Identification of Antiglycative Compounds in Japanese Red Water Pepper (Red Leaf Variant of the Persicaria hydropiper Sprout)
Abstract
1. Introduction
2. Results and Discussion
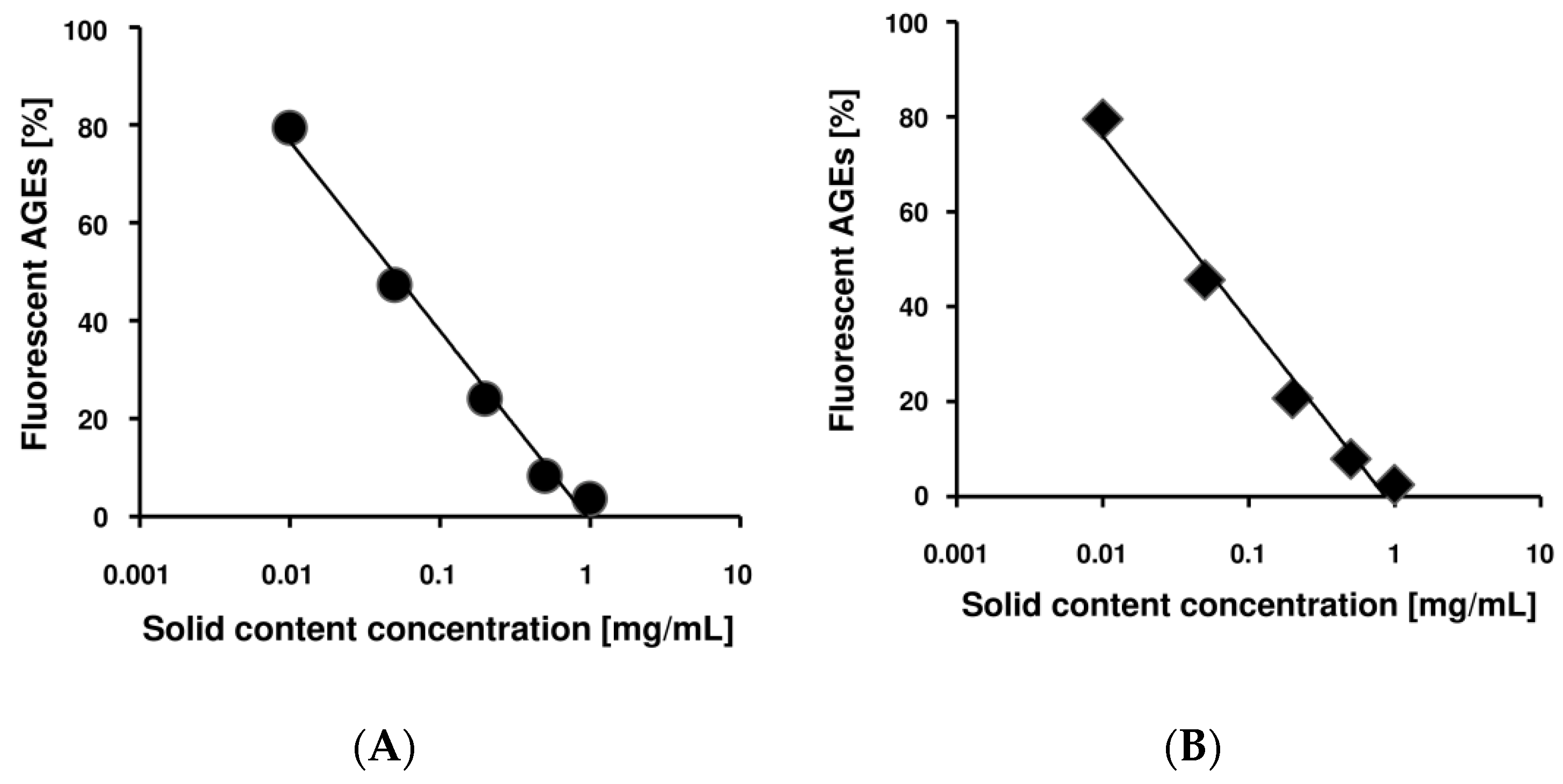
2.1. Benitade Extracts Inhibited Formation of Fluorescent AGEs in a Dose-Dependent Manner
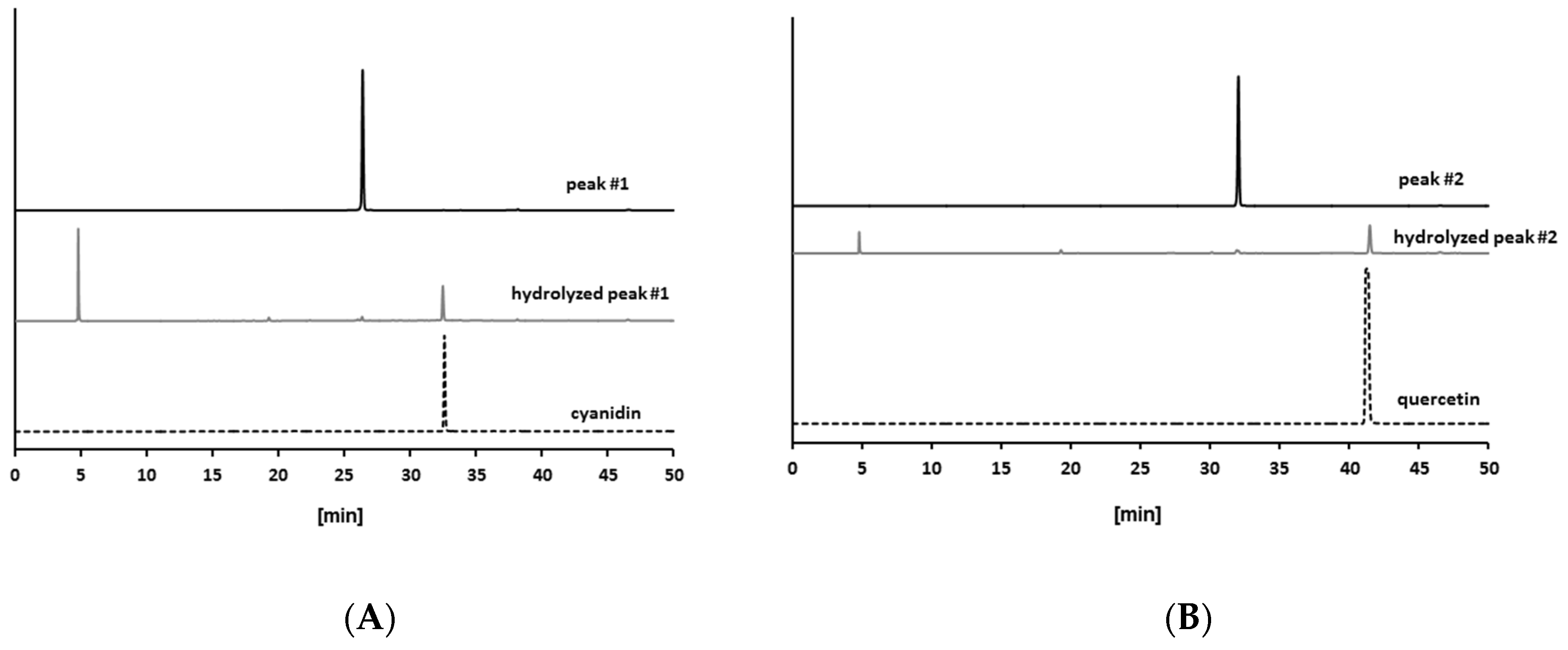
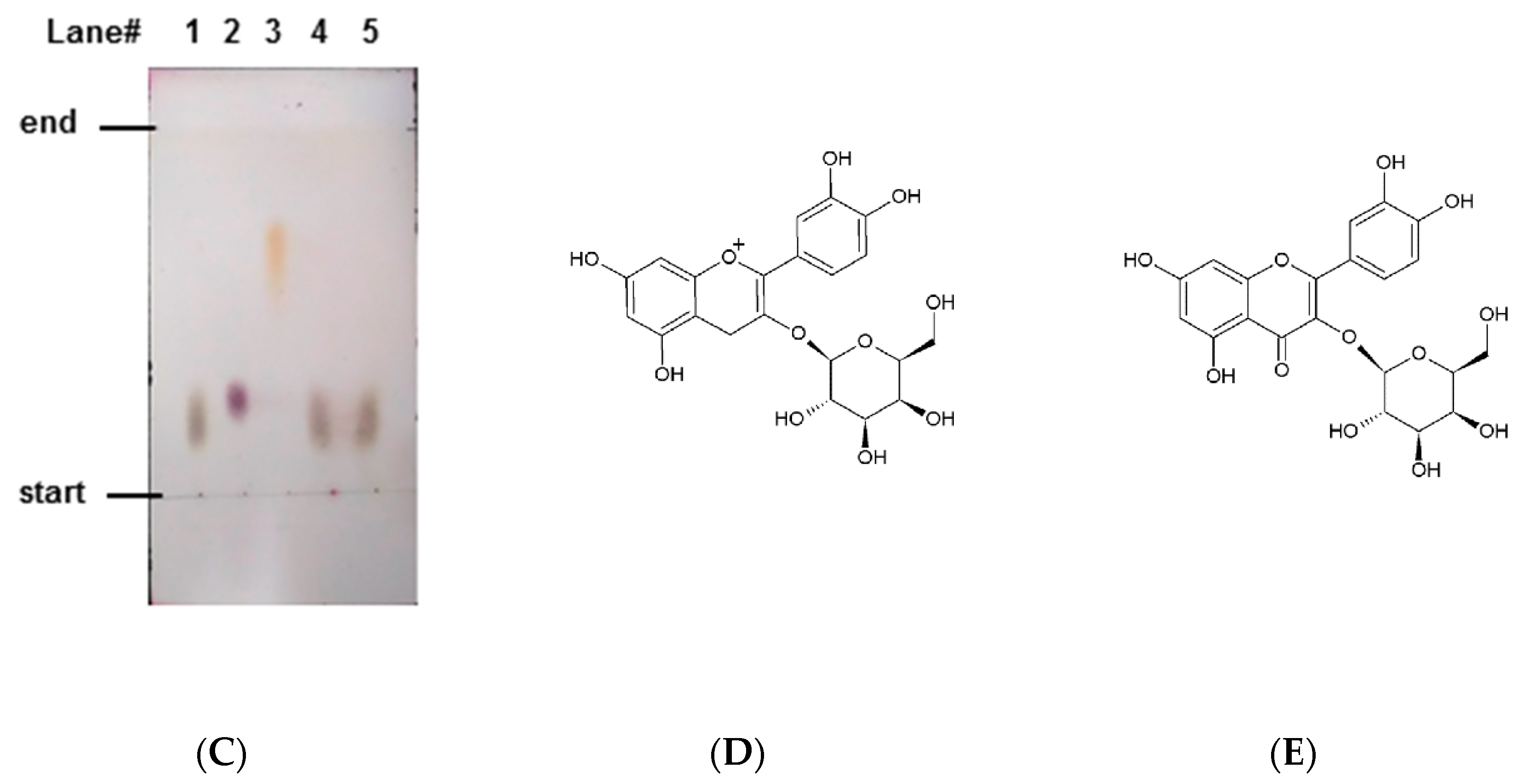
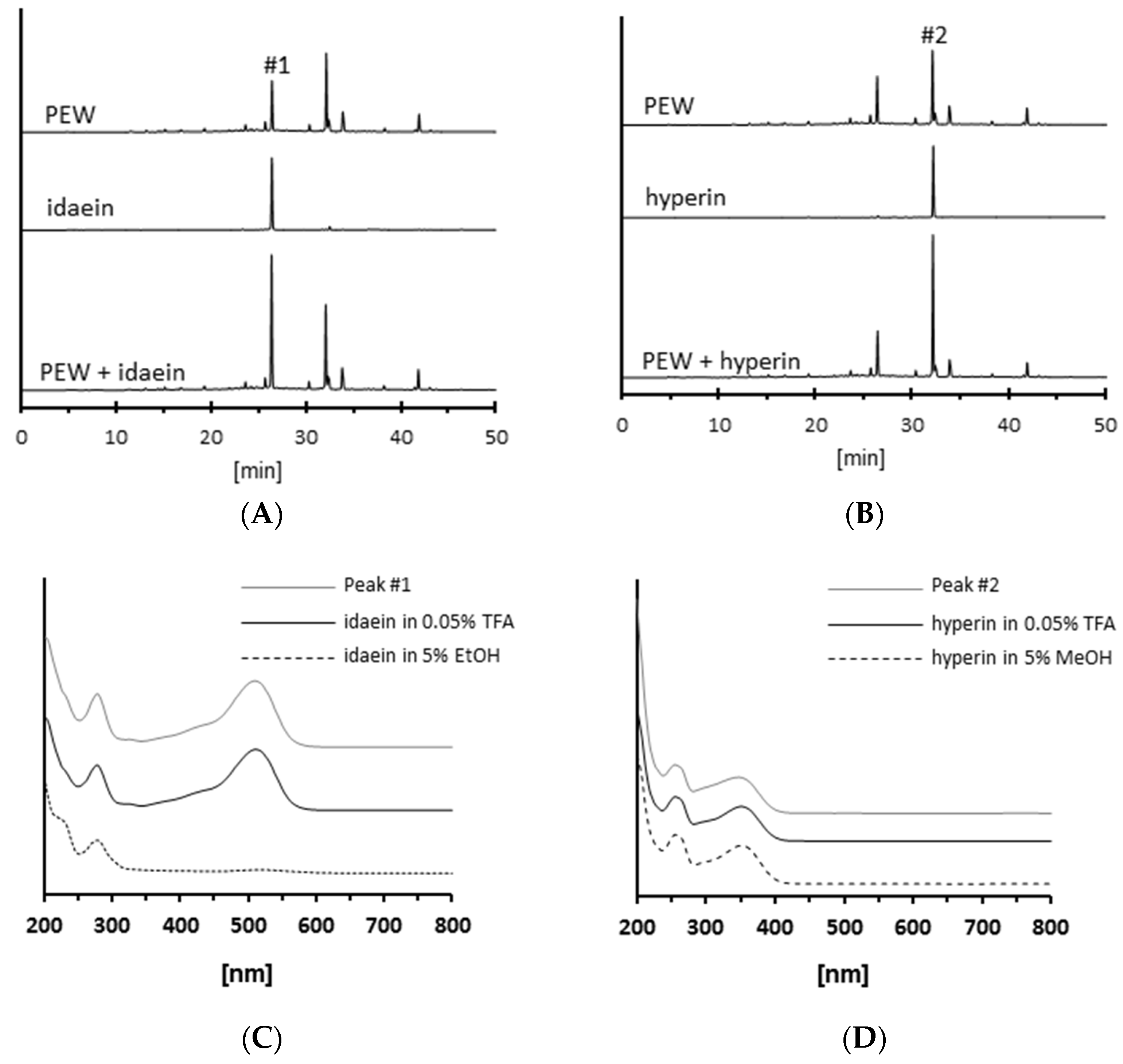
2.2. Isolation of Antiglycative Compounds in Benitade Extract
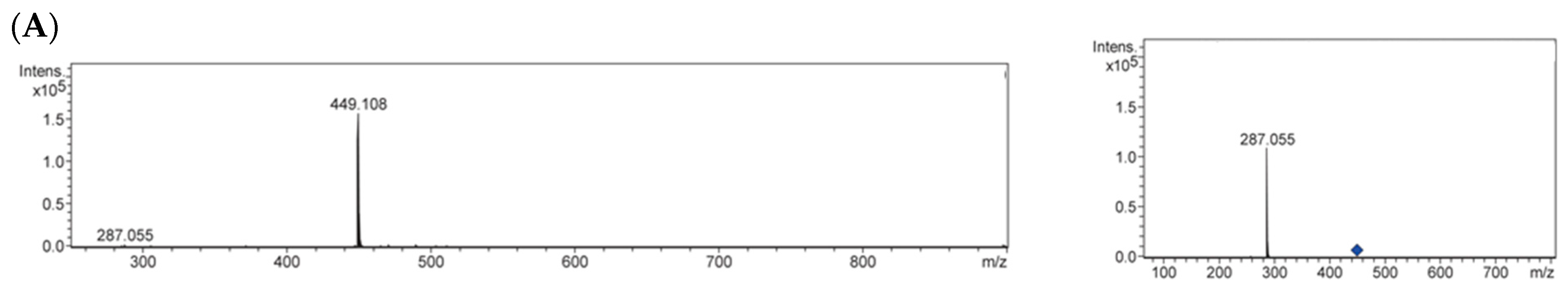
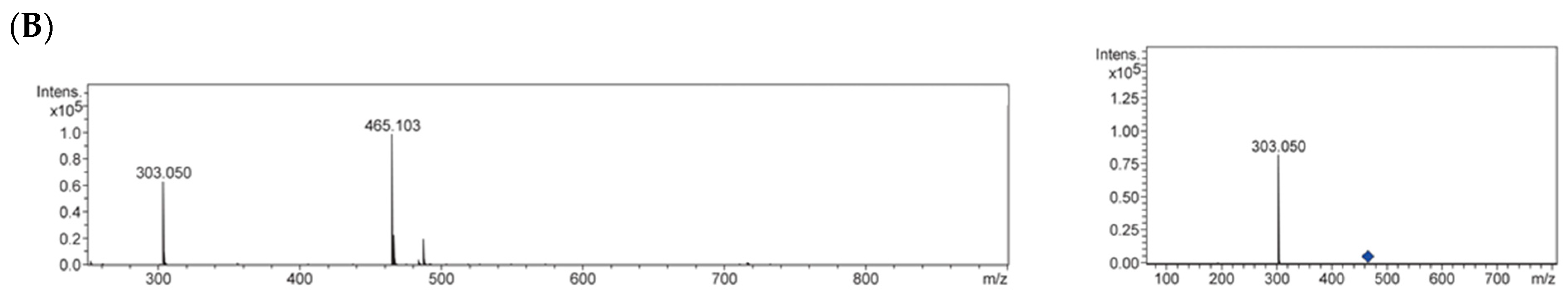
2.3. Identification of Antiglycative Compounds in Benitade Extract
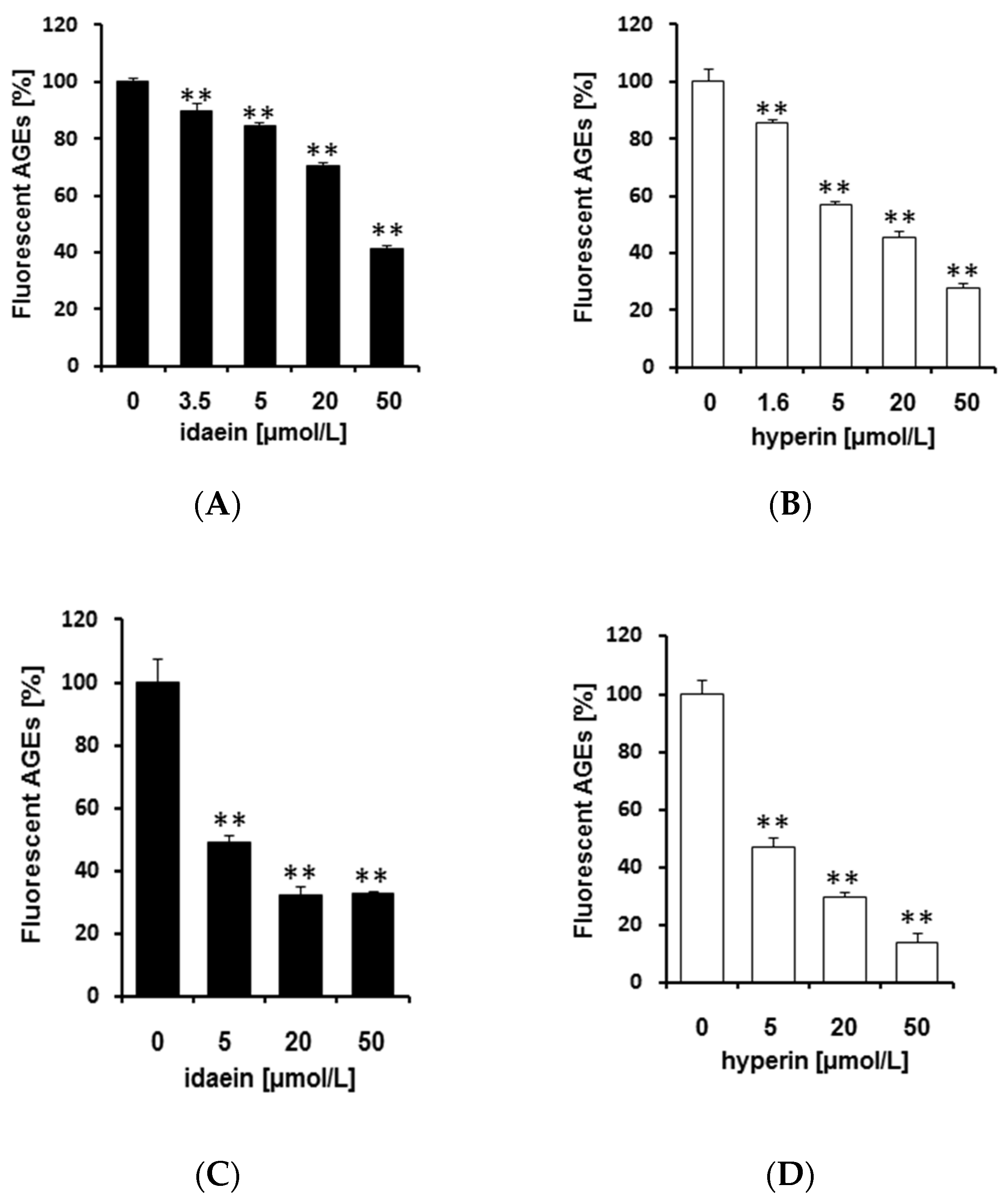
2.4. Idaein and Hyperin and Their Aglycones Inhibited Formation of Fluorescent AGEs in a Dose-Dependent Manner
3. Materials and Methods
3.1. Materials
3.2. Preparation of Persicaria Hydropiper Extract
3.3. Measurement of Antiglycative Effect of Benitade Eextract
3.3.1. Preparation of Glycated Proteins
3.3.2. Measurement of AGE-Derived Fluorescence
3.4. Isolation of Compounds in Benitade Extract
3.4.1. HPLC-UV System for Analyses
3.4.2. Fractionation of Benitade Extract by HPLC
3.5. Hydrolysis of Glycosides
3.6. Thin-Layer Chromatography for Saccharides
3.7. Measurement of UV-Vis Absorption Spectrum
3.8. UHPLC-QqTOF-MS Analysis
3.9. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. Ser. A 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced glycation in health and disease: Role of the modern environment. Ann. N. Y. Acad. Sci. 2005, 1043, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Rabbani, N.; Thornalley, P.J. Glyoxalase in ageing. Semin. Cell. Dev. Biol. 2011, 22, 293–301. [Google Scholar] [CrossRef]
- Kan, H.; Yamagishi, S.; Ojima, A.; Fukami, K.; Ueda, S.; Takeuchi, M.; Hyogo, H.; Aikata, H.; Chayama, K. Elevation of Serum Levels of Advanced Glycation End Products in Patients With Non-B or Non-C Hepatocellular Carcinoma. J. Clin. Lab. Anal. 2015, 29, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, G.E. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol. Metab. Clin. N. Am. 2013, 42, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets 2011, 12, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.N.; El-Bassossy, H.M.; Barakat, W. Targeting AGEs Signaling Ameliorates Central Nervous System Diabetic Complications in Rats. Adv. Pharmacol. Sci. 2015, 346259. Available online: https://www.hindawi.com/journals/aps/2015/346259/ (accessed on 26 July 2018).
- Takeuchi, M.; Yamagishi, S. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.S.; Fortheringham, A.K.; Cooper, M.E.; Forbes, J.M. Targeting advanced glycation endproducts and mitochondrial dysfunction in cardiovascular disease. Curr. Opin. Pharmacol. 2013, 13, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, M.; Kuroda, T.; Tanaka, S.; Saito, M.; Fukunaga, M.; Nakamura, T. Non-enzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J. Bone Miner. Metabol. 2008, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Knecht, K.J.; Dunn, J.A.; McFarland, K.F.; McCance, D.R.; Lyons, T.J.; Thorpe, S.R.; Baynes, J.W. Effect of diabetes and aging on carboxymethyllysine levels in human urine. Diabetes 1991, 40, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Hartog, J.W.; de Vries, A.P.; Lutgers, H.L.; Meerwaldt, R.; Huisman, R.M.; van Son, W.J.; de Jong, P.E.; Smit, A.J. Accumulation of advanced glycation end products, measured as skin autofluorescence, in renal disease. Ann. N. Y. Acad. Sci. 2005, 1043, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, B.T.; Smit, A.J. Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconj. J. 2016, 33, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- Monnier, V.M.; Kohn, R.R.; Cerami, A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1984, 81, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Séro, L.; Sanguinet, L.; Blanchard, P.; Dang, B.T.; Morel, S.; Richomme, P.; Séraphin, D.; Derbré, S. Tuning a 96-well microtiter plate fluorescence-based assay to identify AGE inhibitors in crude plant extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef]
- Farrar, J.L.; Hartle, D.K.; Hargrove, J.L.; Greenspan, P. Inhibition of protein glycation by skins and seeds of the muscadine grape. Biofactors 2007, 30, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Premakumara, G.A.S.; Abeysekera, W.K.S.M.; Ratnasooriya, W.D.; Chandrasekharan, N.V.; Bentota, A.P. Antioxidant, anti-amylase and anti-glycation potential of brans of some Sri Lankan traditional and improved rice (Oryza sativa L.) varieties. J. Cereal Sci. 2013, 58, 451–456. [Google Scholar] [CrossRef]
- Parengkuan, L.; Yagi, M.; Matsushima, M.; Ogura, M.; Hamada, U.; Yonei, Y. Anti-glycation activity of various fruits. Anti-Aging. Med. 2013, 10, 70–76. Available online: http://www.anti-aging.gr.jp/english/pdf/2013/10(4)7076.pdf (accessed on 26 July 2018).
- Ishioka, Y.; Yagi, M.; Ogura, M.; Yonei, Y. Antiglycation effect of various vegetables: Inhibition of advanced glycation end product formation in glucose and human serum albumin reaction system. Glycative Stress Res. 2015, 2, 22–34. [Google Scholar] [CrossRef]
- Ishioka, Y.; Yagi, M.; Ogura, M.; Yonei, Y. Polyphenol content of various vegetables: Relationship to antiglycation activity. Glycative Stress Res. 2015, 2, 41–51. [Google Scholar] [CrossRef]
- Otake, K.; Yagi, M.; Takabe, W.; Yonei, Y. Effect of tea (Camellia sinensis) and herbs on advanced glycation endproduct formation and the influence of post-fermentation. Glycative Stress Res. 2015, 2, 156–162. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Parengkuan, L.; Yagi, M.; Yonei, Y. Effect of proteins, sugars and extraction methods on the anti-glycation activity of spices. Glycative Stress Res. 2015, 2, 129–139. [Google Scholar] [CrossRef]
- Takabe, W.; Kitagawa, K.; Yamada, K.; Noda, Y.; Yamamoto, R.; Yamaguchi, T.; Kannan, R.; Yagi, M.; Yonei, Y. Anti-glycative effect of vegetables and fruit extract on multiple glycation models. Glycative Stress Res. 2017, 4, 71–79. [Google Scholar]
- Hartwell, J.L. Plants used against cancer. A survey. Lloydia 1970, 33, 288–392. [Google Scholar] [PubMed]
- Barnes, C.; Loder, J. The structure of polygodial, a new sesquiterpene dialdehyde from Polygonum hydropiper L. Aust. J. Chem. 1962, 15, 322–327. [Google Scholar] [CrossRef]
- De Almeida Alves, T.M.; Ribeiro, F.L.; Kloos, H.; Zani, C.L. Polygodial, the fungitoxic component from the Brazilian medicinal plant Polygonum punctatum. Mem. Inst. Oswaldo Cruz. 2001, 96, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.S.; Kubo, I. Effect of polygodial on the mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2000, 44, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.F.; Strack, D.; Baumert, A.; Subramaniam, R.; Goh, N.K.; Chia, T.F.; Tan, S.N.; Chia, L.S. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003, 62, 219–228. [Google Scholar] [CrossRef]
- Haraguchi, H.; Hashimoto, K.; Yagi, A. Antioxidative substances in leaves of. J. Agric. Food Chem. 1992, 40, 1349–1351. [Google Scholar] [CrossRef]
- Yagi, M.; Yonei, Y. Glycative stress and anti-aging 4. The evaluation of glycative Stress: Evaluation for anti-glycative effect. Glycative Stress Res. 2017, 4, 87–92. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; McIntyre, K.; Saleem, A.; Haddad, P.S.; Arnason, J.T. The relationship between antiglycation activity and procyanidin and phenolic content in commercial grape seed products. Can. J. Physiol. Pharmacol. 2012, 90, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Kim, J.H.; Tanaka, T.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol. Pharm. Bull. 2011, 34, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, J.F.; Muzio, B.P.; Rosa, C.M.; Nascimento, A.F.; Sugizaki, M.M.; Fernandes, A.A.; Cicogna, A.C.; Padovani, C.R.; Okoshi, M.P.; Okoshi, K. Rutin administration attenuates myocardial dysfunction in diabetic rats. Cardiovasc. Diabetol. 2015, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Yoshitama, K.; Hisada, M.; Ishikura, N. Distribution Pattern of Anthocyanins in the Polygonaceae. Bot. Mag. 1984, 97, 31–38. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Ioku, K.; Tsushida, T.; Takei, Y.; Nakatani, N.; Terao, J. Antioxidative activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochim. Biophys. Acta 1995, 1234, 99–104. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PEW is available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takabe, W.; Yamaguchi, T.; Hayashi, H.; Sugimura, N.; Yagi, M.; Yonei, Y. Identification of Antiglycative Compounds in Japanese Red Water Pepper (Red Leaf Variant of the Persicaria hydropiper Sprout). Molecules 2018, 23, 2319. https://doi.org/10.3390/molecules23092319
Takabe W, Yamaguchi T, Hayashi H, Sugimura N, Yagi M, Yonei Y. Identification of Antiglycative Compounds in Japanese Red Water Pepper (Red Leaf Variant of the Persicaria hydropiper Sprout). Molecules. 2018; 23(9):2319. https://doi.org/10.3390/molecules23092319
Chicago/Turabian StyleTakabe, Wakako, Taiki Yamaguchi, Hideharu Hayashi, Natsuhiko Sugimura, Masayuki Yagi, and Yoshikazu Yonei. 2018. "Identification of Antiglycative Compounds in Japanese Red Water Pepper (Red Leaf Variant of the Persicaria hydropiper Sprout)" Molecules 23, no. 9: 2319. https://doi.org/10.3390/molecules23092319
APA StyleTakabe, W., Yamaguchi, T., Hayashi, H., Sugimura, N., Yagi, M., & Yonei, Y. (2018). Identification of Antiglycative Compounds in Japanese Red Water Pepper (Red Leaf Variant of the Persicaria hydropiper Sprout). Molecules, 23(9), 2319. https://doi.org/10.3390/molecules23092319



