Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities
Abstract
:1. Introduction
2. Results
2.1. Total Flavonoid and Total Phenol Contents of SBR Extracts
2.2. Free Radical Scavenging and Antioxidant Activities
2.2.1. DPPH Radical Scavenging Activity
2.2.2. Antioxidant Activity of SBR Extracts and Their Superoxide (O2•−), Hydroxyl (−OH) and Hydroperoxide (H2O2) Scavenging Activities
2.2.3. Ferrous Ion-chelating Activity
2.3. Inhibitory Effects of SBR Extracts on LHP-induced LPO
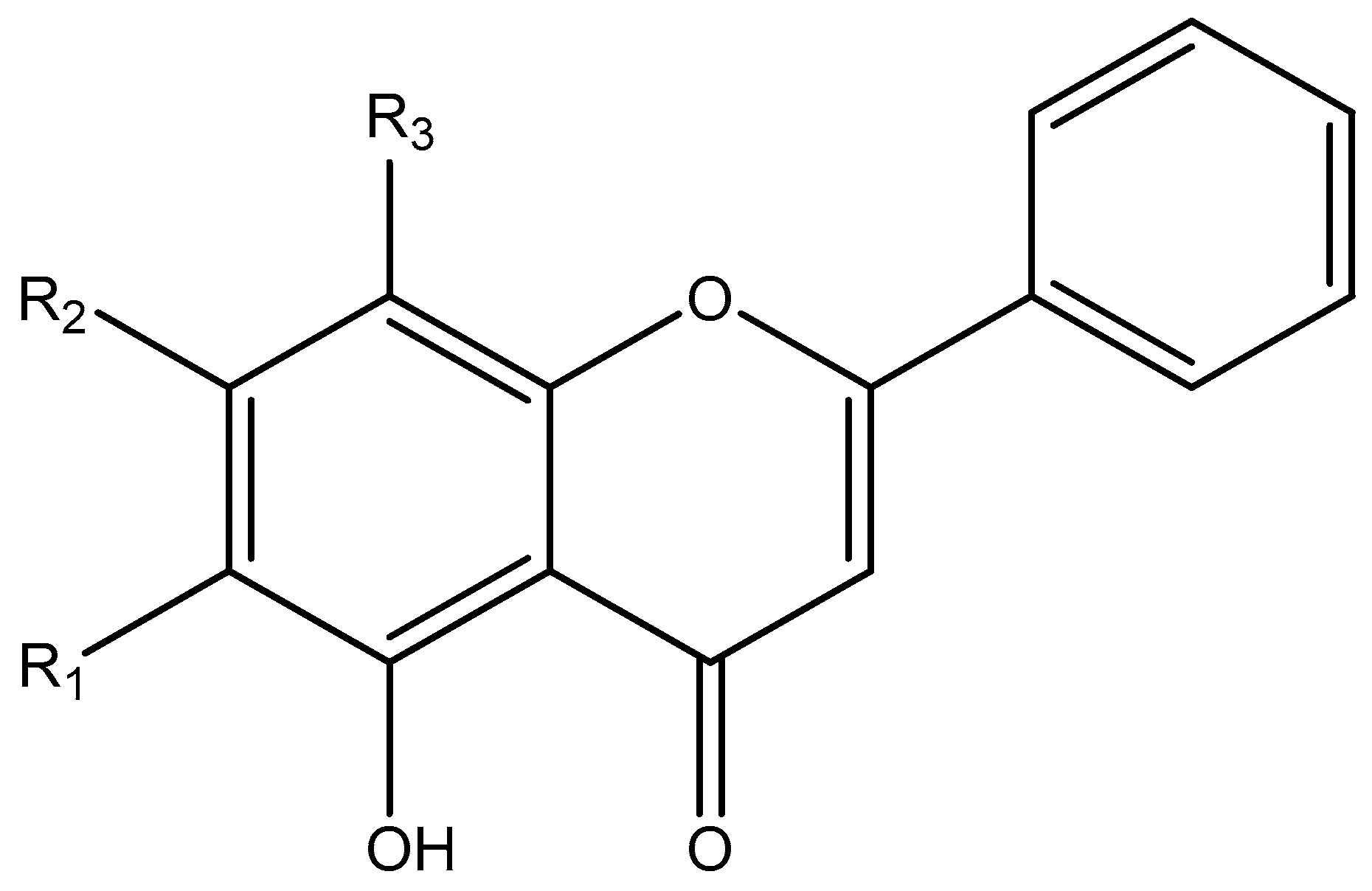
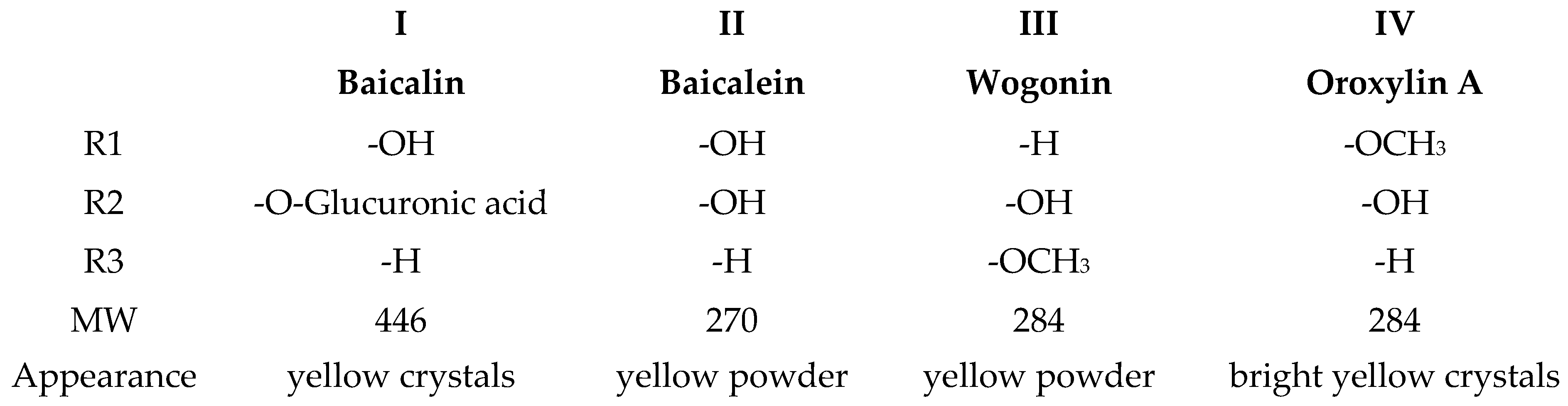
2.4. Phytochemicals Isolated from High-potential Extract and Structure Identification
2.5. LPO Inhibition, free Radical Scavenging and Antioxidant Activities by Main Components of the EtOAc Extract
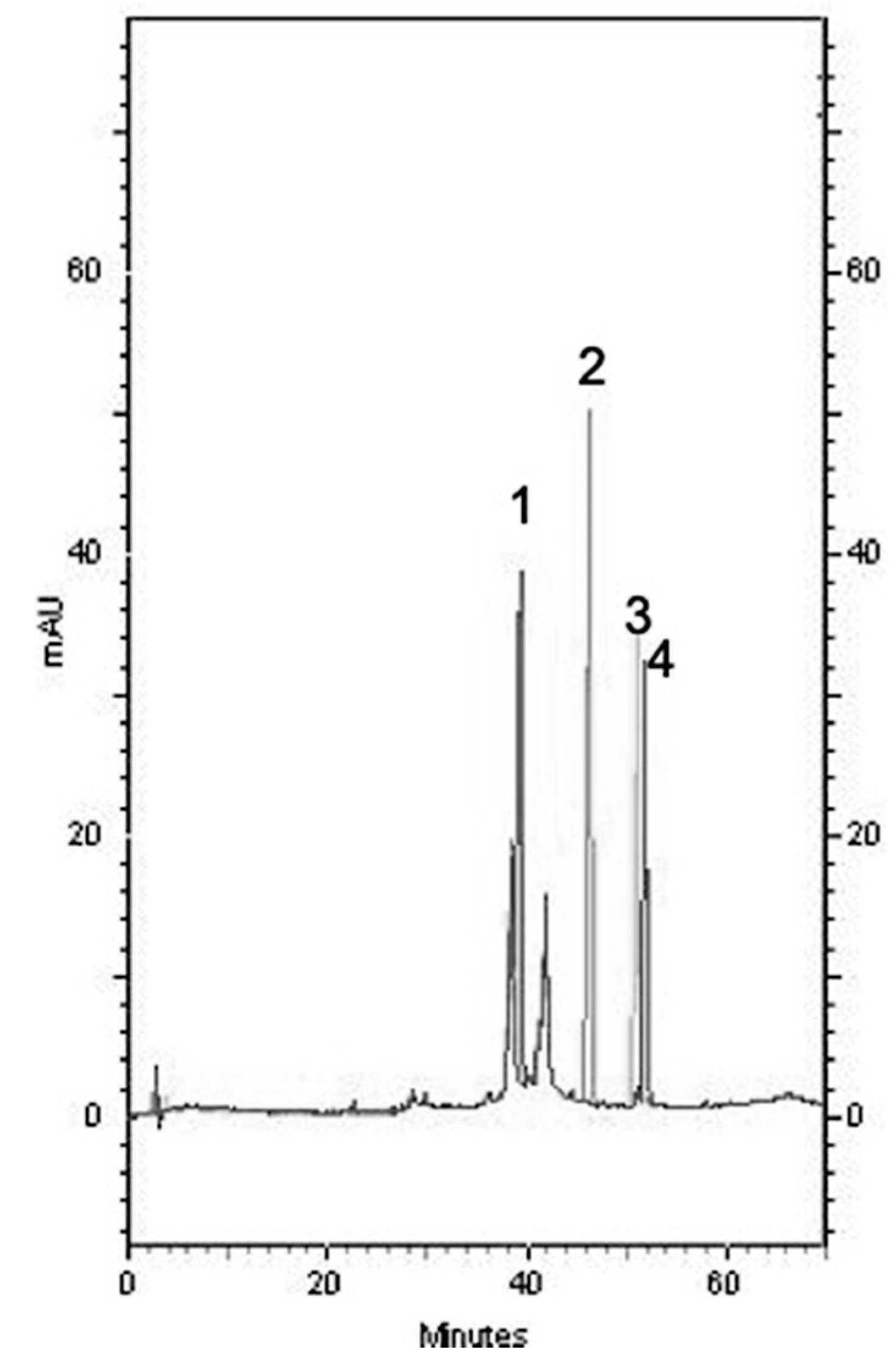
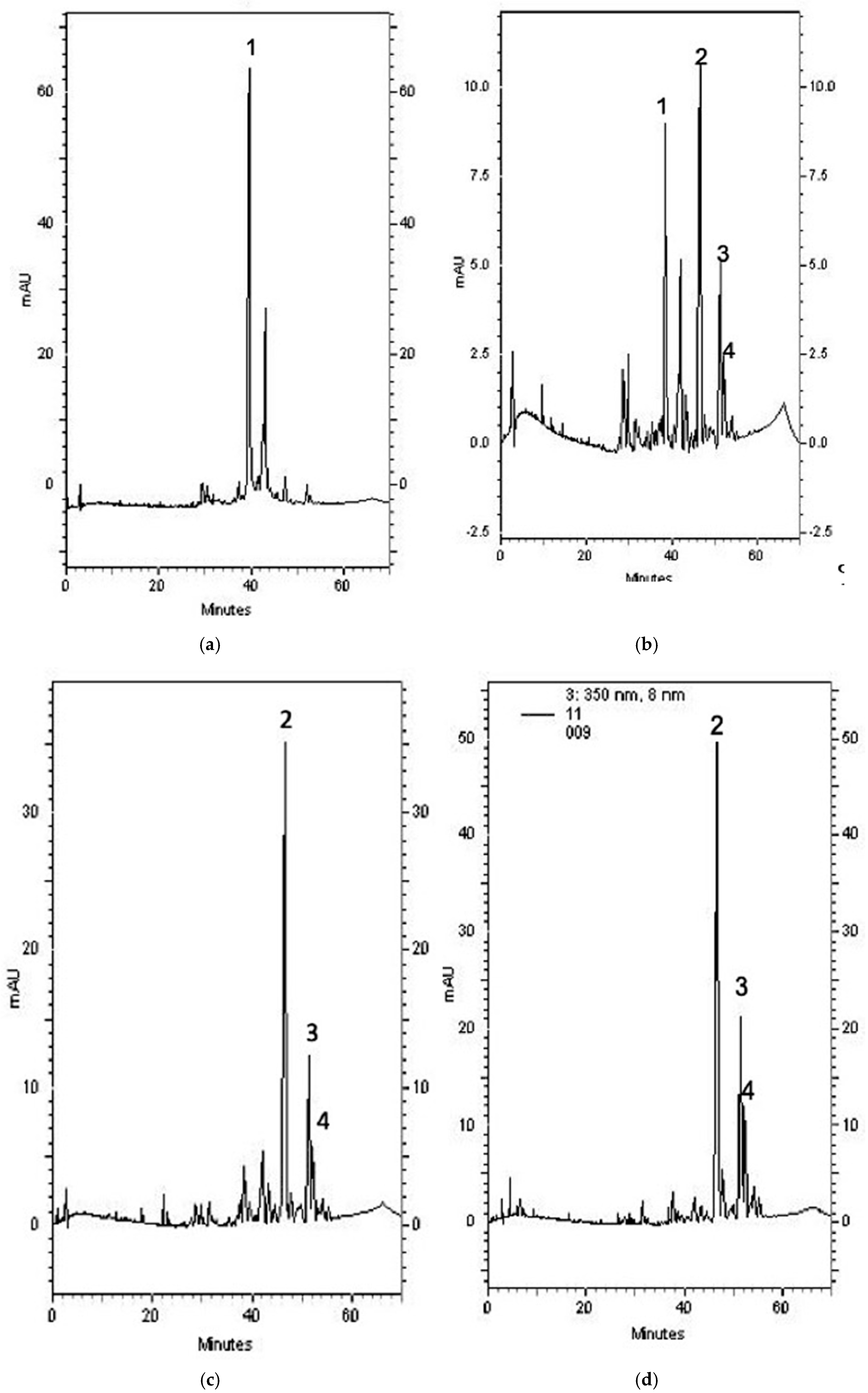
2.6. Composition of Each SBR Extract
3. Discussion
4. Materials and Methods
4.1. Extraction and Sample Preparation
4.2. Chemicals and Reagents
4.3. Experimental Animals
4.4. Free Radical Scavenging and Antioxidantactivity Measurement
4.4.1. Total Phenol Content
4.4.2. Total Flavonoid Content
4.4.3. Diphenylpicrylhydrazyl Radical Scavenging Activity
extract/absorbance intensity of control)] × 100%
4.4.4. Ferric Reducing/antioxidant Power Activity
4.4.5. Superoxide Anion Radical (O2•−) Scavenging Activity
control at 560 nm)] × 100%
4.4.6. Hydroxyl Radical Scavenging Activity
(absorbance of sample − absorbance of control)] × 100%
4.4.7. H2O2 Scavenging Activity
control at 610 nm)] × 100%
4.4.8. Ferrous Ion-chelating Activity
at 562 nm)] × 100%
4.5. Determining the Effect of Lipid Peroxidative Product of SBR Extracts on Rat Lung Tissues
4.5.1. Preparation of Rat Lung Mitochondria
4.5.2. Protein Content Determination
4.5.3. Synthesis of Linoleic Acid Hydroperoxide
4.5.4. LHP-induced LPO Inhibition Assay
532 nm)/ (absorbance of control at 532 nm − absorbance of blank) × 100%
4.6. Phytochemical Isolation from the EtOAc Extract
4.7. Phytochemical Analyses
4.8. Data Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| LPO | lipid peroxidation |
| LHP | linoleic acid hydroperoxide |
| DPPH | diphenylpicrylhydrazyl |
| COPD | chronic obstructive pulmonary disease |
| SBR | Scutellaria baicalensis root |
| EtOAc | ethyl acetate |
| EtOH | ethanol |
| O2•− | superoxide |
| ROO• | alkoxy peroxyl radical |
| RO• | alkoxy radical |
| −OH | hydroxyl |
| H2O2 | hydroperoxide |
| Fe2+ | ferrous ion |
| NBT | nitroblue tetrazolium |
| HRPase | horseradish peroxidase |
| SOD | superoxide dismutase |
| TCA | trichloroacetic acid |
| TBA | thiobarbituric acid |
| NAD(P) H | nicotine adenine dinucleotide phosphate |
References
- Hüls, A.; Vierkötter, A.; Sugiri, D.; Abramson, M.J.; Ranft, U.; Krämer, U.; Schikowski, T. The role of air pollution and lung function in cognitive impairment. Eur. Respir. J. 2018, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Patil, G.; Khan, M.I.; Patel, D.K.; Sultana, S.; Prasad, R.; Ahmad, L. Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic responses of micro- and nano-particles of dolomite on human lung epithelial cells A549. Environ. Toxicol. Phar. 2012, 34, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.X.; Bai, C.; Song, Y. Particulate matter-induced epigenetic changes and lung cancer. Clin. Respir. J. 2017, 11, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Shie, R.H.; Chan, C.C.; Lin, H.H. Burden of disease attributable to ambient fine particulate matter exposure in Taiwan. J. Formo. Med. Assoc. 2017, 116, 32–40. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases (Review). Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, J. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- MacNee, W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 50–60. [Google Scholar] [CrossRef]
- Rezaeetalab, F.; Hamidi, A.D.; Dalili, A. Oxidative stress in COPD, pathogenesis and therapeutic views. Rev. Clin. Med. 2014, 1, 115–124. [Google Scholar] [CrossRef]
- Kwon, B.E.; Song, J.H.; Song, H.H. Antiviral Activity of Oroxylin A against Coxsackievirus B3 Alleviates Virus-Induced Acute Pancreatic Damage in Mice. PLoS ONE 2016, 11, e0155784. [Google Scholar] [CrossRef]
- Lu, Y.; Joerger, R.; Wu, C. Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2011, 59, 10934–10942. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kim, M.H.; Gwak, N.G. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J. Ethnopharmacol. 2012, 141, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Li, W.F.; Li, W.W.; Ren, K.H.; Fan, C.M.; Chen, Y.Y.; Shen, Y.L. Protective effects of the aqueous extract of Scutellaria baicalensis against acrolein-induced oxidative stress in cultured human umbilical vein endothelial cells. Pharm. Biol. 2011, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Shin, Y.C.; Park, J.S.; Park, S.; Park, J.S.; Kim, N.; Um, J.Y.; Go, H.; Sun, S.; Lee, S.; et al. Ethanol extract of Scutellaria baicalensis Georgi prevents oxidative damage and neuroinflammation and memorial impairments in artificial senescense mice. J. Biomed. Sci. 2011, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Liu, P.F.; Han, F.G.; Duan, B.B.; Deng, T.S.; Hou, X.L.; Zhao, M.Q. Purification and antioxidant activities of baicalin isolated from the root of huangqin (Scutellaria baicalensis gcorsi). J. Food Sci. Technol. 2013, 50, 615–619. [Google Scholar] [CrossRef]
- Huang, W.H.; Chien, P.Y.; Yang, C.H.; Lee, A.R. Novel Synthesis of Flavonoids of Scutellaria baicalensis GEORGI. Chem. Pharm. Bull. 2003, 51, 339–340. [Google Scholar] [CrossRef]
- Trang, D.H.T.; Son, H.L.; van Trung, P. Investigation on the in vitro antioxidant capacity of methanol extract, fractions and flavones from Oroxylum indicum Linn bark. Braz. J. Pharm. Sci. 2018, 54, e17178. [Google Scholar] [CrossRef]
- Ozen, G.; Gummustas, M.; Satanakara, H.E.; Gunden Goger, N.; Uslu, B.; Ozakan, S.A. Quantitative determination of isoconazole nitrate and diflucortolone valerate in pharmaceutical creams by UPLC, HPLC and improved derivative uv spectrophotometry. Rev. Roum. Chim. 2015, 60, 527–535. [Google Scholar]
- Raghavan, S.; Kristinsson, H.G.; Leeuwenburgh, C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J. Agric. Food Chem. 2008, 56, 10359–10367. [Google Scholar] [CrossRef]
- Montiel, T.; Quiroz-Baez, R.; Massieu, L.; Arias, C. Role of oxidative stress on beta-amyloid neurotoxicity elicited during impairment of energy metabolism in the hippocampus: Protection by antioxidants. Exp. Neurol. 2006, 200, 496–508. [Google Scholar] [CrossRef]
- Tong, L.; Wan, M.; Zhang, L.; Zhu, Y.; Sun, H.; Bia, K. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 70, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Jiang, Y.; Chen, F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; pp. 77–152. [Google Scholar]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar] [PubMed]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar] [PubMed]
- Sekiya, K.; Okuda, H. Selective inhibition of platelet lipoxygenase by baicalein. Biosci. Biotec. Biochem. 1982, 105, 1090–1095. [Google Scholar] [CrossRef]
- Woo, K.J.; Lim, J.H.; Suh, S.I.; Kwon, Y.K.; Shin, S.W.; Kim, S.C.; Choi, Y.H.; Park, J.W.; Kwon, T.K. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPβ DNA-binding activity. Immunobiology 2006, 211, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ueng, Y.F.; Shyu, C.C.; Lin, Y.L.; Park, S.S.; Liao, J.F.; Chen, C.F. Effects of baicalein and wogonin on drug-metabolizing enzymes in C57BL/6J mice. Life Sci. 2000, 67, 2189–2200. [Google Scholar] [CrossRef]
- Boušová, I.; Hájek, J.; Dršata, J.; Skálová, L. Naturally occurring flavonoids as inhibitors of purified cytosolic glutathione S-transferase. Xenobiotica 2012, 42, 872–879. [Google Scholar] [CrossRef]
- Iio, M.; Kawaguchi, H.; Sakota, Y.; Otonari, J.; Nitahara, H. Effects of polyphenols, including flavonoids, on glutathione S-transferase and glutathione reductase. Biosci. Biotec. Biochem. 1993, 57, 1678–1680. [Google Scholar] [CrossRef]
- Farley, K.S.; Wang, L.; Mehta, S. Septic pulmonary microvascular endothelial cell injury: Role of alveolar macrophage NADPH oxidase. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kornbrust, D.J.; Mavis, R.D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Li, R.R.; Liu, Z.J.; Chen, Z.F.; Ouyang, Y.; Hu, Y.J. Structure-activity relationship study between baicalein and wogonin by spectrometry, molecular docking and microcalorimetry. Food Chem. 2016, 208, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huang, C.C.; Chang, H.Y.; Li, P.Y.; Liang, Y.C.; Deng, J.S.; Huang, S.S.; Huang, G.J. Scutellaria baicalensis Ameliorates Acute Lung Injury by Suppressing Inflammation In Vitro and In Vivo. Am. J. Chin. Med. 2017, 45, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.J.; Zhu, H.Y.; Zhang, Y.Y.; Lu, Y.; Li, H.; Chen, D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Choi, J.; Jung, H.K.; Kim, B.; Kim, C.; Park, S.Y.; Seol, J.W. Baicalein inhibits tumor progression by inhibiting tumor cell growth and tumor angiogenesis. Oncol. Rep. 2017, 38, 3011–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiartivich, S.; Wei, Y.; Liu, J.; Soiampornkul, R.; Li, M.; Zhang, H.; Dong, J. Regulation of cytotoxicity and apoptosis-associated pathways contributes to the enhancement of efficacy of cisplatin by baicalein adjuvant in human A549 lung cancer cells. Oncol. Lett. 2017, 13, 2799–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total Antioxidant Capacity of Teas by the Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, J. Flavonoids are scavengers of superoxide anions. J. Biochem. Pharmacol. 1998, 37, 83–88. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Sagisaka, S. Abrupt Increase in the Level of Hydrogen Peroxide in Leaves of Winter Wheat Is Caused by Cold Treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Archives. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Forr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J. Lipid Res. 1978, 19, 1053–1057. [Google Scholar]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O., Jr.; Leach, C.N., Jr.; Sunderman, F.W. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1978, 33, 214–220. [Google Scholar]
Sample Availability: Not available. |
| In vitro Antioxidant Activity | SBR Extracts of Different Solvent | |||
|---|---|---|---|---|
| Water | EtOH | Acetone | EtOAc | |
| Total phenol content (µg/mg dry weight) | 160.29 ± 6.82 | 252.81 ± 3.27 | 284.98 ± 14.93 | 518.80 ± 34.23 |
| Total flavonoid content (µg/mg dry weight) | 884.92 ± 5.72 | 1076.46 ± 13.31 | 999.21 ± 13.84 | 1903.44 ± 69.41 |
| DPPH scavenging activity (IC50, µg/mL) | 18.17 ± 0.79 | 24.89 ± 0.33 | 24.31 ± 0.72 | 13.08 ± 0.44 |
| Total antioxidant power (TEAC µM on 50 µg/mL) | 16.87 ± 0.68 | 10.37 ± 0.24 | 19.31 ± 0.44 | 23.44 ± 0.83 |
| O2•− scavenging activity (IC50, µg/mL) | 81.78 ± 5.33 | 138.23 ± 12.22 | 95.46 ± 5.06 | 66.05 ± 5.11 |
| −OH scavenging activity (IC50, µg/mL)) | 2.92 ± 0.03 | 2.77 ± 0.07 | 3.74 ± 0.03 | 3.11 ± 0.03 |
| H2O2 scavenging activity (rate% on 1 mg/mL) | 48.73 ± 2.57 | 31.00 ± 1.17 | 41.73 ± 3.34 | 47.00 ± 3.50 |
| Fe2+ chelating activity (rate% on 1 mg/mL) | 78.75 ± 0.73 | 44.35 ± 2.14 | 13.10 ± 0.91 | 1.92 ± 2.90 |
| SBR Extracts | Inhibit Rate (%) (200 µg /mL) | IC50 (µg /mL) | Baicalein Content (µg /mL) |
|---|---|---|---|
| Water | 52.50 | 24.00 ± 3.87 | 13.31 ± 0.96 |
| Ethanol | 59.17 | 24.71 ± 4.02 | 62.29 ± 1.96 |
| Acetone | 55.00 | 2.95 ± 5.28 | 100.77 ± 1.71 |
| Ethyl acetate | 72.50 | 1.71 ± 5.26 | 248.05 ± 32.11 |
| Positive control | |||
| Trolox | 76.67 | 20.12 ± 1.44 | - |
| Compounds | IC50 (µg /mL) |
|---|---|
| Baicalin | 6.84 ± 0.11 |
| Baicalein | 0.20 ± 0.04 |
| Wogonin | 3.30 ± 0.08 |
| Oroxylin A | 105.82 ± 0.80 |
| Positive control | |
| Trolox | 20.12 ± 1.44 |
| In vitro Antioxidant Activity | Pure Compound Isolated from SBR | |||
|---|---|---|---|---|
| Baicalin | Baicalein | Wogonin | Oroxylin A | |
| DPPH scavenging activity (IC50, µg/mL)) | 6.93±0.08 | 2.80 ± 0.05 | >100 | >100 |
| Antioxidant power ability (TEAC µM on 30 µg/mL) | 19.49±0.57 | 10.18 ± 0.30 | 0.43 ± 0.09 | 0.28 ± 0.03 |
| O2•− scavenging activity (IC50, µg/mL) | >100 | 43.99 ± 1.66 | >100 | >100 |
| −OH scavenging activity (IC50, µg/mL)) | 0.69 ± 0.02 | >20 | >20 | >20 |
| H2O2 scavenging activity (rate% on 100 µg/mL) | 14.67 ± 1.39 | 11.69 ± 1.29 | 0 | 0 |
| Fe2+ chelating activity (rate% on 100 µg /mL) | 20.07 ± 1.07 | 2.38 ± 0.69 | 2.32 ± 0.71 | 1.32 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liau, P.R.; Wu, M.S.; Lee, C.K. Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules 2019, 24, 2143. https://doi.org/10.3390/molecules24112143
Liau PR, Wu MS, Lee CK. Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules. 2019; 24(11):2143. https://doi.org/10.3390/molecules24112143
Chicago/Turabian StyleLiau, Pei Ru, Ming Shun Wu, and Ching Kuo Lee. 2019. "Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities" Molecules 24, no. 11: 2143. https://doi.org/10.3390/molecules24112143
APA StyleLiau, P. R., Wu, M. S., & Lee, C. K. (2019). Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules, 24(11), 2143. https://doi.org/10.3390/molecules24112143





