Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications
Abstract
Index
| 1. Introduction to Cholesterol-Based Compounds | 1 |
| 2. Drug Delivery Applications…………………………………………………………………… | 3 |
| 3. Anticancer, Antimicrobial, and Antioxidant Compounds………………………………… | 15 |
| 4. Cholesterol-Based Liquid Crystals…………………………………………………………… | 27 |
| 5. Cholesterol-Based Gelators……………………………………………………………………. | 35 |
| 6. Bioimaging Applications………………………………………………………………………. | 41 |
| 7. Synthetic Applications…………………………………………………………………………. | 48 |
| 8. Miscellaneous…………………………………………………………………………………… | 56 |
| 9. Conclusions……………………………………………………………………………………… | 59 |
| Funding……………………………………………………………………………………………….. | 60 |
| Conflicts of Interest…………………………………………………………………………………. | 60 |
| Abbreviations List……………………………………………………………………………………. | 60 |
| References……………………………………………………………………………………………… | 62 |
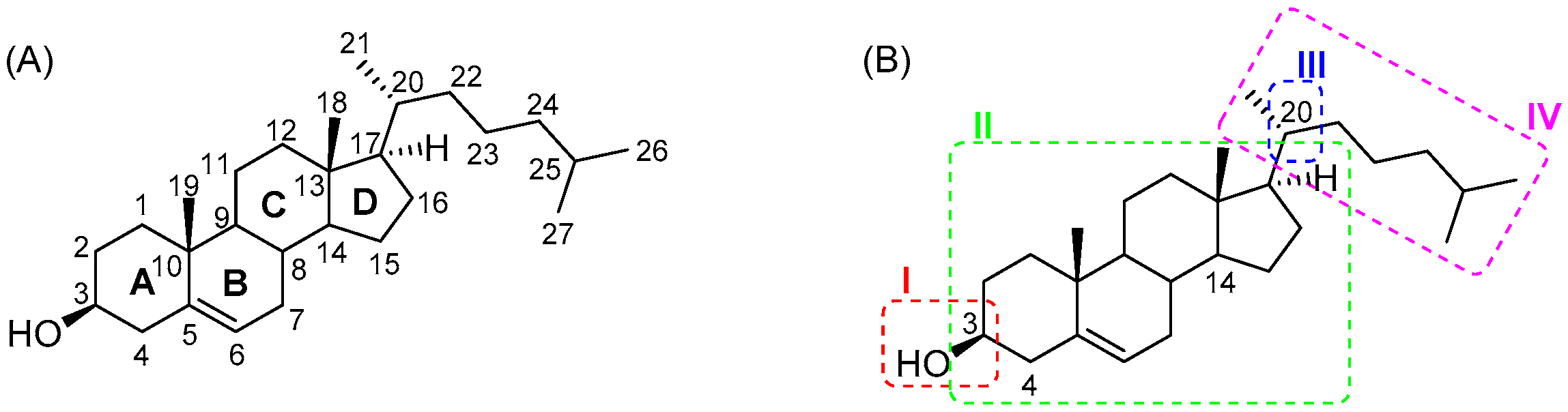
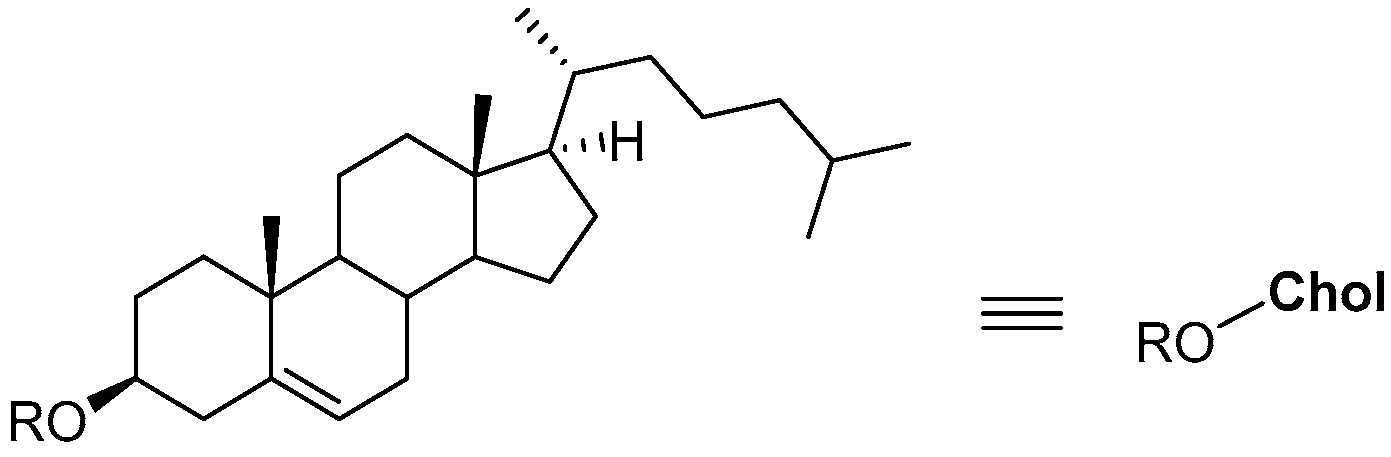
1. Introduction to Cholesterol-Based Compounds
2. Drug Delivery Applications
3. Anticancer, Antimicrobial, and Antioxidant Compounds
4. Cholesterol-Based Liquid Crystals
5. Cholesterol-Based Gelators
6. Bioimaging Applications
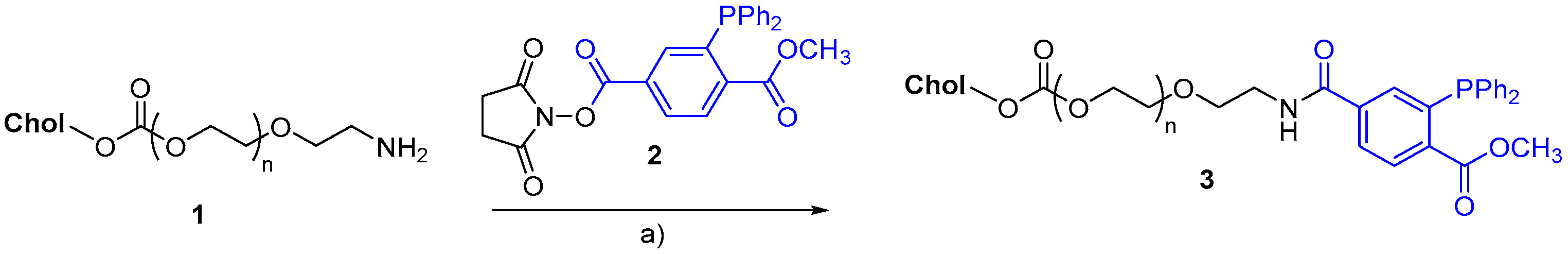
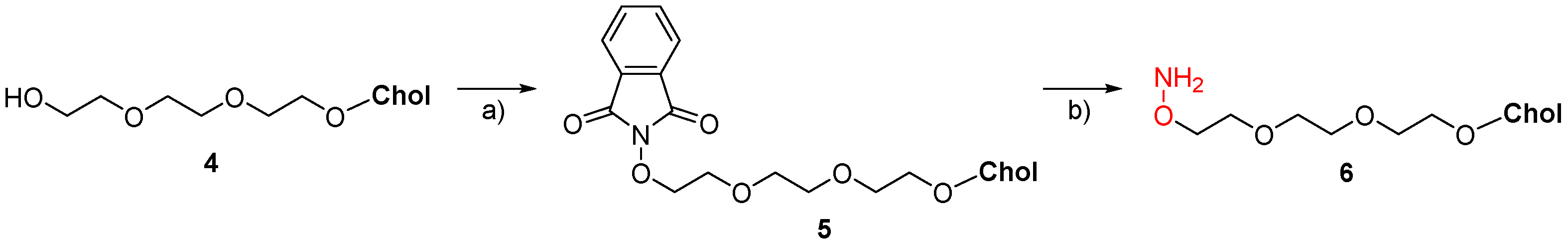
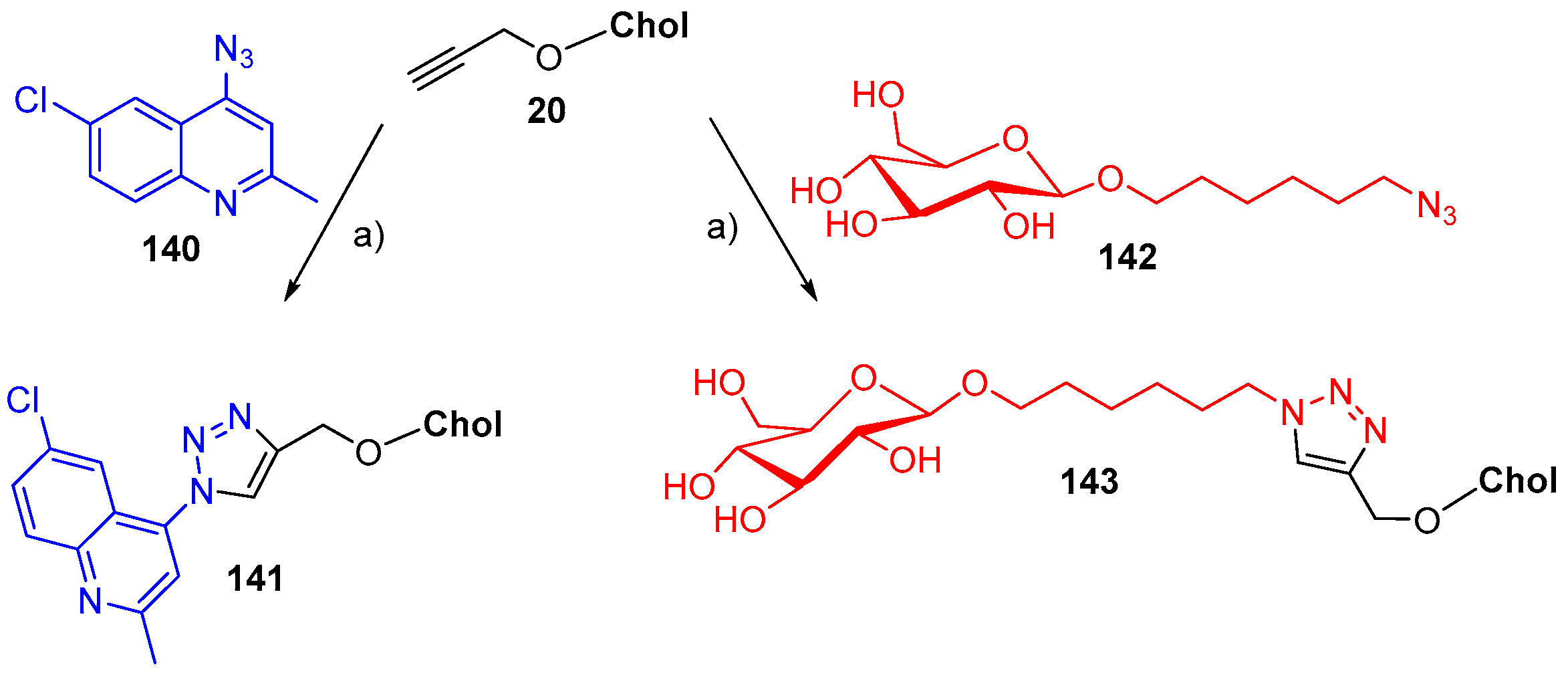
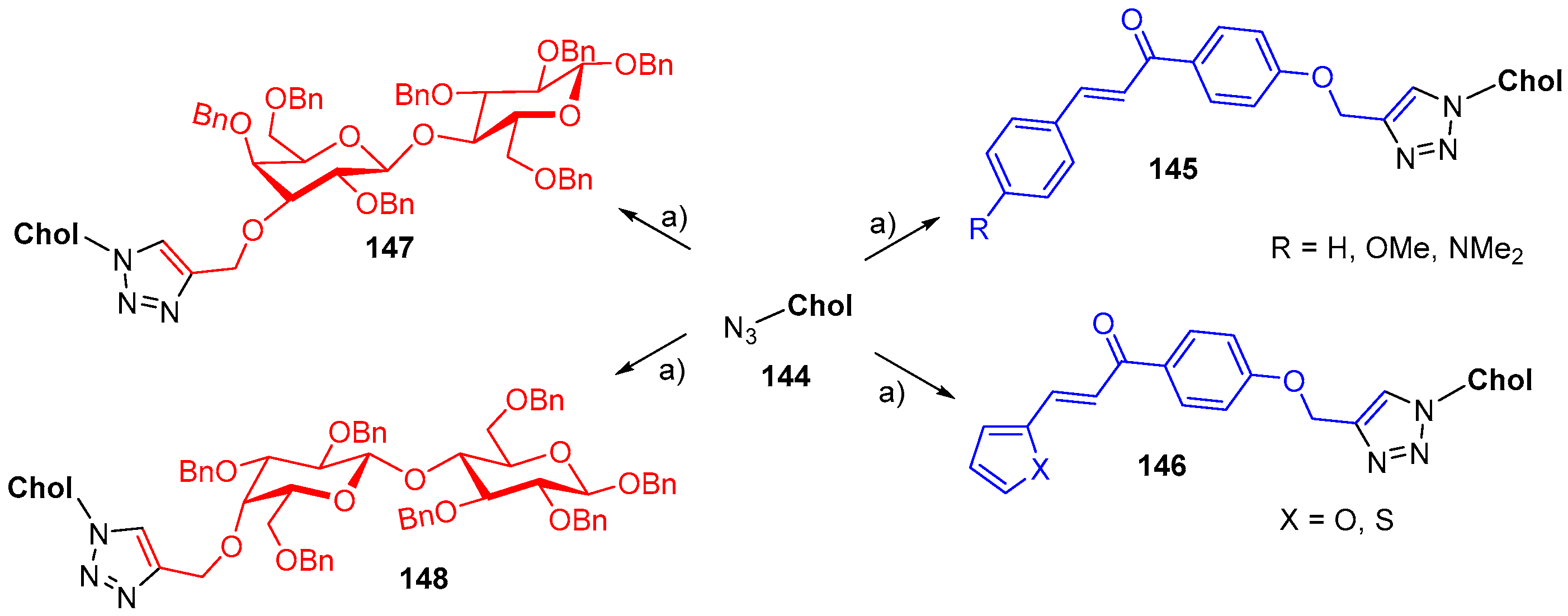
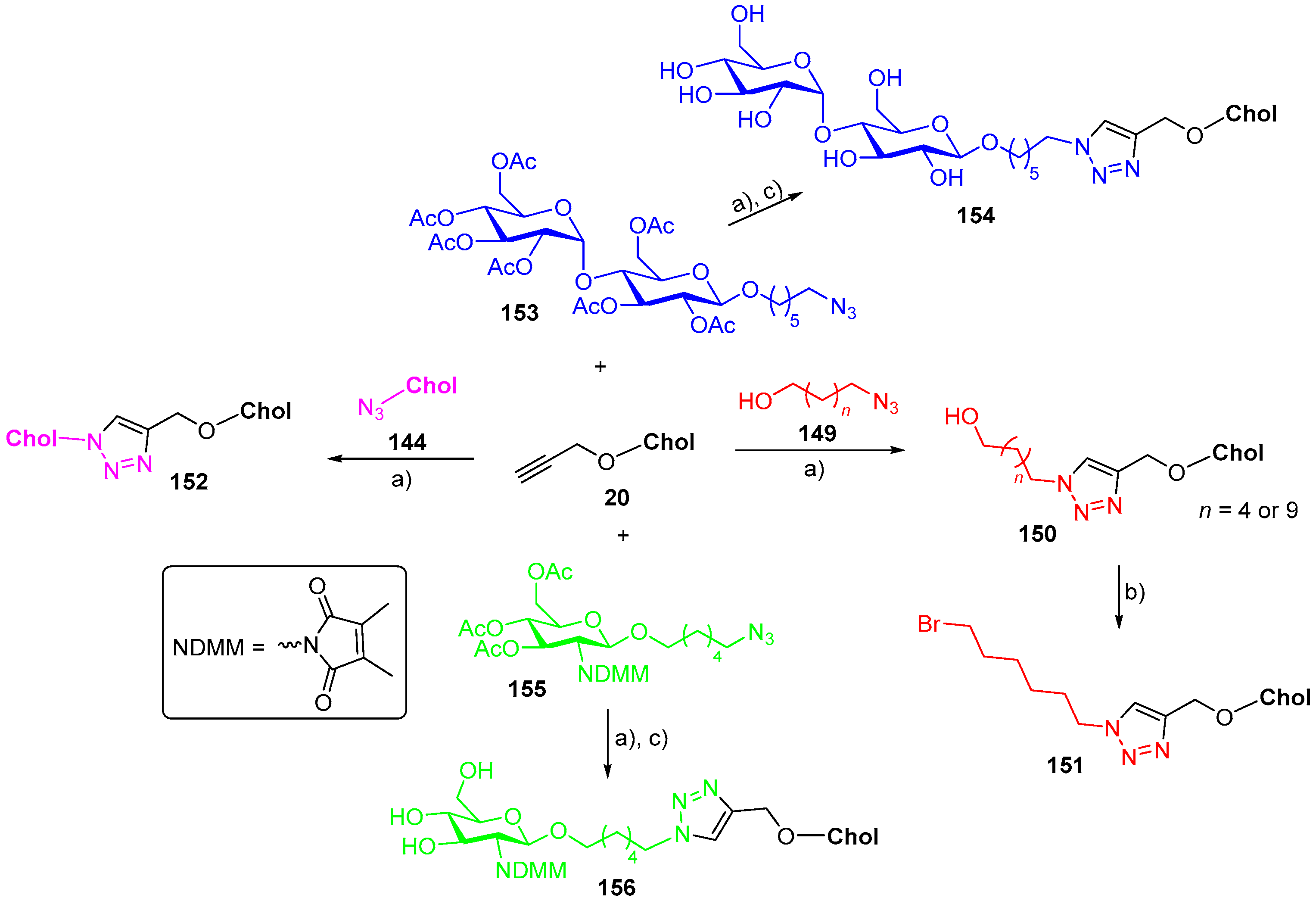
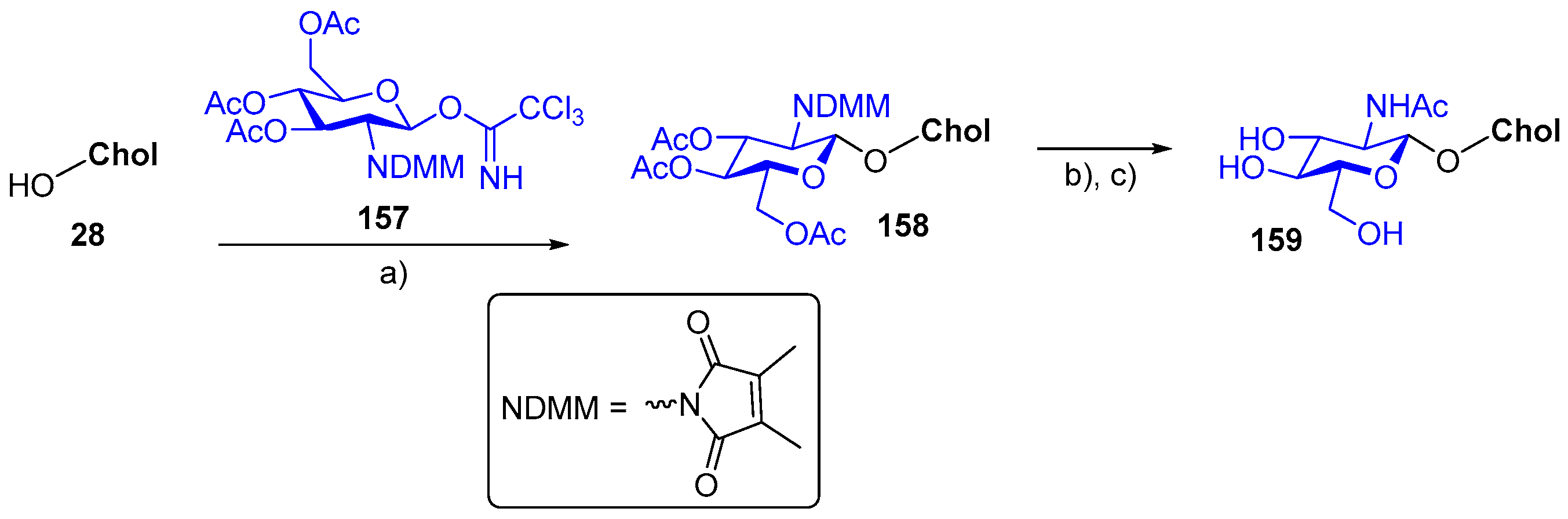
7. Synthetic Applications
8. Miscellaneous
9. Conclusions
Funding
Conflicts of Interest
Abbreviations List
| Ac | acetyl |
| Ac2O | acetic anhydride |
| AcOH | acetic acid |
| AG | arabinogalactan |
| AIBN | 2,2′-azobis(2-methylpropionitrile) |
| AIEE | aggregation induced enhanced emission |
| AL | β-alanine |
| AscONa | sodium ascorbate |
| ATRP | atom transfer radical polymerization |
| BBN | bombesin |
| Bn | benzyl |
| Boc2O | di-tert-butyl decarbonate |
| BODIPY | boron dipyrromethene |
| BtOH | N-hydroxybenzotriazole |
| Bz | benzoyl |
| CAE | cholesterol-arginine ester |
| β-CD | β-cyclodextrin |
| β-CD-NSP | β-cyclodextrin nanosponge |
| CDI | carbonyldiimidazole |
| CF | 5,6-carboxyfluorescein |
| Chol | cholesterol |
| Chol-OA | oxyamine-terminated cholesterol |
| CHS | cholesterol hydrogen succinate |
| Ch-T | chloramine-T |
| CL | conventional liposomes |
| CuAAC | copper(I)-catalyzed azide-alkyne cycloaddition |
| CVS | crystal violet staining |
| Cyclen | 1,4,7,10-tetraazacyclododecane |
| CYS | cystamine |
| DAIN | 4-(diarylmethylene)imidazolinone |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DCC | N,N′-dicyclohexylcarbodiimide |
| DCVJ | 9-(2,2-dicyanovinyl)julolidine |
| DHPC | dihexanoylphosphatidylcholine |
| DIAD | diisopropyl azodicarboxylate |
| DIPEA | N,N-diisopropylethylamine |
| DMAP | dimethylaminopyridine |
| DMF | dimethylformamide |
| DMPC | dimyristoylphosphatidylcholine |
| DMSO | dimethyl sulfoxide |
| DMTAP | dimyristoyltrimethylammonium propane |
| DNA | deoxyribonucleic acid |
| DOPC | dioleoylphosphatidilcholine |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOX | doxorubicin |
| DOX-NPs | doxorubicin loaded nanoparticles |
| DPPA | diphenylphosphoryl azide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| DTX | docetaxel |
| EDAC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| EDCl | N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| Et2N | diethylamine |
| Et3N | triethylamine |
| Et2O | diethyl ether |
| EtOAc | ethyl acetate |
| EtOH | ethanol |
| GFP | green fluorescent protein |
| GSH | glutathione |
| HA | hyaluronic acid |
| hbPG | linear-hyperbranched amphiphilic polyglycerol |
| HOBt | N-hydroxybenzotriazole |
| HSA | human serum albumin |
| IC50 | half-maximal inhibitory concentration |
| ICT | intramolecular charge transfer |
| l-AA | l-ascorbic acid |
| LC | liquid crystal |
| LMGs | low-molecular-weight gelators |
| MeCN | acetonitrile |
| MeOTf | methyl triflate |
| m-CPBA | m-chloroperoxybenzoic acid |
| min | minutes |
| MPP | mitochondria-penetrating peptide |
| MTT | 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide |
| MW | microwave irradiation |
| NaOAc | sodium acetate |
| NaOMe | sodium methoxide |
| NBD | nitrobenzoxadiazole |
| NBS | N-bromosuccinimide |
| NHS | N-hydroxysuccinimide |
| NOAC | nitrile oxide alkyne cycloaddition |
| NPs | nanoparticles |
| PBS | phosphate-buffered saline |
| PDC | pyridinium dichromate |
| PEG | polyethylene glycol |
| PET | positron emission tomography |
| pHPMA | poly[N-(2-hydroxypropyl)-methacrylamide] |
| PMDETA | N,N,N′,N″,N″-pentamethyldiethylenetriamine |
| PPA | phenylpropanolamine |
| PPh3 | triphenylphosphine |
| p-TsCl | p-toluenesulfonyl chloride |
| PTX | paclitaxel |
| PyBOP | benzotriazole-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate |
| RAFT | reversible addition fragmentation chain transfer |
| rt | room temperature |
| SA | succinic anhydride |
| SCID | severe combined immunodeficient |
| SML | surface-modified liposomes |
| SubPcs | subphthalocyanines |
| TACN | 1,4,7-triazacyclononane |
| Tb | trilobolide |
| TBAB | tetrabutylammonium bromide |
| TBAF | tetrabutylammonium fluoride |
| TBAH | tetrabutylammonium hydroxide |
| TBAITBDMS | tetrabutylammonium iodidetert-butyldimethylsilyl |
| TBDMSCl | tert-butyldimethylsilyl chloride |
| Tb-N3VA | trilobolide 8-O-azidovalerate |
| TBTA | tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine |
| t-BuOH | tert-butanol |
| t-BuOK | potassium tert-butoxide |
| t-BuOOH | tert-butyl hydroperoxide |
| TE | transfection efficiency |
| TEG | tetraethylene glycol |
| TFA | trifluoroacetic acid |
| TFAA | trifluoroacetic anhydride |
| THF | tetrahydrofuran |
| TIS | triisopropylsilane |
| TMSCl | trimethylsilyl chloride |
| TMSITMSOTf | iodotrimethylsilanetrimethylsilyl trifluoromethanesulfonate |
| TOAB | tetraoctylammonium bromide |
| TsCl | p-toluenesulfonyl chloride |
References
- Cerqueira, N.M.F.S.A.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D. Biosynthesis of Cholesterol and Other Sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef] [PubMed]
- Habchi, J.; Chia, S.; Galvagnion, C.; Michaels, T.C.T.; Bellaiche, M.M.J.; Ruggeri, F.S.; Sanguanini, M.; Idini, I.; Kumita, J.R.; Sparr, E.; et al. Cholesterol catalyses Aβ42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes. Nat. Chem. 2018, 10, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Morzycki, J.W. Recent advances in cholesterol chemistry. Steroids 2014, 83, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Vabbilisetty, P.; Sun, X.-L. Liposome surface functionalization based on different anchoring lipids via Staudinger ligation. Org. Biomol. Chem. 2014, 12, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Q.; Zhang, Z.; Petretic, A.; Gradinaru, C.C.; Macdonald, P.M. Single Lipid Bilayer Deposition on Polymer Surfaces Using Bicelles. Biomacromolecules 2015, 16, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.O.; Nagarsenker, M.S.; Barhate, C.R.; Padhye, S.G.; Dhawan, V.V.; Bhattacharyya, D.; Viswanathan, C.L.; Steiniger, F.; Fahr, A. Cholesterol anchored arabinogalactan for asialoglycoprotein receptor targeting: Synthesis, characterization, and proof of concept of hepatospecific delivery. Carbohydr. Res. 2015, 408, 33–43. [Google Scholar] [CrossRef]
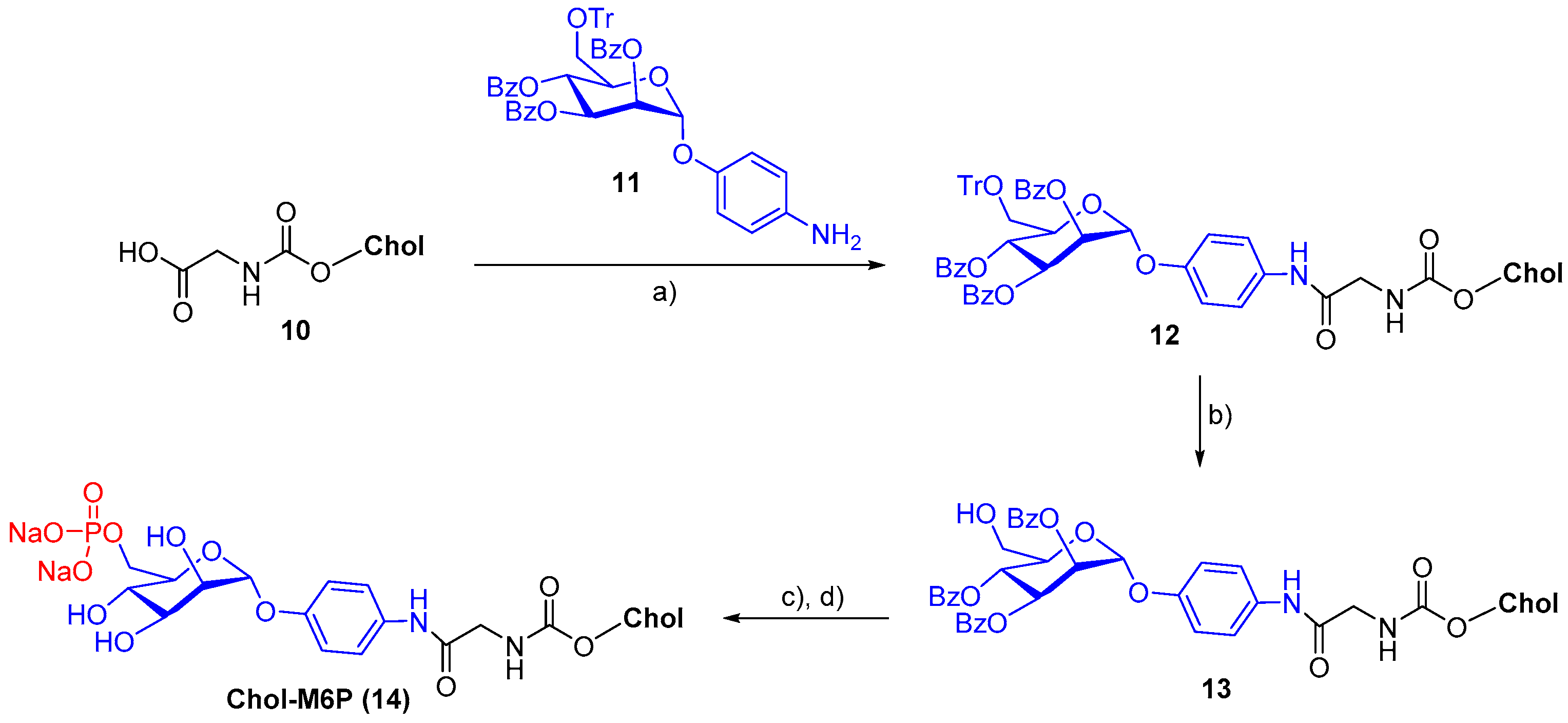
- Crucianelli, E.; Bruni, P.; Frontini, A.; Massaccesi, L.; Pisani, M.; Smorlesi, A.; Mobbili, G. Liposomes containing mannose-6-phosphate-cholesteryl conjugates for lysosome-specific delivery. RSC Adv. 2014, 4, 58204–58207. [Google Scholar] [CrossRef]
- Silva, A.T.M.; Maia, A.L.C.; de Oliveira Silva, J.; de Barros, A.L.B.; Soares, D.C.F.; de Magalhaes, M.T.Q.; Jose Alves, R.; Ramaldes, G.A. Synthesis of cholesterol-based neoglycoconjugates and their use in the preparation of liposomes for active liver targeting. Carbohydr. Res. 2018, 465, 52–57. [Google Scholar] [CrossRef]
- Škorpilová, L.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Lokajová, J.; Effenberg, R.; Slepička, P.; Ruml, T.; Kmoníčková, E.; Drašar, P.B.; Wimmer, Z. BODIPY-based fluorescent liposomes with sesquiterpene lactone trilobolide. Beilstein J. Org. Chem. 2017, 13, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
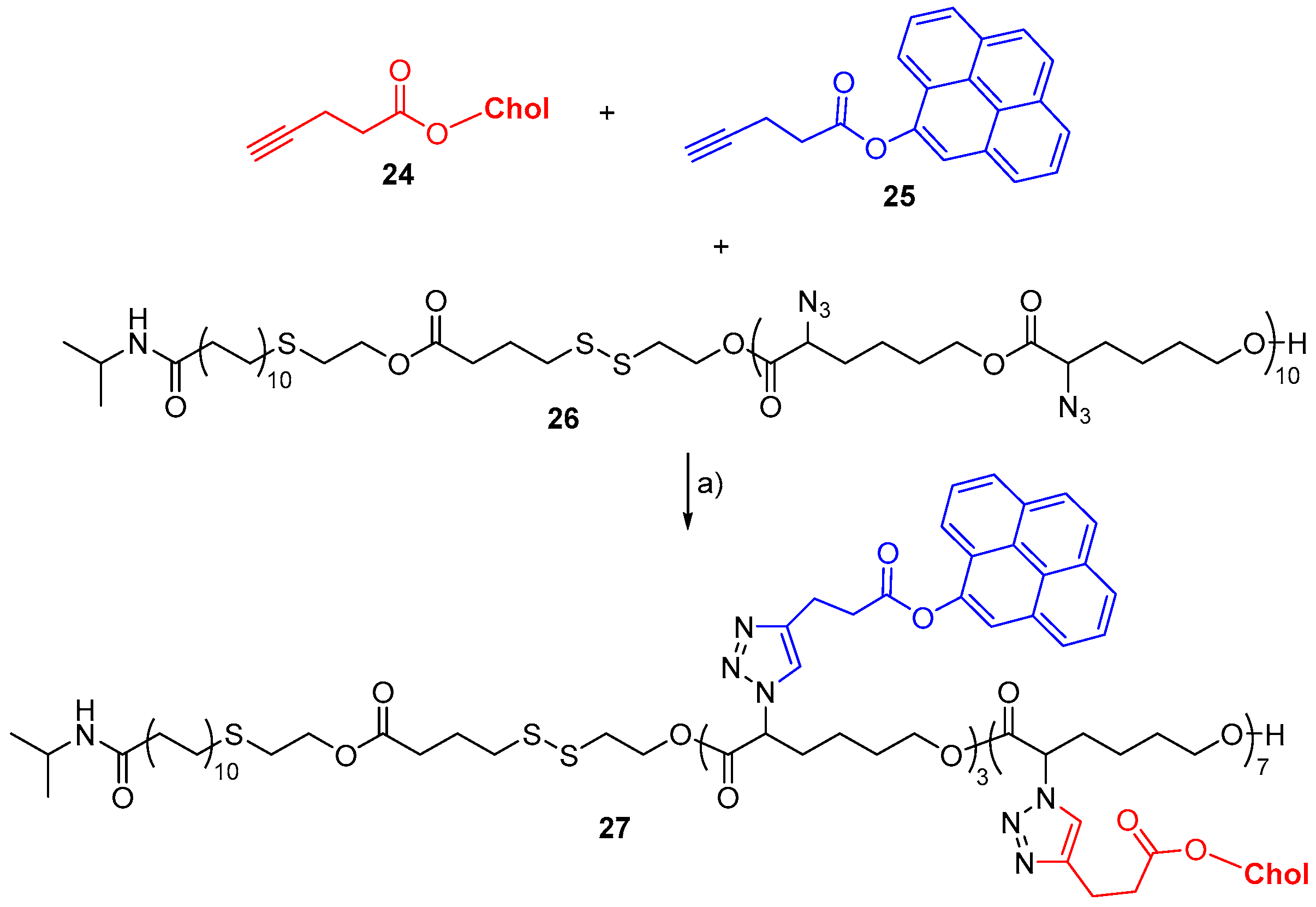
- Lin, Y.-K.; Fang, J.-Y.; Wang, S.-W.; Lee, R.-S. Synthesis and characterization of triple-responsive PNiPAAm-S-S-P(αN3CL-g-alkyne) copolymers bearing cholesterol and fluorescence monitor. React. Funct. Polym. 2018, 130, 29–42. [Google Scholar] [CrossRef]
- Alberti, D.; Toppino, A.; Geninatti Crich, S.; Meraldi, C.; Prandi, C.; Protti, N.; Bortolussi, S.; Altieri, S.; Aime, S.; Deagostino, A. Synthesis of a carborane-containing cholesterol derivative and evaluation as a potential dual agent for MRI/BNCT applications. Org. Biomol. Chem. 2014, 12, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
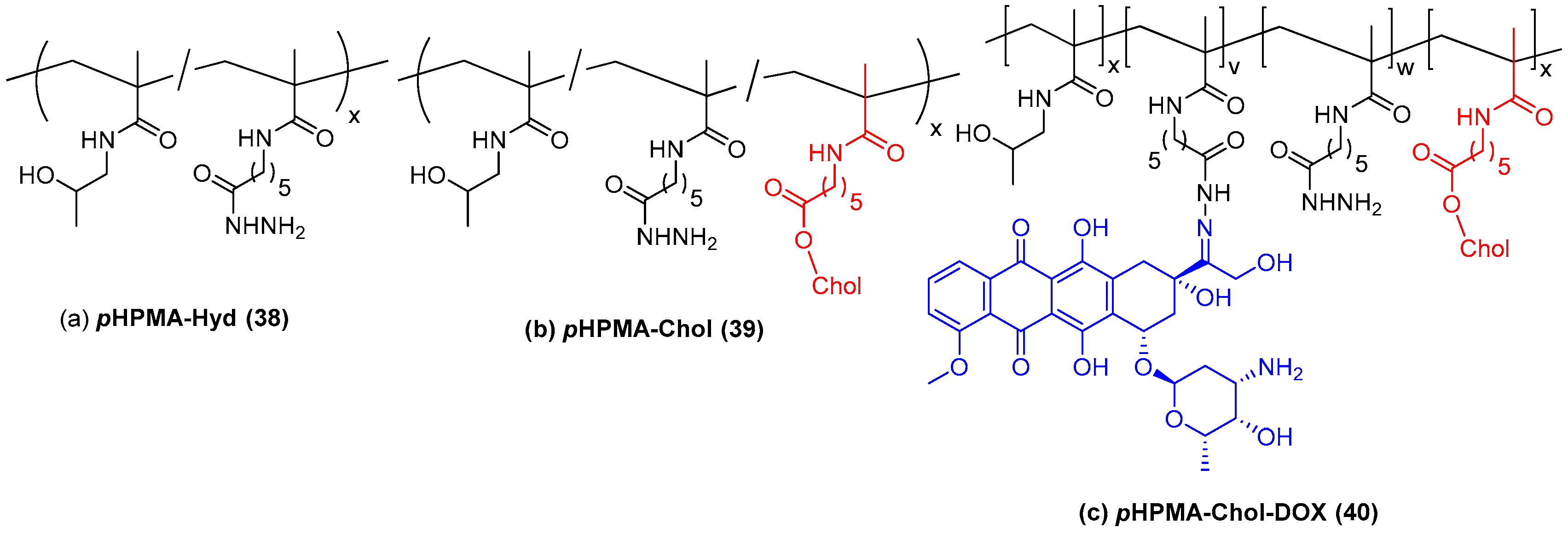
- Zhang, X.; Niebuur, B.-J.; Chytil, P.; Etrych, T.; Filippov, S.K.; Kikhney, A.; Wieland, D.C.F.; Svergun, D.I.; Papadakis, C.M. Macromolecular pHPMA-Based Nanoparticles with Cholesterol for Solid Tumor Targeting: Behavior in HSA Protein Environment. Biomacromolecules 2018, 19, 470–480. [Google Scholar] [CrossRef] [PubMed]
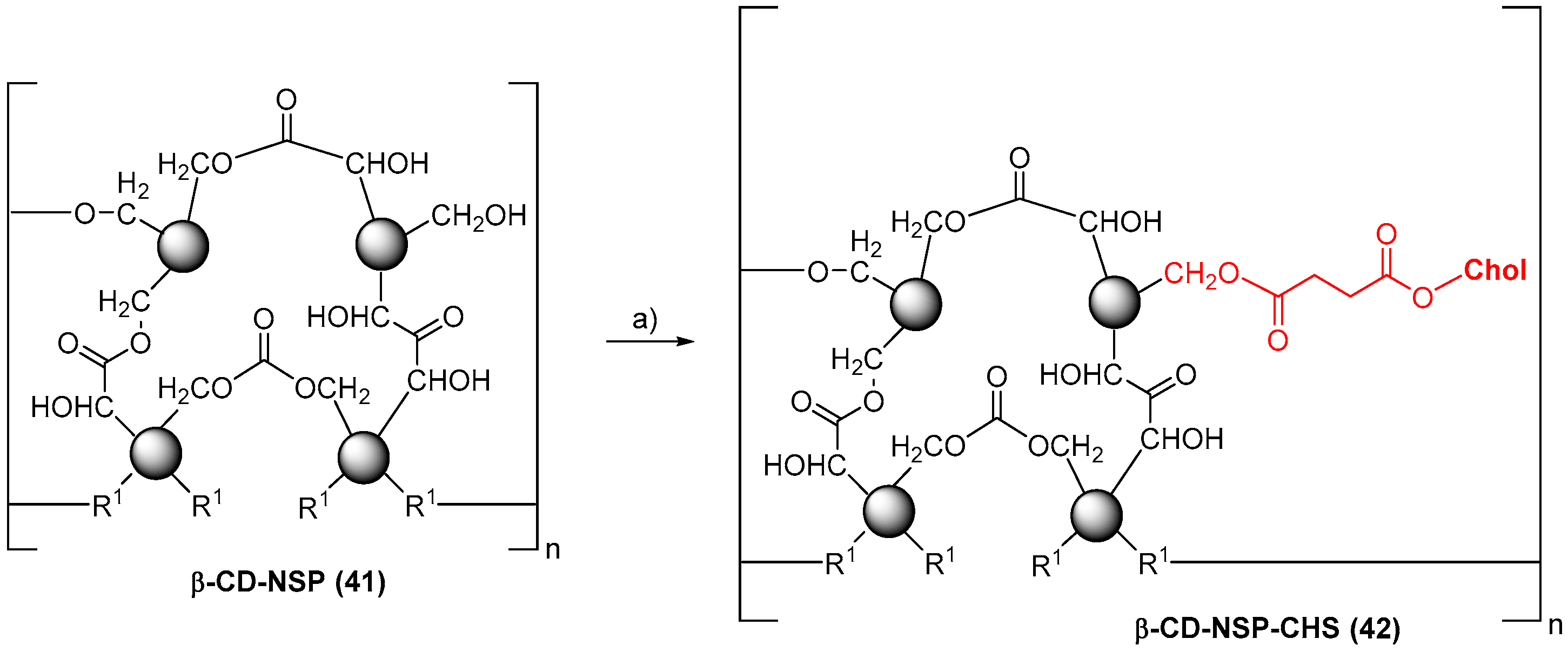
- Singh, P.; Ren, X.; Guo, T.; Wu, L.; Shakya, S.; He, Y.; Wang, C.; Maharjan, A.; Singh, V.; Zhang, J. Biofunctionalization of β-cyclodextrin nanosponges using cholesterol. Carbohydr. Pol. 2018, 190, 23–30. [Google Scholar] [CrossRef] [PubMed]
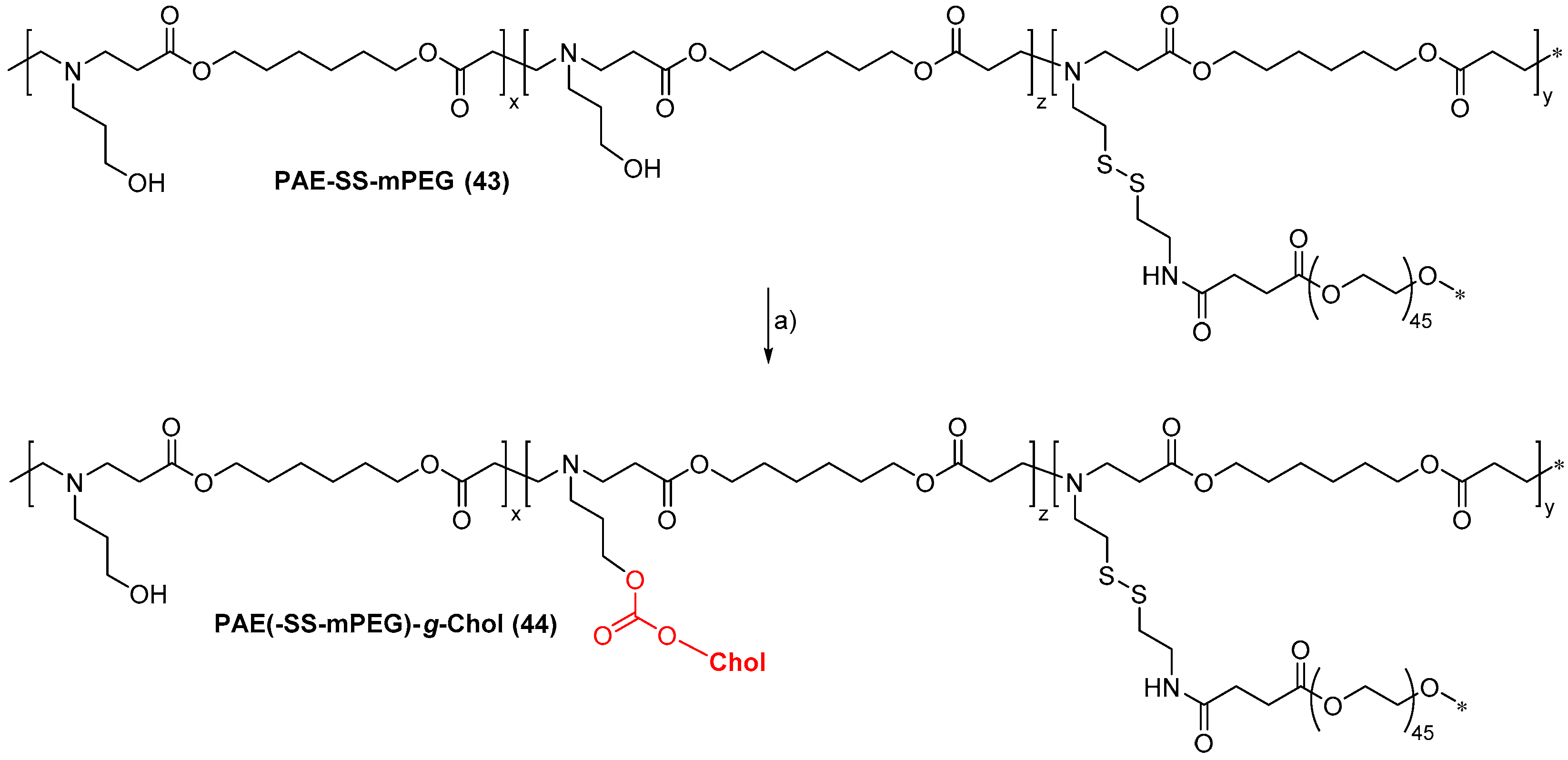
- Li, J.; Ma, Y.J.; Wang, Y.; Chen, B.Z.; Guo, X.D.; Zhang, C.Y. Dual redox/pH-responsive hybrid polymer-lipid composites: Synthesis, preparation, characterization and application in drug delivery with enhanced therapeutic efficacy. Chem. Eng. J. 2018, 341, 450–461. [Google Scholar] [CrossRef]
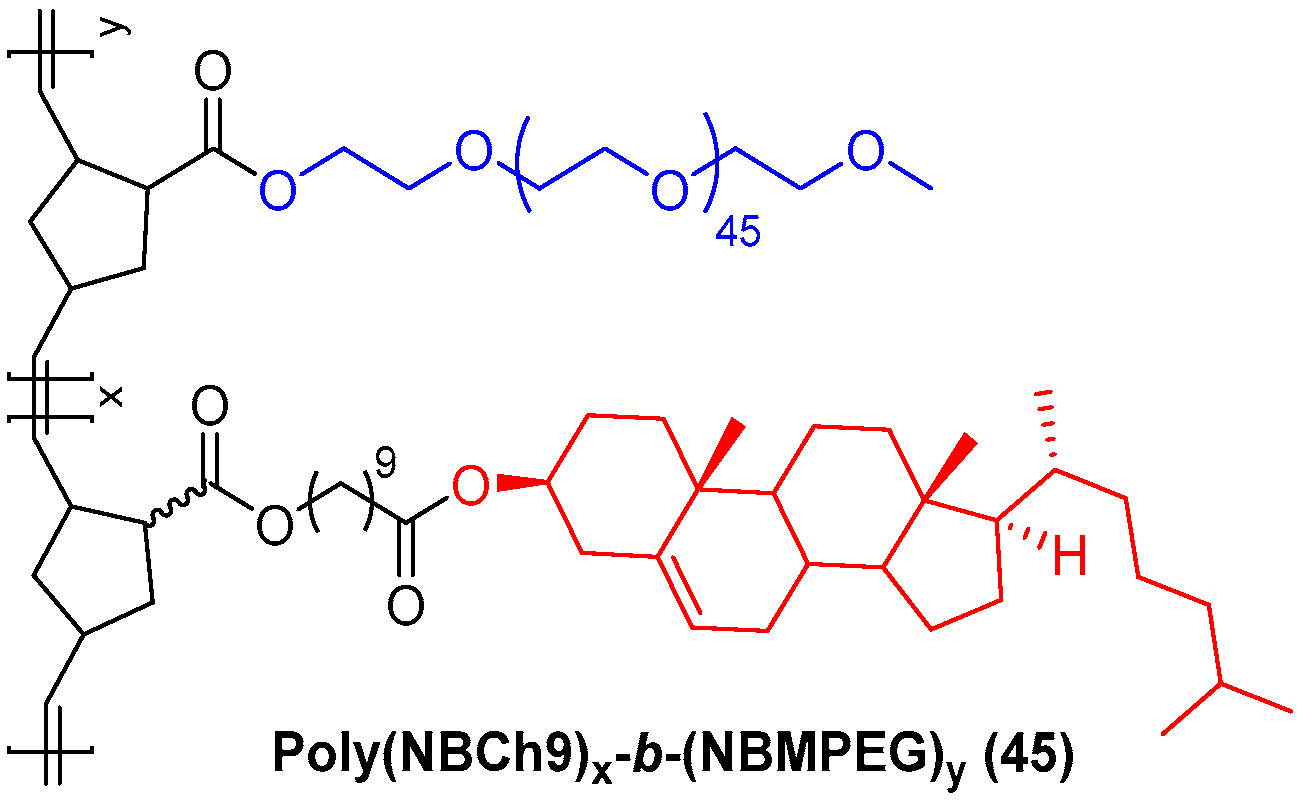
- Tran, T.-H.; Nguyen, C.T.; Gonzalez-Fajardo, L.; Hargrove, D.; Song, D.; Deshmukh, P.; Mahajan, L.; Ndaya, D.; Lai, L.; Kasi, R.M.; et al. Long Circulating Self-Assembled Nanoparticles from Cholesterol-Containing Brush-Like Block Copolymers for Improved Drug Delivery to Tumors. Biomacromolecules 2014, 15, 4363–4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, T.; Sheng, R.; Li, H.; Sun, J.; Cao, A. Amphiphilic Diblock Terpolymer PMAgala-b-P(MAA-co-MAChol)s with Attached Galactose and Cholesterol Grafts and Their Intracellular pH-Responsive Doxorubicin Delivery. Biomacromolecules 2016, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Cui, D.; Bignon, J.; Di Cicco, A.; Wdzieczak-Bakala, J.; Liu, J.; Li, M.-H. Reduction-Responsive Cholesterol-Based Block Copolymer Vesicles for Drug Delivery. Biomacromolecules 2014, 15, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Dolor, A.; Kierstead, P.; Dai, Z.; Szoka, F.C. Sterol-modified PEG lipids: Alteration of the bilayer anchoring moiety has an unexpected effect on liposome circulation. Chem. Commun. 2018, 54, 11949–11952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, D.; Li, Y.; Yang, X.; Pan, W. In vitro–in vivo evaluation of hyaluronic acid-based amphiphilic copolymers for tumour targeted delivery: The role of hydrophobic groups. RSC Adv. 2017, 7, 23942–23953. [Google Scholar] [CrossRef]
- Mallick, S.; Thuy, L.T.; Lee, S.; Park, J.I.; Choi, J.S. Liposomes containing cholesterol and mitochondria-penetrating peptide (MPP) for targeted delivery of antimycin A to A549 cells. Colloids Surf. B 2018, 161, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Huan, M.-L.; Wan, N.; Hou, Y.-L.; Ma, X.-X.; Jia, Y.-Y.; Li, C.; Zhou, S.-Y.; Zhang, B.-L. Cholesterol derived cationic lipids as potential non-viral gene delivery vectors and their serum compatibility. Bioorg. Med. Chem. Lett. 2016, 26, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Vulugundam, G.; Kumar, K.; Kondaiah, P.; Bhattacharya, S. Efficacious redox-responsive gene delivery in serum by ferrocenylated monomeric and dimeric cationic cholesterols. Org. Biomol. Chem. 2015, 13, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; He, X.; Zhang, Y.; Zhang, J.; Liu, Y.-H.; Yu, X.-Q. Hydroxyl-containing non-viral lipidic gene vectors with macrocyclic polyamine headgroups. RSC Adv. 2015, 5, 59417–59427. [Google Scholar] [CrossRef]
- Puchkov, P.A.; Perevoshchikova, K.A.; Kartashova, I.A.; Luneva, A.S.; Kabilova, T.O.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Polycationic amphiphiles based on triethylenetetramine and their transfection efficacy. Russ. J. Bioorg. Chem. 2017, 43, 561–569. [Google Scholar] [CrossRef]
- Monpara, J.; Kanthou, C.; Tozer, G.M.; Vavia, P.R. Rational Design of Cholesterol Derivative for Improved Stability of Paclitaxel Cationic Liposomes. Pharm. Res. 2018, 35, 90–107. [Google Scholar] [CrossRef]
- van Elk, M.; Deckers, R.; Oerlemans, C.; Shi, Y.; Storm, G.; Vermonden, T.; Hennink, W.E. Triggered Release of Doxorubicin from Temperature-Sensitive Poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) Grafted Liposomes. Biomacromolecules 2014, 15, 1002–1009. [Google Scholar] [CrossRef]
- Asayama, S.; Nagashima, K.; Negishi, Y.; Kawakami, H. Byproduct-Free Intact Modification of Insulin by Cholesterol End-Modified Poly(ethylene glycol) for in Vivo Protein Delivery. Bioconj. Chem. 2018, 29, 67–73. [Google Scholar] [CrossRef]
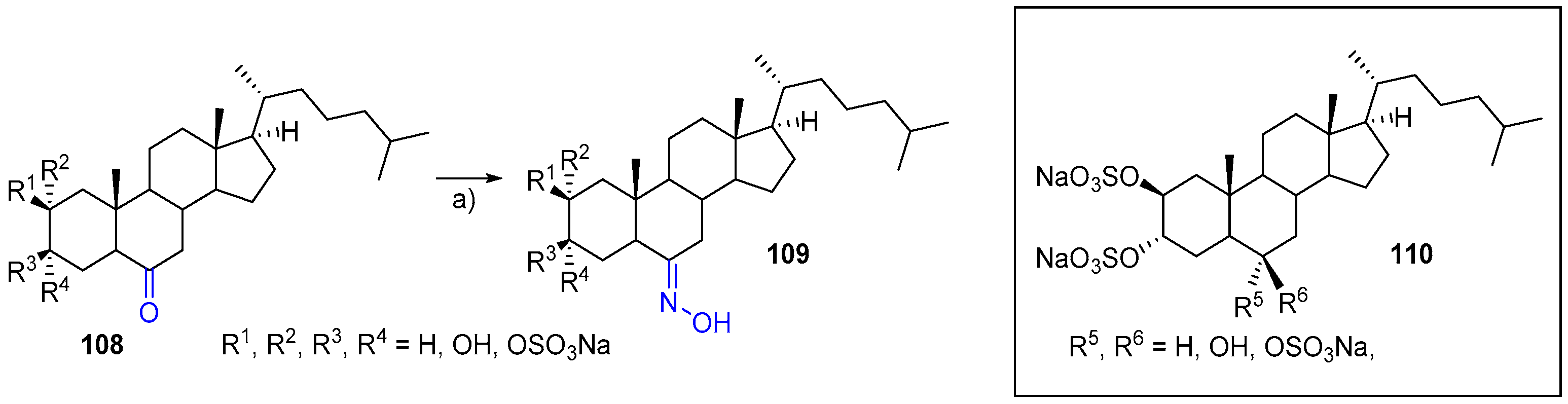
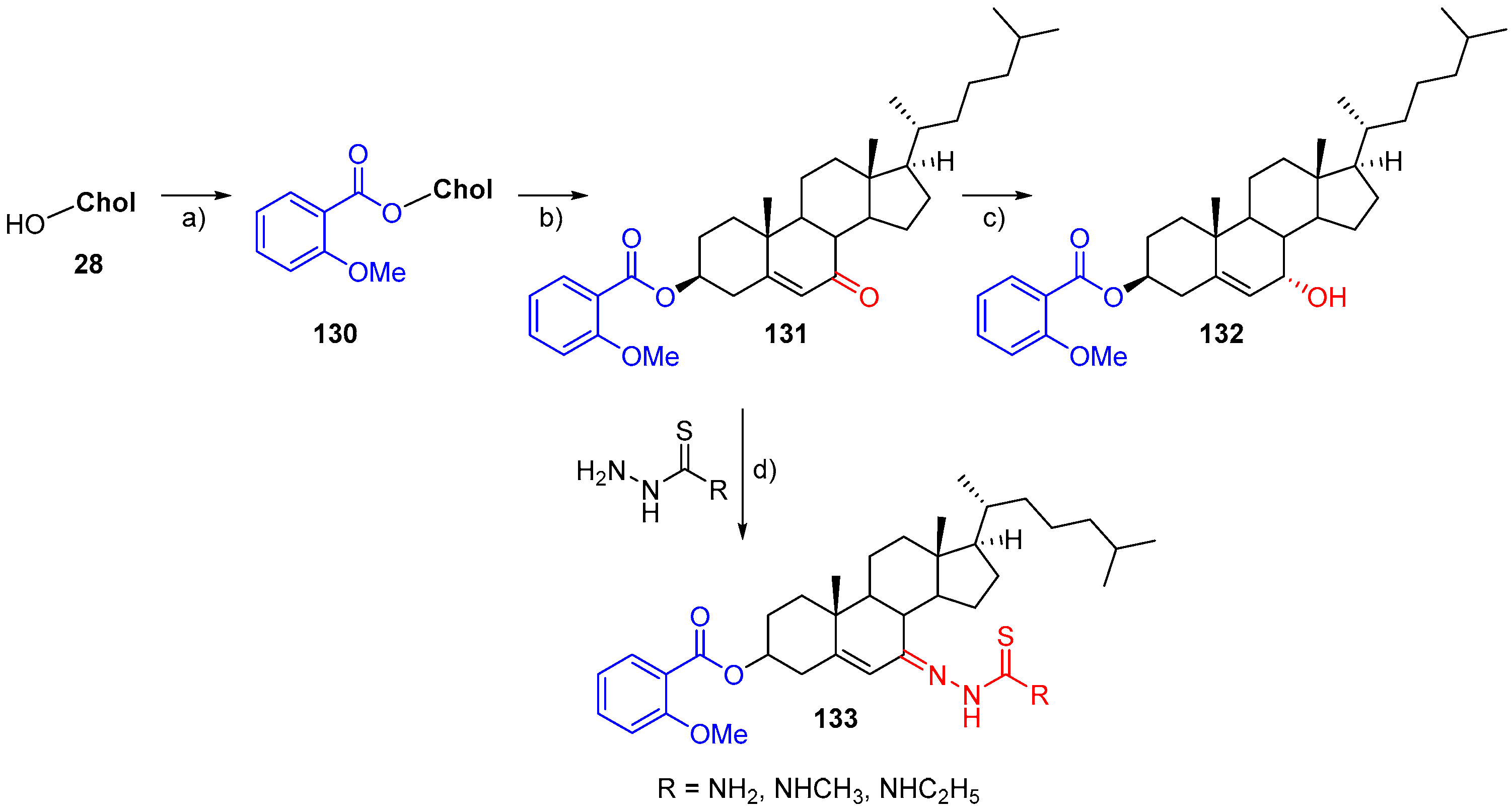
- Rega, M.; Jiménez, C.; Rodríguez, J. 6E-Hydroximinosteroid homodimerization by cross-metathesis processes. Steroids 2007, 72, 729–735. [Google Scholar] [CrossRef]
- Richmond, V.; Careaga, V.P.; Sacca, P.; Calvo, J.C.; Maier, M.S. Synthesis and cytotoxic evaluation of four new 6E-hydroximinosteroids. Steroids 2014, 84, 7–10. [Google Scholar] [CrossRef] [PubMed]
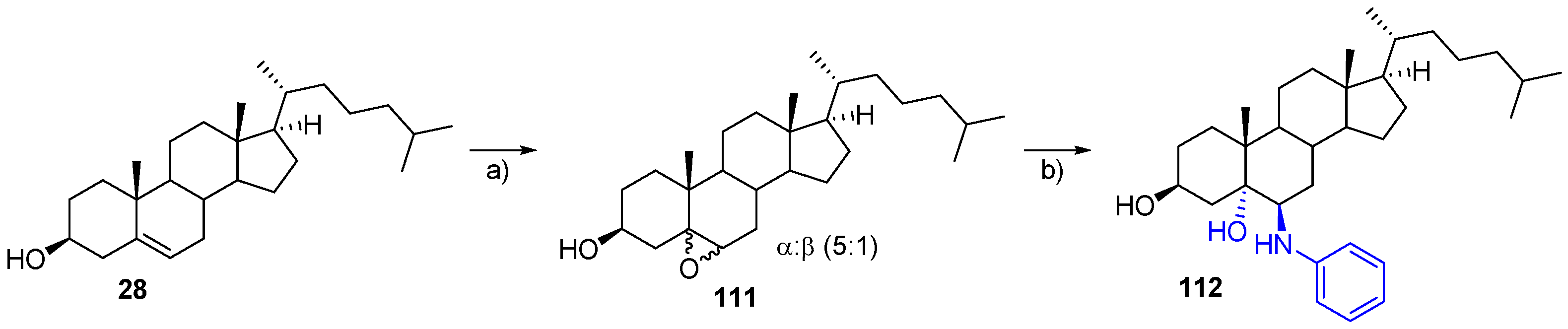
- Soto-Castro, D.; Lara Contreras, R.C.; Pina-Canseco, M.D.S.; Santillan, R.; Hernandez-Huerta, M.T.; Negron Silva, G.E.; Perez-Campos, E.; Rincon, S. Solvent-free synthesis of 6β-phenylamino-cholestan-3β,5α-diol and (25R)-6β-phenylaminospirostan-3β,5α-diol as potential antiproliferative agents. Steroids 2017, 126, 92–100. [Google Scholar] [CrossRef] [PubMed]
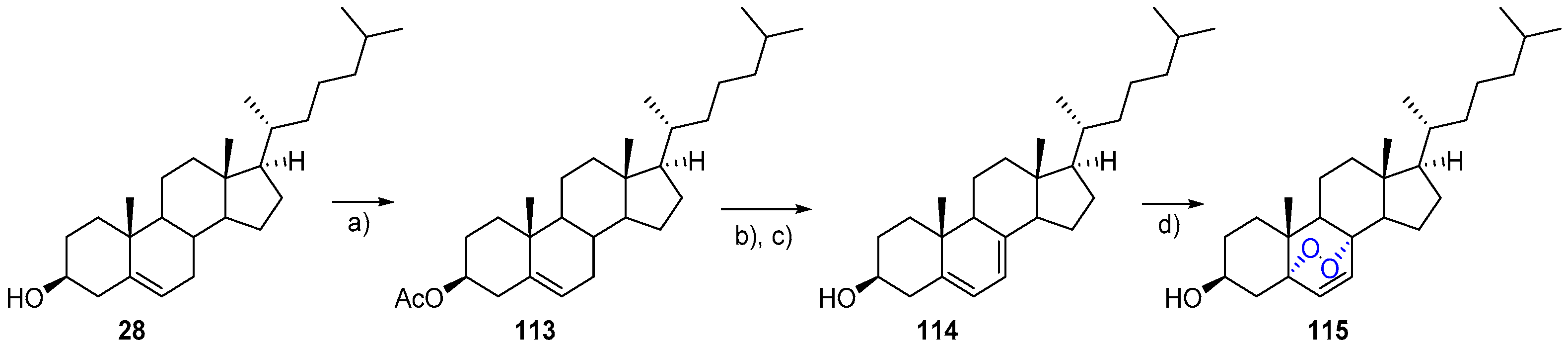
- Bu, M.; Cao, T.; Li, H.; Guo, M.; Yang, B.B.; Zhou, Y.; Zhang, N.; Zeng, C.; Hu, L. Synthesis and biological evaluation of novel steroidal 5α,8α-endoperoxide derivatives with aliphatic side-chain as potential anticancer agents. Steroids 2017, 124, 46–53. [Google Scholar] [CrossRef] [PubMed]
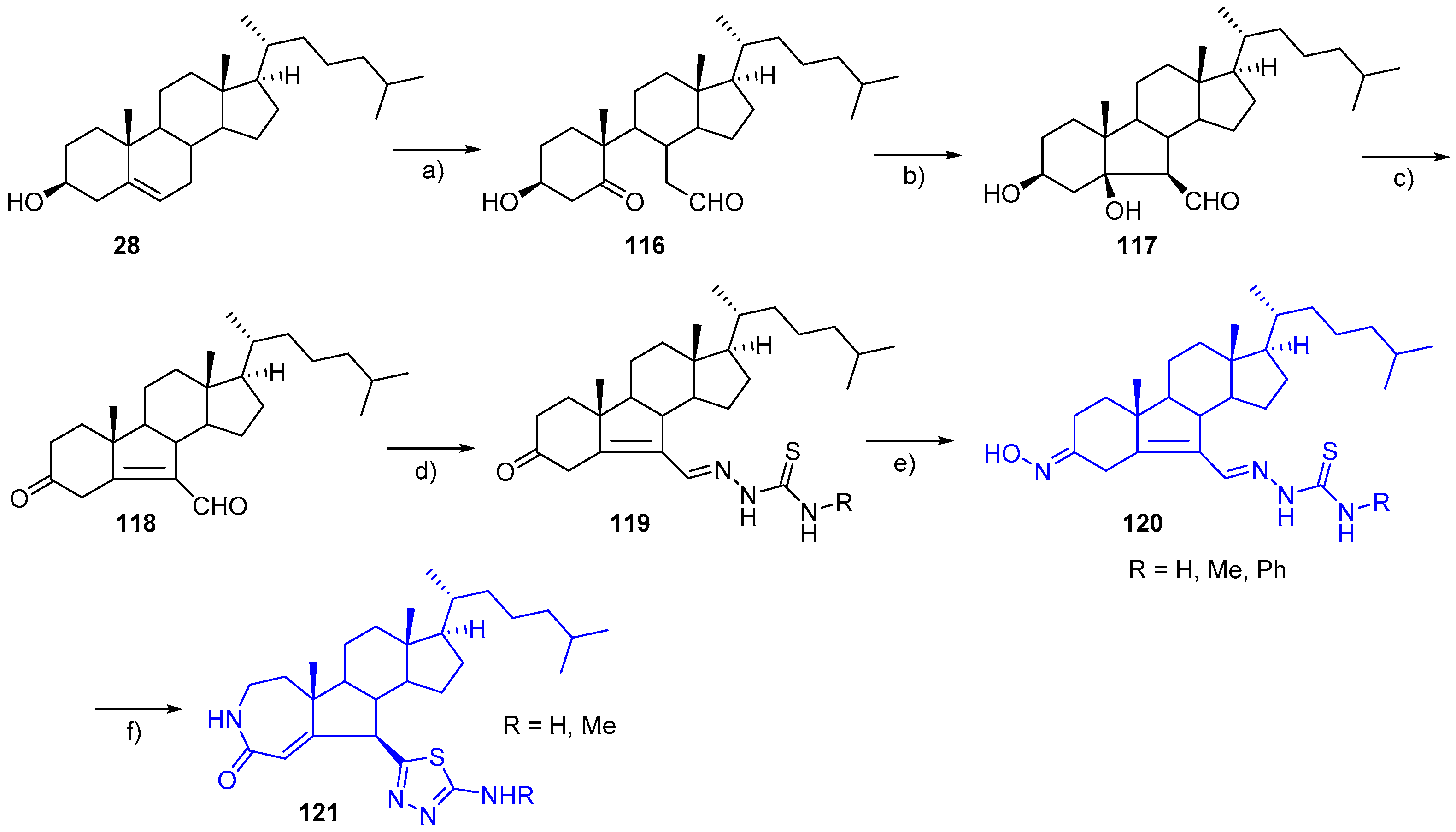
- Huang, Y.; Yang, C.; Zhan, J.; Gan, C.; Liu, Z.; Pang, C.; Chen, H.; Cui, J. Synthesis and antiproliferative activity of novel A-homo-B-norsteroid thiadiazole derivatives. Tetrahedron Lett. 2017, 58, 2952–2954. [Google Scholar] [CrossRef]
- Martínez-Pascual, R.; Meza-Reyes, S.; Vega-Baez, J.L.; Merino-Montiel, P.; Padrón, J.M.; Mendoza, Á.; Montiel-Smith, S. Novel synthesis of steroidal oximes and lactams and their biological evaluation as antiproliferative agents. Steroids 2017, 122, 24–33. [Google Scholar] [CrossRef]
- D’Yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry V: Synthesis of hybrid molecules based on steroid oximes and (5Z,9Z)-tetradeca-5,9-dienedioic acid as potential anticancer agents. Steroids 2018, 138, 14–20. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, H.; Zheng, J.; Gan, C.; Pang, L.; Pang, C.; Liu, X.; Zhan, J.; Cui, J. Synthesis, characterization, and biological evaluations of some steryl 2-methoxybenzoates as anticancer agents. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef]
- Peters, A.D.; McCallion, C.; Booth, A.; Adams, J.A.; Rees-Unwin, K.; Pluen, A.; Burthem, J.; Webb, S.J. Synthesis and biological activity of a CXCR4-targeting bis(cyclam) lipid. Org. Biomol. Chem. 2018, 16, 6479–6490. [Google Scholar] [CrossRef]
- Aly, M.R.E.S.; Saad, H.A.; Mohamed, M.A.M. Click reaction based synthesis, antimicrobial, and cytotoxic activities of new 1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2015, 25, 2824–2830. [Google Scholar] [CrossRef]
- Aly, M.R.E.S.; Saad, H.A.; Abdel-Hafez, S.H. Synthesis, antimicrobial and cytotoxicity evaluation of new cholesterol congeners. Beilstein J. Org. Chem. 2015, 11, 1922–1932. [Google Scholar] [CrossRef]
- Shamsuzzaman; Khanam, H.; Dar, A.M.; Siddiqui, N.; Rehman, S. Synthesis, characterization, antimicrobial and anticancer studies of new steroidal pyrazolines. J. Saudi Chem. Soc. 2016, 20, 7–12. [Google Scholar] [CrossRef]
- Shamsuzzaman; Mashrai, A.; Khanam, H.; Asif, M.; Ali, A.; Sherwani, A.; Owais, M. Green synthesis and biological evaluation of steroidal 2H-pyrans as anticancer and antioxidant agents. J King Saud Univ. Sci. 2015, 27, 1–6. [Google Scholar] [CrossRef]
- Ali, A.; Asif, M.; Alam, P.; Jane Alam, M.; Asif Sherwani, M.; Hasan Khan, R.; Ahmad, S.; Shamsuzzaman. DFT/B3LYP calculations, in vitro cytotoxicity and antioxidant activities of steroidal pyrimidines and their interaction with HSA using molecular docking and multispectroscopic techniques. Bioorg. Chem. 2017, 73, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.; Kaishap, P.P.; Goswami, J.; Singh, A.K.; Deka Boruah, H.P.; Gogoi, S.; Boruah, R.C. Synthesis of steroidal and nonsteroidal vicinal heterocyclic alcohols, N-(1-cycloalkenyl)heterocycles and their antibacterial studies. Steroids 2014, 84, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Morake, M.; Coertzen, D.; Ngwane, A.; Wentzel, J.F.; Wong, H.N.; Smit, F.J.; Birkholtz, L.M.; Pietersen, R.D.; Baker, B.; Wiid, I.; et al. Preliminary Evaluation of Artemisinin-Cholesterol Conjugates as Potential Drugs for the Treatment of Intractable Forms of Malaria and Tuberculosis. ChemMedChem 2018, 13, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.R.E.S.; El Azab, I.H.; Gobouri, A.A. Synthesis, antimicrobial and photoelectric potency of new ferrocene-based congeners. Mon. Chem. 2018, 149, 505–517. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Rauf, M.A.; Owais, M.; Shamsuzzaman. Facile one-pot multicomponent synthesis and molecular docking studies of steroidal oxazole/thiazole derivatives with effective antimicrobial, antibiofilm and hemolytic properties. Steroids 2018, 134, 22–36. [Google Scholar] [CrossRef]
- Begum, A.; Borah, P.; Chowdhury, P. Microwave (MW) promoted high yield expedient synthesis of steryl ferulates—A class of novel biologically active compounds: A comparative study of their antioxidant activity with that of naturally occurring γ-oryzanol. Steroids 2016, 107, 37–44. [Google Scholar] [CrossRef]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; Laschat, S. Discotic Liquid Crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar] [CrossRef]
- Hiremath, U.S. Synthesis and characterization of novel chiral dimers exhibiting highly frustrated liquid crystal phases. Tetrahedron 2014, 70, 4745–4753. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Alshargabi, A.; Mahmood, W.A.K.; Han, C.-C.; Lin, H.-C.; Santo, M.; Ito, M.M. Synthesis, characterization and molecular organization for induced smectic phase of triazole ring in non-symmetric liquid crystalline dimer. Tetrahedron 2015, 71, 3939–3945. [Google Scholar] [CrossRef]
- Guo, H.; Yang, F.; Liu, W.; Lai, J. Novel supramolecular liquid crystals: Synthesis and mesomorphic properties of calix[4]arene-cholesterol derivatives. Tetrahedron Lett. 2015, 56, 866–870. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Yang, F.; Yuan, J. Ion complexation-controlled columnar mesophase of calix[4]arene–cholesterol derivatives with Schiff-base bridges. Tetrahedron Lett. 2016, 57, 905–909. [Google Scholar] [CrossRef]
- Xiong, J.; Lin, X.; Guo, H.; Yang, F.; Lai, J. Liquid crystalline oligomers derived from cholesterol: Synthesis and columnar mesomorphism. Liq. Cryst. 2018, 45, 362–369. [Google Scholar] [CrossRef]
- Gupta, M.; Pal, V.; Pal, S.K. Photo-responsive liquid crystals derived from azobenzene centered cholesterol-based tetramers. New J. Chem. 2018, 42, 8765–8772. [Google Scholar] [CrossRef]
- Gupta, S.K.; Setia, S.; Sidiq, S.; Gupta, M.; Kumar, S.; Pal, S.K. New perylene-based non-conventional discotic liquid crystals. RSC Adv. 2013, 3, 12060–12065. [Google Scholar] [CrossRef]
- Zhu, M.; Guo, H.; Yang, F.; Wang, Z. Synthesis, mesomorphic and photophysical properties of novel triads and pentads of perylene liquid crystals with cholesterol units at the bay-position. RSC Adv. 2017, 7, 4320–4328. [Google Scholar] [CrossRef]
- Chen, S.; Hong, B.; Guo, H.; Yang, F. The mesomorphic and photophysical properties of perylene liquid crystals with different bay-rigid spacers. Liq. Cryst. 2018, 45, 793–800. [Google Scholar] [CrossRef]
- Champagne, P.-L.; Ester, D.; Aldosari, S.; Williams, V.E.; Ling, C.-C. Synthesis and comparison of mesomorphic behaviour of a cholesterol-based liquid crystal dimer and analogous monomers. Liq. Cryst. 2018, 45, 1164–1176. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Xie, Y.; Chen, Z.; Hu, J.; Yang, L. Synthesis and liquid crystal behavior of new side chain aliphatic polycarbonates based on cholesterol. J. Mol. Liq. 2018, 259, 350–358. [Google Scholar] [CrossRef]
- Xiong, Y.; Zheng, S.; Zhu, L.; Guo, H.; Yang, F. Novel liquid crystals with high fluorescence: Synthesis, mesomorphic and photophysical properties of cholesterol-triazine-BODIPY trimers. J. Mol. Struct. 2018, 1164, 311–316. [Google Scholar] [CrossRef]
- Ooi, Y.-H.; Yeap, G.-Y. λ-Shaped liquid crystal trimers with dual terminal cholesteryl moieties: Synthesis and concomitant of N*, SmA and cholesteric glassy phases. Liq. Cryst. 2018, 45, 204–218. [Google Scholar] [CrossRef]
- Frizon, T.E.; Jamal, R.; Sumbal, S.; Bechtold, I.H.; Gallardo, H.; Braga, A.L. Synthesis of Functionalized Organoselenium Materials: Selenides and Diselenides Containing Cholesterol. Eur. J. Org. Chem. 2015, 2015, 3470–3476. [Google Scholar] [CrossRef]
- Beaulieu, R.; Gottis, S.; Meyer, C.; Grand, E.; Deveaux, V.; Kovensky, J.; Stasik, I. Cholesteryl and diosgenyl glycosteroids: Synthesis and characterization of new smectic liquid crystals. Carbohydr. Res. 2015, 404, 70–78. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Devi, M.; Dhir, A.; Dhir, A.; Pooja; Pradeep, C.P. New triangular steroid-based A(LS)3 type gelators for selective fluoride sensing application. RSC Adv. 2014, 4, 27098–27105. [Google Scholar] [CrossRef]
- Geng, L.; Feng, G.; Wang, S.; Yu, X.; Xu, Z.; Zhen, X.; Wang, T. Fluoride-responsive organogel containing azobenzyl and cholesterol units. J. Fluorine Chem. 2015, 170, 24–28. [Google Scholar] [CrossRef]
- Ghosh, K.; Panja, S. Coumarin-based supramolecular gelator: A case of selective detection of F− and HP2O73−. RSC Adv. 2015, 5, 12094–12099. [Google Scholar] [CrossRef]
- Pang, X.; Yu, X.; Xie, D.; Li, Y.; Geng, L.; Ren, J.; Zhen, X. Tunable multicolor emissions in a monocomponent gel system by varying the solvent, temperature and fluoride anion. Org. Biomol. Chem. 2016, 14, 11176–11182. [Google Scholar] [CrossRef]
- Panja, S.; Ghosh, S.; Ghosh, K. Pyridine/pyridinium symmetrical bisamides as functional materials: Aggregation, selective sensing and drug release. New J. Chem. 2018, 42, 6488–6497. [Google Scholar] [CrossRef]
- Huibin, S.; Shujuan, L.; Qiang, Z.; Wei, H. Multiple-Stimuli Responsive Luminescent Gels Based on Cholesterol Containing Benzothiadiazole Fluorophores. Chin. J. Chem. 2015, 33, 1140–1144. [Google Scholar]
- Panja, A.; Ghosh, K. Selective sensing of Hg2+ via sol–gel transformation of a cholesterol-based compound. Supramol. Chem. 2018, 30, 722–729. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Diaminomalenonitrile-decorated cholesterol-based supramolecular gelator: Aggregation, multiple analyte (hydrazine, Hg2+ and Cu2+) detection and dye adsorption. New J. Chem. 2018, 42, 13718–13725. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Cholesterol-based diazine derivative: Selective sensing of Ag+ and Fe3+ ions through gelation and the performance of metallogels in dye and picric acid adsorption from water. Mater. Chem. Front. 2018. [Google Scholar] [CrossRef]
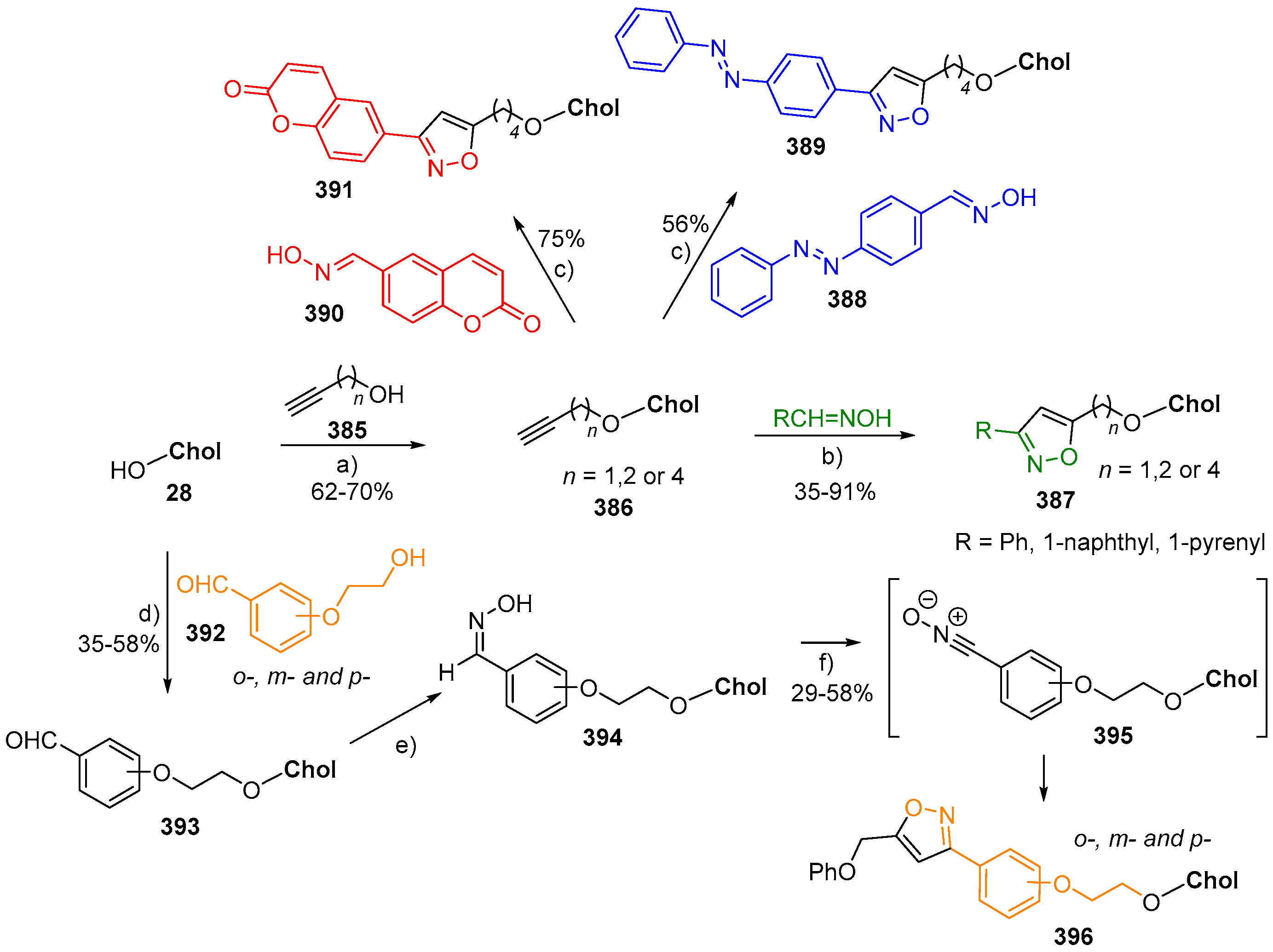
- Ren, Y.; Wang, B.; Zhang, X. Synthesis of photoresponsive cholesterol-based azobenzene organogels: Dependence on different spacer lengths. Beilstein J. Org. Chem. 2015, 11, 1089–1095. [Google Scholar] [CrossRef]
- Tan, X.; Li, Z.; Xia, M.; Cheng, X. Reversible photoresponsive chiral liquid crystal and multistimuli responsive organogels based on a cholesterol-azobenzene dimesogen. RSC Adv. 2016, 6, 20021–20026. [Google Scholar] [CrossRef]
- Yu, X.; Chen, H.; Shi, X.; Albouy, P.-A.; Guo, J.; Hu, J.; Li, M.-H. Liquid crystal gelators with photo-responsive and AIE properties. Mater. Chem. Front. 2018. [Google Scholar] [CrossRef]
- Shimasaki, T.; Okamiya, Y.; Sato, R.; Hara, K.; Nakamura, T.; Teramoto, N.; Shibata, M. Synthesis, structure and properties of cholesterol-based A(LS)2- and A(LS)3-type gelators without hydrogen bond linkers. Tetrahedron 2016, 72, 1517–1523. [Google Scholar] [CrossRef]
- Yao, C.; Sun, Q.; Xia, W.; Zhang, J.; Lin, C.; Wang, L. Ferrocenyl-guest tunable organogel constructed from a Pillar[6]arene-functionalized cholesterol derivative. J. Organomet. Chem. 2017, 847, 68–73. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-Healing Supramolecular Self-Assembled Hydrogels Based on Poly(l-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef] [PubMed]
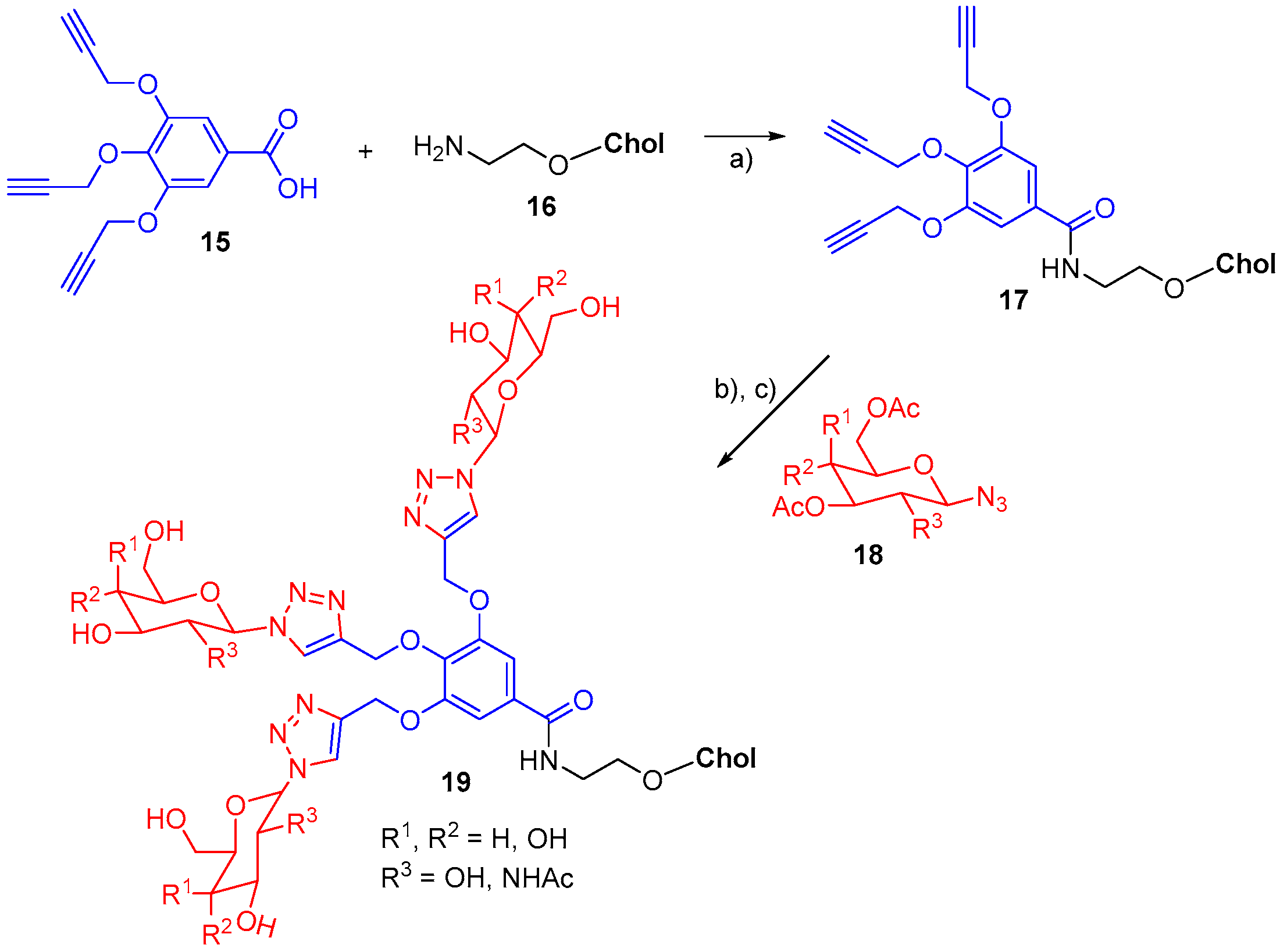
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.P.; Saha, T.; Lahiri, M.; Talukdar, P. BODIPY based ‘click on’ fluorogenic dyes: Application in live cell imaging. Tetrahedron Lett. 2014, 55, 244–247. [Google Scholar] [CrossRef]
- Byrd, K.M.; Arieno, M.D.; Kennelly, M.E.; Estiu, G.; Wiest, O.; Helquist, P. Design and synthesis of a crosslinker for studying intracellular steroid trafficking pathways. Bioorg. Med. Chem. 2015, 23, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Seu, Y.-B.; Choi, J.-S.; Park, J.-W.; Doh, K.-O. Synthesis and validation of novel cholesterol-based fluorescent lipids designed to observe the cellular trafficking of cationic liposomes. Bioorg. Med. Chem. Lett. 2015, 25, 3893–3896. [Google Scholar] [CrossRef] [PubMed]
- Reibel, A.T.; Müller, S.S.; Pektor, S.; Bausbacher, N.; Miederer, M.; Frey, H.; Rösch, F. Fate of Linear and Branched Polyether-Lipids In Vivo in Comparison to Their Liposomal Formulations by 18F-Radiolabeling and Positron Emission Tomography. Biomacromolecules 2015, 16, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Palakollu, V.; Kanvah, S. Cholesterol-tethered AIEE fluorogens: Formation of self-assembled nanostructures. RSC Adv. 2015, 5, 33049–33057. [Google Scholar] [CrossRef]
- Wercholuk, A.N.; Thuman, J.M.; Stanley, J.L.; Sargent, A.L.; Anderson, E.S.; Allen, W.E. Incorporation of fluorophore-cholesterol conjugates into liposomal and mycobacterial membranes. Bioorg. Med. Chem. 2016, 24, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
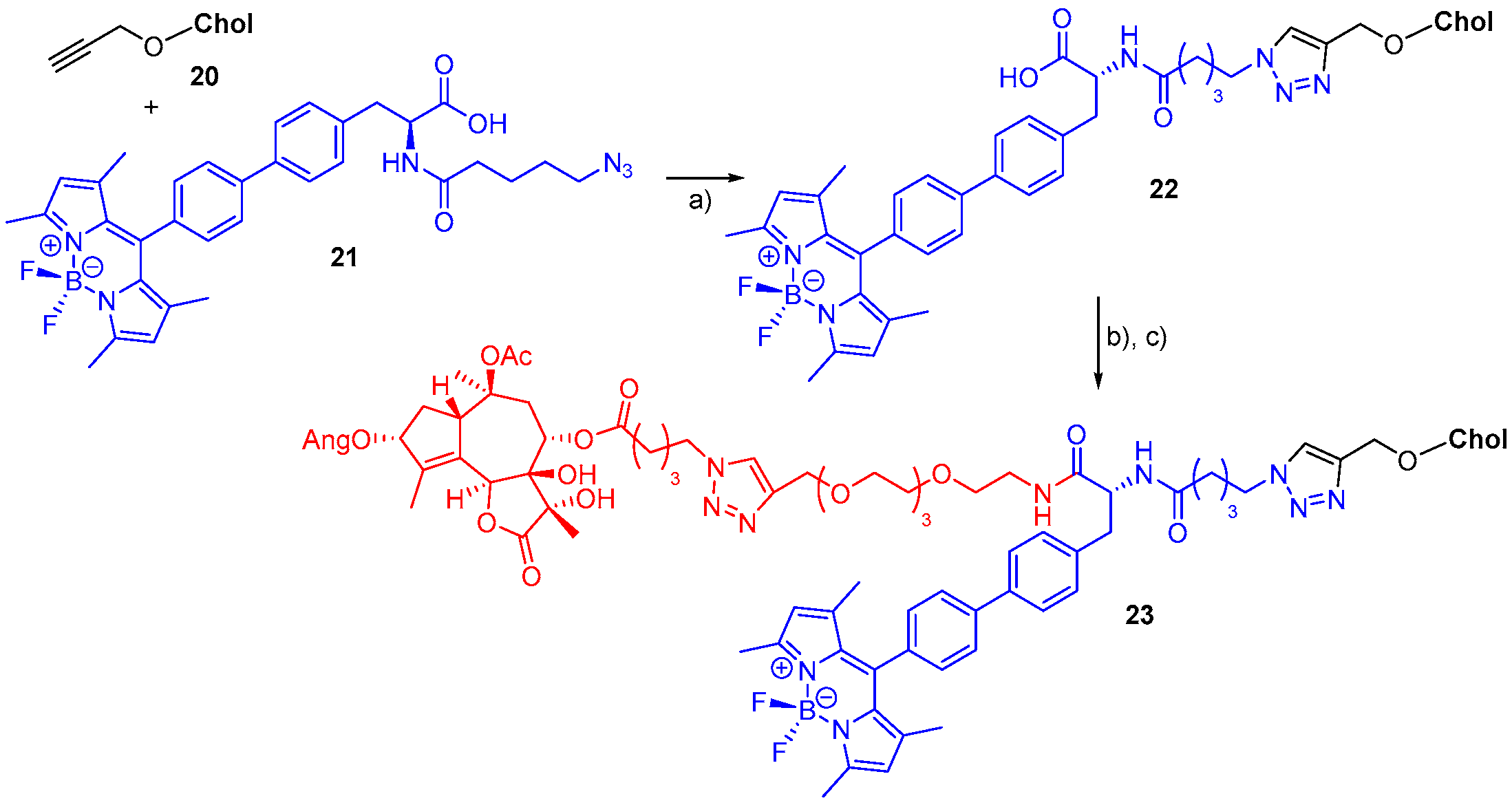
- Bernhard, Y.; Gigot, E.; Goncalves, V.; Moreau, M.; Sok, N.; Richard, P.; Decréau, R.A. Direct subphthalocyanine conjugation to bombesin vs. indirect conjugation to its lipidic nanocarrier. Org. Biomol. Chem. 2016, 14, 4511–4518. [Google Scholar] [CrossRef]
- Ikejiri, M.; Mori, K.; Miyagi, R.; Konishi, R.; Chihara, Y.; Miyashita, K. A hybrid molecule of a GFP chromophore analogue and cholestene as a viscosity-dependent and cholesterol-responsive fluorescent sensor. Org. Biomol. Chem. 2017, 15, 6948–6958. [Google Scholar] [CrossRef]
- Tomkiel, A.M.; Kowalski, J.; Płoszyńska, J.; Siergiejczyk, L.; Łotowski, Z.; Sobkowiak, A.; Morzycki, J.W. Electrochemical synthesis of glycoconjugates from activated sterol derivatives. Steroids 2014, 82, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Tomkiel, A.M.; Biedrzycki, A.; Ploszynska, J.; Narog, D.; Sobkowiak, A.; Morzycki, J.W. 3α,5α-Cyclocholestan-6β-yl ethers as donors of the cholesterol moiety for the electrochemical synthesis of cholesterol glycoconjugates. Beilstein J. Org. Chem. 2015, 11, 162–168. [Google Scholar] [CrossRef] [PubMed]
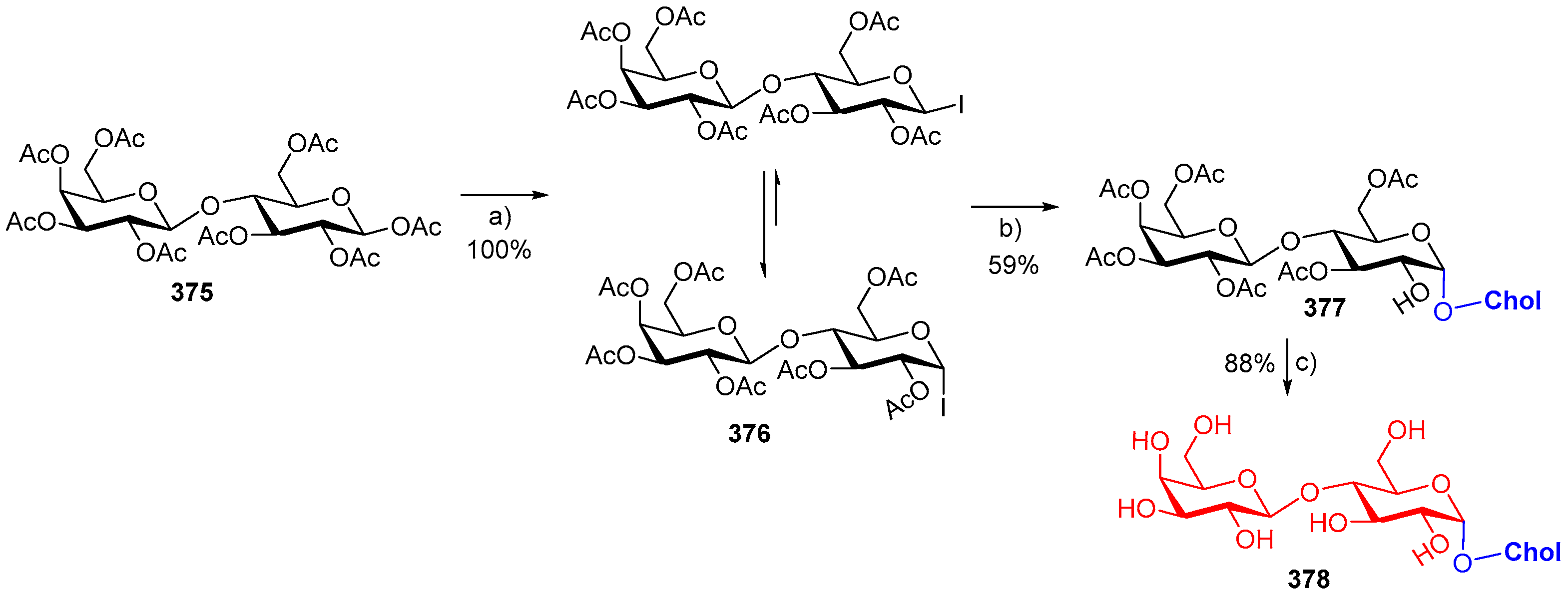
- Davis, R.A.; Fettinger, J.C.; Gervay-Hague, J. Tandem Glycosyl Iodide Glycosylation and Regioselective Enzymatic Acylation Affords 6-O-Tetradecanoyl-α-d-cholesterylglycosides. J. Org. Chem. 2014, 79, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.-Z.; Guo, F.; Xiong, D.-C.; Li, Q.; Duan, J.; Ye, X.-S. Photoinduced C-S Bond Cleavage of Thioglycosides and Glycosylation. Org. Lett. 2015, 17, 5606–5609. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Fettinger, J.C.; Gervay-Hague, J. Synthesis of cholesteryl-α-d-lactoside via generation and trapping of a stable β-lactosyl iodide. Tetrahedron Lett. 2015, 56, 3690–3694. [Google Scholar] [CrossRef] [PubMed]
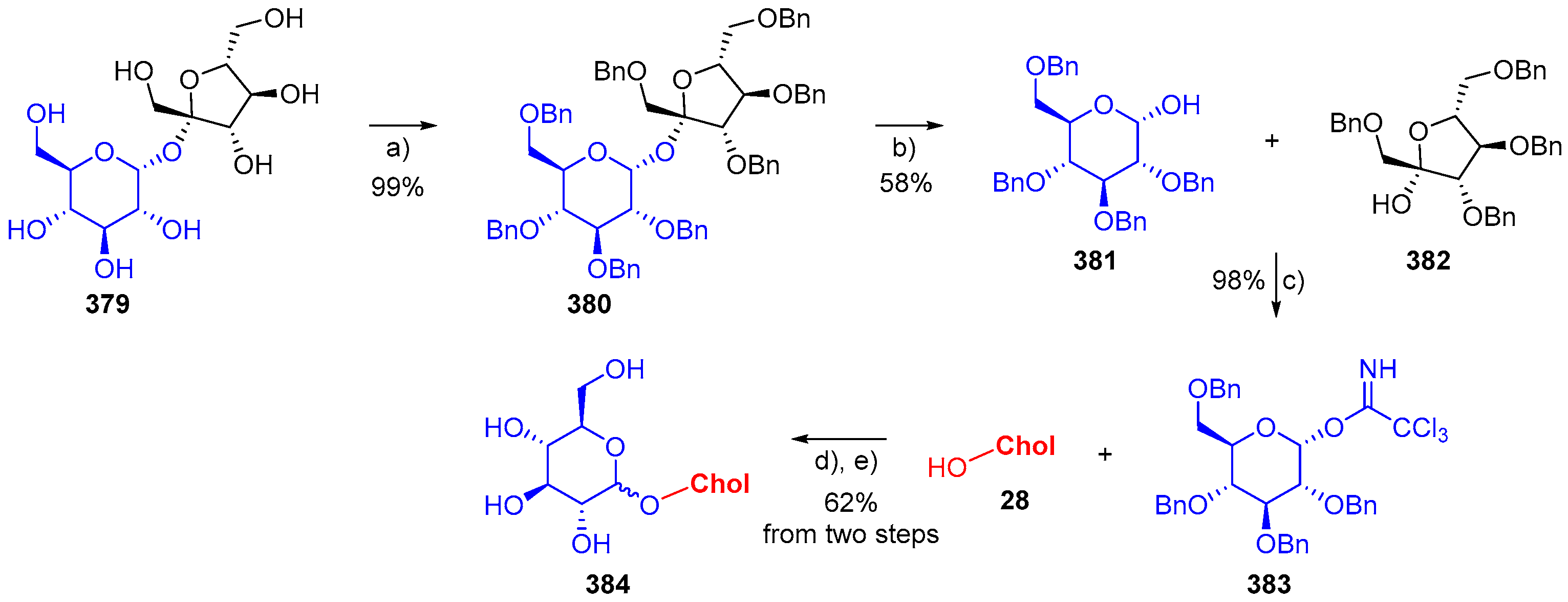
- Weiss, S.; Neu, P.M.; Ludwig, C.; Schober, S.; Mittelbach, M. Novel Method for the Synthesis of Cholesteryl Glucosides starting from Disaccharides. Eur. J. Lipid Sci. Technol. 2018, 120, 1700389. [Google Scholar] [CrossRef]
- Algay, V.; O’Sullivan, J.; Heaney, F. C-3β-Tethered Functional Cholesterol Conjugates by Nitrile Oxide Alkyne Cycloaddition (NOAC). Eur. J. Org. Chem. 2014, 2014, 2522–2532. [Google Scholar] [CrossRef]
- Alarcon-Manjarrez, C.; Arcos-Ramos, R.; Alamo, M.F.; Iglesias-Arteaga, M.A. Synthesis, NMR and crystal characterization of dimeric terephthalates derived from epimeric 4,5-seco-cholest-3-yn-5-ols. Steroids 2016, 109, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Watanabe, T.; Urano, Y.; Takabe, W.; Noguchi, N.; Kitagishi, H. Synthesis of 24(S)-hydroxycholesterol esters responsible for the induction of neuronal cell death. Bioorg. Med. Chem. 2016, 24, 2559–2566. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, J.; Ghosh, P. p-TsOH-Catalyzed one-pot transformation of di- and trihydroxy steroids towards diverse A/B-ring oxo-functionalization. New J. Chem. 2017, 41, 9051–9060. [Google Scholar] [CrossRef]
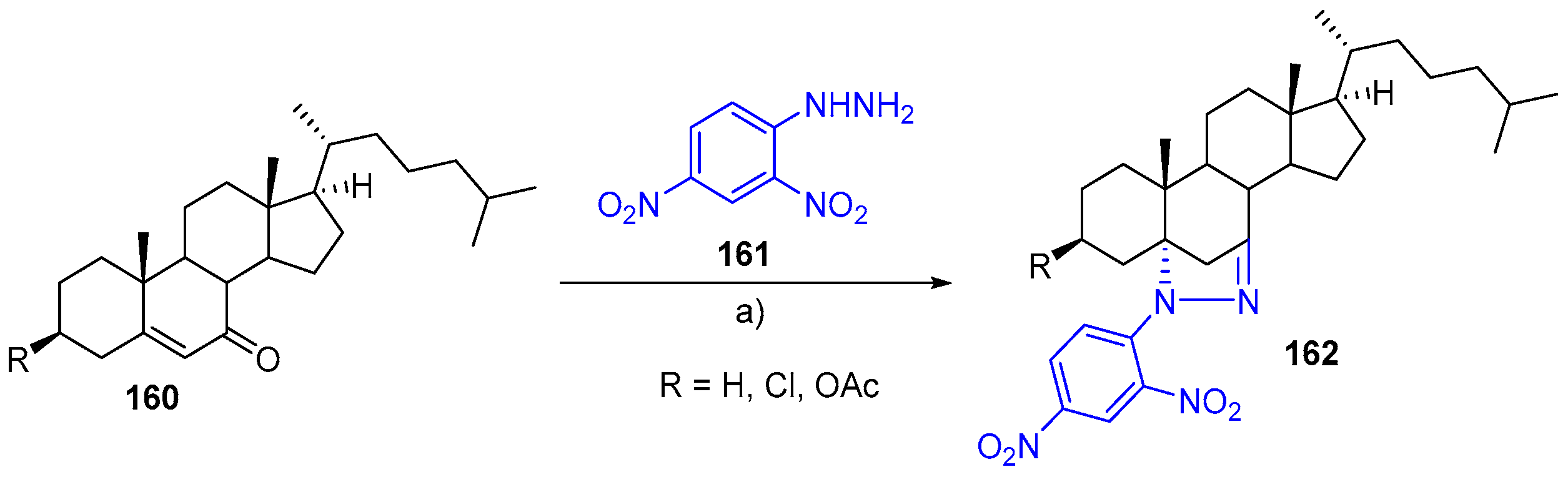
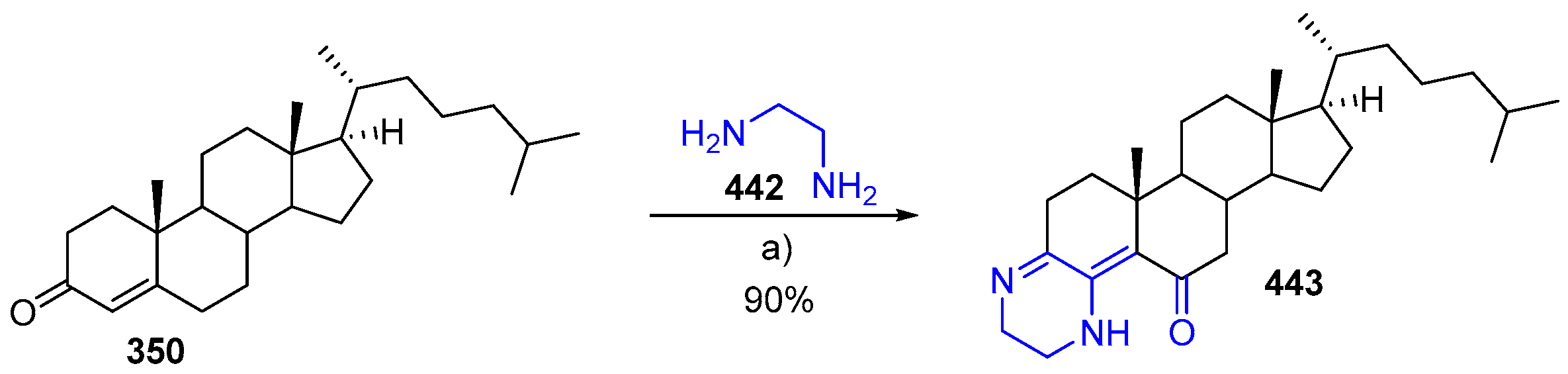
- Borthakur, M.; Barthakur, M.G.; Boruah, R.C. Microwave promoted one-pot synthesis of novel A-ring fused steroidal dehydropiperazines. Steroids 2008, 73, 539–542. [Google Scholar] [CrossRef] [PubMed]
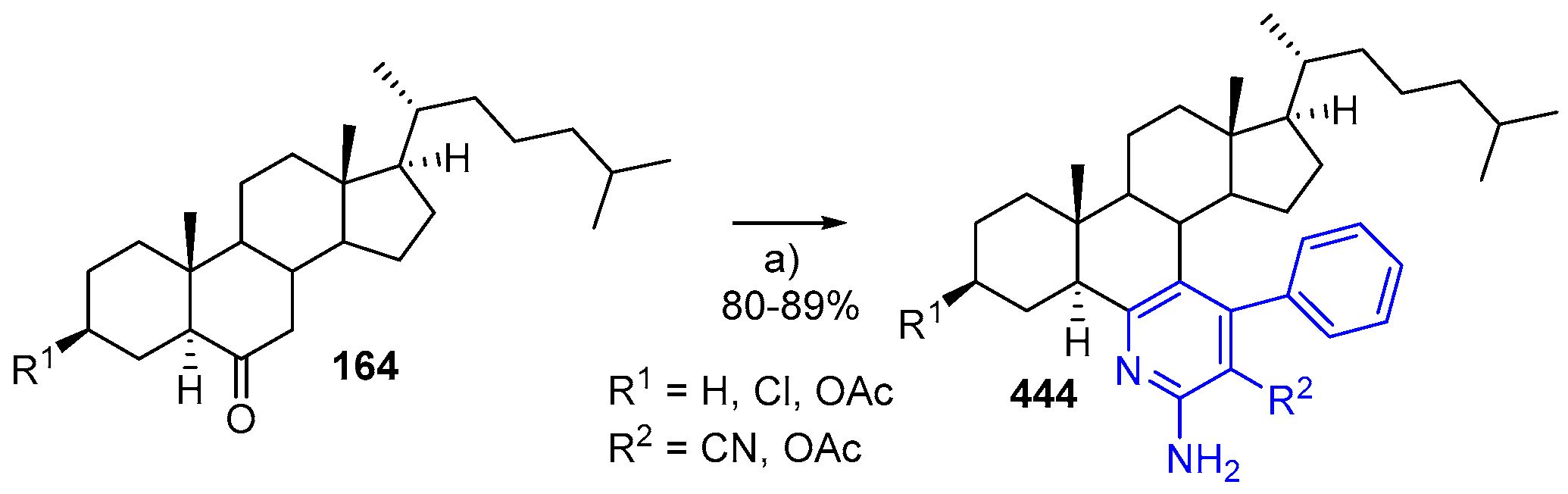
- Ansari, A.; Ali, A.; Asif, M. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
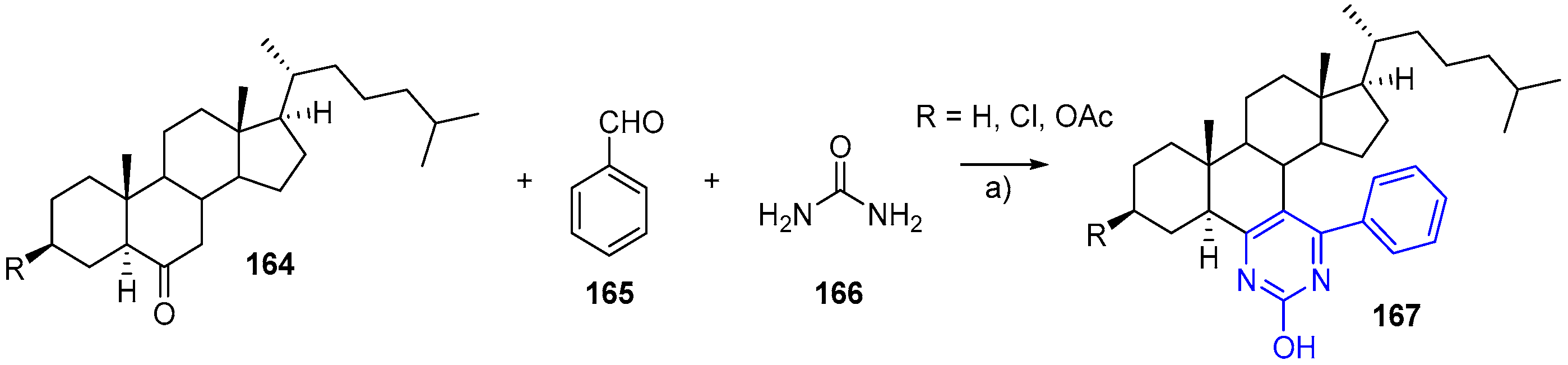
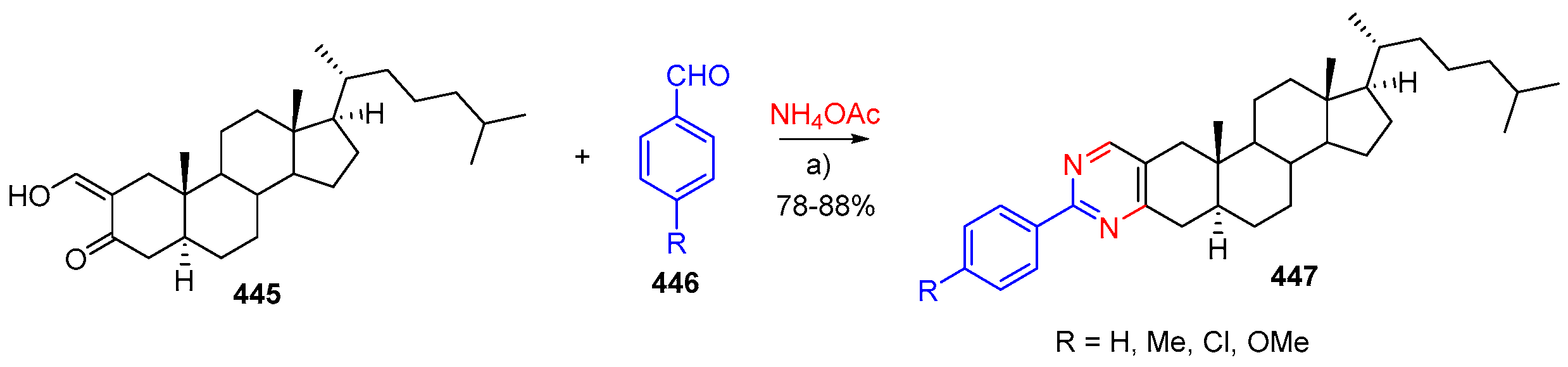
- Barthakur, M.G.; Gogoi, S.; Dutta, M.; Boruah, R.C. A facile three-component solid phase synthesis of steroidal A-ring fused pyrimidines under microwave irradiation. Steroids 2009, 74, 730–734. [Google Scholar] [CrossRef] [PubMed]
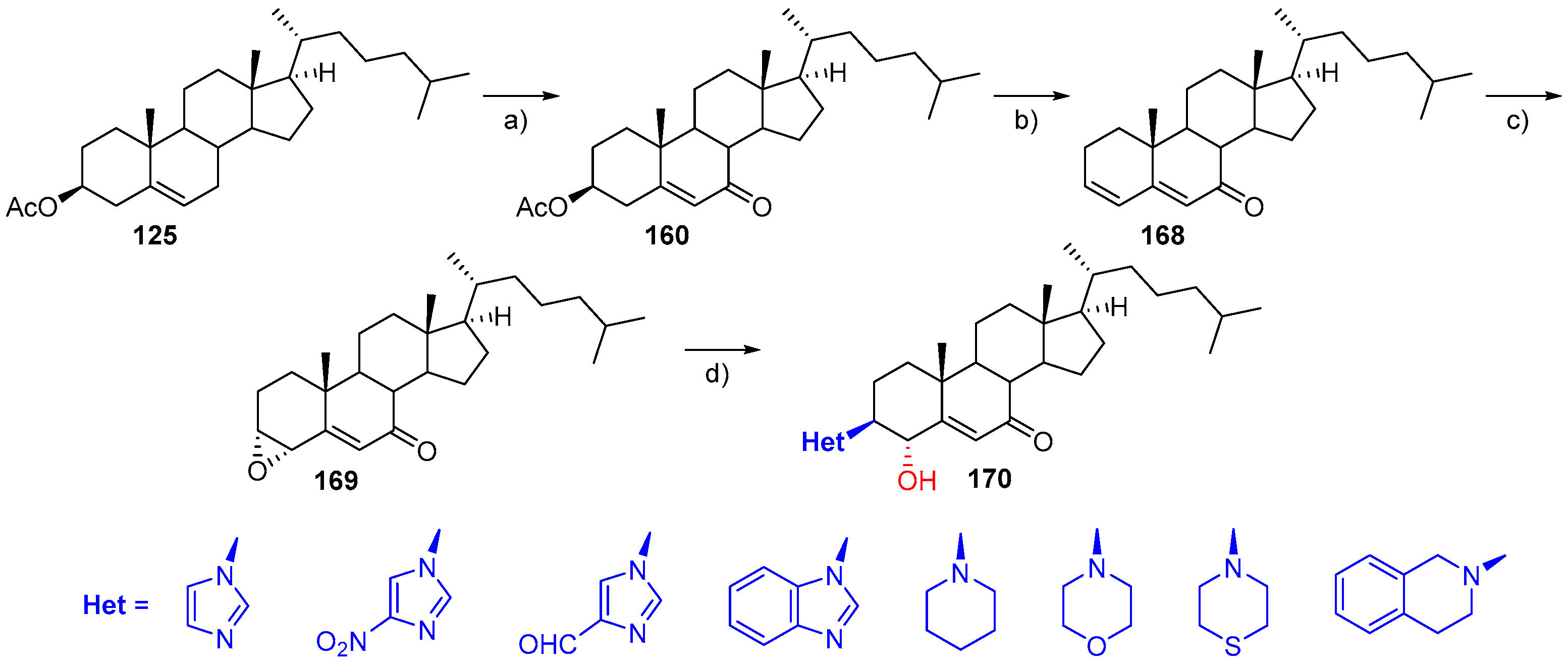
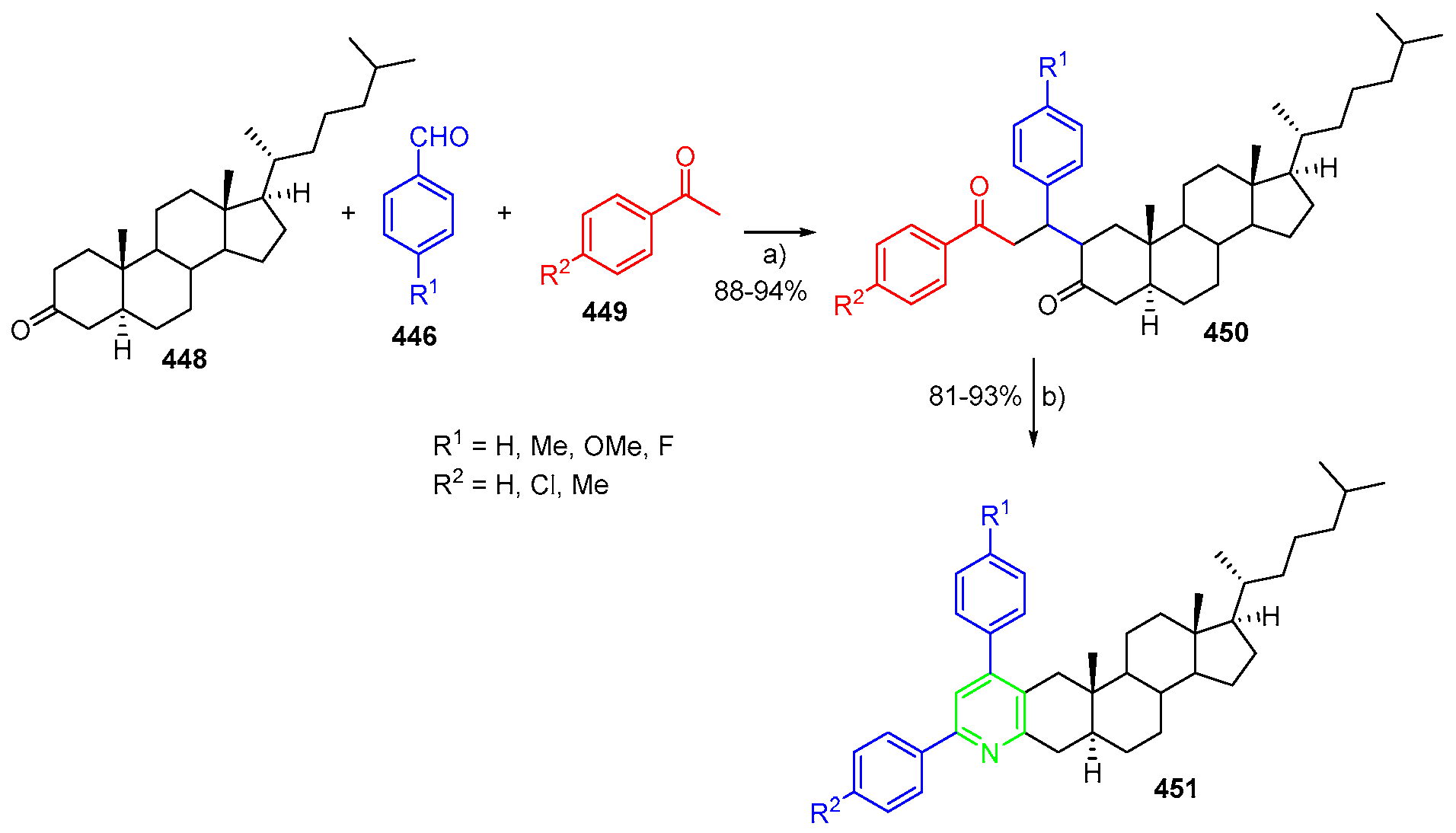
- Dutta, M.; Saikia, P.; Gogoi, S.; Boruah, R.C. Microwave-promoted and Lewis acid catalysed synthesis of steroidal a- and d-ring fused 4,6-diarylpyridines. Steroids 2013, 78, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Scott, D.E.; Scherer, A.; Hampel, F.; Hamilton, R.J.; Gray, M.R.; Tykwinski, R.R.; Stryker, J.M. Steroid-Derived Naphthoquinoline Asphaltene Model Compounds: Hydriodic Acid Is the Active Catalyst in I2-Promoted Multicomponent Cyclocondensation Reactions. Org. Lett. 2015, 17, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
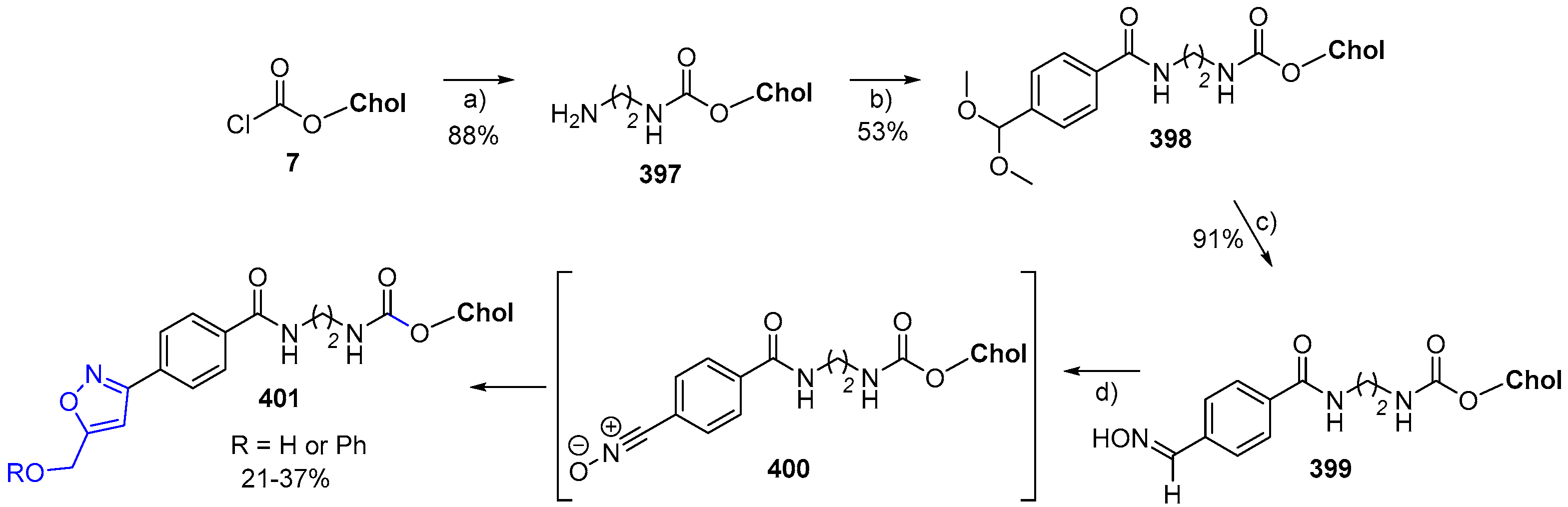
- Berg, M.; Nozinovic, S.; Engeser, M.; Lützen, A. A Cholesterol Containing pH-Sensitive Bistable [2]Rotaxane. Eur. J. Org. Chem. 2015, 2015, 5966–5978. [Google Scholar] [CrossRef]
- Venkataraman, S.; Mineart, K.P.; Prabhu, V.M.; Hedrick, J.L.; Yang, Y.Y. Cholesterol functionalized aliphatic N-substituted 8-membered cyclic carbonate. Polym. Chem. 2018, 9, 2434–2437. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R.; Wei, J.; Cheng, C.; Zheng, Y.; Pan, Y.; He, X.; Ding, M.; Tan, H.; Fu, Q. Conformation-Directed Micelle-to-Vesicle Transition of Cholesterol-Decorated Polypeptide Triggered by Oxidation. J. Am. Chem. Soc. 2018, 140, 6604–6610. [Google Scholar] [CrossRef] [PubMed]
- Kozanecka, W.; Mrowczynska, L.; Pospieszny, T.; Jasiewicz, B.; Gierszewski, M. Synthesis, spectroscopy, theoretical and biological studies of new gramine-steroids salts and conjugates. Steroids 2015, 98, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenböck, C.; Schaffer, A.; Pahl, P.; Nelson, P.J.; Huss, R.; Rieger, B. Precise synthesis of thermoresponsive polyvinylphosphonate-biomolecule conjugates via thiol–ene click chemistry. Polym. Chem. 2018, 9, 284–290. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, X.; Cai, Y.; Liu, L.; Zhao, H. Coassembly of Lysozyme and Amphiphilic Biomolecules Driven by Unimer–Aggregate Equilibrium. J. Phys. Chem. B 2018, 122, 3900–3907. [Google Scholar] [CrossRef] [PubMed]







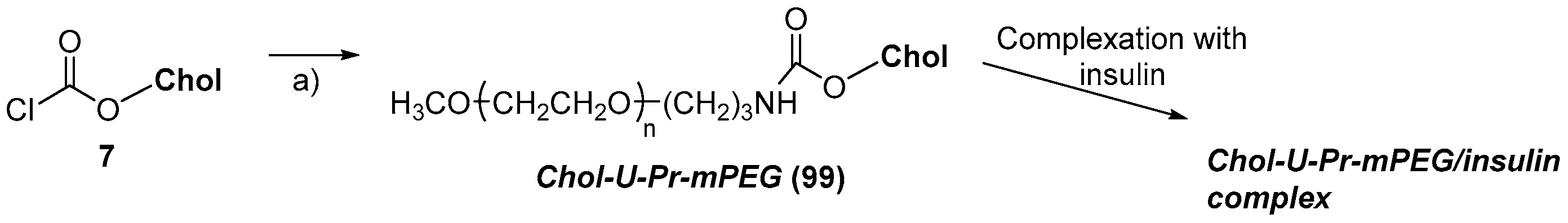




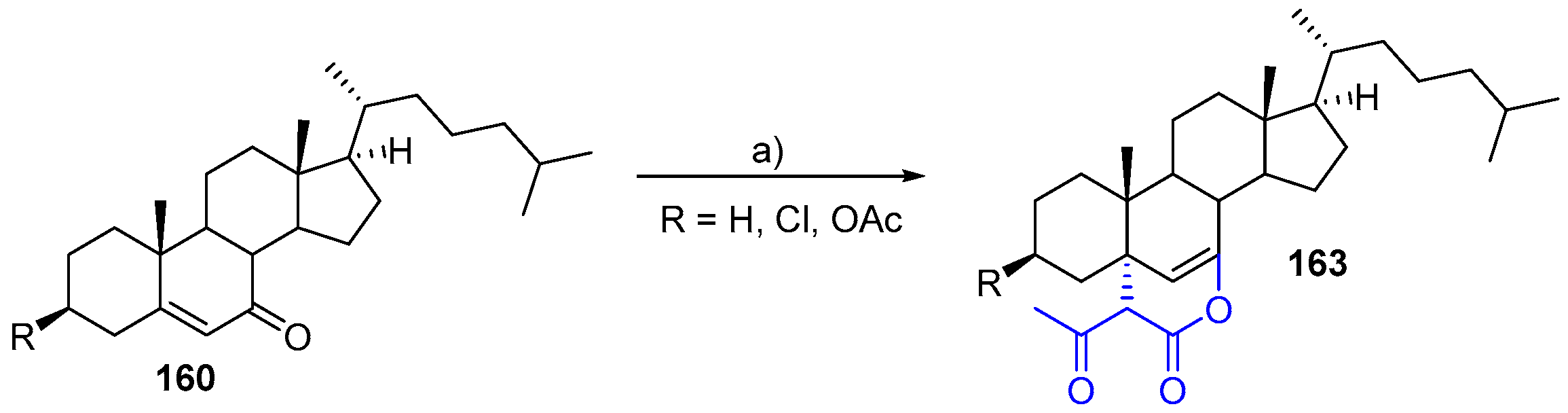


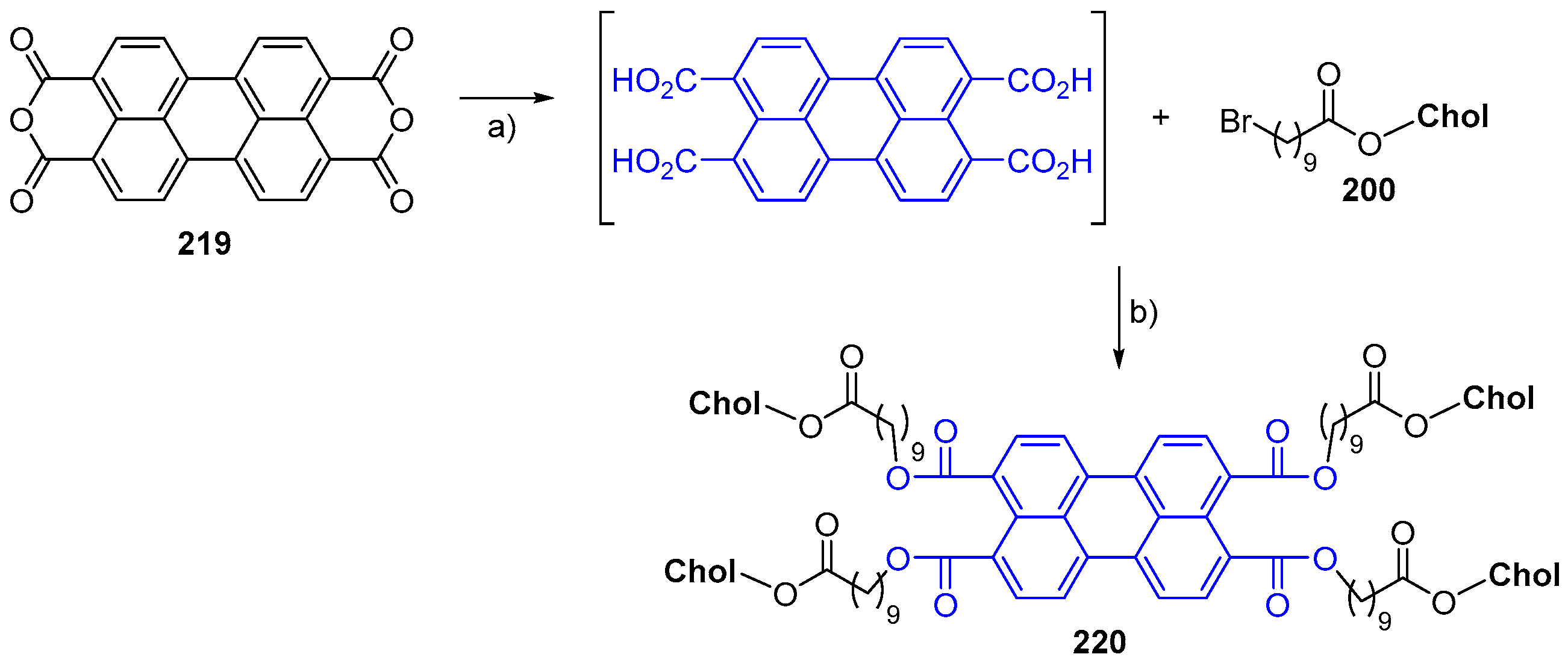
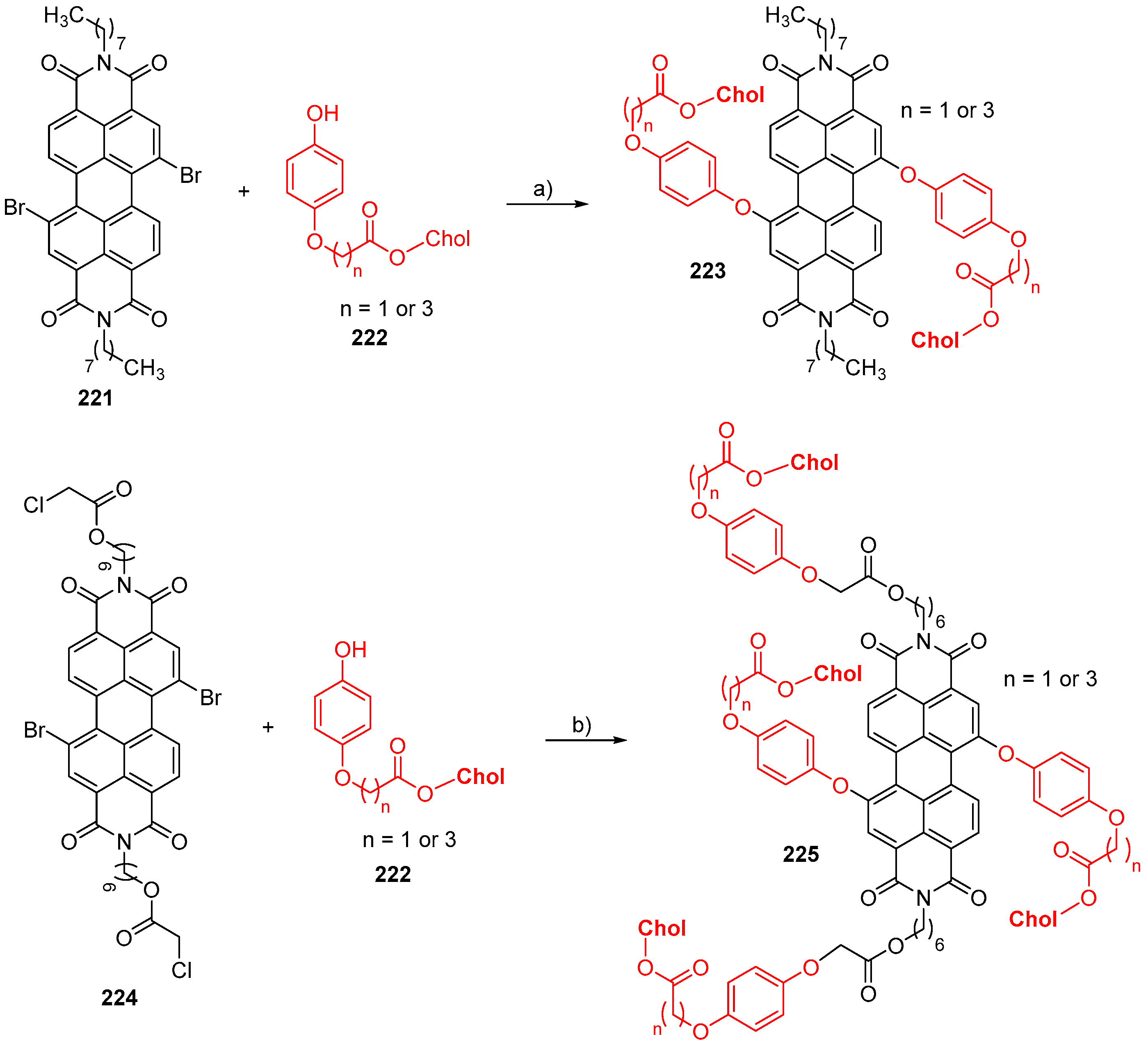
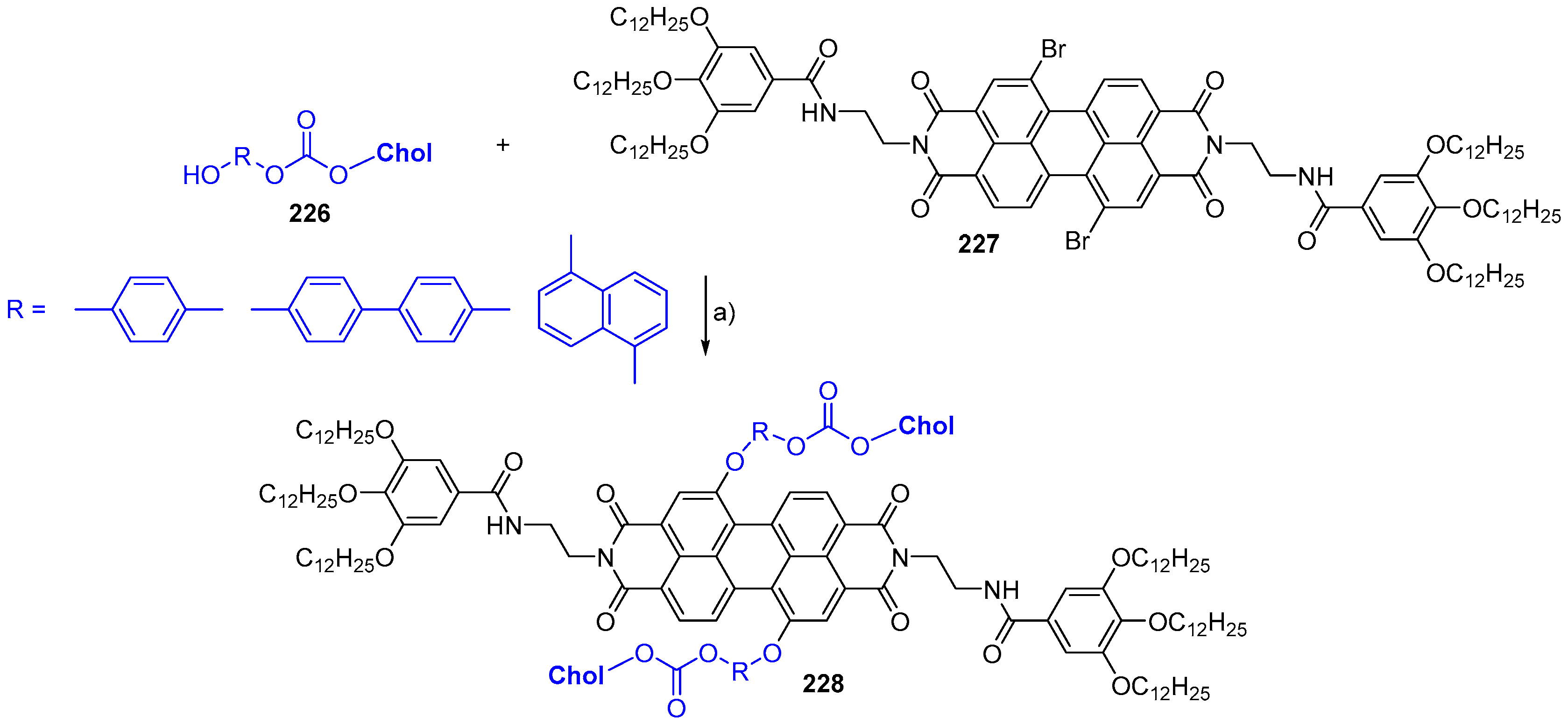
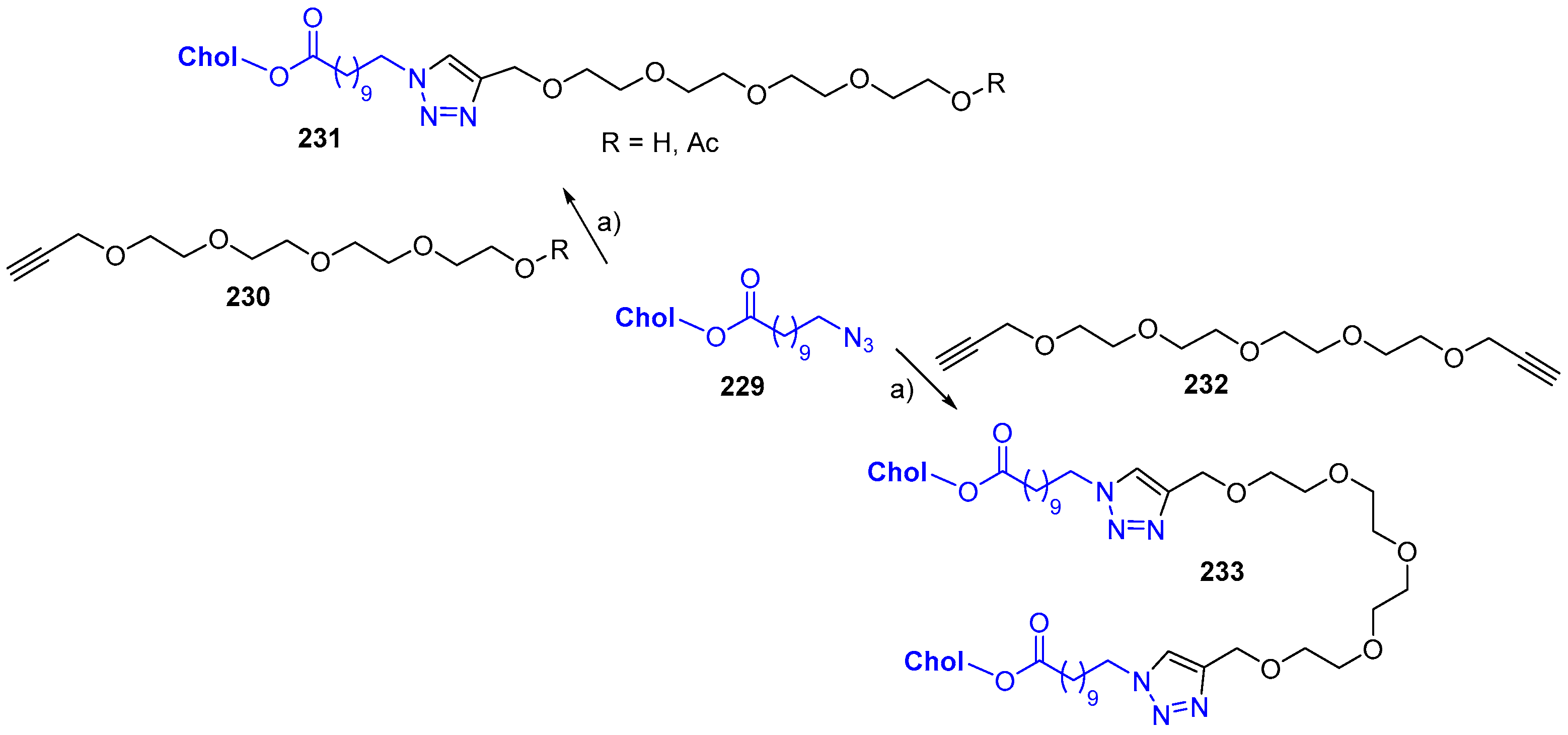
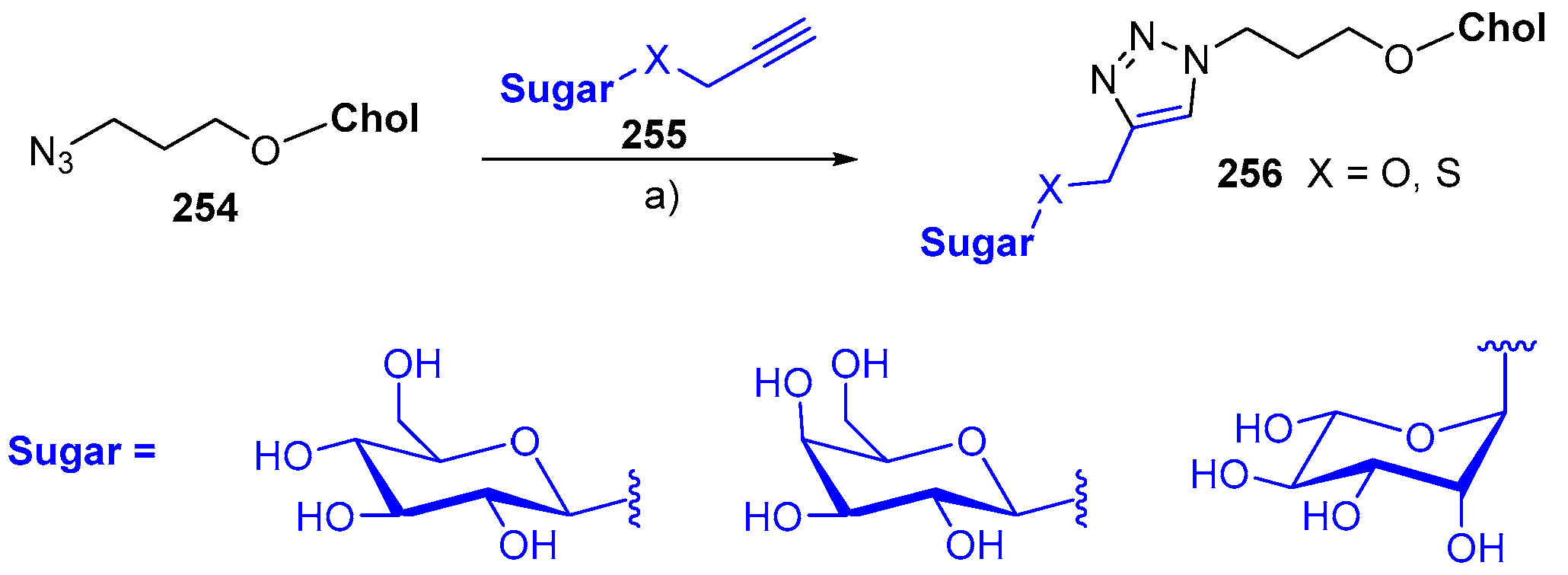
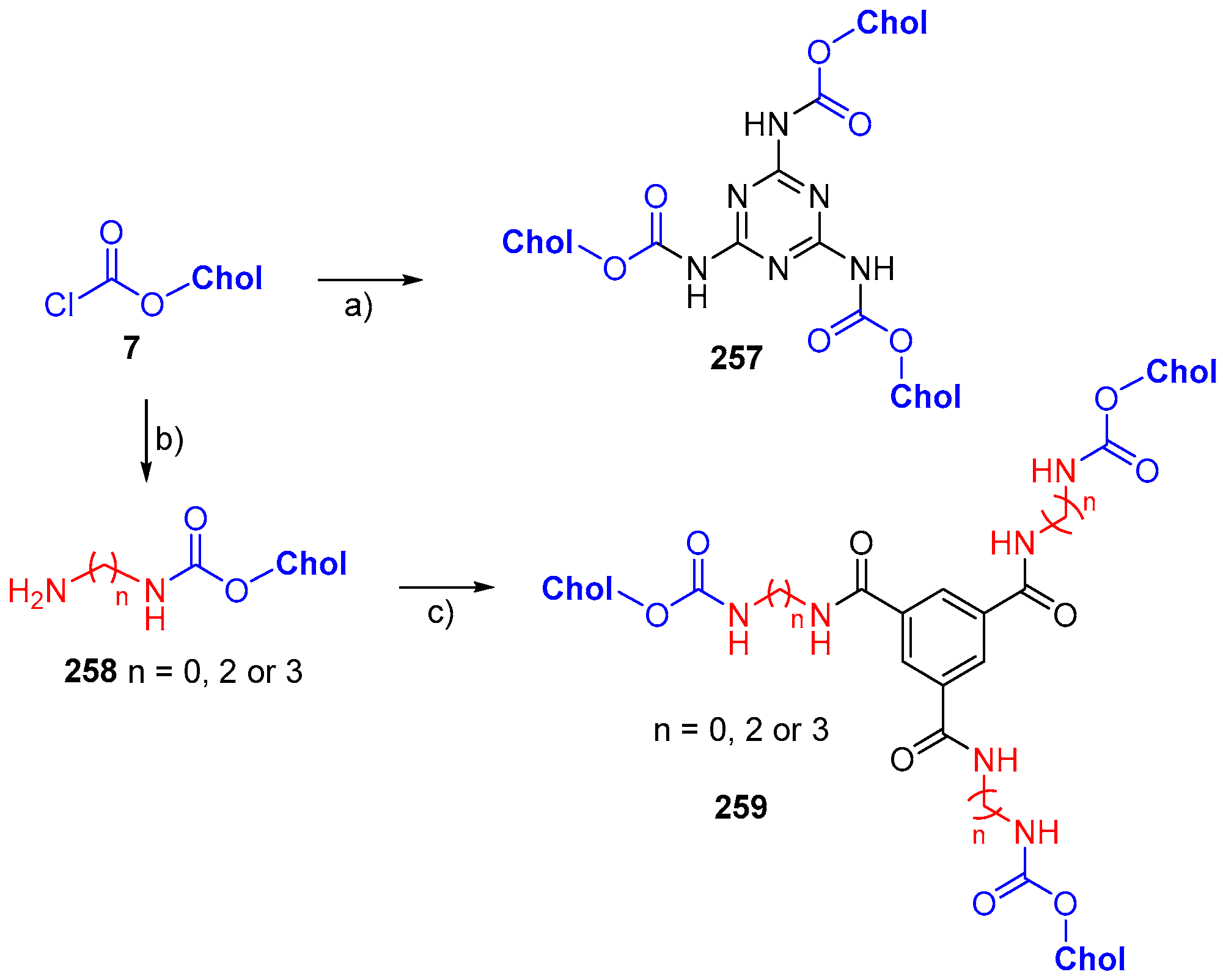
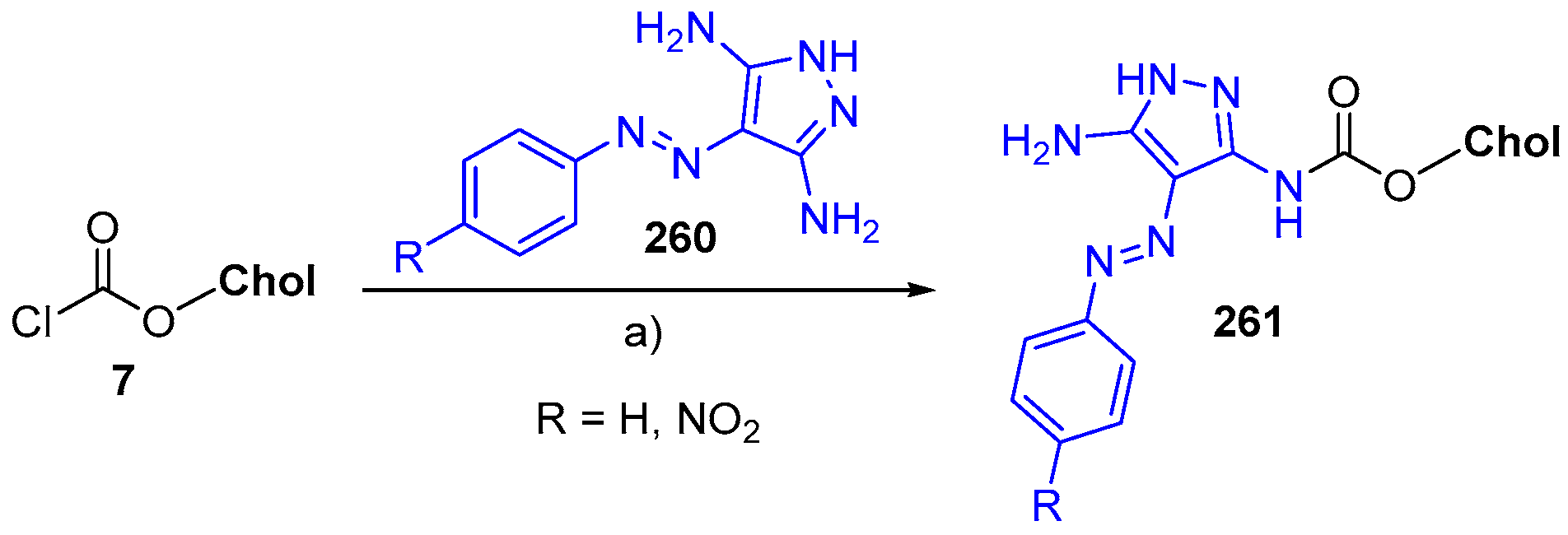

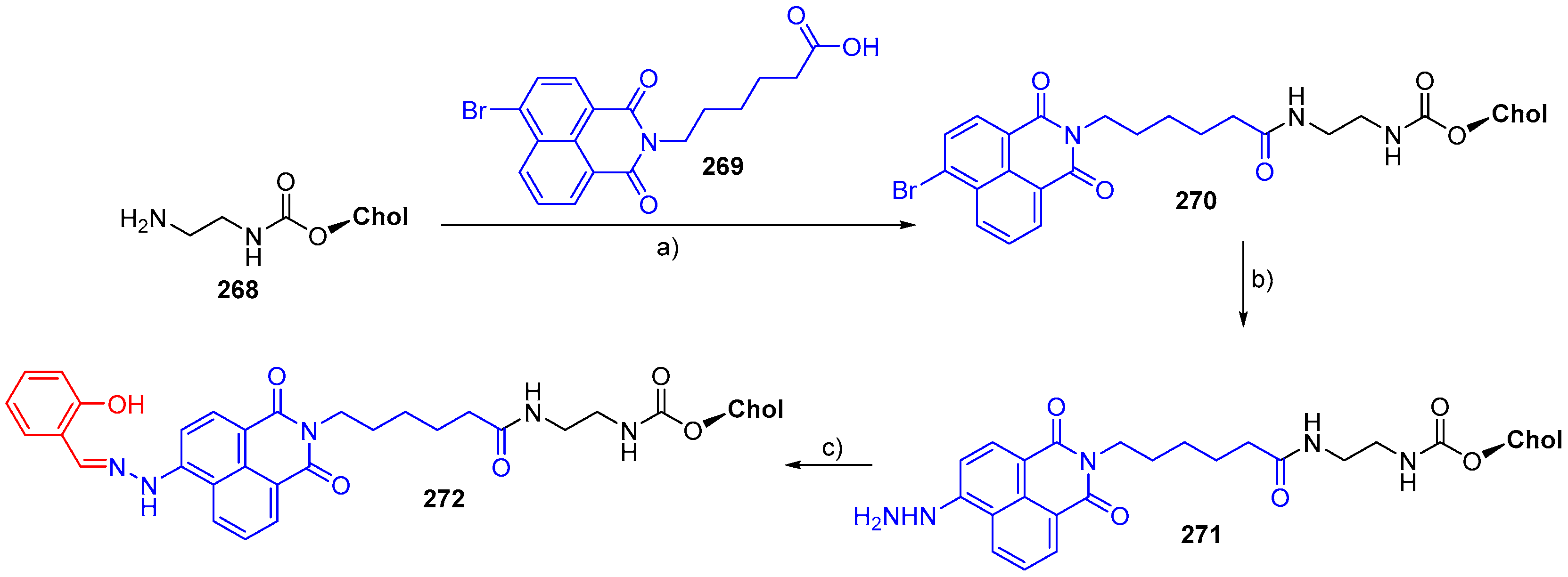


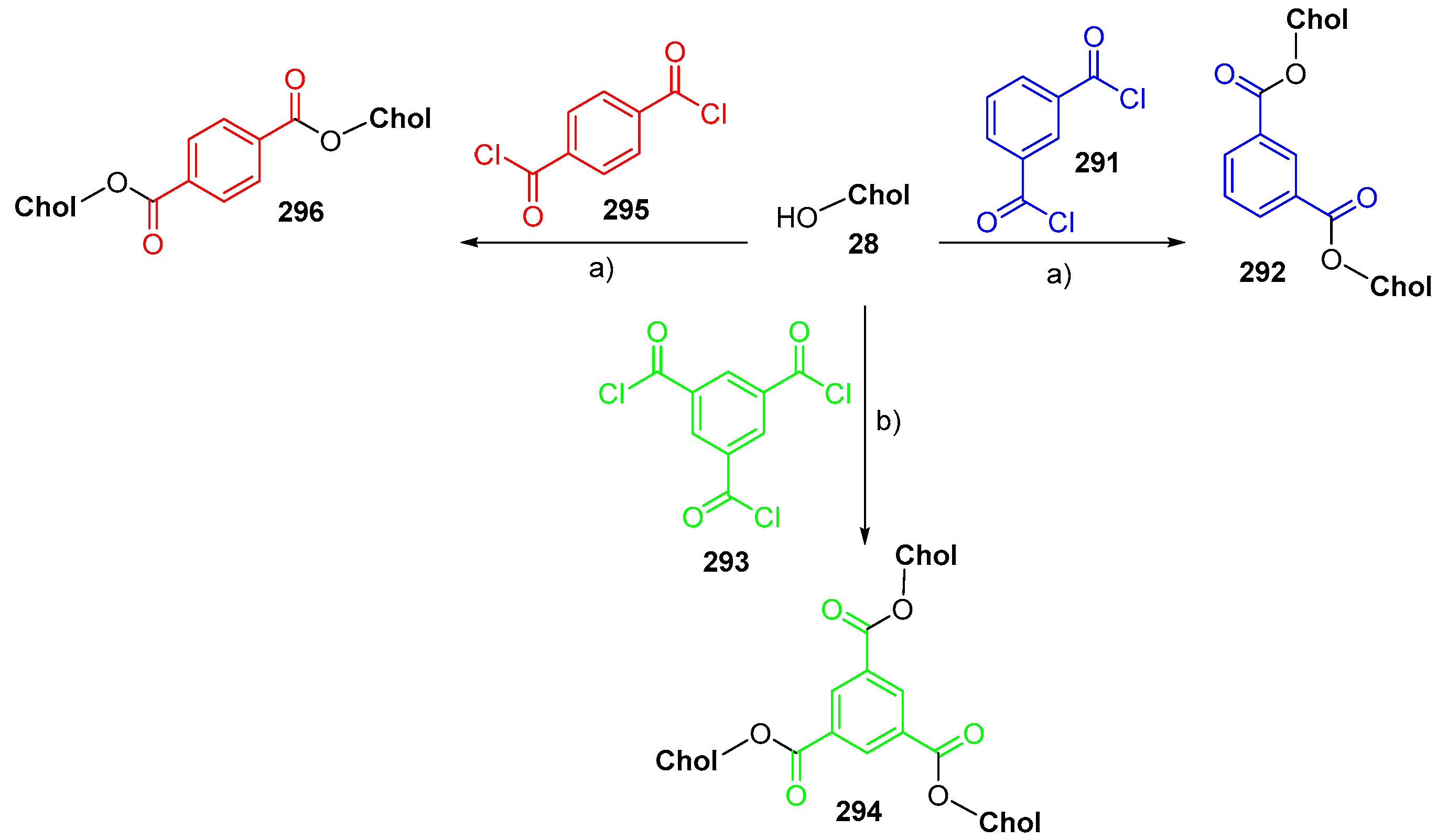
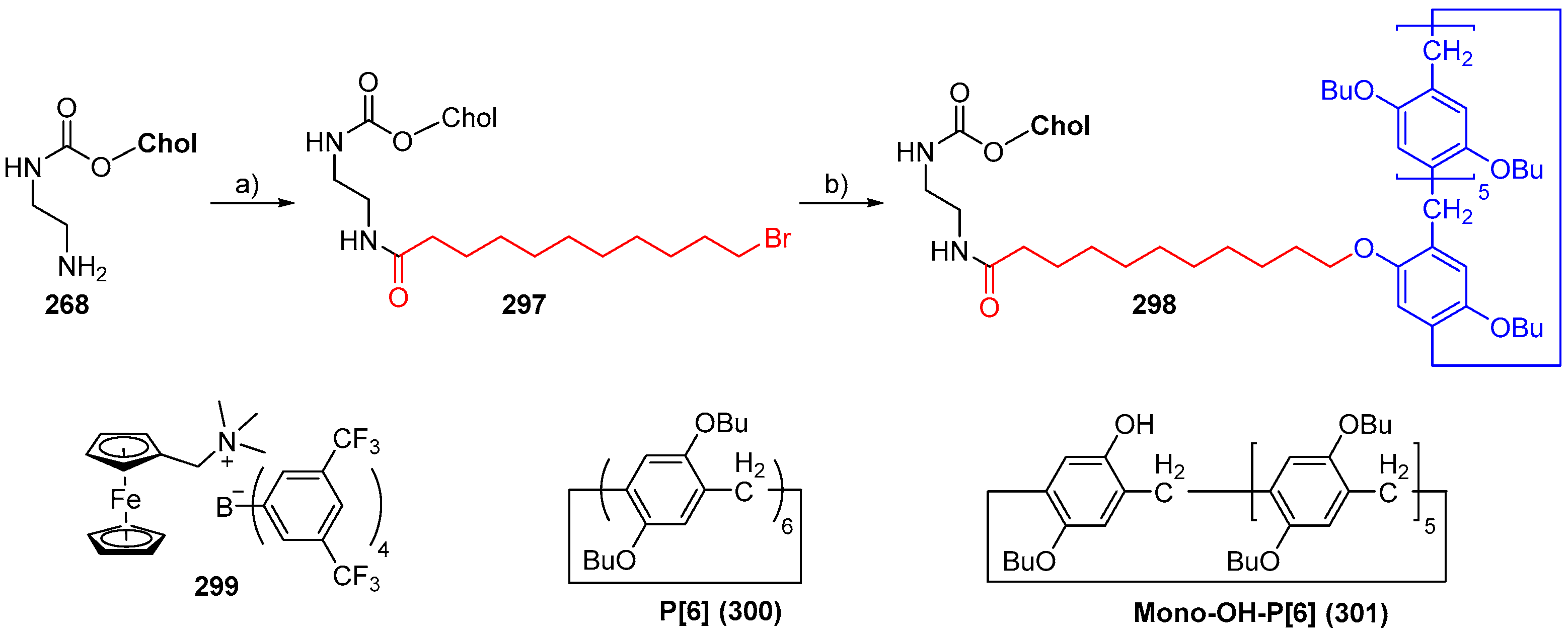
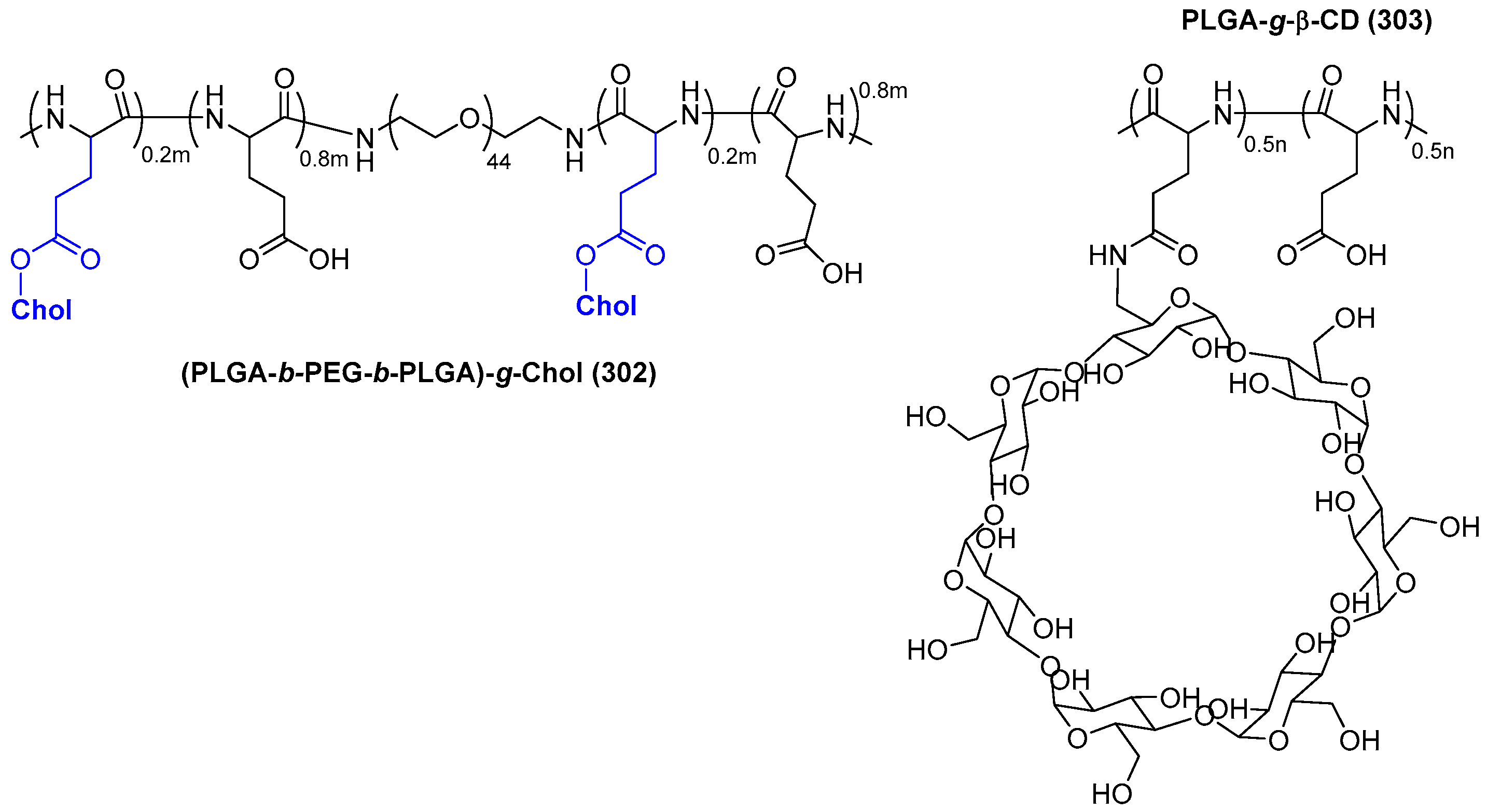
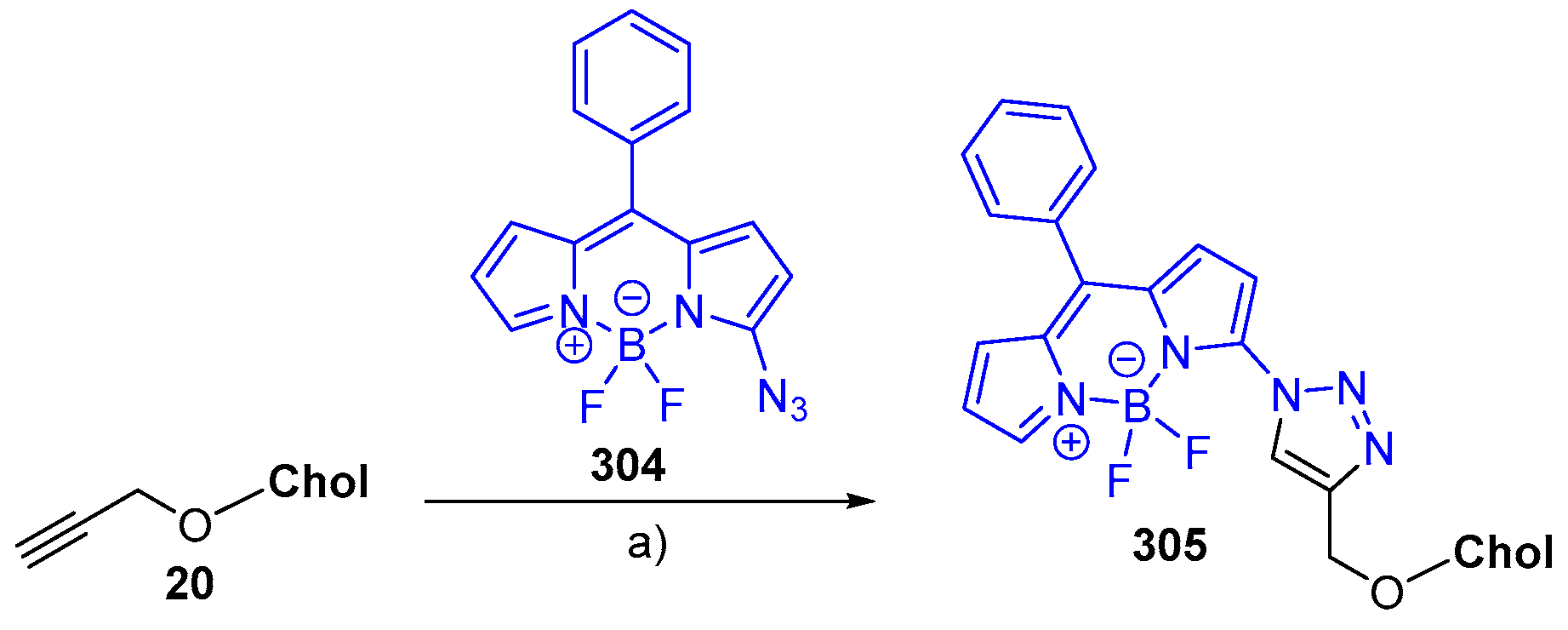

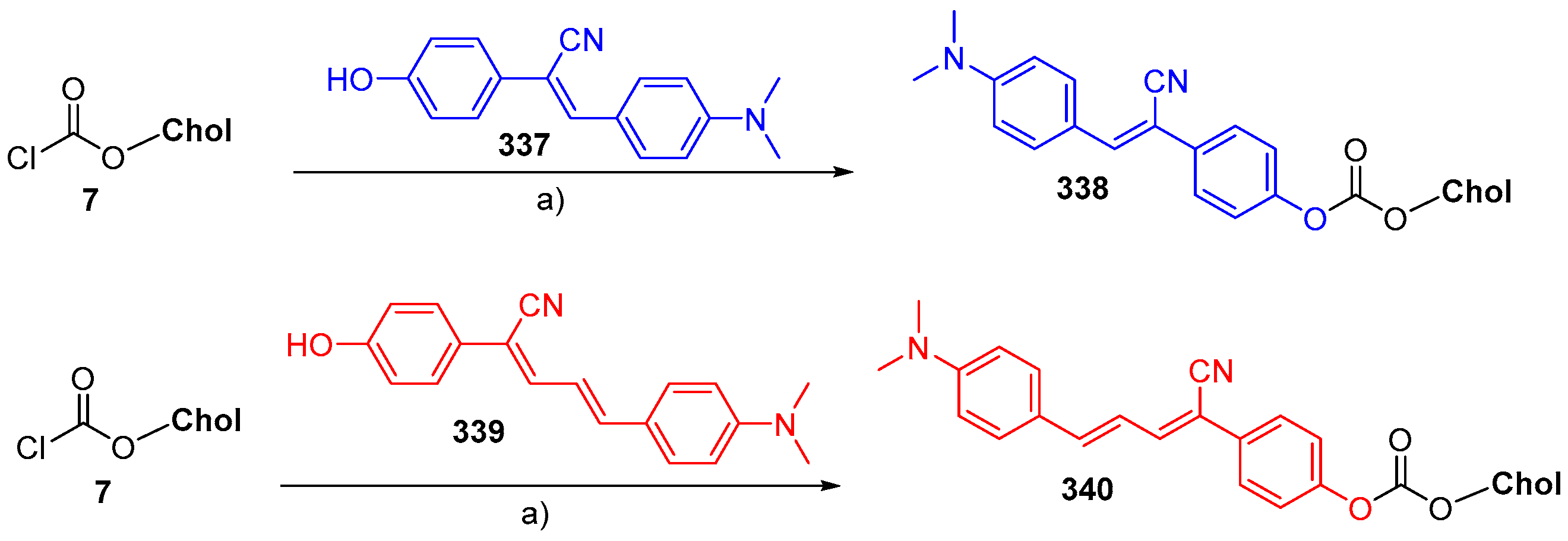
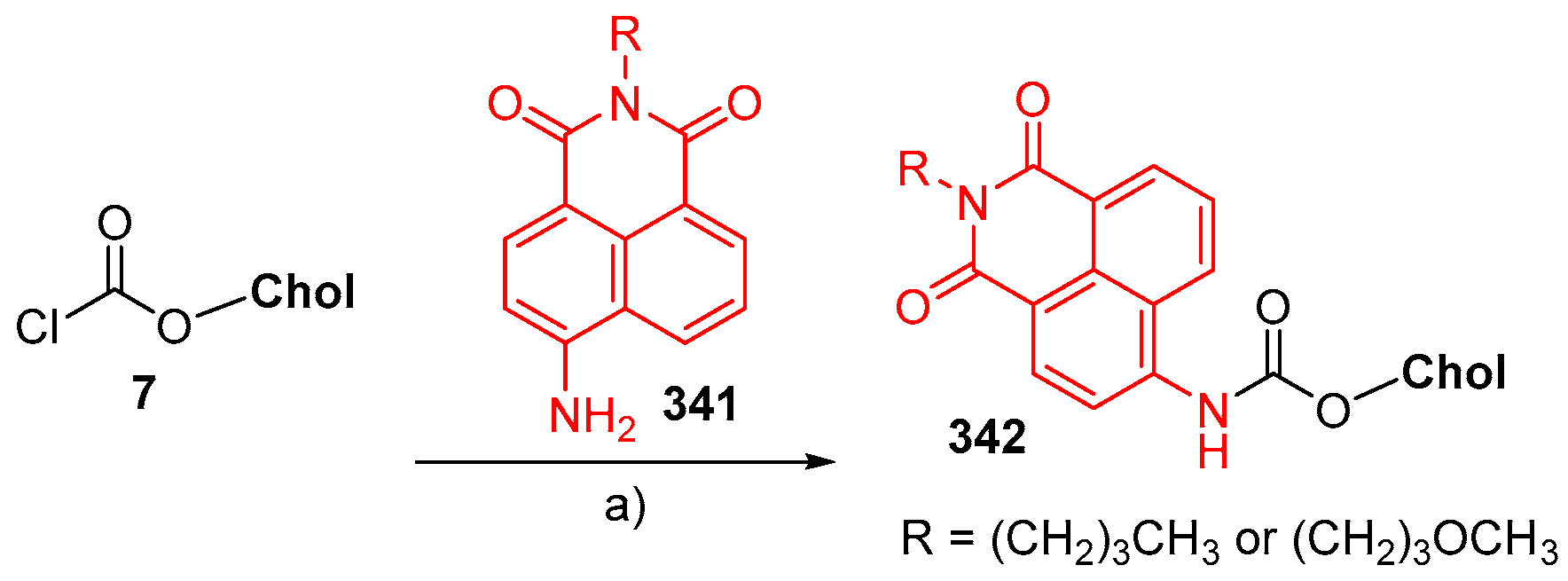
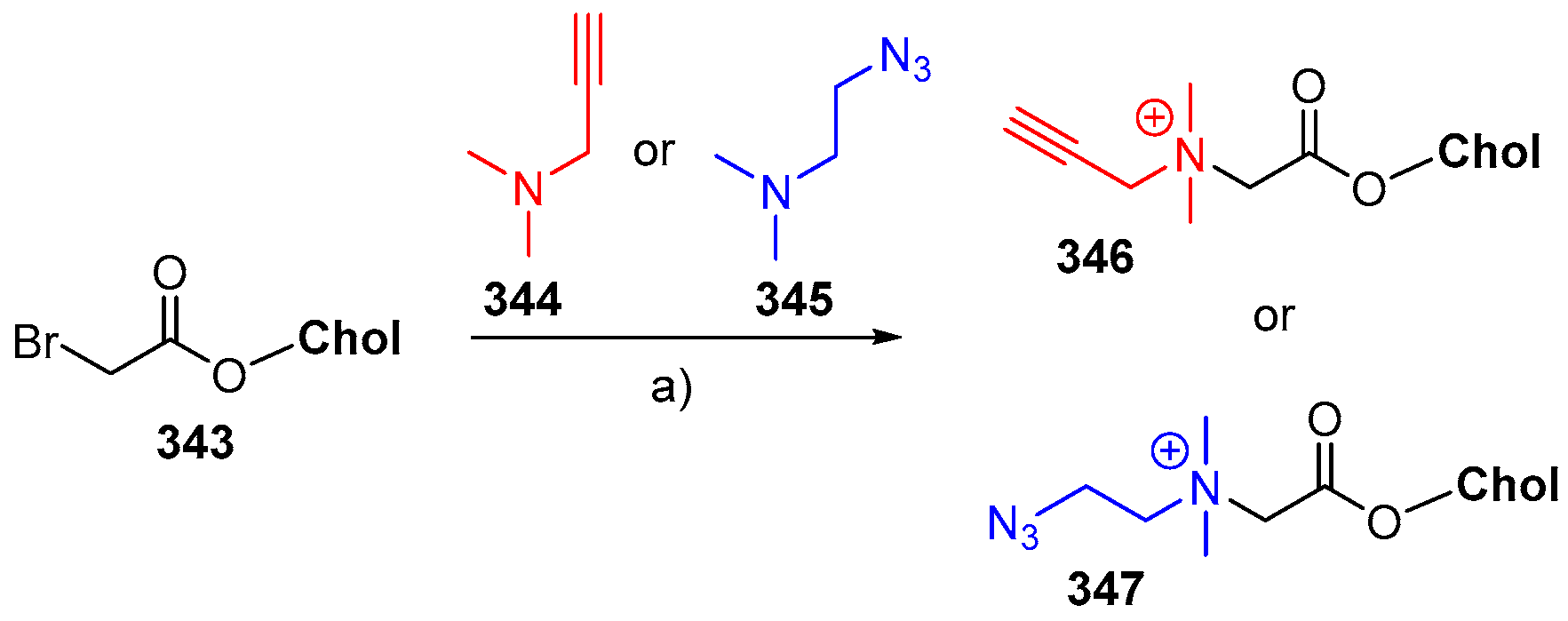
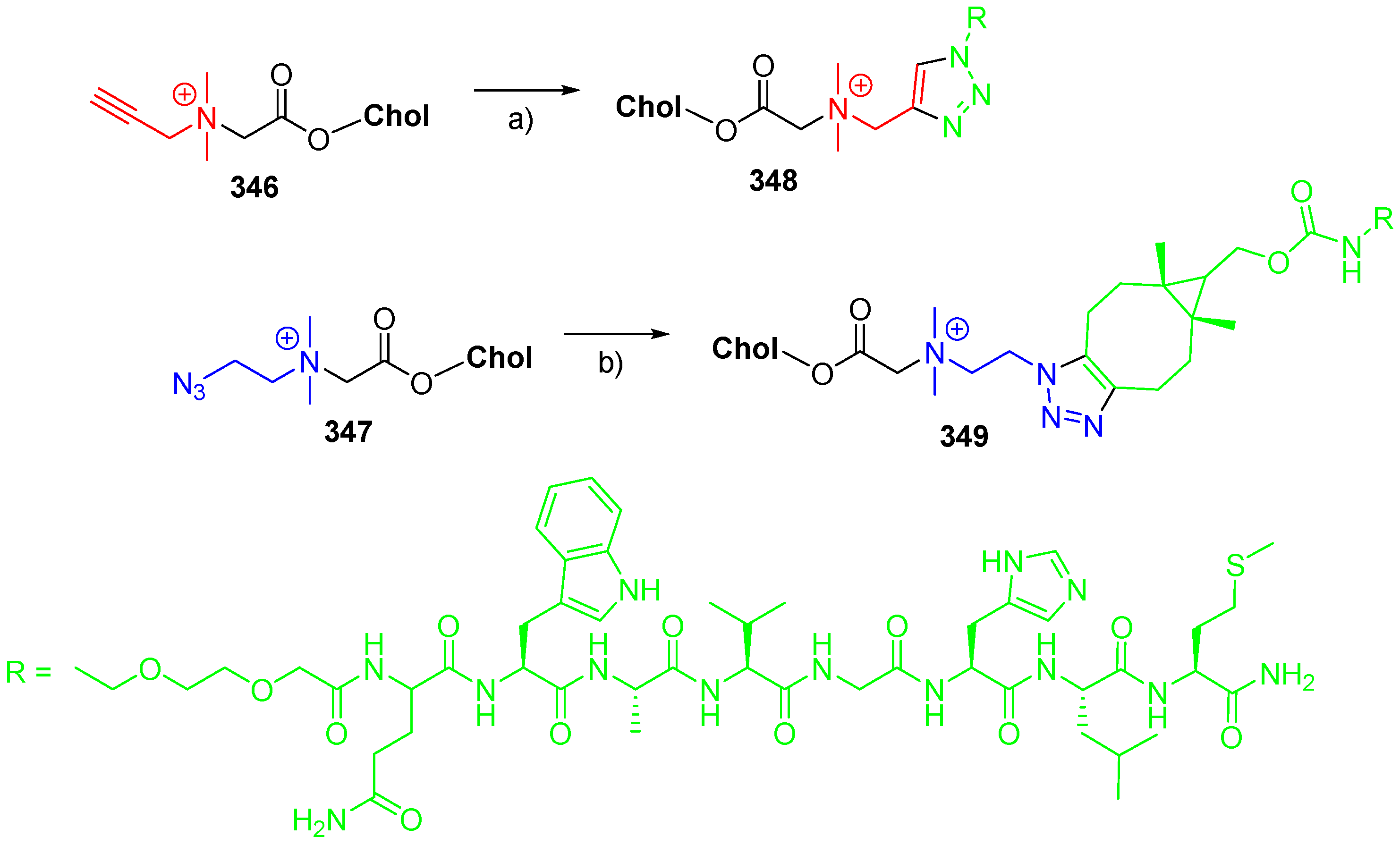




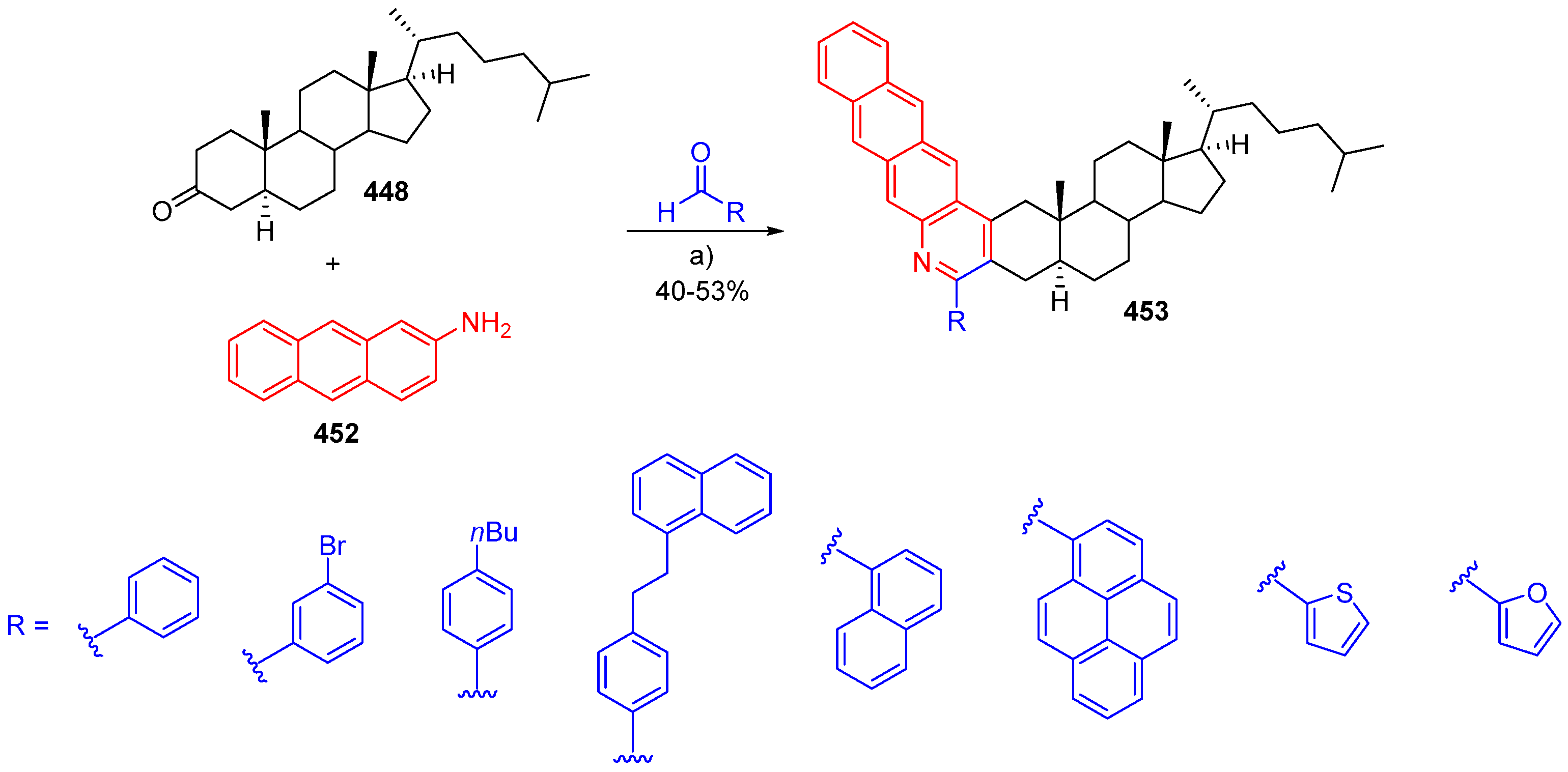
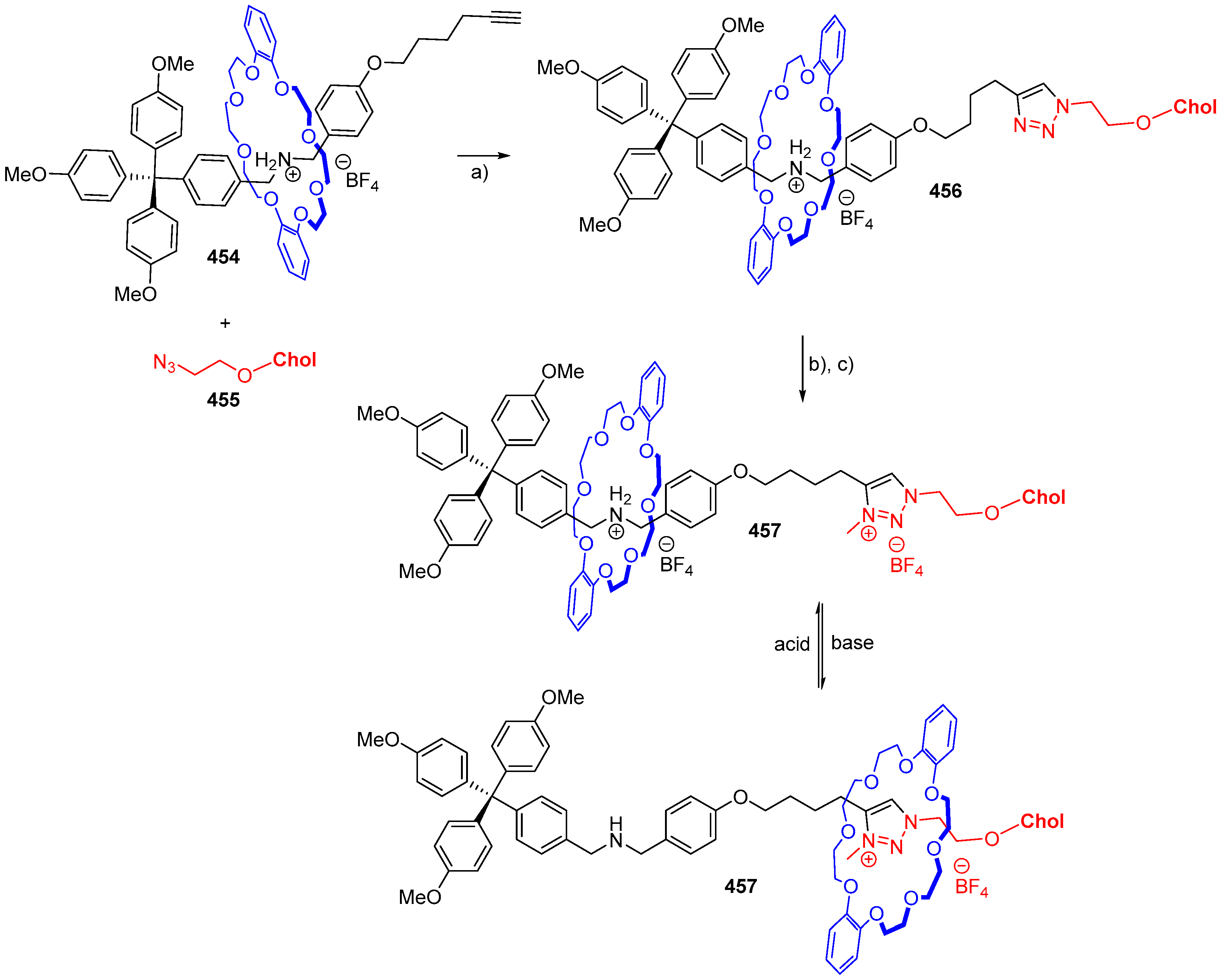
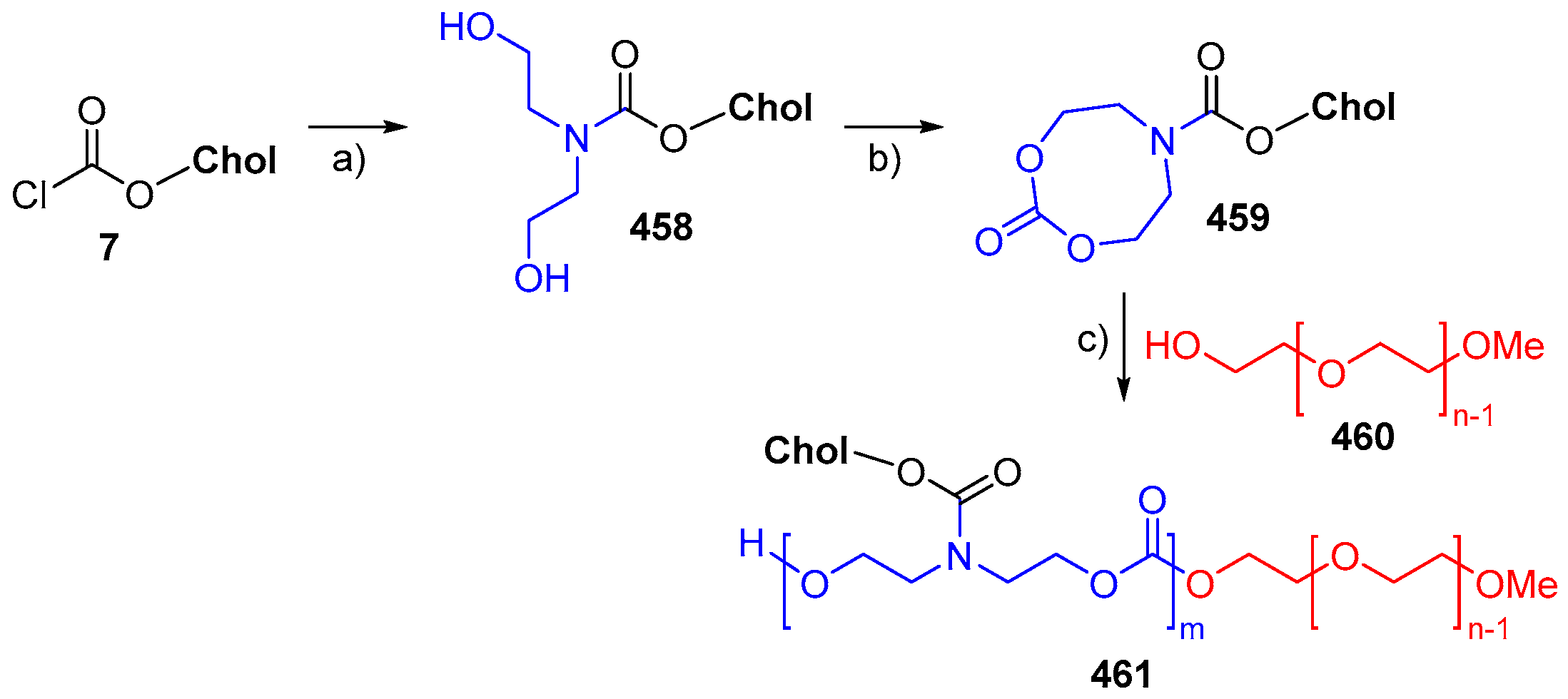
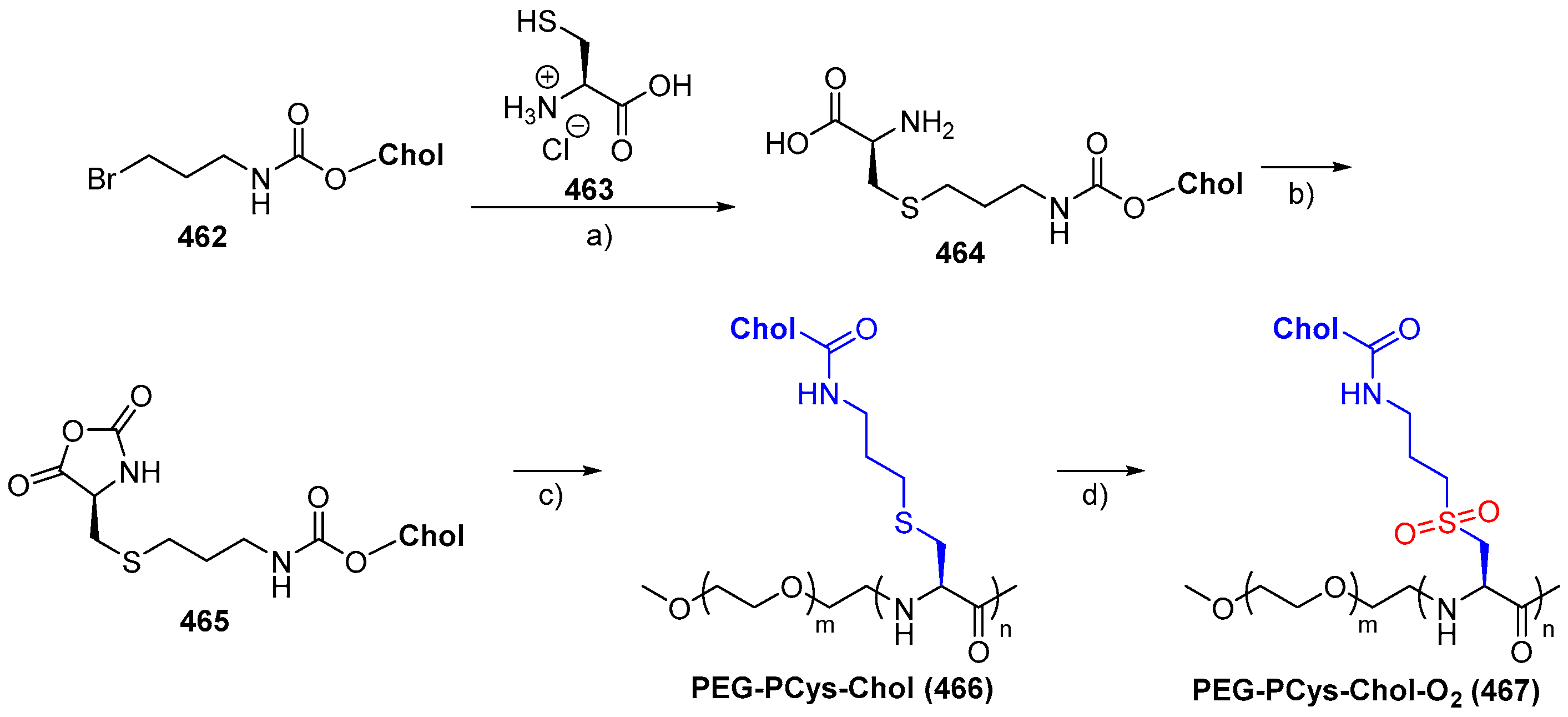
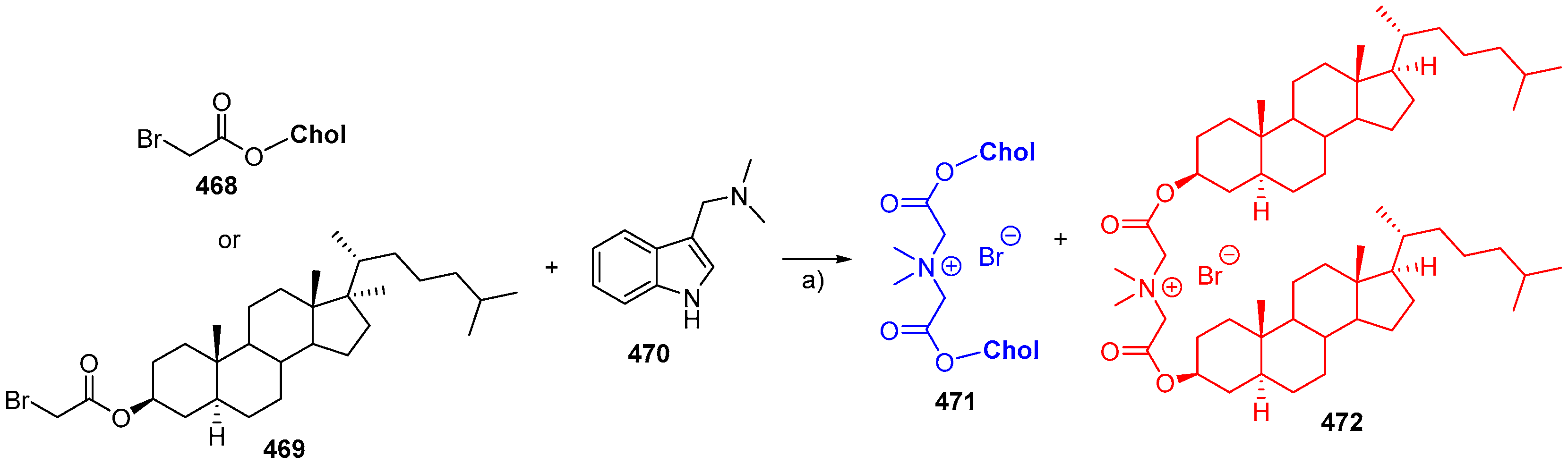
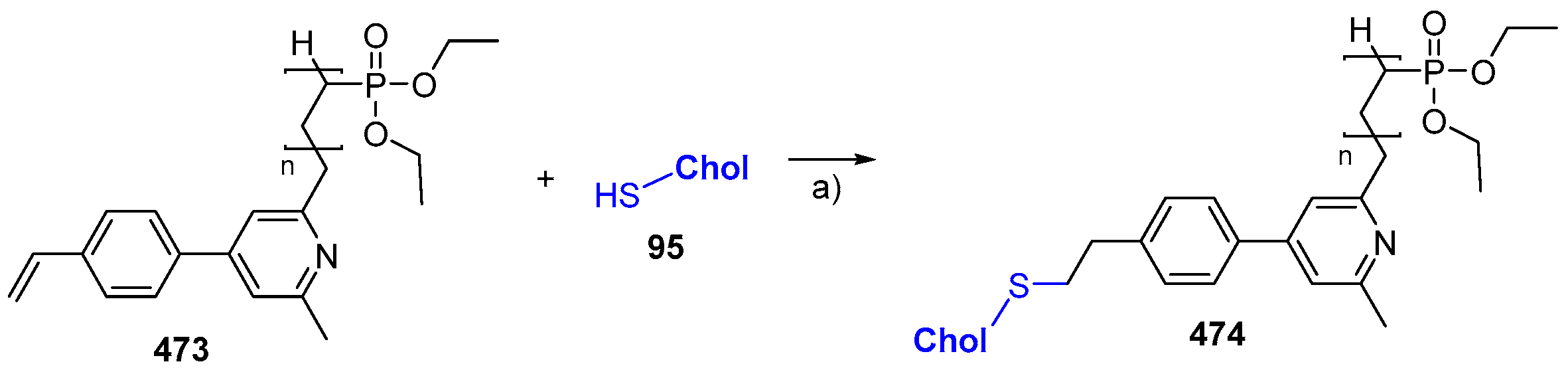
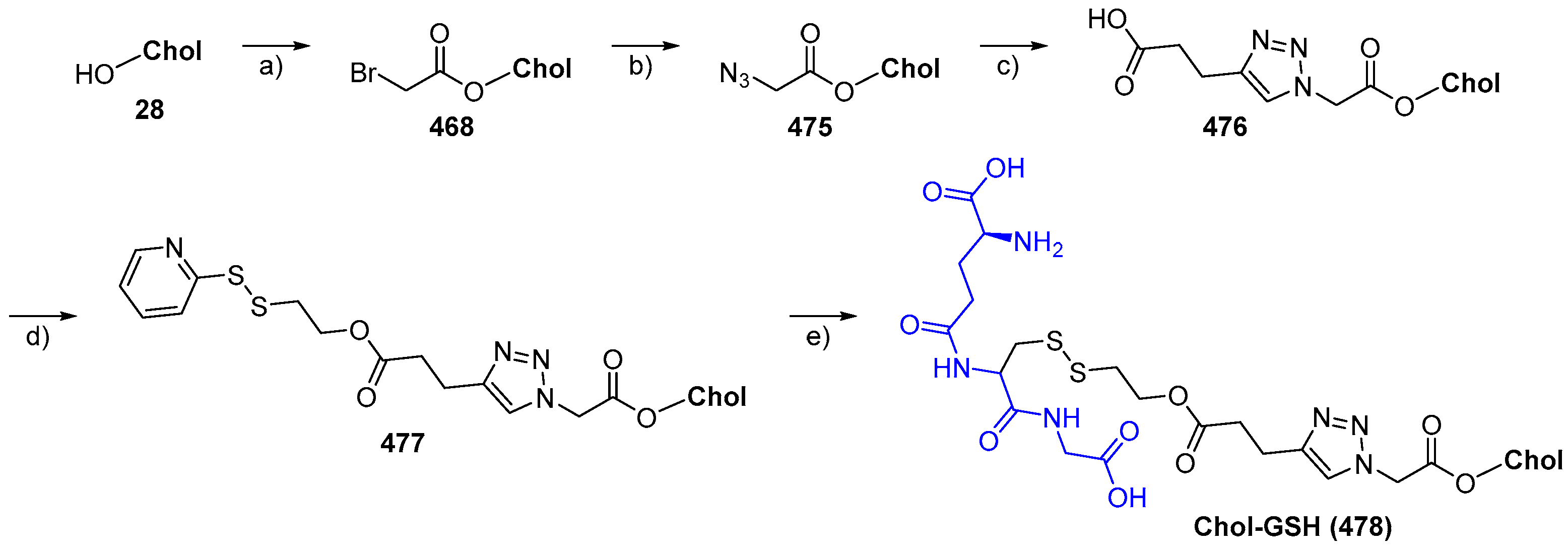
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, H.M.T.; Santos, C.M.M.; Silva, A.M.S. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2019, 24, 116. https://doi.org/10.3390/molecules24010116
Albuquerque HMT, Santos CMM, Silva AMS. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules. 2019; 24(1):116. https://doi.org/10.3390/molecules24010116
Chicago/Turabian StyleAlbuquerque, Hélio M. T., Clementina M. M. Santos, and Artur M. S. Silva. 2019. "Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications" Molecules 24, no. 1: 116. https://doi.org/10.3390/molecules24010116
APA StyleAlbuquerque, H. M. T., Santos, C. M. M., & Silva, A. M. S. (2019). Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules, 24(1), 116. https://doi.org/10.3390/molecules24010116







