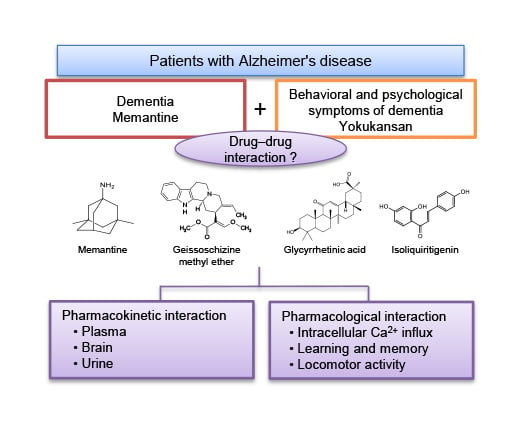
Basic Study of Drug-Drug Interaction between Memantine and the Traditional Japanese Kampo Medicine Yokukansan
Abstract
1. Introduction
2. Results and Discussion
2.1. Pharmacokinetic Interaction
2.1.1. Plasma and Brain Concentrations of Memantine and Yokukansan-Derived Ingredients
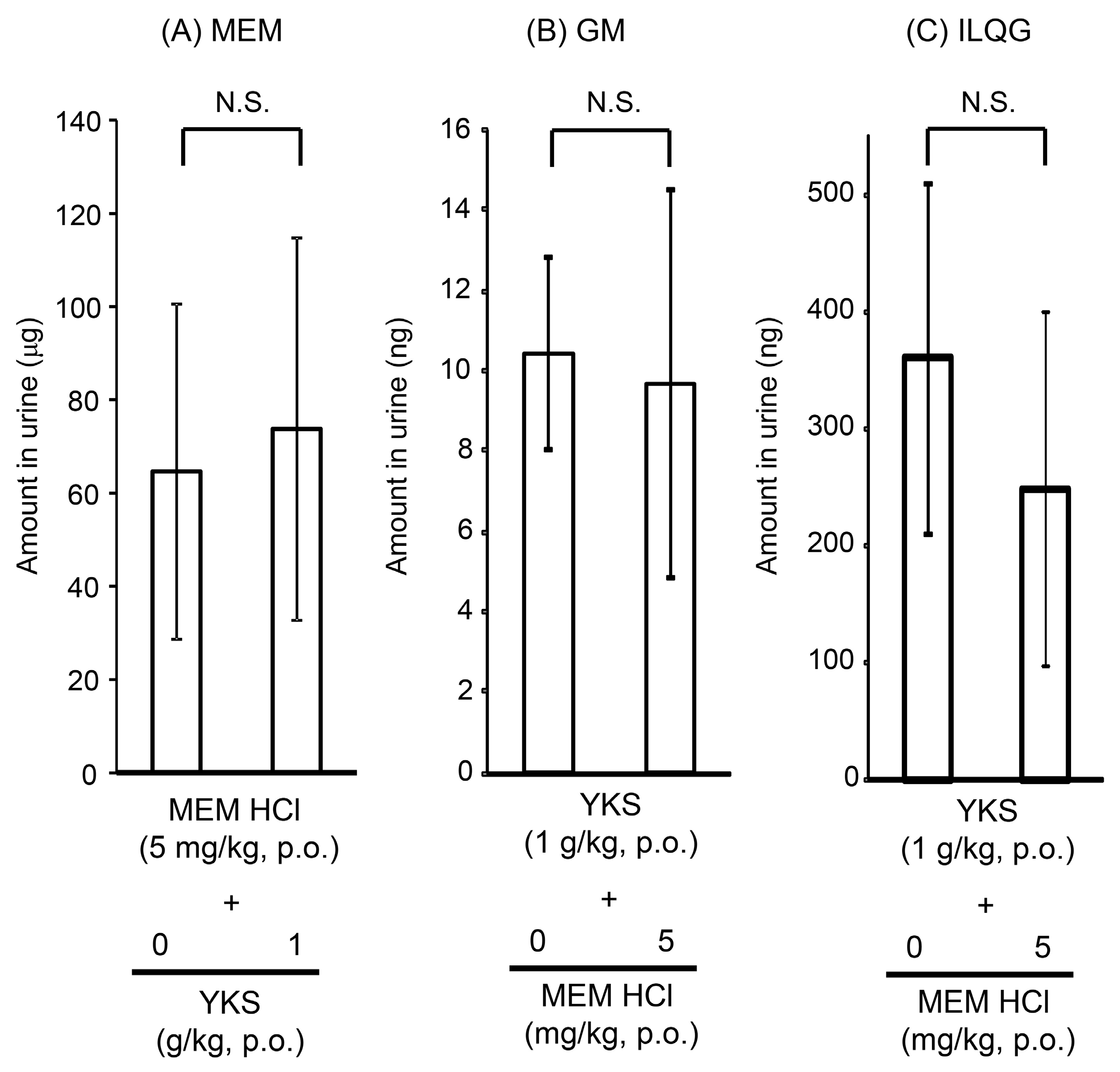
2.1.2. Urine Concentrations of Memantine and Yokukansan-Derived Ingredients
2.2. Pharmacological Interaction
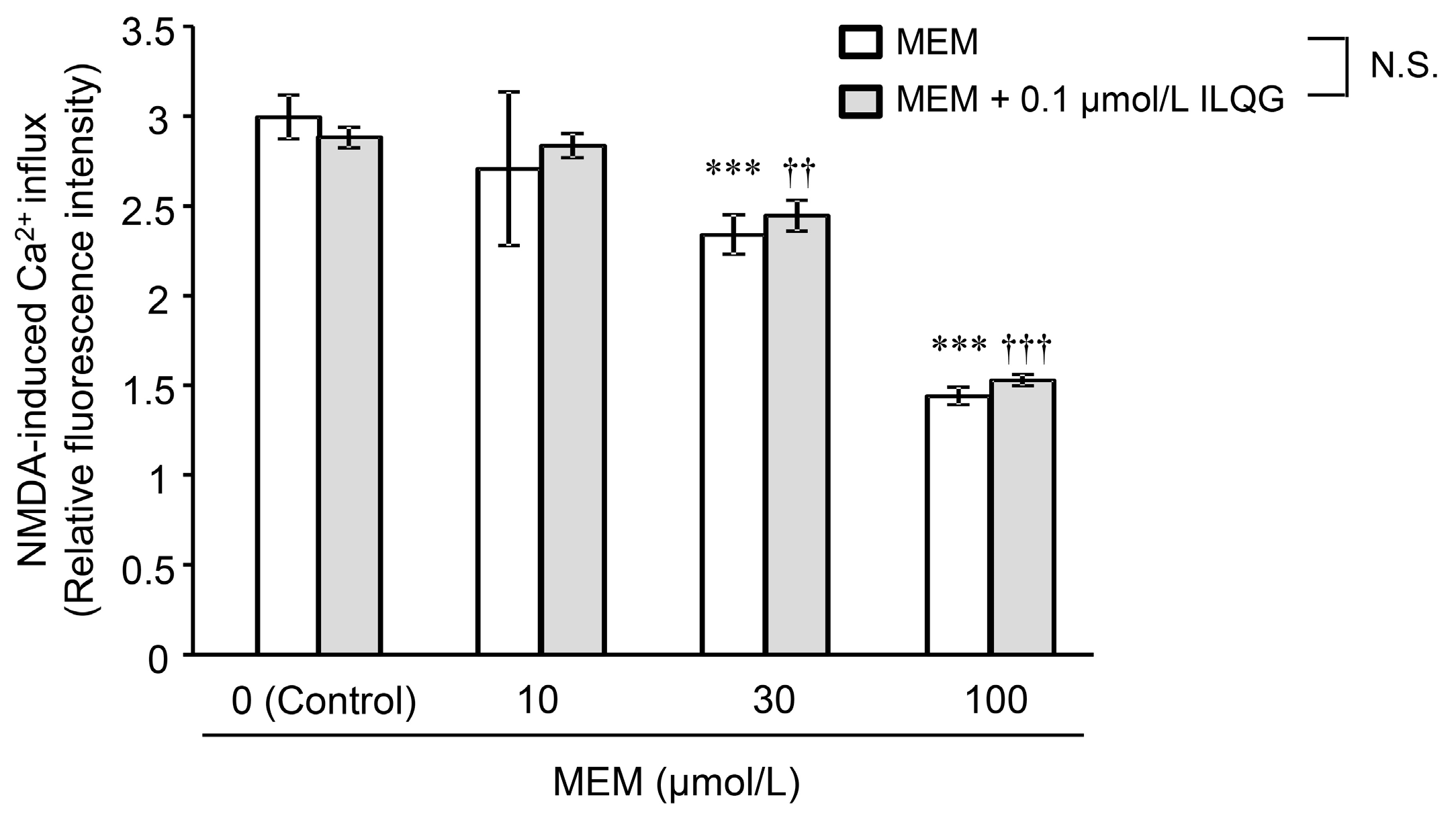
2.2.1. N-Methyl-d-Aspartic Acid-Induced Intracellular Ca2+ Influx
2.2.2. Learning and Memory Functions
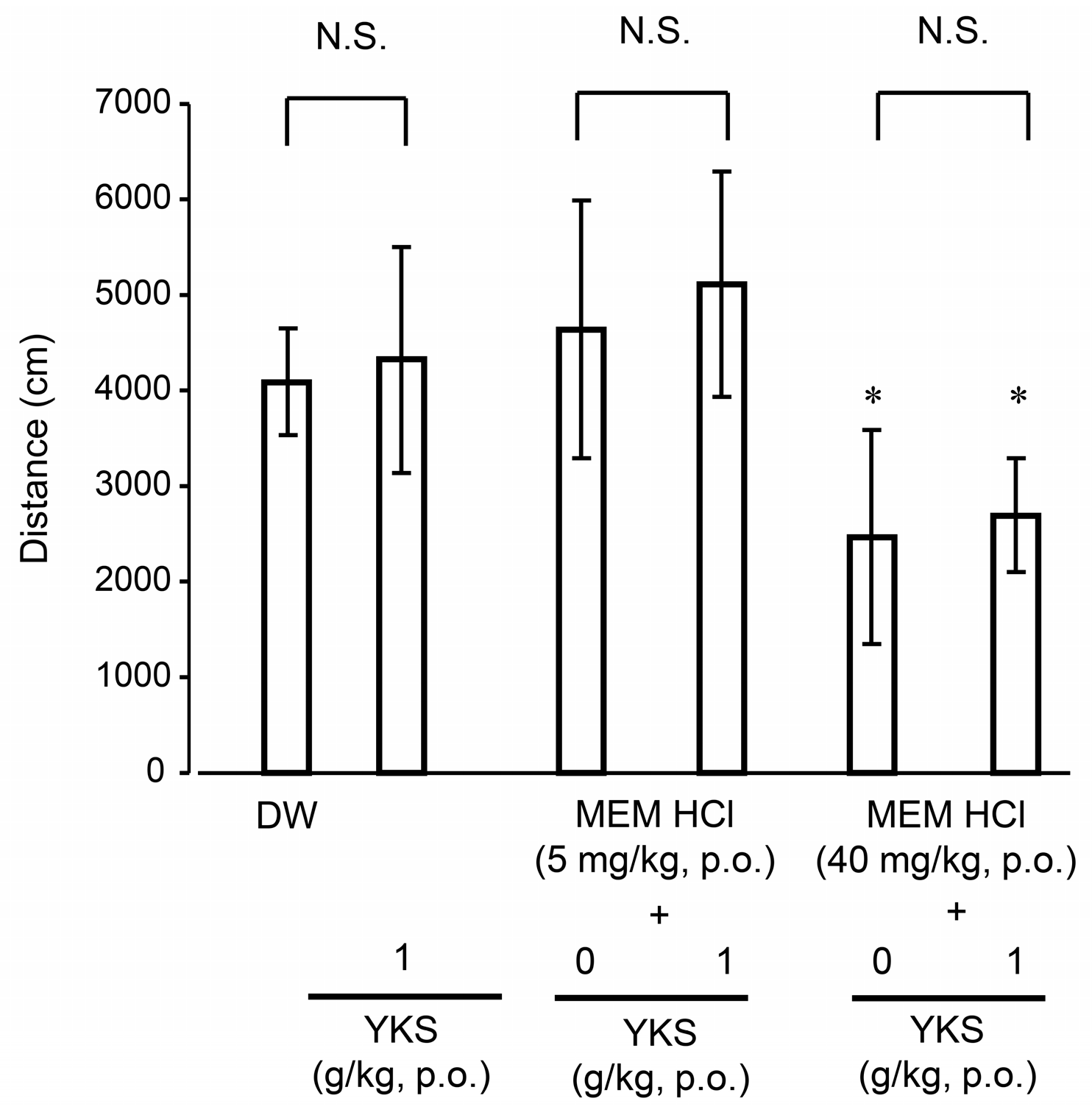
2.2.3. Locomotor Activity
3. Materials and Methods
3.1. Test Drugs
3.2. Animals
3.3. Pharmacokinetic Analyses
3.4. Pharmacological Experiments
3.4.1. Measurement of Ca2+ Influx in Cultured Neurons
3.4.2. Step-Through Passive Avoidance Task
3.4.3. Open-Field Test
3.5. Statistical Analysis
4. Conclusions
Supplementary materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Memantine [package insert]. Merz Pharmaceuticals GmbH & Co., frankfurt am main, germany. 2002. Available online: https://ec.europa.eu/health/documents/community-register/2002/200205175237/anx_5237_en.pdf (accessed on 15 November 2018).
- Parsons, C.G.; Gruner, R.; Rozental, J.; Millar, J.; Lodge, D. Patch clamp studies on the kinetics and selectivity of N-methyl-d-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology 1993, 32, 1337–1350. [Google Scholar] [CrossRef]
- Frankiewicz, T.; Potier, B.; Bashir, Z.I.; Collingridge, G.L.; Parsons, C.G. Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. Br. J. Pharmacol. 1996, 117, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Poritis, N. Memantine in severe dementia: Results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine). Int. J. Geriatr. Psychiatry 1999, 14, 135–146. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer's disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Murayama, N.; Noshita, T.; Katsuragi, R.; Ohno, T. Cognitive dysfunction induced by sequential injection of amyloid-β and ibotenate into the bilateral hippocampus; protection by memantine and MK-801. Eur. J. Pharmacol. 2006, 548, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zajaczkowski, W.; Frankiewicz, T.; Parsons, C.G.; Danysz, W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology 1997, 36, 961–971. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Alvarez, X.A.; Cacabelos, R.; Quack, G. Neuroprotection by memantine against neurodegeneration induced by β-amyloid(1-40). Brain Res. 2002, 958, 210–221. [Google Scholar] [CrossRef]
- Creeley, C.; Wozniak, D.F.; Labruyere, J.; Taylor, G.T.; Olney, J.W. Low doses of memantine disrupt memory in adult rats. J. Neurosci. 2006, 26, 3923–3932. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Kishi, T.; Shibayama, H.; Iwata, N. Yokukansan in the treatment of behavioral and psychological symptoms of dementia: A systematic review and meta-analysis of randomized controlled trials. Hum. Psychopharmacol. 2013, 28, 80–86. [Google Scholar] [CrossRef]
- Mizukami, K.; Asada, T.; Kinoshita, T.; Tanaka, K.; Sonohara, K.; Nakai, R.; Yamaguchi, K.; Hanyu, H.; Kanaya, K.; Takao, T.; et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int. J. Neuropsychopharmacolog. 2009, 12, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Kosaka, K.; Mori, H.; Okitsu, R.; Furukawa, K.; Manabe, Y.; Yoshita, M.; Kanamori, A.; Ito, N.; Wada, K.; et al. Improvement in delusions and hallucinations in patients with dementia with Lewy bodies upon administration of yokukansan, a traditional Japanese medicine. Psychogeriatrics 2012, 12, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Ikarashi, Y. Multiple psychopharmacological effects of the traditional Japanese kampo medicine yokukansan, and the brain regions it affects. Front Pharmacol. 2017, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, Y.; Mizoguchi, K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016, 166, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Ikarashi, Y. Cellular pharmacological effects of the traditional Japanese kampo medicine yokukansan on brain cells. Front Pharmacol. 2017, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Z.; Ikarashi, Y.; Kase, Y. Glycyrrhizin and its metabolite 18β-glycyrrhetinic acid in glycyrrhiza, a constituent herb of yokukansan, ameliorate thiamine deficiency-induced dysfunction of glutamate transport in cultured rat cortical astrocytes. Eur. J. Pharmacol. 2010, 626, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Kawakami, Z.; Kanno, H.; Omiya, Y.; Mizoguchi, K.; Yamamoto, M. Yokukansan, a traditional Japanese medicine, enhances the glutamate transporter GLT-1 function in cultured rat cortical astrocytes. Evid. Based Complement Alternat. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Miyagishi, H.; Tsuji, M.; Saito, A.; Miyagawa, K.; Takeda, H. Inhibitory effect of yokukansan on the decrease in the hippocampal excitatory amino acid transporter EAAT2 in stress-maladaptive mice. J. Tradit. Complement Med. 2017, 7, 371–374. [Google Scholar] [CrossRef]
- Kawakami, Z.; Kanno, H.; Ueki, T.; Terawaki, K.; Tabuchi, M.; Ikarashi, Y.; Kase, Y. Neuroprotective effects of yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxicity in cultured cells. Neuroscience 2009, 159, 1397–1407. [Google Scholar] [CrossRef]
- Kawakami, Z.; Ikarashi, Y.; Kase, Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell Mol. Neurobiol. 2011, 31, 1203–1212. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Sekiguchi, K.; Mizoguchi, K. Serotonin receptor binding characteristics of geissoschizine methyl ether, an indole alkaloid in Uncaria hook. Curr. Med. Chem. 2018, 25, 1036–1045. [Google Scholar] [CrossRef]
- Sani, G.; Serra, G.; Kotzalidis, G.D.; Romano, S.; Tamorri, S.M.; Manfredi, G.; Caloro, M.; Telesforo, C.L.; Caltagirone, S.S.; Panaccione, I.; et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS Drugs 2012, 26, 663–690. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, G.; Tognini, S.; Calsolaro, V.; Polini, A.; Monzani, F. Potential drug-drug interactions in Alzheimer patients with behavioral symptoms. Clin. Interv. Aging 2015, 10, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Beconi, M.G.; Howland, D.; Park, L.; Lyons, K.; Giuliano, J.; Dominguez, C.; Munoz-Sanjuan, I.; Pacifici, R. Pharmacokinetics of memantine in rats and mice. PLoS Curr. 2011, 3, RRN1291. [Google Scholar] [CrossRef] [PubMed]
- Kushida, H.; Fukutake, M.; Tabuchi, M.; Katsuhara, T.; Nishimura, H.; Ikarashi, Y.; Kanitani, M.; Kase, Y. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria hook in rat plasma and brain after oral administration of the traditional Japanese medicine yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2013, 27, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Imamura, S.; Kawakami, Z.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of 18β-glycyrrhetinic acid, a major metabolite of glycyrrhizin in Glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol. Neurobiol. 2012, 32, 1139–1146. [Google Scholar] [CrossRef]
- Noetzli, M.; Guidi, M.; Ebbing, K.; Eyer, S.; Wilhelm, L.; Michon, A.; Thomazic, V.; Alnawaqil, A.M.; Maurer, S.; Zumbach, S.; et al. Population pharmacokinetic study of memantine: Effects of clinical and genetic factors. Clin. Pharmacokinet. 2013, 52, 211–223. [Google Scholar] [CrossRef]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef]
- Kushida, H.; Matsumoto, T.; Igarashi, Y.; Nishimura, H.; Watanabe, J.; Maemura, K.; Kase, Y. Metabolic profiling of the Uncaria hook alkaloid geissoschizine methyl ether in rat and human liver microsomes using high-performance liquid chromatography with tandem mass spectrometry. Molecules 2015, 20, 2100–2114. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kushida, H.; Maruyama, T.; Nishimura, H.; Watanabe, J.; Maemura, K.; Kase, Y. In vitro identification of human cytochrome P450 isoforms involved in the metabolism of geissoschizine methyl ether, an active component of the traditional Japanese medicine yokukansan. Xenobiotica 2016, 46, 325–334. [Google Scholar] [CrossRef]
- Soraoka, H.; Oniki, K.; Matsuda, K.; Ono, T.; Taharazako, K.; Uchiyashiki, Y.; Kamihashi, R.; Kita, A.; Takashima, A.; Nakagawa, K.; et al. The effect of yokukansan, a traditional herbal preparation used for the behavioral and psychological symptoms of dementia, on the drug-metabolizing enzyme activities in healthy male volunteers. Biol. Pharm. Bull. 2016, 39, 1468–1474. [Google Scholar] [CrossRef]
- Gai, Y.; Chen, H.; Liu, W.; Feng, F.; Xie, N. The metabolism of Yi-Gan-San and subsequent pharmacokinetic evaluation of four metabolites in rat based on liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 972, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Satoh, T.; Watanabe, Y.; Ikarashi, N.; Asano, T.; Morita, T.; Sugiyama, K. Effects of Kampo medicines on CYP and P-gp activity in vitro. Biol. Pharm. Bull. 2008, 31, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Takasaki, A.; Ikarashi, N.; Watanabe, J.; Kanitani, M.; Sugiyama, K. Effects of Kampo medicines on Cyp3a and P-glycoprotein activity in vivo. J. Trad. Med. 2009, 26, 131–135. [Google Scholar]
- Ichisawa, S.; Ito, K.; Ikarashi, N.; Watanabe, J.; Kanitani, M.; Kase, Y.; Sugiyama, K. A study of the interactions between donepezil and yokukansan, cimetidine, and ketoconazole in rats. J. Trad. Med. 2010, 27, 84–89. [Google Scholar]
- Imamura, S.; Tabuchi, M.; Kushida, H.; Nishi, A.; Kanno, H.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol. Neurobiol. 2011, 31, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Munekage, M.; Ichikawa, K.; Fukudome, I.; Munekage, E.; Takezaki, Y.; Matsumoto, T.; Igarashi, Y.; Hanyu, H.; Hanazaki, K. Pharmacokinetics of active components of yokukansan, a traditional Japanese herbal medicine after a single oral administration to healthy Japanese volunteers: A cross-over, randomized study. PLoS ONE 2015, 10, e0131165. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Hayashi, T.; Kobashi, K.; Kanaoka, M.; Kato, H.; Kobayashi, M.; Takeda, S.; Oyama, T. Intestinal bacterial hydrolysis is indispensable to absorption of 18β-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J. Pharm. Pharmacol. 1994, 46, 135–137. [Google Scholar] [CrossRef]
- Jing, J.; Ren, W.; Chen, X.; Wang, Y.; Yu, Q.; Wang, G.; Davey, A.K.; Wang, J.; Jing, N. Glucuronide-sulfate diconjugate as a novel metabolite of glycyrrhetic acid in rat bile. Drug Metab. Pharmacokinet. 2008, 23, 175–180. [Google Scholar] [CrossRef]
- Ploeger, B.; Mensinga, T.; Sips, A.; Seinen, W.; Meulenbelt, J.; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef]
- Mogami, S.; Sadakane, C.; Nahata, M.; Mizuhara, Y.; Yamada, C.; Hattori, T.; Takeda, H. CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress-induced anorexia. Sci. Rep. 2016, 6, 27516. [Google Scholar] [CrossRef]
- Muller, F.; Weitz, D.; Derdau, V.; Sandvoss, M.; Mertsch, K.; Konig, J.; Fromm, M.F. Contribution of MATE1 to renal secretion of the NMDA receptor antagonist memantine. Mol. Pharm. 2017, 14, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Makino, T. 3-Monoglucuronyl glycyrrhretinic acid is a possible marker compound related to licorice-induced pseudoaldosteronism. Biol. Pharm. Bull. 2014, 37, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Munekage, M.; Matsumoto, T.; Sadakane, C.; Fukutake, M.; Aoki, K.; Watanabe, J.; Maemura, K.; Hattori, T.; Kase, Y.; et al. Pharmacokinetic profiles of active ingredients and its metabolites derived from rikkunshito, a ghrelin enhancer, in healthy Japanese volunteers: A cross-over, randomized study. PLoS ONE 2015, 10, e0133159. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Takayama, S.; Iwasaki, K.; Tabuchi, M.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kudo, Y.; Kase, Y.; Arai, H.; et al. Yokukansan, a traditional Japanese medicine, ameliorates memory disturbance and abnormal social interaction with anti-aggregation effect of cerebral amyloid β proteins in amyloid precursor protein transgenic mice. Neuroscience 2011, 180, 305–313. [Google Scholar] [CrossRef]
- Misztal, M.; Danysz, W. Comparison of glutamate antagonists in continuous multiple-trial and single-trial dark avoidance. Behav. Pharmacol. 1995, 6, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, Y.; Iizuka, S.; Imamura, S.; Yamaguchi, T.; Sekiguchi, K.; Kanno, H.; Kawakami, Z.; Yuzurihara, M.; Kase, Y.; Takeda, S. Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biol. Pharm. Bull. 2009, 32, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Yamaguchi, T.; Iizuka, S.; Imamura, S.; Ikarashi, Y.; Kase, Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer’s disease. J. Ethnopharmacol. 2009, 122, 157–162. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Shoji, H.; Tanaka, Y.; Tabira, T. Ameliorative effect of traditional Japanese medicine yokukansan on age-related impairments of working memory and reversal learning in rats. Neuroscience 2011, 177, 127–137. [Google Scholar] [CrossRef]
- Nishi, A.; Yamaguchi, T.; Sekiguchi, K.; Imamura, S.; Tabuchi, M.; Kanno, H.; Nakai, Y.; Hashimoto, K.; Ikarashi, Y.; Kase, Y. Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin1A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience 2012, 207, 124–136. [Google Scholar] [CrossRef]
- Kos, T.; Popik, P. A comparison of the predictive therapeutic and undesired side-effects of the NMDA receptor antagonist, memantine, in mice. Behav. Pharmacol. 2005, 16, 155–161. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Yamaguchi, T.; Tabuchi, M.; Ikarashi, Y.; Kase, Y. Effects of yokukansan, a traditional Japanese medicine, on aggressiveness induced by intracerebroventricular injection of amyloid β protein into mice. Phytother. Res. 2009, 23, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Sekiguchi, K.; Kawakami, Z.; Watanabe, J.; Mizoguchi, K.; Ikarashi, Y.; Yamamoto, M. Basic Study of Drug-Drug Interaction between Memantine and the Traditional Japanese Kampo Medicine Yokukansan. Molecules 2019, 24, 115. https://doi.org/10.3390/molecules24010115
Matsumoto T, Sekiguchi K, Kawakami Z, Watanabe J, Mizoguchi K, Ikarashi Y, Yamamoto M. Basic Study of Drug-Drug Interaction between Memantine and the Traditional Japanese Kampo Medicine Yokukansan. Molecules. 2019; 24(1):115. https://doi.org/10.3390/molecules24010115
Chicago/Turabian StyleMatsumoto, Takashi, Kyoji Sekiguchi, Zenji Kawakami, Junko Watanabe, Kazushige Mizoguchi, Yasushi Ikarashi, and Masahiro Yamamoto. 2019. "Basic Study of Drug-Drug Interaction between Memantine and the Traditional Japanese Kampo Medicine Yokukansan" Molecules 24, no. 1: 115. https://doi.org/10.3390/molecules24010115
APA StyleMatsumoto, T., Sekiguchi, K., Kawakami, Z., Watanabe, J., Mizoguchi, K., Ikarashi, Y., & Yamamoto, M. (2019). Basic Study of Drug-Drug Interaction between Memantine and the Traditional Japanese Kampo Medicine Yokukansan. Molecules, 24(1), 115. https://doi.org/10.3390/molecules24010115