Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya
Abstract
:1. Introduction
2. Results and Discussion
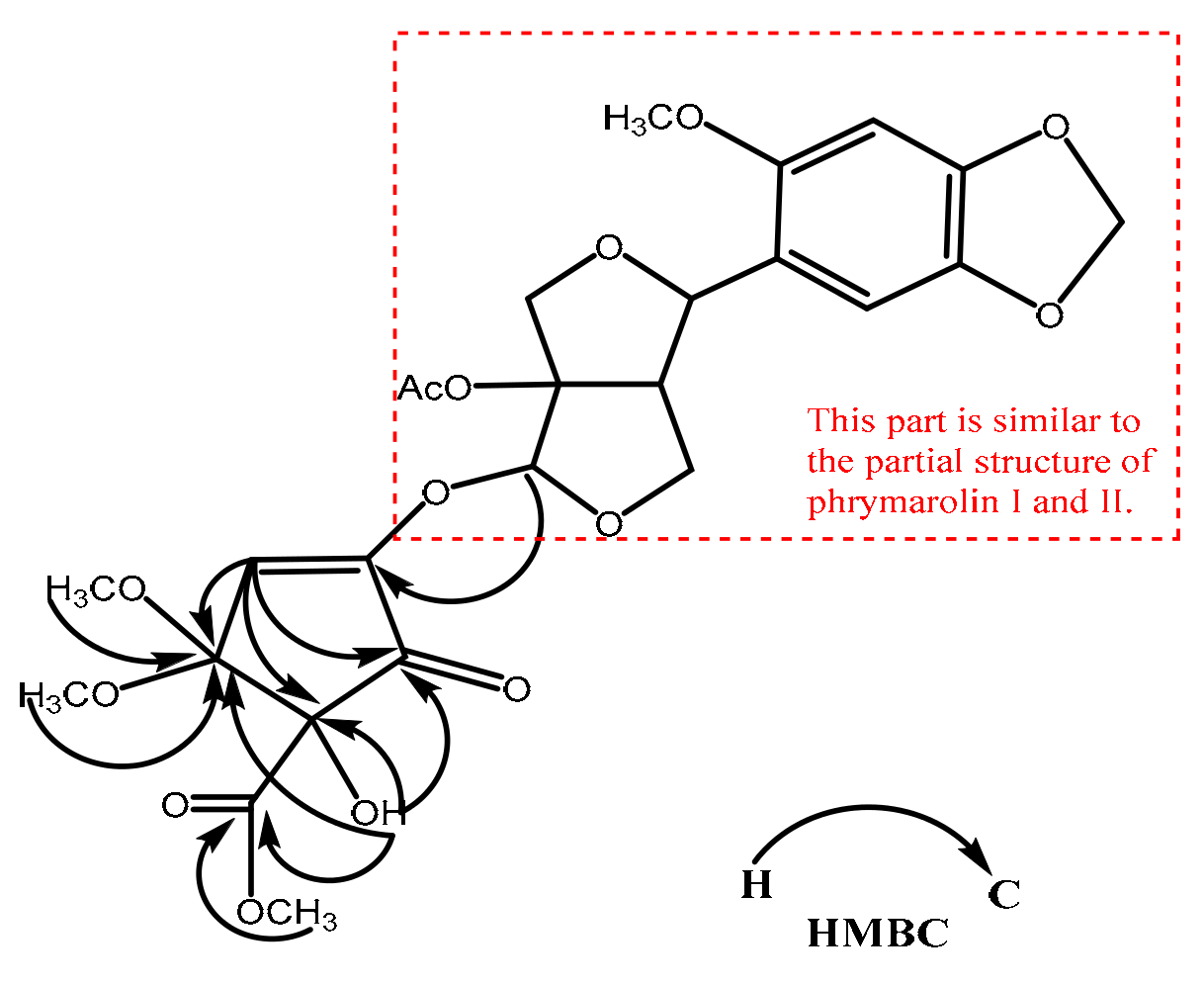
2.1. Structural Elucidation
2.2. Insecticidal Activity
3. Materials and Methods
3.1. Instruments
3.2. Plant Materials
3.3. Extraction and Purification
3.4. Bioassay of Insecticidal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.M.; Min, B.S.; Kho, Y.H. Brine shrimp lethality of the compounds from Phryma leptostachya L. Arch. Pharm. Res. 2002, 25, 652–654. [Google Scholar] [CrossRef]
- Endo, Y.; Miyauchi, T. Thermonasty of young main stems of Phryma leptostachya (Phrymaceae). J. Plant Res. 2006, 119, 449–457. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C.; Kim, C.S.; Lee, H.J.; Choi, W.S.; Ahn, Y.J. Larvicidal activity of lignans identified in Phryma leptostachya Var. asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar]
- Jung, H.J.; Cho, Y.W.; Lim, H.W.; Choi, H.; Ji, D.J.; Lim, C.J. Anti-inflammatory, antioxidant, anti-angiogenic and skin whitening activities of Phryma leptostachya var. asiatica Hara extract. Biomol. Ther. 2013, 21, 72–78. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, E. Synthesis and absolute configuration of the insecticidal sesquilignan (+)-haedoxan A. Phytochemistry 1998, 2, 613–622. [Google Scholar] [CrossRef]
- Taniguchi, E.; Oshima, Y. Phrymarolin-I, a novel lignan from Phryma leptostachya L. Agric. Biol. Chem. 1972, 36, 1013–1025. [Google Scholar] [CrossRef]
- Taniguchi, E.; Oshima, Y. Structure of phrymarolin-II. Agric. Biol. Chem. 1972, 36, 1489–1496. [Google Scholar] [CrossRef]
- Xiao, X.M.; Hu, Z.N.; Ji, Z.Q.; Shi, B.J.; Zhang, J.W.; Wei, S.P.; Wu, W.J. Isolation, structure identification and bioactivity of active ingredients from Phryma leptostachya. Chin. J. Pestic. Sci. 2012, 14, 583–586. [Google Scholar]
- Chen, C.M.; Zhu, H.C.; Zhao, D.; Deng, J. Lignans from Phryma leptostachya L. Helv. Chim. Acta 2012, 95, 333–338. [Google Scholar] [CrossRef]
- Xiao, X.M.; Hu, Z.N.; Shi, B.J.; Wei, S.P.; Wu, W.J. Larvicidal activity of lignans from Phryma leptostachya L. against Culex pipiens pallens. Parasitol. Res. 2012, 110, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.M.; Ji, Z.Q.; Zhang, J.W.; Shi, B.J.; Wei, S.P.; Wu, W.J. A new lignan from Phryma leptostachya. Chem. Nat. Compd. 2013, 49, 21–23. [Google Scholar] [CrossRef]
- Xiao, X.M.; Hu, Z.N.; Ji, Z.Q.; Wei, S.P.; Lv, M.; Shi, B.J.; Wu, W.J. Ultrastructure and Na+-K+-ATPase, Ca2+-ATPase activities on parietal muscle of Mythimna separata larvae treated by haedoxane E. Chin. J. Pestic. Sci. 2017, 19, 512–517. [Google Scholar]
- Seo, S.M.; Park, I.K. Larvicidal activity of medicinal plant extracts and lignan identified in Phryma leptostachya var. asiatica roots against housefly (Musca domestica L.). Parasitol. Res. 2012, 110, 1849–1853. [Google Scholar] [PubMed]
- Zhang, J.W.; Hu, Z.; Gao, P.; Wang, J.R.; Hu, Z.N.; Wu, W.J. Synthesis and larvicidal activity against Culex pipiens pallens of new triazole derivatives of phrymarolin from Phryma leptostachya L. Int. J. Mol. Sci. 2013, 14, 24064–24073. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.N.; Du, Y.Z.; Xiao, X.M.; Dong, K.; Wu, W.J. Insight into the mode of action of haedoxan A from Phryma leptostachya. Toxins 2016, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.; Imamura, K.; Ishibashi, F.; Matsui, T.; Nishio, A. Structure of the novel insecticidal sesquilignan, haedoxan A. Agric. Biol. Chem. 1989, 53, 631–643. [Google Scholar]
- Nakamura, Y.; Hirata, M.; Kuwano, E.; Taniguchi, E. Enantioselective synthesis of a (+)-(2R, 3R)-1,4-benzodioxane-7-carbaldehyde derivative, a key intermediate in the total synthesis of haedoxan analogs. Biosci. Biotechnol. Biochem. 1998, 62, 1550–1554. [Google Scholar] [CrossRef]
- Okazaki, M.; Ishibashi, F.; Shuto, Y.; Taniguchi, E. Total synthesis of (+)-phrymarolon I from (+)-malic acid. Biosci. Biotechnol. Biochem. 1997, 61, 660–663. [Google Scholar] [CrossRef]
- Hirata, M.; Taniguchi, E. Synthesis of (+)-(2s, 3s)-benzodioxane. J. Fac. Agric. Kyushu Univ. 1997, 42, 101–112. [Google Scholar]
- Hirata, M.; Taniguchi, E. A new lignan having a chromene moiety: synthesis of haedoxan analogue with precocene I skeleton. J. Fac. Agric. Kyushu Univ. 1996, 41, 113–124. [Google Scholar]
- Velasques, J.; Cardoso, M.H.; Abrantes, G.; Frihling, B.E.; Franco, O.L.; Migliolo, L. The rescue of botanical insecticides: A bioinspiration for new niches and needs. Pest. Biochem. Physiol. 2017, 143, 14–25. [Google Scholar] [CrossRef]
- Li, Y.K.; Aioub, A.; Lv, B.; Hu, Z.N.; Wu, W.J. Antifungal activity of pregnane glycosides isolated from Periploca sepium root barks against various phytopathogenic fungi. Ind. Crop. Prod. 2019, 132, 150–155. [Google Scholar] [CrossRef]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.L.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Ind. Crop. Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Chen, N.C. Pesticide Bioassay Technology; China Agricultutural University Press: Beijing, China, 1991; pp. 68–71. [Google Scholar]
- Hu, M.Y.; Klocke, J.A.; Chiu, S.F.; Kubo, I. Response of five insect species to botanical insecticide, Rhodojaponin III. J. Econ. Entomol. 1993, 86, 706–711. [Google Scholar]
- Feng, Y.T.; Wu, Q.J.; Wang, S.L.; Chang, X.L.; Xie, W.; Xu, B.Y.; Zhang, Y.J. Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in B-biotype Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Nagata, S.; Taniguchi, E. Effect on insecticidal activity of substituents at the 1,4-benzodioxanyl moiety of haedoxan. Biosci. Biotechnol. Biochem. 1992, 56, 1193–1197. [Google Scholar] [CrossRef]
- Yamauchi, S.; Taniguchi, E. Influence on insecticidal activity of the 3-(3,4-methylenedioxyphenyl) group in the 1,4-benzodioxanyl moiety of haedoxans. Biosci. Biotechnol. Biothem. 1992, 56, 1744–1750. [Google Scholar] [CrossRef]
- Yamauchi, S.; Taniguchi, E. Synthesis and insecticidal activity of sesquilignan analogs with 2-alkyl-6-methoxy-3-(3,4-methylenedioxyphenyl)-1,4-benzodioxanyl group. Biosci. Biotechnol. Biochem. 1992, 56, 1751–1759. [Google Scholar] [CrossRef]
- Pavela, R.; Waffo-Teguo, P.; Waffo-Teguo, B.; Richard, T.; Merillon, J.M. Vitis vinifera canes, a source of stilbenoids against Spodoptera littoralis larvae. J. Pest Sci. 2017, 90, 961–970. [Google Scholar]
- Gabaston, J.; El Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Merillon, J.M.; Pavela, R. Stilbenes from grapevine root: a promising natural insecticide against Leptinotarsa decemlineata. J. Pest Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Wu, W.J.; Tu, Y.Q.; Liu, H.X.; Zhu, J.B. Celangulins II, III, and IV: new insecticidal sesquiterpenoids from Celastrus angulatus. J. Nat. Prod. 1992, 55, 1294–1298. [Google Scholar] [CrossRef]
- Wu, W.J. The Experimental Techniques of Plant Protection; Shaanxi Science and Technology Press: Xi’an, China, 1987; pp. 23–25. [Google Scholar]
Sample Availability: Samples of the compounds T1–T4 are available from the authors. |

| Position | δC (ppm) | δH (ppm, J in Hz) |
|---|---|---|
| 1 | 179.43 | |
| 2 | 108.31 | 5.79 (s, 1H) |
| 3 | 103.47 | |
| 4 | 86.57 | |
| 5 | 195.81 | |
| 6 | 103.74 | 6.07 (s, 1H) |
| 7 | 97.05 | |
| 8 | 76.01 | 3.85 (d, J = 11.2 Hz, 1H); 4.55 (d, J = 11.1 Hz, 1H) |
| 1′ | 122.45 | |
| 2′ | 106.49 | 6.97 (s, 1H) |
| 3′ | 142.23 | |
| 4′ | 148.60 | |
| 5′ | 95.45 | 6.71 (s, 1H) |
| 6′ | 152.43 | |
| 7′ | 84.04 | 4.84 (d, J = 6.7 Hz, 1H) |
| 8′ | 56.82 | 2.83 (s, 1H) |
| 9′ | 71.33 | 4.16–4.29 (m, 2H) |
| 10′ | 102.30 | 5.95 (s, 2H) |
| 3-OMe | 52.64 | 3.39 (s, 3H) |
| 52.05 | 3.35 (s, 3H) | |
| 4-OH | 5.00 (s, 1H) | |
| 4-C′OOCH3 | 171.31 | |
| 4-COOC′H3 | 53.03 | 3.69 (s, 3H) |
| 7-OC′OCH3 | 170.07 | |
| 7-OCOC’H3 | 20.89 | 2.05 (s, 3H) |
| 6′-OMe | 57.04 | 3.83 (s, 3H) |
| Compounds a | Mode of Action | 4 h Knockdown Rate (%) | 8 h Knockdown Rate (%) | 24 h Corrected Mortality (%) |
|---|---|---|---|---|
| T1 | ST b | 66.7 | 0.0 | 0.0 |
| TT c | 8.3 | 0.0 | 0.0 | |
| T2 | ST | 0.0 | 0.0 | 0.0 |
| TT | 0.0 | 0.0 | 0.0 | |
| T3 | ST | 100.0 | 100.0 | 100.0 |
| TT | 100.0 | 100.0 | 100.0 | |
| T4 | ST | 0.0 | 95.8 | 25.0 |
| TT | 0.0 | 0.0 | 0.0 |
| Compounds | Time (h) | Mode of Action | Toxicity Regession Equation (y = a + bx) | r | KD50/LC50 a (95% Confidence Interval) mg/L |
|---|---|---|---|---|---|
| T1 | 4 | ST b | y = −1.6517 + 1.8801x | 0.9885 | 3450.21 (2568.05–4635.56) |
| TT c | - | - | >10,000 | ||
| T3 | 24 | ST | y = 2.9026 + 1.7027x | 0.9814 | 17.06 (12.30–23.65) |
| TT | y = −2.8244 + 2.5651x | 0.9775 | 1123.14 (885.25–1425.01) | ||
| T4 | 8 | ST | y = −2.7981 + 2.2615x | 0.9819 | 2807.10 (2180.02–3614.31) |
| TT | - | - | >10,000 | ||
| Indoxacarb | 24 | ST | y = 1.9652 + 2.3051x | 0.9896 | 20.73 (16.83–25.52) |
| TT | y = 4.5199 + 2.2861x | 0.9902 | 1.62 (1.15–2.29) |
| Compounds a | Mode of Action | 24 h Corrected Mortality (%) | 48 h Corrected Mortality (%) |
|---|---|---|---|
| T1 | ST b | 0.0 | 50.0 |
| TT c | 0.0 | 0.0 | |
| T2 | ST | 0.0 | 0.0 |
| TT | 0.0 | 0.0 | |
| T3 | ST | 10.0 | 60.0 |
| TT | 0.0 | 0.0 | |
| T4 | ST | 0.0 | 0.0 |
| TT | 0.0 | 0.0 |
| Compounds | Time (h) | Mode of Action | Toxicity Regession Equation (y = a + bx) | r | LC50 a (95% Confidence Interval) mg/L |
|---|---|---|---|---|---|
| T1 | 48 | ST b | y = −0.2142 + 1.6521x | 0.9813 | 1432.05 (1051.58–1952.23) |
| T3 | 48 | ST | y = −0.9181 + 2.0178x | 0.9772 | 857.28 (663.69–1108.28) |
| Indoxacarb | 48 | ST | y = 2.9102 + 2.3246x | 0.9836 | 7.92 (6.25–10.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wei, J.; Fang, J.; Lv, W.; Ji, Y.; Aioub, A.A.A.; Zhang, J.; Hu, Z. Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya. Molecules 2019, 24, 1976. https://doi.org/10.3390/molecules24101976
Li Y, Wei J, Fang J, Lv W, Ji Y, Aioub AAA, Zhang J, Hu Z. Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya. Molecules. 2019; 24(10):1976. https://doi.org/10.3390/molecules24101976
Chicago/Turabian StyleLi, Yankai, Jiaqi Wei, Jiameng Fang, Wenbo Lv, Yufei Ji, Ahmed A.A. Aioub, Jiwen Zhang, and Zhaonong Hu. 2019. "Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya" Molecules 24, no. 10: 1976. https://doi.org/10.3390/molecules24101976
APA StyleLi, Y., Wei, J., Fang, J., Lv, W., Ji, Y., Aioub, A. A. A., Zhang, J., & Hu, Z. (2019). Insecticidal Activity of Four Lignans Isolated from Phryma leptostachya. Molecules, 24(10), 1976. https://doi.org/10.3390/molecules24101976







