Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings
Abstract
:1. Introduction
2. Results and Discussion
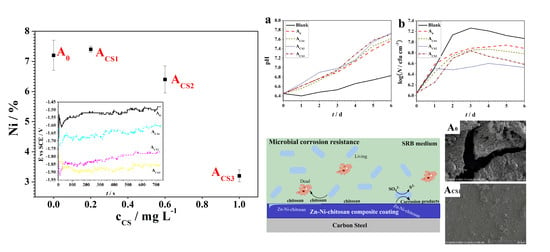
2.1. Influence of the Chitosan on the Coating Electrodeposition
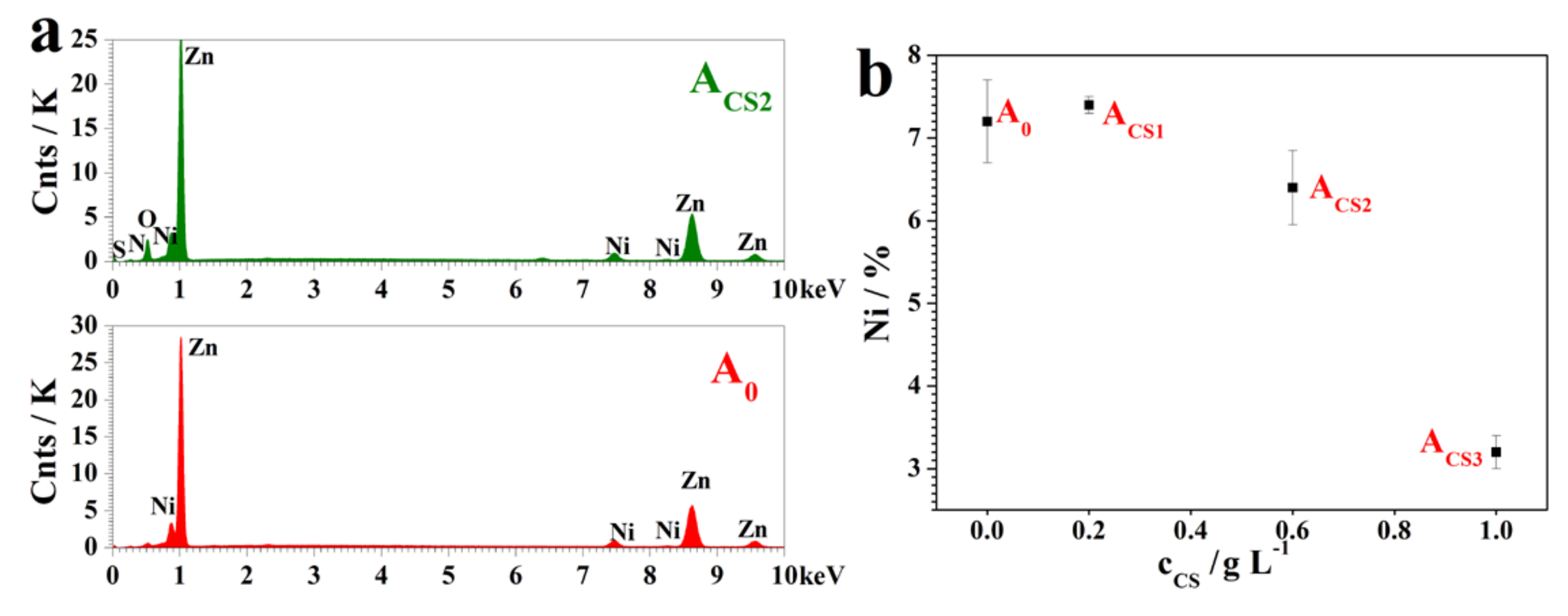
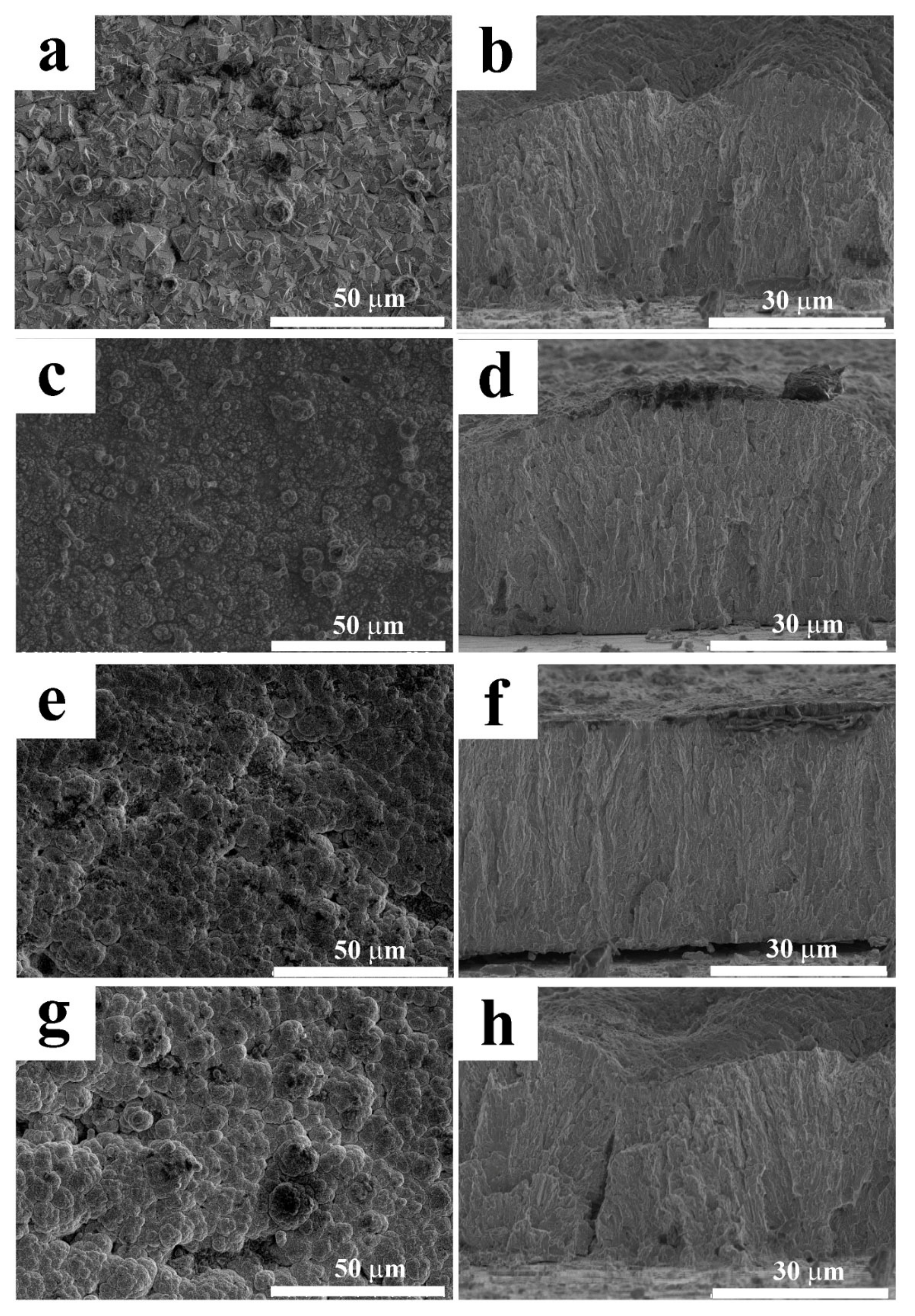
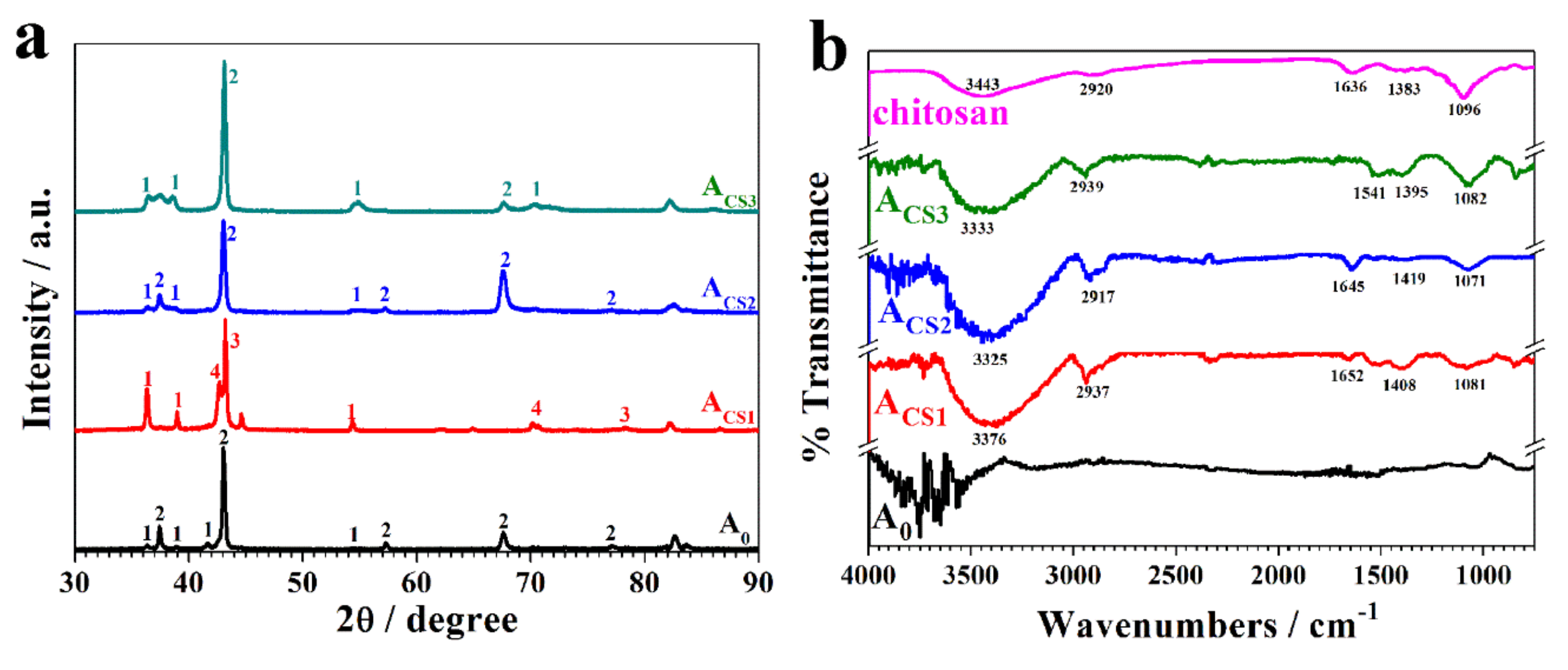
2.2. The Influence of Chitosan on the Coating Structure
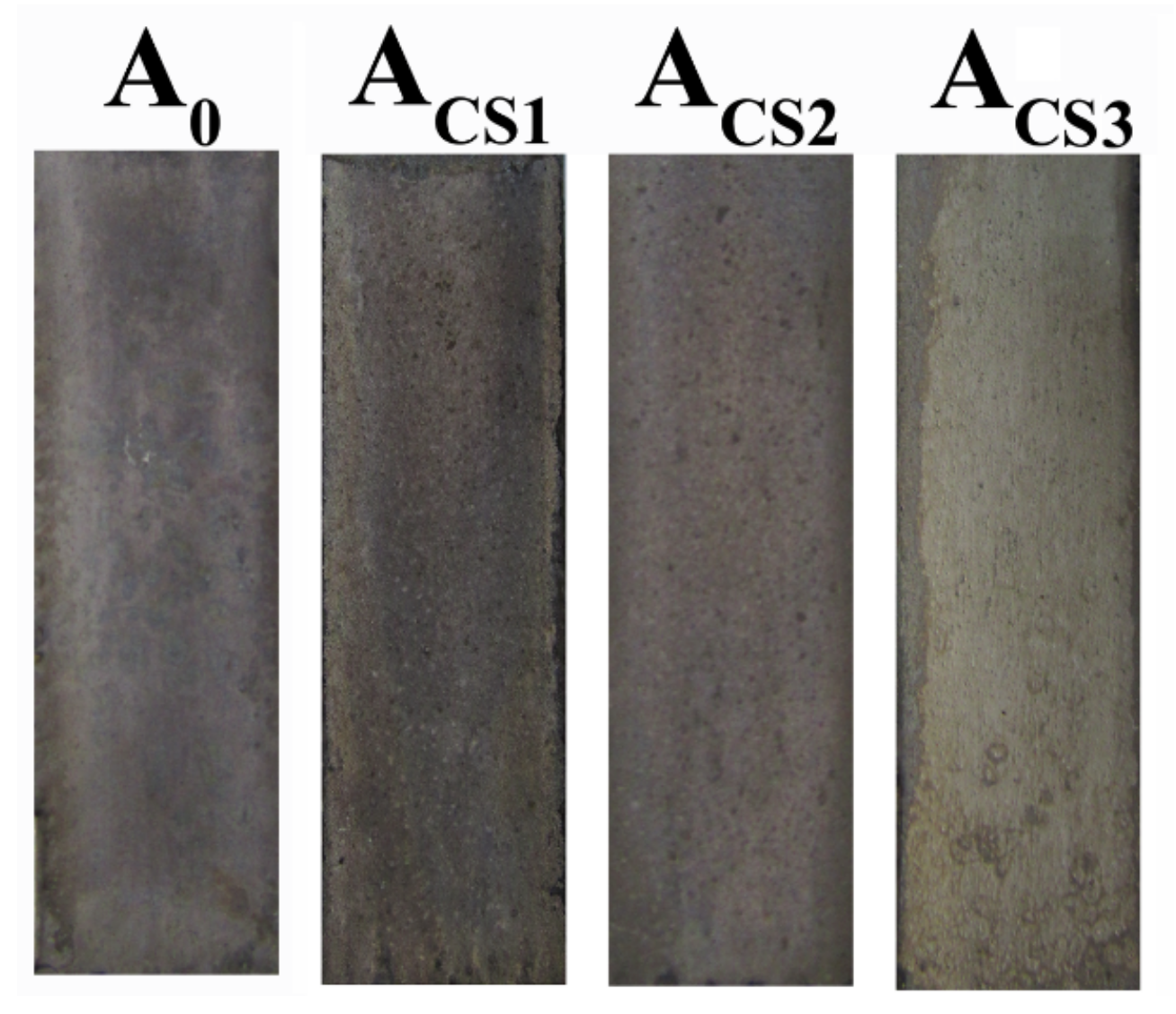
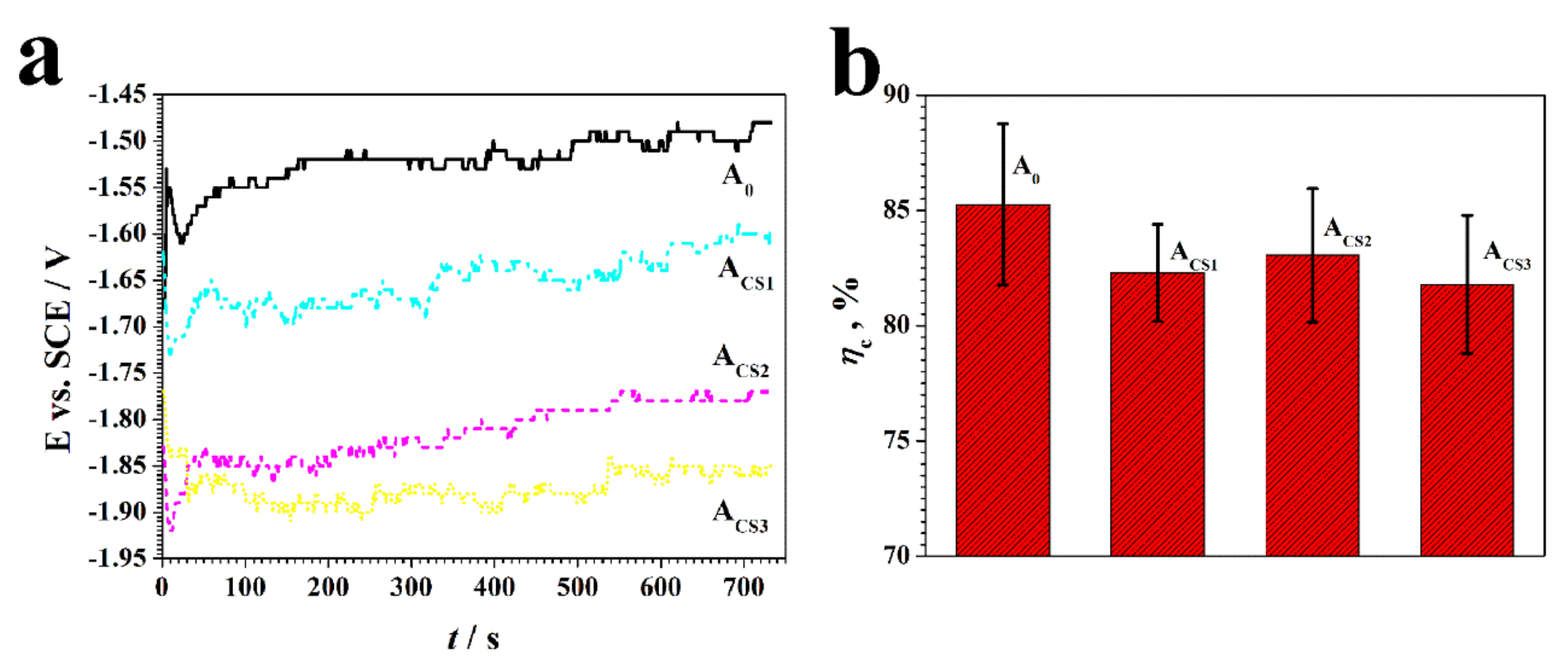
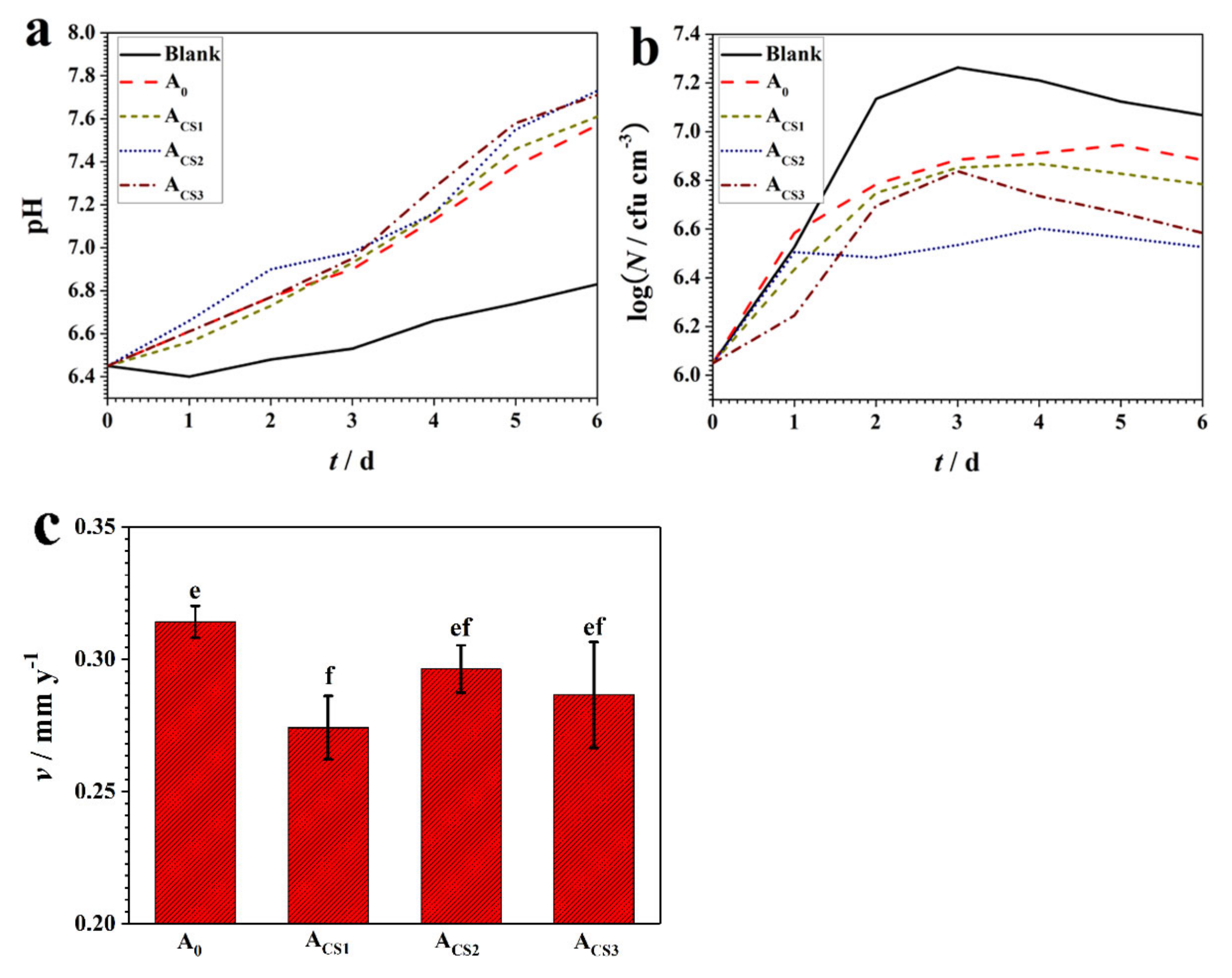
2.3. Corrosion Resistance of the Coatings
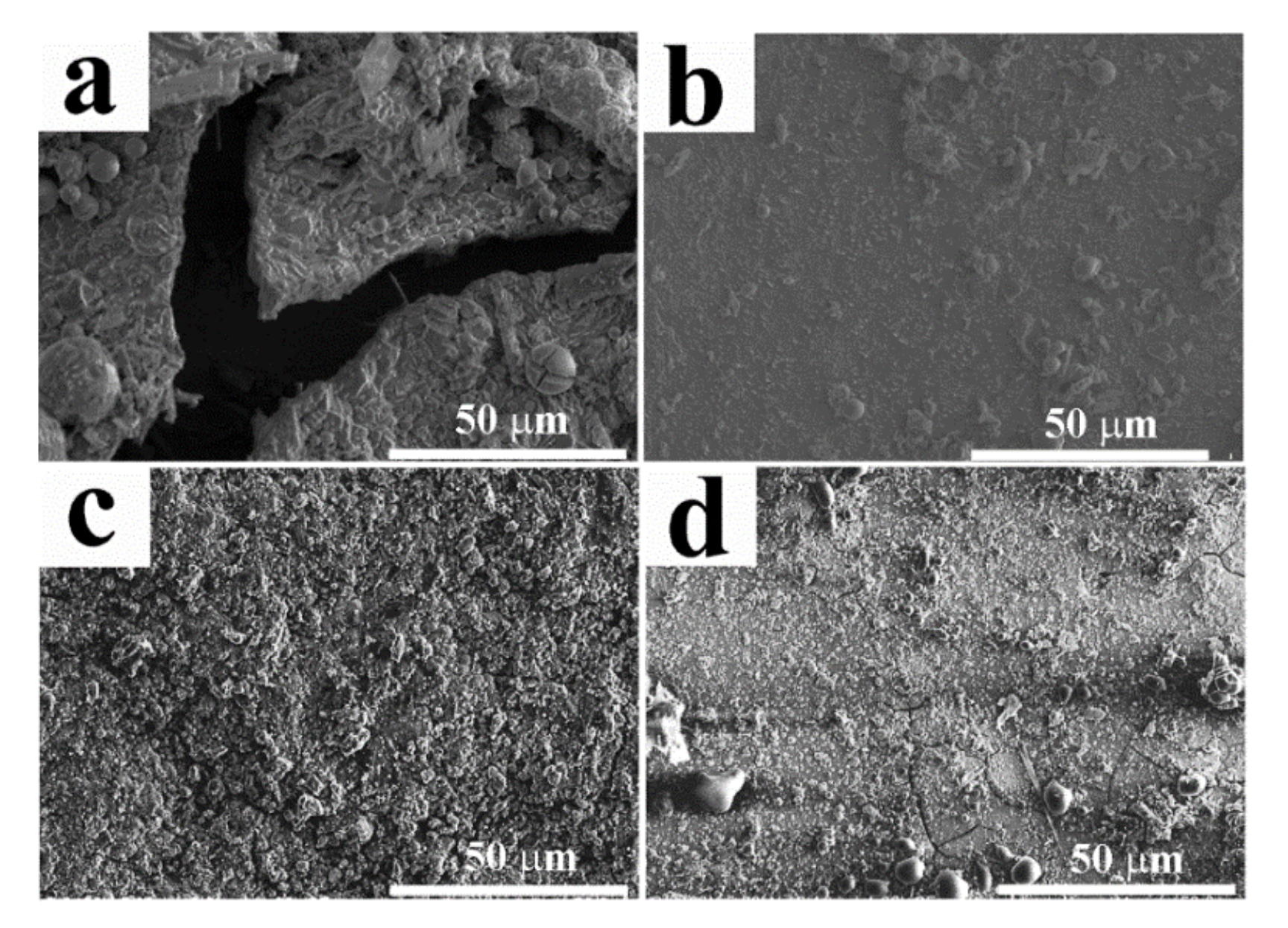
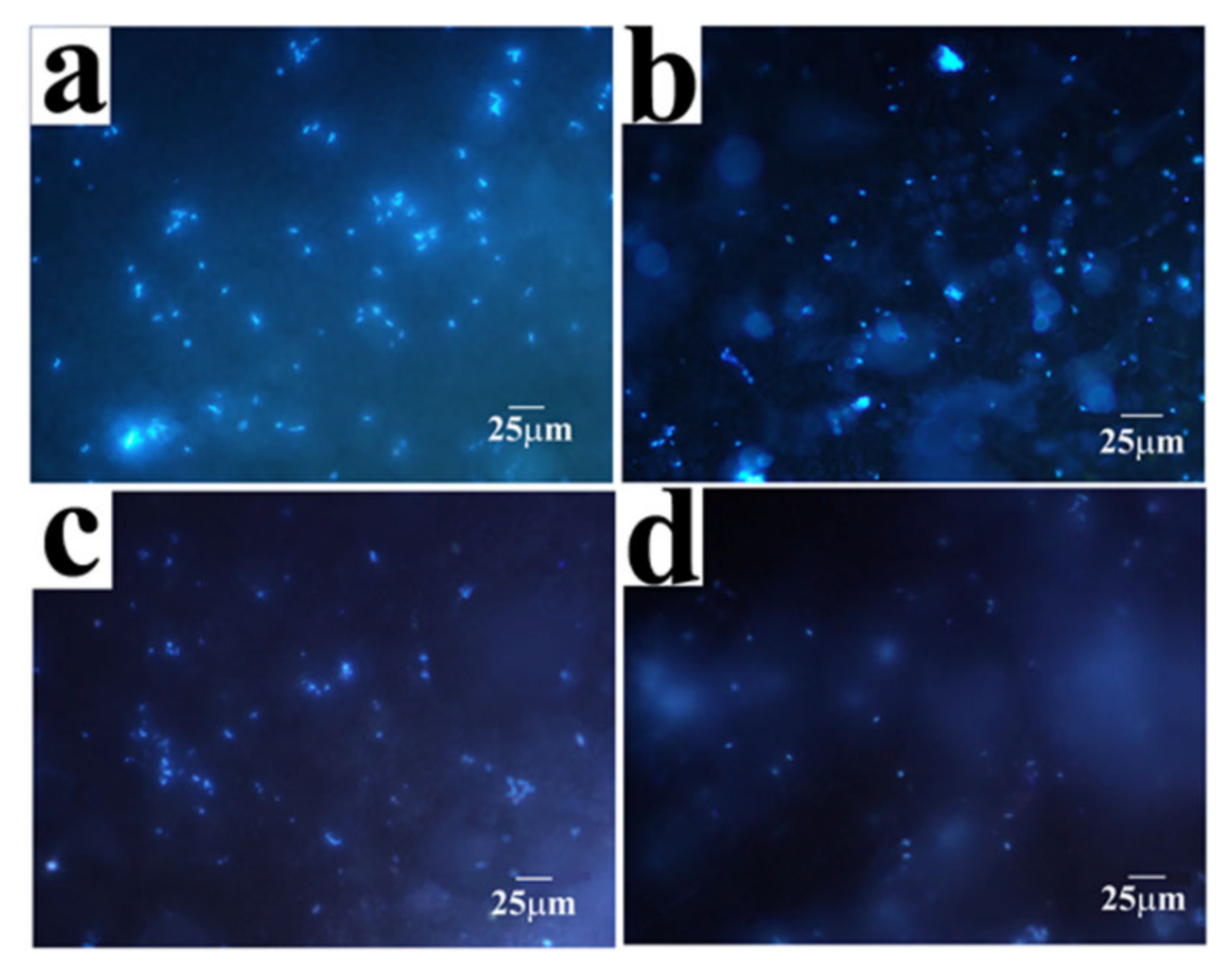
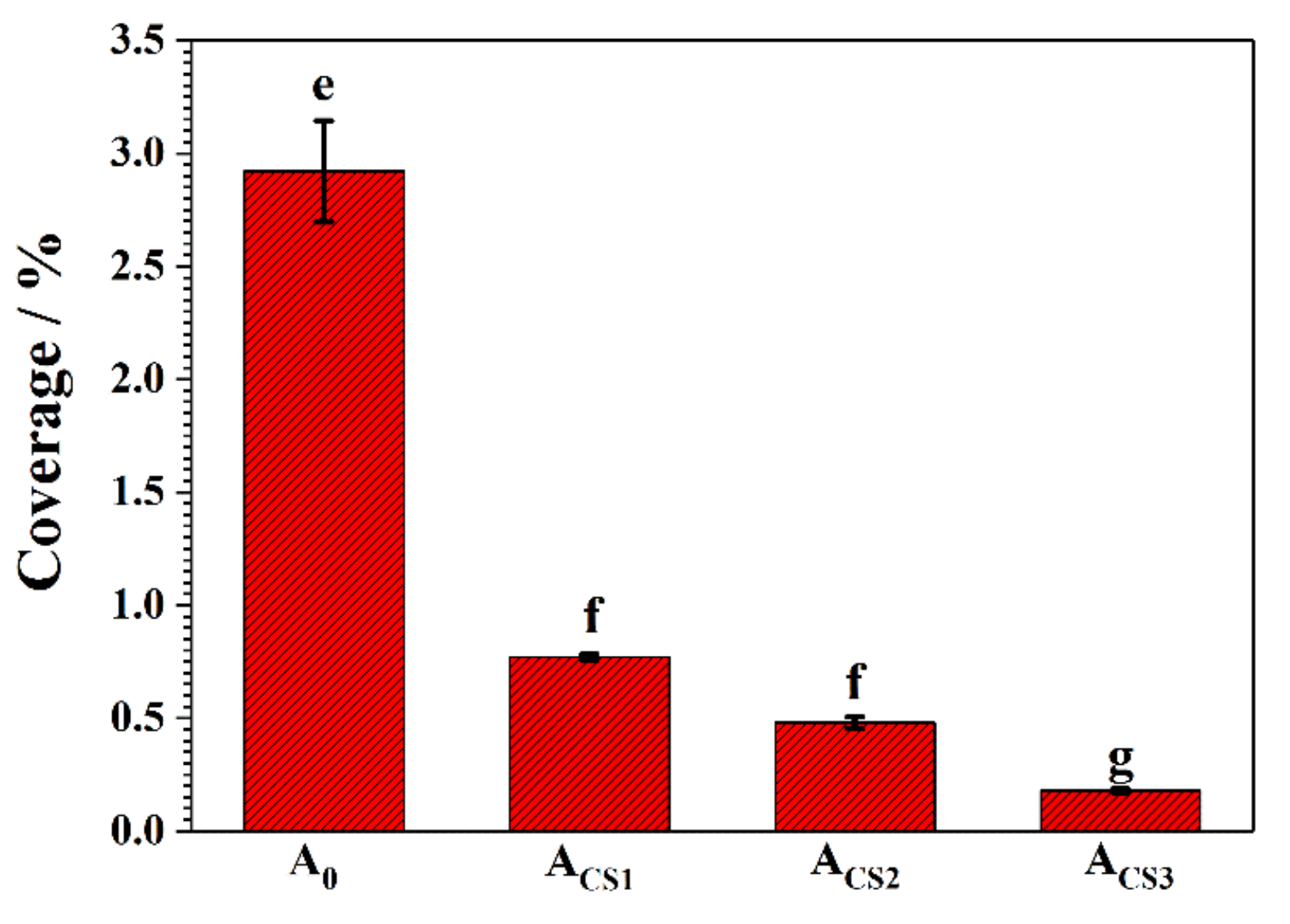
2.4. Antibacterial Properties of the Coatings
3. Materials and Methods
3.1. Electrodeposition of Zn–Ni–chitosan Coatings on Carbon Steel
3.2. Characterization of the Zn–Ni–chitosan Coatings
3.3. Investigation of the Interaction Between the Zn–Ni–chitosan Coatings and the SRB Medium
3.4. Antibacterial Tests on Zn–Ni–chitosan Coatings in E. coli Media
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Abou-Krisha, M.M. Effect of pH and current density on the electrodeposition of Zn-Ni-Fe alloys from a sulfate bath. J. Coat. Technol. Res. 2012, 9, 775–783. [Google Scholar] [CrossRef]
- Eliaz, N.; Venkatakrishna, K.; Hegde, A.C. Electroplating and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co alloys. Surf. Coat. Technol. 2010, 205, 1969–1978. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Characterization of corrosion resistance of electrodeposited Zn–Ni Zn and Cd coatings. Electrochim. Acta 2013, 105, 314–323. [Google Scholar] [CrossRef]
- Duan, J.; Wu, S.; Zhang, X.; Huang, G.; Du, M.; Hou, B. Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim. Acta 2008, 54, 22–28. [Google Scholar] [CrossRef]
- Ilhan-Sungur, E.; Cansever, N.; Cotuk, A. Microbial corrosion of galvanized steel by a freshwater strain of sulphate reducing bacteria (Desulfovibrio sp.). Corros. Sci. 2007, 49, 1097–1109. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Little, B.; Wagner, P.; Mansfeld, F. An overview of microbiologically influenced corrosion. Electrochim. Acta 1992, 37, 2185–2194. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, E.; Zhao, Y.; Li, H.; Liu, Z.; Zhang, D.; Yang, C.; Lin, H.; Li, X.; Yang, K. Enhanced resistance of 2205 Cu-bearing duplex stainless steel towards microbiologically influenced corrosion by marine aerobic Pseudomonas aeruginosa biofilms. J. Mater. Sci. Technol. 2018, 34, 1325–1336. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Xia, J.; Yang, C.; Xu, D.; Sun, D.; Nan, L.; Sun, Z.; Li, Q.; Gu, T.; Yang, K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Hip Int. J. Clin. Expe. Res. Hip Pathol. Ther. 2015, 31, 481–492. [Google Scholar]
- Nan, L.; Xu, D.; Gu, T.; Song, X.; Yang, K. Microbiological influenced corrosion resistance characteristics of a 304L-Cu stainless steel against Escherichia coli. Mater. Sci. Eng. C 2015, 48, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef]
- Li, P.; Zhou, C.; Rayatpisheh, S.; Ye, K.; Poon, Y.F.; Hammond, P.T.; Duan, H.; Chan-Park, M.B. Cationic Peptidopolysaccharides Show Excellent Broad-Spectrum Antimicrobial Activities and High Selectivity. Adv. Mater. 2012, 24, 4130–4137. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Shahzadi, L.; Mahmood, N.; Siddiqi, S.A.; Rauf, A.; Manzoor, F.; Chaudhry, A.A.; Rehman, I. u.; Yar, M. A new synthetic methodology for the preparation of biocompatible and organo-soluble barbituric- and thiobarbituric acid based chitosan derivatives for biomedical applications. Mater. Sci. Eng. C 2016, 66, 156–163. [Google Scholar] [CrossRef]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interf. 2014, 6, 10005–10013. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Liu, C.; Feng, X.; Wei, L.; Shao, L. Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater. Design 2016, 92, 471–479. [Google Scholar] [CrossRef]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.Z.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zhang, J.Y.; Wang, Y.B.; Li, C.M.; Lu, Z.S.; Hu, X.F.; Xu, L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater. Sci. Eng. C 2019, 98, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Electrochemically deposited chitosan/Ag complex coatings on biomedical NiTi alloy for antibacterial application. Surf. Coat. Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, X.; Cheng, K.; Weng, W.; Song, C.; Du, P.; Shen, G.; Han, G. Release behaviors of drug loaded chitosan/calcium phosphate coatings on titanium. Thin Solid Films 2011, 519, 4658–4662. [Google Scholar] [CrossRef]
- Đošić, M.; Eraković, S.; Janković, A.; Vukašinović-Sekulić, M.; Matić, I.Z.; Stojanović, J.; Rhee, K.Y.; Mišković-Stanković, V.; Park, S.-J. In vitro investigation of electrophoretically deposited bioactive hydroxyapatite/chitosan coatings reinforced by graphene. J. Ind. Eng. Chem. 2017, 47, 336–347. [Google Scholar] [CrossRef]
- Kääriäinen, M.L.; Weiss, C.K.; Ritz, S.; Pütz, S.; Cameron, D.C.; Mailänder, V.; Landfester, K. Zinc release from atomic layer deposited zinc oxide thin films and its antibacterial effect on Escherichia coli. Appl. Surf. Sci. 2013, 287, 375–380. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhao, Y.; Su, H.; Tan, T. The behavior of active bactericidal and antifungal coating under visible light irradiation. Appl. Surf. Sci. 2014, 292, 756–763. [Google Scholar] [CrossRef]
- Roventi, G.; Cecchini, R.; Fabrizi, A.; Bellezze, T. Electrodeposition of nickel–zinc alloy coatings with high nickel content. Surf. Coat. Technol. 2015, 276, 1–7. [Google Scholar] [CrossRef]
- De Carvalho, M.F.; Barbano, E.P.; Carlos, I.A. Influence of disodium ethylenediaminetetraacetate on zinc electrodeposition process and on the morphology, chemical composition and structure of the electrodeposits. Electrochim. Acta 2013, 109, 798–808. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Gudavičiūtė, L.; Kosenko, A.; Girčienė, O.; Selskis, A. Structural and corrosion characterisation of pulse plated Zn and Zn–Ni alloy coatings. T. I. Met. Finish. 2012, 90, 237–245. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. Electrodeposition of single gamma phased Zn–Ni alloy coatings from additive-free acidic bath. Appl. Surf. Sci. 2014, 311, 635–642. [Google Scholar] [CrossRef]
- Fratesi, R.; Roventi, G. Electrodeposition of zinc-nickel alloy coatings from a chloride bath containing NH4Cl. J. Biol. Chem. 2005, 280, 41332–41341. [Google Scholar]
- Yu, L.; Duan, J.; Zhao, W.; Huang, Y.; Hou, B. Characteristics of hydrogen evolution and oxidation catalyzed by Desulfovibrio caledoniensis biofilm on pyrolytic graphite electrode. Electrochim. Acta 2011, 56, 9041–9047. [Google Scholar] [CrossRef]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Component | Content |
|---|---|
| KH2PO4 | 0.5 g |
| NH4Cl | 1 g |
| CaCl2•6H2O | 0.06 g |
| MgSO4•7H2O | 0.06 g |
| 70% Sodium lactate | 6 mL |
| Yeast extract | 1 g |
| Sodium citrate | 0.3 g |
| Filtered seawater | 1 L |
| Component | Content |
|---|---|
| NaCl | 10 g |
| Peptone from fish | 10 g |
| Yeast extract | 5 g |
| Distilled water | 1 L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, X.; Ren, Y.; Wang, N.; Guan, F.; Agievich, M.; Duan, J.; Hou, B. Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings. Molecules 2019, 24, 1974. https://doi.org/10.3390/molecules24101974
Zhai X, Ren Y, Wang N, Guan F, Agievich M, Duan J, Hou B. Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings. Molecules. 2019; 24(10):1974. https://doi.org/10.3390/molecules24101974
Chicago/Turabian StyleZhai, Xiaofan, Yadong Ren, Nan Wang, Fang Guan, Maria Agievich, Jizhou Duan, and Baorong Hou. 2019. "Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings" Molecules 24, no. 10: 1974. https://doi.org/10.3390/molecules24101974
APA StyleZhai, X., Ren, Y., Wang, N., Guan, F., Agievich, M., Duan, J., & Hou, B. (2019). Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings. Molecules, 24(10), 1974. https://doi.org/10.3390/molecules24101974