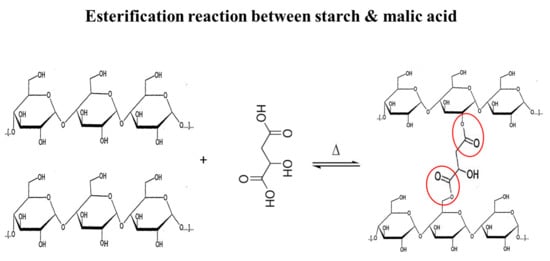
Structural Characteristics and In Vitro Digestibility of Malic Acid-Treated Corn Starch with Different pH Conditions
Abstract
:1. Introduction
2. Results and Discussion
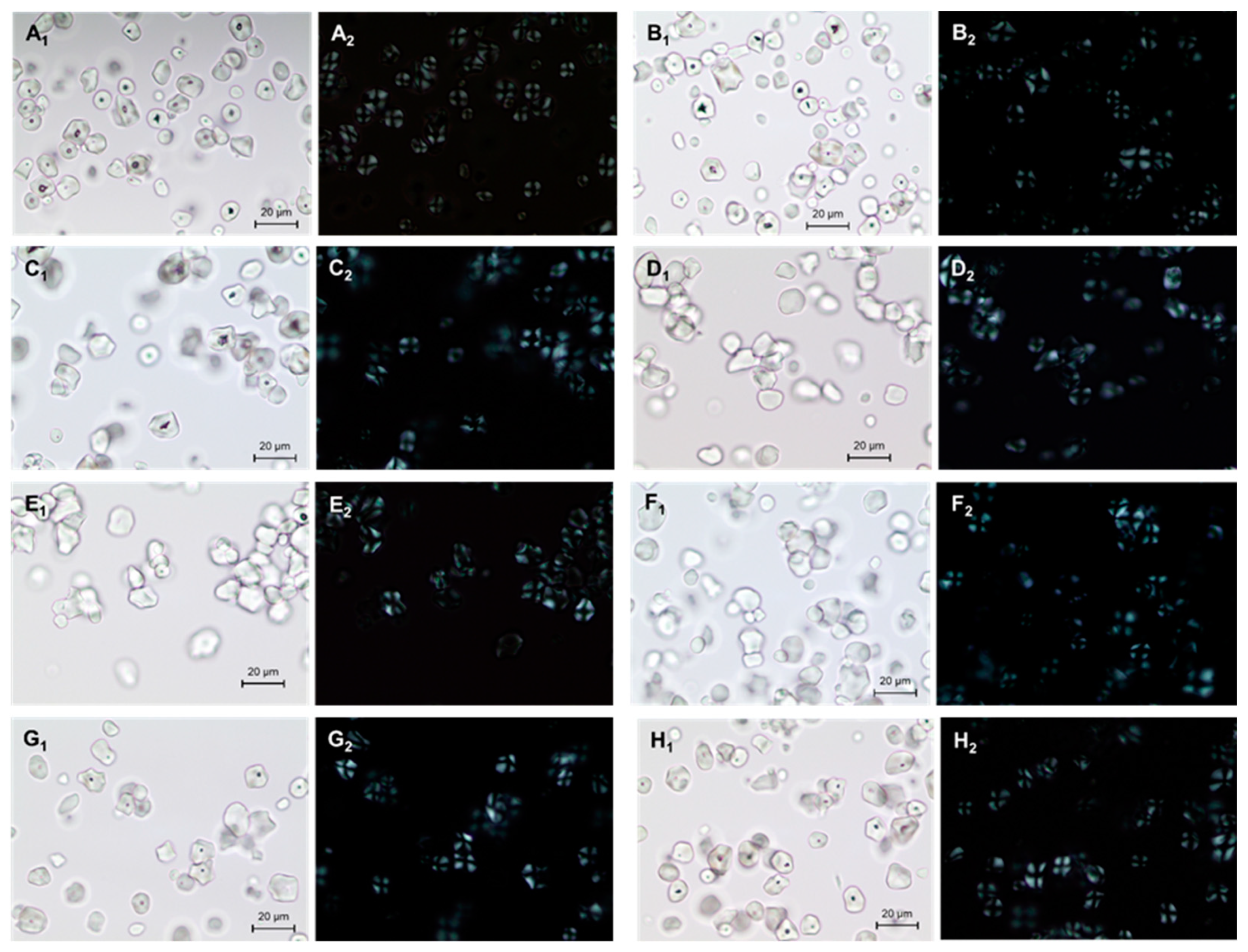
2.1. Light Microscope
2.2. Degree of Substitution (DS)
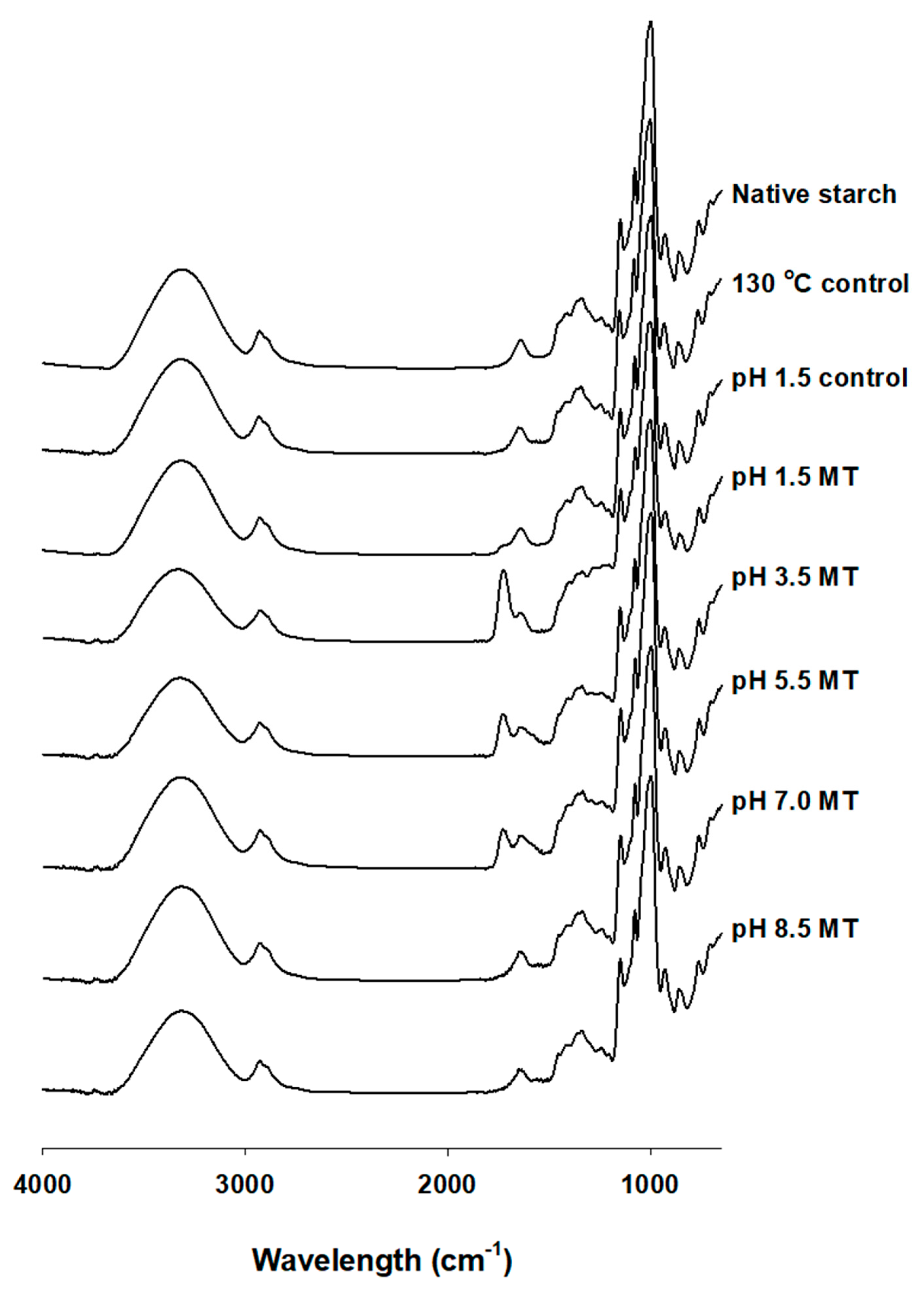
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
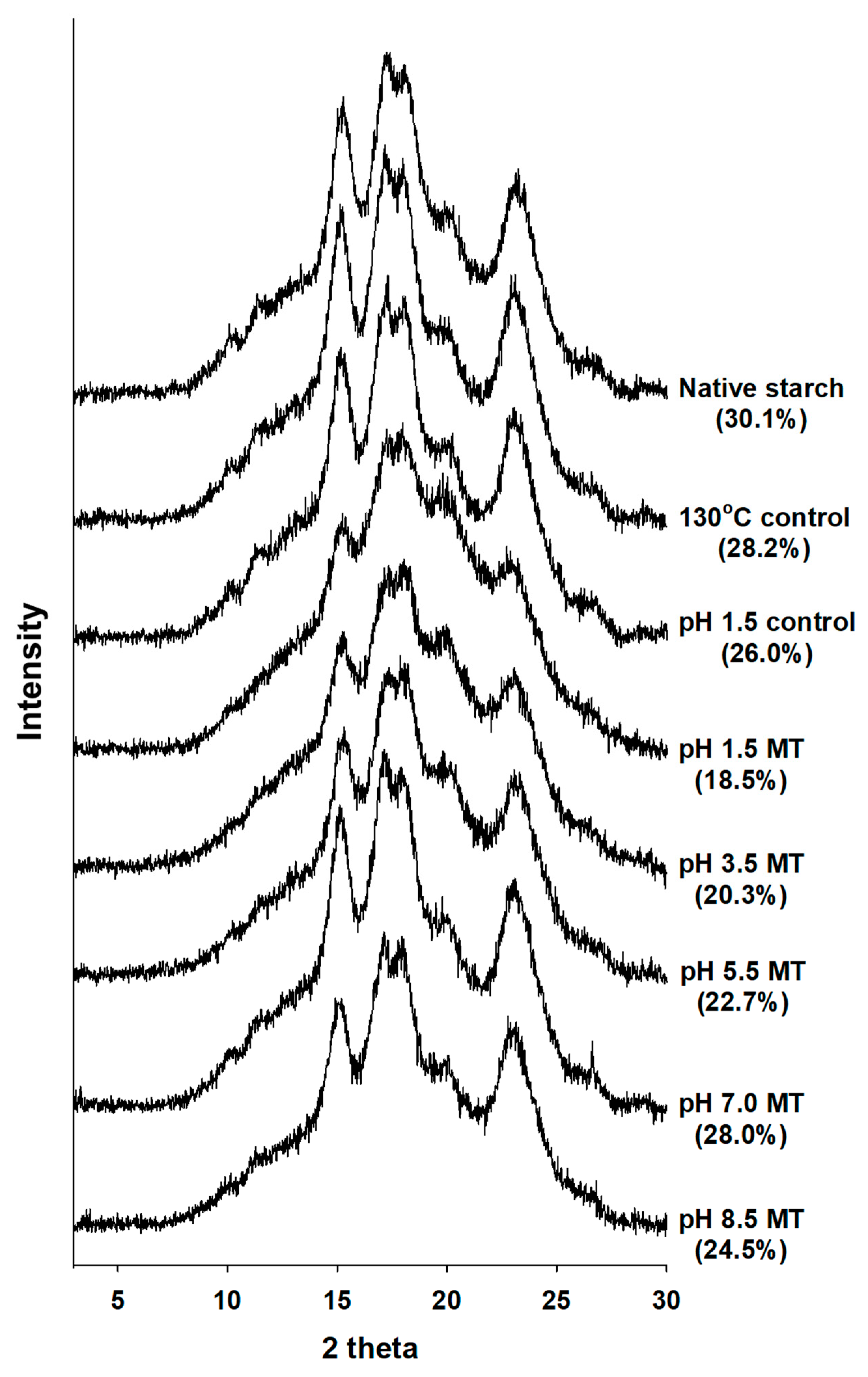
2.4. X-ray Diffraction
2.5. In Vitro Digestibility
3. Materials and Methods
3.1. Materials
3.2. Preparation of Malic Acid-Treated Corn Starch
3.3. Light Microscopy
3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.5. Degree of Substitution (DS)
3.6. In Vitro Digestibility
3.7. X-ray Diffraction
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mun, S.H.; Shin, M.S. The effects of annealing on resistant starch contents of cross-linked maize starches. Korean J. Food Sci. Technol. 2002, 34, 431–436. [Google Scholar]
- Tie, J.; Lee, E.S.; Hong, S.T.; Ryu, G.H. Manufacturing of Goami flakes by using extrusion process. Korean J. Food Sci. Technol. 2007, 39, 146–151. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–a review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Oh, S.H.; Shin, M.S.; Choi, I.S. The effect of resistant starch on physio-nutrition availability in human. Korean J. Nutr. 2002, 35, 932–942. [Google Scholar]
- Bravo, L.; Englyst, H.N.; Hudson, G.J. Nutritional evaluation of carbohydrates in the Spanish diet: Non-starch polysaccharides and in vitro starch digestibility of breads and breakfast products. Food Res. Int. 1998, 31, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.U.; Maningat, C.C.; Woo, K.; Seib, P.A. In vitro digestion of RS4-type resistant wheat and potato starches, and fermentation of indigestible fractions. Cereal Chem. 2011, 88, 72–79. [Google Scholar] [CrossRef]
- Xie, X.S.; Liu, Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch Starke 2004, 56, 364–370. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, K.Y.; Lee, H.G. Effect of different pH conditions on the in vitro digestibility and physicochemical properties of citric acid-treated potato starch. Int. J. Biol. Macromol. 2018, 107, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Shin, M. Effects of organic acids and starch water ratios on the properties of retrograded maize starches. Food Sci. Biotechnol. 2011, 20, 1013–1019. [Google Scholar] [CrossRef]
- Klaushofer, H.; Berghofer, E.; Steyrer, W. Stärkecitrate–Produktion und anwendungs-technische Eigenschaften. Starch Starke 1978, 30, 47–51. [Google Scholar] [CrossRef]
- Lakso, A.N.; Kliewer, W.M. The influence of temperature on malic acid metabolism in grape berries: I. Enzyme responses. Plant Physiol. 1975, 56, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- Junistia, L.; Sugih, A.K.; Manurung, R.; Picchioni, F.; Janssen, L.P.; Heeres, H.J. Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch-Starke 2008, 60, 667–675. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, S. Physicochemical properties, molecular structure, and uses of sweetpotato starch. Trends Food Sci. Technol. 2014, 36, 68–78. [Google Scholar] [CrossRef]
- Tian, S.J.; Rickard, J.E.; Blanshard, J.M.V. Physicochemical properties of sweet potato starch. J. Sci. Food Agric. 1991, 57, 459–491. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.J.; Shin, S.I.; Sohn, M.R.; Lee, C.J.; Kim, Y.; Cho, W.II.; Moon, T.W. Resistant glutarate starch from adlay: Preparation and properties. Carbohydr. Polym. 2008, 74, 787–796. [Google Scholar] [CrossRef]
- Błaszczak, W.; Fornal, J.; Valverde, S.; Garrido, L. Pressure-induced changes in the structure of corn starches with different amylose content. Carbohydr. Polym. 2005, 61, 132–140. [Google Scholar] [CrossRef]
- Xia, H.; Li, Y.; Gao, Q. Preparation and properties of RS4 citrate sweet potato starch by heat-moisture treatment. Food Hydrocoll. 2016, 55, 172–178. [Google Scholar] [CrossRef]
- Bajpai, A.; Shrivastava, J. In vitro enzymatic degradation kinetics of polymeric blends of crosslinked starch and carboxymethyl cellulose. Polym. Int. 2005, 54, 1524–1536. [Google Scholar] [CrossRef]
- Zarski, A.; Ptak, S.; Siemion, P.; Kapusniak, J. Esterification of potato starch by a biocatalysed reaction in an ionic liquid. Carbohydr. Polym. 2016, 137, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.N.; Ali, F.; Goswami, T.; Ghosh, A.C. Esterification of high α-cellulose extracted from Hibiscus cannabinus L. Ind. Crops Prod. 1995, 4, 233–239. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Jane, J.L.; Wong, K.S.; McPherson, A.E. Branch-structure difference in starches of A-and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr. Res. 1997, 300, 219–227. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. I. Structural aspects provide insight into crystallinity characteristics and gelatinisation behaviour of granular starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Woo, K.S.; Seib, P.A. Cross-linked resistant starch: Preparation and properties. Cereal Chem. 2002, 79, 819–825. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and characterization of starch acetates with high substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Shin, S.I.; Lee, C.J.; Kim, D.I.; Lee, H.A.; Cheong, J.J.; Chung, K.M.; Baik, M.-Y.; Park, C.S.; Kim, C.H.; Moon, T.W. Formation, characterization, and glucose response in mice to rice starch with low digestibility produced by citric acid treatment. J. Cereal Sci. 2007, 45, 24–33. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample | Degree of Substitution |
|---|---|
| Native starch | 0.0004 ± 0.0002 a |
| 130 °C control | 0.0002 ± 0.0001 a |
| pH 1.5 control | 0.0383 ± 0.0067 b |
| pH 1.5 MT | 0.2156 ± 0.0103 c |
| pH 3.5 MT | 0.1979 ± 0.0035 d |
| pH 5.5 MT | 0.0897 ± 0.0064 e |
| pH 7.0 MT | 0.0366 ± 0.0071 b |
| pH 8.5 MT | 0.0349 ± 0.0032 b |
| Sample | Uncooked Starch * | Cooked Starch | ||||
|---|---|---|---|---|---|---|
| RDS (%) | SDS (%) | RS (%) | RDS (%) | SDS (%) | RS (%) | |
| Native starch | 8.20 ± 0.73 a | 67.1 ± 1.29 f | 24.7 ± 1.99 a | 72.5 ± 0.39 d | 8.47 ± 0.59 a | 19.1 ± 0.69 b |
| 130 °C control | 8.62 ± 2.06 a | 68.4 ± 1.78 f | 23.0 ± 1.92 a | 76.8 ± 1.07 e | 6.70 ± 0.34 a | 16.5 ± 0.76 a |
| pH 1.5 control | 29.0 ± 1.45 c | 45.2 ± 1.78 d | 25.7 ± 0.83 ab | 71.6 ± 1.60 d | 6.52 ± 0.99 a | 21.9 ± 0.69 c |
| pH 1.5 MT | 9.12 ± 0.68 a | 3.01 ± 0.53 a | 87.9 ± 1.21 f | 17.4 ± 1.78 a | 7.83 ± 1.02 a | 74.8 ± 1.34 f |
| pH 3.5 MT | 36.7 ± 0.50 d | 11.7 ± 0.66 c | 51.9 ± 1.57 e | 38.6 ± 1.99 b | 12.6 ± 0.94 b | 48.8 ± 1.05 e |
| pH 5.5 MT | 46.2 ± 1.06 e | 7.54 ± 1.32 b | 46.4 ± 1.09 d | 49.0 ± 1.89 c | 4.32 ± 0.77 a | 46.8± 1.15 d |
| pH 7.0 MT | 23.0 ± 1.66 b | 47.4 ± 1.36 de | 29.6 ± 1.71 c | 70.9 ± 1.17 d | 7.64 ± 2.14 a | 21.5 ± 1.25 c |
| pH 8.5 MT | 22.4 ± 1.22 b | 48.9 ± 1.44 e | 28.6 ± 0.26 b | 74.0 ± 1.59 de | 7.88 ± 1.64 a | 18.2 ± 0.63 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.J.; Na, J.H.; Park, J.-Y.; Chang, P.-S. Structural Characteristics and In Vitro Digestibility of Malic Acid-Treated Corn Starch with Different pH Conditions. Molecules 2019, 24, 1900. https://doi.org/10.3390/molecules24101900
Lee CJ, Na JH, Park J-Y, Chang P-S. Structural Characteristics and In Vitro Digestibility of Malic Acid-Treated Corn Starch with Different pH Conditions. Molecules. 2019; 24(10):1900. https://doi.org/10.3390/molecules24101900
Chicago/Turabian StyleLee, Chang Joo, Jong Hee Na, Jun-Young Park, and Pahn-Shick Chang. 2019. "Structural Characteristics and In Vitro Digestibility of Malic Acid-Treated Corn Starch with Different pH Conditions" Molecules 24, no. 10: 1900. https://doi.org/10.3390/molecules24101900
APA StyleLee, C. J., Na, J. H., Park, J.-Y., & Chang, P.-S. (2019). Structural Characteristics and In Vitro Digestibility of Malic Acid-Treated Corn Starch with Different pH Conditions. Molecules, 24(10), 1900. https://doi.org/10.3390/molecules24101900