Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes
Abstract
1. Introduction
2. Results and Discussion
2.1. Cell Migration
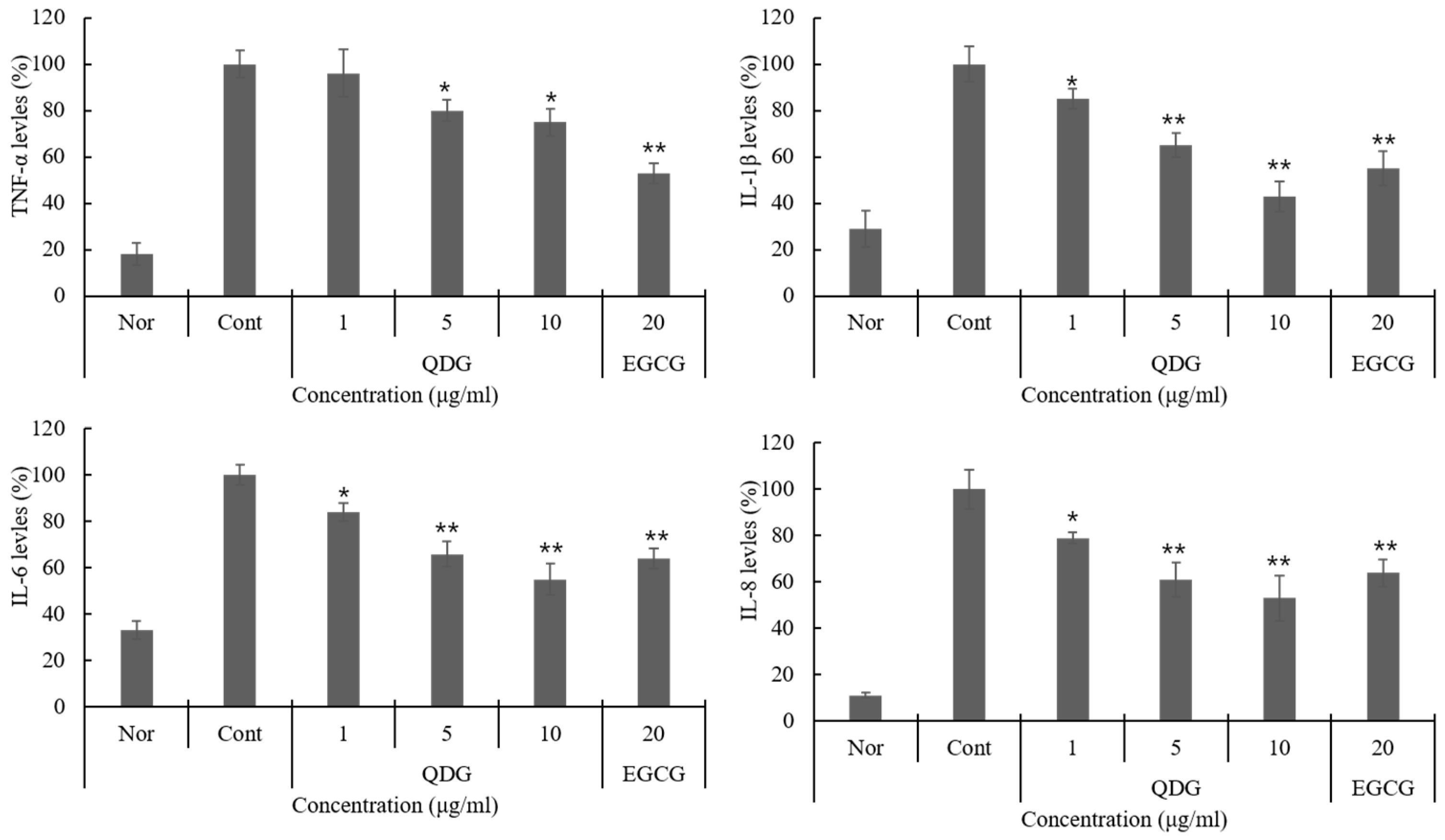
2.2. QDG’s Inhibitory Effect on Cytokine Production
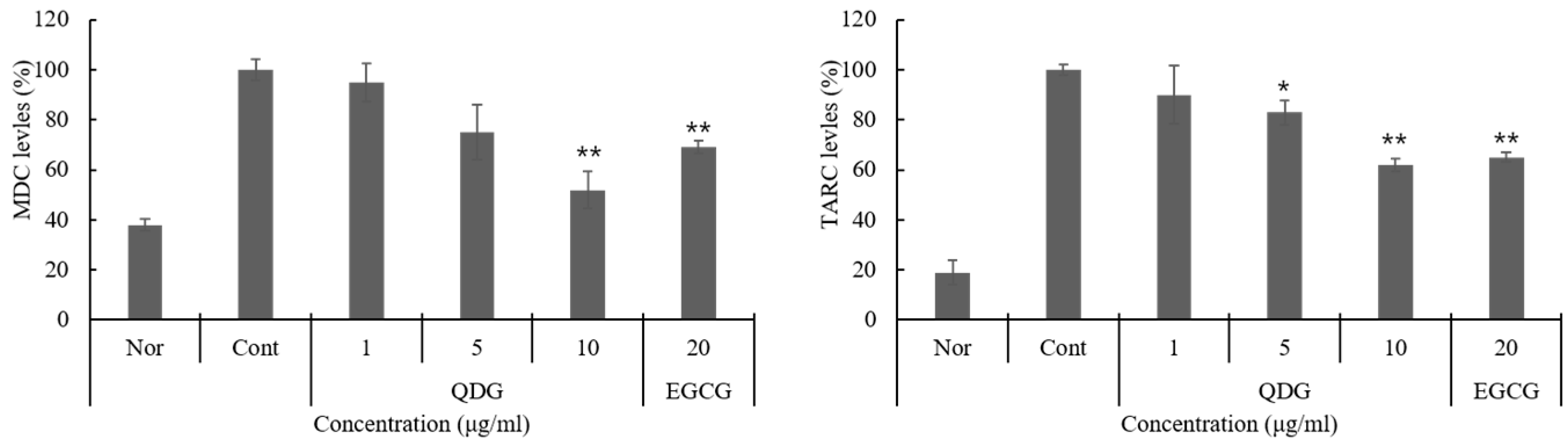
2.3. QDG’s Inhibitory Effect on Chemokine Production
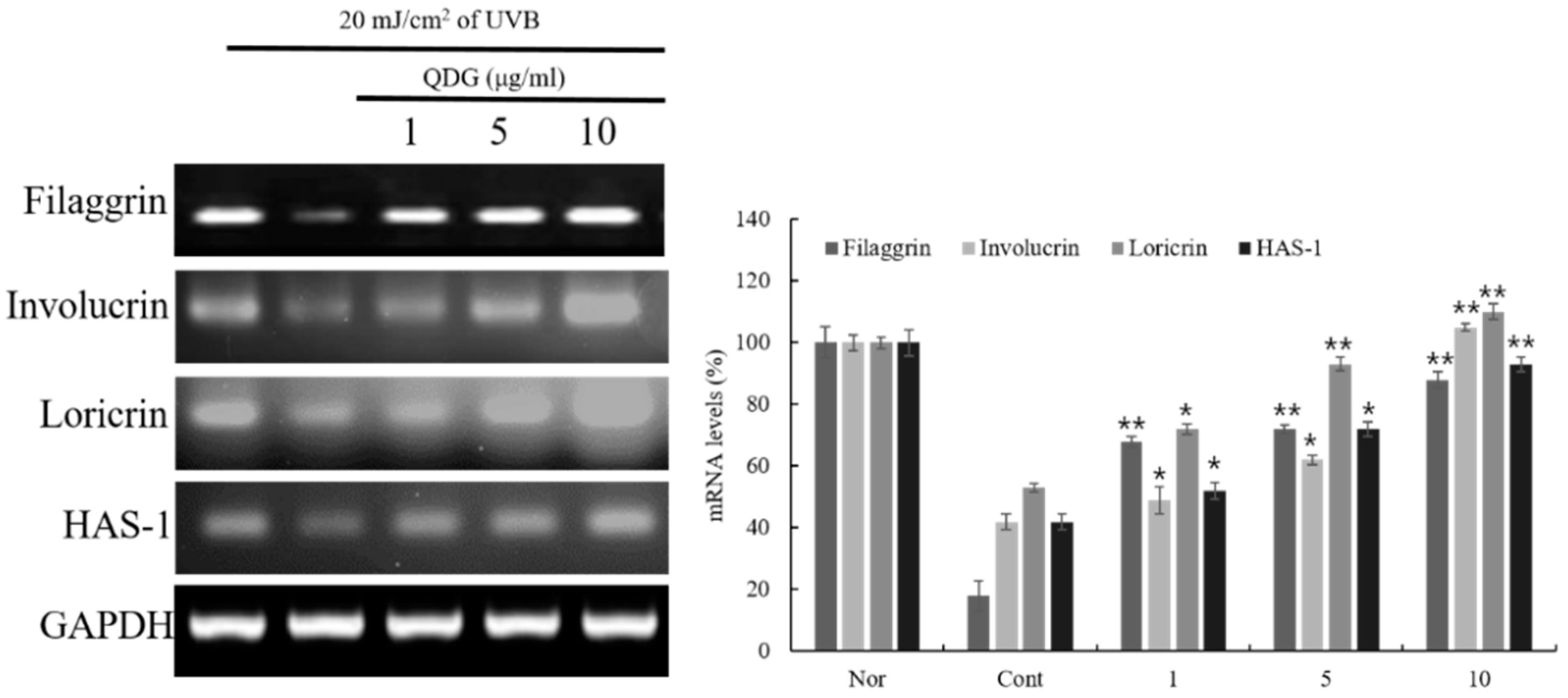
2.4. QDG’s Effect on the Skin Barrier and Hyaluronic Acid Synthase Production
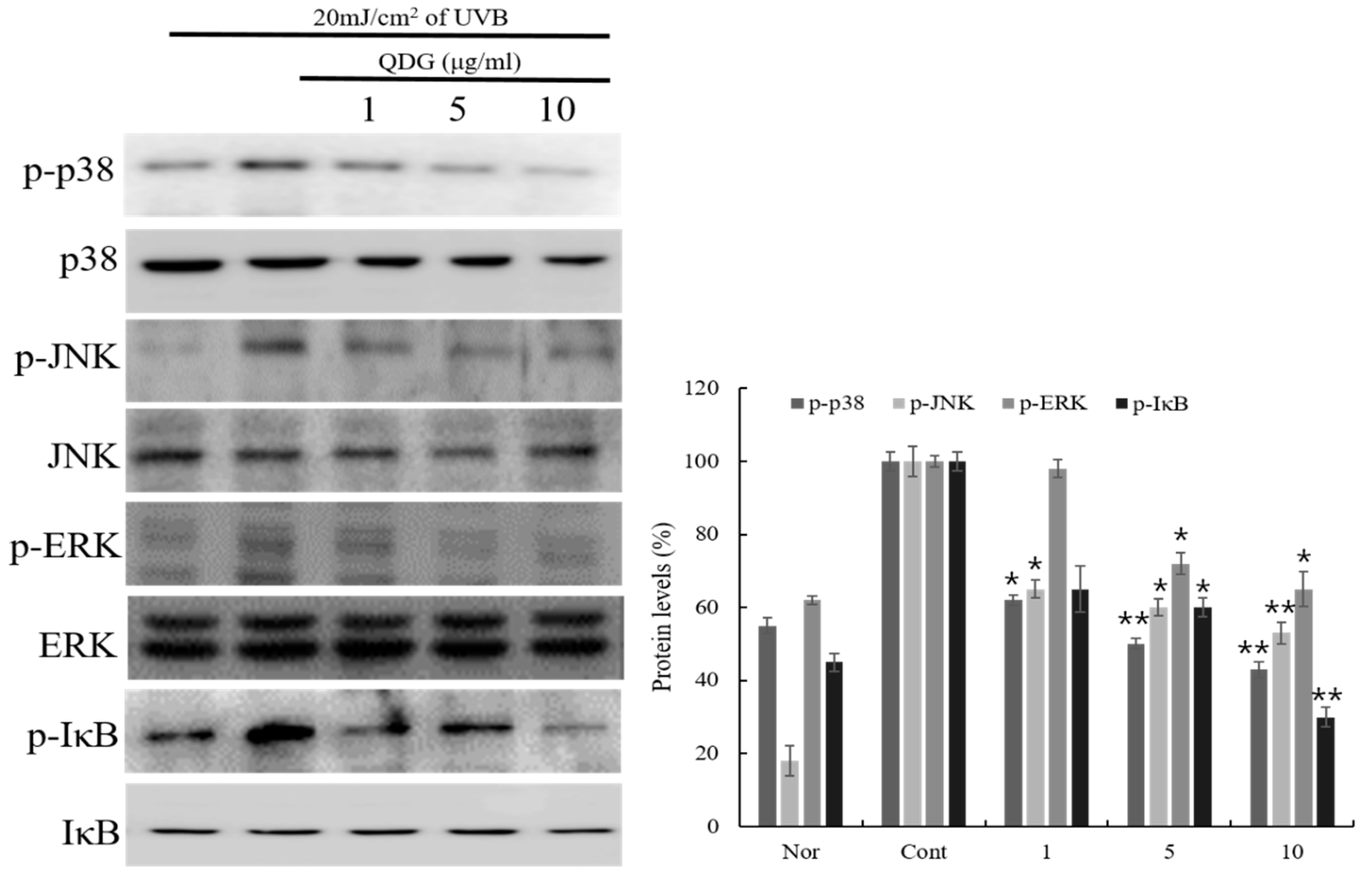
2.5. Phosphorylation of p38/JNK/ERK/IκB
2.6. Signaling Pathways Leading to the Activation of NF-κB
3. Materials and Methods
3.1. General Procedures
3.2. Reagents
3.3. Plant Material
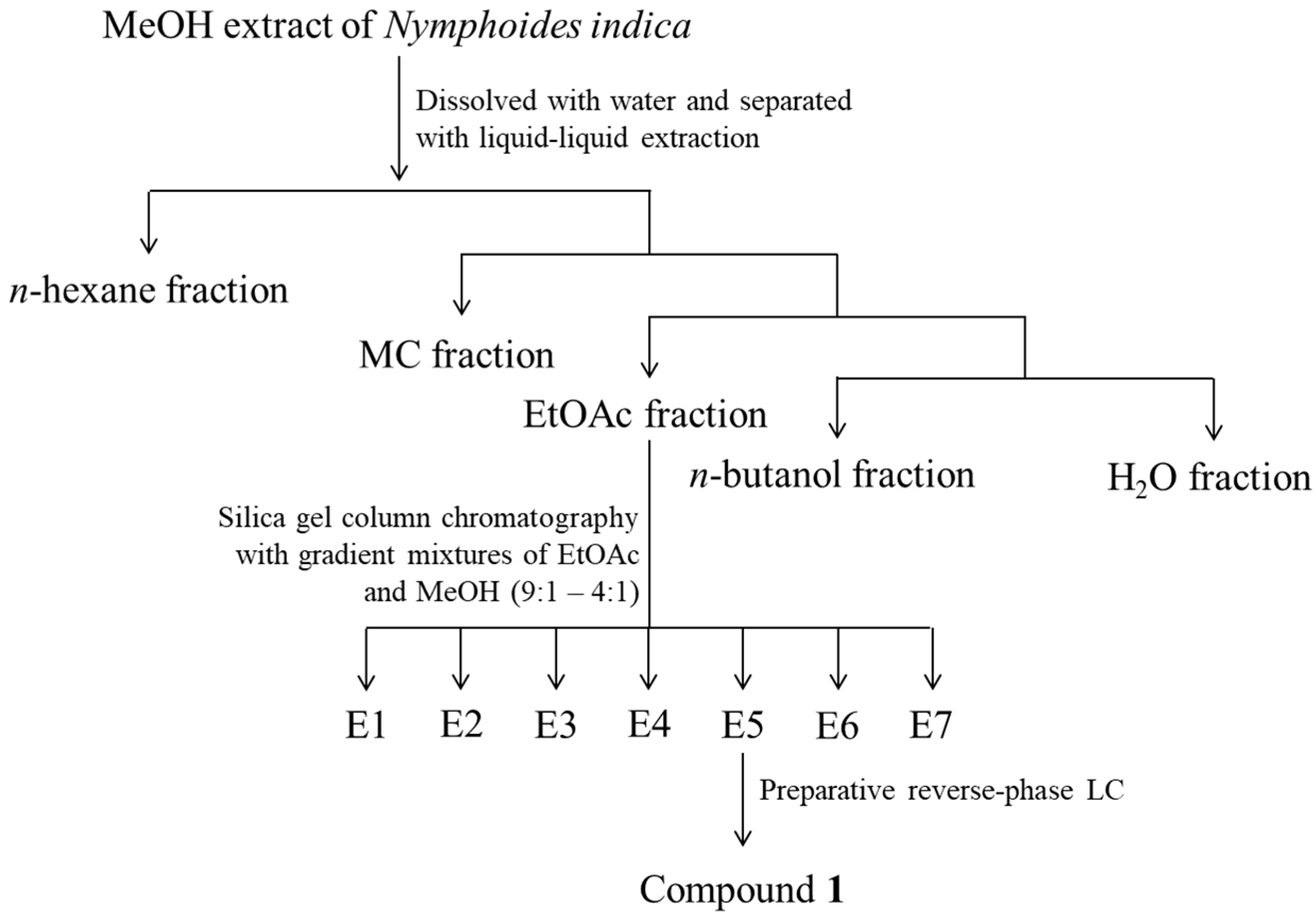
3.4. Isolation and Structure Determination of Compound 1
3.5. Cell Culture and UVB Irradiation
3.6. Cell Migration
3.7. Immunoassays for Cytokines and Chemokines
3.8. Measurement of Skin Barrier Peptide and Hyaluronic Acid
3.9. Preparation of Cytosolic and Nuclear Extracts
3.10. Western Blot Assay
3.11. Immunofluorescence
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miodovnik, M.; Koren, R.; Ziv, E.; Ravid, A. The inflammatory response of keratinocytes and its modulation by Vitamin D: The role of MAPK signaling pathways. J. Cell. Physiol. 2012, 227, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; De, P.O.; Girolomoni, G. Resident skin cells in psoriasis: A special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 2007, 25, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, J.L.; Luster, M.I. Chemical induction of interleukin-8, a pro-inflammatory chemokine, in human epidermal keratinocyte cultures and its relation to cytogenetic toxicity. Cell Biol. Toxicol. 1995, 11, 37–50. [Google Scholar] [PubMed]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Joydeep, D.; Jyotirmoy, G.; Prasenjit, M.; Parames, C.S. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem. Pharmacol. 2011, 81, 891–909. [Google Scholar]
- Henkel, T.; Machleidt, T.; Alkalay, I.; Krönke, M.; Ben-Neriah, Y.; Baeuerle, P.A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 1993, 365, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Steelman, L.S.; Shelton, J.G.; Lee, J.T.; Nayolanic, P.M.; Blalock, W.L.; Franklin, R.; McCubrey, J.A. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway. Int. J. Oncol. 2003, 22, 469–480. [Google Scholar] [PubMed]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Jnawali, H.N.; Lee, E.; Shin, A.; Park, Y.G.; Kim, Y. Effect of quercetin in the UV-irradiated human keratinocyte HaCaT cells and a model of its binding to p38 MAPK. Bull. Korean Chem. Soc. 2014, 35, 2787–2790. [Google Scholar] [CrossRef]
- Lee, C.S.; Jeong, E.B.; Kim, Y.J.; Lee, M.S.; Seo, S.J.; Park, K.H.; Lee, M.W. Quercetin-3-O-(2″-galloyl)-α-l-rhamnopyranoside inhibits TNF-α-activated NF-κB-induced inflammatory mediator production by suppressing ERK activation. Int. Immunopharmacol. 2013, 15, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- González, S.; Fernández-Lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin. Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Wang, M.; Yang, L.; Jiang, J.G.; Zhao, J.W.; Zhu, W. Flavonoid glycosides from Rubus chingii Hu fruits display anti-inflammatory activity through suppressing MAPKs activation in macrophages. J. Funct. Foods 2015, 18, 235–243. [Google Scholar] [CrossRef]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-d-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.P.; Akkol, E.K.; Yalcın, F.N.; Koca, U.; Keles, H.; Yesilada, E. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J. Ethnopharmacol. 2010, 129, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Upadhyay, A.; Zafar, M.; Cos, P.; Maes, L.; Apers, S.; Exarchou, V.; Pieters, L. Antibacterial, antifungal, cytotoxic, antioxidant and antidiabetic compounds from Nymphoides indica the first comprehensive phytochemical and pharmacological study. Planta Med. 2016, 80, P1L115. [Google Scholar] [CrossRef]
- Madhavan, V.; Shilpi, A.; Anita, M.; Yoganarasimhan, S.N. Anti-convulsant activity of aqueous and alcohol extracts of roots and rhizomes of Nymphoides indica (L.) Kuntze in Swiss albino mice. J. Nat. Remed. 2009, 9, 68–73. [Google Scholar]
- Kim, D.H.; Kim, Y.A.; Yu, J.M.; Park, C.B.; Park, B.J.; Park, T.S. Inhibitory effect of Nymphoides indica extract on α-MSH induced melanin synthesis. J. Appl. Biol. Chem. 2017, 60, 327–332. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Woo, S.O.; Lee, I.K.; Lee, M.L.; Lee, M.Y.; Baek, H.H.; Bae, K.H. Studies on the antimicrobial effect of collected bee venom using electric shock method (I). Korean J. Apic. 2005, 20, 53–58. [Google Scholar]
- Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.H.; Nguyen, T.T.T.; Park, J.S.; Hwang, H.M.; Song, M.S.; Lee, D.H.; Chai, K.Y. Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules 2012, 17, 2992–3007. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postep. Derm. Alergol. 2014, 31, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.H.; Yang, E.J.; Jin, M.; Lee, J.Y.; Choi, Y.A.; Park, P.H.; Lee, S.R.; Kim, S.U.; Shin, T.Y.; Kwon, T.K.; et al. Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int. Immunopharm. 2018, 59, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Takehara, K.; Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jahnz-Rozyk, K.; Targowski, T.; Paluchowska, E.; Owczarek, W.; Kucharczyk, A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy 2005, 60, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, P.T.; Hägg, P.M.; Peltonen, S. Reevaluation of the normal epidermal calcium gradient, and analysis of calcium levels and ATP receptors in Hailey–Hailey and Darier epidermis. J. Investig. Dermatol. 2009, 129, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Dhitavat, J.; Cobbold, C.; Leslie, N.; Burge, S.; Hovnanian, A. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J. Investig. Dermatol. 2003, 121, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Ahn, S.K.; Denda, M.; Brown, B.E.; Crumrine, D.; Kimutai, L.K.; Komuves, L.; Lee, S.H.; Feingold, K.R. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J. Investig. Dermatol. 2002, 119, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, E.H.; Yoo, S.G.; Hong, Y.H.; Han, S.Y.; Jeong, S.G.; Jeong, D.O.; Kim, J.H.; Cho, J.Y.; Park, J.S. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng Res. 2018, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Davidovici, B.; Krueger, J.G. New insights in the immunologic basis of psoriasis. Semin. Cutan. Med. Surg. 2010, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G. Psoriasis: Evolution of pathogenic concepts and new therapies through phases of translational research. Br. J. Dermatol. 2007, 157, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Schieven, G.L. The biology of p38 kinase: A central role in inflammation. Curr. Top. Med. Chem. 2005, 5, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.U.; Chao, Y.W.; Lee, W.F.; Chen, S.H.; Tsai, Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.H.; Kang, I.J.; Won, M.H.; Yi, J.S.; et al. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-pentaO-galloyl-b-d-glucose via blockade of NF-jB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.F.; Teng, Y.C.; Yoon, Y.S.; Kim, D.H.; Cai, D.Q.; Lee, K.J. Reactive oxygen species are involved in the IFN-c-stimulated production of Th2 chemokines in HaCaT keratinocytes. J. Cell. Physiol. 2011, 226, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Komine, M.; Kakinuma, T.; Kagami, S.; Hanakawa, Y.; Hashimoto, K.; Tamaki, K. Mechanism of thymus- and activation-regulated chemokine (TARC)/CCL17 production and its modulation by roxithromycin. J. Investig. Dermatol. 2005, 125, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Li, J.H.; Song, S.B.; Jin, D.; Huang, X.Z.; Teng, Y.C.; Lee, K.J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/ CCL22 production through p38 MAPK and NF-κB in HaCaT keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Delhalle, S.; Blasius, R.; Dicato, M.; Diederich, M. A Beginner’s guide to NF-κB signaling pathways. Ann. N. Y. Acad. Sci. 2004, 1030, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Enk, R.; Ehehalt, R.; Graham, J.E.; Bierhaus, A.; Remppis, A.; Greten, H.J. Differential effect of Rhizoma coptidis and its main alkaloid compound berberine on TNF-alpha induced NFkappaB translocation in human keratinocytes. J. Ethnopharmacol. 2007, 109, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yang, J.W. Suppressive effects of ethanol extract of Aralia elata on UVB-induced oxidative stress in human keratinocytes. J. Nutr. Health 2016, 49, 135–143. [Google Scholar] [CrossRef]
- Tang, S.C.; Liao, P.Y.; Hung, S.J.; Ge, J.S.; Chen, S.M.; Lai, J.C.; Hsiao, Y.P.; Yang, J.H. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 2007, 86, 238–248. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are available from the corresponding author. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.A.; Kim, D.H.; Park, C.B.; Park, T.S.; Park, B.J. Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes. Molecules 2018, 23, 2342. https://doi.org/10.3390/molecules23092342
Kim YA, Kim DH, Park CB, Park TS, Park BJ. Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes. Molecules. 2018; 23(9):2342. https://doi.org/10.3390/molecules23092342
Chicago/Turabian StyleKim, You Ah, Dong Hee Kim, Chae Bin Park, Tae Soon Park, and Byoung Jun Park. 2018. "Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes" Molecules 23, no. 9: 2342. https://doi.org/10.3390/molecules23092342
APA StyleKim, Y. A., Kim, D. H., Park, C. B., Park, T. S., & Park, B. J. (2018). Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes. Molecules, 23(9), 2342. https://doi.org/10.3390/molecules23092342




