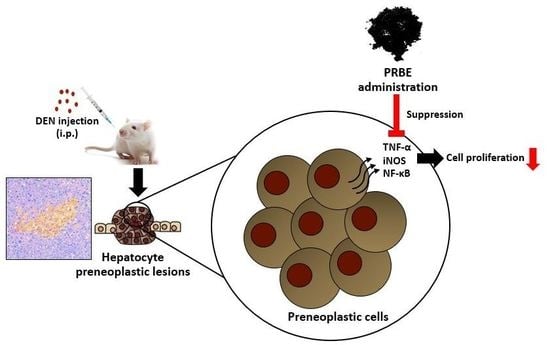
Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemical Constituents in Defatted Rice Bran Extracts
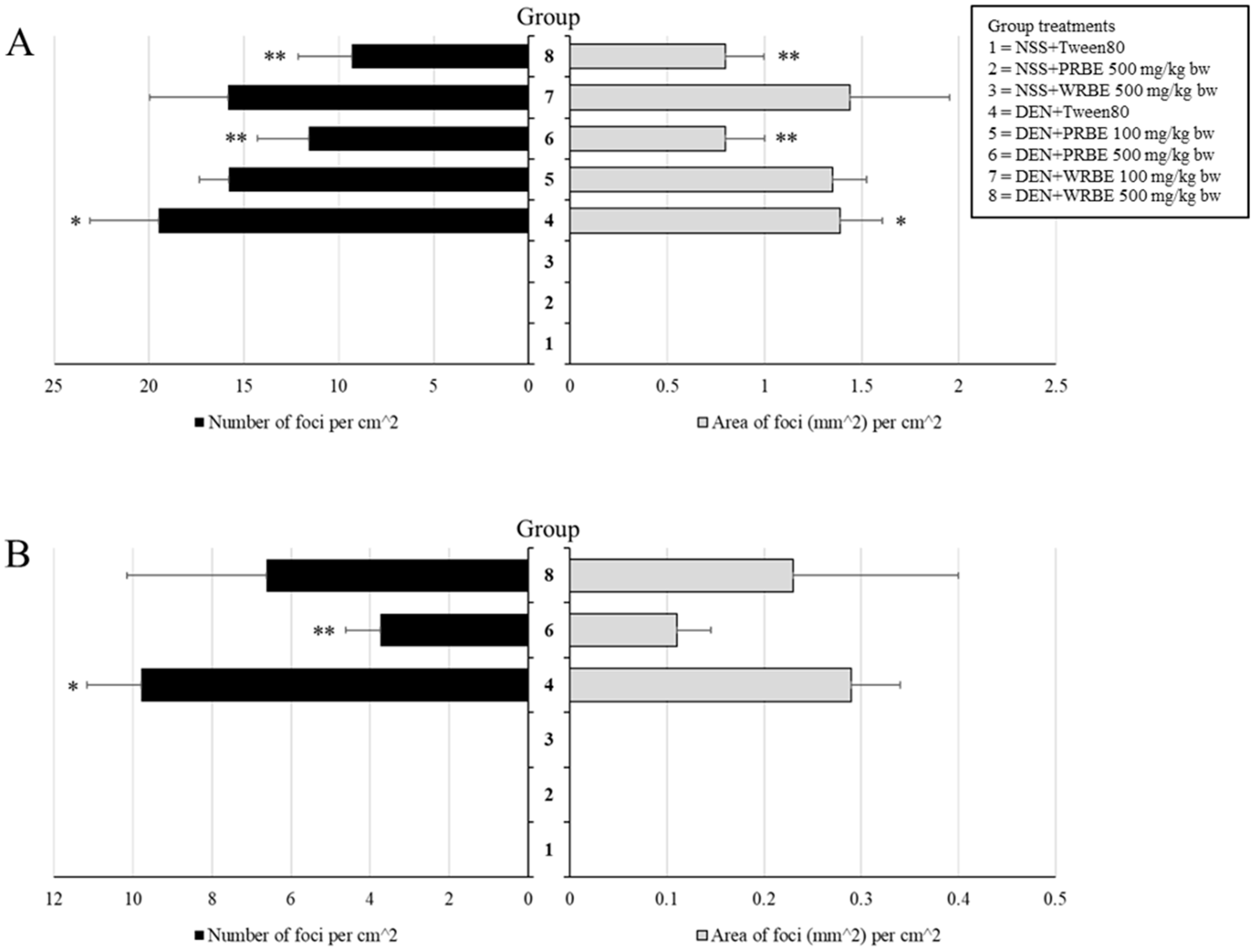
2.2. Effect of 15-Week Administration of Rice Bran Extracts on Promotion Stage of Hepatocarcinogenesis
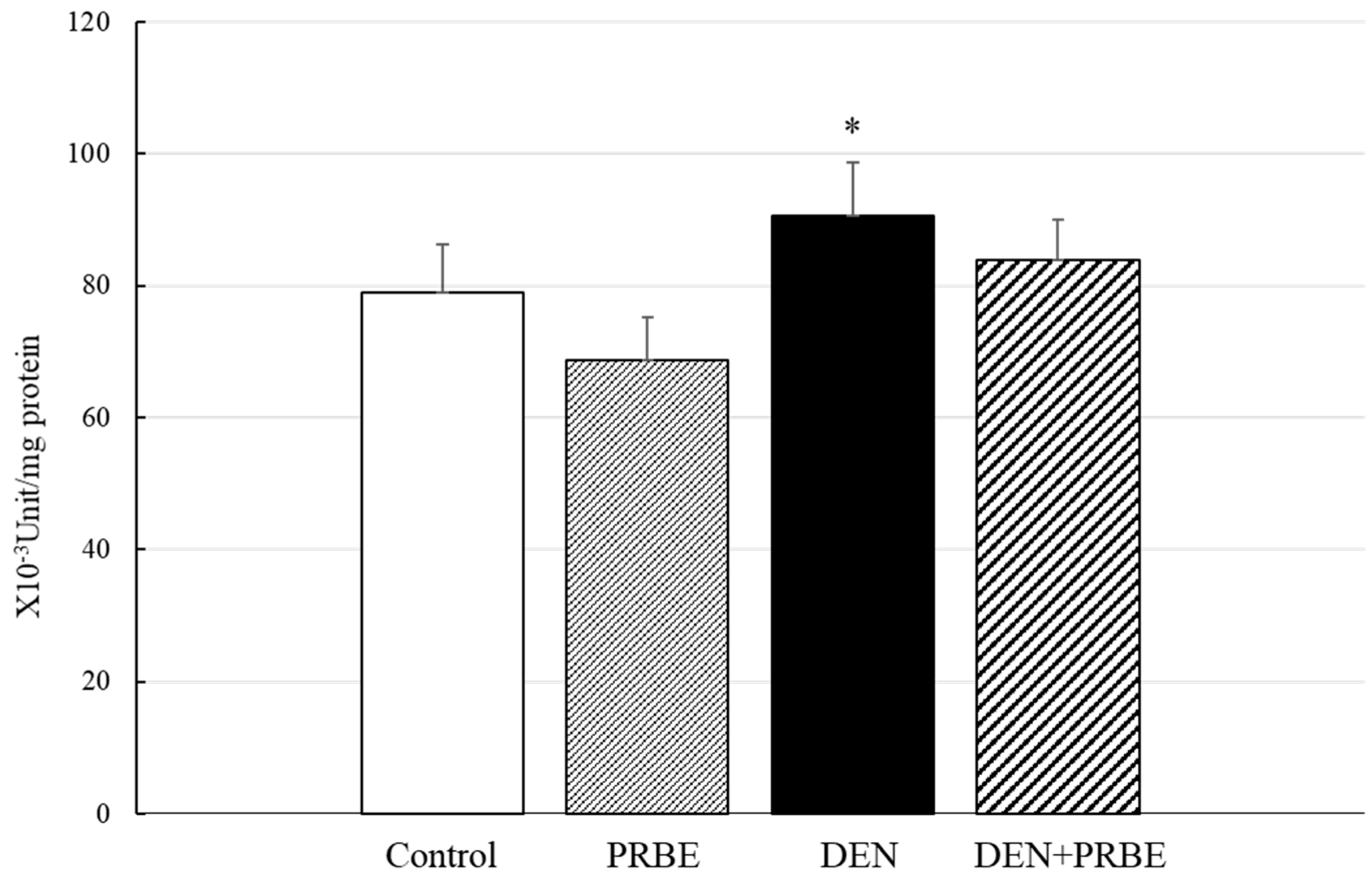
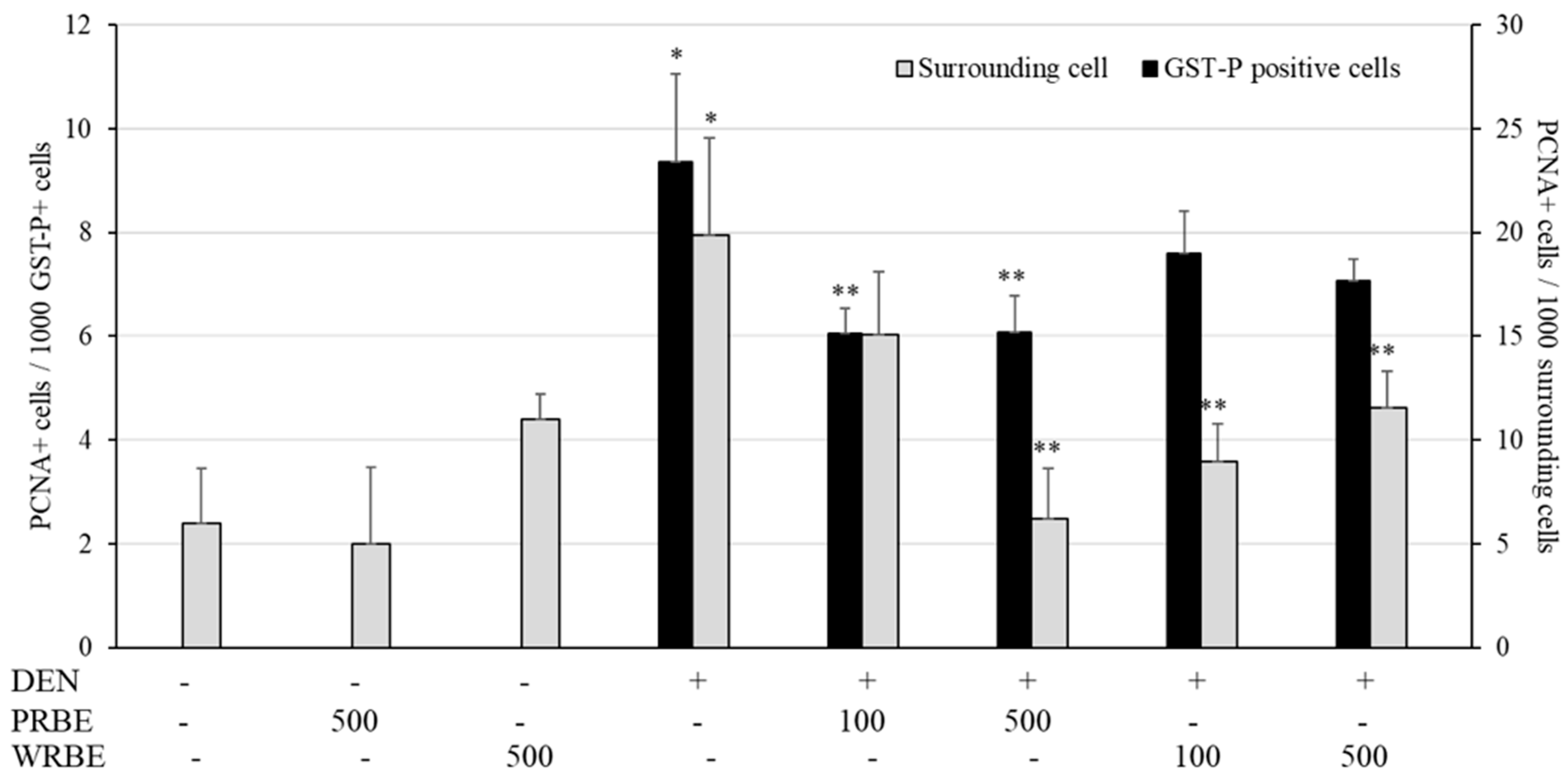
2.3. Effect of Five-Week Administration of Rice Bran Extracts on Initiation Stage of Hepatocarcinogenesis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Methanol Extract from Sticky Rice Bran
4.3. Determination of Chemical Constituents in Sticky Rice Brans Extracts
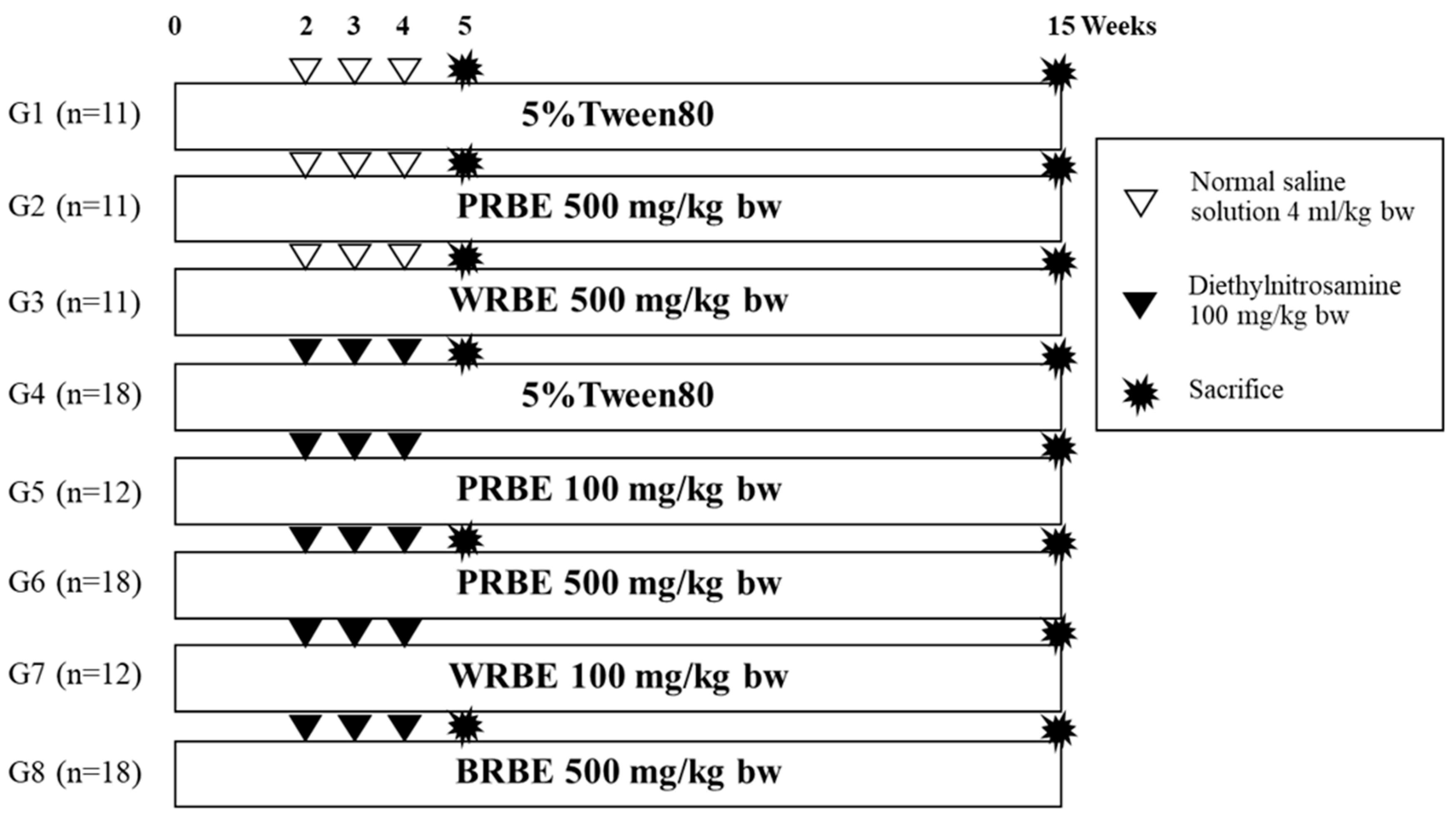
4.4. Anticarcinogenicity of Methanol Extracts of Sticky Rice Bran on DEN-treated Rats
4.5. Evaluation of Glutathione S-Transferase Placental form Positive Foci in Liver Tissues
4.6. Determination of Cell Proliferation in Liver Tissues by Double Staining Immunohistochemistry
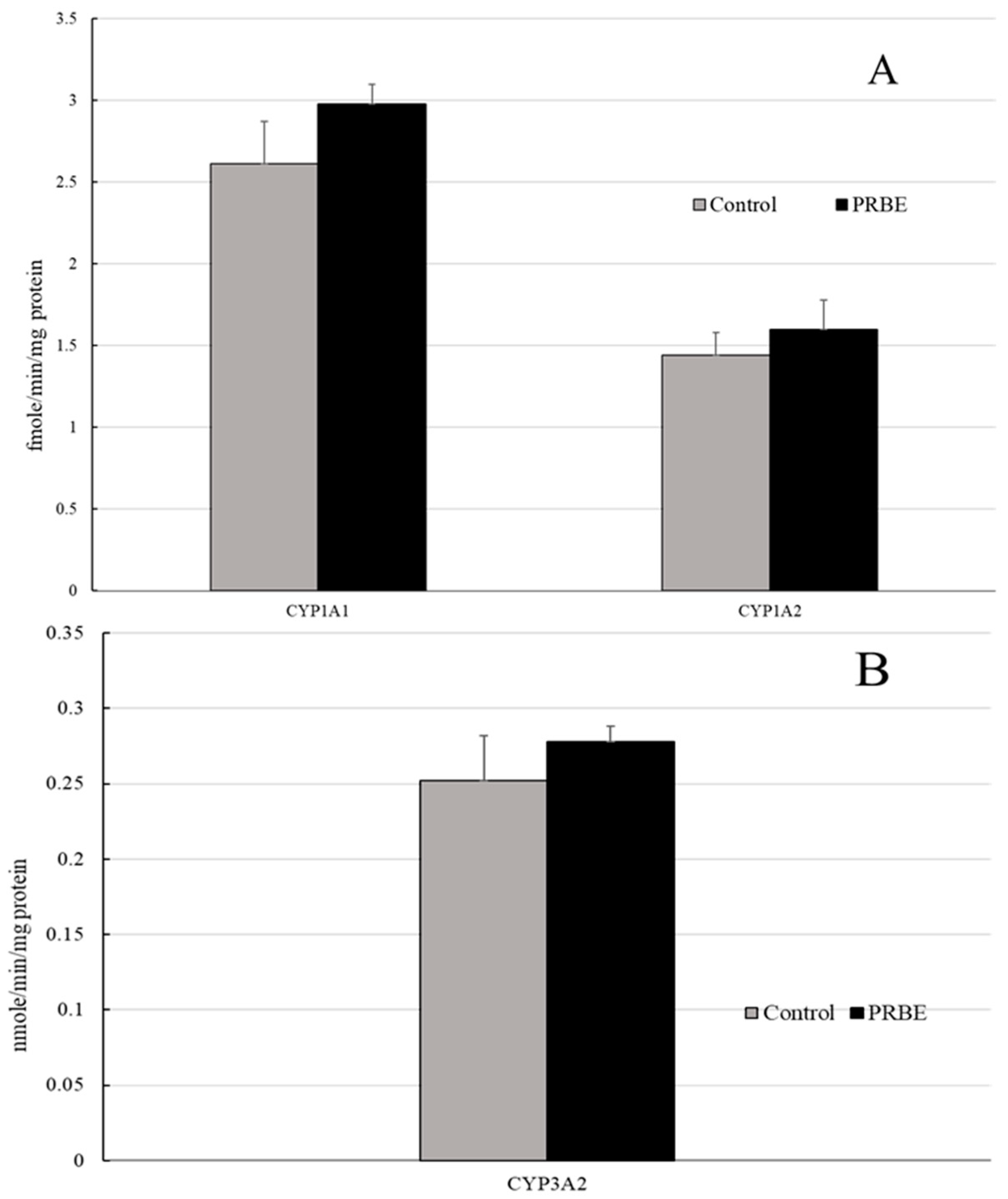
4.7. Determination of Phases I and II Xenobiotic Metabolizing Enzyme Activities
4.8. Determination of Pro-Inflammatory Cytokine Gene Expression by Real-time PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, 35–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibited cancer cell metastasis involving the down-regulation of Ras/Akt/NF-kB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, J.P. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.I.; Song, G.S.; Youn, Y.; Kim, Y.S. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J. Food Sci. 2012, 77, 759–764. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Kozukue, N.; Friedman, M. Antioxidative, antimutagenic and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J. Agric. Food Chem. 2005, 53, 816–822. [Google Scholar] [CrossRef]
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J. Agric. Food Chem. 2003, 51, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai Northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar] [CrossRef] [PubMed]
- Suwannakul, N.; Punvittayagul, C.; Jarukamjorn, K.; Wongpoomchai, R. Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pac. J. Cancer Prev. 2015, 16, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 1163–1170. [Google Scholar] [PubMed]
- Schiffer, E.; Housset, C.; Cacheux, W.; Wendum, D.; Desbois-Mouthon, C.; Rey, C.; Clergue, F.; Poupon, R.; Barbu, V.; Rosmorduc, O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 2005, 41, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Matsuda, T.; Otoshi, T.; Yano, Y.; Otani, S.; Hasegawa, T.; Nakae, D.; Konishi, Y.; Fukushima, S. Enhancement by organosulfur compounds from garlic and onions of diethylnitrosamine-induced glutathione S-transferase positive foci in the rat liver. Cancer Res. 1994, 54, 2895–2899. [Google Scholar] [PubMed]
- Nikolaou, K.; Sarris, M.; Talianidis, I. Molecular pathways: The complex roles of inflammation pathways in the development and treatment of liver cancer. Clin. Cancer Res. 2013, 19, 2810–2816. [Google Scholar] [CrossRef]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB pathway and cancer stem cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef]
- Roderburg, C.; Gautheron, J.; Luedde, T. TNF-dependent signaling pathways in liver cancer: Promising targets for therapeutic strategies. J. Dig. Dis. 2012, 30, 500–507. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef]
- Calvisi, F.D.; Pinna, F.; Ladu, S.; Pellegrino, R.; Muroni, M.R.; Simile, M.M.; Frau, M.M.; Tomasi, M.L.; De Miglio, M.R.; Seddaiu, M.A.; et al. Aberrant iNOS signaling is under genetic control in rodent liver cancer and potentially prognostic for the human disease. Carcinogenesis 2008, 29, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Taya, S.; Punvittayagul, C.; Inboot, W.; Fukushima, S.; Wongpoomchai, R. Cleistocalyx nervosum extract ameliorates chemical-Induced oxidative stress in early stages of rat hepatocarcinogenesis. Asian Pac. J. Cancer Prev. 2014, 15, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, R.J.; Bhatia, D.; Barnes, K.F.; Háznagy-Radnai, E.; Hohmann, J.; Darvesh, A.S.; Bishayee, A. Black currant anthocyanins abrogate oxidative stress through Nrf2-mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr. Cancer Drug Targets 2012, 12, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008, 28, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, D.; Wang, L.S.; Bei, D.; Wang, J.; See, W.A.; Mallery, S.R.; Stoner, G.D.; Liu, Z. Pharmacokinetics of protocatechuic acid in mouse and its quantification in human plasma using LC–tandem mass spectrometry. J. Chromatogr. B 2012, 908, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ayano, I.; Katsuhiro, I.; Masuo, K.; Masaya, K.; Masakuzu, K.; Makoto, T.; Kiyohito, Y. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin A-induced liver injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef]
- Nilnumkhum, A.; Punvittayagul, C.; Chariyakornkul, A.; Wongpoomchai, R. Effects of hydrophilic compounds in purple rice husk on AFB1-induced mutagenesis. Mol. Cell Toxicol. 2017, 13, 171–178. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential cancer chemoprevention activity of protocatechuic acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, R.; Gupta, S.C.; Kim, J.H.; Aggarwal, B.B. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012, 7, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.; Arnold, O.M. Grapefruit–medication interactions: Forbidden fruit or avoidable consequences. Can. Med. Assoc. J. 2013, 185, 309–316. [Google Scholar] [CrossRef]
- Ma, Y.T.; Cheung, P.C. Spectrophotometric determination of phenolic compounds by enzymatic and chemical methods—A comparison of structure-activity relationship. J. Agric. Food Chem. 2007, 55, 4222–4228. [Google Scholar] [CrossRef]
- Maksimovic, Z.; Malencic, D.; Kovacevic, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, C.; Saghai Maroof, M.A.; Biyashev, R.M.; Grabau, E.A.; Kwanyuen, P.; Burton, J.W.; Buss, G.R. A modified colorimetric method for phytic acid analysis in soybean. Crop. Sci. 2007, 47, 1797–1803. [Google Scholar] [CrossRef]
- Inácio, M.R.; De Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; De Almeida Teixeira, G.S. Total anthocyanin content determination in intact açaí (Euterpe oleracea Mart.) and palmitero-juçara (Euterpe edulis Mart.) fruit using near infrared spectroscopy (NIR) and multivariate calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef]
- Insuan, O.; Chariyakornkul, A.; Rungrote, Y.; Wongpoomchai, R. Antimutagenic and antioxidant activities of Thai rice brans. J. Cancer Prev. 2017, 22, 89–97. [Google Scholar] [CrossRef]
- Thumvijit, T.; Taya, S.; Punvittayagul, C.; Peerapornpisai, Y.; Wongpoomchai, R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kützing on diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian Pac. J. Cancer Prev. 2014, 15, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Sankam, P.; Punvittayagul, C.; Sringam, K.; Chaiyasut, C.; Wongpoomchai, R. Antimutagenicity and anticlastogenicity of glutinous purple rice hull using in vitro and in vivo testing systems. Mol. Cell Toxicol. 2013, 9, 169–176. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Wang, S.; Guo, H.; Dong, S.; Wang, T.; Zhang, L.; Jiang, Z. Emodin ameliorates hepatic steatosis through endoplasmic reticulum–stress sterol regulatory element-binding protein 1c pathway in liquid fructose-feeding rats. Hepatol. Res. 2016, 46, 105–117. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Compounds (Per Gram Extract) | Purple Rice Bran | White Rice Bran |
|---|---|---|
| Spectrophotometry analysis | ||
| Total phenolic compounds (mg) | 74.2 ± 3.6 * | 16.5 ± 1.0 |
| Total flavonoids (mg) | 50.7 ± 1.4 * | 12.7 ± 1.8 |
| Total anthocyanins (μg) | 29.7 ± 0.2 * | ND |
| Total phytic acids (mg) | 9.71 ± 2.4 | 7.39 ± 1.9 |
| HPLC method | ||
| Total γ-oryzanol (mg) | 3.71 ± 0.02 | 5.05 ± 0.04 * |
| Total vitamin E (μg) | 321.4 ± 9.3 * | 274.7 ± 4.4 |
| α-tocopherol (μg) | 24.5 ± 0.3 | 13.6 ± 0.1 |
| β-tocopherol (μg) | ND | 26.1 ± 0.3 |
| γ-tocopherol (μg) | 44.1 ± 1.9 | 39.4 ± 0.3 |
| δ-tocopherol (μg) | 5.6 ± 0.1 | ND |
| α-tocotrienol (μg) | 14.1 ± 0.1 | ND |
| γ-tocotrienol (μg) | 202.6 ± 6.9 | 167.3 ± 5.1 |
| δ-tocotrienol (μg) | 30.5 ± 0.4 | 28.0 ± 0.2 |
| p-Coumaric acid (mg) | 0.35 ± 0.00 | 0.84 ± 0.02 * |
| Protocatechuic acid (mg) | 7.12 ± 0.03 * | ND |
| Vanillic acid (mg) | 4.73 ± 0.02 * | ND |
| 4-Hydroxybenzoic acid (mg) | ND | 0.35 ± 0.01 * |
| Cyanidin-3-glucoside (μg) | 5.7 ± 0.0 * | ND |
| Peonidin-3-glucoside (μg) | 3.6 ± 0.0 * | ND |
| Group | Chemical | Treatment | Gene Expression Relative to β-Actin (Fold Change) | |||
|---|---|---|---|---|---|---|
| TNF-α | IL-1β | iNOS | NF-κB | |||
| 1 | NSS | 5% Tween80 | 1.00 ± 0.20 | 1.00 ± 0.29 | 1.00 ± 0.40 | 0.99 ± 0.27 |
| 2 | NSS | PRBE 500 mg/kg bw | 1.00 ± 0.17 | 0.98 ± 0.15 | 0.79 ± 0.44 | 0.99 ± 0.11 |
| 3 | DEN | 5% Tween80 | 2.31 ± 0.55 * | 3.12 ± 1.10 * | 7.73 ± 4.56 * | 2.10 ± 0.99 * |
| 4 | DEN | PRBE 500 mg/kg bw | 1.76 ± 0.45 ** | 2.60 ± 0.78 | 2.23 ± 1.15 ** | 0.89 ± 0.13 ** |
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | 5′-AAATGGCCCTCTCATCAGTCC-3′ | 5′-TCTGCTTGGTGGTTTGCTACGAC-3′ |
| IL-1β | 5′-CACCTCTCAAGCAGAGCACAG-3′ | 5′-GGGTTCCATGGTGAAGTCAAC-3′ |
| iNOS | 5′-CAGGTGCTATTCCCAGCCCAACA-3′ | 5′-CATTCTGTGCAGTCCCAGTGAGGAA-3′ |
| NF-κB | 5′-GGCATGCGTTTCCGTTACAA-3′ | 5′-TGATCTTGATGGTGGGGTGC-3′ |
| β-actin | 5′-ACAGGATGCAGAAGGAGATTAC-3′ | 5′-AGAGTGAGGCCAGGATAGA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul, P.; Wongpoomchai, R. Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats. Molecules 2019, 24, 2142. https://doi.org/10.3390/molecules24112142
Dokkaew A, Punvittayagul C, Insuan O, Limtrakul P, Wongpoomchai R. Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats. Molecules. 2019; 24(11):2142. https://doi.org/10.3390/molecules24112142
Chicago/Turabian StyleDokkaew, Aphisit, Charatda Punvittayagul, Orapin Insuan, Pornngarm Limtrakul (Dejkriengkraikul), and Rawiwan Wongpoomchai. 2019. "Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats" Molecules 24, no. 11: 2142. https://doi.org/10.3390/molecules24112142
APA StyleDokkaew, A., Punvittayagul, C., Insuan, O., Limtrakul, P., & Wongpoomchai, R. (2019). Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats. Molecules, 24(11), 2142. https://doi.org/10.3390/molecules24112142