Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples
Abstract
1. Introduction
2. Results and Discussion
2.1. Weight Loss of Cut Apple Cubes
2.2. Microscopic Examination of Coating
2.3. Color Changes for Storage
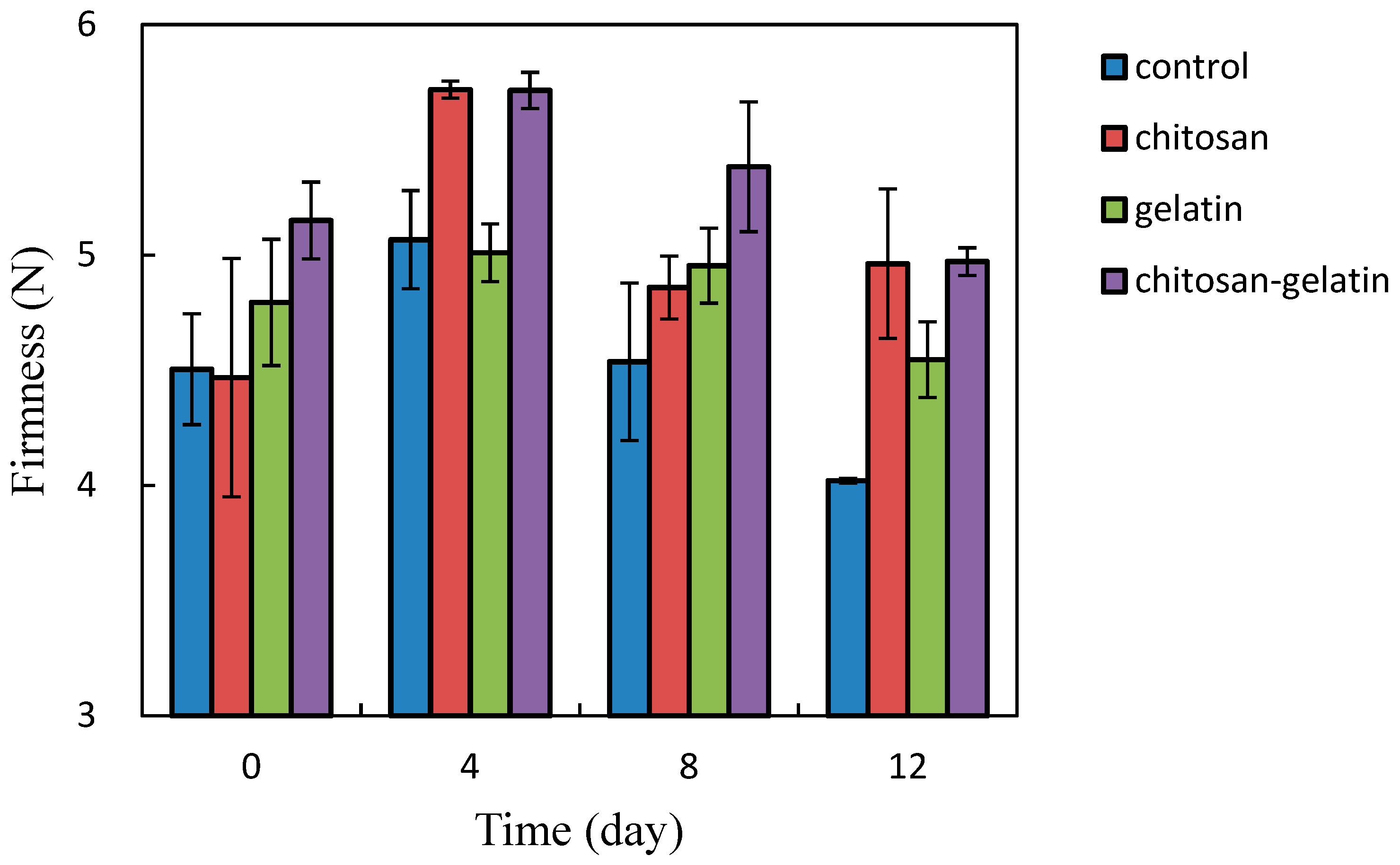
2.4. Firmness of Cut Apple Cubes
2.5. pH of the Cut Apple Cubes
2.6. Total Soluble Solids (TSS) And Total Titratable Acidity (TTA) of Cut Apple Cubes
2.7. Ascorbic Acid Content of Cut Apple Cubes
2.8. Microbiological Analysis of Stored Cut Apple Cubes
2.9. Sensory Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan-Gelatin Coating
3.3. Weight Loss of Apple Cubes
3.4. Color Measurement
3.5. Firmness of Cut Apple Cubes
3.6. pH of Cut Apple Cubes
3.7. Total Soluble Solids (TSS) and Total Titratable Acidity (TTA) of the Cut Apple Cubes
3.8. Vitamin C Content of the Cut Apple Cubes
3.9. Microbiological Analysis
3.10. Microscopic Examination of the Cut Apple Cubes
3.11. Sensory Evaluation
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roller, R.S.; Chism, G.W. Physiological consequences of minimally processed fruits and vegetables. J. Food Qual. 1987, 10, 157–177. [Google Scholar] [CrossRef]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending shelf-life of fresh-cut ‘Fuji’ apples with chitosan-coatings. Innov. Food Sci. Emerg. Technol. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef] [PubMed]
- Albanese, D.; Cinquanta, L.; Matteo, M.D. Effects of an innovative dipping treatment on the cold storage of minimally processed Annurca apples. Food Chem. 2007, 105, 1054–1060. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Hatoum, D.; Buts, K.; Hertog, M.L.A.T.M.; Geeraerd, A.H.; Schenk, A.; Vercammen, J.; Nicolai, B.M. Effects of pre- and postharvest factors on browning in Braeburn. Hort. Sci. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Zhou, R.; Mo, Y.; Li, Y.; Zhao, Y.; Zhang, G.; Hu, Y. Quality and internal characteristics of Huanghua peers (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol. Technol. 2008, 49, 171–179. [Google Scholar] [CrossRef]
- Park, H.J. Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Che, Z.; Lin, H. Chitosan-based coating with antimicrobial agents preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym. Sci. 2016, 1–24. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Lee, J.J.; Lee, W.Y. Combined effect of chitosan coating and modified atmosphere packaging on fresh-cut cucumber. Food Sci. Nutr. 2019, 7, 1043–1052. [Google Scholar] [CrossRef]
- Hagenmaier, R.D. A comparison of ethane, ethylene and CO2 peel permeance for fruit with different coatings. Postharvest Biol. Technol. 2005, 37, 56–64. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Rocchetti, R. Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; Demarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and insulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Preserving apple (Malus domestica var. Anna) fruit bioactive substances using olive wastes extract-chitosan film coating. Inform. Proc. Agric. 2017, 4, 90–99. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Di Venere, D.; Salerno, M. Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1866. [Google Scholar] [CrossRef]
- Jovanovic, G.D.; Klaus, A.S.; Niksic, M.P. Antimicrobial activity of chitosan coatings and films against Listeria monocytogenes on black radish. Rev. Argent Microbial. 2016, 48, 128–136. [Google Scholar] [CrossRef]
- Valdes, A.; Ramos, M.; Beltran, A.; Jimenez, A.; Garrigos, M.C. state of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Simonaitiene, D.; Brink, I.; Sipailiene, A.; Leskauskaite, D. The effect of chitosan and whey proteins-chitosan films on the growth of Penicillium expansum in apples. J. Sci. Food Agric. 2015, 95, 1475–1481. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Tarsi, R.; Fillippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.R. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.W.; Li, C.F.; Shih, D.Y.C. Antifungal activity of chitosan and its preservative effect on low-sugar candied kumwuat. J. Food Prot. 1994, 56, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Bilideris, C.G. Thermophysical properties of chitosan-starch and chitosan-pullulan films near the glass transition. Carbohydr. Polym. 2002, 48, 179–190. [Google Scholar] [CrossRef]
- Malinowska-Pancyzk, E.; Staroszczyk, H.; Gottfried, K.; Kolodziejska, I.; Wojtasz-Pajak, A. Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan films. Polimery 2015, 60, 11–12. [Google Scholar]
- Foegeding, E.A.; Lantier, T.C.; Hultin, H.O. Collagen. In Fennema OP (3rd ed) Food Chemistry; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 902–906. [Google Scholar]
- Amiri, S.; Akhavan, H.R.; Zare, N.; Radi, M. Effect of gelatin-based edible coatings incorporated with Aloe Vera and green tea extracts on the shelf-life of fresh-cut apple. Ital. J. Food Sci. 2018, 30, 61–74. [Google Scholar]
- Samsi, M.S.; Kamari, A.; Fatimah, I.; Sunardi, S.; Najiah, S.; Yusoff, M. Synthesis, characterisation and application of gelatin-chitosan blend films for fruit preservation. Fresen Environ. Bull. 2019, 28, 30–43. [Google Scholar]
- Wangtueai, S.; Noomhorm, A. Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT Food Sci. Technol. 2009, 42, 825–834. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Canovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, M.; Ansarifar, E.; Hasanpour, N.; Amiryousefi, M.R. Suitability of aloe vera and gum tragacanth as edible coatings for extending the shelf life of button mushroom. Food Bioproc. Technol. 2011, 5, 3193–3202. [Google Scholar] [CrossRef]
- Kim, K.M.; Ko, J.A.; Lee, J.S.; Park, H.J.; Hama, M.A. Effect of modified atmosphere packaging on the shelf-life of coated, whole and sliced mushrooms. LWT Food Sci. Technol. 2006, 39, 364–371. [Google Scholar] [CrossRef]
- Rojas-Grau, M.A.; Tapia, M.S.; Rodriguez, F.J.; Carmona, A.J.; Martin-Belloso, O. Aliginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocolloid. 2007, 21, 118–127. [Google Scholar] [CrossRef]
- Moreira, M.R.; Tomadoni, B.; Martin-Belloso, O.; Soliva-Fortuny, R. Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT Food Sci. Technol. 2015, 64, 1130–1137. [Google Scholar] [CrossRef]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K.B. Quality change of apple slice coated with Aloe vera gel during storage. J. Food Sci. 2013, 78, c817–c822. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hu, C. The new technology in agricultural products processing and the quality analysis/detection technology for food/agricultural products. In Proceedings of the ICAE International Conference on New Technology of Agricultural Engineering, Zibo, China, 27–29 May 2011. [Google Scholar]
- Pan, X.J.; Tu, K. Comparison of texture properties of post-harvested apples using texture profile analysis. Trans. CSAE 2005, 21, 166–170. (In Chinese) [Google Scholar]
- Jackmen, R.L.; Stanley, D.W. Perspectives in the textural evaluation of plant foods. Trends Food Sci. Technol. 1995, 6, 187–194. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Brasil, I.M.; Gomes, C.; Puerta-Gomez, A.; Castell-perez, M.E.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT Food Sci. Tech. 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Bett, K.L.; Ingram, D.A.; Grimm, C.C.; Lloyd, S.W.; Spanier, A.M.; Gross, K.C.; Baldwin, E.A.; Vinyard, B.T. Flavor of fresh-cut Gala apples in barrier film packaging as affected by storage time. J. Food Qual. 2001, 24, 141–156. [Google Scholar] [CrossRef]
- Durango, A.M.; Soares, N.F.F.; Andrade, N.J. Microbiological evaluation of an edible antimicrobial coating on minimally processed carrots. Food Control 2006, 17, 336–341. [Google Scholar] [CrossRef]
- Coma, V.; Martial-Giros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002, 67, 1162–1168. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.; Piette, G.; Begin, A.; Holley, R.A. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int. J. Food Microbiol. 2000, 62, 139–148. [Google Scholar] [CrossRef]
- Seyed, F.H.; Masoud, R.; Mojag, Z.; Farhid, F.G. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
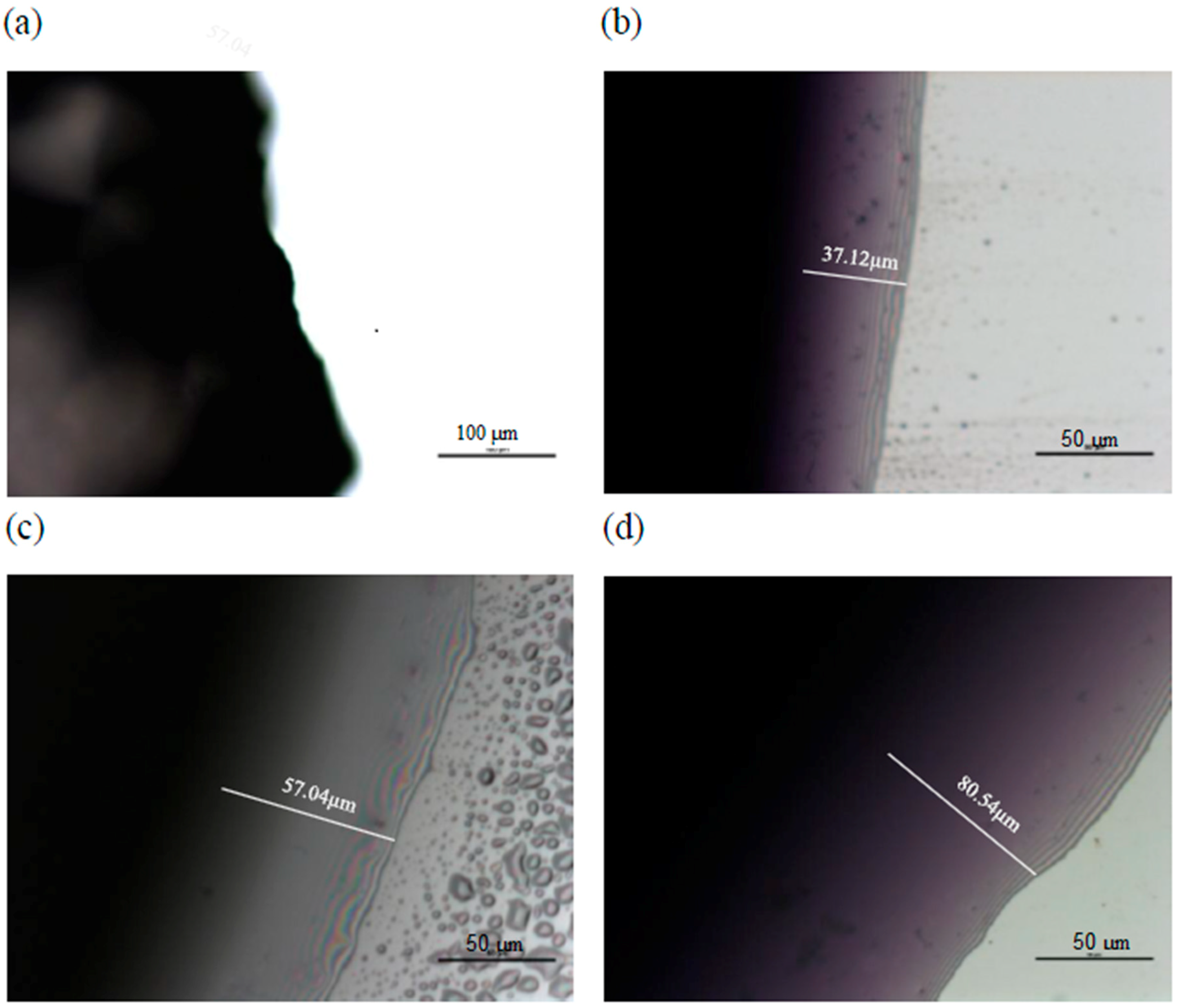

| L* Value | Sample Treatment | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| 22 °C | Control | 68.23 ± 0.41 A | 51.22 ± 1.12 B | - * | - |
| Chitosan | 65.29 ± 0.01 A | 49.75 ± 0.00 B | 39.46 ± 0.00 C | 42.02 ± 0.38 C | |
| Gelatin | 67.26 ± 0.72 A | 44.49 ± 1.86 C | - | - | |
| Chitosan+Gelatin | 65.92 ± 1.03 A | 48.60 ± 3.01 B | - | - | |
| 5 °C | Control | 67.70 ± 1.32 A | 63.58 ± 0.86 A | 64.00 ± 0.78 A | 64.90 ± 1.73 A |
| Chitosan | 67.84 ± 1.78 A | 62.08 ± 3.00 A | 62.39 ± 1.86 A | 55.98 ± 0.50 B | |
| Gelatin | 67.51 ± 1.60 A | 59.19 ± 3.47 A | 57.30 ± 0.85 B | 54.41 ± 1.70 B | |
| Chitosan+Gelatin | 65.05 ± 0.56 A | 60.39 ± 1.31 A | 60.41 ± 0.64 A | 62.92 ± 1.79 A | |
| Browning Index | Sample Treatment | 0 | 4 | 8 | 12 |
|---|---|---|---|---|---|
| 22 °C | Control | 80.84 ± 0.18 AB | 95.74 ± 2.39 AB | - * | - |
| Chitosan | 87.13 ± 4.48 AB | 86.03 ± 6.10 AB | 80.82 ± 17.46 A | 83.10 ± 4.11 A | |
| Gelatin | 112.98 ± 5.32 A | 119.76 ± 3.36 A | - | - | |
| Chitosan+Gelatin | 85.79 ± 0.89 AB | 93.74 ± 9.72 AB | - | - | |
| 5 °C | Control | 88.29 ± 1.64 AB | 94.66 ± 6.52 AB | 89.02 ± 5.86 A | 86.87 ± 1.23 A |
| Chitosan | 75.98 ± 0.07 B | 90.32 ± 0.00 B | 88.18 ± 0.00 A | 82.53 ± 4.35 A | |
| Gelatin | 78.18 ± 2.46 AB | 91.81 ± 3.13 B | 88.71 ± 3.66 A | 96.95 ± 6.62 A | |
| Chitosan+Gelatin | 80.03 ± 1.22 AB | 89.95 ± 7.08 B | 87.56 ± 0.96 A | 82.32 ± 1.40 A |
| pH | Sample Treatment | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| 22 °C | Control | 3.83 ± 0.24 A | 3.86 ± 0.18 BC | - * | - |
| Chitosan | 4.18 ± 0.15 A | 4.59 ± 0.19 AC | 4.64 ± 0.23 A | 4.86 ± 0.02 AD | |
| Gelatin | 3.94 ± 0.27 A | 3.89 ± 0.21 BC | - | - | |
| Chitosan+Gelatin | 4.06 ± 0.03 A | 4.06 ± 0.21 AB | - | - | |
| 5 °C | Control | 3.86 ± 0.24 A | 4.07 ± 0.32 AB | 3.96 ± 0.45 A | 4.18 ± 0.13 BC |
| Chitosan | 4.21 ± 0.31 A | 4.36 ± 0.37 AB | 4.52 ± 0.27 A | 4.48 ± 0.23 BD | |
| Gelatin | 3.88 ± 0.29 A | 4.01 ± 0.37 AB | 3.89 ± 0.10 A | 4.04 ± 0.06 CD | |
| Chitosan+Gelatin | 4.04 ± 0.30 A | 4.15 ± 0.31 AB | 4.04 ± 0.41 A | 4.31 ± 0.25 BC | |
| TSS (°Brix) | Sample Treatment | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| 22 °C | Control | 10.5 ± 1.0 A | 10.8 ± 0.4 AC | - * | - |
| Chitosan | 10.6 ± 0.8 A | 10.8 ± 0.1 AC | 10.7 ± 0.0 A | 10.5 ± 0.1 AD | |
| Gelatin | 10.3 ± 0.8 A | 11.5 ± 0.1 AC | - | - | |
| Chitosan+Gelatin | 11.0 ± 1.6 A | 10.4 ± 0.6 AA | - | - | |
| 5 °C | Control | 11.1 ± 1.1 A | 10.8 ± 0.0 A | 11.2 ± 0.7 A | 11.4 ± 0.5 AA |
| Chitosan | 10.6 ± 0.8 A | 10.8 ± 0.4 A | 10.6 ± 1.2 A | 10.2 ± 0.6 AD | |
| Gelatin | 10.1 ± 1.6 A | 10.9 ± 0.4 A | 10.8 ± 0.7 A | 10.6 ± 0.2 AD | |
| Chitosan+Gelatin | 10.6 ± 1.5 A | 10.4 ± 1.3 A | 10.6 ± 0.6 A | 10.9 ± 0.2 AA | |
| Microbiological Analysis | Sample Treatment | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| Total aerobic bacteria | Control | <1 | <1 | <1 | 4.59 Aa |
| Chitosan | <1 | <1 | <1 | <1 | |
| Gelatin | <1 | 1.92 Ab | 2.23 Ab | 5.34 Aa | |
| Chitosan+Gelatin | <1 | <1 | <1 | <1 | |
| Yeast and mold | Control | <1 | <1 | 2.36 Ab | 5.02 Aa |
| Chitosan | <1 | <1 | <1 | <1 | |
| Gelatin | <1 | <1 | 3.03 Ab | 5.64 Aa | |
| Chitosan+Gelatin | <1 | <1 | <1 | <1 | |
| Coliform | Control | <1 | <1 | <1 | 4.81 Aa |
| Chitosan | <1 | <1 | <1 | <1 | |
| Gelatin | <1 | 2.55 Aab | 2.72 Aab | 5.30 Aa | |
| Chitosan+Gelatin | <1 | <1 | <1 | <1 | |
| Sensory Scores | Sample Treatment | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| Color | Control | 4.15 ± 1.28 Ba | 3.00 ± 1.00 Ab | 2.87 ± 1.06 Cb | 2.93 ± 1.33 BbA |
| Chitosan | 4.38 ± 1.12 Ba | 3.77 ± 1.30 Aa | 4.60 ± 0.99 Aa | 4.14 ± 1.56 AaA | |
| Gelatin | 5.77 ± 1.17 Aa | 3.92 ± 1.55 Ab | 3.87 ± 1.25 ABb | 3.27 ± 1.10 ABb | |
| Chitosan + Gelatin | 3.85 ± 1.46 Ba | 3.62 ± 1.56 Aa | 3.33 ± 1.11 BCa | 4.07 ± 1.00 AaA | |
| Odor | Control | 4.92 ± 1.26 Aa | 4.31 ± 1.25 Aaa | 4.13 ± 1.25 AaA | 4.00 ± 1.41 AaA |
| Chitosan | 3.46 ± 1.27 Ba | 3.62 ± 1.19 Aaa | 3.00 ± 0.93 Bab | 2.57 ± 0.76 BbA | |
| Gelatin | 5.54 ± 1.27 Aa | 4.54 ± 0.97 Aab | 3.87 ± 1.13 ABb | 4.33 ± 1.76 AbA | |
| Chitosan + Gelatin | 3.54 ± 1.13 Ba | 3.62 ± 0.96 Aaa | 3.33 ± 1.23 ABa | 3.50 ± 1.29 ABa | |
| Texture | Control | 3.38 ± 1.45 Aa | 2.92 ± 1.12 Baa | 3.00 ± 1.00 Ba | 3.29 ± 1.54 Baa |
| Chitosan | 3.77 ± 0.93 Ab | 4.00 ± 1.41 ABab | 4.80 ± 0.86 Aa | 4.57 ± 0.76 Aab | |
| Gelatin | 3.77 ± 1.83 Aa | 3.62 ± 1.33 ABaa | 3.33 ± 1.23 Ba | 3.27 ± 1.10 Baa | |
| Chitosan + Gelatin | 3.77 ± 1.83 Aa | 3.62 ± 1.33 ABaa | 3.33 ± 1.23 Ba | 3.27 ± 1.10 Baa | |
| Flavor | Control | 4.15 ± 1.68 Aa | 3.46 ± 1.05 Aaa | 4.27 ± 0.96 Aaa | 3.29 ± 1.68 ABaa |
| Chitosan | 4.31 ± 1.25 Aa | 3.54 ± 1.27 Aab | 3.60 ± 0.99 ABab | 3.14 ± 1.23 BbaA | |
| Gelatin | 4.46 ± 1.56 Aa | 3.54 ± 0.97 Aaa | 4.13 ± 1.64 ABa | 4.20 ± 0.86 AaaA | |
| Chitosan + Gelatin | 4.38 ± 1.56 Aa | 3.77 ± 1.01 Aab | 3.27 ± 1.16 Bba | 3.86 ± 1.03 ABab | |
| Overall quality | Control | 3.69 ± 1.18 Aa | 3.31 ± 0.95 Baa | 3.87 ± 0.64 ABa | 3.21 ± 1.37 Aaa |
| Chitosan | 3.92 ± 1.26 Aa | 4.00 ± 1.00 ABa | 4.07 ± 0.70 AaA | 3.29 ± 0.83 Aaa | |
| Gelatin | 4.23 ± 1.30 Aa | 3.77 ± 1.17 ABa | 4.13 ± 1.41 AaA | 4.00 ± 0.93 Aaa | |
| Chitosan + Gelatin | 4.15 ± 1.21 Aa | 4.23 ± 0.83 Aaa | 3.27 ± 1.10 BbA | 3.79 ± 1.05 Aab | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyu, Y.-S.; Chen, G.-W.; Chiang, S.-C.; Sung, W.-C. Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules 2019, 24, 2008. https://doi.org/10.3390/molecules24102008
Shyu Y-S, Chen G-W, Chiang S-C, Sung W-C. Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules. 2019; 24(10):2008. https://doi.org/10.3390/molecules24102008
Chicago/Turabian StyleShyu, Yung-Shin, Guan-Wen Chen, Shao-Ching Chiang, and Wen-Chieh Sung. 2019. "Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples" Molecules 24, no. 10: 2008. https://doi.org/10.3390/molecules24102008
APA StyleShyu, Y.-S., Chen, G.-W., Chiang, S.-C., & Sung, W.-C. (2019). Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules, 24(10), 2008. https://doi.org/10.3390/molecules24102008






