offer interesting development opportunities as potential therapeutic agents for chikungunya treatment.
Funding
This work was supported by an “Investissement d′Avenir” grant managed by Agence Nationale de la Recherche (CEBA, ANR- 10-LABX-25-01).
Acknowledgments
The authors would like to thank Cécile Apel for carefully reading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, S.K.; Unni, S.K. Chikungunya virus: Host pathogen interaction. Rev. Med. Virol. 2011, 21, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Neyts, J.; Delang, L. Towards antivirals against chikungunya virus. Antivir. Res. 2015, 121, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and Pharmacological Research of the Plants in Genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. Diterpenes from European Euphorbia species serving as prototypes for natural-product-based drug discovery. Eur. J. Org. Chem. 2012, 27, 5115–5130. [Google Scholar] [CrossRef]
- Appendino, G. Ingenane Diterpenoids. In Progress in the Chemistry of Organic Natural Products; Springer: Columbus, OH, USA, 2016; Volume 35, pp. 1–90. [Google Scholar]
- Webb, B.L.J.; Hirst, S.J.; Giembycz, M.A. Protein kinase C isoenzymes: A review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br. J. Pharm. 2000, 130, 1433–1452. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Collado, I.G.; Hernández-Galán, R. Biologically active diterpenes containing a gem-dimethylcyclopropane subunit: An intriguing source of PKC modulators. Nat. Prod. Rep. 2014, 31, 940–952. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Cardellina, J.H.; McMahon, J.B.; Gulakowski, R.J.; Ishitoya, J.; Szallasi, Z.; Lewin, N.; Blumberg, P.; Weislow, O.S.; Beutler, J.A.; et al. A Nonpromoting Phorbol from the Samoan Medicinal Plant Homalanthus Nutans Inhibits Cell Killing by HIV-1. J. Med. Chem. 1992, 35, 1978–1986. [Google Scholar] [CrossRef]
- Kinghorn, A.D. Plant-derived anti-HIV agents. In Anti-AIDS Drug Development; Mohan, P., Masanori, B., Eds.; CRC Press: Chur, Switzerland, 1995; pp. 211–237. [Google Scholar]
- Erickson, K.L.; Beutler, J.A.; Cardellina, J.H.; McMahon, J.B.; Newman, D.J.; Boyd, M.R. A Novel phorbol ester from excoecaria agallocha. J. Nat. Prod. 1995, 58, 769–772. [Google Scholar] [CrossRef]
- Hossain Chowdhury, M.I.; Koyanagi, Y.; Kobayashi, S.; Hamamoto, Y.; Yoshiyama, H.; Yoshida, T.; Yamamoto, N. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: Possible involvement of protein kinase C pathway. Virology 1990, 176, 126–132. [Google Scholar] [CrossRef]
- Gulakowski, R.J.; McMahon, J.B.; Buckheit, R.W.; Gustafson, K.R.; Boyd, M.R. Antireplicative and anticytopathic activities of prostratin, a non-tumor-promoting phorbol ester, against human immunodeficiency virus (HIV). Antivir. Res. 1997, 33, 87–97. [Google Scholar] [CrossRef]
- Bocklandt, S.; Blumberg, P.M.; Hamer, D.H. Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate. Antivir. Res. 2003, 59, 89–98. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.F.; Pannecouque, C.; Renucci, F.; Delang, L.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. Antiviral Activity of Diterpene Esters on Chikungunya Virus and HIV Replication. J. Nat. Prod. 2015, 78, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Zhang, X.; Ma, Q.Y.; Peng, H.; Zheng, Y.T.; Hu, J.M.; Dai, H.F.; Zhou, J.; Zhao, Y.X. Anti-HIV-1 tigliane diterpenoids from Excoecaria acertiflia Didr. Fitoterapia 2014, 95, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, Y.; Yu, Z.; Yamamoto, N.; Mori, M.; Saito, H.; Takeuchi, M.; Ito, M.; Nemoto, H. Synthesis of new phorbol derivatives having ethereal side chain and evaluation of their anti-HIV activity. Bioorg. Med. Chem. 2005, 13, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Duffhues, G.; Q. Vo, M.; Perez, M.; A. Calzado, M.; Moreno, S.; Appendino, G.; Munoz, E. Activation of Latent HIV-1 Expression by Protein Kinase C Agonists. A Novel Therapeutic Approach to Eradicate HIV-1 Reservoirs. Curr. Drug Targets 2012, 12, 348–356. [Google Scholar] [CrossRef]
- Hezareh, M.; Moukil, M.A.; Szanto, I.; Pondarzewski, M.; Mouche, S.; Cherix, N.; Brown, S.J.; Carpentier, J.L.; Foti, M. Mechanisms of HIV receptor and co-receptor down-regulation by prostratin: Role of conventional and novel PKC isoforms. Antivir. Chem. Chemother. 2004, 15, 207–222. [Google Scholar] [CrossRef]
- Bourjot, M.; Delang, L.; Nguyen, V.H.; Neyts, J.; Guéritte, F.; Leyssen, P.; Litaudon, M. Prostratin and 12-O-tetradecanoylphorbol 13-acetate are potent and selective inhibitors of chikungunya virus replication. J. Nat. Prod. 2012, 75, 2183–2187. [Google Scholar] [CrossRef]
- Bourjot, M.; Leyssen, P.; Neyts, J.; Dumontet, V.; Litaudon, M. Trigocherrierin A, a potent inhibitor of chikungunya virus replication. Molecules 2014, 19, 3617–3627. [Google Scholar] [CrossRef]
- Allard, P.M.; Leyssen, P.; Martin, M.T.; Bourjot, M.; Dumontet, V.; Eydoux, C.; Guillemot, J.C.; Canard, B.; Poullain, C.; Guéritte, F.; et al. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry 2012, 84, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; Smadja, J.; Rasoanaivo, P.; Gurib-Fakim, A.; Mahomoodally, M.F.; Canard, B.; Guillemot, J.C.; Litaudon, M.; Guéritte, F. Biodiversity as a Source of Potent and Selective Inhibitors of Chikungunya Virus Replication. In Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics, 1st ed.; Gurib-Fakim, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 151–161. [Google Scholar]
- Corlay, N.; Delang, L.; Girard-Valenciennes, E.; Neyts, J.; Clerc, P.; Smadja, J.; Guéritte, F.; Leyssen, P.; Litaudon, M. Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. Fitoterapia 2014, 97, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Boutet-Mercey, S.; Cachet, X.; De La Torre, E.; Laboureur, L.; Gallard, J.F.; Retailleau, P.; Brunelle, A.; Dorrestein, P.C.; Costa, J.; et al. Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia semiperfoliata. J. Nat. Prod. 2017, 80, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Palenzuela, H.; Girard-Valenciennes, E.; Neyts, J.; Pannecouque, C.; Roussi, F.; Grondin, I.; Leyssen, P.; Litaudon, M. Antiviral Activity of Flexibilane and Tigliane Diterpenoids from Stillingia lineata. J. Nat. Prod. 2015, 78, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Allard, P.M.; Koval, A.; Righi, D.; Genta-Jouve, G.; Neyts, J.; Apel, C.; Pannecouque, C.; Nothias, L.F.; Cachet, X.; et al. Bioactive Natural Products Prioritization Using Massive Multi-Informational Molecular Networks. Acs Chem. Biol. 2017, 12, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Nothias, L.F.; Retailleau, P.; Costa, J.; Roussi, F.; Neyts, J.; Leyssen, P.; Touboul, D.; Litaudon, M.; Paolini, J. Isolation of Premyrsinane, Myrsinane, and Tigliane Diterpenoids from Euphorbia pithyusa Using a Chikungunya Virus Cell-Based Assay and Analogue Annotation by Molecular Networking. J. Nat. Prod. 2017, 80, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Nothias-Esposito, M.; Da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef]
- Nothias-Esposito, M.; Nothias, L.F.; Da Silva, R.R.; Retailleau, P.; Zhang, Z.; Leyssen, P.; Roussi, F.; Touboul, D.; Paolini, J.; Dorrestein, P.C.; et al. Investigation of Premyrsinane and Myrsinane Esters in Euphorbia cupanii and Euphobia pithyusa with MS2LDA and Combinatorial Molecular Network Annotation Propagation. J. Nat. Prod. 2019. [Google Scholar] [CrossRef]
- Remy, S.; Olivon, F.; Desrat, S.; Blanchard, F.; Eparvier, V.; Leyssen, P.; Neyts, J.; Roussi, F.; Touboul, D.; Litaudon, M. Structurally Diverse Diterpenoids from Sandwithia guyanensis. J. Nat. Prod. 2018, 81, 901–912. [Google Scholar] [CrossRef]
- Schmidt, R.; Hecker, E. Autoxidation of Phorbol Esters under Normal Storage Conditions. Cancer Res. 1975, 35, 1375–1377. [Google Scholar]
- Warrilow, D.; Gardner, J.; Darnell, G.A.; Suhrbier, A.; Harrich, D. HIV Type 1 Inhibition by Protein Kinase C Modulatory Compounds. Aids Res. Hum. Retrovir. 2006, 22, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Márquez, N.; Calzado, M.A.; Sánchez-Duffhues, G.; Pérez, M.; Minassi, A.; Pagani, A.; Appendino, G.; Diaz, L.; Muñoz-Fernández, M.Á.; Muñoz, E. Differential effects of phorbol-13-monoesters on human immunodeficiency virus reactivation. Biochem. Pharm. 2008, 75, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cik, M.; Appendino, G.; Puyvelde, L.; Leysen, J.; Kimpe, N. Daphnane-Type Diterpene Orthoesters and their Biological Activities. Mini-Rev. Med. Chem. 2005, 2, 185–200. [Google Scholar] [CrossRef]
- Olivon, F.; Remy, S.; Grelier, G.; Apel, C.; Eydoux, C.; Guillemot, J.C.; Neyts, J.; Delang, L.; Touboul, D.; Roussi, F.; et al. Antiviral compounds from Codiaeum peltatum targeted by a multi-informative molecular networks approach. J. Nat. Prod. 2019, 82, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.M.; Martin, M.T.; Tran Huu Dau, M.E.; Leyssen, P.; Guéritte, F.; Litaudon, M. Trigocherrin a, the first natural chlorinated daphnane diterpene orthoester from Trigonostemon cherrieri. Org. Lett. 2012, 14, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Litaudon, M.; Nothias, L.; Allard, P.; Bourjot, M.; Dumontet, V.; Guéritte, F.; Delang, L.; Pannecouque, C.; Leyssen, P. Diterpenoids from Euphorbiaceae with Potent Anti-CHIKV and Anti-HIV Activities: Are these Antiviral Properties Correlated? Planta Med. 2013, 79, 866–867. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.F.; Retailleau, P.; Paolini, J.; Pannecouque, C.; Neyts, J.; Dumontet, V.; Roussi, F.; Leyssen, P.; Costa, J.; Litaudon, M. Jatrophane diterpenes as inhibitors of chikungunya virus replication: Structure-activity relationship and discovery of a potent lead. J. Nat. Prod. 2014, 77, 1505–1512. [Google Scholar] [CrossRef]
- Esposito, M.; Nothias, L.F.; Nedev, H.; Gallard, J.F.; Leyssen, P.; Retailleau, P.; Costa, J.; Roussi, F.; Iorga, B.I.; Paolini, J.; et al. Latex as a Source of Jatrophane Esters: Isolation, Structural Analysis, Conformational Study, and Anti-CHIKV Activity. J. Nat. Prod. 2016, 79, 2873–2882. [Google Scholar] [CrossRef]
- Wittenberg, R.; Beier, C.; Dräger, G.; Jas, G.; Jasper, C.; Monenschein, H.; Kirschning, A. Towards the total synthesis of tonantzitlolone - Preparation of key fragments and the complete carbon backbone. Tetrahedron Lett. 2004, 45, 4457–4460. [Google Scholar] [CrossRef]
- Dräger, G.; Jeske, F.; Kunst, E.; Lopez, E.G.; Sanchez, H.V.; Tsichritzis, F.; Kirschning, A.; Jakupovic, J. Tonantzitlolone and other diterpenes from Stillingia sanguinolenta. Eur. J. Org. Chem. 2007, 5020–5026. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Amrun, S.N.; Ng, L.F.P.; Leyssen, P.; Neyts, J.; Delang, L. Protein kinases C as potential host targets for the inhibition of chikungunya virus replication. Antivir. Res. 2017, 139, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, D.; Braiman-Wiksman, L.; Daum, N.; Denning, M.F.; Tennenbaum, T. Protein kinase C family: On the crossroads of cell signaling in skin and tumor epithelium. J. Cancer Res. Clin. Oncol. 2007, 133, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Corbalán-García, S.; Gómez-Fernández, J.C. Protein kinase C regulatory domains: The art of decoding many different signals in membranes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 633–654. [Google Scholar] [CrossRef] [PubMed]
- McKernan, L.N.; Momjian, D.; Kulkosky, J. Protein Kinase C: One Pathway towards the Eradication of Latent HIV-1 Reservoirs. Adv. Virol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matias, D.; Bessa, C.; Fátima Simões, M.; Reis, C.P.; Saraiva, L.; Rijo, P. Natural Products as Lead Protein Kinase C Modulators for Cancer Therapy. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2016; Volume 50, pp. 45–79. [Google Scholar]
- Xu, R.X.; Pawelczyk, T.; Xia, T.H.; Brown, S.C. NMR structure of a protein kinase C-γ phorbol-binding domain and study of protein-lipid micelle interactions. Biochemistry 1997, 36, 10709–10717. [Google Scholar] [CrossRef]
- Kraft, A.S.; Anderson, W.B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature 1983, 301, 621–623. [Google Scholar] [CrossRef]
- Wender, P.A.; Koehler, K.F.; Sharkey, N.A.; Dell’Aquila, M.L.; Blumberg, P.M. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc. Natl. Acad. Sci. USA 1986, 83, 4214–4218. [Google Scholar] [CrossRef]
- Silinsky, E.M.; Searl, T.J. Phorbol esters and neurotransmitter release: More than just protein kinase C? Br. J. Pharm. 2003, 138, 1191–1201. [Google Scholar] [CrossRef]
- Jeffrey, A.M.; Liskamp, R.M. Computer-assisted molecular modeling of tumor promoters: Rationale for the activity of phorbol esters, teleocidin B, and aplysiatoxin. Proc. Natl. Acad. Sci. USA 1986, 83, 241–245. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Mateos, E.; Díaz-Gil, G.; Gómez-Esquer, F.; Del Cojo, M.S.; Alcamí, J.; Coiras, M. Protein kinase Cθ is a specific target for inhibition of the HIV type 1 replication in CD4 + T lymphocytes. J. Biol. Chem. 2011, 286, 27363–27377. [Google Scholar] [CrossRef]
- Trushin, S.A.; Bren, G.D.; Asin, S.; Pennington, K.N.; Paya, C.V.; Badley, A.D. Human Immunodeficiency Virus Reactivation by Phorbol Esters or T-Cell Receptor Ligation Requires both PKC and PKC. J. Virol. 2005, 79, 9821–9830. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Koyanagi, Y.; Nakashima, H.; Kobayashi, N.; Yamamoto, N. Tumor promoter, TPA, enhances replication of HTLV-III/LAV. Virology 1986, 154, 249–258. [Google Scholar] [CrossRef]
- Jiang, G.; Dandekar, S. Targeting NF-κB Signaling with Protein Kinase C Agonists as An Emerging Strategy for Combating HIV Latency. Aids Res. Hum. Retrovir. 2014, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Horwitz, M.S.; Fife, B.T.; Lester, R.; Cameron, D.W. Landscape review of current HIV “kick and kill” cure research-some kicking, not enough killing. Bmc Infect. Dis. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kazanietz, M.G.; Blumberg, P.M.; Hurley, J.H. Crystal structure of the Cys2 activator-binding domain of protein kinase Cδ in complex with phorbol ester. Cell 1995, 81, 917–924. [Google Scholar] [CrossRef]
- Newton, A.C.; Brognard, J. Reversing the Paradigm: Protein Kinase C as a Tumor Suppressor. Trends Pharm. Sci. 2017, 38, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, L.; Fresco, P.; Pinto, E.; Portugal, H.; Gonçalves, J. Differential Activation by Daphnetoxin and Mezerein of PKC-Isotypes α, βI, δ and ζ. Planta Med. 2001, 67, 787–790. [Google Scholar] [CrossRef]
- Hou, J.J.; She, Y.; Yang, Z.; Fang, L.; Cai, L.Y.; Yao, S.; Long, H.L.; Wu, W.Y.; Guo, D.A. Anti-proliferation activity of terpenoids isolated from Euphorbia kansui in human cancer cells and their structure-activity relationship. Chin. J. Nat. Med. 2017, 15, 766–774. [Google Scholar] [CrossRef]
- Rosen, R.H.; Gupta, A.K.; Tyring, S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Derm. 2012, 66, 486–493. [Google Scholar] [CrossRef]
- Miller, J.; Campbell, J.; Blum, A.; Reddell, P.; Gordon, V.; Schmidt, P.; Lowden, S. Dose Characterization of the Investigational Anticancer Drug Tigilanol Tiglate (EBC-46) in the Local Treatment of Canine Mast Cell Tumors. Front. Vet. Sci. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- QBiotics. Available online: https://qbiotics.com (accessed on 27 May 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).