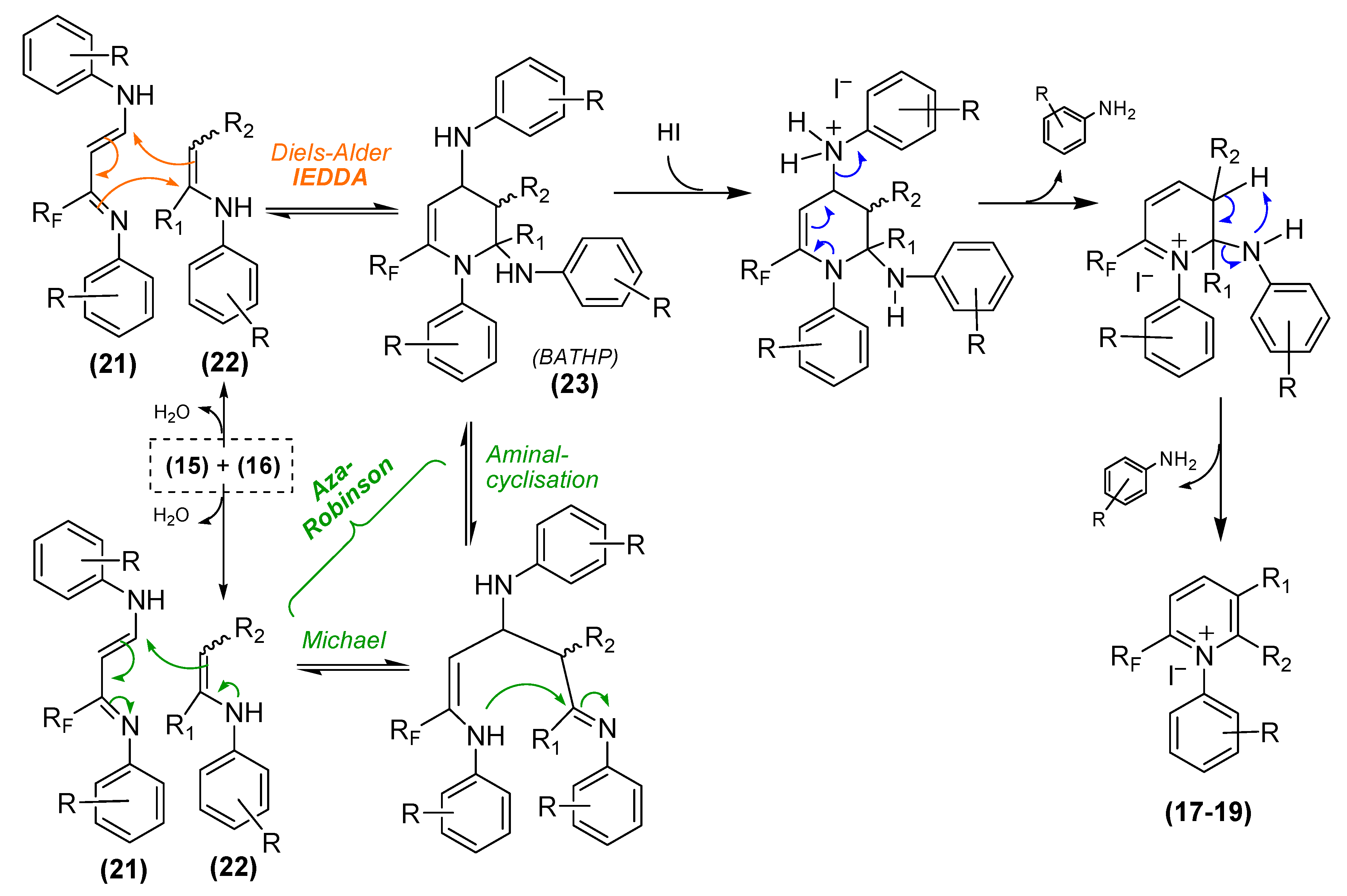
3. Materials and Methods
3.1. General Methods
All reaction solvents were purchased from commercial suppliers and distilled before use. All synthetic reactions were performed in oven-dried glassware, and their progress was monitored by thin layer chromatography (TLC) using silica gel plates, and by 19F-NMR spectroscopy of aliquots. Chromatographic column purifications were performed on silica gel (40–63 μm). 1H, 13C, and 19F-NMR spectra were recorded in either CDCl3 or DMSO-d6 solution on an Avance 300 (300 MHz) or an Avance 400 (400 MHz) spectrometer (Bruker, Billerica, MA, USA). Chemical shifts are reported in ppm, using the solvent signal chemical shift as a reference. Abbreviations used in the description of NMR spectra: s: singlet, d: doublet, t: triplet, q: quadruplet, bs: broad signal. Coupling constants (J) are given in Hertz. Mass spectra (either low- or high-resolution) were recorded on a SX102 mass spectrometer (JEOL, Tokyo, Japan) in FAB+ mode, using 3-nitrobenzyl alcohol matrix. Elemental analyses were carried out on a Flash 2000 elemental analyzer (Thermo Finnigan, Waltham, MA, USA).
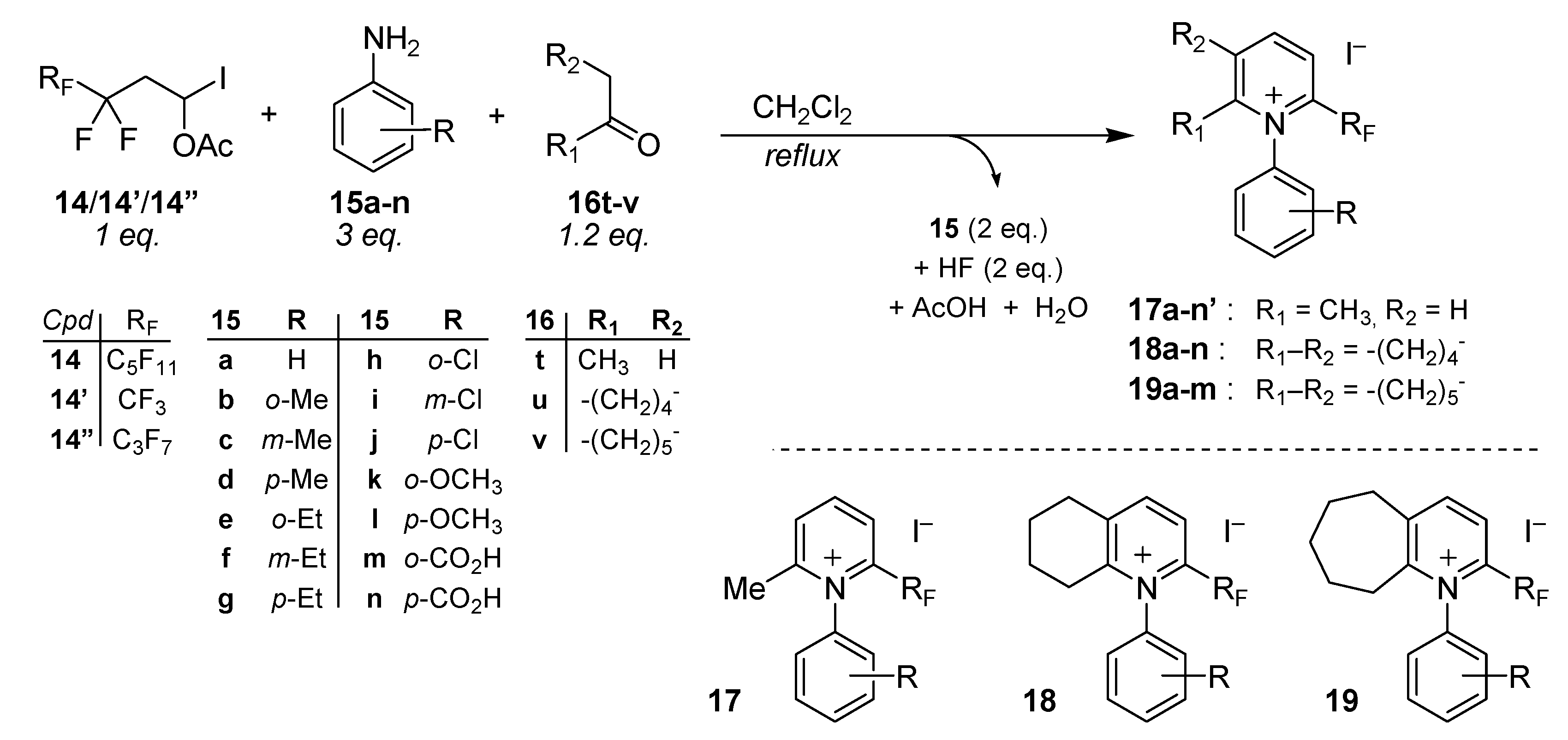
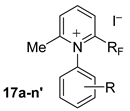
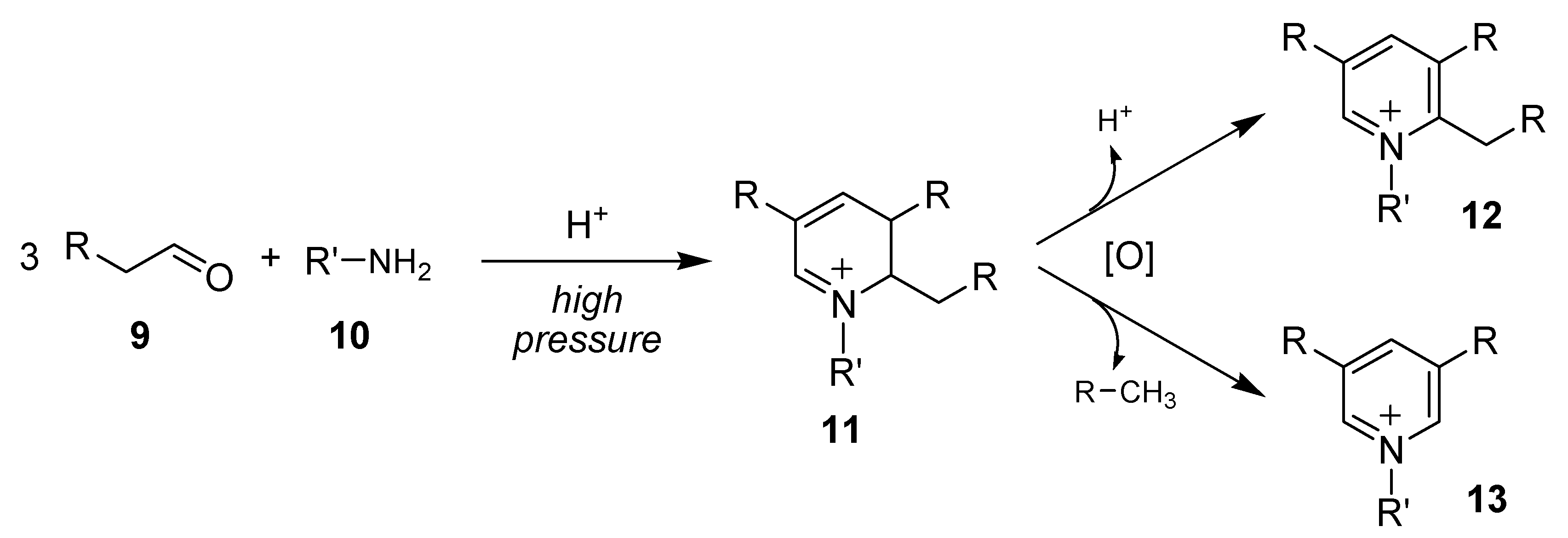
3.2. General Procedure for the Synthesis 2-Trifluoromethylated and 2-Perfluoroalkylated 6-Methyl-N-(R-Phenyl)Pyridinium Iodides (17a–l), N-(R-Phenyl)-5,6,7,8-Tetrahydroquinolinium Iodides (18a–l) and N-(R-Phenyl)-6,7,8,9-Tetrahydro-5H-Cyclohepta[B]Pyridinium Iodides (19a–l) (All examples except R = CO2H or R = M-Ome)
To a stirred solution of 1-acetoxy-1-iodo-perfluoroalkylethane compounds
14 (1 equiv.) in anhydrous dichloromethane (10 mL DCM for 1g of
14), was added 3 equiv. of the corresponding substituted aniline
15 and 1.2 equiv. of ketone
16. The mixture was stirred under reflux for the desired time (4–12 h,
Table 1,
Table 2 and
Table 3) until complete consumption of
14 (monitored by TLC eluent petroleum ether/ethyl acetate: 80/20
v/
v and
19F-NMR spectroscopy of aliquots). When the reaction was completed, the mixture was allowed to cool to r.t. then the brown precipitate accumulated during the reaction was separated by vacuum filtration (it was subsequently identified as anilinium salts by NMR and MS). Then ethyl ether was added to the filtrate and the corresponding pyridinium iodides
17a–
l, 18a–
l, and
19a–
l precipitate instantly and were isolated by vacuum filtration as amorphous solids.
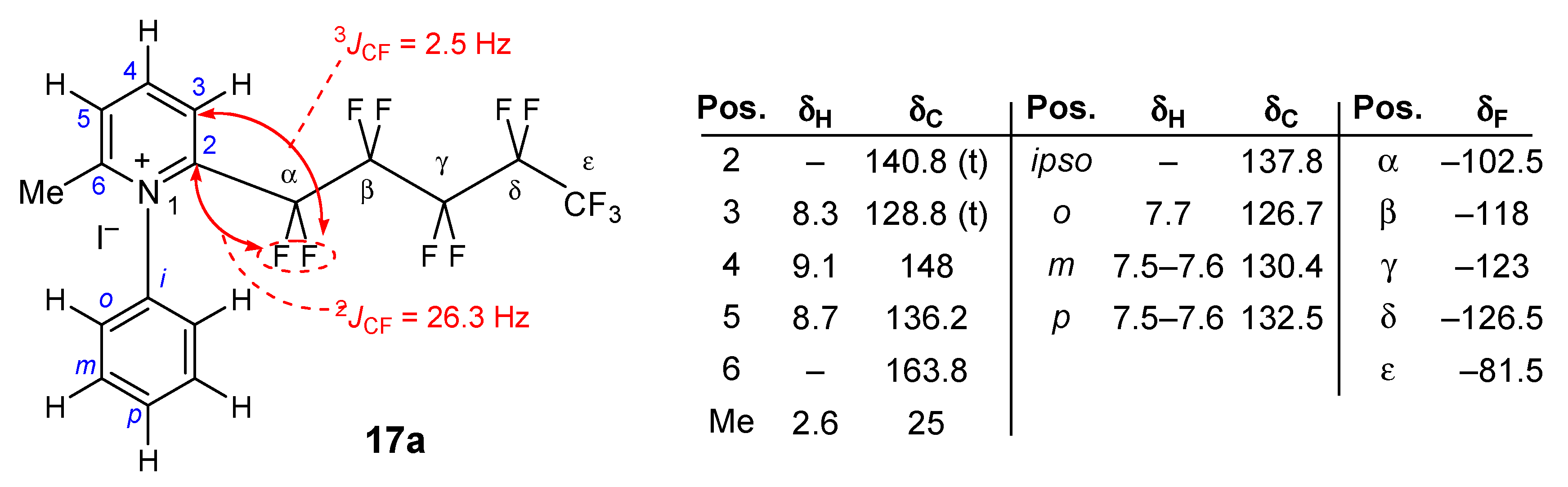
2-Perfluoropentyl-6-methyl-N-phenylpyridinium iodide (17a). Aniline 15a (5.24 g, 5.63 × 10−2 mole) and acetone (16t, 1.65 mL, 1.3 g, 2.25 × 10−2 mole) were added to a solution of 14 (RF = C5F11, 10 g, 1.87 × 10−2 mole) in dry dichloromethane (100 mL). The mixture was stirred for 2 h at reflux to afford 9.55 g of the title product 17a, total yield 90%. 1H-NMR (400.13 MHz, CDCl3) δ 2.6 (s, 3H, CH3), 7.5–7.6 (m, 3H, Ph-H), 7.7 (d, J = 7.1 Hz, 2H, Ph-H), 8.3 (d, J = 8 Hz, 1H, Py-H), 8.7 (d, J = 8 Hz, 1H, Py-H), 9.1 (t, J = 8.1 Hz, 1H, Py-H); 1H-NMR (400.13 MHz, CDCl3+D2O) δ 7.5–7.6 (m, 3H, Ph-H), 7.7 (d, J = 7.2 Hz, 2H, Ph-H), 8.3 (d, J = 8 Hz, 1H, Py-H), 8.7 (d, J = 8.1 Hz, 1H, Py-H), 9.1 (t, J = 8.1 Hz, 1H, Py-H); 13C-NMR (100.6 MHz, CDCl3) δ 25 (s, CH3), 126.7, 128.8 (t, 3JCF = 2.5 Hz), 130.4, 132.5, 136.2, 137.8, 140.8 (t, 2JCF = 26.3 Hz), 148, 163.8; 19F-NMR (235.3 MHz, CDCl3) δ −126.5 (m 2F, CF2-CF2-CF2-CF2-CF3), −123 (m 2F, CF2-CF2-CF2-CF2-CF3), −118 (m 2F, CF2-CF2-CF2-CF2-CF3), −102.5 (m 2F, CF2-CF2-CF2-CF2-CF3), −81.5 (m 3F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 438 ([M − I]+, 100). HRMS calcd. for C17H11F11N+ 438.0716, found 438.0720. Anal. Calcd. for C17H11F11NI: C, 36.13; H, 1.96; N, 2.48. Found: C, 36.15; H, 1.95; N, 2.46.
2-Trifluoromethyl-6-methyl-N-phenylpyridinium iodide (17a’). Aniline 15a (8.4 g, 9 × 10−2 mol) and acetone (16t, 2.7 mL, 2 g, 3.61 × 10−2 mol) were added to a solution of 14’ (RF = CF3, 10 g, 3 × 10−2 mol) in dry dichloromethane (100 mL). The mixture was stirred for 2 h at reflux to give 9.9 g of the title product 17a’, total yield 90%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2.6 (s, 3H, CH3), 7.5–7.6 (m, 3H), 7.8 (d, J = 9 Hz, 2H), 8.5 (dd, J = 9 and 3 Hz, 1H), 8.6 (dd, J = 9 and 3 Hz, 1H), 9 (t, J = 9 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 24.1 (s, CH3), 118.6 (q, CF3, 1JCF = 276.9 Hz), 125.6 (q, 3JCF = 5.2 Hz), 125.7, 130.1, 132.3, 135.1, 136.7, 140.9 (q, C-CF3, 2JCF = 35.4 Hz), 148, 162.5; 19F-NMR (282.4 MHz, CDCl3) δ −59.8 (s 3F, CF3). MS (m/z): 238 ([M − I]+, 100). HRMS calcd. for C13H11F3N+: 238.0844, found 238.0848. Anal. Calcd. for C13H11F3NI: C, 42.76; H, 3.04; N, 3.84. Found: C, 42.78; H, 3.05; N, 3.82.
2-Perfluoropropyl-6-methyl-N-phenylpyridinium iodide (17a’’). Aniline 15a (6.45 g, 6.94 × 10−2 mol) and acetone (16t, 1.6 mL, 2 g, 2.77 × 10−2 mol) were added to a solution of 14’’ (RF = C3F7, 10 g, 2.31 × 10−2 mol) in dry dichloromethane (100 mL). The mixture was stirred for 2 h at reflux, affording 9.69 g of the title product 17a’’, total yield 91%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.6 (s, 3H, CH3), 7.5–7.6 (m, 3H), 7.7 (d, J = 7 Hz, 2H), 8.3 (d, J = 8.1 Hz, 1H), 8.8 (d, J = 8 Hz, 1H), 9 (t, J = 8 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 25.1 (s, CH3), 126.5, 128.7 (t, 3JCF = 2.5 Hz), 130.4, 132.2, 136.1, 137.7, 140.9 (t, 2JCF = 26.5 Hz), 148, 163.7; 19F-NMR (235.3 MHz, CDCl3) δ −127 (m 2F, CF2), −102 (m 2F, CF2), −80.5 (m 3F, CF3). MS (m/z): 338 ([M − I]+, 100). HRMS calcd. for C15H11F7N+: 338.0780, found 338.0782. Anal. Calcd. for C15H11F7NI: C, 38.73; H, 2.38; N, 3.01. Found: C, 38.75; H, 2.39; N, 3.04.
2-Perfluoropentyl-6-methyl-N-(2-methylphenyl)pyridinium iodide (17b). 2-Methylaniline (15b, 6 g, 5.63 × 10−2 mol) and acetone (16t, 1.66 mL, 1.3 g, 2.25 × 10−2 mol) were added to a solution of 14 (RF = C5F11, 10 g, 1.87 × 10−2 mol) in dry dichloromethane (100 mL). The mixture was stirred for 4 h at reflux, to give 8.7 g of the title product 17b, total yield 80%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.5 (s, 3H, CH3), 2.6 (s, 3H, CH3), 7.4–7.6 (m, 4H), 7.9 (t, J = 7.2 Hz, 1H), 8.2 (d, J = 8.1 Hz, 1H), 8.9 (d, J = 8.2 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 20.1 (s, CH3), 25 (s, CH3), 127, 128.5 (t, 3JCF = 2 Hz), 130.5, 131.9, 136.1, 138, 141.1 (t, 2JCF = 26 Hz), 148, 163.5; 19F-NMR (282.4 MHz, CDCl3) δ −125.7 (m 2F, CF2), −122.1 (m 2F, CF2), −118.2 (AB system, 2JFF = 338.8 Hz, 1F, CF2-CF2-CF2), −116 (AB system, 2JFF = 338.8 Hz, 1F, CF2-CF2-CF2), −108.5 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2), −98 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2), −80.6 (m 3F, CF3). MS (m/z): 452 ([M − I]+, 100). HRMS calcd. for C18H13F11N+: 452.0872, found 452.0877. Anal. Calcd. for C18H13F11NI: C, 37.33; H, 2.26; N, 2.42. Found: C, 37.35; H, 2.30; N, 2.40.
2-Perfluoropentyl-6-methyl-N-(3-methylphenyl)pyridinium iodide (17c). 3-Methylaniline (15c, 6.34 g, 5.92 × 10−2 mol) and acetone (16t, 1.75 mL, 1.37 g, 2.36 × 10−2 mol) were added to a solution of 14 (RF = C5F11, 0.5 g, 1.97 × 10−2 mol) in dry dichloromethane (105 mL). The mixture was stirred for 4 h at reflux to give 9.7 g of the title product 17c, total yield 85%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.6 (s, 3H, CH3), 2.7 (s, 3H, CH3), 7.4–7.6 (m, 4H), 8.3 (d, J = 7.2 Hz, 1H), 8.8 (d, J = 8.1 Hz, 1H), 9.2 (t, J = 8.1 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 22 (s, CH3), 26 (s, CH3), 121, 123, 128 (t, 3JCF = 2 Hz), 130.5, 132, 136.1, 137, 139 (t, 2JCF = 25 Hz), 145, 148, 161; 19F-NMR (282.4 MHz, CDCl3) δ −126 (s 2F, CF2), −122.4 (s 2F, CF2), −117.2 (s 2F, CF2), −103.5 (AB system, 2JFF = 310.6 Hz, 1F, CF2-CF2), −101.1 (AB system, 2JFF = 310.6 Hz, 1F, CF2-CF2), −80.7 (m 3F, CF3). MS (m/z): 452 ([M − I]+, 95). HRMS calcd. for C18H13F11N+: 452.0872, found 452.0875. Anal. Calcd. for C18H13F11NI: C, 37.33; H, 2.26; N, 2.42. Found: C, 37.36; H, 2.28; N, 2.40.
2-Perfluoropentyl-6-methyl-N-(4-methylphenyl)pyridinium iodide (17d). 4-Methylaniline (15d, 5.74 g, 5.35 × 10−2 mol) and acetone (16t, 1.58 mL, 1.24 g, 2.14 × 10−2 mol) were added to a solution of 14 (RF = C5F11, 9.5 g, 1.78 × 10−2 mol) in dry dichloromethane (95 mL). The mixture was stirred for 4 h at reflux to afford 8.79 g of the title product 17d, total yield 85%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.65 (s, 3H, CH3), 2.7 (s, 3H, CH3), 7.4 (d, J = 8 Hz, 2H), 7.7 (d, J = 8 Hz, 2H), 8.3 (d, J = 8.2 Hz, 1H), 8.8 (d, J = 8.3 Hz, 1H), 9 (t, J = 8.2 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 25 (s, CH3), 26.1 (s, CH3), 122.7, 126.5, 127.7 (t, 3JCF = 1.5 Hz), 129.9, 130, 135.1, 137.8 (t, 2JCF = 27.2 Hz), 142.5, 146.6, 150.2, 167.5; 19F-NMR (282.4 MHz, CDCl3) δ −126 (s 2F, CF2), −122.4 (s 2F, CF2), −117.2 (s 2F, CF2), −101.6 (s, 2F, CF2-CF2), −80.7 (m 3F, CF3). MS (m/z): 452 ([M − I]+, 100). HRMS calcd. for C18H13F11N+: 452.0872, found 452.0878. Anal. Calcd. for C18H13F11NI: C, 37.33; H, 2.26; N, 2.42. Found: C, 37.37; H, 2.26; N, 2.39.
2-Trifluoromethyl-6-methyl-N-(4-methylphenyl)pyridinium iodide (17d’). 4-Methylaniline (15d, 8.81 g, 8.22 × 10−2 mol) and acetone (16t, 2.43 mL, 1.9 g, 3.28 × 10−2 mol) were added to a solution of 14’ (RF = CF3, 9.1 g, 2.74 × 10−2 mol) in dry dichloromethane (91 mL). The mixture was stirred for 4 h at reflux to yield 9.14 g of the title product 17d’, total yield 88%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.6 (s, 3H, CH3), 2.7 (s, 3H, CH3), 7.4 (d, J = 8.1 Hz, 2H), 7.7 (d, J = 8 Hz, 2H), 8.3 (d, J = 8 Hz, 1H), 8.7 (d, J = 8.1 Hz, 1H), 9.2 (t, J = 8.1 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 24.2 (s, CH3), 25 (s, CH3), 118.4 (q, CF3, 1JCF = 276.8 Hz), 124.8 (q, 3JCF = 5 Hz), 122.5, 125.6, 130, 132.3, 140.9 (q, C-CF3, 2JCF = 36 Hz), 147.2, 150, 162.5; 19F-NMR (282.4 MHz, CDCl3) δ −59.9 (s 3F, CF3). MS (m/z): 252 ([M − I]+, 95). HRMS calcd. for C14H13F3N+: 252.100, found 252.1010. Anal. Calcd. for C14H13F3NI: C, 44.35; H, 3.46; N, 3.69. Found: C, 44.38; H, 3.47; N, 3.70.
2-Perfluoropentyl-6-methyl-N-(2-ethylphenyl)pyridinium iodide (17e). 2-Ethylaniline (15e, 7 g, 5.8 × 10−2 mol) and acetone (16t, 1.7 mL, 1.34 g, 2.32 × 10−2 mol) were added to a solution of 14 (RF = C5F11, 10.3 g, 1.93 × 10−2 mol) in dry dichloromethane (103 mL). The mixture was stirred for 6 h at reflux to produce 8 g of the title product 17e, total yield 70%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.6 (q, J = 8 Hz, 2H, CH2-CH3), 2.8 (s, 3H, CH3), 7.4–7.6 (m, 3H), 8 (s, 1H), 8.2 (d, J = 8 Hz, 1H), 8.9 (d, J = 8.1 Hz, 1H); 9.2 (t, J = 8.1 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 13.2 (s, CH2-CH3), 24.3 (s, CH2-CH3), 27.1 (s, CH3), 124.2, 125.9, 127.8 (t, 3JCF = 2.1 Hz), 129.6, 131, 136.1, 137.5 (t, 2JCF = 25 Hz), 146.5, 150.1, 167.7; 19F-NMR (282.4 MHz, CDCl3) δ −126 (m 2F, CF2), −122.9 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.8 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −118.7 (AB system, 2JFF = 300.9 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −115.9 (AB system, 2JFF = 300.9 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −108.5 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −98.8 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.7 (m 3F, CF3). MS (m/z): 466 ([M − I]+, 95). HRMS calcd. for C19H15F11N+: 466.1029, found 466.1033. Anal. Calcd. for C19H15F11NI: C, 38.47; H, 2.55; N, 2.36. Found: C, 38.48; H, 2.56; N, 2.40.
2-Perfluoropentyl-6-methyl-N-(3-ethylphenyl)pyridinium iodide (17f). 3-Ethylaniline (15f, 7.17 g, 5.92 × 10−2 mol) and acetone (16t, 1.75 mL, 1.37 g, 2.36 × 10−2 mol) were added to a solution of 14 (RF = C5F11, 10.5 g, 1.97 × 10−2 mol) in dry dichloromethane (105 mL). The mixture was stirred for 4 h at reflux to obtain. 9.36 g of the title product 17f, total yield 80%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.7 (s, 3H, CH3), 2.8 (q, J = 8 Hz, 2H, CH2-CH3), 7.4–7.7 (m, 4H), 8.4 (d, J = 6.7 Hz, 1H), 8.9 (d, J = 7.1 Hz, 1H), 9.2 (t, J = 7.9 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 13 (s, CH2-CH3), 23 (s, CH2-CH3), 27 (s, CH3), 121.5, 123.5, 127 (t), 128, 130, 134, 135, 139 (t, 2JCF = 25 Hz), 145, 146, 162; 19F-NMR (282.4 MHz, CDCl3) δ −126 (s 2F, CF2), −122.4 (s 2F, CF2), −117.2 (s 2F, CF2), −102.5 (AB system, 2JFF = 315.5 Hz, 1F, CF2-CF2), −101.5 (AB system, 2JFF = 315.5 Hz, 1F, CF2-CF2), −80.7 (m 3F, CF3). MS (m/z): 466 ([M − I]+, 100). HRMS calcd. for C19H15F11N+: 466.1029, found 466.1035. Anal. Calcd. for C19H15F11NI: C, 38.47; H, 2.55; N, 2.36. Found: C, 38.49; H, 2.56; N, 2.33.
2-Perfluoropentyl-6-methyl-N-(4-ethylphenyl)pyridinium iodide (17g). 6.9 g of 4-ethylaniline 15g (5.69 × 10−2 mol) and 1.68 mL or 1.32 g (2.27 × 10−2 mol) of acetone 16t were added to a solution of 10.1 g (1.89 × 10−2 mol) of 14 (RF = C5F11) in 101 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.34 g of the title product 17g were obtained, total yield 83%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.7 (s, 3H, CH3), 2.8 (q, J = 8 Hz, 2H, CH2-CH3), 7.1 (d, J = 8.4 Hz, 2H), 7.7 (d, J = 8.3 Hz, 2H), 8.4 (d, J = 7.5 Hz, 1H), 8.8 (d, J = 7.8 Hz, 1H), 9.1 (t, J = 7.8 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 13 (s, CH2-CH3), 24 (s, CH2-CH3), 26.8 (s, CH3), 113.5, 126.5, 127.5 (t, 3JCF = 2 Hz), 129.5, 130, 135, 139 (t, 2JCF = 25 Hz), 147, 159, 162; 19F-NMR (282.4 MHz, CDCl3) δ −126.9 (s 2F, CF2), −123.3 (s 2F, CF2), −117.3 (s 2F, CF2), −101.8 (s, 2F, CF2-CF2), −80.7 (m 3F, CF3). MS (m/z): 466 ([M − I]+, 100). HRMS calcd. for C19H15F11N+: 466.1029, found 466.1030. Anal. Calcd. for C19H15F11NI: C, 38.47; H, 2.55; N, 2.36. Found: C, 38.48; H, 2.56; N, 2.35.
2-Trifluoromethyl-6-methyl-N-(4-ethylphenyl)pyridinium iodide (17g’). 9.85 g of 4-ethylaniline 15g (8.13 × 10−2 mol) and 2.4 mL or 1.88 g (3.25 × 10−2 mol) of acetone 16t were added to a solution of 9 g (2.71 × 10−2 mol) of 14’ (RF = CF3) in 90 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9 g of the title product 17g’ were obtained, total yield 85%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.7 (s, 3H, CH3), 2.8 (q, J = 8 Hz, 2H, CH2-CH3), 7.1 (d, J = 8 Hz, 2H), 7.7 (d, J = 8 Hz, 2H), 8.2 (d, J = 8 Hz, 1H), 8.7 (d, J = 8 Hz, 1H), 9 (t, J = 8.1 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 13.2 (s, CH2-CH3), 23.9 (s, CH2-CH3), 27 (s, CH3), 118.1 (q, CF3, 1JCF = 277.6 Hz), 125 (q, 3JCF = 4.8 Hz), 122.4, 125.7, 130.2, 132, 147.2 (q, C-CF3, 2JCF = 35.5 Hz), 149.9, 162; 19F-NMR (282.4 MHz, CDCl3) δ −58.9 (s 3F, CF3). MS (m/z): 266 ([M − I]+, 100). HRMS calcd. for C15H15F3N+: 266.1157, found 266.1160. Anal. Calcd. for C15H15F3NI: C, 45.82; H, 3.85; N, 3.56. Found: C, 45.85; H, 3.86; N, 3.52.
2-Perfluoropentyl-6-methyl-N-(2-chlorophenyl)pyridinium iodide (17h). 7.4 g of 2-chloroaniline 15h (5.8 × 10−2 mol) and 1.7 mL or 1.34 g (2.32 × 10−2 mol) of acetone 16t were added to a solution of 10.3 g (1.93 × 10−2 mol) of 14 (RF = C5F11) in 103 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 6.96 g of the title product 17h were obtained, total yield 60%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.8 (s, 3H, CH3), 7.7–7.9 (m, 3H), 8.4 (m, 1H), 8.5 (d, J = 8 Hz, 1H), 8.9 (d, J = 8.1 Hz, 1H); 9.4 (t, J = 8 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 24.2 (s, CH3), 128.5, 129.1, 130.1, 130.8, 133.8, 135, 136.2, 140.5 (t, 2JCF = 24.1 Hz), 148.8, 163.8; 19F-NMR (282.4 MHz, CDCl3) δ −126.5 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.7 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −122.9 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.6 (AB system, 2JFF = 289.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −118.7 (AB system, 2JFF = 299.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −115.9 (AB system, 2JFF = 299.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −108.2 (AB system, 2JFF = 288.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −98.8 (AB system, 2JFF = 288.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.7 (m 3F, CF3). MS (m/z): 472 ([M − I]+, 90). HRMS calcd. for C17H10ClF11N+: 472.0326, found 472.0329. Anal. Calcd. for C17H10ClF11NI: C, 34.05; H, 1.68; N, 2.34. Found: C, 34.08; H, 1.67; N, 2.33.
2-Perfluoropentyl-6-methyl-N-(3-chlorophenyl)pyridinium iodide (17i). 7.55 g of 3-chloroaniline 15i (5.92 × 10−2 mol) and 1.75 mL or 1.37 g (2.36 × 10−2 mol) of acetone 16t were added to a solution of 10.5 g (1.97 × 10−2 mol) of 14 (RF = C5F11) in 105 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 8.28 g of the title product 17i were obtained, total yield 70%. Spectral data: 1H-NMR (400.13 MHz, CDCl3) δ 2.7 (s, 3H, CH3), 7.3 (s, 1H), 7.4–7.6 (m, 2H), 7.7 (m, 1H), 8.3 (d, J = 8 Hz, 1H), 8.5 (d, J = 8 Hz, 1H), 8.8 (t, J = 8 Hz, 1H); 13C-NMR (100.6 MHz, CDCl3) δ 27.3 (s, CH3), 127.9, 129.6, 131.4, 134.1, 135.5, 138.5 (t, 2JCF = 25 Hz), 141.1, 150.6, 166.4; 19F-NMR (282.4 MHz, CDCl3) δ −121.5 (s 2F, CF2), −117.5 (s 2F, CF2), −112.7 (s 2F, CF2), −97.4 (AB system, 2JFF = 105.3 Hz, 1F, CF2-CF2), −97.1 (AB system, 2JFF = 105.3 Hz, 1F, CF2-CF2), −75.9 (m 3F, CF3). MS (m/z): 472 ([M − I]+, 90). HRMS calcd. for C17H10ClF11N+: 472.0326, found 472.0330. Anal. Calcd. for C17H10ClF11NI: C, 34.05; H, 1.68; N, 2.34. Found: C, 34.11; H, 1.65; N, 2.36.
2-Perfluoropentyl-6-methyl-N-(4-chlorophenyl)pyridinium iodide (17j). 7.19 g of 4-chloroaniline 15j (5.63 × 10−2 mol) and 1.66 mL or 1.3 g (2.25 × 10−2 mol) of acetone 16t were added to a solution of 10 g (1.87 × 10−2 mol) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 9.58 g of the title product 17j were obtained, total yield 85%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2.5 (s, 3H, CH3), 7.9 (d, J = 8 Hz, 2H), 8.2 (d, J = 8.1 Hz, 2H), 8.6 (d, J = 8 Hz, 1H), 8.8 (d, J = 7.9 Hz, 1H), 9 (t, J = 7.9 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 26 (s, CH3), 113.2, 126, 127.4 (t, 3JCF = 2.2 Hz), 130.2, 136, 139.2 (t, 2JCF = 24.8 Hz), 147.2, 158, 162.3; 19F-NMR (282.4 MHz, CDCl3) δ −125.8 (s 2F, CF2), −122.3 (s 2F, CF2), −117.7 (s 2F, CF2), −101.8 (s, 2F, CF2-CF2), −80.2 (m 3F, CF3); MS (m/z): 472 ([M − I]+, 90). HRMS calcd. for C17H10ClF11N+: 472.0326, found 472.0324. Anal. Calcd. for C17H10ClF11NI: C, 34.05; H, 1.68; N, 2.34. Found: C, 34.10; H, 1.68; N, 2.35.
2-Perfluoropentyl-6-methyl-N-(2-methoxyphenyl)pyridinium iodide (17k). 6.8 g of 2-methoxyaniline 15k (5.52 × 10−2 mol) and 1.63 mL or 1.28 g (2.21 × 10−2 mol) of acetone 16t were added to a solution of 9.8 g (1.84 × 10−2 mol) of 14 (RF = C5F11) in 98 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 7.67 g of the title product 17k were obtained, total yield 70%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2.8 (s, 3H, CH3), 3.7 (s, 3H, OCH3), 7.1 (d, J = 8.3 Hz, 1H), 7.2 (t, J = 7.6 Hz, 1H), 7.7 (t, J = 8.8 Hz, 1H), 8 (m, 1H), 8.3 (d, J = 8.1 Hz, 1H), 8.8 (d, J = 8.2 Hz, 1H), 9 (d, J = 8.2 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 22.2 (s, CH3), 54 (s, OCH3), 113.1, 128.5, 129.1, 130.8, 133.7, 136.2, 139.9 (t, 2JCF = 25 Hz), 147, 160, 162.2; 19F-NMR (282.4 MHz, CDCl3) δ −126.5 (AB system, 2JFF = 295 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.5 (AB system, 2JFF = 295 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −122.9 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.5 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −119 (AB system, 2JFF = 288 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −116.5 (AB system, 2JFF = 288 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −107 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −99.5 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.69 (m 3F, CF3). MS (m/z): 468 ([M − I]+, 100). HRMS calcd. for C18H13F11NO+: 468.0821, found 468.0827. Anal. Calcd. for C18H13F11INO: C, 36.32; H, 2.20; N, 2.35. Found: C, 36.35; H, 2.21; N, 2.34.
2-Perfluoropentyl-6-methyl-N-(4-methoxyphenyl)pyridinium iodide (17l). 6.94 g of 4-methoxyaniline 15l (5.63 × 10−2 mol) and 1.66 mL or 1.3 g (2.25 × 10−2 mol) of acetone 16t were added to a solution of 10 g (1.87 × 10−2 mol) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.62 g of the title product 17l were obtained, total yield 86%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2.7 (s, 3H, CH3), 3.9 (s, 3H, OCH3), 7.2 (d, J = 8.5 Hz, 2H), 7.7 (d, J = 8.4 Hz, 2H), 8.4 (d, J = 7.5 Hz, 1H), 8.8 (d, J = 7.8 Hz, 1H), 9.1 (t, J = 7.8 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 22.3 (s, CH3), 54.2 (s, OCH3), 113, 128.7 (t, 3JCF = 2 Hz), 129.4, 130.5, 133, 136.1, 140 (t, 2JCF = 25 Hz), 147, 160.2, 162.5; 19F-NMR (282.4 MHz, CDCl3) δ −126.1 (s 2F, CF2), −122.3 (s 2F, CF2), −117.1 (s 2F, CF2), −101.8 (s, 2F, CF2-CF2), −80.7 (m 3F, CF3). MS (m/z): 468 ([M − I]+, 100). HRMS calcd. for C18H13F11NO+: 468.0821, found 468.0830. Anal. Calcd. for C18H13F11INO: C, 36.32; H, 2.20; N, 2.35. Found: C, 36.39; H, 2.21; N, 2.38.
2-Perfluoropentyl-N-(phenyl)-5,6,7,8-tetrahydroquinolinium iodide (18a). 5.24 g of aniline 15a (5.63 × 10−2 mole) and 2.21 g (2.25 × 10−2 mole) of cyclohexanone 16u were added to a solution of 10 g (1.87 × 10−2 mole) of 14 (RF = C5F11) in 50 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10 g of the title product 18a were obtained, total yield 88%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2 (m, 4H), 2.7 (m, 2H), 3.3 (m, 2H), 7.5–7.6 (m, 3H), 7.7 (d, J = 7.1 Hz, 2H), 8.2 (d, J = 8.1 Hz, 1H), 8.7 (d, J = 8.2 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 19.5, 21, 29.5, 31.2, 115, 125.1, 127, 128.1, 129.2, 139 (t, 2JCF = 27 Hz), 146, 148.1, 163.1; 19F-NMR (235.3 MHz, CDCl3) δ −126.2 (m 2F, CF2), −122.4 (m 2F, CF2), −117.3 (m 2F, CF2), −101.8 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 478 ([M − I]+, 95). HRMS calcd. for C20H15F11N+ 478.1029, found 478.1033. Anal. Calcd. for C20H15F11NI: C, 39.69; H, 2.50; N, 2.31. Found: C, 39.72; H, 2.51; N, 2.29.
2-Trifluoromethyl-N-(phenyl)-5,6,7,8-tetrahydroquinolinium iodide (18a’). 7.14 g of aniline 15a (7.68 × 10−2 mol) and 3 g (3.07 × 10−2 mol) of cyclohexanone 16u were added to a solution of 8.5 g (2.56 × 10−2 mol) of 14’ (RF = CF3) in 85 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.23 g of the title product 18a’ were obtained, total yield 89%. 1H-NMR (300.13 MHz, CDCl3) δ 1.9 (m, 4H, CH2, cyclohexyl group), 2.6 (m, 2H, CH2, cyclohexyl group), 3.3 (m, 2H, CH2, cyclohexyl group), 7.5–7.6 (m, 3H, Ph-H), 7.8 (d, J = 7.5 Hz, 2H, Ph-H), 8.2 (d, J = 8 Hz, 1H, Py-H), 8.8 (d, J = 8.1 Hz, 1H, Py-H); 13C-NMR (75.46 MHz, CDCl3) δ 19.8, 21, 29.6, 32.2, 115, 118.6 (q, CF3, 1JCF = 282.5 Hz), 125.2, 127.1, 128.8, 129.2, 139.5, 146.4, 148.5 (q, C-CF3, 2JCF = 34.5 Hz), 163; 19F-NMR (282.4 MHz, DMSO-d6) δ −59.8 (s 3F, CF3). MS (m/z): 278 ([M − I]+, 95). HRMS calcd. for C16H15F3N+: 278.1157, found 278.1160. Anal. Calcd. for C16H15F3NI: C, 47.43; H, 3.73; N, 3.46. Found: C, 47.45; H, 3.74; N, 3.44.
2-Perfluoropentyl-N-(2-methylphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18b). 5.86 g of 2-methylaniline 15b (5.46 × 10−2 mol) and 2.14 g (2.18 × 10−2 mol) of cyclohexanone 16u were added to a solution of 9.7 g (1.82 × 10−2 mol) of 14 (RF = C5F11) in 97 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 8.12 g of the title product 18b were obtained, total yield 72%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.8 (bs, 2H), 1.9 (s, 3H, CH3), 2.1 (m, 3H), 2.9 (m, 1H), 3.3 (m, 1H), 3.4 (m, 1H), 7.3–7.5 (m, 3 H), 8 (t, J = 7.2 Hz, 1H), 8.2 (d, J = 8.1 Hz, 1H), 8.8 (d, J = 8.1 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 19.9, 21, 26, 29.5, 32.1, 115.2, 126.5, 127.8, 128, 129.5, 136.5, 138 (t, 2JCF = 24.2 Hz), 148.1, 150.5, 161.1, 163; 19F-NMR (282.4 MHz, DMSO-d6) δ −126.3 (AB system, 2JFF = 285.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.4 (AB system, 2JFF = 285.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −123.1 (AB system, 2JFF = 310 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.7 (AB system, 2JFF = 310 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −118.7 (AB system, 2JFF = 299.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −117.6 (AB system, 2JFF = 299.6 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −107.1 (AB system, 2JFF = 290.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −98.2 (AB system, 2JFF = 290.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.2 (m 3F, CF3). MS (m/z): 492 ([M − I]+, 100). HRMS calcd. for C21H17F11N+: 492.1185, found 492.1190. Anal. Calcd. for C21H17F11NI: C, 40.73; H, 2.77; N, 2.26. Found: C, 40.75; H, 2.76; N, 2.25.
2-Perfluoropentyl-N-(3-methylphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18c). 6.4 g of 3-methylaniline 15c (5.97 × 10−2 mol) and 2.34 g (2.39 × 10−2 mol) of cyclohexanone 16u were added to a solution of 10.6 g (1.99 × 10−2 mol) of 14 (RF = C5F11) in 53 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.2 g of the title product 18c were obtained, total yield 83%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H), 1.9 (m, 2H), 2.1 (s, 3H, CH3), 2.9 (m, 2H), 3.5 (m, 2H), 7.5 (s, 1H), 7.6 (m, 3H), 8.2 (d, J = 8.1 Hz, 1H), 8.7 (d, J = 8.1 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 19.8, 21.1, 26.3, 29.5, 31, 117.2, 126.1, 127.8, 128.1, 129.2, 136.8, 138.5 (t, 2JCF = 25.8 Hz), 148.2, 150.5, 163.1; 19F-NMR (235.3 MHz, CDCl3) δ −126.1 (m 2F, CF2), −122 (m 2F, CF2), −117 (m 2F, CF2), −103.1 (AB system, 2JFF = 275.6 Hz, 1F, CF2-CF2), −101.2 (AB system, 2JFF = 275.6 Hz, 1F, CF2-CF2), −80.1 (m 3F, CF3). MS (m/z): 492 ([M − I]+, 100). HRMS calcd. for C21H17F11N+: 492.1185, found 492.1187. Anal. Calcd. for C21H17F11NI: C, 40.73; H, 2.77; N, 2.26. Found: C, 40.76; H, 2.77; N, 2.27.
2-Perfluoropentyl-N-(4-methylphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18d). 5.74 g of 4-methylaniline 15d (5.35 × 10−2 mol) and 2.1 g (2.14 × 10−2 mol) of cyclohexanone 16u were added to a solution of 9.5 g (1.78 × 10−2 mol) of 14 (RF = C5F11) in 95 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 8.84 g of the title product 18d were obtained, total yield 80%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.9 (m, 4H), 2 (s, 3H, CH3), 2.5 (m, 2H), 3.4 (m, 2H), 7.4 (d, J = 8 Hz, 2H), 7.7 (d, J = 8.1 Hz, 2H), 8.2 (d, J = 8.1 Hz, 1H), 8.7 (d, J = 8.1 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 19.5, 21, 26.2, 29.5, 31.2, 116.2, 125.1, 127, 128.1, 129.2, 135.1, 137.8 (t, 2JCF = 26 Hz), 146, 148.1, 150, 163.1; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.5 (m 2F, CF2), −117.5 (m 2F, CF2), −101.2 (m 2F, CF2), −80.6 (m 3F, CF3). MS (m/z): 492 ([M − I]+, 100). HRMS calcd. for C21H17F11N+: 492.1185, found 492.1189. Anal. Calcd. for C21H17F11NI: C, 40.73; H, 2.77; N, 2.26. Found: C, 40.75; H, 2.78; N, 2.25.
2-Perfluoropentyl-N-(4-ethylphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18g). 7.24 g of 4-ethylaniline 15g (5.97 × 10−2 mol) and 2.34 g (2.39 × 10−2 mol) of cyclohexanone 16u were added to a solution of 10.6 g (1.99 × 10−2 mol) of 14 (RF = C5F11) in 106 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.72 g of the title product 18g were obtained, total yield 85%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 1.9 (m, 4H), 2.6 (m, 2H), 2.8 (q, J = 8 Hz, 2H, CH2-CH3), 3.3 (m, 2H), 7.1 (d, J = 8.2 Hz, 2H), 7.7 (d, J = 8.3 Hz, 2H), 8.2 (d, J = 8.1 Hz, 1H), 8.6 (d, J = 8.1 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 13.1, 19.5, 21, 24.1, 29.5, 31.2, 113.2, 126.1, 127, 129.6, 129.8, 135.1, 138.8 (t, 2JCF = 26.2 Hz), 148.1, 152, 162.9; 19F-NMR (235.3 MHz, CDCl3) δ −126.9 (m 2F, CF2), −123.5 (m 2F, CF2), −117.5 (m 2F, CF2), −101.8 (m 2F, CF2), −80.6 (m 3F, CF3). MS (m/z): 506 ([M − I]+, 95). HRMS calcd. for C22H19F11N+: 506.1342, found 506.1346. Anal. Calcd. for C22H19F11NI: C, 41.72; H, 3.02; N, 2.21. Found: C, 41.75; H, 3.04; N, 2.19.
2-Perfluoropentyl-N-(3-chlorophenyl)-5,6,7,8-tetrahydroquinolinium iodide (18i). 7.33 g of 3-chloroaniline 15i (5.75 × 10−2 mol) and 2.25 g (2.3 × 10−2 mol) of cyclohexanone 16u were added to a solution of 10.2 g (1.91 × 10−2 mol) of 14 (RF = C5F11) in 102 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.32 g of the title product 18i were obtained, total yield 76%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H), 1.9 (m, 2H), 2.9 (m, 2H), 3.5 (m, 2H), 7.4 (s, 1H), 7.5–7.6 (m, 2H), 7.7 (m, 1H), 8.2 (d, J = 8 Hz, 1H), 8.7 (d, J = 8 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 19.6, 21, 26, 29.5, 31.5, 118.2, 127.1, 127.8, 128.1, 130.1, 137.8, 138.8 (t, 2JCF = 24.8 Hz), 147.2, 150.6, 165.6; 19F-NMR (235.3 MHz, CDCl3) δ −125.6 (m 2F, CF2), −117.6 (m 2F, CF2), −113.5 (m 2F, CF2), −97.9 (AB system, 2JFF = 150.2 Hz, 1F, CF2-CF2), −98.2 (AB system, 2JFF = 150.2 Hz, 1F, CF2-CF2), −79.1 (m 3F, CF3). MS (m/z): 512 ([M − I]+, 90). HRMS calcd. for C20H14F11NCl+: 512.0639, found 512.0643. Anal. Calcd. for C20H14F11NICl: C, 37.55; H, 2.21; N, 2.19. Found: C, 37.58; H, 2.22; N, 2.17.
2-Perfluoropentyl-N-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18l). 7.36 g of 4-anisidine 15l (5.97 × 10−2 mol) and 2.34 g (2.39 × 10−2 mol) of cyclohexanone 16u were added to a solution of 10.6 g (1.99 × 10−2 mol) of 14 (RF = C5F11) in 106 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 11.39 g of the title product 18l were obtained, total yield 90%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2 (m, 4H), 2.7 (m, 2H), 3.3 (m, 2H), 3.9 (s, 3H, OCH3), 7.1 (d, J = 8.8 Hz, 2H), 7.7 (d, J = 8.6 Hz, 2H), 8.2 (d, J = 8.1 Hz, 1H), 8.8 (d, J = 8.2 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 20.1, 22, 29, 31.1, 56, 117, 125, 127, 128.2, 129.9, 139.5 (t, 2JCF = 27.2 Hz), 146.2, 148.1, 162, 164.5; 19F-NMR (235.3 MHz, CDCl3) δ −126.9 (m 2F, CF2), −123.5 (m 2F, CF2), −117.5 (m 2F, CF2), −101.7 (m 2F, CF2), −80.75 (m 3F, CF3). MS (m/z): 508 ([M − I]+, 100). HRMS calcd. for C21H17F11NO+: 508.1134, found 508.1138. Anal. Calcd. for C21H17F11NIO: C, 39.70; H, 2.70; N, 2.20. Found: C, 39.72; H, 2.71; N, 2.19.
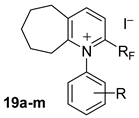
2-Perfluoropentyl-N-(phenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19a). 5.24 g of aniline 15a (5.63 × 10−2 mole) and 2.52 g (2.25 × 10−2 mole) of cycloheptanone 16v were added to a solution of 10 g (1.87 × 10−2 mole) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.54 g of the title product 19a were obtained, total yield 82%. 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H, CH2, cycloheptyl group), 1.9 (m, 4H, CH2, cycloheptyl group), 3.1 (m, 2H, CH2, cycloheptyl group), 3.4 (m, 2H, CH2, cycloheptyl group), 7.5–7.7 (m, 5H, Ph-H), 8.2 (d, J = 8.2 Hz, 1H, Py-H), 8.9 (d, J = 8 Hz, 1H, Py-H); 13C-NMR (75.46MHz, CDCl3) δ 24, 25.3, 30.7, 33.9, 35.4, 124.2, 125.9, 127.7, 129.6, 131.5, 137.7 (t, 2JCF = 27.1 Hz), 146.8, 150.3, 167.4; 19F-NMR (282.4 MHz, CDCl3) δ −126 (m 2F, CF2-CF2 CF2 CF2-CF3), −122.4 (m 2F, CF2-CF2-CF2-CF2-CF3), −117.3 (m 2F, CF2-CF2-CF2-CF2-CF3), −101.6 (m 2F, CF2-CF2-CF2-CF2-CF3), −80.7 (m 3F, CF2-CF2-CF2 CF2-CF3). MS (m/z): 492 ([M − I]+, 95). HRMS calcd. for C21H17F11N+ 492.1185, found 492.1190. Anal. Calcd. for C21H17F11NI: C, 40.73; H, 2.77; N, 2.26. Found: C, 40.75; H, 2.78; N, 2.25.
2-Perfluoropentyl-N-(3-methylphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19c). 5.92 g of 3-methylaniline 15c (5.52 × 10−2 mol) and 2.47 g (2.21 × 10−2 mol) of cycloheptanone 16v were added to a solution of 9.8 g (1.84 × 10−2 mol) of 14 (RF = C5F11) in 98 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.68 g of the title product 19c were obtained, total yield 83%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H), 1.9 (m, 4H), 2.45 (s, 3H, CH3), 3 (d, J = 8 Hz, 2H), 3.4 (m, 2H), 7.4 (m, 2H), 7.6 (m, 2H), 8.2 (d, J = 8.4 Hz, 1H), 8.9 (d, J = 8 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 20.7, 21.5, 24.3, 25.3, 30.7, 33.9, 35.7, 122.5, 126.2, 127.7, 129.5, 131, 137.6 (t, 2JCF = 27 Hz), 146.6, 150.2, 167.8; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.5 (m 2F, CF2), −117 (m 2F, CF2), −101.7 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 506 ([M − I]+, 95). HRMS calcd. for C22H19F11N+: 506.1342, found 506.1346. Anal. Calcd. for C22H19F11NI: C, 41.72; H, 3.02; N, 2.21. Found: C, 41.75; H, 3.06; N, 2.19.
2-Perfluoropentyl-N-(4-methylphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19d). 6.34 g of 4-methylaniline 15d (5.92 × 10−2 mol) and 2.65 g (2.36 × 10−2 mol) of cycloheptanone 16v were added to a solution of 10.5 g (1.97 × 10−2 mol) of 14 (RF = C5F11) in 105 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.62 g of the title product 19d were obtained, total yield 85%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.6 (m, 2H), 1.9 (m, 4H), 2.4 (s, 3H, CH3), 3 (m, 2H), 3.3 (m, 2H), 7.3 (d, J = 8 Hz, 2H), 7.6 (d, J = 8 Hz, 2H), 8.2 (d, J = 8.4 Hz, 1H), 8.8 (d, J = 8.4 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 20.8, 21.3, 24.2, 25.3, 30.6, 33.9, 35.4, 122.7, 126.6, 127.7, 129.9, 130.2, 135.1, 137.8 (t, 2JCF = 27.1 Hz), 142.5, 146.6, 150.2, 167.7; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.4 (m 2F, CF2), −117.2 (m 2F, CF2), −101.6 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 506 ([M − I]+, 95). HRMS calcd. for C22H19F11N+: 506.1342, found 506.1348. Anal. Calcd. for C22H19F11NI: C, 41.72; H, 3.02; N, 2.21. Found: C, 41.76; H, 3.05; N, 2.23.
2-Perfluoropentyl-N-(3-ethylphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19f). 6.69 g of 3-ethylaniline 15f (5.52 × 10−2 mol) and 2.47 g (2.21 × 10−2 mol) of cycloheptanone 16v were added to a solution of 9.8 g (1.84 × 10−2 mol) of 14 (RF = C5F11) in 98 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.5 g of the title product 19f were obtained, total yield 88%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.2 (t, J = 8 Hz, 3H, CH2-CH3), 1.6 (s, 2H), 1.9 (m, 4H), 2.7 (q, J = 8 Hz, 2H, CH2-CH3), 3 (m, 2H), 3.3 (m, 2H), 7.5 (m, 3 H), 7.6 (m, 1H), 8.2 (d, J = 8.4 Hz, 1H), 8.9 (d, J = 8 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 15.1, 24.3, 25.4, 28.4, 30.6, 33.9, 35.4, 124.1, 125.9, 127.7, 129.6, 131.5, 137.6 (t, 2JCF = 26.7 Hz), 146.7, 150.2, 167.4; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.4 (m 2F, CF2), −117.2 (m 2F, CF2), −102.2 (AB system, 2JFF = 263.3 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −101.1 (AB system, 2JFF = 263.3 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.7 (m 3F, CF3). MS (m/z): 520 ([M − I]+, 100). HRMS calcd. for C23H21F11N+: 520.1498, found 520.1501. Anal. Calcd. for C23H21F11NI: C, 42.68; H, 3.27; N, 2.16. Found: C, 42.70; H, 3.28; N, 2.14.
2-Perfluoropentyl-N-(4-ethylphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19g). 6.96 g of 4-ethylaniline 15g (5.75 × 10−2 mol) and 2.58 g (2.3 × 10−2 mol) of cycloheptanone 16v were added to a solution of 10.2 g (1.91 × 10−2 mol) of 14 (RF = C5F11) in 102 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.3 g of the title product 19g were obtained, total yield 83%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.3 (t, J = 7.6 Hz, 3H, CH2-CH3), 1.6 (s, 2H), 1.8 (m, 4H), 2.71 (q, J = 7.6 Hz, 2H, CH2-CH3), 3 (m, 2H), 3.4 (m, 2H), 7.3 (d, J = 8.4 Hz, 2H), 7.6 (d, J = 8.4 Hz, 2H), 8.1 (d, J = 8 Hz, 1H), 8.8 (d, J = 8 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 14.9, 24.2, 25.3, 28.5, 30.6, 33.9, 35.4, 126.7, 127.7, 128.9, 135.2, 137.8 (t, 2JCF = 27.1 Hz), 146.6, 148.6, 150.2, 167.7; 19F-NMR (235.3 MHz, CDCl3) δ −126.1 (m 2F, CF2), −122.5 (m 2F, CF2), −117.2 (m 2F, CF2), −101.6 (m 2F, CF2), −80.8 (m 3F, CF3). MS (m/z): 520 ([M − I]+, 100). HRMS calcd. for C23H21F11N+: 520.1498, found 520.1499. Anal. Calcd. for C23H21F11NI: C, 42.68; H, 3.27; N, 2.16. Found: C, 42.69; H, 3.29; N, 2.15.
2-Perfluoropentyl-N-(3-chlorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19i). 6.4 g of 3-chloroaniline 15i (5.01 × 10−2 mol) and 2.25 g (2.10−2 mol) of cycloheptanone 16v were added to a solution of 8.9 g (1.67 × 10−2 mol) of 14 (RF = C5F11) in 89 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 7.65 g of the title product 19i were obtained, total yield 70%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.6–2.2 (m, 6H), 3.1 (m, 2H), 3.4 (s, 2H), 7.6 (t, J = 8.1 Hz, 1H), 7.7 (d, J = 8.2 Hz, 1H), 7.9 (s, 1H), 8.1 (d, J = 7.9 Hz, 1H), 8.3 (d, J = 8.1 Hz, 1H), 8.9 (d, J = 8.2 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 24.1, 25.2, 30.7, 34, 34.3, 35.5, 127.9, 128.5, 129.8, 136.1, 137.7 (t, 2JCF = 26.8 Hz), 138.5, 146.8, 150.5, 167.7; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.4 (m 2F, CF2), −117.2 (m 2F, CF2), −101.5 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 526 ([M − I]+, 100). HRMS calcd. for C21H16F11NCl+: 526.0796, found 526.0799. Anal. Calcd. for C21H16F11NICl: C, 38.58; H, 2.47; N, 2.14. Found: C, 38.61; H, 2.48; N, 2.14.
2-Perfluoropentyl-N-(4-chlorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19j). 7.26 g of 4-chloroaniline 15j (5.69 × 10−2 mol) and 2.55 g (2.27 × 10−2 mol) of cycloheptanone 16v were added to a solution of 10.1 g (1.89 × 10−2 mol) of 14 (RF = C5F11) in 101 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 9.68 g of the title product 19j were obtained, total yield 78%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H), 1.9 (m, 4H), 3 (m, 2H), 3.3 (m, 2H), 7.5 (d, J = 8.8 Hz, 2H), 7.8 (d, J = 8.8 Hz, 2H), 8.2 (d, J = 8 Hz, 1H), 8.8 (d, J = 8.3 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 24, 25.2, 30.7, 33.9, 34.2, 35.4, 127.9, 128.4, 129.9, 136.1, 137.5 (t, 2JCF = 26.8 Hz), 138.3, 146.8, 150.5, 167.6; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.3 (m 2F, CF2), −117.3 (m 2F, CF2), −101.5 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 526 ([M − I]+, 100). HRMS calcd. for C21H16F11NCl+: 526.0796, found 526.0797. Anal. Calcd. for C21H16F11NICl: C, 38.58; H, 2.47; N, 2.14. Found: C, 38.60; H, 2.47; N, 2.13.
2-Perfluoropentyl-N-(2-methoxyphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19k). 6.18 g of 2-anisidine 15k (5.01 × 10−2 mol) and 2.25 g (2 × 10−2 mol) of cycloheptanone 16v were added to a solution of 8.9 g (1.67 × 10−2 mol) of 14 (RF = C5F11) in 45 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 7.6 g of the title product 19k were obtained, total yield 70%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.5 (m, 1H), 1.7–2 (m, 4H), 2.1 (m, 1H), 2.9 (m,1H), 3.2–3.5 (m, 3H), 3.8 (s, 3H, OCH3), 7.2 (d, J = 8.3 Hz, 1H), 7.3 (t, J = 7.6 Hz, 1H), 7.7 (t, J = 8.8 Hz, 1H), 8 (s, 1H), 8.3 (d, J = 8.1 Hz, 1H), 9 (d, J = 8.2 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 24, 25.1, 30.7, 34.4, 34.3, 35.6, 127.8, 128.5, 130, 136.1, 137.8 (t, 2JCF = 26.9 Hz), 138.8, 147, 151.5, 167.5; 19F-NMR (282.4 MHz, DMSO-d6) δ −126.5 (AB system, 2JFF = 284.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.5 (AB system, 2JFF = 284.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −123 (AB system, 2JFF = 355.2 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.8 (AB system, 2JFF = 355.2 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −119.1 (AB system, 2JFF = 380.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −116.5 (AB system, 2JFF = 380.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −107.1 (AB system, 2JFF = 350 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −99.5 (AB system, 2JFF = 350 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.69 (m 3F, CF3). MS (m/z): 522 ([M − I]+, 100). HRMS calcd. for C22H19F11NO+: 522.1291, found 522.1297. Anal. Calcd. for C22H19F11NIO: C, 40.70; H, 2.95; N, 2.16. Found: C, 40.73; H, 2.96; N, 2.16.
2-Perfluoropentyl-N-(4-methoxyphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19l). 6.66 g of 4-anisidine 15l (5.41 × 10−2 mol) and 2.42 g (2.16 × 10−2 mol) of cycloheptanone 16v were added to a solution of 9.6 g (1.8 × 10−2 mol) of 14 (RF = C5F11) in 96 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 10.31 g of the title product 19l were obtained, total yield 88%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 1.7 (m, 2H), 1.9 (m, 4H), 3 (m, 2H), 3.3 (m, 2H), 3.8 (s, 3H, OCH3), 7 (d, J = 8.8 Hz, 2H), 7.6 (d, J = 8.4 Hz, 2H), 8.1 (d, J = 8.4 Hz, 1H), 8.7 (d, J = 8 Hz, 1H); 13C-NMR (75.46MHz, CDCl3) δ 24.3, 25.3, 30.7, 33.9, 35.5, 55.8, 114.6, 127.6, 128.2, 130, 138.2, 146.5, 150.2, 161.7, 168.2; 19F-NMR (235.3 MHz, CDCl3) δ −126 (m 2F, CF2), −122.3 (m 2F, CF2), −117.2 (m 2F, CF2), −101.5 (m 2F, CF2), −80.7 (m 3F, CF3). MS (m/z): 522 ([M − I]+, 100). HRMS calcd. for C22H19F11NO+: 522.1291, found 522.1295. Anal. Calcd. for C22H19F11NIO: C, 40.70; H, 2.95; N, 2.16. Found: C, 40.74; H, 2.95; N, 2.13.
3.3. General Procedure for the Synthesis of 2-Trifluoromethylated and 2-Perfluoroalkylated 6-Methyl-N-(o-/p-Carboxyphenyl)Pyridinium Iodides (17m–n’), N-(O-/P-Carboxyphenyl)-5,6,7,8-Tetrahydroquinolinium Iodides (18m–n) and N-(O-Carboxyphenyl)-6,7,8,9-Tetrahydro-5H-Cyclohepta[b]Pyridinium Iodide (19m)
To a stirred solution of 1-acetoxy-1-iodo-perfluoroalkylethane compounds
14 or
14’ (1 equiv.) in anhydrous dichloromethane (5 mL DCM for 1 g of
14–
14’), was added three equiv. of the corresponding aminobenzoic acid
15m–
n and 1.2 equiv. of ketone
16t–
v. The mixture was stirred under reflux for 12 h (
Table 1,
Table 2 and
Table 3) until complete consumption of
14–
14’ (monitored by TLC eluent petroleum ether/ethyl acetate: 80/20
v/
v, and
19F-NMR of aliquots). When the reaction was completed, the mixture was allowed to cool to r.t. then the brown precipitate accumulated during the reaction was separated by vacuum filtration (it was subsequently identified as anilinium salts by NMR and MS). Then a mixture of petroleum ether and ethyl ether (40/60
v/
v) was added to the filtrate and the corresponding pyridinium iodides
17m–n’, 18m–n and
19m instantly precipitate and were isolated by vacuum filtration as amorphous solids.
2-Perfluoropentyl-6-methyl-N-(2-carboxyphenyl)pyridinium iodide (17m). 7.34 g of 2-aminobenzoic acid 15m (5.35 × 10−2 mole) and 1.58 mL or 1.24 g (2.14 × 10−2 mole) of acetone 16t were added to a solution of 9.5 g (1.78 × 10−2 mole) of 14 (RF = C5F11) in 48 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 5.43 g of the title product 17m were obtained, total yield 50%. 1H-NMR (300.13 MHz, DMSO-d6) δ 2.4 (s, 3H, CH3), 7.7–7.9 (m, 3 H), 8.2 (d, J = 8 Hz, 1H), 8.7 (m, 2H), 9 (t, J = 8 Hz, 1H), 13.9 (bs, 1H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6) δ 22.9 (s, CH3), 127.9, 128.6, 129.4, 132.8, 133.6, 134.4, 137, 139.7 (t, 2JCF = 25.1 Hz), 148.2, 163.4, 165.1 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6) δ −126.7 (AB system, 2JFF = 288.7 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.7 (AB system, 2JFF = 288.7 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −122.8 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.8 (AB system, 2JFF = 289 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −119.2 (AB system, 2JFF = 310.2 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −116.3 (AB system, 2JFF = 310.2 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −107.5 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −99.1 (AB system, 2JFF = 282.5 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.69 (m 3F, CF3). MS (m/z): 482 ([M − I]+, 100). HRMS calcd. for C18H11F11NO2+ 482.0614, found 482.0622. Anal. Calcd. for C18H11F11INO2: C, 35.49; H, 1.82; N, 2.30. Found: C, 35.51; H, 1.82; N, 2.33.
2-Trifluoromethyl-6-methyl-N-(2-carboxyphenyl)pyridinium iodide (17m’). 11.02 g of 2-aminobenzoic acid 15m (8.04 × 10−2 mol) and 2.37 mL or 1.86 g (3.21 × 10−2 mol) of acetone 16t were added to a solution of 8.9 g (2.68 × 10−2 mol) of 14’ (RF = CF3) in 89 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 5.7 g of the title product 17m’ were obtained, total yield 52%. Spectral data: 1H-NMR (300.13 MHz, DMSO-d6) δ 2.3 (s, 3H, CH3), 7.7–7.8 (m, 3 H), 8 (d, J = 8.1 Hz, 1H), 8.8 (m, 2H), 9 (t, J = 8 Hz, 1H), 14.2 (bs, 1H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6) δ 21.9 (s, CH3), 118.5 (q, CF3, 1JCF = 280.2 Hz), 127.5 (q, 3JCF = 3.9 Hz), 128, 133.7, 140.2, 140.7, 146, 148.1 (q, C-CF3, 2JCF = 36.6 Hz), 163, 167 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6) δ −60.3 (s 3F, CF3). MS (m/z): 282 ([M − I]+, 100). HRMS calcd. for C14H11F3NO2+: 282.0742, found 282.0745. Anal. Calcd. for C14H11F3INO2: C, 41.10; H, 2.71; N, 3.42. Found: C, 41.13; H, 2.72; N, 3.40.
2-Perfluoropentyl-6-methyl-N-(4-carboxyphenyl)pyridinium iodide (17n). 8.27 g of 4-aminobenzoic acid 15n (6.03 × 10−2 mol) and 1.78 mL or 1.39 g (2.41 × 10−2 mol) of acetone 16t were added to a solution of 10.7 g (2.01 × 10−2 mol) of 14 (RF = C5F11) in 107 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 7.35 g of the title product 17n were obtained, total yield 60%. 1H-NMR (300.13 MHz, DMSO-d6) δ 2.5 (s, 3H, CH3), 8 (d, J = 8.1 Hz, 2H, Ph-H), 8.3 (d, J = 8.1 Hz, 2H, Ph-H), 8.8 (m, 2H, Py-H), 9 (t, J = 8 Hz, 1H, Py-H), 13.8 (bs, 1H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6) δ 22.9 (s, CH3), 127.1, 128.7, 130.5, 133.8, 139.7, 140.7, 146, 147.6, 163.1, 165.9 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6) δ −125.8 (s 2F, CF2-CF2-CF2-CF2-CF3), −122.3 (s 2F, CF2-CF2-CF2-CF2-CF3), −117.7 (s 2F, CF2-CF2-CF2-CF2-CF3), −101.8 (s, 2F, CF2-CF2-CF2-CF2-CF3), −80.2 (m 3F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 482 ([M − I]+, 100). HRMS calcd. for C18H11F11NO2+: 482.0614, found 482.0620. Anal. Calcd. for C18H11F11INO2: C, 35.49; H, 1.82; N, 2.30. Found: C, 35.52; H, 1.83; N, 2.28.
2-Trifluoromethyl-6-methyl-N-(4-carboxyphenyl)pyridinium iodide (17n’).12.63 g of 4-aminobenzoic acid 15n (9.21 × 10−2 mol) and 2.72 mL or 2.13 g (3.68 × 10−2 mol) of acetone 16t were added to a solution of 10.2 g (3.07 × 10−2 mol) of 14’ (RF = CF3) in 102 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 7.54 g of the title product 17n’ were obtained, total yield 60%. Spectral data: 1H-NMR (300.13 MHz, DMSO-d6) δ 2.5 (s, 3H, CH3), 8.1 (d, J = 8 Hz, 2H), 8.3 (d, J = 8 Hz, 2H), 8.7 (m, 2H), 9.1 (t, J = 8 Hz, 1H), 14 (bs, 1H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6) δ 22.7 (s, CH3), 118 (q, CF3, 1JCF = 279 Hz), 127.1 (q, 3JCF = 4.2 Hz), 128, 130.4, 133.7, 140, 140.7, 146, 147.6 (q, C-CF3, 2JCF = 35.5 Hz), 163, 166 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6) δ −59.9 (s 3F, CF3). MS (m/z): 282 ([M − I]+, 100). HRMS calcd. for C14H11F3NO2+: 282.0742, found 282.0747. Anal. Calcd. for C14H11F3INO2: C, 41.10; H, 2.71; N, 3.42. Found: C, 41.14; H, 2.70; N, 3.40.
2-Perfluoropentyl-N-(2-carboxyphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18m). 7.88 g of 2-aminobenzoic acid 15m (5.75 × 10−2 mol) and 2.25 g (2.3 × 10−2 mole) of cyclohexanone 16u were added to a solution of 10.2 g (1.91 × 10−2 mol) of 14 (RF = C5F11) in 51 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 8.96 g of the title product 18m were obtained, total yield 72%. 1H-NMR (300.13 MHz, CDCl3) δ 1.8 (s, 2H, CH2, cyclohexyl group), 2 (s, 2H, CH2, cyclohexyl group), 2.4 (m, 1H, CH2, cyclohexyl group), 2.7 (m, 1H, CH2, cyclohexyl group), 3.1 (m, 1H, CH2, cyclohexyl group), 3.3 (m, 1H, CH2, cyclohexyl group), 6.9 (bs, 1H, CO2H), 7.8 (m, 3H, Ph-H), 8.2 (d, J = 7.4 Hz, 1H, Ph-H), 8.4 (d, J = 8.1 Hz, 1H, Py-H), 8.9 (d, J = 8.2 Hz, 1H, Py-H); 1H-NMR (400.13 MHz, DMSO-d6) δ 1.6–1.8 (m, 4H, CH2, cyclohexyl group), 2.3 (m, 1H, CH2, cyclohexyl group), 2.6 (m, 1H, CH2, cyclohexyl group), 3.2 (m, 2H, CH2, cyclohexyl group), 7.8–8 (m, 3H, Ph-H), 8.3 (d, J = 8 Hz, 1H, Ph-H), 8.6 (d, J = 8 Hz, 1H, Py-H), 8.9 (d, J = 8 Hz, 1H, Py-H); 13C-NMR (75.46 MHz, CDCl3) δ 19.9, 21.4, 29.5, 30.5, 126.5, 126.7, 130.6, 132.2, 132.7, 133.2, 136.4, 139.3 (t, 2JCF = 25 Hz), 144.4, 147.5, 161.6, 165.8 (s, CO2H); 13C-NMR (100.6 MHz, DMSO-d6) δ 20, 21.4, 29.1, 30.3, 127.2 (t, 3JCF = 4 Hz, =C-H, Py), 128.5, 132.6, 133, 134.6, 136.6, 137.2, 137.4 (t, 2JCF = 22.13 Hz, =C-CF2, Py), 144.7, 148, 162.2, 164.9 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6) δ −126.5 (AB system, 2JFF = 282 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.3 (AB system, 2JFF = 282 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −123 (AB system, 2JFF = 367 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −121.9 (AB system, 2JFF = 367 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −118.9 (AB system, 2JFF = 338 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −117 (AB system, 2JFF = 338 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −107 (AB system, 2JFF = 310 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −98.5 (AB system, 2JFF = 310 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.31 (m 3F, CF3). MS (m/z): 522 ([M − I]+, 98). HRMS calcd. for C21H15F11NO2+ 522.0927, found 522.0933. Anal. Calcd. for C21H15F11NIO2: C, 38.85; H, 2.33; N, 2.16. Found: C, 38.88; H, 2.34; N, 2.15.
2-Perfluoropentyl-N-(4-carboxyphenyl)-5,6,7,8-tetrahydroquinolinium iodide (18n). 8.27 g of 4-aminobenzoic acid 15n (6.03 × 10−2 mol) and 2.36 g (2.41 × 10−2 mol) of cyclohexanone 16u were added to a solution of 10.7 g (2.01 × 10−2 mol) of 14 (RF = C5F11) in 53.5 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 9.14 g of the title product 18n were obtained, total yield 70%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 2 (m, 4H), 2.6 (m, 2H), 3.4 (m, 2H), 7.9 (d, J = 8 Hz, 2H), 8.2 (d, J = 8 Hz, 2H), 8.8 (d, J = 8.1 Hz, 1H), 9 (d, J = 8.1 Hz, 1H) 12.9 (bs, 1H, CO2H); 13C-NMR (75.46MHz, CDCl3) δ 20.8, 21, 29.2, 31.5, 125.5, 127, 128.4, 129.5, 133.7, 139.7 (t, 2JCF = 24.3 Hz), 146, 147.2, 163.1, 166 (s, CO2H); 19F-NMR (235.3 MHz, CDCl3) δ −125.9 (m 2F, CF2), −122.5 (m 2F, CF2), −117.7 (m 2F, CF2), −101.5 (m 2F, CF2), −80.4 (m 3F, CF3). MS (m/z): 522 ([M − I]+, 90). HRMS calcd. for C21H15F11NO2+: 522.0927, found 522.0931. Anal. Calcd. for C21H15F11NIO2: C, 38.85; H, 2.33; N, 2.16. Found: C, 38.89; H, 2.34; N, 2.15.
2-Perfluoropentyl-N-(2-carboxyphenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridinium (19m). 8.35 g of 2-aminobenzoic acid 15m (6.09 × 10−2 mol) and 2.73 g (2.43 × 10−2 mole) of cycloheptanone 16v were added to a solution of 10.8 g (2.03 × 10−2 mole) of 14 (RF = C5F11) in 54 mL of dry dichloromethane. The mixture was stirred for 6 h at reflux. 9.15 g of the title product 19m were obtained, total yield 68%. 1H-NMR (300.13 MHz, CDCl3) δ 1.4–2 (m, 6H, CH2, cycloheptyl group), 2.7 (m, 1H, CH2, cycloheptyl group), 3 (m, 1H, CH2, cycloheptyl group), 3.3–3.4 (m, 2H, CH2, cycloheptyl group), 7.7 (bs, 1H, CO2H), 7.77 (m, 3H, Ar-H), 8.1 (d, J = 8 Hz, 1H, Ar-H), 8.3 (d, J = 8 Hz, 1H, Py-H), 8.9 (d, J = 8 Hz, 1H, Py-H); 1H-NMR (300.13 MHz, DMSO-d6) δ 1.4 (m, 1H, CH2, cycloheptyl group), 1.6–1.9 (m, 5H, CH2, cycloheptyl group), 2.8 (m, 2H, CH2, cycloheptyl group), 3.3 (m, 2H, CH2, cycloheptyl group), 7.9 (m, 2H, Ar-H), 8 (m, 1H, Ar-H), 8.4 (d, J = 6 Hz, 1H, Ar-H), 8.6 (d, J = 6.15 Hz, 1H, Py-H), 8.9 (d, J = 6.18 Hz, 1H, Py-H); 13C-NMR (75.46MHz, DMSO-d6) δ 24, 25.5, 30.4, 33.4, 35.3, 127.2, 127.5, 129.7, 132.2, 132.6, 132.9, 137.1, 138.2 (t, 2JCF = 20.1 Hz), 147.1, 149.2, 165.1, 166.6; 19F-NMR (282.4 MHz, DMSO-d6) δ −126.6 (AB system, 2JFF = 293.4 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −125.5 (AB system, 2JFF = 293.4 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −123.4 (AB system, 2JFF = 297.1 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −122.3 (AB system, 2JFF = 297.1 Hz, 1F, CF2-CF2-CF2-CF2-CF3), -119 (AB system, 2JFF = 300.96 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −116.5 (AB system, 2JFF = 300.96 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −108.2 (AB system, 2JFF = 285.9 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −97.9 (AB system, 2JFF = 285.9 Hz, 1F, CF2-CF2-CF2-CF2-CF3), −80.86 (m 3F, CF3). MS (m/z): 536 ([M − I]+, 95). HRMS calcd. for C22H17F11NO2+ 536.1084, found 536.1089. Anal. Calcd. for C22H17F11NIO2: C, 39.84; H, 2.58; N, 2.11. Found: C, 39.86; H, 2.59; N, 2.10.
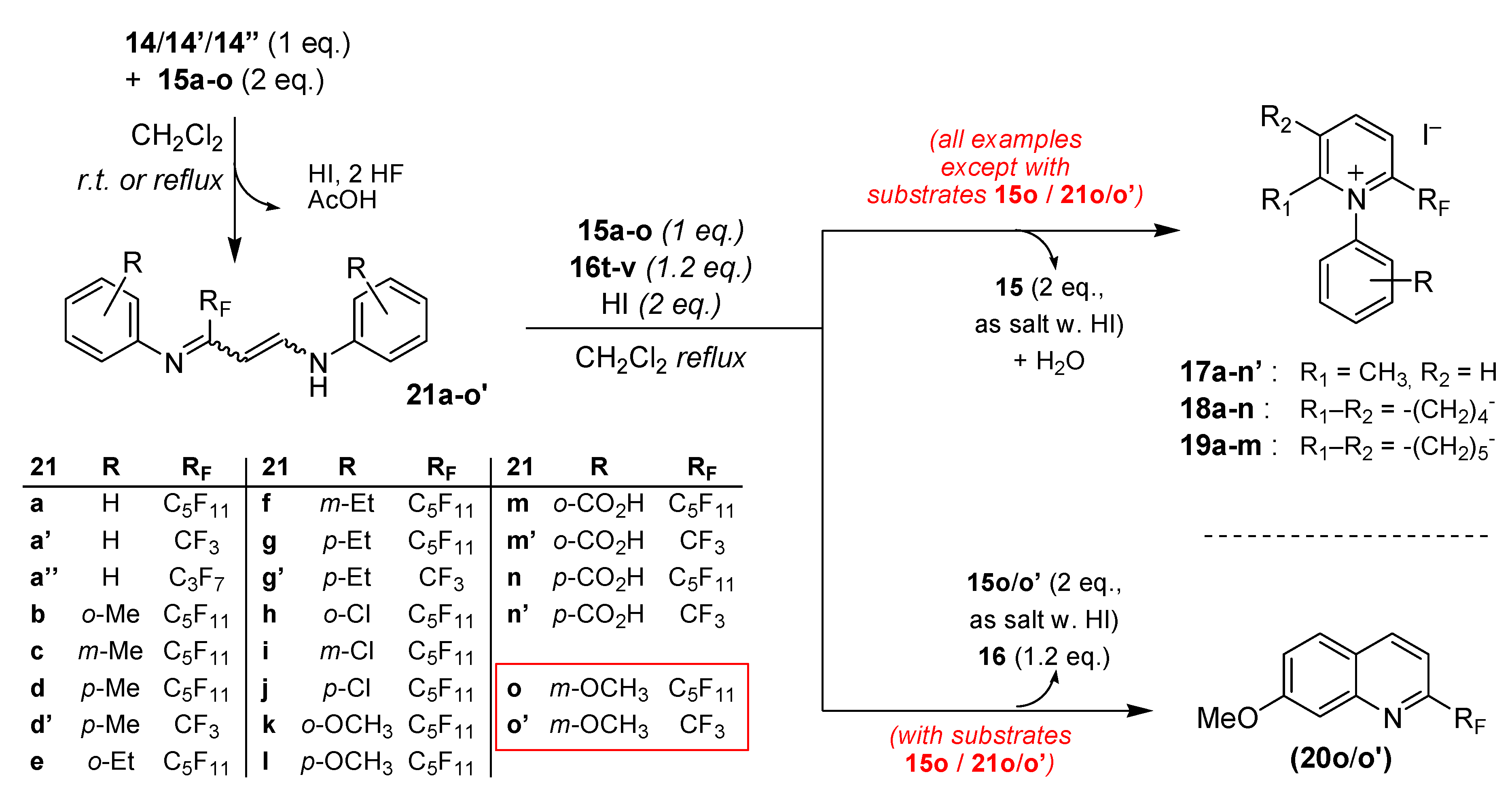
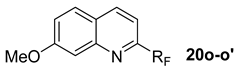
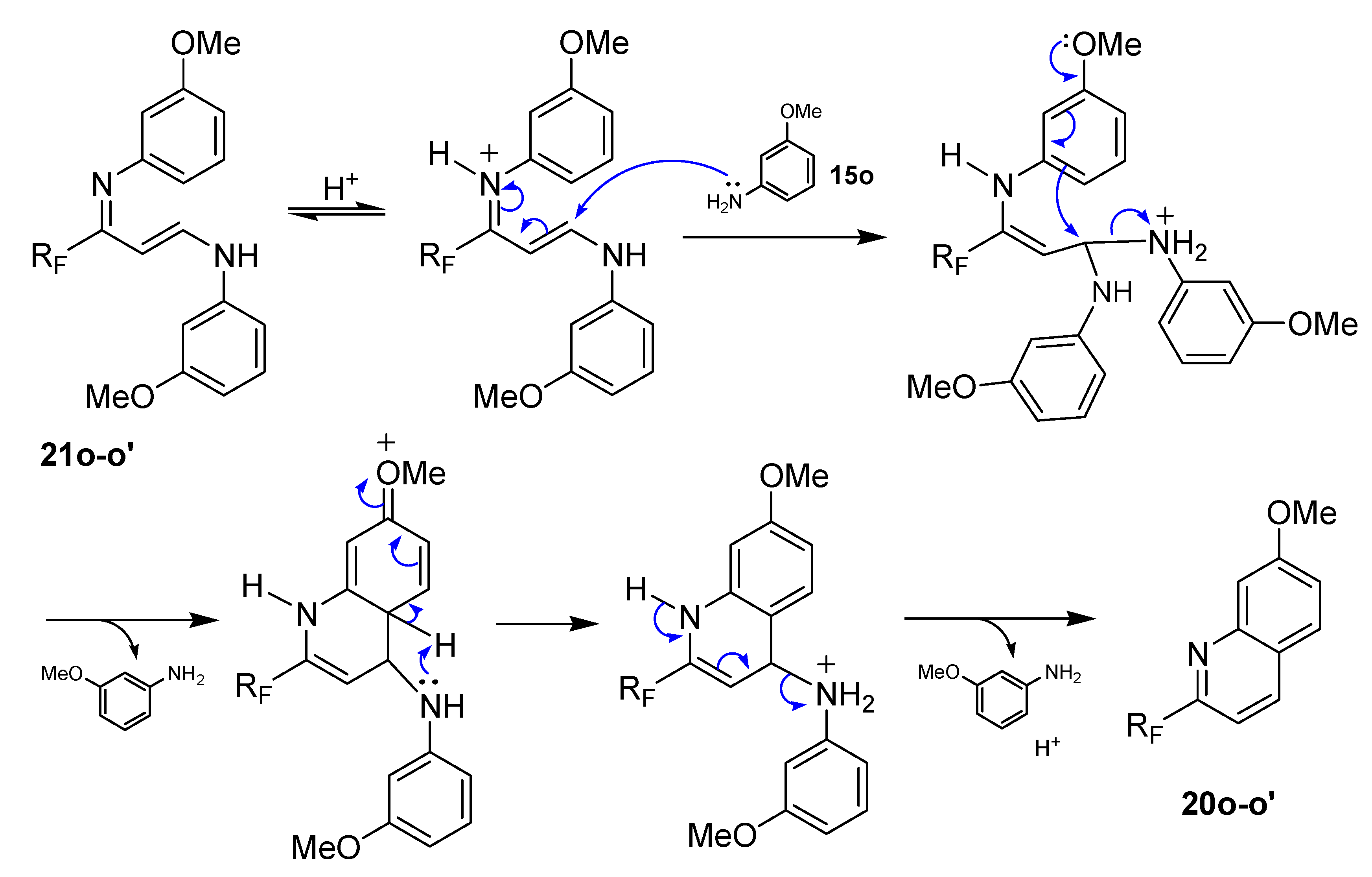
3.4. General Procedure for the Synthesis 2-Trifluoromethyl or 2-Perfluoroalkyl-7-Methoxyquinolines 20o–o’
To a stirred solution of 1-acetoxy-1-iodo-perfluoroalkylethane compounds
14 or
14’ (1 equiv.) in anhydrous dichloromethane (10 mL DCM for 1g of
14–
14’), was added 3 equiv. of
meta-anisidine
15o. The mixture was stirred under reflux for the desired time (12 h,
Table 4) until complete consumption of
14–
14’ (monitored by TLC eluent petroleum ether/ethyl acetate: 80/20
v/
v, and
19F-NMR of aliquots). When the reaction was completed, the mixture was concentrated under reduced pressure and then stirred with diethyl ether. An excess of petroleum ether was added; the precipitate that had formed was eliminated by vacuum filtration and washed three times with petroleum ether. The filtrate was concentrated in vacuo to give a brown oil. Chromatography over silica gel column (eluent, petroleum ether/ethyl acetate 98/2
v/
v) left a yellow oil which was crystallized from methanol/water to give pure samples of the corresponding quinolines
20o–
o’.
3.5. General Procedure for the Synthesis 2-Trifluoromethyl or 2-Perfluoroalkyl-7-Methoxyquinolines 20o–o’ in the Presence of Ketones 16t–v
To a stirred solution of 1-acetoxy-1-iodo-perfluoroalkylethane compounds
14 or
14’ (1 equiv.) in anhydrous dichloromethane (10 mL DCM for 1 g of
14–
14’), was added three equiv. of
meta-anisidine
15o and 1.2 equiv. of ketone
16t–
v. The mixture was stirred under reflux for desired time (12 h,
Table 4) until complete consumption of
14–
14’ (monitored by TLC eluent petroleum ether/ethyl acetate: 80/20
v/
v, and
19F-NMR of aliquots). When the reaction was completed, the mixture was concentrated under reduced pressure and then stirred with a mixture of petroleum ether/ethyl acetate. Chromatography over silica gel column (eluent, petroleum ether/ethyl acetate 98/2
v/
v) left a yellow oil which was crystallized from methanol/water to give pure samples of the corresponding quinolines
20o–
o’. We were able to isolate unreacted ketones
16t–
v from the corresponding reactions (1.2 equivalent) which were identified and characterized by NMR and mass spectroscopy
2-perfluoropentyl-6-methoxyquinoline (20o). 6.25 g of meta-anisidine 15o (5.07 × 10−2 mol) and 2.03 × 10−2 mole of the corresponding ketone (1.17 g of acetone 16t or 1.99 g of cyclohexanone 16u or 2.27 g of cylcoheptanone 16v) were added to a solution of 9 g (1.69 × 10−2 mole) of 14 (RF = C5F11) in 90 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. In the presence of acetone: 6.1 g of 20o (85% yield) and 0.5 g of 16t. Or in the presence of cyclohexanone: 5.9 g of 20o (82% yield) and 1.9 g of 16u. Or in the case of cycloheptanone: 5.9 g of 20o (82% yield) and 2.25 g of 16u were obtained respectively.
Or 6.8 g of meta-anisidine 15o (5.52 × 10−2 mol) were added to a solution of 9.8 g (1.84 ×10−2 mol) of 14 (RF = C5F11) in 98 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 6.6 g (84% yield) of quinoline 20o were obtained respectively. 1H-NMR (300.13 MHz, CDCl3) δ 3.8 (s, 3H, OCH3), 7.5 (d, J = 8.5 Hz, 1H), 7.8 (d, J = 8.6 Hz, 1H), 8 (m, 2H), 8.6 (d, J = 8.6 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 55.6 (s, OCH3), 117.6, 126.5, 130.5, 130.7, 135.2, 138.7, 139.8, 146.3, 147.8 (t, 2JCF = 26 Hz, C-CF2); 19F-NMR (282.4 MHz, CDCl3) δ −126.5 (m 2F, CF2-CF2-CF2-CF2-CF3), −122.5 (m 2F, CF2-CF2-CF2-CF2-CF3), −122 (m 2F, CF2-CF2-CF2-CF2-CF3), −114.5 (m, 2F, CF2-CF2-CF2-CF2-CF3), −81.2 (m 3F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 428 [M + H]+. HRMS m/z [M + H]+ calcd. for C15H9F11NO+: 428.0508, found: 428.0510. Anal calcd. for C15H8F11NO: C, 42.17; H, 1.89; N, 3.28, found: C, 42.19; H, 1.88; N, 3.26.
2-Trifluoromethyl-6-methoxyquinoline (20o’). 10.9 g of 3-anisidine 15o (8.85 × 10−2 mol) and 2.6 mL or 2 g (3.54 × 10−2 mol) of acetone 16t were added to a solution of 9.8 g (2.95 × 10−2 mol) of 14’ (R’F = C2F5) in 98 mL of dry dichloromethane. The mixture was stirred for 12 h at reflux. 5.9 g of the quinoline 20o’ were obtained, total yield 88%. Spectral data: 1H-NMR (300.13 MHz, CDCl3) δ 3.8 (s, 3H, OCH3), 7.6 (d, J = 8.4 Hz, 1H), 7.8 (d, J = 8.5 Hz, 1H), 7.9–8 (m, 2H), 8.5 (d, J = 8.5 Hz, 1H); 13C-NMR (75.46 MHz, CDCl3) δ 55.5 (s, OCH3), 116.5 (q, 3JCF = 2.1 Hz, CH-C-CF3), 122.8 (q, 1JCF = 275.6 Hz, CF3), 128, 128.5, 129.3, 132.1, 136, 139.1, 142.6, 148.5 (q, 2JCF = 34 Hz, C-CF3); 19F-NMR (282.4 MHz, CDCl3) δ − 67.8 (m 3F, CF3). MS (m/z): 228 [M + H]+. HRMS m/z [M + H]+ calcd. for C11H9F3NO+: 228.0636, found: 228.0643. Anal calcd. for C11H8F3NO: C, 58.15; H, 3.55; N, 6.17, found: C, 58.17; H, 3.56; N, 6.15.
3.6. General Procedure for the Preparation and Isolation of 2-Perfluoroalkyl- and 2-Trifluoromethyl-1-(R-phenyl)amino-3-(R-phenyl)iminopropene Intermediates 21a–l (all examples except R = CO2H or R = m-OMe)
A mixture of one equivalent of gem-iodoacetate compounds 14 or 14’ or 14” and two equivalents of the corresponding anilines 15a–l in dichloromethane (10 mL DCM for 1 g of 14–14”) was stirred at room temperature until disappearance of 19F-NMR signals corresponding to the starting products 14–14” (2–6 h). At the end of the reaction, a solution of 10% sodium thiosulfate was added to the reaction mixture and the product was extracted three times with ether. The combined extracts were washed several times with aqueous 0.5 M hydrochloric solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give a bright yellow oil. Chromatography over silica gel column (eluent: petroleum ether/ethyl acetate 98/2 v/v) yielded the pure compounds 21a–l as yellow liquids.
3-Perfluoropenthyl-1-phenylamino-3-phenyliminopropene (21a). 3.49 g of aniline 15a (3.75 × 10−2 mol) were added to a solution of 10 g (1.87 × 10−2 mol) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 8.28 g of the title product 21a were obtained, total yield 90%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21a) δ 5.5 (d, 3JHH = 13 Hz, 1H, CH=CH-NH), 6.8 (t (AB system), J = 8.7 Hz, 4Hortho, Ph-H), 6.9 (t, J = 7.4 Hz, 1Hpara, Ph-H), 7.1 (t, J = 7.4 Hz, 1Hpara, Ph-H), 7.2 (t, J = 8.1 Hz, 2Hmeta, Ph-H), 7.4 (t, J = 7.8 Hz, 2Hmeta, Ph-H), 7.45 (m, 1H, CH=CH-NH), 9.9 (d, 3JHNH = 12.8 Hz, CH=CH-NH); 1H-NMR (300.13 MHz, DMSO-d6/D2O, EEE-21a) δ 5.4 (d, 3JHH = 13 Hz, 1H, CH=CH-NH), 6.7 (d, J = 8.5 Hz, 4Hortho, Ph-H), 6.9 (t, J = 7.5 Hz, 1Hpara, Ph-H), 7.1 (t, J = 7.4 Hz, 1Hpara, Ph-H), 7.2 (t, J = 8 Hz, 2Hmeta, Ph-H), 7.4 (t, J = 8 Hz, 2Hmeta, Ph-H), 7.45 (m, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21a) δ 91.5, 114.6, 118.5, 122.1, 123.5, 129.1, 129.5, 130, 140.5, 141.5 (t, 3JCF = 5.4 Hz, CF2-(C=N)-CH=CH-NH), 149.9, 153.6 (t, 2JC1F = 22.5 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21a) δ −80.2 (t, J = 8.4 Hz, 3F, CF2-CF2-CF2-CF2-CF3), −109.5 (t, J = 11 Hz, 2F, CF2-CF2-CF2-CF2-CF3), −120.4 (m, 2F, CF2-CF2-CF2-CF2-CF3), −121 (q, J = 5.6 Hz, 2F, CF2-CF2-CF2-CF2-CF3), −125.8 (t, J = 11.2 Hz, 2F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 491 [M + H]+. HRMS calcd. for C20H14F11N2+: 491.0981, found: 491.0985; Anal calcd. for C20H13F11N2: C, 48.99; H, 2.67; N, 5.71, found C, 48.97; H, 2.66; N, 5.74.
3-Trifluoromethyl-1-phenylamino-3-phenyliminopropene (21a’). 3.64 g of aniline 15a (3.91 × 10−2 mol) were added to a solution of 6.5 g (1.95 × 10−2 mol) of 14’ (RF = CF3) in 65 mL of dry dichloromethane. The mixture was stirred for 3 h at room temperature. 4.65 g of the title product 21a’ were obtained, total yield 82%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21a’) δ 5.5 (d, J = 13.7 Hz, 1H, CH=CH-NH), 6.8 (d, J = 7.5 Hz, 2H), 7 (m, 3H), 7.15 (t, J = 7.4 Hz, 1H), 7.3 (t, J = 7.8 Hz, 2H), 7.4 (t, J = 7.7 Hz, 2H), 7.6 (t, J = 13 Hz, 1H, CH=CH-NH), 9.9 (d, J = 12.3 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21a’) δ 90.8, 115.1, 119.4, 120.5 (q, CF3, 1JCF = 279.2 Hz), 122.2, 123.5, 129.1, 129.5, 140.64, 140.8 (q, 3JCF = 3 Hz, CF3-(C=N)-CH=CH-NH), 149.5, 153.3 (q, 2JCF = 30.5 Hz, CF3-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21a’) δ −65.7 (s, 3F). MS (m/z): 291 [M + H]+. HRMS calcd. for C16H14F3N2+: 291.1109, found 291.1110. Anal. Calcd. for C16H13F3N2: C, 66.20; H, 4.51; N, 9.65. Found: C, 66.19; H, 4.48; N, 9.55.
3-perfluoropropyl-1-phenylamino-3-phenyliminopropene (21a”). 2.36 g of aniline 15a (2.54 × 10−2 mol) were added to a solution of 5.5 g (1.27 × 10−2 mol) of 14” (RF = C3F7) in 55 mL of dry dichloromethane. The mixture was stirred for 3 h at room temperature. 4.22 g of the title product 21a” were obtained, total yield 85%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21a”) δ 5.5 (d, J = 13.5 Hz, 1H, CH=CH-NH), 6.8 (d, J = 7.4 Hz, 2H), 7 (m, 3H), 7.2 (t, J = 7.4 Hz, 1H), 7.3 (t, J = 7.7 Hz, 2H), 7.4 (t, J = 7.7 Hz, 2H), 7.7 (t, J = 13.1 Hz, 1H, CH=CH-NH), 9.9 (d, J = 12.1 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21a’’) δ 90.5, 115, 118.5, 122, 123.5, 129.3, 129.7, 130, 140.5, 141 (t, 3JCF = 5 Hz, CF2-(C=N)-CH=CH-NH), 150, 153.6 (t, 2JCF = 21.5 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21a”) δ −80.5 (m, 3F), −109.1 (t, J = 11 Hz, 2F), −126 (m, 2F). MS (m/z): 391 [M + H]+. HRMS calcd. for C18H14F7N2+: 391.1045, found: 391.1055; Anal calcd. for C18H13F7N2: C, 55.39; H, 3.36; N, 7.18, found C, 55.41; H, 3.35; N, 7.15.
3-perfluoropentyl-1-(2-methylphenylamino)-3-(2-methylphenylimino)-propene (21b). 3.26 g of 2-methylaniline 15b (3.04 × 10−2 mol) were added to a solution of 8.1 g (1.52 × 10−2 mol) of 14 (RF = C5F11) in 81 mL of dry dichloromethane. The mixture was stirred for 6 h at room temperature. 5.52 g of the title product 21b were obtained, total yield 70%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21b) δ 2.1 (s, 3H), 2.3 (s, 3H), 5.6 (d, J = 13.1 Hz, CH=CH-NH), 6.5–7.3 (m, 8H, HAr), 7.6 (m, 1H, CH=CH-NH), 9.3 (bs, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21b) δ 18.8, 18.9, 89, 113.1, 117.7, 121.5, 123.5, 129, 129.3, 130, 140.5, 141.5 (t, 3JCF = 4.8 Hz, CF2-(C=N)-CH=CH-NH), 150.1, 153.5 (t, 2JC1F = 20.5 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21b) δ −80.5 (m, 3F), −109 (m, 2F), −120.5 (m, 2F), −121 (m, 2F), −125.5 (m, 2F). MS (m/z): 519 [M + H]+. HRMS calcd. for C22H18F11N2+: 519.1294, found: 519.1298; Anal calcd. for C22H17F11N2: C, 50.97; H, 3.31; N, 5.40, found C, 50.99; H, 3.30; N, 5.37.
3-perfluoropentyl-1-(3-methylphenylamino)-3-(3-methylphenylimino)-propene (21c). 3.22 g of 3-methylaniline 15c (3.10−2 mol) were added to a solution of 8 g (1.5 × 10−2 mol) of 14 (RF = C5F11) in 80 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 6.23 g of the title product 21c were obtained, total yield 80%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21c) δ 2.2 (s, 3H), 2.3 (s, 3H), 5.4 (d, J = 13.6 Hz, 1H, CH=CH-NH), 6.6 (m, 2H), 6.7 (m, 3H), 6.9 (d, J = 7.6 Hz, 1H), 7.1 (m, 1H), 7.2 (t, J = 7.6 Hz, 1H), 7.5 (t, J = 12.5 Hz, 1H, CH=CH-NH), 9.7 (d, J = 12.5 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21c) δ 20.5, 20.9, 89, 113.5, 117.5, 121.5, 123.5, 129, 129.5, 130.2, 140, 141.1 (t, 3JCF = 5.3 Hz, CF2-(C=N)-CH=CH-NH), 150, 153 (t, 2JC1F = 21.5 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21c) δ −80.5 (m, 3F), −109.6 (m, 2F), −120.2 (m, 2F), −121.5 (m, 2F), −125.5 (m, 2F). MS (m/z): 519 [M + H]+. HRMS calcd. for C22H18F11N2+: 519.1294, found: 519.1295; Anal calcd. for C22H17F11N2: C, 50.97; H, 3.31; N, 5.40, found C, 50.99; H, 3.28; N, 5.38
3-perfluoropentyl-1-(4-methylphenylamino)-3-(4-methylphenylimino)-propene (21d). 3.62 g of 4-methylaniline 15d (3.38 × 10−2 mol) were added to a solution of 9 g (1.69 × 10−2 mol) of 14 (RF = C5F11) in 90 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7.71 g of the title product 21d were obtained, total yield 88%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21d) δ 2.2 (s, 3H), 2.3 (s, 3H), 5.4 (d, J = 13 Hz, 1H, CH=CH-NH), 6.7 (d, J = 8 Hz, 2H), 6.8 (d, J = 8.2 Hz, 2H), 7.1 (d, J = 8.2 Hz, 2H), 7.2 (d, J = 8 Hz, 2H), 7.5 (t, J = 12.5 Hz, 1H, CH=CH-NH), 9.7 (d, J = 12.4 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21d) δ 20.1, 20.4, 90.2, 115.5, 117.5, 122, 124.5, 129.2, 129.5, 140, 141.9 (t, 3JCF = 3.5 Hz, CF2-(C=N)-CH=CH-NH), 150.5, 152.9 (t, 2JC1F = 31.1 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21d) δ −80.3 (m, 3F), −109.5 (m, 2F), −120.1 (m, 2F), −121.5 (m, 2F), −125 (m, 2F). MS (m/z): 519 [M + H]+. HRMS calcd. for C22H18F11N2+: 519.1294, found: 519.1290; Anal calcd. for C22H17F11N2: C, 50.97; H, 3.31; N, 5.40, found C, 50.97; H, 3.29; N, 5.42
3-Trifluoromethyl-1-(4-methylphenylamino)-3-(4-methylphenylimino)-propene (21d’). 5.48 g of 4-methylaniline 15d (5.12 × 10−2 mol) were added to a solution of 8.5 g (2.56 × 10−2 mol) of 14’ (RF = CF3) in 85 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7 g of the title product 21d’ were obtained, total yield 86%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21d’) δ 2.2 (s, 3H), 2.3 (s, 3H), 5.4 (d, J = 13.8 Hz, 1H, CH=CH-NH), 6.7 (d, J = 8 Hz, 2H), 6.8 (d, J = 8.2 Hz, 2H), 7.1 (d, J = 8.2 Hz, 2H), 7.2 (d, J = 8 Hz, 2H), 7.5 (t, J = 12.9 Hz, 1H, CH=CH-NH), 9.7 (d, J = 12.4 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21d’) δ 20.1, 20.3, 90.4, 115, 119.1, 120.6 (q, 1JCF = 279.5 Hz, CF3), 129.1, 129.7, 129.9, 130.8, 131.1, 132.5, 138.2, 140.8 (q, 3JCF = 3.1 Hz, CF3-(C=N)-CH=CH-NH), 147, 153.4 (q, 2JCF = 30.8 Hz, CF3-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21d’) δ −65.7 (s, 3F). MS (m/z): 319 [M + H]+. HRMS calcd. for C18H18F3N2+: 319.1422, found: 319.1425; Anal calcd. for C18H17F3N2: C, 67.91; H, 5.38; N, 8.80, found C, 67.95; H, 5.37; N, 8.82.
3-perfluoropentyl-1-(2-ethylphenylamino)-3-(2-ethylphenylimino)-propene (21e). 3.59 g of 2-ethylaniline 15e (2.96 × 10−2 mol) were added to a solution of 7.9 g (1.48 × 10−2 mol) of 14 (RF = C5F11) in 79 mL of dry dichloromethane. The mixture was stirred for 6 h at room temperature. 5.43 g of the title product 21e were obtained, total yield 67%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21e) δ 1.2 (t, J = 8 Hz, 3H, CH2-CH3), 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.5 (q, J = 8 Hz, 2H, CH2-CH3), 2.7(q, J = 8 Hz, 2H, CH2-CH3), 5.6 (d, J = 12.8 Hz, CH=CH-NH), 6.5–7.3 (m, 8H, HAr), 7.5 (m, 1H, CH=CH-NH), 9.1 (bs, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21e) δ 13.2 (s, CH2-CH3), 13.3 (s, CH2-CH3), 24.1 (s, CH2-CH3), 24.3 (s, CH2-CH3), 89, 112.9, 117.5, 121.2, 129.3, 129.4, 130.5, 140.5, 141 (t, 3JCF = 4.9 Hz, CF2-(C=N)-CH=CH-NH), 149.9, 153.1 (t, 2JC1F = 21.5 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21e) δ −80.6 (m, 3F), −109.2 (m, 2F), −120.1 (m, 2F), −121.1 (m, 2F), −125 (m, 2F). MS (m/z): 547 [M + H]+. HRMS calcd. for C24H22F11N2+: 547.1607, found: 547.1610; Anal calcd. for C24H21F11N2: C, 52.75; H, 3.87; N, 5.13, found C, 52.76; H, 3.88; N, 5.10.
3-perfluoropentyl-1-(3-ethylphenylamino)-3-(3-ethylphenylimino)-propene (21f). 3.87 g of 3-ethylaniline 15f (3.19 × 10−2 mol) were added to a solution of 8.5 g (1.59 × 10−2 mol) of 14 (RF = C5F11) in 85 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 6.71 g of the title product 21f were obtained, total yield 77%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21f) δ 1.1 (t, J = 8 Hz, 3H, CH2-CH3), 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.6 (q, J = 8 Hz, 2H, CH2-CH3), 2.7 (q, J = 8 Hz, 2H, CH2-CH3), 5.4 (d, J = 13.1 Hz, 1H, CH=CH-NH), 6.6–6.8 (m, 5H), 6.9 (d, J = 7.5 Hz, 1H), 7.1 (m, 1H), 7.2 (t, J = 7.6 Hz, 1H), 7.5 (t, J = 12.5 Hz, 1H, CH=CH-NH), 9.6 (d, J = 12.8 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21f) δ 13.1 (s, CH2-CH3), 13.3 (s, CH2-CH3), 24 (s, CH2-CH3), 24.3 (s, CH2-CH3), 90.2, 114.1, 117.8, 122, 123.1, 129, 129.2, 130.2, 140, 141.1 (t, 3JCF = 5.9 Hz, CF2-(C=N)-CH=CH-NH), 151.1, 153.5 (t, 2JC1F = 22.1 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21f) δ −81.5 (m, 3F), −109.1 (m, 2F), −120.2 (m, 2F), −121.5 (m, 2F), −126.1 (m, 2F). MS (m/z): 547 [M + H]+. HRMS calcd. for C24H22F11N2+: 547.1607, found: 547.1611; Anal calcd. for C24H21F11N2: C, 52.75; H, 3.87; N, 5.13, found C, 52.78; H, 3.88; N, 5.14.
3-perfluoropentyl-1-(4-ethylphenylamino)-3-(4-ethylphenylimino)-propene (21g). 4 g of 4-ethylaniline 15g (3.3 × 10−2 mol) were added to a solution of 8.8 g (1.65 × 10−2 mol) of 14 (RF = C5F11) in 88 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7.67 g of the title product 21g were obtained, total yield 85%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21g) δ 1.2 (t, J = 8 Hz, 3H, CH2-CH3), 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.6 (q, J = 8 Hz, 2H, CH2-CH3), 2.7 (q, J = 8 Hz, 2H, CH2-CH3), 5.6 (d, J = 13.4 Hz, 1H, CH=CH-NH), 6.6 (d, J = 8.1 Hz, 2H), 6.8 (d, J = 8.2 Hz, 2H), 7.1 (d, J = 8.2 Hz, 2H), 7.2 (d, J = 8.1 Hz, 2H), 7.5 (t, J = 12.8 Hz, 1H, CH=CH-NH), 9.6 (d, J = 12.7 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21g) δ 13.3 (s, CH2-CH3), 13.4 (s, CH2-CH3), 24.3 (s, CH2-CH3), 24.5 (s, CH2-CH3), 90.2, 115.5, 117.5, 123.1, 125.2, 129.1, 129.3, 140.5, 141.9 (t, 3JCF = 4.2 Hz, CF2-(C=N)-CH=CH-NH), 151.5, 153.4 (t, 2JC1F = 32.2 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21g) δ −80.7 (m, 3F), −109.5 (m, 2F), −120.7 (m, 2F), −122.4 (m, 2F), −125.8 (m, 2F). MS (m/z): 547 [M + H]+. HRMS calcd. for C24H22F11N2+: 547.1607, found: 547.1609; Anal calcd. for C24H21F11N2: C, 52.75; H, 3.87; N, 5.13, found C, 52.77; H, 3.87; N, 5.14.
3-Trifluoromethyl-1-(4-ethylphenylamino)-3-(4-ethylphenylimino)-propene (21g’). 6.56 g of 4-ethylaniline 15g (5.42 × 10−2 mol) were added to a solution of 9 g (2.71 × 10−2 mol) of 14’ (RF = CF3) in 90 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7.97 g of the title product 21g’ were obtained, total yield 85%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21g’) δ 1.2 (t, J = 7.8 Hz, 3H, CH2-CH3), 1.3 (t, J = 8 Hz, 3H, CH2-CH3), 2.5 (q, J = 7.9 Hz, 2H, CH2-CH3), 2.7 (q, J = 8.1 Hz, 2H, CH2-CH3), 5.5 (d, J = 13.6 Hz, 1H, CH=CH-NH), 6.7 (d, J = 7.9 Hz, 2H), 6.8 (d, J = 8 Hz, 2H), 7.1 (d, J = 8.1 Hz, 2H), 7.2 (d, J = 8 Hz, 2H), 7.5 (t, J = 12.5 Hz, 1H, CH=CH-NH), 9.6 (d, J = 12.4 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21g’) δ 13.3 (s, CH2-CH3), 13.4 (s, CH2-CH3), 24.3 (s, CH2-CH3), 24.5 (s, CH2-CH3), 90.6, 116.2, 120.1, 120.5 (q, 1JCF = 280.5 Hz, CF3), 129.7, 129.9, 130.8, 131, 132.5, 138.3, 140.8 (q, 3JCF = 4.2 Hz, CF3-(C=N)-CH=CH-NH), 147.5, 153.1 (q, 2JCF = 29.9 Hz, CF3-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21g’) δ −65.5 (s, 3F). MS (m/z): 347 [M + H]+. HRMS calcd. for C20H22F3N2+: 347.1735, found: 347.1740; Anal calcd. for C20H21F3N2: C, 69.35; H, 6.11; N, 8.09, found C, 69.41; H, 6.10; N, 8.11.
3-perfluoropentyl-1-(2-chlorophenylamino)-3-(2-chlorophenylimino)-propene (21h). 4.84 g of 2-chloroaniline 15h (3.79 × 10−2 mol) were added to a solution of 10.1 g (1.89 × 10−2 mol) of 14 (RF = C5F11) in 101 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 6.57 g of the title product 21h were obtained, total yield 62%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21h) δ 5.6 (d, J = 13.8 Hz, 1H, CH=CH-NH), 6.9 (d, J = 7.71 Hz, 1HAr), 7.1–7.4 (m, 7HAr), 7.5 (d, J = 7.9 Hz, 1H, CH=CH-NH), 9.5 (bs, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21h) δ 90.1, 114.2, 115.1, 117.1, 121.2, 129.3, 129.4, 130.5, 140.5, 149.9, 153.2 (t, 2JC1F = 29.8 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21h) δ −80.6 (m, 3F), −109.5 (m, 2F), −120.3 (m, 2F), −121.1 (m, 2F), −125 (m, 2F). MS (m/z): 560 [M + H]+. HRMS calcd. for C20H12Cl2F11N2+: 559.0202, found: 559.0210; Anal calcd. for C20H11Cl2F11N2: C, 42.96; H, 1.98; N, 5.01, found C, 42.95; H, 1.96; N, 5.10.
3-perfluoropentyl-1-(3-chlorophenylamino)-3-(3-chlorophenylimino)-propene (21i). 4.79 g of 3-chloroaniline 15i (3.75 × 10−2 mol) were added to a solution of 10 g (1.87 × 10−2 mol) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7.35 g of the title product 21i were obtained, total yield 70%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21i) δ 5.6 (d, J = 13.6 Hz, 1H, CH=CH-NH), 6.7–6.9 (m, 6H), 7.2–7.3 (m, 2H), 7.5 (t, J = 13.2 Hz, 1H, CH=CH-NH), 9.7 (d, J = 12.8 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21i) δ 90.4, 112.2, 115.1, 116.4, 117.1, 121.4, 129.1, 129.5, 130.6, 141.5, 150.2, 153.2 (t, 2JC1F = 30.2 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21i) δ −80.5 (m, 3F), −109.1 (m, 2F), −120.1 (m, 2F), −121.1 (m, 2F), −125 (m, 2F). MS (m/z): 560 [M + H]+. HRMS calcd. for C20H12Cl2F11N2+: 559.0202, found: 559.0205; Anal calcd. for C20H11Cl2F11N2: C, 42.96; H, 1.98; N, 5.01, found C, 42.98; H, 1.97; N, 5.00.
3-perfluoropentyl-1-(4-chlorophenylamino)-3-(4-chlorophenylimino)-propene (21j). 4.6 g of 4-chloroaniline 15j (3.6 × 10−2 mol) were added to a solution of 9.6 g (1.8 × 10−2 mol) of 14 (RF = C5F11) in 96 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 7.76 g of the title product 21j were obtained, total yield 77%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21j) δ 5.4 (d, J = 13.6 Hz, 1H, CH=CH-NH), 6.8 (d, J = 8 Hz, 2H), 7 (d, J = 8.1 Hz, 2H), 7.3 (d, J = 8.2 Hz, 2H), 7.4 (d, J = 8 Hz, 2H), 7.6 (t, J = 13.2 Hz, 1H, CH=CH-NH), 9.8 (d, J = 12.9 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21j) δ 90.3, 116.1, 117.5, 123, 125.2, 129.2, 129.3, 140.6, 141.5 (t, 3JCF = 5.1 Hz, CF2-(C=N)-CH=CH-NH), 151.6, 153.5 (t, 2JC1F = 31.1 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21j) δ −80.6 (m, 3F), −109.2 (m, 2F), −120.5 (m, 2F), −122.5 (m, 2F), −125.2 (m, 2F). MS (m/z): 560 [M + H]+. HRMS calcd. for C20H12Cl2F11N2+: 559.0202, found: 559.0209; Anal calcd. for C20H11Cl2F11N2: C, 42.96; H, 1.98; N, 5.01, found C, 42.99; H, 1.98; N, 5.03.
3-perfluoropentyl-1-(2-methoxyphenylamino)-3-(2-methoxyphenylimino)-propene (21k). 3.93 g of 2-methoxyaniline 15k (3.19 × 10−2 mol) were added to a solution of 8.5 g (1.59 × 10−2 mol) of 14 (RF = C5F11) in 85 mL of dry dichloromethane. The mixture was stirred for 6 h at room temperature. 5.97 g of the title product 21k were obtained, total yield 68%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21k) δ 3.7 (s, 3H, OCH3), 3.8 (s, 3H, OCH3), 5.5 (d, J = 12.8 Hz, CH=CH-NH), 6.5–7.4 (m, 8H, HAr), 7.5 (m, 1H, CH=CH-NH), 9.1 (bs, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21k) δ 55.5 (s, OCH3), 55.6 (s, OCH3), 89.2, 112.5, 116.9, 120.8, 129.1, 129.2, 130.2, 132.1, 140.6, 141.2 (t, 3JCF = 4.5 Hz, CF2-(C=N)-CH=CH-NH), 149.2, 152.9 (t, 2JC1F = 20.1 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21k) δ −80.6 (m, 3F), −109.1 (m, 2F), −120.1 (m, 2F), −121.3 (m, 2F), −125.5 (m, 2F). MS (m/z): 551 [M + H]+. HRMS calcd. for C22H18F11N2O2+: 551.1193, found: 551.1199; Anal calcd. for C22H17F11N2O2: C, 48.01; H, 3.11; N, 5.09, found C, 48.08; H, 3.10; N, 5.11.
3-perfluoropentyl-1-(4-methoxyphenylamino)-3-(4-methoxyphenylimino)-propene (21l). 4.25 g of 4-anisidine 15l (3.45 × 10−2 mol) were added to a solution of 9.2 g (1.72 × 10−2 mol) of 14 (RF = C5F11) in 92 mL of dry dichloromethane. The mixture was stirred for 4 h at room temperature. 6.84 g of the title product 21l were obtained, total yield 72%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21l) δ 3.7 (s, 3H, OCH3), 3.8 (s, 3H, OCH3), 5.6 (d, J = 13.1 Hz, 1H, CH=CH-NH), 6.6 (d, J = 7.9 Hz, 2H), 6.8 (d, J = 8 Hz, 2H), 7.1 (d, J = 8 Hz, 2H), 7.2 (d, J = 8.1 Hz, 2H), 7.5 (t, J = 12.5 Hz, 1H, CH=CH-NH), 9.4 (d, J = 12.7 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21l) δ 55.5 (s, OCH3), 55.7 (s, OCH3), 89.8, 114.8, 115.6, 117.52, 123.1, 125, 129, 129.3, 139.8, 141.5 (t, 3JCF = 3.9 Hz, CF2-(C=N)-CH=CH-NH), 151.1, 154.2 (t, 2JC1F = 29.6 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21l) δ −80.5 (m, 3F), −109 (m, 2F), −120.3 (m, 2F), −122.2 (m, 2F), −125.5 (m, 2F). MS (m/z): 551 [M + H]+. HRMS calcd. for C22H18F11N2O2+: 551.1193, found: 551.1195; Anal calcd. for C22H17F11N2O2: C, 48.01; H, 3.11; N, 5.09, found C, 48.05; H, 3.12; N, 5.08.
3.7. General Procedure for the Isolation of 2-Perfluoropentyl- and 2-Trifluoromethyl-1-((2-/4-)-Carboxy-phenyl)Amino-3-((2-/4-)-Carboxyphenyl)Iminopropene Intermediates 21m–n’
A mixture of one equivalent of gem-iodoacetate compounds 14 or 14’ and two equivalents of the corresponding aminobenzoic acid 15m–n in dichloromethane (10 mL DCM for 1 g of 14–14’) was stirred at reflux until disappearance of 19F-NMR signals corresponding to the starting products 14–14’ (2–4 h). The reaction mixture was concentrated in vacuo and then diluted with diethyl ether. An excess of petroleum ether was added, and the precipitate that had formed was eliminated by vacuum filtration. The filtrate was concentrated under reduced pressure to give brown oil. Chromatography over silica gel column (eluent, petroleum ether/ethyl acetate 90/10 v/v) then purification over a plate chromatography (eluent petroleum ether/ethyl acetate 70/30 v/v) yielded pure compounds 21m-n’ as yellow amorphous solids.
3-perfluoropentyl-1-(2-carboxyphenylamino)-3-(2-carboxyphenylimino)-propene (21m). 5.15 g of 2-aminobenzoic acid 15m (3.75 × 10−2 mol) were added to a solution of 10 g (1.87 × 10−2 mol) of 14 (RF = C5F11) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 4.88 g of the title product 21m were obtained after column chromatography, yield 45%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21m) δ 5.7 (d, 3JHH = 13.7 Hz, CH=CH-NH), 6.9–7.45 (m, 8H, HAr), 7.6 (m, 1H, CH=CH-NH), 9.6 (bs, 1H, CH=CH-NH), 14 (bs, 2H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21m) δ 90.1, 114.5, 115, 118.1, 120.8, 129.1, 129.4, 131.2, 141.5, 150.5, 153.1 (t, 2JC1F = 25.6 Hz, CF2-(C=N)-CH=CH-NH), 170.5 (s, CO2H), 170.7 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21m) δ −80.3 (m, 3F), −109.68 (m, 2F), −120.4 (m, 2F), −121.05 (m, 2F), −125.83 (m, 2F). MS (m/z): 579 [M + H]+. HRMS calcd. for C22H14F11N2O4+: 579.0778, found: 579.0783 Anal calcd. for: C22H13F11N2O4: C, 45.69; H, 2.27; N, 4.84, found C, 45.72; H, 2.26; N,4.82.
3-Trifluoromethyl-1-(2-carboxyphenylamino)-3-(2-carboxyphenylimino)-propene (21m’). 6.44 g of 2-aminobenzoic acid 15m (4.69 × 10−2 mol) were added to a solution of 7.8 g (2.34 × 10−2 mol) of 14’ (RF = CF3) in 78 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 3.99 g of the title product 21m’ were obtained after column chromatography, yield 45%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21m’) δ 5.7 (d, J = 13.6 Hz, CH=CH-NH), 6.8–7.5 (m, 8H, HAr), 7.6 (m, 1H, CH=CH-NH), 9.7 (bs, 1H, CH=CH-NH), 14.1 (bs, 2H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21m’) δ 91.4, 115.2, 119.5, 121 (q, 1JCF = 277.7 Hz, CF3), 129.2, 129.7, 130.1, 130.8, 131.5, 132.5, 138.1, 140.7 (q, 3JCF = 2.8 Hz, CF3-(C=N)-CH=CH-NH), 147.1, 153.5 (q, 2JCF = 30.1 Hz, CF3-(C=N)-CH=CH-NH), 170.3 (s, CO2H), 170.5 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21m’) δ −65.6 (s, 3F). MS (m/z): 379 [M + H]+. HRMS calcd. for C18H14F3N2O4+: 379.0906, found: 379.0911 Anal calcd. for: C18H13F3N2O4: C, 57.15; H, 3.46; N, 7.41, found C, 57.19; H, 3.45; N,7.40.
3-Perfluoropenthyl-1-(4-carboxyphenyl)amino-3-(4-carboxyphenyl)iminopropene (21n). 5.15 g of 4-aminobenzoic acid (3.75 × 10−2 mole) were added to a solution of 10 g (1.87 × 10−2 mole) of 14 (R’F = C6F13) in 100 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 5.21 g of the title product 21n were obtained after column chromatography, yield 48%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21n) δ 5.4 (d, 3JHH = 12.9 Hz, 1H, CH=CH-NH), 6.8 (d, 3JHH = 8.1 Hz, 2H, Ar-H), 7 (d, 3JHH = 8.3 Hz, 2H, Ar-H), 7.3 (d, 3JHH = 8.3 Hz, 2H, Ar-H), 7.4 (d, 3JHH = 8.1 Hz, 2H, Ar-H), 7.6 (t (dd), 3JHH = 3JHNH = 12.5 Hz, 1H, CH=CH-NH), 9.9 (d, 3JHNH = 12.2 Hz, CH=CH-NH), 13.5 (bs, 2H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21n) δ 91, 115.5, 118.5, 121.9, 123.5, 129.2, 129.5, 130.1, 142.5, 141.9 (t, 3JCF = 4.9 Hz, CF2-(C=N)-CH=CH-NH), 149.8, 153.8 (t, 2JC1F = 20.5 Hz, CF2-(C=N)-CH=CH-NH); 170.1 (s, CO2H), 170.4 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21n) δ −80.5 (m, 3F, CF2-CF2-CF2-CF2-CF3), −109.2 (t, J = 11.2 Hz, 2F, CF2-CF2-CF2-CF2-CF3), −120.4 (m, 2F, CF2-CF2-CF2-CF2-CF3), −121.5 (m, 2F, CF2-CF2-CF2-CF2-CF3), −125.5 (m, 2F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 579 [M + H]+. HRMS calcd. for C22H14F11N2O4+: 579.0778, found: 579.0782 Anal calcd. for: C22H13F11N2O4: C, 45.69; H, 2.27; N, 4.84, found C, 45.71; H, 2.26; N,4.82.
3-Trifluoromethyl-1-(4-carboxyphenylamino)-3-(4-carboxyphenylimino)-propene (21n’). 8.67 g of 4-aminobenzoic acid 15n (6.32 × 10−2 mol) were added to a solution of 10.5 g (3.16 × 10−2 mol) of 14 (RF = CF3) in 105 mL of dry dichloromethane. The mixture was stirred for 4 h at reflux. 6.57 g of the title product 21n’ were obtained after column chromatography, yield 55%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21n’) δ 5.5 (d, 3JHH = 13 Hz, 1H, CH=CH-NH), 6.8 (d, J = 8 Hz, 2H), 7.1 (d, J = 8.1 Hz, 2H), 7.3 (d, J = 8 Hz, 2H), 7.4 (d, J = 8.1 Hz, 2H), 7.6 (t, J = J = 12.8 Hz, 1H, CH=CH-NH), 9.8 (d, J = 12.5 Hz, CH=CH-NH), 13.8 (bs, 2H, CO2H); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21n’) δ 91.1, 115.5, 119.8, 121.5 (q, CF3, 1JCF = 278.2 Hz), 123.1, 123.5, 128.9, 129.2, 140.5, 141.2 (q, 3JCF = 2.1 Hz, CF3-(C=N)-CH=CH-NH), 150.2, 153.5 (q, 2JCF = 27.5 Hz, CF3-(C=N)-CH=CH-NH), 170 (s, CO2H), 170.2 (s, CO2H); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21n’) δ −65.5 (s, 3F). MS (m/z): 379 [M + H]+. HRMS calcd. for C18H14F3N2O4+: 379.0906, found: 379.0910 Anal calcd. for: C18H13F3N2O4: C, 57.15; H, 3.46; N, 7.41, found C, 57.19; H, 3.45; N,7.42.
3.8. General Procedure for the Preparation and Isolation of 2-Perfluoropentyl- and 2-Trifluoromethyl-1-(3-Methoxyphenyl)Amino-3-(3-Methoxyphenyl)Iminopropene Intermediates 21o–o’
A mixture of one equivalent of gem-iodoacetate compounds 14 or 14’ and two equivalents of 3-anisidine 15o in dichloromethane (20 mL DCM for 1 g of 14–14’) was stirred at room temperature. The evolution of the reaction was monitored by TLC (eluent petroleum ether/ethyl acetate: 80/20 v/v), and 19F-NMR spectroscopy of aliquots. After four h of stirring, a solution of 10% sodium thiosulfate was added to the reaction mixture and the product was extracted three times with ether. The combined extracts were washed two times with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give a yellow oil. Chromatography over silica gel column (eluent: petroleum ether/ethyl acetate 98/2 v/v), then purification over a plate chromatography (eluent petroleum ether/ethyl acetate 85/15 v/v) yielded the pure compounds 21o–o’ as yellow liquids.
3-Perfluoropenthyl-1-(3-methoxyphenyl)amino-3-(3-methoxyphenyl)iminopropene (21o). 3.98 g of 3-anisidine 15o (3.23 × 10−2 mole) were added to a solution of 8.6 g (1.61 × 10−2 mole) of 14 (RF = C5F11) in 172 mL of dry dichloromethane. 4 g of the title product 21o were obtained after column chromatography, yield 45%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21o) δ 3.7 (s, 3H, OCH3), 3.8 (s, 3H, OCH3), 5.4 (d, J = 13.1 Hz, 1H, CH=CH-NH), 6.6–6.9 (m, 6H, Ph-H), 7.1 (t, J = 7.4 Hz, 1H, Ph-H), 7.3 (t, J = 7.4 Hz, 1H, Ph-H), 7.6 (t, J = 12.9 Hz, 1H, CH=CH-NH), 9.6 (d, J = 12.8 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21o) δ 55.5 (s, OCH3), 55.6 (s, OCH3), 89.5, 113.5, 115.5, 117.5, 121.5, 123.5, 129.1, 129.4, 130.6, 140, 140.8 (t, 3JCF = 4.2 Hz, CF2-(C=N)-CH=CH-NH), 150.5, 153.2 (t, 2JC1F = 26.7 Hz, CF2-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21o) δ −80.5 (m, 3F, CF2-CF2-CF2-CF2-CF3), −109.5 (m, 2F, CF2-CF2-CF2-CF2-CF3), −120.5 (m, 2F, CF2-CF2-CF2-CF2-CF3), −121.3 (m, 2F, CF2-CF2-CF2-CF2-CF3), −125.6 (m, 2F, CF2-CF2-CF2-CF2-CF3). MS (m/z): 551 [M + H]+. HRMS calcd. for C22H18F11N2O2+: 551.1193, found: 551.1198; Anal calcd. for C22H17F11N2O2: C, 48.01; H, 3.11; N, 5.09, found C, 48.03; H, 3.11; N, 5.10.
3-Trifluoromethyl-1-(3-methoxyphenyl)amino-3-(3-methoxyphenyl)iminopropene (21o’). 6.67 g of 3-anisidine 15o (5.42 × 10−2 mol) were added to a solution of 9 g (2.71 × 10−2 mol) of 14’ (RF = CF3) in 180 mL of dry dichloromethane. 4.55 g of the title product 21o’ were obtained after column chromatography, yield 48%. 1H-NMR (300.13 MHz, DMSO-d6, EEE-21o’) δ 3.7 (s, 3H, OCH3), 3.8 (s, 3H, OCH3), 5.4 (d, J = 13 Hz, 1H, CH=CH-NH), 6.7–6.9 (m, 6H, Ph-H), 7.1 (t, J = 7.3 Hz, 1H, Ph-H), 7.3 (t, J = 7.4 Hz, 1H, Ph-H), 7.7 (t, J = 12.7 Hz, 1H, CH=CH-NH), 9.6 (d, J = 12.6 Hz, 1H, CH=CH-NH); 13C-NMR (75.46 MHz, DMSO-d6, EEE-21o’) δ 55.5 (s, OCH3), 55.6 (s, OCH3), 90.2, 113.1, 115.5, 116.5, 119.4, 120.7 (q, 1JCF = 278.7 Hz, CF3), 123.5, 124.2, 129.6, 138.7, 139.1, 140.5, 141 (q, 3JCF = 2.8 Hz, CF3-(C=N)-CH=CH-NH), 149.6, 153.5 (q, 2JCF = 30.1 Hz, CF3-(C=N)-CH=CH-NH); 19F-NMR (282.4 MHz, DMSO-d6, EEE-21o’) δ −65.6 (s, 3F, CF3). MS (m/z): 351 [M + H]+. HRMS calcd. for C18H18F3N2O2+: 351.1320, found: 351.1322; Anal calcd. for C18H17F3N2O2: C, 61.71; H, 4.89; N, 8.00, found C, 61.75; H, 4.88; N, 8.01.
3.9. Reaction of N,N’-Diaryl-2-(Perfluoroalkyl)-1,5-Diazapentadienes 21a–o’ with Anilines 15a–o and Ketones 16t–v: Formation of 2-Trifluoromethyl-/2-Perfluoroalkyl-N-Arylpyridinium Derivatives 17–19 or 2-Trifluoromethyl-/2-Perfluoroalkyl-7-Methoxyquinolines 20o–o’
To a stirred solution of N,N’-diaryl-2-(perfluoroalkyl)-1.5-diazapentadienes 21a–o’ (1 equiv.) in anhydrous dichloromethane (10 mL DCM for 1g of 21), was added one equiv. of the corresponding substituted aniline 15a–o and 1.2 equiv. of ketone 16t–v. The mixture was stirred under reflux for desired time (4–12 h) until complete consumption of 21a–o’ (monitored by TLC eluent petroleum ether/ethyl acetate: 80/20 v/v, and 19F-NMR of aliquots). When the reaction was completed, the mixture was allowed to cool to r.t. then the brown precipitate accumulated during the reaction was separated by vacuum filtration (it was subsequently identified as anilinium salts by NMR and MS).
Then in the cases of pyridinium iodides 17a–l, 18a–l and 19a–l, ethyl ether was added to the filtrate. However, in the cases of pyridinium iodides 17m–n’, 18m–n and 19m, a mixture of petroleum ether and ethyl ether (40/60 v/v) was added to the corresponding filtrates and the expected pyridiniums 17a–n’, 18a–n and 19a–m precipitate instantly and were isolated by vacuum filtration as amorphous solids.
In the case of 3-anisidine (15o) at the end of the reaction, the mixture was concentrated under reduced pressure and then stirred with diethyl ether. An excess of petroleum ether was added; the precipitate that had formed was eliminated by vacuum filtration and washed three times with petroleum ether. The filtrate was concentrated in vacuo to give a yellow oil. Chromatography over silica gel column (eluent, petroleum ether/ethyl acetate 98/2 v/v) left a yellow oil which was crystallized from methanol/water to give pure samples of the corresponding quinolines 20o–o’. All the isolated pyridiniums 17a–n’, 18a–n, 19a–m and quinolines 20o–o’ were fully characterized and found identical to the previously obtained products.