The Mutation of Conservative Asp268 Residue in the Peptidoglycan-Associated Domain of the OmpA Protein Affects Multiple Acinetobacter baumannii Virulence Characteristics
Abstract
1. Introduction
2. Results
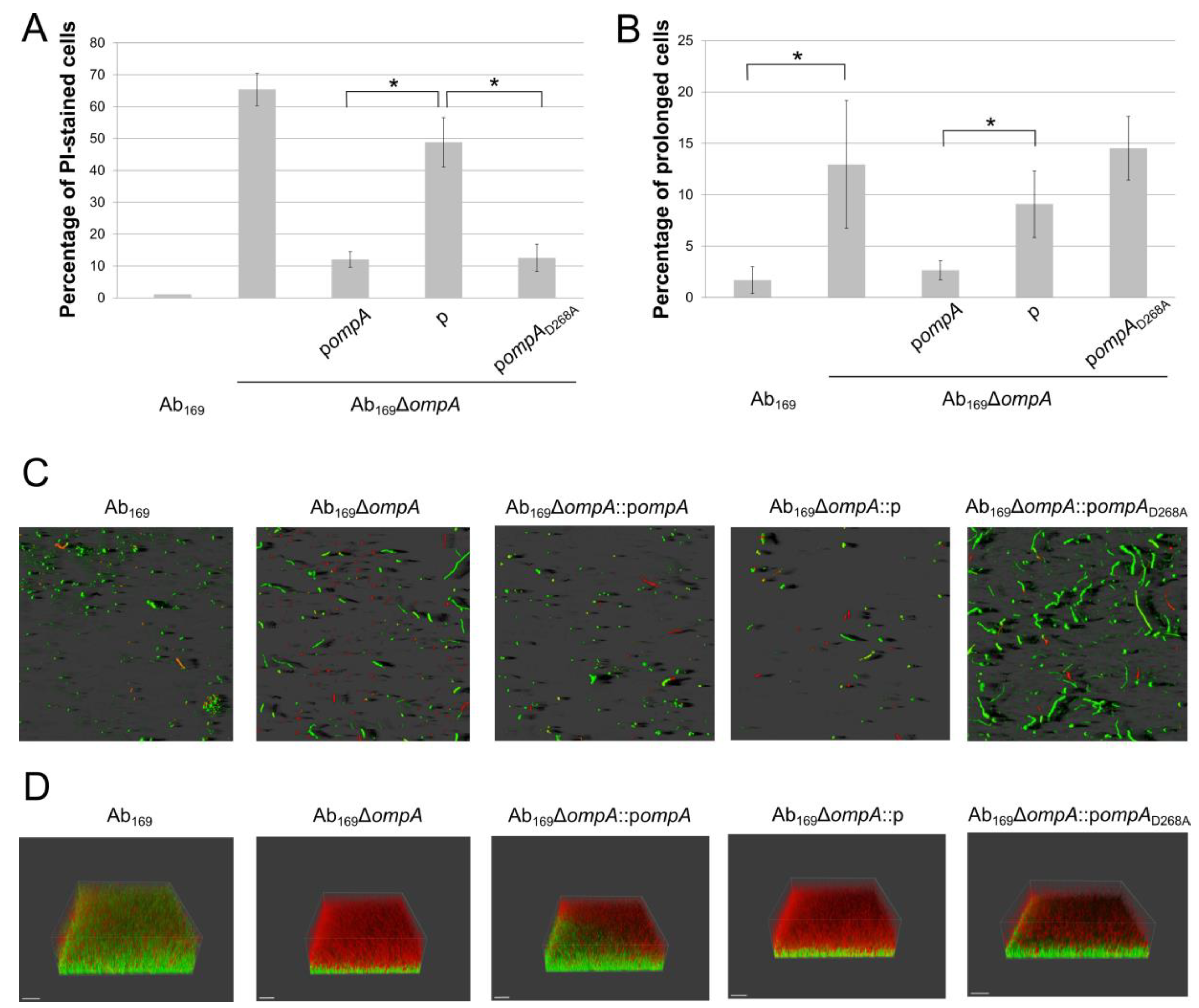
2.1. Effect of OmpA D268A Substitution on A. baumannii Biofilm Morphology
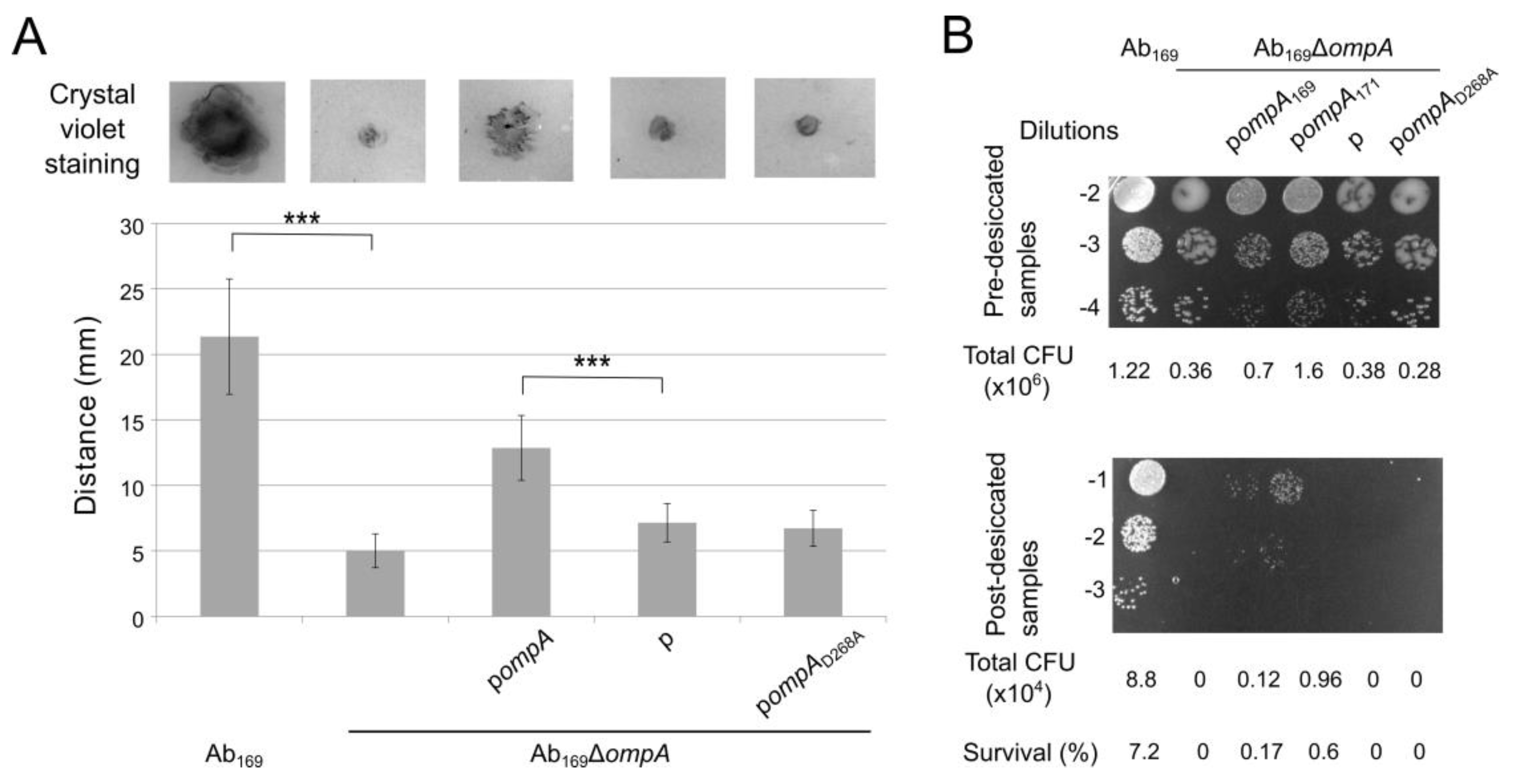
2.2. OmpA D268A Substitution Reduces A. baumannii Motility and Resistance to Desiccation
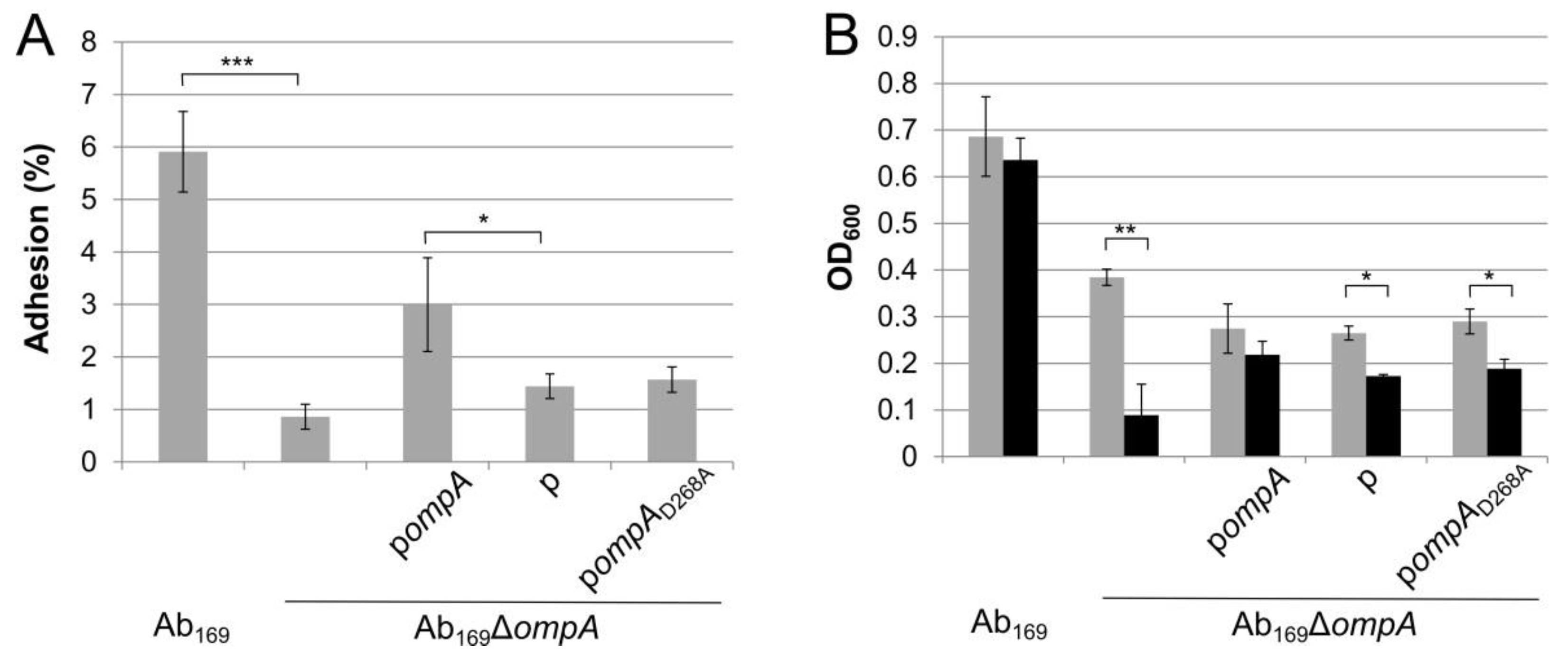
2.3. Effect of OmpA D268A Substitution on A. baumannii Adhesion to Lung Epithelial Cells and Resistance to Serum-Mediated Killing
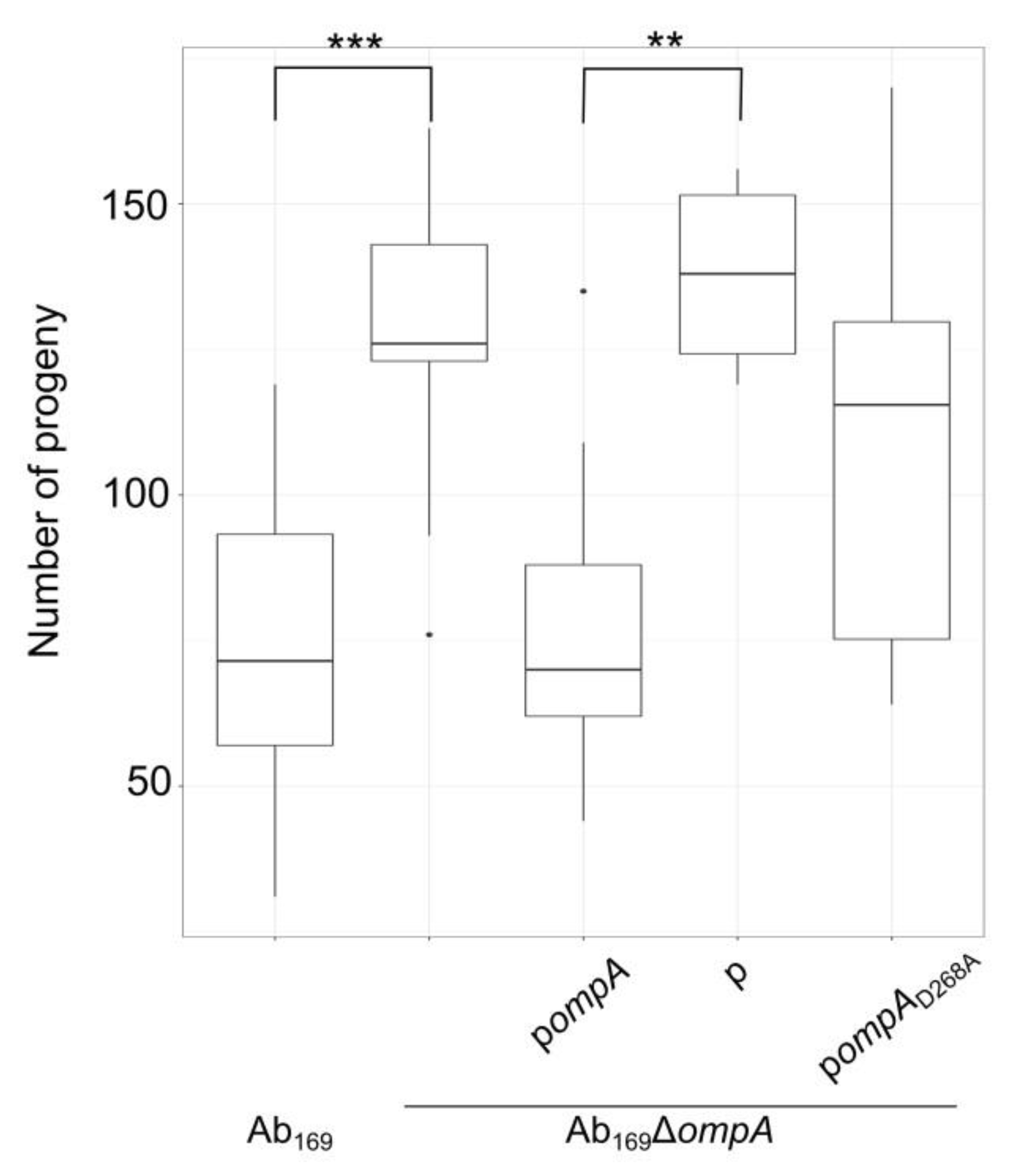
2.4. Effect of OmpA D268A Substitution on A. baumannii Virulence in Caenorhabditis elegans Infection Model
2.5. The OmpA Association to Peptidoglycan Is Dispensable for Ampicillin Inactivation by Outer Membrane Vesicles
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Motility Assays
4.3. Desiccation Assay
4.4. Confocal Laser Scanning Microscopy (CLSM)
4.5. Isolation of Outer Membrane Vesicles
4.6. Transmission Electron Microscopy (TEM)
4.7. A. baumannii Growth Assays
4.8. Generation of ΔompA Deletion Mutant, Complemented Strains and Site-Directed Mutagenesis
4.9. SDS-PAGE and Immunoassay
4.10. Cell Culture Assays
4.11. Caenorhabditis elegans Fertility Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glance, L.G.; Stone, P.W.; Mukamel, D.B.; Dick, A.W. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg. 2011, 146, 794–801. [Google Scholar] [CrossRef]
- Tiwari, P.; Rohit, M. Assessment of Costs Associated with Hospital-Acquired Infections in a Private Tertiary Care Hospital in India. Value Health Reg. Issues 2013, 2, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmier, J.K.; Hulme-Lowe, C.K.; Semenova, S.; Klenk, J.A.; DeLeo, P.C.; Sedlak, R.; Carlson, P.A. Estimated hospital costs associated with preventable health care-associated infections if health care antiseptic products were unavailable. Clin. Outcomes Res. 2016, 8, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, A.; Calà, C.; Fasciana, T.; Dowzicky, M.J. Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial. mSphere 2017, 2, e00310-16. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; and Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Weber, B.S.; Harding, C.M.; Feldman, M.F. Pathogenic Acinetobacter: From the Cell Surface to Infinity and Beyond. J. Bacteriol. 2016, 198, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front Cell Infect Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Sugawara, E.; Nikaido, H. OmpA is the principal nonspecific slow porin of Acinetobacter baumannii. J. Bacteriol. 2012, 194, 4089–4096. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, E.Y.; Lee, Y.C.; Park, T.I.; Kim, H.J.; Hyun, S.H.; Kim, S.A.; Lee, S.K.; Lee, J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005, 7, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- Kim, S.W.; Moon, D.C.; Jin, J.S.; Lee, J.H.; Shin, J.H.; Kim, J.M.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Lee, J.C. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol. Lett. 2009, 301, 224–231. [Google Scholar] [CrossRef]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Jahangiri, A.; Rasooli, I.; Owlia, P.; Fooladi, A.A.; Salimian, J. In silico design of an immunogen against Acinetobacter baumannii based on a novel model for native structure of Outer membrane protein A. Microb. Pathog. 2017, 105, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Moussa, S.H.; Durand-Réville, T.F.; Tommasi, R.; Miller, A. Acinetobacter baumannii OmpA Is a Selective Antibiotic Permeant Porin. Acs. Infect. Dis. 2018, 4, 373–381. [Google Scholar] [CrossRef]
- Samsudin, F.; Ortiz-Suarez, M.L.; Piggot, T.J.; Bond, P.J.; Khalid, S. OmpA: A Flexible Clamp for Bacterial Cell Wall Attachment. Structure 2016, 24, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.C.; Choi, C.H.; Lee, J.H.; Choi, C.W.; Kim, H.Y.; Park, J.S.; Kim, S.I.; Lee, J.C. Acinetobacter baumannii outer membrane protein A modulates the biogenesis of outer membrane vesicles. J. Microbiol. 2012, 50, 155–160. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, W.C.; Yeo, K.J.; Ryu, K.S.; Kumarasiri, M.; Hesek, D.; Lee, M.; Mobashery, S.; Song, J.H.; Kim, S.I.; et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012, 26, 219–228. [Google Scholar] [CrossRef]
- Kwon, H.I.; Kim, S.; Oh, M.H.; Na, S.H.; Kim, Y.J.; Jeon, Y.H.; Lee, J.C. Outer membrane protein A contributes to antimicrobial resistance of Acinetobacter baumannii through the OmpA-like domain. J. Antimicrob. Chemother. 2017, 72, 3012–3015. [Google Scholar] [CrossRef] [PubMed]
- Aaron, M.; Charbon, G.; Lam, H.; Schwarz, H.; Vollmer, W.; Jacobs-Wagner, C. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol. Microbiol. 2007, 64, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Arrigucci, R.; Pozzi, G. Identification of the chain-dispersing peptidoglycan hydrolase LytB of Streptococcus gordonii. PloS ONE 2017, 12, e0176117. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Peters, K.; Casanova, M.; Palacios, P.; VanNieuwenhze, M.; Breukink, E.; Vicente, M.; Vollmer, W. Z-ring membrane anchors associate with cell wall synthases to initiate bacterial cell division. Nat. Commun. 2018, 9, 5090. [Google Scholar] [CrossRef] [PubMed]
- Ha, R.; Frirdich, E.; Sychantha, D.; Biboy, J.; Taveirne, M.E.; Johnson, J.G.; DiRita, V.J.; Vollmer, W.; Clarke, A.J.; Gaynor, E.C. Accumulation of Peptidoglycan O-Acetylation Leads to Altered Cell Wall Biochemistry and Negatively Impacts Pathogenesis Factors of Campylobacter jejuni. J. Biol. Chem. 2016, 291, 22686–22702. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Byeon, J.H.; Jang, H.A.; Kim, J.K.; Yoo, J.W.; Kikuchi, Y.; Lee, B.L. Bacterial cell motility of Burkholderia gut symbiont is required to colonize the insect gut. FEBS Lett. 2015, 589, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Skerniškytė, J.; Krasauskas, R.; Péchoux, C.; Kulakauskas, S.; Armalytė, J.; Sužiedėlienė, E. Surface-Related Features and Virulence Among Acinetobacter baumannii Clinical Isolates Belonging to International Clones I and II. Front. Microbiol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Chiang, S.R.; Jung, F.; Tang, H.J.; Chen, C.H.; Chen, C.C.; Chou, H.Y.; Chuang, Y.C. Desiccation and ethanol resistances of multidrug resistant Acinetobacter baumannii embedded in biofilm: The favorable antiseptic efficacy of combination chlorhexidine gluconate and ethanol. J. Microbiol. Immunol. Infect. 2018, 51, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Luke, N.R.; Beanan, J.M.; Olson, R.; Sauberan, S.L.; MacDonald, U.; Schultz, L.W.; Umland, T.C.; Campagnari, A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010, 78, 3993–4000. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Confer, A.W.; Ayalew, S. The OmpA family of proteins: Roles in bacterial pathogenesis and immunity. Vet. Microbiol. 2013, 163, 207–222. [Google Scholar] [CrossRef]
- Smith, S.G.; Mahon, V.; Lambert, M.A.; Fagan, R.P. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 2007, 273, 1–11. [Google Scholar] [CrossRef]
- Maruvada, R.; Kim, K.S. Extracellular loops of the Eschericia coli outer membrane protein A contribute to the pathogenesis of meningitis. J. Infect. Dis. 2011, 203, 131–140. [Google Scholar] [CrossRef]
- Mittal, R.; Prasadarao, N.V. Outer membrane protein A expression in Escherichia coli K1 is required to prevent the maturation of myeloid dendritic cells and the induction of IL-10 and TGF-beta. J. Immunol. 2008, 181, 2672–2682. [Google Scholar] [CrossRef]
- Whitelegge, J. Gas-phase structure of the E. coli OmpA dimer. Structure 2014, 22, 666–667. [Google Scholar] [CrossRef]
- Tan, K.; Deatherage, K.B.L.; Wu, R.; Cuff, M.; Fan, Y.; Bigelow, L.; Jedrzejczak, R.P.; Adkins, J.N.; Cort, J.R.; Babnigg, G.; et al. Insights into PG-binding, conformational change, and dimerization of the OmpA C-terminal domains from Salmonella enterica serovar Typhimurium and Borrelia burgdorferi. Protein Sci. 2017, 26, 1738–1748. [Google Scholar] [CrossRef]
- Marcoux, J.; Politis, A.; Rinehart, D.; Marshall, D.P.; Wallace, M.I.; Tamm, L.K.; Robinson, C.V. Mass spectrometry defines the C-terminal dimerization domain and enables modeling of the structure of full-length OmpA. Structure 2014, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.U.; Park, J.S.; Bae, S.H.; Kim, H.Y.; Yeo, K.J.; Hwang, E.; Lee, K.Y.; Jee, J.G.; Cheong, H.K.; Jeon, Y.H. Ligand-Mediated Folding of the OmpA Periplasmic Domain from Acinetobacter baumannii. Biophys. J. 2017, 112, 2089–2098. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertson, T.G.; Elizabeth, D.R.C.; Nicole, D.; Christine, I.; Murat, B.; Jean, C.; Michael, V.N. FTT0831c/FTL_0325 Contributes to Francisella tularensis Cell Division, Maintenance of Cell Shape, and Structural Integrity. Infect. Immun. 2014, 82, 2935–2948. [Google Scholar] [CrossRef]
- Egan, A.J.F. Bacterial outer membrane constriction. Mol. Microbiol. 2018, 107, 676–687. [Google Scholar] [CrossRef]
- Wojdyla, J.A.; Cutts, E.; Kaminska, R.; Papadakos, G.; Hopper, J.T.; Stansfeld, P.J.; Staunton, D.; Robinson, C.V.; Kleanthous, C. Structure and function of the Escherichia coli Tol-Pal stator protein TolR. J. Biol. Chem. 2015, 290, 26675–26687. [Google Scholar] [CrossRef]
- Boes, A.; Olatunji, S.; Breukink, E.; Terrak, M. Regulation of the peptidoglycan polymerase activity of PBP1b by antagonist actions of the core divisome proteins FtsBLQ and FtsN. mBio 2019. [Google Scholar] [CrossRef]
- Ginez, L.D.; Osorio, A.; Poggio, S. Localization of the outer membrane protein OmpA2 in Caulobacter crescentus depends on the position of the gene in the chromosome. J. Bacteriol. 2014, 196, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, C.H.; Kim, J.W.; Lee, J.C. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J. Microbiol. 2010, 48, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Tomás, M.; Fernández, M.E.; Soares, N.C.; Carvajal, M.; Santillana, E.; Beceiro, A.; Romero, A.; Bou, G. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect. Immun. 2014, 82, 4666–4680. [Google Scholar] [CrossRef] [PubMed]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzún, D.P.; Barrera, B.; Gil, F.; Calderón, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Karthikeyan, R.; Gayathri, P.; RameshBabu, B.; Ahmed, G.; Jagannadham, M.V. Studies on the mechanism of multidrug resistance of Acinetobacter baumannii by proteomic analysis of the outer membrane vesicles of the bacterium. J. Proteins Proteom. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. Outer membrane vesicles from β-lactam-resistant Escherichia coli enable the survival of β-lactam-susceptible E. coli in the presence of β-lactam antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chavez, J.D.; Schweppe, D.K.; Zheng, C.; Weisbrod, C.R.; Eng, J.K.; Murali, A.; Lee, S.A.; Ramage, E.; Gallagher, L.A.; et al. In vivo protein interaction network analysis reveals porin-localized antibiotic inactivation in Acinetobacter baumannii strain AB5075. Nat. Commun. 2016, 7, 13414. [Google Scholar] [CrossRef] [PubMed]
- Poquet, I.; Saujet, L.; Canette, A.; Monot, M.; Mihajlovic, J.; Ghigo, J.M.; Soutourina, O.; Briandet, R.; Martin-Verstraete, I.; Dupuy, B. Clostridium difficile Biofilm: Remodeling Metabolism and Cell Surface to Build a Sparse and Heterogeneously Aggregated Architecture. Front. Microbiol. 2018, 9, 2084. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, J.C.; Kim, J.; Choi, C.H.; and Han, K. Simple Method for Markerless Gene Deletion in Multidrug-Resistant Acinetobacter baumannii. Appl. Environ. Microbiol. 2015, 81, 3357–3368. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; CSH Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
Sample Availability: All data generated and/or materials during this study are included in this article and are available from the corresponding author on reasonable request. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skerniškytė, J.; Karazijaitė, E.; Deschamps, J.; Krasauskas, R.; Briandet, R.; Sužiedėlienė, E. The Mutation of Conservative Asp268 Residue in the Peptidoglycan-Associated Domain of the OmpA Protein Affects Multiple Acinetobacter baumannii Virulence Characteristics. Molecules 2019, 24, 1972. https://doi.org/10.3390/molecules24101972
Skerniškytė J, Karazijaitė E, Deschamps J, Krasauskas R, Briandet R, Sužiedėlienė E. The Mutation of Conservative Asp268 Residue in the Peptidoglycan-Associated Domain of the OmpA Protein Affects Multiple Acinetobacter baumannii Virulence Characteristics. Molecules. 2019; 24(10):1972. https://doi.org/10.3390/molecules24101972
Chicago/Turabian StyleSkerniškytė, Jūratė, Emilija Karazijaitė, Julien Deschamps, Renatas Krasauskas, Romain Briandet, and Edita Sužiedėlienė. 2019. "The Mutation of Conservative Asp268 Residue in the Peptidoglycan-Associated Domain of the OmpA Protein Affects Multiple Acinetobacter baumannii Virulence Characteristics" Molecules 24, no. 10: 1972. https://doi.org/10.3390/molecules24101972
APA StyleSkerniškytė, J., Karazijaitė, E., Deschamps, J., Krasauskas, R., Briandet, R., & Sužiedėlienė, E. (2019). The Mutation of Conservative Asp268 Residue in the Peptidoglycan-Associated Domain of the OmpA Protein Affects Multiple Acinetobacter baumannii Virulence Characteristics. Molecules, 24(10), 1972. https://doi.org/10.3390/molecules24101972






