Hydrogen Desorption Properties of LiBH4/xLiAlH4 (x = 0.5, 1, 2) Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
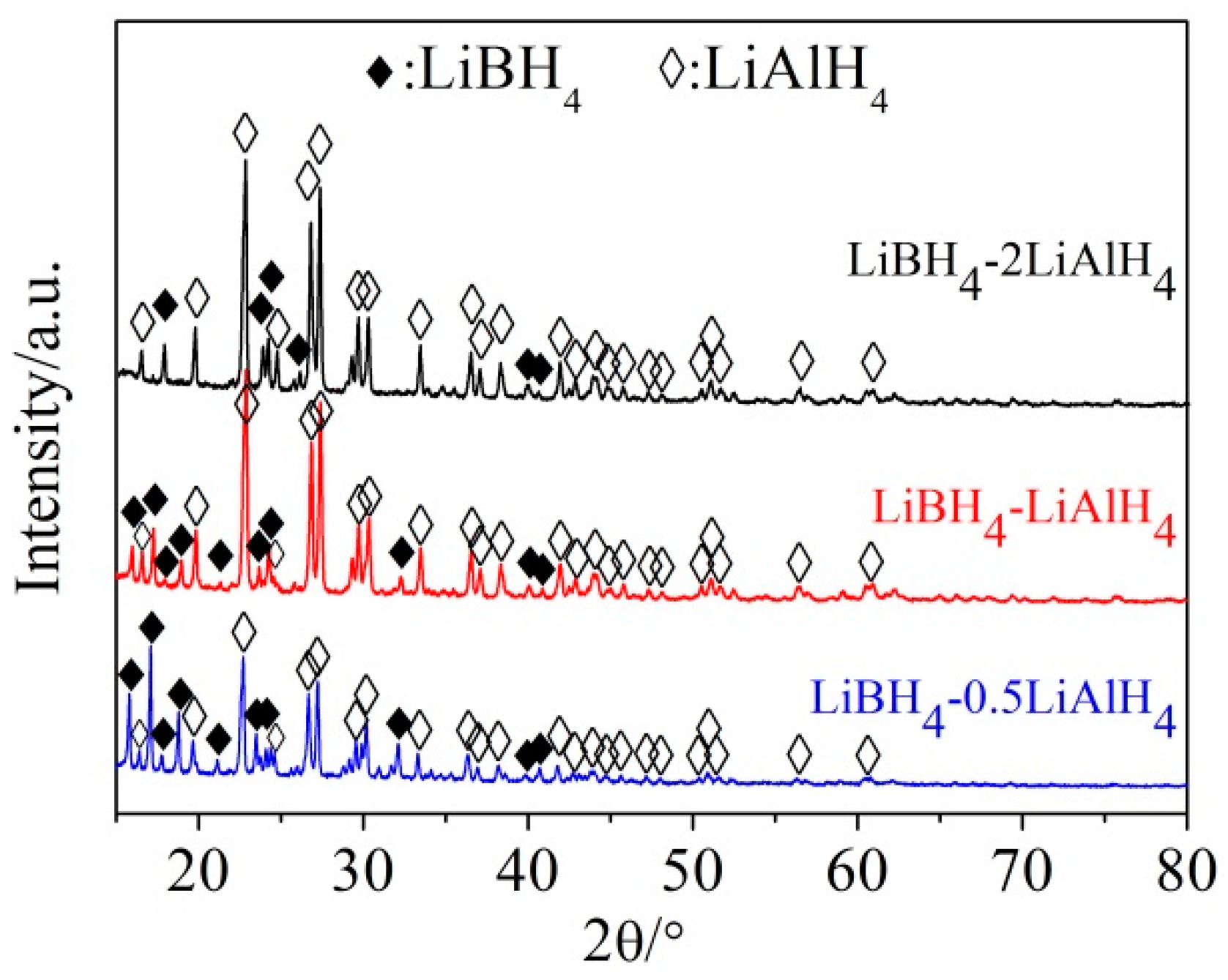
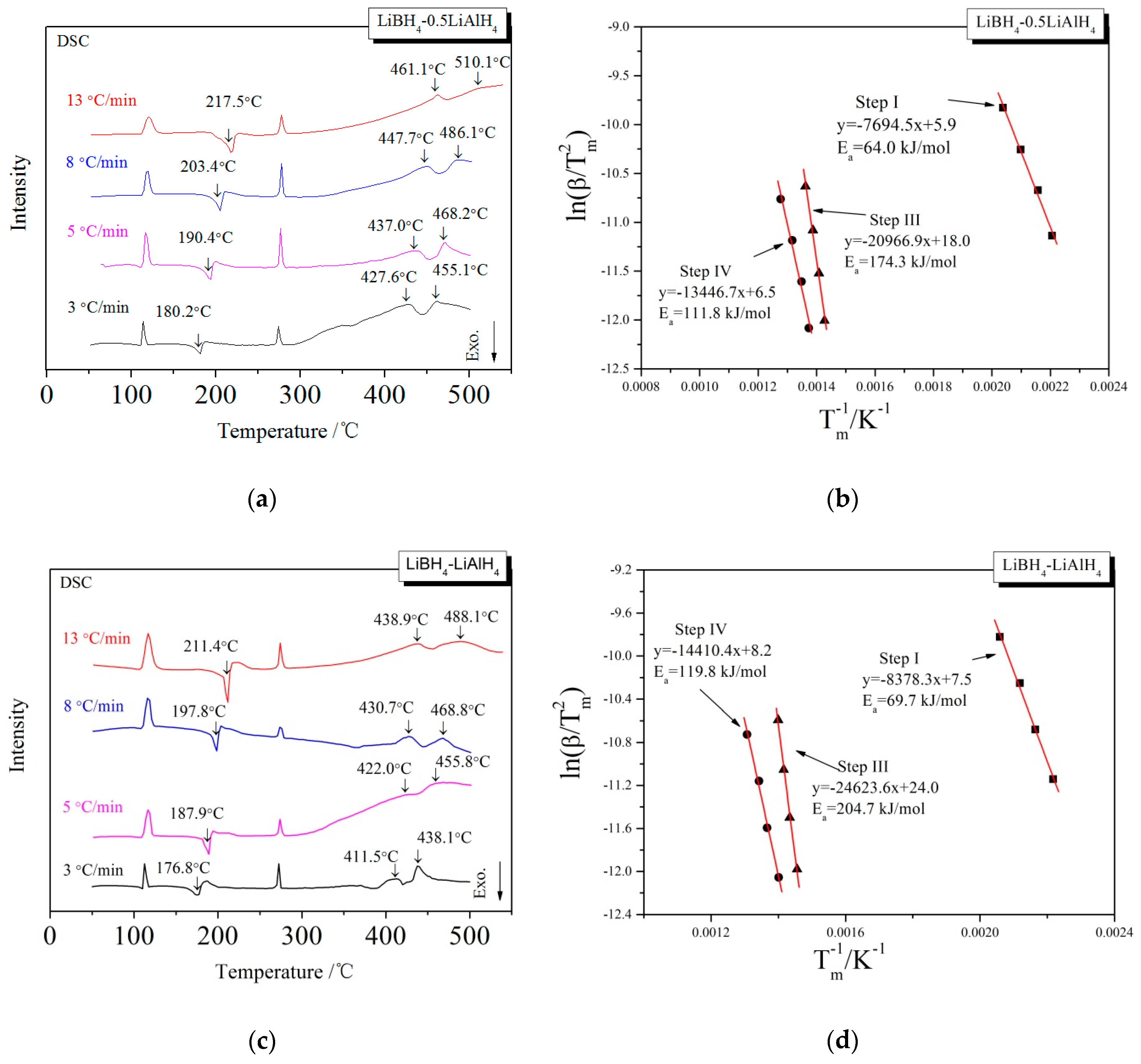
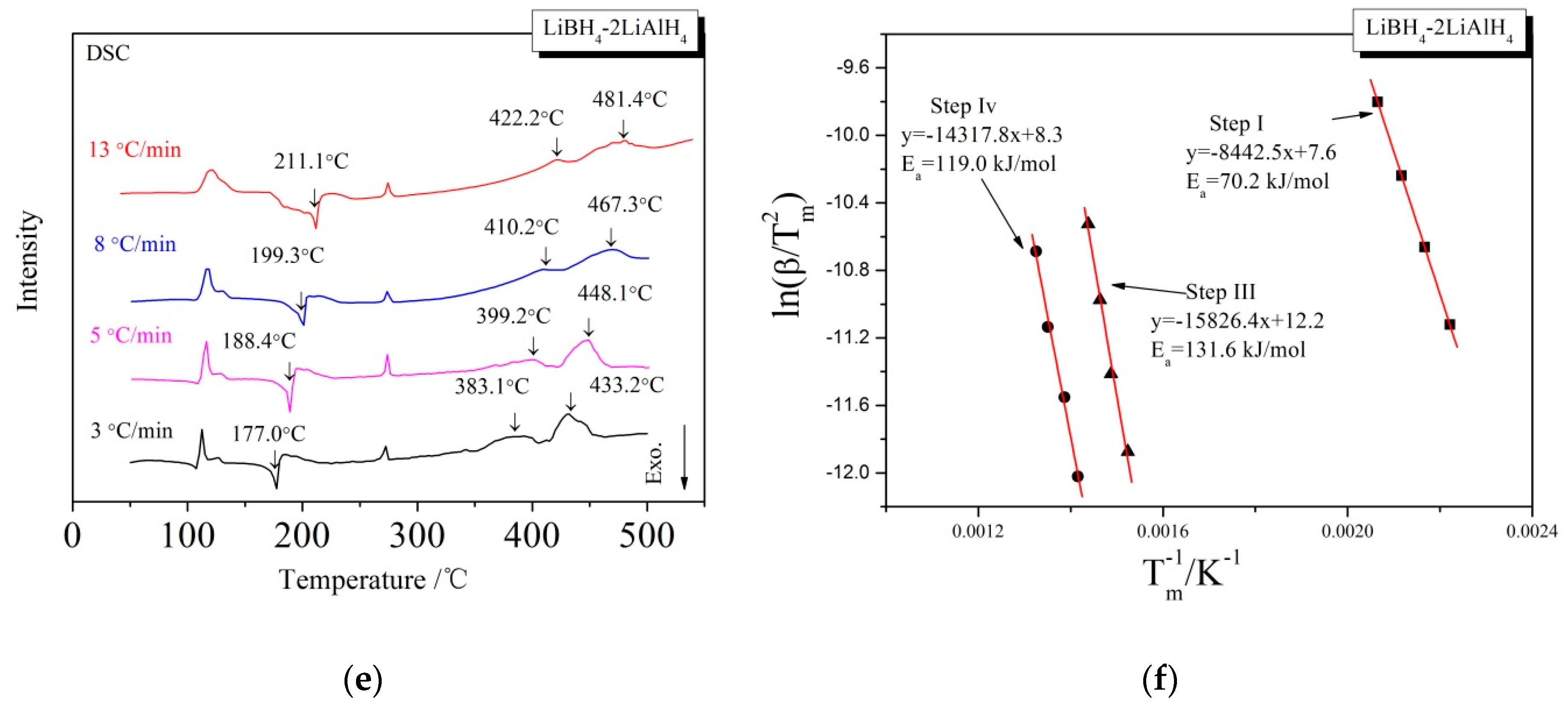
3.1. Dehydrogenation Performance of LiBH4/xLiAlH4 Composites
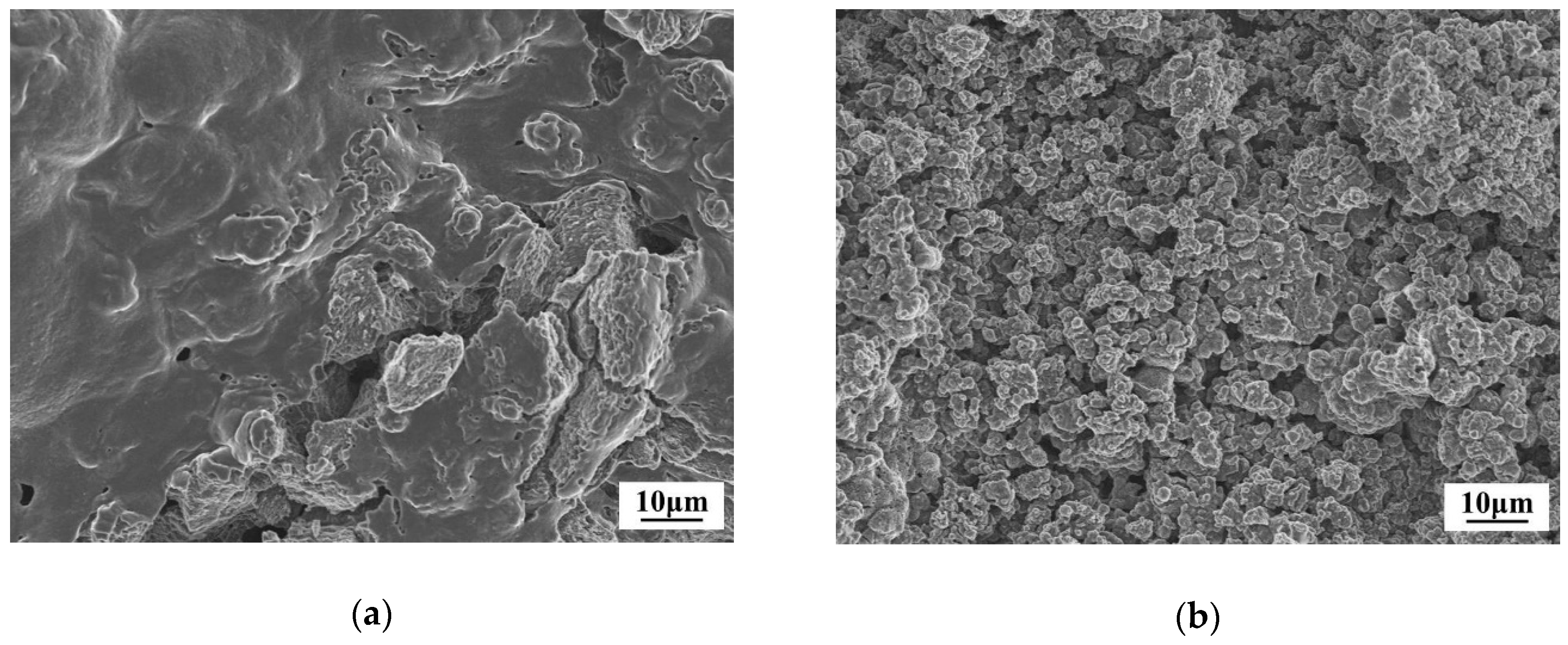
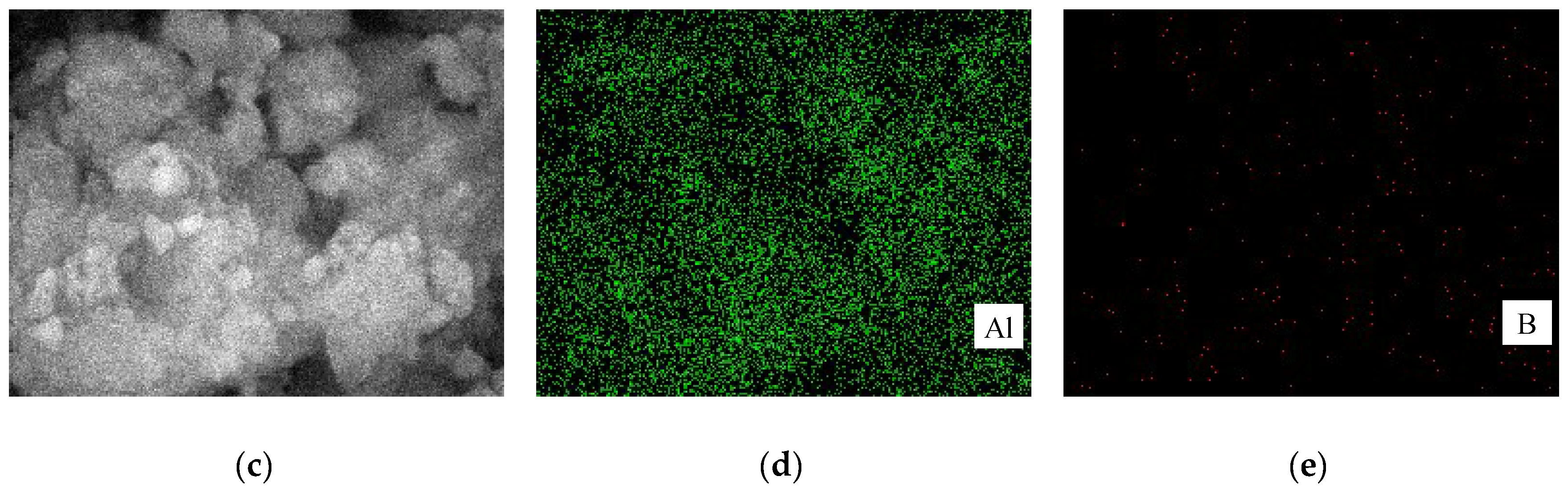
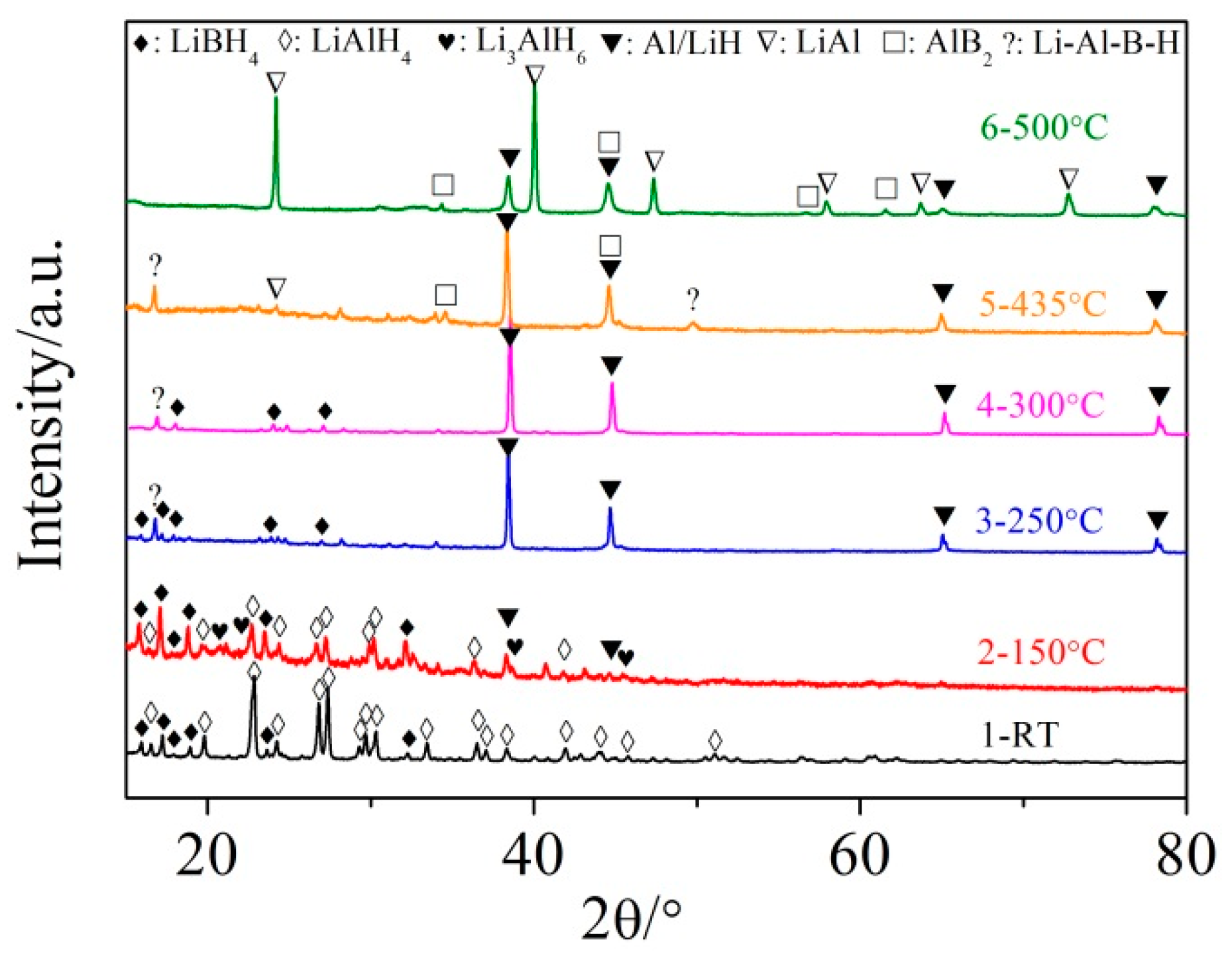
3.2. Mechanism of the Dehydrogenation Process
3.3. Kinetic Investigations of the Dehydrogenation Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schaub, T.; Trincado, M.; Grützmacher, H.; Prechtl, M.H.; Vogt, M.; Suárez, A.; Campos, J.; Feller, M.; Broere, D.; Ke, Z.; et al. Hydrogen Storage: Based on Hydrogenation and Dehydrogenation Reactions of Small Molecules; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2019. [Google Scholar]
- Romanos, J.; Beckner, M.; Prosniewski, M.; Rash, T.; Lee, M.; Robertson, J.D.; Firlej, L.; Kuchta, B.; Pfeifer, P. Boron-neutron Capture on Activated Carbon for Hydrogen Storage. Sci. Rep. 2019, 9, 2971. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Samantaray, S.S.; Mangisetti, S.; Ramaprabhu, S. Investigation of room temperature hydrogen storage in biomass derived activated carbon. J. Alloys Compd. 2019, 789, 800–804. [Google Scholar] [CrossRef]
- Carraro, P.M.; Sapag, K.; Oliva, M.I.; Eimer, G.A. Comparative study of hydrogen storage on metal doped mesoporous materials. Chem. Phys. Lett. 2018, 701, 93–97. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Rahali, S.; Belhocine, Y.; Seydou, M.; Maurel, F.; Tangour, B. Multiscale study of the structure and hydrogen storage capacity of an aluminum metal-organic framework. Int. J. Hydrogen Energy 2017, 42, 15271–15282. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.L.; Wei, H.W.; Li, W.Q.; Zhou, X.; Tian, W.Q. Hydrogen storage of dual-Ti-doped single-walled carbon nanotubes. Int. J. Hydrogen Energy 2019, 44, 2960–2975. [Google Scholar] [CrossRef]
- Rajaura, R.S.; Srivastava, S.; Sharma, P.K.; Mathur, S.; Shrivastava, R.; Sharma, S.S.; Vijay, Y.K. Structural and surface modification of carbon nanotubes for enhanced hydrogen storage density. Nano-Struct. Nano-Objects 2018, 14, 57–65. [Google Scholar] [CrossRef]
- Panigrahi, P.; Naqvi, S.R.; Hankel, M.; Ahuja, R.; Hussain, T. Enriching the hydrogen storage capacity of carbon nanotube doped with polylithiated molecules. Appl. Surf. Sci. 2018, 444, 467–473. [Google Scholar] [CrossRef]
- Gaboardi, M.; Amadé, N.S.; Aramini, M.; Milanese, C.; Magnani, G.; Sanna, S.; Riccò, M.; Pontiroli, D. Extending the hydrogen storage limit in fullerene. Carbon 2017, 120, 77–82. [Google Scholar] [CrossRef]
- Bi, L.; Yin, J.; Huang, X.; Ren, S.; Yan, G.; Wu, Q.; Wang, Y.; Yang, Z. Lithium decoration of boron-doped hybrid fullerenes and nanotubes as a novel 3D architecture for enhanced hydrogen storage: A DFT study. Int. J. Hydrogen Energy 2019, 44, 2934–2942. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Cheng, X.; Tang, Y. Ti-decorated B38 fullerene: A high capacity hydrogen storage material. Int. J. Hydrogen Energy 2016, 41, 19123–19128. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Aguey-Zinsou, K.F. Hydrogen storage properties of nanoconfined aluminium hydride (AlH3). Chem. Eng. Sci. 2019, 194, 64–70. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage-new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Von Colbe, J.B.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Cai, W.; Hou, J.; Huang, S.; Chen, J.; Yang, Y.; Tao, P.; Ouyang, L.; Wang, H.; Yang, X. Altering the chemical state of boron towards the facile synthesis of LiBH4 via hydrogenating lithium compound-metal boride mixture. Renew. Energy 2019, 134, 235–240. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, Y.; Peng, D.; Zhang, L.; Zhao, Y.; Li, Y.; Han, S. Effects of the hierarchical pyrolysis polyaniline on reversible hydrogen storage of LiBH4. Prog. Nat. Sci. 2018, 28, 529–533. [Google Scholar] [CrossRef]
- Ianni, E.; Sofianos, M.V.; Rowles, M.R.; Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Synthesis of NaAlH4/Al composites and their applications in hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 17309–17317. [Google Scholar] [CrossRef]
- Tian, M.; Shang, C. Mg-based composites for enhanced hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 338–344. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Utke, R. Confined LiBH4-LiAlH4 in nanopores of activated carbon nanofibers. Int. J. Hydrogen Energy 2015, 40, 7083–7092. [Google Scholar] [CrossRef]
- Blanchard, D.; Shi, Q.; Boothroyd, C.B.; Vegge, T. Reversibility of Al/Ti modified LiBH4. J. Phys. Chem. C 2009, 113, 14059–14066. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a metal (M = Mg, Al, Ti, V, Cr, or Sc) or metal hydride (MH2 = MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Zhao, S.X.; Wang, C.Y.; Liu, D.M.; Tan, Q.J.; Li, Y.T.; Si, T.Z. Destabilization of LiBH4 by SrF2 for reversible hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 5098–5103. [Google Scholar] [CrossRef]
- Weiqing, J.; Shilong, C. Effect of Al on the dehydrogenation of LiBH4 from first-principles calculations. Int. J. Hydrogen Energy 2017, 42, 6181–6188. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Cai, J.; Zang, L.; Zhao, L.; Liu, J.; Wang, Y. Dehydrogenation characteristics of LiAlH4 improved by in-situ formed catalysts. J. Energy Chem. 2016, 25, 868–873. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Ouyang, L.; Liu, J.; Zhu, M. Enhanced dehydrogenation of LiBH4·NH3-LiAlH4 composites. Int. J. Hydrogen Energy 2017, 42, 22406–22410. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Liu, H.K.; Yu, X.B. Reversible hydrogen storage in titanium-catalyzed LiAlH4-LiBH4 system. J. Alloys Compd. 2009, 487, 434–438. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.M.C.E.P.; Sudan, P.H.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- McCarty, M., Jr.; Maycock, J.N.; Verneker, V.R.P. Thermal decomposition of lithium aluminum hydride. J. Phys. Chem. 1968, 72, 4009–4014. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Cao, G.; Li, S.; Ge, H.; Chen, L.; Yan, M. Hydrogen storage properties of LiBH4-Li3AlH6 composites. J. Alloys Compd. 2012, 517, 127–131. [Google Scholar] [CrossRef]
- Hansen, B.R.; Ravnsbæk, D.B.; Skibsted, J.; Jensen, T.R. Hydrogen reversibility of LiBH4-MgH2-Al composites. Phys. Chem. Chem. Phys. 2014, 16, 8970–8980. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Sheng, P.; Liu, S.; Zhao, G.; Wang, B.; Wang, X.; Yan, M. Hydrogen desorption kinetics of the destabilized LiBH4-AlH3 composites. Int. J. Hydrogen Energy 2017, 42, 22358–22365. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Andreasen, A.; Vegge, T.; Pedersen, A.S. Dehydrogenation kinetics of as-received and ball-milled LiAlH4. J. Solid State Chem. 2005, 178, 3672–3678. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| x | Ea of the First Step (kJ/mol) | Ea of the Third Step (kJ/mol) | Ea of the Fourth Step (kJ/mol) |
|---|---|---|---|
| 0.5 | 64.0 | 174.3 | 111.8 |
| 1 | 69.7 | 204.7 | 119.8 |
| 2 | 70.2 | 131.6 | 119.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Zhu, D.; Wu, X.; Dong, D.; Xu, M.; Tong, Z. Hydrogen Desorption Properties of LiBH4/xLiAlH4 (x = 0.5, 1, 2) Composites. Molecules 2019, 24, 1861. https://doi.org/10.3390/molecules24101861
He Q, Zhu D, Wu X, Dong D, Xu M, Tong Z. Hydrogen Desorption Properties of LiBH4/xLiAlH4 (x = 0.5, 1, 2) Composites. Molecules. 2019; 24(10):1861. https://doi.org/10.3390/molecules24101861
Chicago/Turabian StyleHe, Qing, Dongdong Zhu, Xiaocheng Wu, Duo Dong, Meng Xu, and Zhaofei Tong. 2019. "Hydrogen Desorption Properties of LiBH4/xLiAlH4 (x = 0.5, 1, 2) Composites" Molecules 24, no. 10: 1861. https://doi.org/10.3390/molecules24101861
APA StyleHe, Q., Zhu, D., Wu, X., Dong, D., Xu, M., & Tong, Z. (2019). Hydrogen Desorption Properties of LiBH4/xLiAlH4 (x = 0.5, 1, 2) Composites. Molecules, 24(10), 1861. https://doi.org/10.3390/molecules24101861






