Phenolic Compounds in Honey and Their Associated Health Benefits: A Review
Abstract
1. Introduction
2. Chemical and Phytochemical Composition
2.1. Nutrients
2.2. Enzymes and Organic Acids
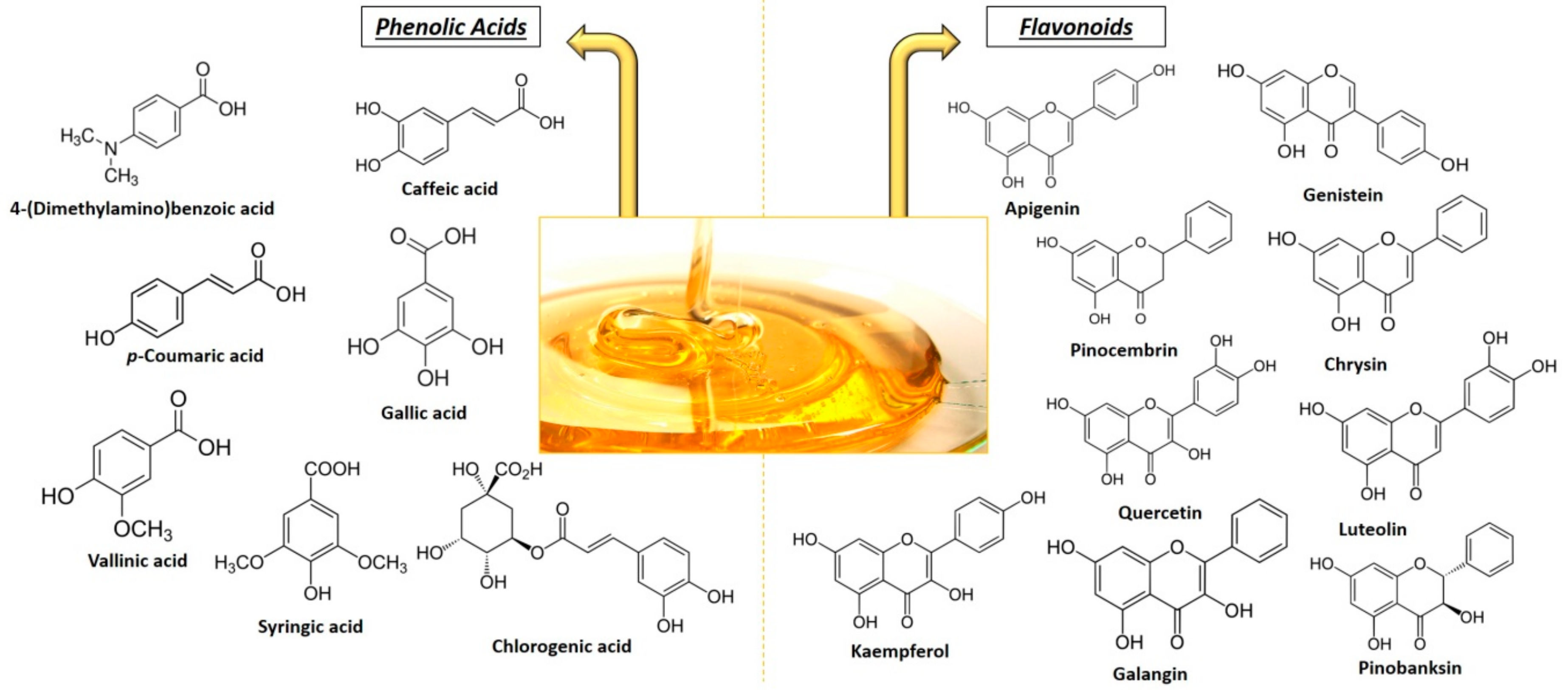
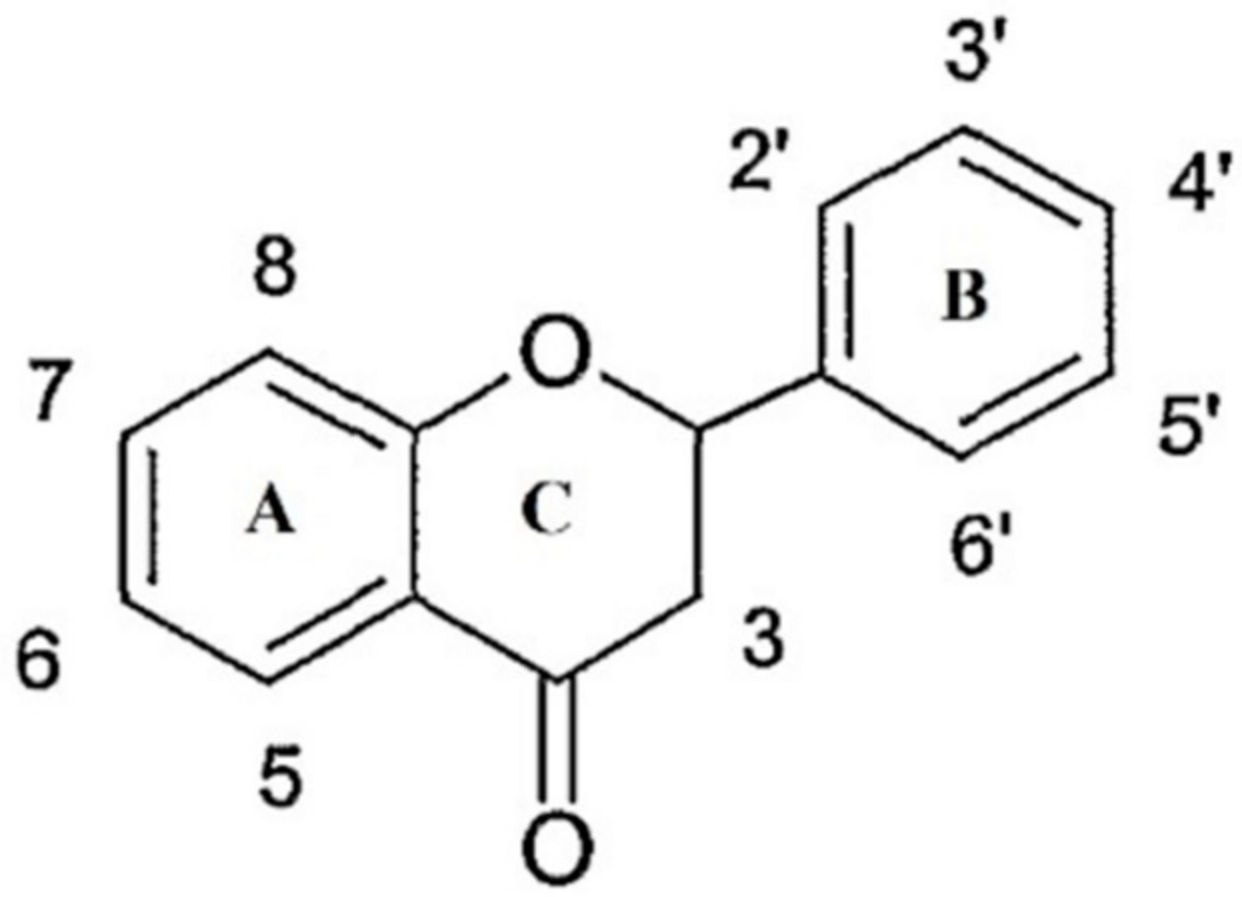
2.3. Phenolic Compounds
3. Metabolism and Bioavailability of Honey Polyphenols
4. Oxidative Stress, Antioxidant Activity, and Inflammation
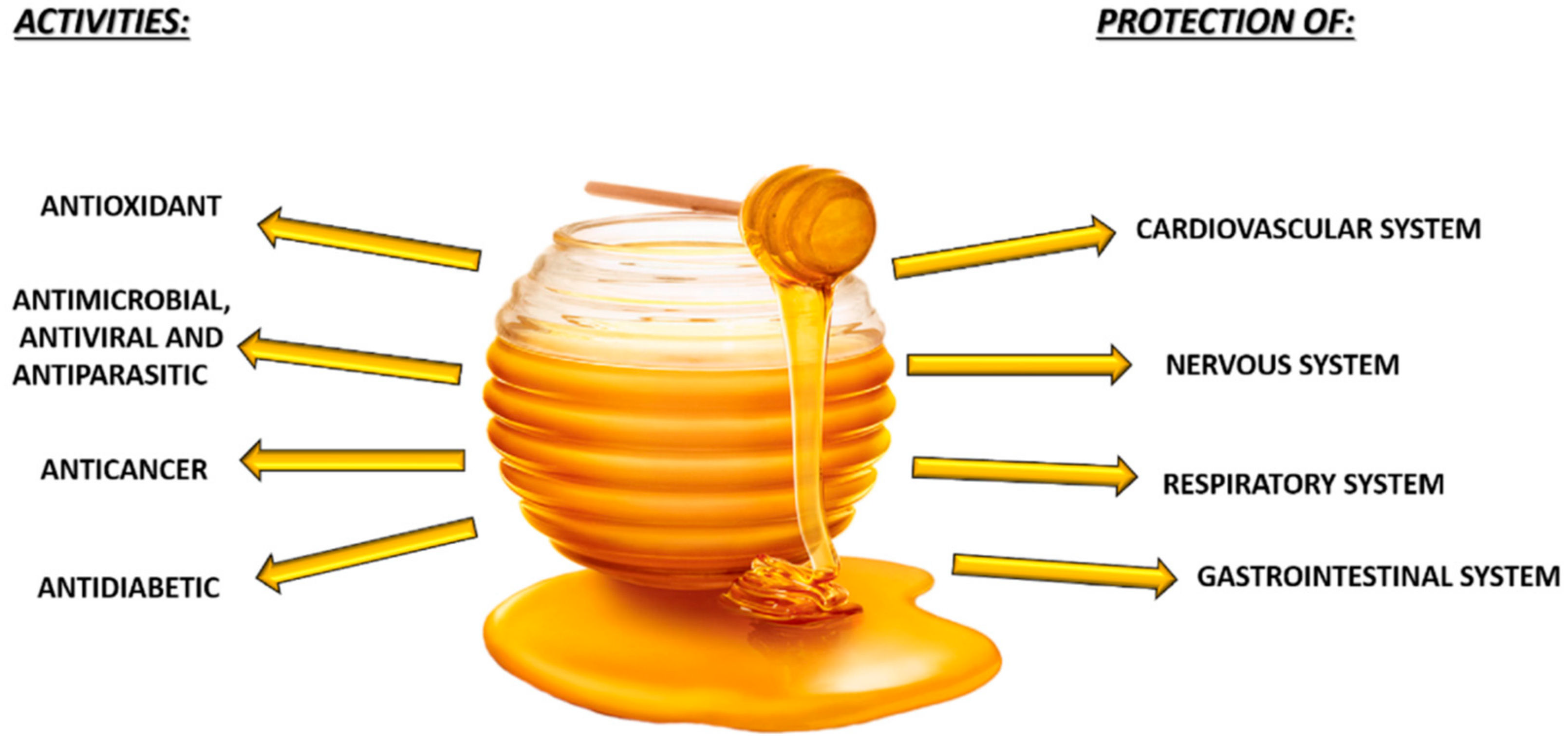
5. Honey and Health and Diseases
5.1. Antimicrobial, Antiviral, and Antifungal Activity
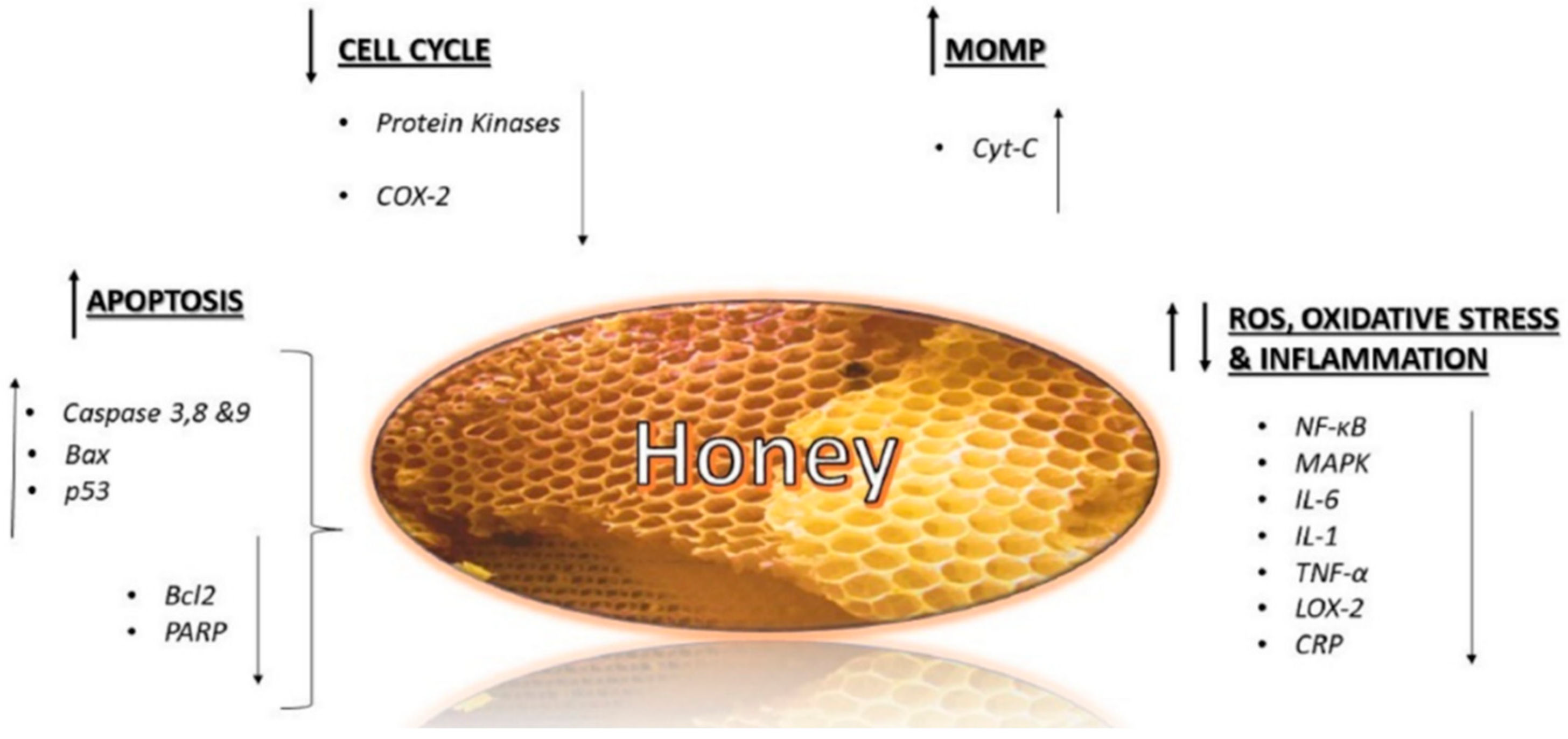
5.2. Anticancer Activity
5.3. Antidiabetic Effect
5.4. Protective Effects of Honey
5.4.1. Cardiovascular System
5.4.2. Nervous System
5.4.3. Respiratory System
5.4.4. Gastrointestinal System
6. Physical Activity, Oxidative Stress, and Honey
7. Infant Botulism and Other Toxic Compounds in Honey
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Faiza, S.; Mohammed, A.; Amjed, A.; Khelod, Y.S.; Ahmad, A.A.L.G. Effects of natural honey on polymicrobial culture of various human pathogens. Arch. Med. Sci. 2014, 10, 246–250. [Google Scholar]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, D.; Gasparrini, M. Forbes-Hernandez, T.Y.; Mazzoni, L.; Afrin, S.; Beltran-Ayala, P.; Gonzales-Paramas, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signaling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar]
- PeternelJ, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Puscas, A.; Hosu, A.; Cimpoiu, C. Application of a newly developed and validated high-performance thin-layer chromatographic method to control honey adulteration. J. Chromatogr. A 2013, 1272, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, R.N. Identification of volatile compounds in solvent extracts of honeys produced in South Africa. Afr. J. Agric. Res. 2011, 6, 4327–4334. [Google Scholar]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Med. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Fallico, B.; Arena, E.; Zappala, M. Degradation of 5-hydroxymethylfurfural in honey. J. Food Sci. 2008, 73, C625–C631. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef] [PubMed]
- White, J.W.; Doner, L.W. Honey Composition and Properties. Beekeep. U.S. Agric. 1980, 335, 82–91. [Google Scholar]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Rapid determination of nonaromatic organic acids in honey by capillary zone electrophoresis with direct ultraviolet detection. J. Agric. Food Chem. 2006, 54, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the flavonoid component of bioactive New Zealand mānuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Kečkeš, J.; Trifković, J.; Andrić, F.; Jovetić, M.; Tešić, Z.; Milojković-Opsenica, D. Amino acids profile of Serbian unifloral honeys. J. Sci. Food Agric. 2013, 93, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersive liquid-liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Ali, F.; Zarei, M.; Md Akim, A.; Hamid, H.A.; Khazaai, H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT-Food Sci. Technol. 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Petretto, G.L.; Cossu, M.; Alamanni, M.C. Phenolic content, antioxidant and physico-chemical properties of Sardinian monofloral honeys. Int. J. Food Sci. Technol. 2015, 50, 482–491. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Ismail, H.M.; Al-Ahwal, A.-M.; Gomaa, N.F. Determination of flavonoid and phenolic Acid contents of Clover, cotton and citrus floral honeys. J. Egy. Public Health Assoc. 2009, 84, 245–259. [Google Scholar]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Tomás-Barberán, F.A.; Soler, C.; García-Viguera, C.; Ortiz, A.; Tomás-Lorente, F. A simple extractive technique for honey flavonoid HPLC analysis. Apidologie 1994, 25, 21–30. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Dispersive liquid-liquid microextraction for the determination of flavonoid aglycone compounds in honey using liquid chromatography with diode array detection and time-of-flight mass spectrometry. Talanta 2015, 131, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Gómez-Caravaca, A.M.; Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds in Rosemary honey using solid-phase extraction by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2006, 41, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Akalın, H.; Bayram, M.; Anlı, R.E. Determination of some individual phenolic compounds and antioxidant capacity of mead produced from different types of honey. J. Inst. Brew. 2016, 123, 167–174. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Yung An, C.; Rao, P.V.; Hawlader, M.N.; Azlan, S.A.; Sulaiman, S.A.; Gan, S.H. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: Determination of antioxidant capacity. BioMed Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues—Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Absorption and metabolism of flavonoids. Free Radict. Biol. Med. 2004, 36, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Kimmich, G.A.; Randles, J. Phloretin-like action of bioflavonoids on sugar accumulation capability of isolated intestinal cells. Membr. Biochem. 1978, 1, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Sign. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural polyphenols: Influence on membrane transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2014, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Wang, X.; Morris, M.E. Flavonoids are inhibitors of human organic anion transporter 1 (OAT1)-mediated transport. Drug Metab. Dispos. 2014, 42, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- North, J.A.; Spector, A.A.; Buettner, G.R. Cell fatty acid composition affects free radical formation during lipid peroxidation. Am. J. Physiol. 1994, 267, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, J.; Sil, P.C. Drug metabolism and oxidative stress: Cellular mechanism and new therapeutic insights. Biochem. Anal. Biochem. 2016, 5, 255. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants-Double-edged swords in cellular redox state. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.B. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2015, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Manisha, D.B.; Shyamapada, M. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Dis. 2011, 2011, 154–160. [Google Scholar]
- Kumar, P.; Sindhu, R.K.; Narayan, S.; Singh, I. Honey collected from different floras of Chandigarh Tricity: A comparative study involving physicochemical parameters and biochemical activities. J. Diet. Suppl. 2010, 7, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, E.O.; Ogunsanya, T.; Aiwerioba, O.I.R. Conventional use of honey as antibacterial agent. Ann. Afr. Med. 2006, 5, 79–81. [Google Scholar]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Salma, M.A.; Al Lenjawi, B.; Abdi, S.; Gouda, Z.; Barakat, N.; Elmahdi, H.; Abraham, S.; Hamza, A.H.; Al Khozaei, D.; et al. The efficacy and safety of natural honey on the healing of foot ulcers: A case series. Wounds 2015, 27, 103–114. [Google Scholar] [PubMed]
- Saha, A.; Chattopadhyay, S.; Azam, M.D.; Sur, P.K. The role of honey in healing of bedsores in cancer patients. South Asian J. Cancer 2012, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Cohrs, R.J. In vitro antiviral activity of honey against varicella zoster virus (VZV): A translational medicine study for potential remedy for shingles. Transl. Biomed. 2012, 3, 2. [Google Scholar] [PubMed]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza viral effects of honey in vitro: Potent high activity of Manuka honey. Med. Res. Arch. 2014, 45, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.-Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Knezevic, A.; Sver, L.; Terzic, S.; Hackenberger, B.K.; Basic, I. Influence of honey bee products on transplantable murine tumours. Vet. Comp. Oncol. 2003, 1, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhi, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 2005, 4, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Othman, N.H. Oral administration of Tualang and Manuka honeys modulates breast cancer progression in Sprague-Dawley rats model. Evid. Based Complement. Altern. Med. 2017, 2017, 5904361. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A comparison with Manuka honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes Hernandez, T.; Cianciosi, D.; Reboredo Rodríguez, P.; Amici, A.; Quiles, J.L.; Battino, M. Inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cells growth. Part 1: Suppression of proliferation, promotion of apoptosis and arrest of cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes Hernandez, T.; Cianciosi, D.; Reboredo Rodríguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. Inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cells growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.; Mandal, M. Honey constituents and its apoptotic effect in colon cancer cells. J. Apiprod. Apimed. Sci. JAAS 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Mabrouk, G.M.; Moselhy, S.S.; Zohny, S.F.; Ali, E.M.; Helal, T.E.; Amin, A.A.; Khalifa, A.A. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J. Exp. Clin. Cancer Res. 2002, 21, 341–346. [Google Scholar] [PubMed]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Mabrouk, G.M.; Shehata, H.H.; Aboelhussein, M.M. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012, 11, 354–363. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.F.; Kandeel, A.A.; El-Aziz, S.F.A.; Ribea, H.M. Anti-tumor effects of bee honey on PCNA and P53 expression in the rat hepatocarcinogenesis. Int. J. Cancer Res. 2012, 8, 130–139. [Google Scholar] [CrossRef]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Honey induces apoptosis in renal cell carcinoma. Pharmacogn. Mag. 2011, 7, 46–52. [Google Scholar] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G.; Canini, A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [PubMed]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; al-Ramadi, B.K. Intravenous administration of Manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS ONE 2013, 8, e5599. [Google Scholar]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Odunola, O.A.; Farooq, A.D.; Rasheed, H.; Mesaik, A.M.; Choudhary, M.I.; Channa, I.S.; Khan, S.A.; Erukainure, O.L. Molecular mechanism of antiproliferation potential of Acacia honey on NCI-H460 cell line. Nutr. Cancer 2013, 65, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Haza, A.I. Antiproliferative and apoptotic effects of spanish honeys. Pharm. J. 2013, 9, 231–237. [Google Scholar]
- Rohlfing, C.L.; Wiedmeyer, H.M.; Little, R.R.; England, J.D.; Tennill, A.; Goldstein, D.E. Defining the relationship between plasma glucose and HbA(1c): Analysis of glucose profiles and HbA(1c) in the diabetes control and complications trial. Diabetes Care 2002, 25, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Fasanmade, A.A.; Alabi, O.T. Differential effect of honey on selected variables in alloxan-induced and fructose induced diabetic rats. Afr. J. Biomed. Res. AJBR 2008, 11, 191–196. [Google Scholar]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.S. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [PubMed]
- Shambaugh, P.; Worthington, V.; Herbert, J.H. Differential effects of honey, sucrose, and fructose on blood sugar levels. J. Manip. Physiol. Ther. 1990, 13, 322–325. [Google Scholar]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Saengsirisuwan, V.; Sloniger, J.A.; Teachey, M.K.; Henriksen, E.J. Oxidant stress and skeletal muscle glucose transport: Roles of insulin signaling and p38 MAPK. Free Radic. Biol. Med. 2006, 41, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Salam, S.K.; Salleh, M.S.; Gurtu, S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2010, 11, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Sulaiman, S.A. The Potential Role of Honey and its Polyphenols in Preventing Heart Diseases: A Review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Khan, R.A.; Azim, M.K.; Saeed, S.A.; Mesaik, M.A.; Ahmed, S.; Imran, I. Effect of natural honey on human platelets and blood coagulation proteins. Pak. J. Pharm. Sci. 2011, 24, 389–397. [Google Scholar] [PubMed]
- Makedou, K.; Iliadis, S.; Kara, E.; Gogou, M.; Feslikidis, T.H.; Papageorgiou, G. Honey and its protective role against oxidation of human low density lipoproteins and total serum lipoproteins. Hippokratia 2012, 16, 287. [Google Scholar] [PubMed]
- Hegazi, A.G.; Abd El-Hady, F.K. Influence of honey on the suppression of Human Low Density Lipoprotein (LDL) peroxidation (In vitro). Evid. Based Complement. Altern. Med. 2009, 6, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Syarifah-Noratiqah, S.; Naina-Mohamed, I.; Zulfarina, M.S.; Qodriyah, H.M. Natural polyphenols in the treatment of Alzheimer’s Disease. Curr. Drug Targets 2018, 19, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, M.A.; Olowookere, T.A.; Atunwa, S.A.; Ibrahim, B.O.; Lamidi, O.F.; Adams, P.A.; Ajimuda, B.O.; Adeyemo, L.E. Neuropharmacological effects of Nigerian honey in mice. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Aziz, C.B.A.; Nazariah Ismail, C.A.; Hussin, C.M.C.; Mohamed, M. The antinociceptive effects of Tualang Honey in male Sprague-Dawley rats: A preliminary study. J. Tradit. Complement. Med. 2014, 4, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Bâcvarov, V.I. Treatment of chronic bronchitis and bronchial asthma with honey. Ther. Ggw. 1970, 109, 260–268. [Google Scholar] [PubMed]
- Kamaruzaman, N.A.; Sulaiman, S.A.; Kaur, G.; Yahaya, B. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement. Altern. Med. 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Asha’ari, Z.A.; Ahmad, M.Z.; Jihan, W.S.; Che, C.M.; Leman, I. Ingestion of honey improves the symptoms of allergic rhinitis: Evidence from a randomized placebo-controlled trial in the East Coast of Peninsular Malaysia. Ann. Saudi Med. 2013, 33, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Haffejee, I.E.; Moosa, A. Honey in the treatment of infantile gastroenteritis. BMJ 1985, 290, 1866–1867. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, O.; Yfanti, C. Antioxidants in Athlete’s Basic Nutrition: Considerations towards a Guideline for the Intake of Vitamin C and Vitamin E. In Source Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Krisnanda, D.A. The effect of honey supplementation before physical activity towards the plasma malondialdehyde level in male wistar rats (rattus norvegicus). In Proceedings of the 1st Yogyakarta International Seminar on Health, Physical Education, and Sports Science, Yogyakarta, Indonesia, 14 October 2017. [Google Scholar]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Jurcău, R.; Jurcău, I. Effect of Manuka honey administration on malondialdehyde, in intense exercise. Palest. Third Millenn. Civ. Sport 2017, 18, 201–205. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H. Correlation between seminal oxidative stress biomarkers and antioxidants with sperm DNA damage in elite athletes and recreationally active men. Clin. J. Sport Med. 2012, 22, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Abdul Aziz, A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose-Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Dembek, Z.F.; Smith, L.A.; Rusnak, J.M. Botulism: Cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med. Public Health Prep. DMPHP 2007, 1, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Cagan, E.; Peker, E.; Dogan, M.; Caksen, H. Infant Botulism. Eurasian J. Med. 2010, 42, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, C.O.; Ayubi, A.; Zulfiquer, F.; Santhanam, G.; Ahmed, M.A.; Deeb, J. Infant botulism following honey ingestion. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Küplülü, Ö.; Göncüoğlu, M.; Özdemir, H.; Koluman, A. Incidence of Clostridium botulinum spores in honey in Turkey. Food Control 2006, 17, 222–224. [Google Scholar] [CrossRef]
- Gücükoğlu, A.; Terzi, G.; Çadirci, Ö.; Alişarli, M.; Kevenk, O.; Uyanik, T. Detection of C. botulinum types in honey by mPCR. J. Food Sci. 2014, 9, M600–M603. [Google Scholar] [CrossRef] [PubMed]
- Rall, V.L.; Bombo, A.J.; Lopes, T.F.; Carvalho, L.R.; Silva, M.G. Honey consumption in the state of São Paulo: A risk to human health? Anaerobe 2003, 9, 299–303. [Google Scholar] [CrossRef]
- Midura, T.F.; Snowden, S.; Wood, R.M.; Arnon, S.S. Isolation of Clostridium botulinum from honey. Clin. Microbiol. 1979, 9, 282–283. [Google Scholar]
- Nevas, M.; Hielm, S.; Lindström, M.; Horn, H.; Koivulehto, K.; Korkeala, H. High prevalence of Clostridium botulinum types A and B in honey samples detected by polymerase chain reaction. Int. J. Food Microbiol. 2002, 72, 45–52. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Casula, L.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Scanu, R.; Tapparo, A.; Sanna, G. An RP-HPLC determination of 5-hydroxymethylfurfural in honey: The case of strawberry tree honey. Talanta 2006, 68, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Toxic compounds in honey. J. Appl. Toxicol. 2014, 34, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.A.; Kleerekooper, I.; Hofman, Z.L.; Kappen, I.F.; Stary-Weinzinger, A.; van der Heyden, M.A. Grayanotoxin poisoning: ‘mad honey disease’ and beyond. Cardiovasc. Toxicol. 2012, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Miraldi, E.; Masti, A.; Ferri, S.; Comparini, I.B. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia 2001, 72, 644–648. [Google Scholar] [CrossRef]
- Jaremicz, Z.; Luczkiewicz, M.; Kokotkiewicz, A.; Krolicka, A.; Sowinski, P. Production of tropane alkaloids in Hyoscyamus niger (black henbane) hairy roots grown in bubble-column and spray bioreactors. Biotechnol. Lett. 2014, 36, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ekabo, O.A.; Farnsworth, N.R.; Henderson, T.O.; Mao, G.; Mukherjee, R. Antifungal and molluscicidal saponins from Serjania salzmanniana. J. Nat. Prod. 1996, 59, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Gong, N.; Huang, J.L.; Guo, L.C.; Wang, Y.X. Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait.; exhibits potent and specific antinociception in chronic pain by acting at spinal α3 glycine receptors. Pain 2013, 154, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
| Component | Amount in 100 g of Honey |
|---|---|
| Water | 16.9–18 g |
| Carbohydrates (total) | 64.9–73.1 g |
| Fructose | 35.6–41.8 g |
| Glucose | 25.4–28.1 g |
| Maltose | 1.8–2.7 g |
| Sucrose | 0.23–1.21 g |
| Proteins, vitamins, amino acid and minerals | 0.50–1 g |
| Presence of Phenolic Compounds in Different Honeys | ||
|---|---|---|
| Flavonoids | ||
| Apigenin | C15H10O5 | AH, TH, STH |
| Catechin | C15H14O6 | TH, PH |
| Chrysin | C15H10O4 | MH, AH, TH, HH, THH, RH |
| Galangin | C15H10O5 | MH, AH, STH, HH |
| Genistein | C15H10O5 | AH |
| Isorhamnetin | C16H12O7 | MH |
| Kaempferol | C15H10O6 | MH, AH, TH, STH, THH, RH |
| Luteolin | C15H10O6 | MH, AH, TH, STH, THH, RH |
| Myricetin | C15H10O8 | AH, HH, THH |
| Pinobanksin | C15H12O5 | MH, AH, STH, RH |
| Pinocembrin | C15H12O4 | MH, AH, STH, RH |
| Quercetin | C15H10O7 | MH, AH, CH, THH |
| Rutin | C27H30O16 | STH |
| Phenolic Acids | ||
| 2-cis,4-trans Abscisic acid | C15H20O4 | STH |
| 2-Hydroxycinnamic acid | C9H8O3 | TH |
| Caffeic acid | C9H8O4 | MH, AH, TH, THH |
| Chlorogenic acid | C16H18O9 | AH, HH, THH |
| Cinnamic acid | C9H8O2 | TH, STH, CH, HH, THH |
| Ellagic acid | C14H6O8 | HH |
| Ferulic acid | C10H10O4 | MH, AH, HH, THH |
| Gallic acid | C7H6O5 | MH, AH, TH, HH, THH, PH |
| p-Coumaric acid | C9H8O3 | MH, AH, TH, HH, THH, RH, PH |
| p-Hydroxybenzoic acid | C7H6O3 | CH, HH |
| Protocatechuic acid | C7H6O4 | HH, PH |
| Sinapic acid | C11H12O5 | HH |
| Syringic acid | C9H10O5 | MH, AH, TH, STH, HH, THH |
| Vanillic acid | C8H8O4 | AH, HH |
| Bacterial Strain | Clinical Importance |
|---|---|
| Helicobacter pylori | Peptic ulcer, gastric malignancies, chronic gastritis |
| Pseudomonas aeruginosa | Diabetic foot ulcer, wound infection, urinary infections |
| Escherichia coli | Urinary tract infection, diarrhea, septicemia, wound infections |
| Mycobacterium tuberculosis | Tuberculosis |
| Staphylococcus aureus | Community acquired and nosocomial infection |
| Proteus spp. | Septicemia, urinary infections, wound infections |
| Salmonella enterica | Enteric fever |
| Acinetobacter baumannii | Infection through open wounds, catheters, and breathing tubes |
| Vibrio cholerae | Cholera |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. https://doi.org/10.3390/molecules23092322
Cianciosi D, Forbes-Hernández TY, Afrin S, Gasparrini M, Reboredo-Rodriguez P, Manna PP, Zhang J, Bravo Lamas L, Martínez Flórez S, Agudo Toyos P, et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules. 2018; 23(9):2322. https://doi.org/10.3390/molecules23092322
Chicago/Turabian StyleCianciosi, Danila, Tamara Yuliett Forbes-Hernández, Sadia Afrin, Massimiliano Gasparrini, Patricia Reboredo-Rodriguez, Piera Pia Manna, Jiaojiao Zhang, Leire Bravo Lamas, Susana Martínez Flórez, Pablo Agudo Toyos, and et al. 2018. "Phenolic Compounds in Honey and Their Associated Health Benefits: A Review" Molecules 23, no. 9: 2322. https://doi.org/10.3390/molecules23092322
APA StyleCianciosi, D., Forbes-Hernández, T. Y., Afrin, S., Gasparrini, M., Reboredo-Rodriguez, P., Manna, P. P., Zhang, J., Bravo Lamas, L., Martínez Flórez, S., Agudo Toyos, P., Quiles, J. L., Giampieri, F., & Battino, M. (2018). Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules, 23(9), 2322. https://doi.org/10.3390/molecules23092322













