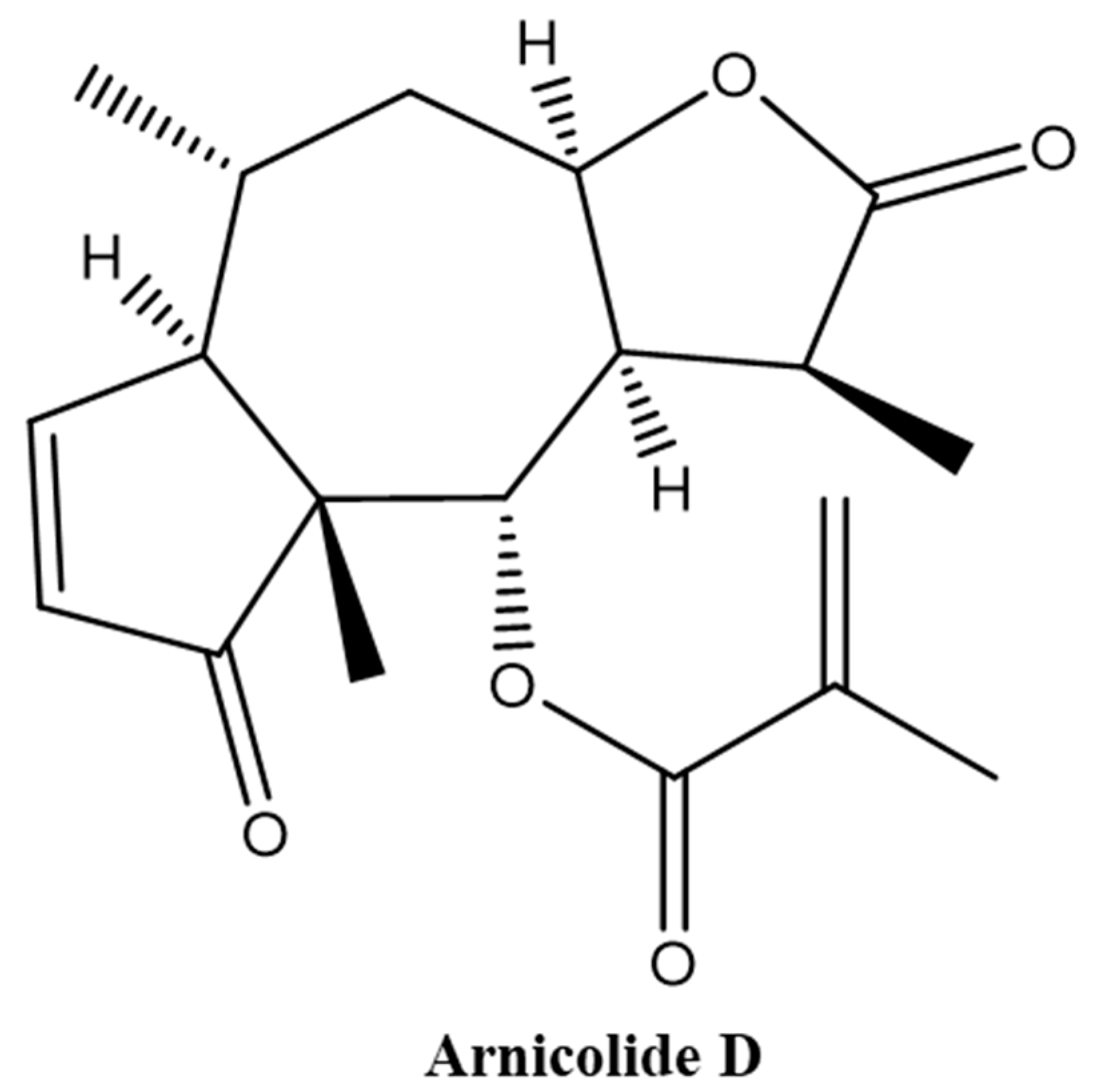
Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Results
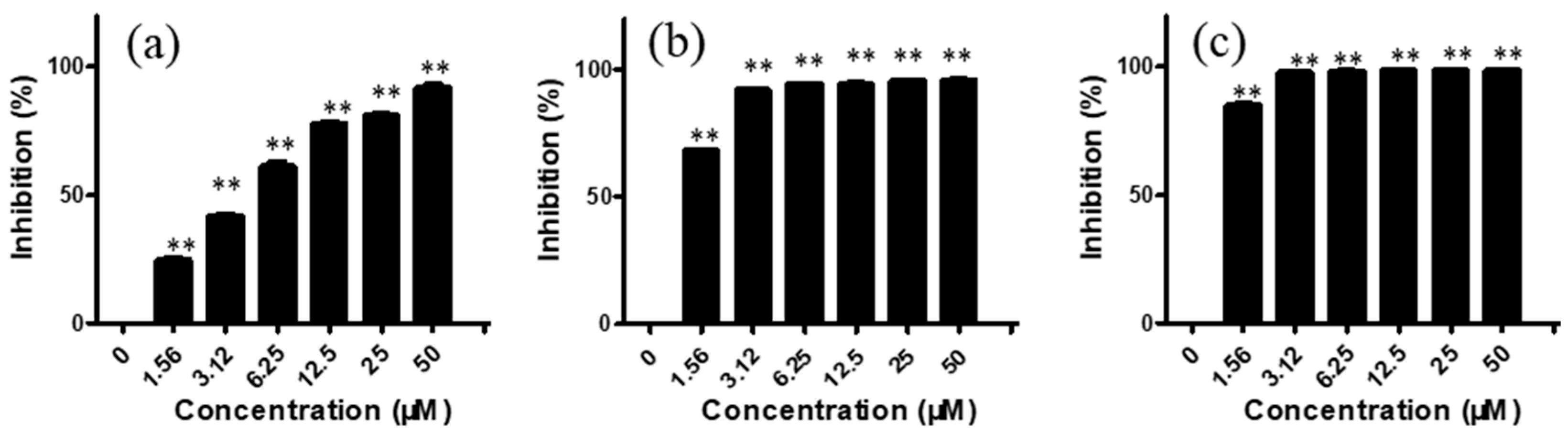
2.1. Arnicolide D Exhibited Cytotoxic Effects on NPC Cells In Vitro
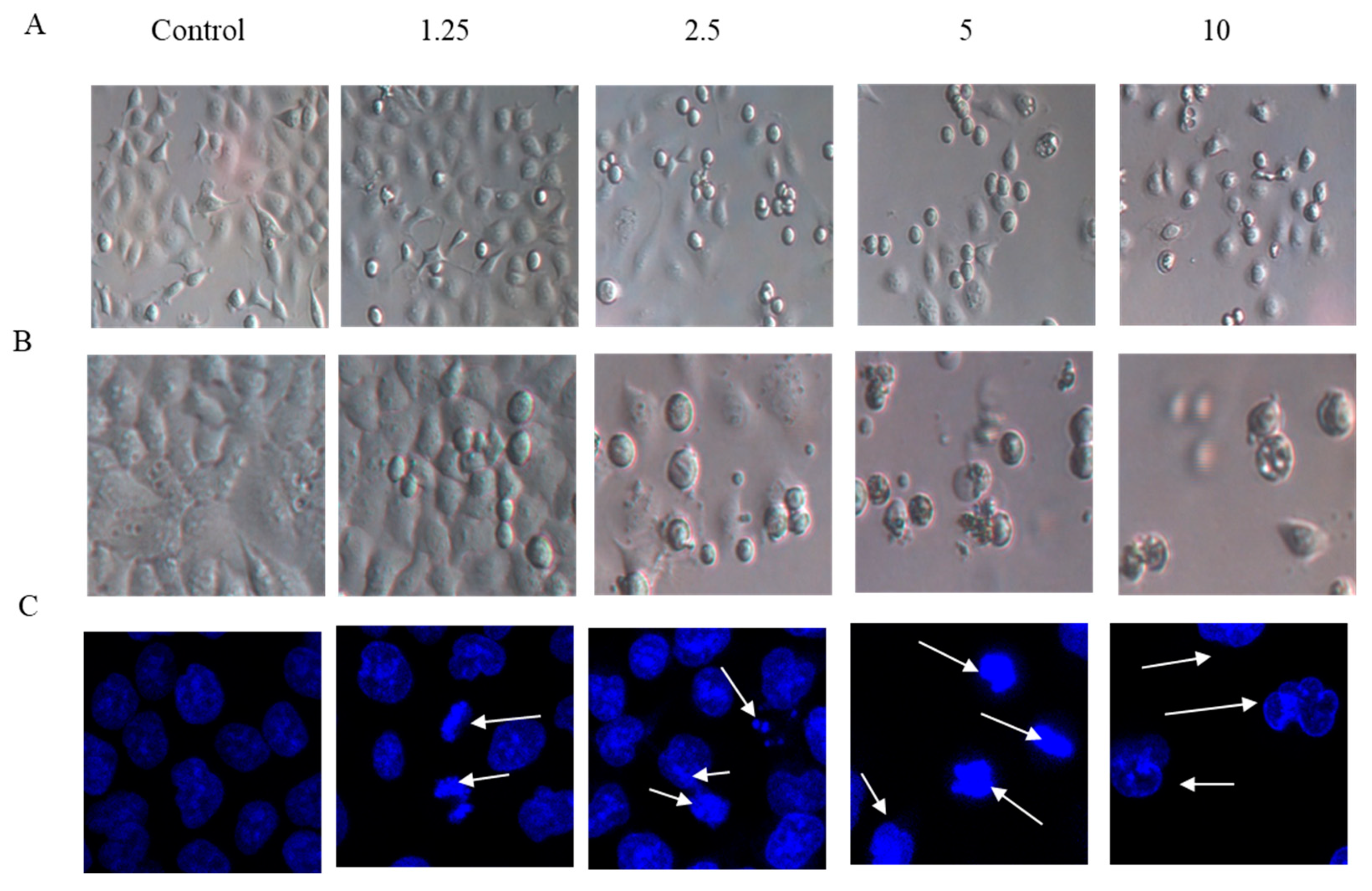
2.2. Cell Morphology
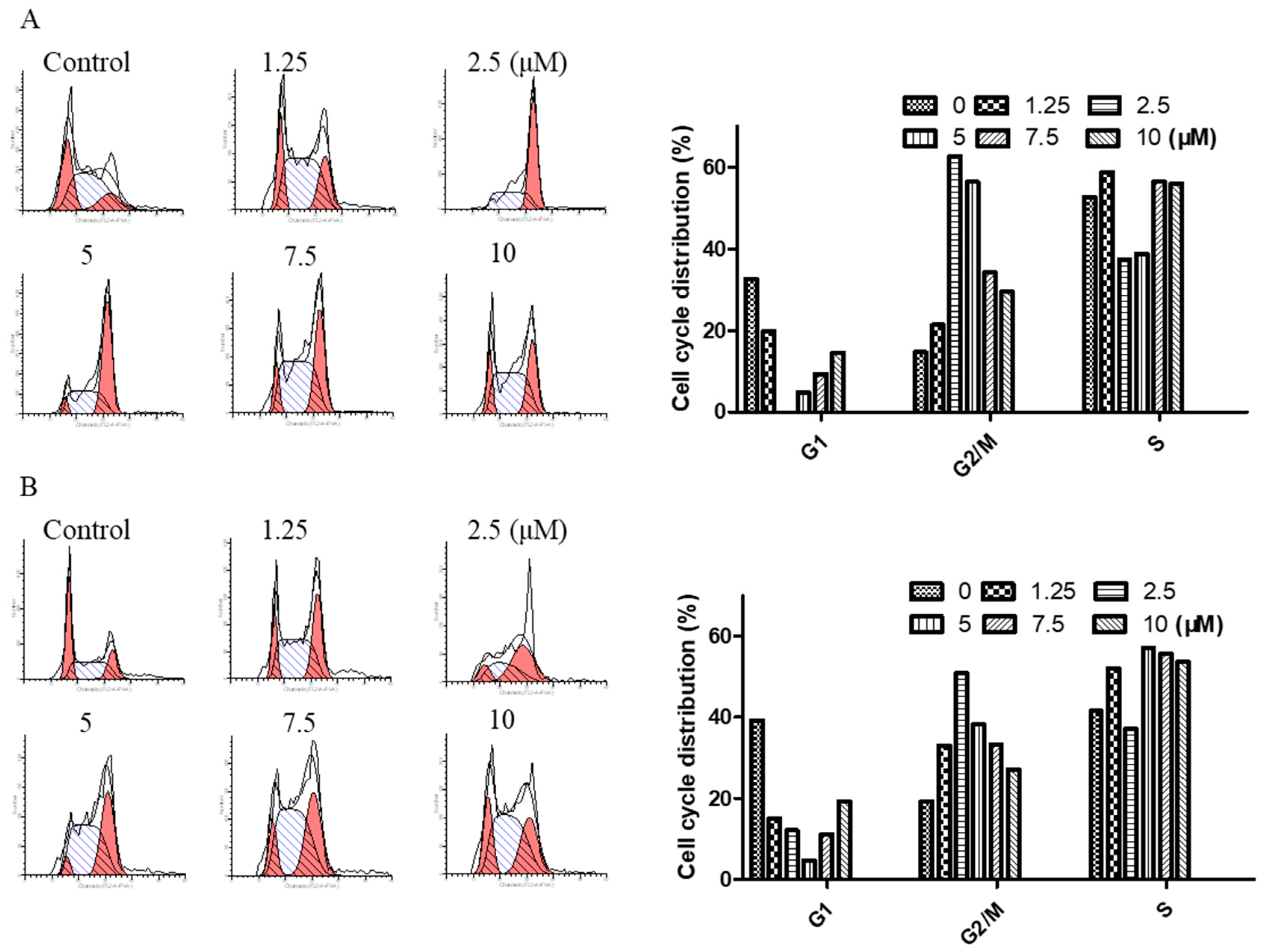
2.3. Arnicolide D Modulated Cell Cycle Distribution in NPC Cells
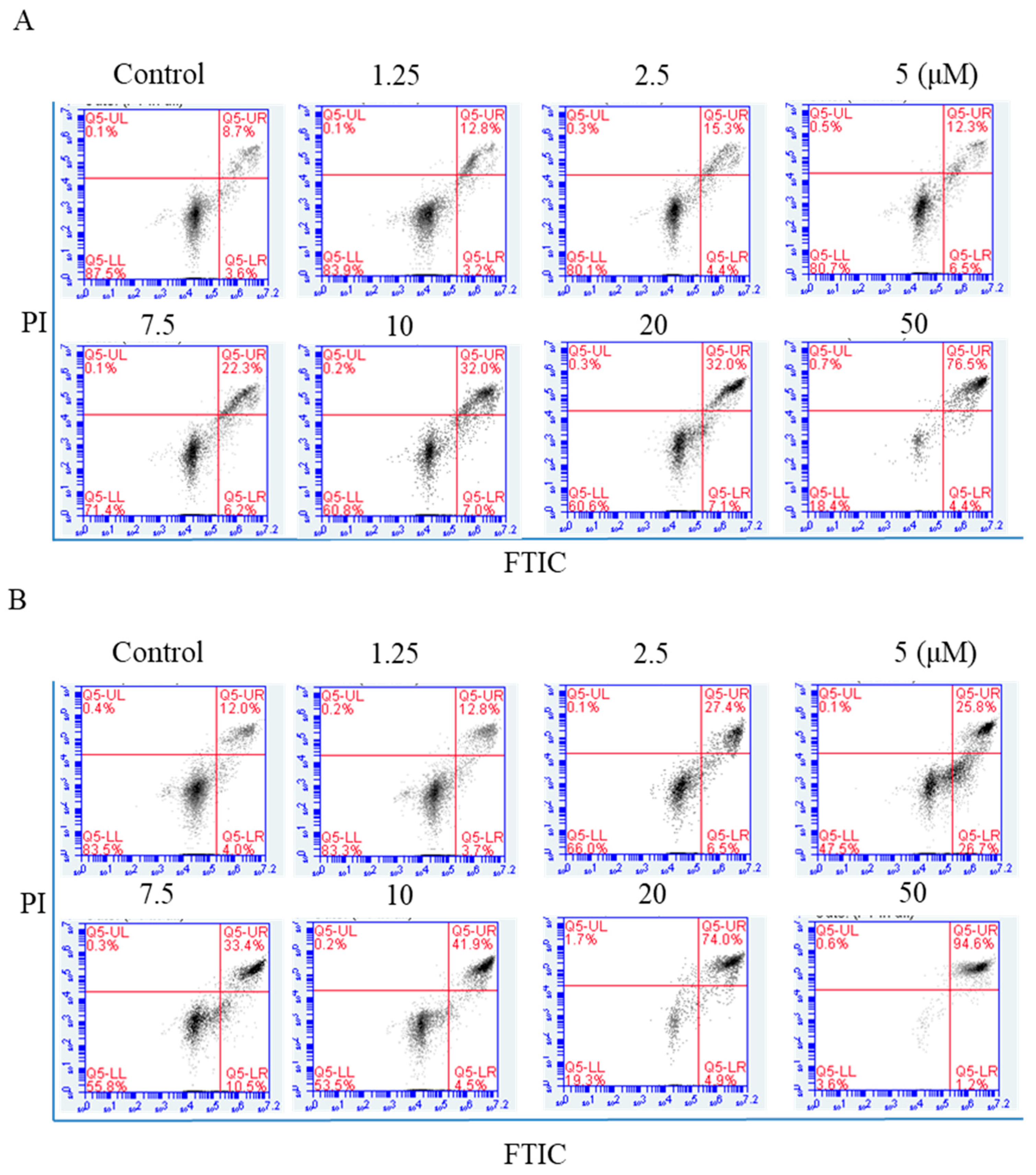
2.4. Flow Cytometric Analysis of Apoptosis
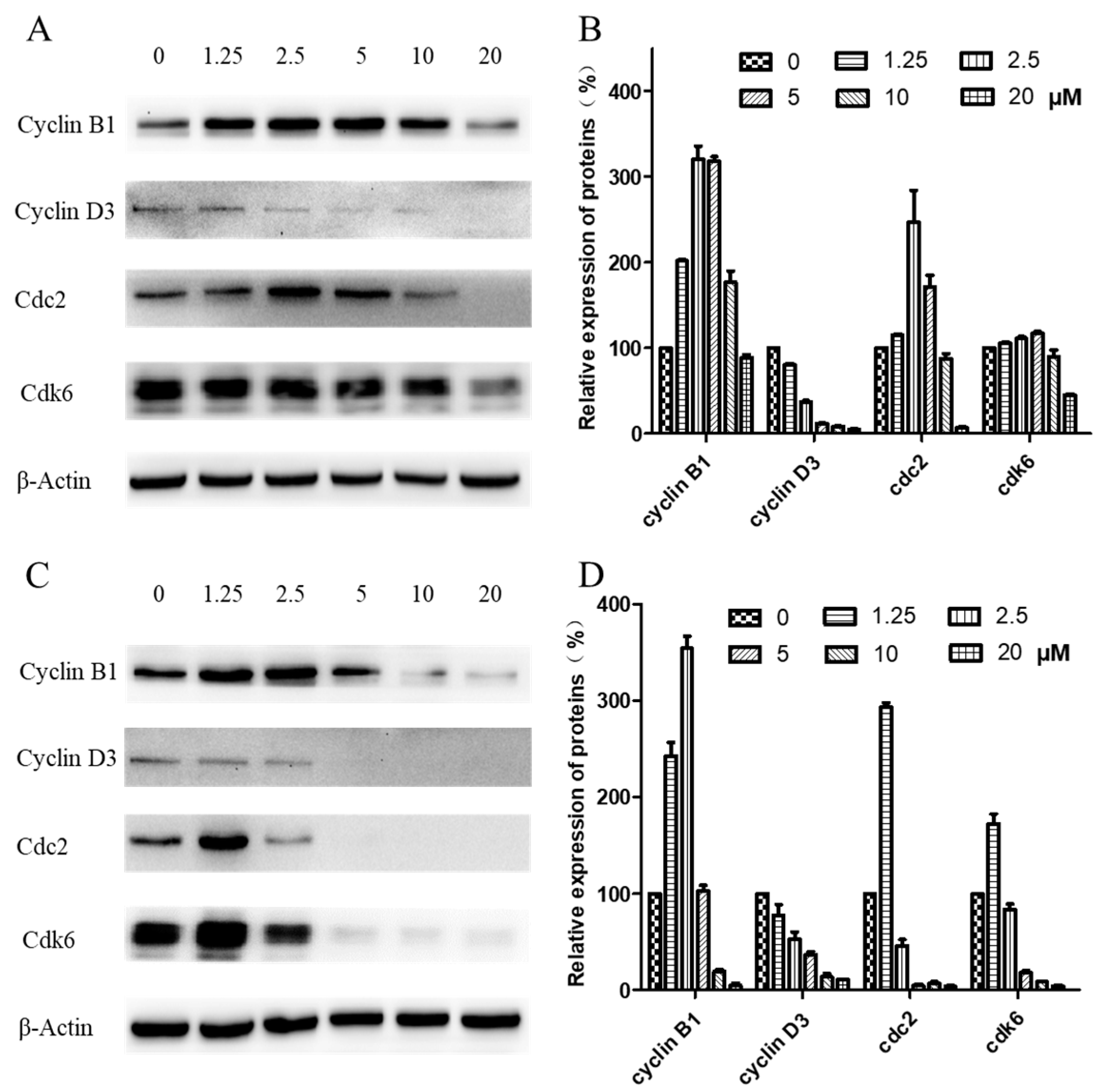
2.5. Western Blot Analysis of Cell-Cycle-Related Proteins
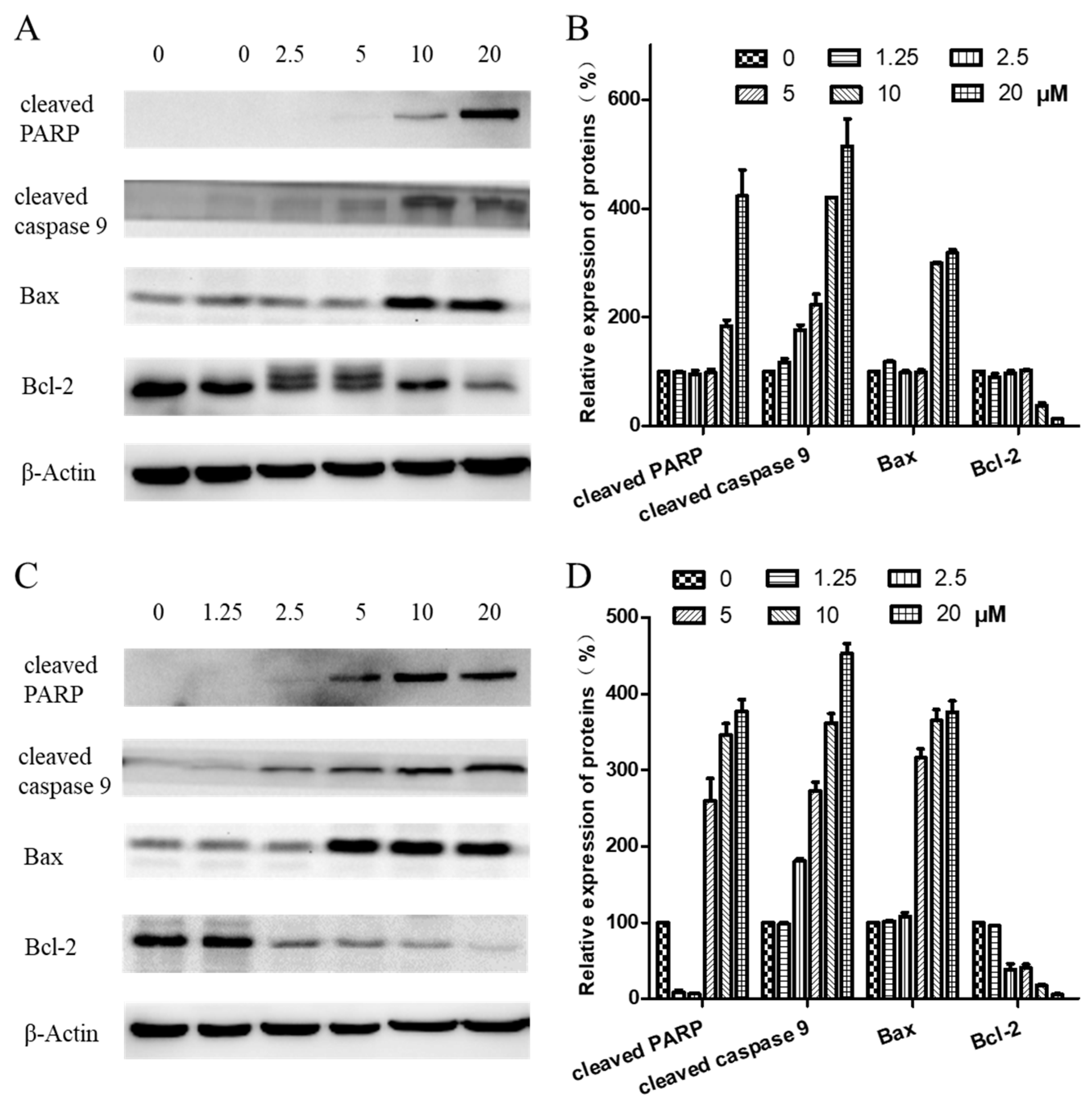
2.6. Expression of Proteins Related to Apoptosis
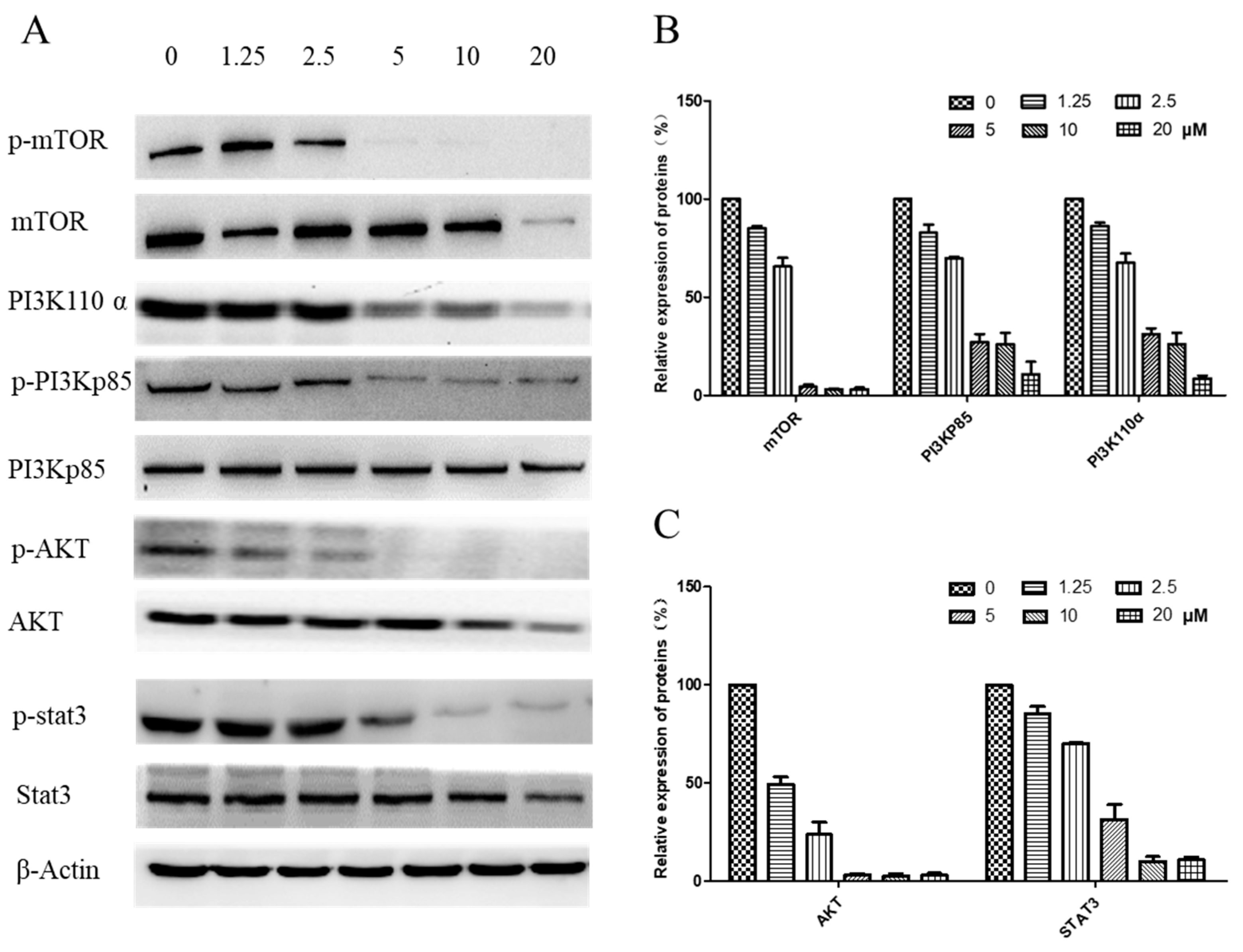
2.7. Regulation of the PI3K/AKT and STAT3 Signaling Pathway
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drugs and Reagents
5.2. Cell Culture and Drug Treatments
5.3. Cell Viability Assay
5.4. Cell Cycle Analysis
5.5. Annexin V-FITC Apoptosis Assay
5.6. Morphological Observation
5.7. Western Blot Analysis of the Cell-Cycle-Related Proteins
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liang, X.; Yang, J.; Gao, T.; Zhang, Z.; Chen, Y.; Zheng, R. Nasopharynx Cancer Epidemiology in China. China Cancer 2016, 25, 835–840. [Google Scholar]
- Dou, H.; Hu, D.; Lam, C.; Liu, Y.; Wang, X.; Zhang, W. Retrospective analysis of results of treatment for nasopharyngeal carcinoma in Macao. Chin. J. Cancer Res. 2014, 26, 148–158. [Google Scholar] [PubMed]
- Wei, K.; Yu, Y.; Yang, Y.; Ji, M.; Yu, B.; Liang, Z.; Ren, X. Epidemiology of Nasopharyngeal Carcinoma in China. Pract. Prev. Med. 2010, 17, 828–830. [Google Scholar]
- Ozoya, O.O.; Sokol, L.; Dalia, S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect. Disord. Drug Targets 2016, 16, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Bravi, F.; Polesel, J.; Bosetti, C.; Negri, E.; Garavello, W.; Taborelli, M.; Serraino, D.; Libra, M.; Montella, M.; et al. Adherence to the Mediterranean diet and nasopharyngeal cancer risk in Italy. Cancer Causes Control 2017, 28, 89–95. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Rong, X.; Peng, Y.; Tang, Y. Neurosurgery and prognosis in patients with radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy: a follow-up study. Radiat. Oncol. 2013, 8, 88. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Li, N.; Bauer, D.; Mendonca, P.; Taka, E.; Darb, M.; Thomas, L.; Williams, H.; Soliman, K. Natural product HTP screening for antibacterial (E.coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement Altern. Med. 2016, 16, 467:1–467:14. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission of People’s Republic of China. Pharmacopoeia of the People’s Republic Volume 1; China Med. Sci. Technol. Press: Beijing, China, 2015; ISBN 978-750-678-929-5. [Google Scholar]
- Chen, H.; Li, C.; Yu, M.; Xu, Y.; Ding, Z. Empirical study on the effect of different extracts of Centipede minima (L.) A. Br.et aschers on the proliferation of cell CNE-2. Chin. Arch. Trad. Chin. Med. 2011, 29, 1621–1623. [Google Scholar]
- Guo, Y.; Wang, W.; Chen, Z.; Chen, S. Proliferation inhibition and apoptosis induction of Centipeda minima extracts on human nasopharyngeal carcinoma cell CNE-1. Chin. J. Bioprocess Eng. 2013, 11, 65–75. [Google Scholar]
- Guo, Y.Q.; Sun, H.Y.; Chan, C.O.; Liu, B.B.; Wu, J.H.; Chan, S.W.; Mok, D.K.; Tse, A.K.; Yu, Z.L.; Chen, S.B. Centipeda minima (Ebushicao) extract inhibits PI3K-Akt-mTOR signaling in nasopharyngeal carcinoma CNE-1 cells. Chin. Med. 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Wu, J.B.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991, 39, 3272–3275. [Google Scholar] [CrossRef]
- Chan, C.O.; Jin, D.P.; Dong, N.P.; Chen, S.B.; Mok, D.K. Qualitative and quantitative analysis of chemical constituents of Centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J. Pharm. Biomed. Anal. 2016, 125, 400–407. [Google Scholar] [PubMed]
- Liu, Y.; Yang, Y.; Wu, H.; Liu, Y. Determination of brevilin in Centipeda minima by RP-HPLC. J. Chin. Med. Mater. 2005, 6, 473–474. [Google Scholar]
- Wang, Y.; Yu, R.Y.; Zhang, J.; Zhang, W.X.; Huang, Z.H.; Hu, H.F.; Li, Y.L.; Li, B.; He, Q.Y. Inhibition of Nrf2 enhances the anticancer effect of 6-O-angeloylenolin in lung adenocarcinoma. Biochem. Pharmacol. 2017, 129, 43–53. [Google Scholar] [CrossRef]
- Huang, X.; Awano, Y.; Maeda, E.; Asada, Y.; Takemoto, H.; Watanabe, T.; Kojima-Yuasa, A.; Kobayashi, Y. Cytotoxic activity of two natural sesquiterpene lactones, isobutyroylplenolin and arnicolide D, on human colon cancer cell line HT-29. Nat. Prod. Res. 2014, 28, 914–916. [Google Scholar] [CrossRef]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! A promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement Altern Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, C.; Butturini, E.; Sestito, R.; Madonna, S.; Cavani, A.; Mariotto, S.; Albanesi, C. Inhibition of inflammatory and proliferative responses of human keratinocytes exposed to the sesquiterpene lactones dehydrocostuslactone and costunolide. PLoS ONE 2014, 9, e107904. [Google Scholar] [CrossRef]
- Ding, L.F.; Liu, Y.; Liang, H.X.; Liu, D.P.; Zhou, G.B.; Cheng, Y.X. Two new terpene glucosides and antitumor agents from Centipeda minima. J. Asian Nat. Prod. Res. 2009, 11, 732–736. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Yang, Y.; Liu, J.; Chen, Z. Sesquiterpene lactone 6-O-angeloylplenolin reverses vincristine resistance by inhibiting YB-1 nuclear translocation in colon carcinoma cells. Oncol. Lett. 2018, 15, 9673–9680. [Google Scholar] [CrossRef]
- Taylor, R.S.L.; Towers, G.H.N. Antibacterial constituents of the nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Nan, J.; Zhang, X.; Qin, X.; Wang, Y.; Hou, J.; Wang, Q.; Yang, J. Brevilin A, a novel natural product, inhibits janus kinase activity and blocks STAT3 signaling in cancer cells. PLoS ONE 2013, 8, e63697. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Zhang, B.; Cheng, Y.X. Small compound 6-O-angeloylplenolin induces caspase-dependent apoptosis in human multiple myeloma cells. Oncol. Lett. 2013, 6, 556–558. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.Q.; Wang, G.; Yang, L.N.; Lu, Y.Z.; Li, X.C.; Zhou, B.; Qu, L.W.; Wang, X.L.; Cheng, Y.X.; et al. Proteomic identification of the oncoprotein STAT3 as a target of a novel Skp1 inhibitor. Oncotarget 2017, 8, 2681–2693. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.Q.; Liang, H.X.; Zhang, F.X.; Zhang, B.; Jin, J.; Chen, Y.L.; Cheng, Y.X.; Zhou, G.B. Small compound 6-O-angeloylplenolin induces mitotic arrest and exhibits therapeutic potentials in multiple myeloma. PLoS ONE 2011, 6, e21930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; Cheng, X.; Lu, Y.Z.; Wang, G.Z.; Li, X.C.; Zhang, J.; Wen, Z.S.; Huang, Z.L.; Gao, Q.L.; et al. Skp1 in lung cancer: clinical significance and therapeutic efficacy of its small molecule inhibitors. Oncotarget 2015, 6, 34953–34967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. Oncol. Targets Ther. 2018, 11, 7031–7040. [Google Scholar] [CrossRef]
- You, P.; Wu, H.; Deng, M.; Peng, J.; Li, F.; Yang, Y. Brevilin A induces apoptosis and autophagy of colon adenocarcinoma cell CT26 via mitochondrial pathway and PI3K/AKT/mTOR inactivation. Biomed. Pharmacother. 2018, 98, 619–625. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Huang, Y.; Yang, Y.; Liu, Y.; Liu, J. 6-O-Angeloylenolin induces apoptosis through a mitochondrial/caspase and NF-κB pathway in human leukemia HL60 cells. Biomed. Pharmacother. 2008, 62, 401–409. [Google Scholar]
- Su, M.; Chung, H.; Li, Y. 6-O-Angeloylenolin induced cell-cycle arrest and apoptosis in human nasopharyngeal cancer cells. Chem.-Biol. Interact. 2011, 189, 167–176. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zhongliang, C. New Guaianolides from Centipeda minima. Chin. Sci. Bull. 1984, 29, 900. [Google Scholar]
- Pu, S.; Guo, Y.; Gao, W. Chemical constituents from Centipeda minima. Zhongguo Zhong Yao Za Zhi 2019, 34, 1520–1522. [Google Scholar]
- Yang, Y.F.; Zhu, Y.P.; Zhang, B.W.; Yan, B.; Wu, H.Z. Determination of two sesquiterpenoids in Centipeda minina during different harvest periods. J. Lishizhen Med. Mater. Med. Res. 2014, 25, 1209–1211. [Google Scholar]
- Cao, S.M.; Chen, S.H.; Qian, C.N.; Liu, Q.; Xia, Y.F. Familial nasopharyngeal carcinomas possess distinguished clinical characteristics in southern China. Chin. J. Cancer Res. 2014, 26, 543–549. [Google Scholar] [PubMed]
- Nasopharyngeal Cancer: Statistics. Available online: http://www.cancer.net/cancer-types/nasopharyngeal-cancer/statistics (accessed on 19 April 2018).
- Bosco, A.; Golsteyn, R.M. Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules 2017, 22. [Google Scholar] [CrossRef]
- King, R.; Jackson, P.; Kirschner, M. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Widrow, R.; Rabinovitch, P.; Cho, K.; Laird, C. Separation of cells at different times within G2 and mitosis by cyclin B1 flow cytometry. Cytometry 1997, 27, 250–254. [Google Scholar] [CrossRef]
- Lin, H.F.; Hsieh, M.J.; Hsi, Y.T.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K.; Chien, S.Y. Celastrol-induced apoptosis in human nasopharyngeal carcinoma is associated with the activation of the death receptor and the mitochondrial pathway. Oncol. Lett. 2017, 14, 1683–1690. [Google Scholar] [CrossRef]
- Mei, Y.P.; Zhou, J.M.; Wang, Y.; Huang, H.; Deng, R.; Feng, G.K.; Zeng, Y.X.; Zhu, X.F. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle 2007, 6, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tian, J.; Lv, Y.; Shi, F.; Kong, F.; Shi, H.; Zhao, L. Leptin induces functional activation of cyclooxygenase-2 through jak2/stat3, mapk/erk, and pi3k/akt pathways in human endometrial cancer cells. Cancer Sci. 2009, 100, 389–395. [Google Scholar] [CrossRef]
- Daniel, M.; Mcleod, H.L. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anti-Cancer Drugs 2005, 16, 797–803. [Google Scholar]
- Liu, Z.; Ma, L.; Wen, Z.S.; Hu, Z.; Wu, F.Q.; Li, W.; Liu, J.; Zhou, G.B. Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis 2014, 35, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Z.; Chen, J.J.; Xue, K.; Wang, Z.G.; Yu, D. Clinicopathologic and prognostic significance of VEGF, JAK2 and STAT3 in patients with nasopharyngeal carcinoma. Cancer Cell Int. 2018, 18, 110:1–110:9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Xu, Y.; Jiang, Z.Z.; Guerram, M.; Wang, B.; Zhu, X.; Zhang, L.Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in SGC-7901 cells and inhibits tumor growth in vivo. Molecules 2015, 20, 1661–1675. [Google Scholar] [CrossRef]
- Luo, M.; Liu, X.; Zu, Y.; Fu, Y.; Zhang, S.; Yao, L.; Efferth, T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2010, 188, 151–160. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The samples from the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Dow Chan, B.; Mok, D.K.-W.; Lee, C.-S.; Tai, W.C.-S.; Chen, S. Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma. Molecules 2019, 24, 1908. https://doi.org/10.3390/molecules24101908
Liu R, Dow Chan B, Mok DK-W, Lee C-S, Tai WC-S, Chen S. Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma. Molecules. 2019; 24(10):1908. https://doi.org/10.3390/molecules24101908
Chicago/Turabian StyleLiu, Rui, Brandon Dow Chan, Daniel Kam-Wah Mok, Chi-Sing Lee, William Chi-Shing Tai, and Sibao Chen. 2019. "Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma" Molecules 24, no. 10: 1908. https://doi.org/10.3390/molecules24101908
APA StyleLiu, R., Dow Chan, B., Mok, D. K.-W., Lee, C.-S., Tai, W. C.-S., & Chen, S. (2019). Arnicolide D, from the herb Centipeda minima, Is a Therapeutic Candidate against Nasopharyngeal Carcinoma. Molecules, 24(10), 1908. https://doi.org/10.3390/molecules24101908







