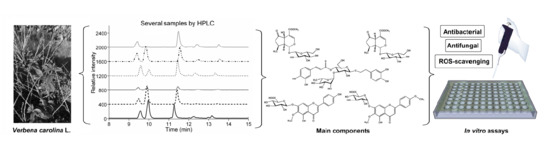
Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae) †
Abstract
:1. Introduction
2. Results and Discussion
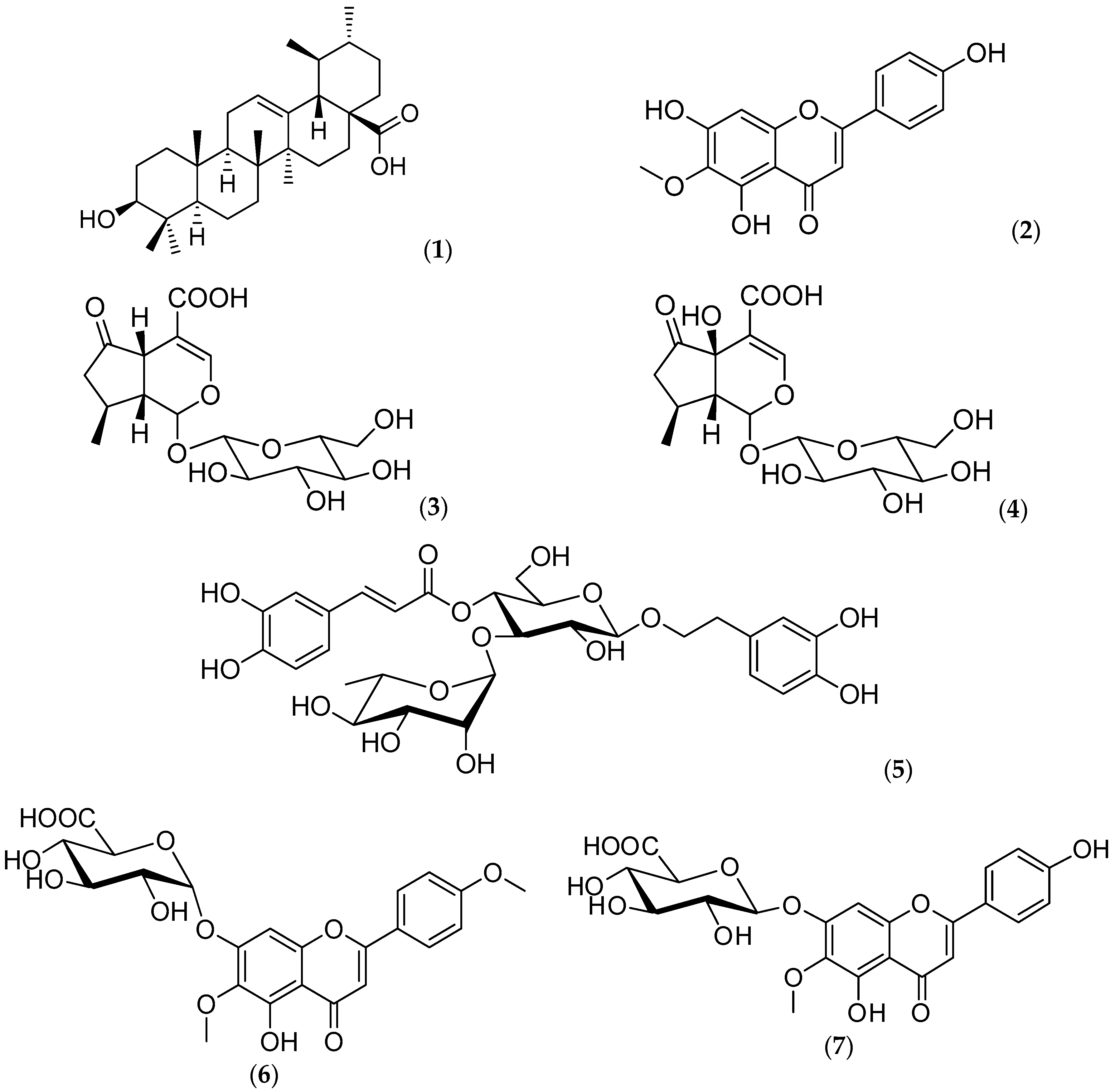
2.1. Chemical Composition
2.2. Phenolic Content of V. carolina Extracts
2.3. Validation of the Liquid Chromatography (LC) Method and Analysis of Samples
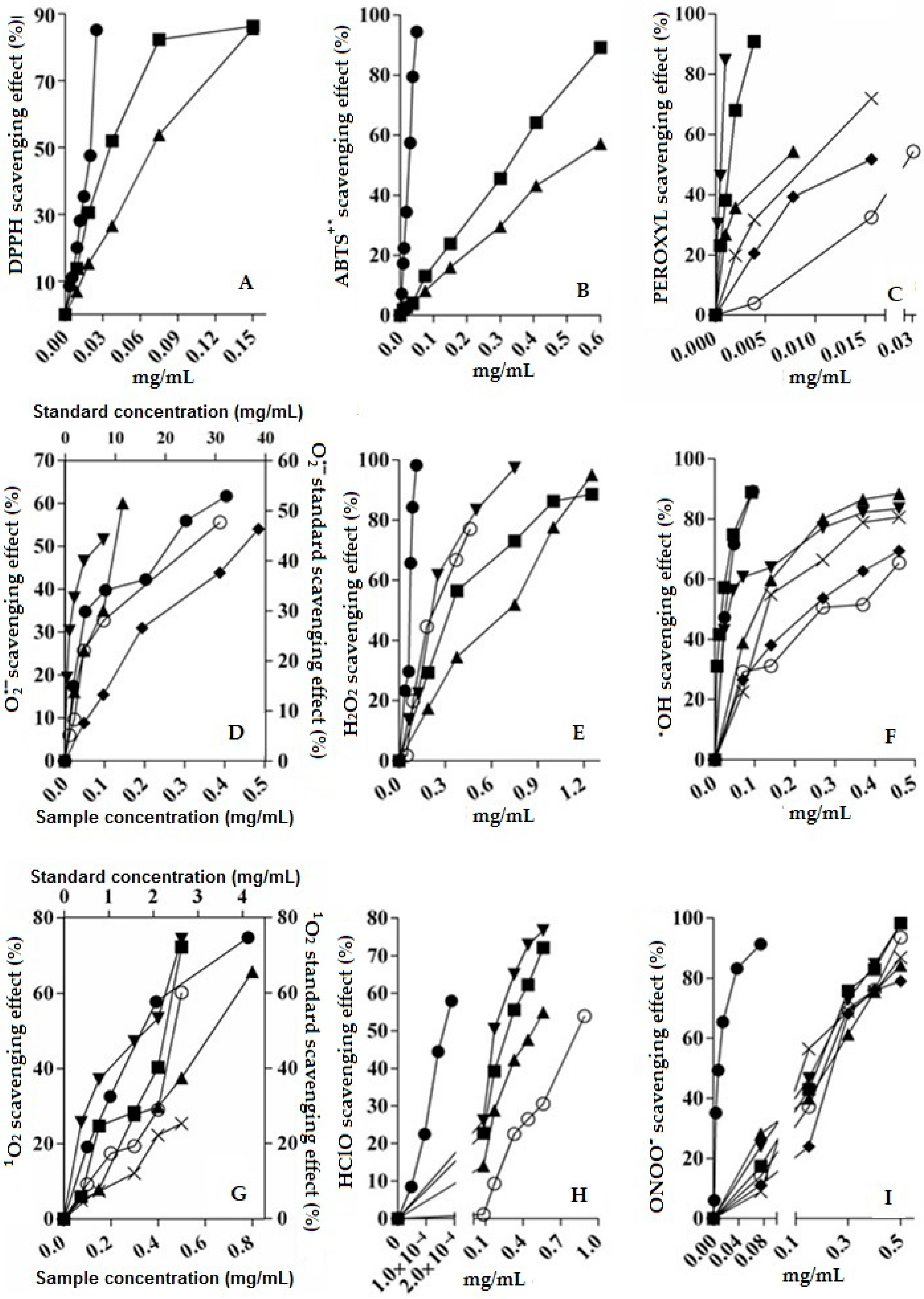
2.4. Scavenging Activity of V. carolina Extracts
2.5. Scavenging Activity of V. carolina Bioactive Compounds: Comparison Against Extracts
2.6. Antimicrobial Activity and Acute Toxicity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Material
3.3. Preparation of the Extracts and Isolation of Compounds
3.4. Total Phenolics Quantitation
3.5. Equipment and Chromatographic Conditions
3.6. Preparation of Stock and Working Solutions for HPLC
3.7. HPLC Analytical Method
3.8. Radical Scavenging Tests
3.9. Reactive Oxygen Species (ROS)-Scavenging Measurements
3.10. Antibacterial and Antifungal Activity
3.11. Animals
3.12. Acute Toxicity Study in Mice
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Márquez, A.C.; Lara, F.; Esquivel, B.; Mata, R. Plantas Medicinales de México II. Composición, Usos Y Actividad Biológica; UNAM: Mexico City, Mexico, 1999; p. 165. [Google Scholar]
- Favari-Perozzi, L.; Nava-Alvarez, R.; Meléndez-Camargo, M.E. Possible protective effect of verbena in rat liver injury induced by carbon tetrachloride. Rev. Mex. Cienc. Farm. 2007, 38, 19–25. [Google Scholar]
- Osamu, S.; Hiroshi, H.; Homare, T.; Satoshi, Y.; Fuminobu, Y. A Skin Care Preparation and Skin Care Composition. Japanese Patent JP2002020232, 23 January 2002. [Google Scholar]
- Castro, G.M.I.; Perez-Guil, R.F.; Madrigal, A.L. Chemical composition, toxic factors, and digestibility of Verbena carolina L. Turrialba 1991, 41, 289–292. [Google Scholar]
- Deepak, M.; Handa, S. Quantitative Determination of the Major Constituents of Verbena officinalis using High Performance Thin Layer Chromatography and High Pressure Liquid Chromatography. Phytochem. Anal. 2000, 11, 351–355. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, A.M.V.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Li, Y.; Matsunaga, K.; Kato, R.; Ohizumi, Y. Verbenachalcone, a novel dimeric dihydrochalcone with potentiating activity on nerve growth factor-action from Verbena littoralis. J. Nat. Prod. 2001, 64, 806–808. [Google Scholar] [CrossRef]
- Mûller, A.; Ganzera, M.; Stuppner, H. Analysis of the aerial parts of Verbena officinalis L. by micellar electrokinetic capillary chromatography. Chromatographia 2004, 60, 193–197. [Google Scholar] [CrossRef]
- Valento, P.; Andrade, P.; Areias, F.; Ferreres, F.; Seabra, R. Analysis of the vervain flavonoids by HPLC/Diod Array Detector Method. Its application to quality control. J. Agric. Food Chem. 1999, 47, 4579–4582. [Google Scholar] [CrossRef]
- Chulasiri, M.; Bunyapraphatsara, N.; Moongkarndi, P. Mutagenicity and antimutagenicity of hispidulin and hortensin, the flavonoids from Millingtonia hortensis L. Environ. Mol. Mutagen. 1992, 20, 307–312. [Google Scholar] [CrossRef]
- Gil, B.; Sanz, M.J.; Terencio, M.C.; Ferrandiz, M.L.; Bustos, G.; Paya, M.; Gunasegaran, R.; Alcaraz, M.J. Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice. Life Sci. 1994, 54, 333–338. [Google Scholar] [CrossRef]
- Tan, R.X.; Lu, H.; Wolfender, J.L.; Yu, T.T.; Zheng, W.F.; Yang, L.; Gafner, S.; Hostettmann, K. Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med. 1999, 65, 64–67. [Google Scholar] [CrossRef]
- Kawadias, D.; Sand, P.; Youdim, K.A.; Qaiser, M.Z.; Rice-Evans, C.; Baur, R.; Sigel, E.; Rausch, W.D. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. Br. J. Pharmacol. 2004, 142, 811–820. [Google Scholar]
- Yuting, C.; Rongliang, Z.; Zhongjian, J.; Yong, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Z.; Ma, C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem. Biophys. 2014, 69, 311–317. [Google Scholar] [CrossRef]
- He, L.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer 2011, 102, 219–225. [Google Scholar] [CrossRef]
- Makino, Y.; Kondo, S.; Nishimura, Y.; Tsukamoto, Y.; Huang, Z.; Urade, Y. Hastatoside and verbenalin are sleep-promoting components in Verbena officinalis. Sleep Biol. Rhythms 2009, 7, 211. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A.; Chandan, B.K.; Anand, K.K.; Suri, O.P. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia 1998, 69, 135–140. [Google Scholar]
- Rimpler, H.; Schäfer, B. Hastatosid, ein neues Iridoid aus Verbena hastata L.und Verbena officinalis L. Z. Naturforschung 1979, 34c, 311–318. [Google Scholar] [CrossRef]
- Scarpati, M.L. Isolamento dal Verbascum sinuatum di due nuovi glucosidi: Il verbascoside e l′isoverbascoside. Ann. Chim. 1963, 53, 356–367. [Google Scholar]
- Andary, C.; Wylde, R.; Laffite, C.; Privat, G.; Winternitz, F. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry 1982, 21, 1123–1127. [Google Scholar] [CrossRef]
- Avila, J.G. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Herbert, J.M.; Mafrand, J.P. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J. Nat. Prod. 1991, 54, 1595–1600. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, H.Y.; Shen, C.C.; Huang, Y.L.; Ou, J.C. Chemical constituents of Verbena bonariensis. J. Chin. Pharm. Sci. 2003, 55, 65–70. [Google Scholar]
- Arisawa, M.; Fukuta, M.; Shimizu, M.; Morita, N. The constituents of the leaves of Comanthosphace japonica S. Moore (Labiatae): Isolation of two new flavones glycosides, comanthosides A and B. Chem. Pharm. Bull. 1979, 27, 1252–1254. [Google Scholar] [CrossRef]
- Murata, T.; Watahiki, M.; Tanaka, Y.; Miyase, T.; Yoshizaki, F. Hyaluronidase inhibitors from Takuran, Lycopus lucidus. Chem. Pharm. Bull. 2010, 58, 394–397. [Google Scholar] [CrossRef]
- Ávila-Reyes, J.A.; Almaraz-Abarca, N.; Chaidez-Ayala, A.I.; Ramírez-Noya, D.; Delgado-Alvarado, E.A.; Torres-Ricario, R.; Naranjo-Jiménez, N.; Alanís-Bañuelos, R.E. Foliar phenolic compounds of ten wild species of Verbenacea as antioxidants and specific chemomarkers. Braz. J. Biol. 2018, 78, 98–107. [Google Scholar] [CrossRef]
- ICH. Text on validation of Analytical Procedures. Harmonized tripartite guideline [Q2(R1)]. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 1–13 November 2005; Available online: http://www.ich.org/fileadmin/public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf (accessed on 27 July 2012).
- Habu, J.B.; Ibeh, B.O. In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract. Biol. Res. 2015, 48, 1–10. [Google Scholar] [CrossRef]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Navarro, V.; Villarreal, M.L.; Rojas, G.; Lozoya, X. Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious deseases. J. Ethnopharmacol. 1996, 53, 143–147. [Google Scholar] [CrossRef]
- Alvarez, L.; Pérez, M.C.; González, J.L.; Navarro, V.; Villarreal, M.L.; Olson, J.O. SC-1, an antimycotic spirostan saponin from Solanum chrysotrichum. Planta Med. 2001, 67, 372–374. [Google Scholar] [CrossRef]
- Zamilpa, A.; Tortoriello, J.; Navarro, V.; Delgado, G.; Alvarez, L. Five new steroidal saponins from Solanum chrysotrichum leaves and their antimicrobial activity. J. Nat. Prod. 2002, 65, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Rivero-Cruz, I.; Arriaga-Alba, M.; Castañeda-Corral, G.; Angeles-López, G.E.; Navarrete, A.; Mata, R. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J. Ethnopharmacol. 2007, 110, 334–342. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverri, J. Antioxidant activity of a type proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Carranza, R.; Cárdenas, N.; Huerta-Gertrudis, B.; Medina-Campos, O.N.; Mendoza-Cruz, M.; Delgado-Lamas, G.; Espinoza-Aguirre, J. Antioxidant activity of Heterotheca inuloides extracts and of some of its metabolites. Toxicology 2010, 276, 41–48. [Google Scholar] [CrossRef]
- Blanco-Ayala, T.; Lugo-Huitrón, R.; Serrano-López, E.M.; Reyes-Chilpa, R.; Rangel-López, E.; Pineda, B.; Medina-Campos, O.N.; Sánchez-Chapul, L.; Pinzón, E.; Trejo-Solis, C.; et al. Antioxidant properties of xanthones from Calophyllum brasiliense: Prevention of oxidative damage induced by FeSO4. BMC Complement. Altern. Med. 2013, 13, 262. [Google Scholar] [CrossRef]
- Floriano-Sánchez, E.; Villanueva, C.; Medina-Campos, O.N.; Rocha, D.; Sánchez-González, D.J.; Cárdenas-Rodríguez, N.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic. Res. 2006, 40, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Rahalison, L.; Hamburger, M.; Monod, M.; Frenk, E.; Hostettmann, K. Antifungal test in phytochemical investigations: Comparison of bioautographic methods using phytopatogenic and human pathogenic fungi. Planta Med. 1994, 60, 41–44. [Google Scholar] [CrossRef]
- Gadhi, C.; Benharref, A.; Jana, M.; Basile, A.; Contet-Audonneau, N.; Fortier, B. Antidermatophic properties of extracts from the leaves of Aristolochia paucinervis Pomel. Phytother. Res. 2001, 15, 79–81. [Google Scholar] [CrossRef]
- Ríos, J.; Recio, M.; Villar, A. Screening methods for natural products with antimicrobial activity: A review of the literature. J. Ethnopharmacol. 1988, 23, 127–149. [Google Scholar] [CrossRef]
- Lorke, D. A New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
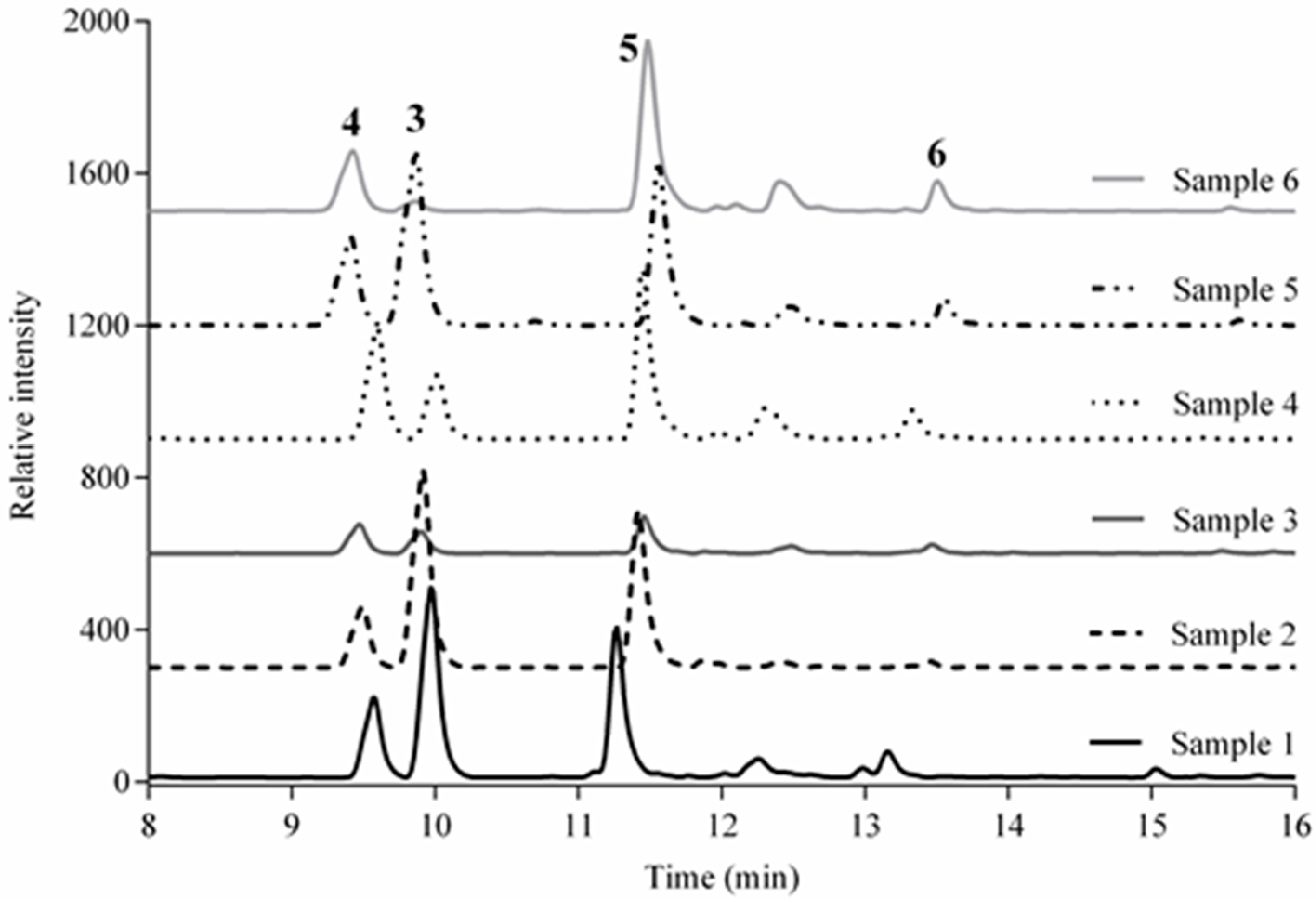
 ), aqueous extract (
), aqueous extract (  ), verbenaline (3) (
), verbenaline (3) (  ), hastatoside (4) (
), hastatoside (4) ( 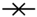 ), verbascoside (5) (
), verbascoside (5) (  ), 7OβGH (6) (
), 7OβGH (6) (  ), reference compounds (
), reference compounds (  ).
).
 ), aqueous extract (
), aqueous extract (  ), verbenaline (3) (
), verbenaline (3) (  ), hastatoside (4) (
), hastatoside (4) (  ), verbascoside (5) (
), verbascoside (5) (  ), 7OβGH (6) (
), 7OβGH (6) (  ), reference compounds (
), reference compounds (  ).
).
| Analyte | Amount Added (μg/mL) | Amount Found (μg/mL) | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Verbenalin (3) | 35 | 35.0943 | 100.26 | 1.98 |
| 70 | 70.5581 | 100.79 | 1.89 | |
| 105 | 106.6606 | 101.58 | 0.60 | |
| Hastatoside (4) | 20 | 20.216 | 101.08 | 0.48 |
| 40 | 40.1022 | 100.25 | 1.15 | |
| 60 | 60.4068 | 100.68 | 0.60 | |
| Verbascoside (5) | 45 | 45.0416 | 100.09 | 1.54 |
| 90 | 90.7351 | 100.81 | 0.67 | |
| 135 | 136.0689 | 100.78 | 1.55 | |
| 7OβGH, (6) | 40 | 39.257 | 98.13 | 0.46 |
| 80 | 80.4062 | 100.5 | 1.25 | |
| 120 | 120.7182 | 100.59 | 0.36 |
| Sample | Concentration (mg/g of Plant) Mean ± RSD | |||
|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |
| S1 | 16.29 ± 0.89 | 11.44 ± 0.52 | 25.64 ± 0.67 | 17.87 ± 0.98 |
| S2 | 17.88 ± 0.05 | 8.97 ± 0.16 | 28.82 ± 0.23 | 3.42 ± 0.49 |
| S3 | 1.7 ± 0.00 | 4.39 ± 0.01 | 7.18 ± 0.03 | 6.17 ± 0.25 |
| S4 | 5.83 ± 0.13 | 17.21 ± 0.37 | 31.4 ± 0.96 | 20.52 ± 0.90 |
| S5 | 17.58 ± 0.07 | 14.99 ± 0.07 | 31.29 ± 0.09 | 23.71 ± 0.41 |
| S6 | 0.68 ± 0.03 | 10.58 ± 0.10 | 33.78 ± 0.36 | 25.15 ± 0.52 |
| DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|
| Compound/Extract | EC50 (mg/mL) | TEAC [µmol trolox/mg extract] | EC50 (mg/mL) | TEAC [µmol trolox/mg extract] | TEAC [µmol trolox/mg extract] | AAEAC [µM AA/mg extract] |
| CAqE | 0.081 ± 0.0001 | 7.50 ± 0.06 | 0.50 ± 0.007 | 7.10 ± 0.05 | 3.88 ± 0.06 | 5.49 ± 0.04 |
| CME | 0.041 ± 0.00009 | 14.20 ± 0.20 | 0.32 ± 0.01 | 10.10 ± 0.11 | 13.18 ± 0.11 | 11.74 ± 0.08 |
| Ascorbic acid | 0.018 ± 0.00003 | 23.12 ± 0.53 | 0.026 ± 0.0001 | 125.13 ± 0.90 | - | - |
| Quercetin | 89.30 ± 0.90 | 117.76 ± 0.61 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Issasi, G.; Salgado, C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Morales, A.; Águila, M.A.; Avilés, M.; Rivero-Cruz, B.E.; Navarro, V.; Ríos-Gómez, R.; et al. Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae). Molecules 2019, 24, 1970. https://doi.org/10.3390/molecules24101970
Lara-Issasi G, Salgado C, Pedraza-Chaverri J, Medina-Campos ON, Morales A, Águila MA, Avilés M, Rivero-Cruz BE, Navarro V, Ríos-Gómez R, et al. Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae). Molecules. 2019; 24(10):1970. https://doi.org/10.3390/molecules24101970
Chicago/Turabian StyleLara-Issasi, Gonzalo, Cecilia Salgado, José Pedraza-Chaverri, Omar N. Medina-Campos, Agustín Morales, Marco A. Águila, Margarita Avilés, Blanca E. Rivero-Cruz, Víctor Navarro, Ramiro Ríos-Gómez, and et al. 2019. "Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae)" Molecules 24, no. 10: 1970. https://doi.org/10.3390/molecules24101970
APA StyleLara-Issasi, G., Salgado, C., Pedraza-Chaverri, J., Medina-Campos, O. N., Morales, A., Águila, M. A., Avilés, M., Rivero-Cruz, B. E., Navarro, V., Ríos-Gómez, R., & Aguilar, M. I. (2019). Antimicrobial, Antioxidant Activities, and HPLC Determination of the Major Components of Verbena carolina (Verbenaceae). Molecules, 24(10), 1970. https://doi.org/10.3390/molecules24101970