Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration
Abstract
1. Introduction
2. Materials and Characterization
2.1. Chemicals
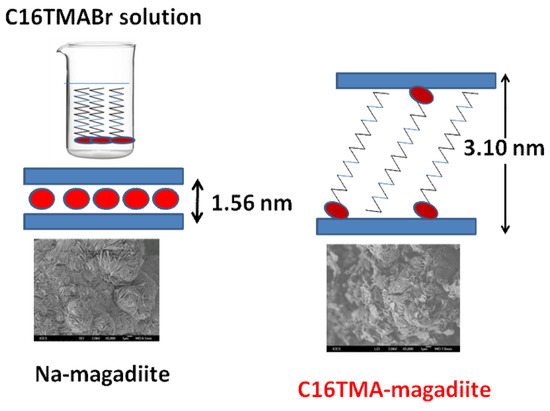
2.2. Na-Magadiite
2.3. Organo-Magadiites
2.4. Chemical Properties
2.5. Eosin Removal
2.6. Regeneration Studies of Spent Organo-Magadiites
2.7. Characterization
3. Results and Discussion
3.1. C, H, and N Elemental Analysis
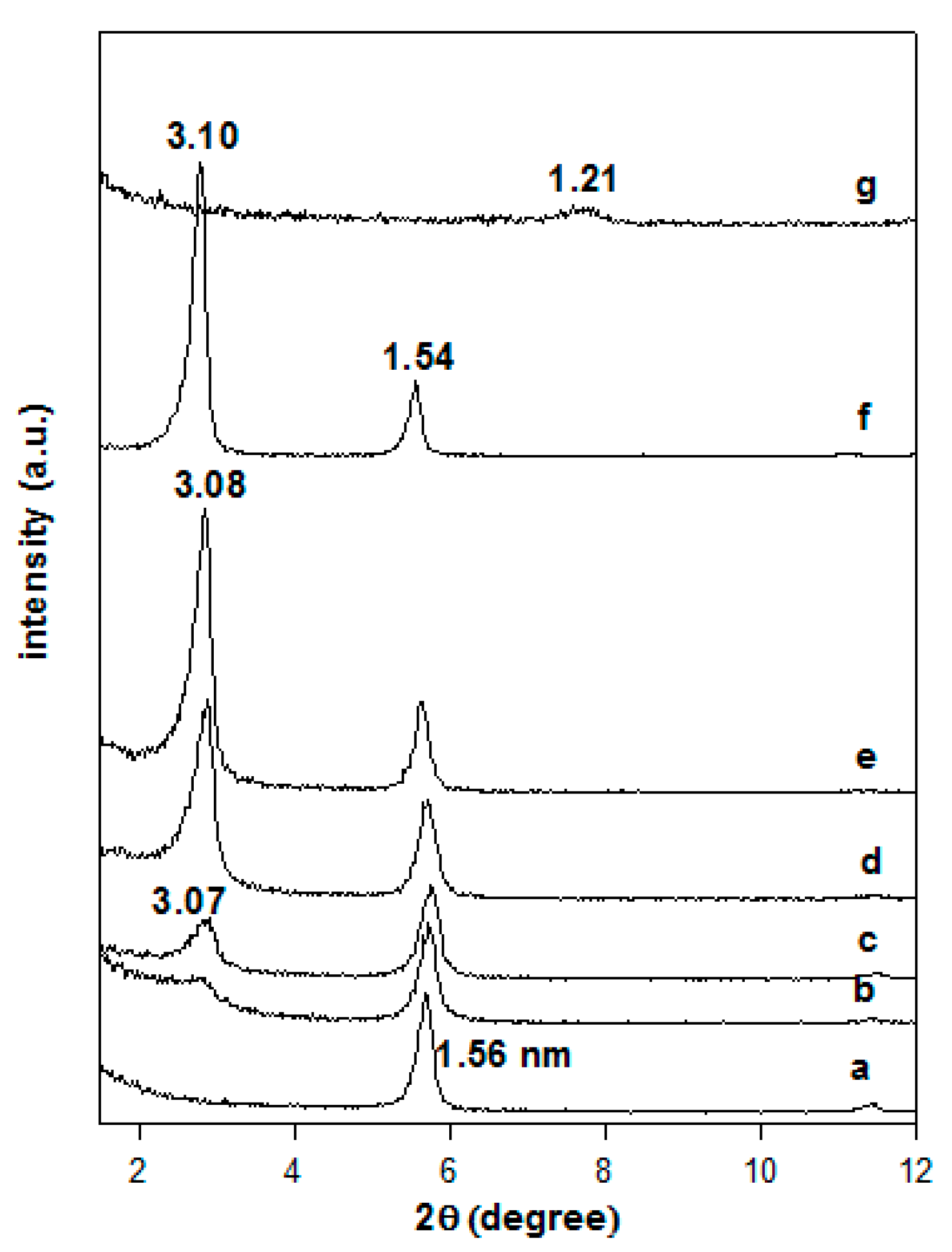
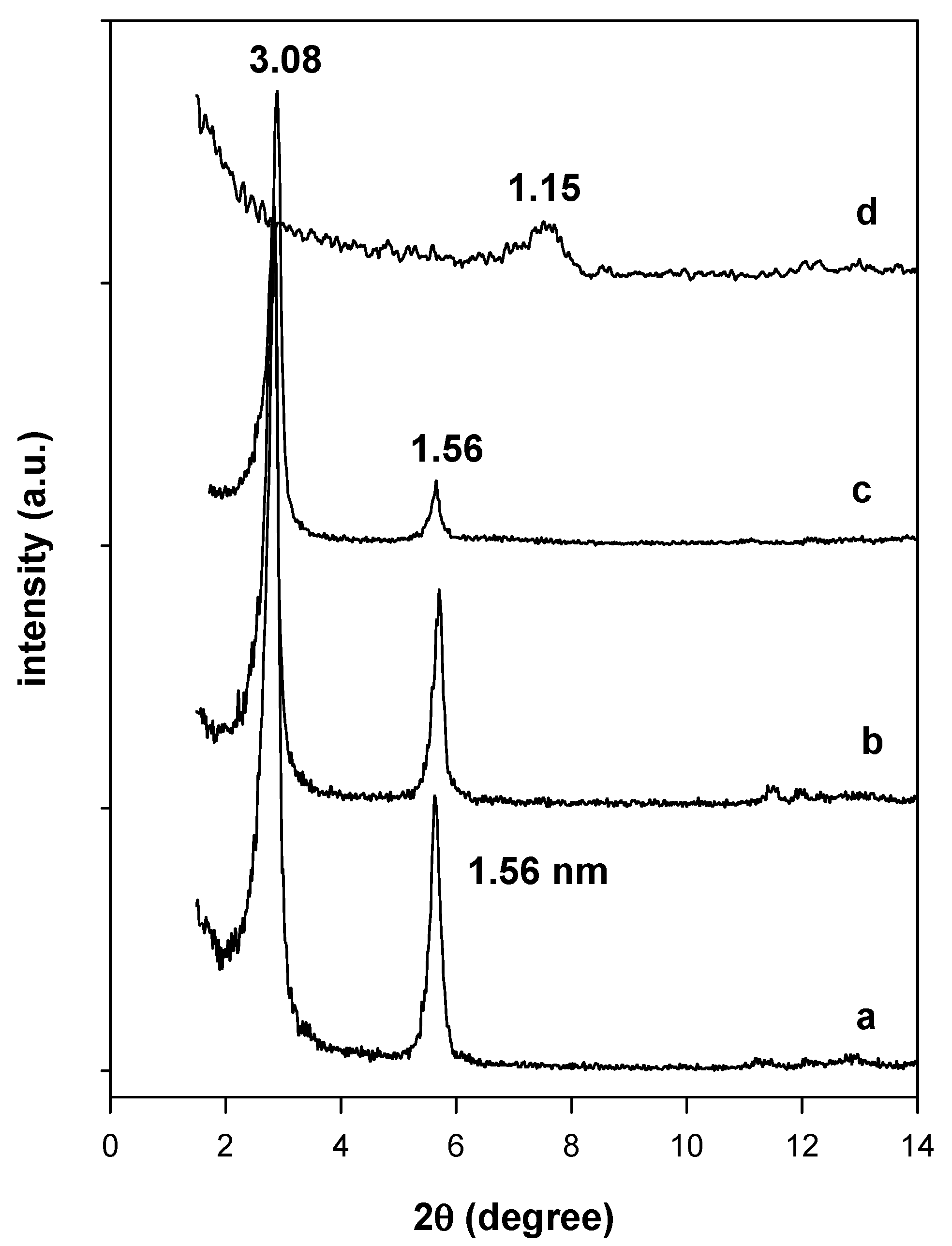
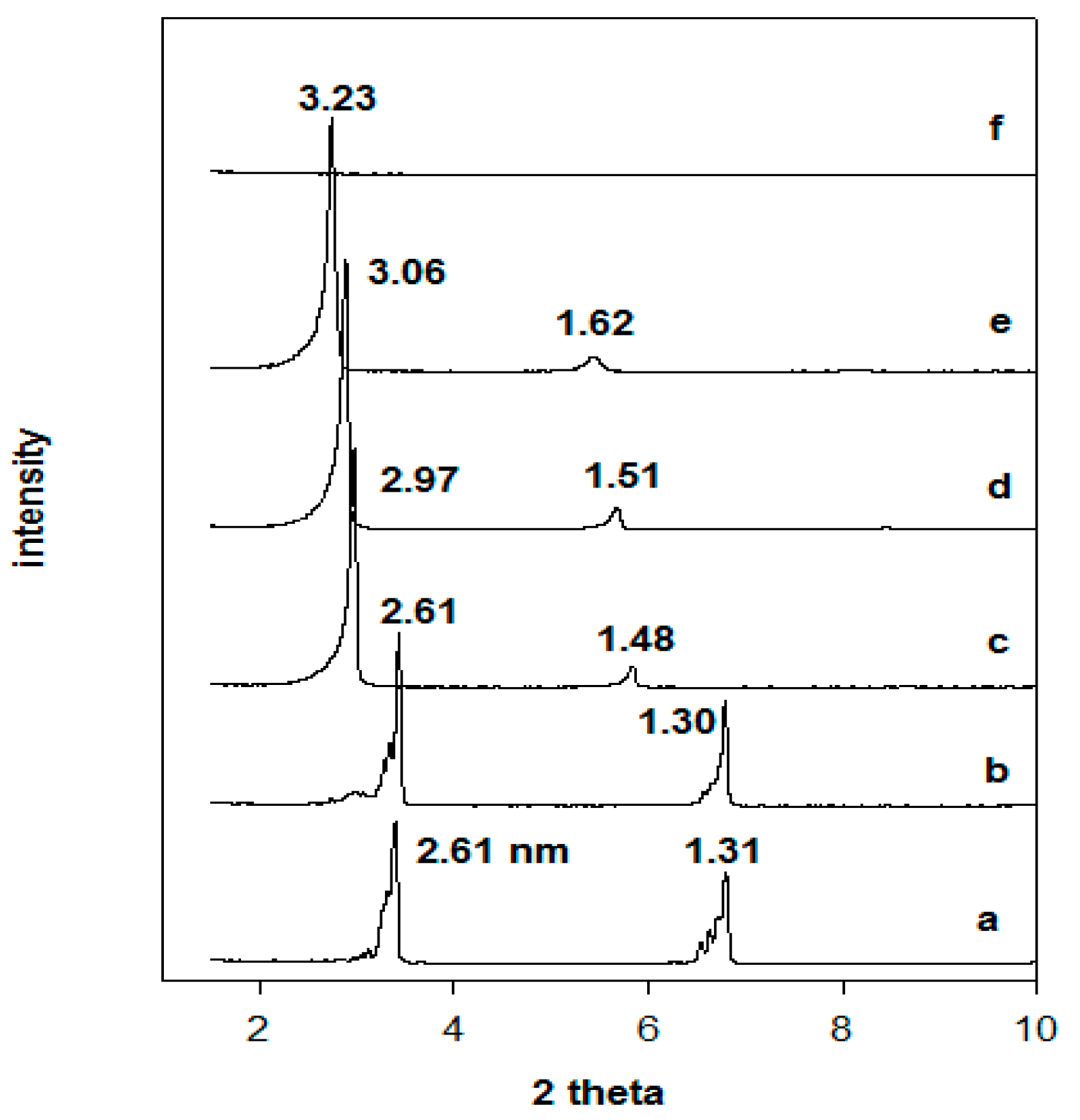
3.2. X-ray Diffraction Data
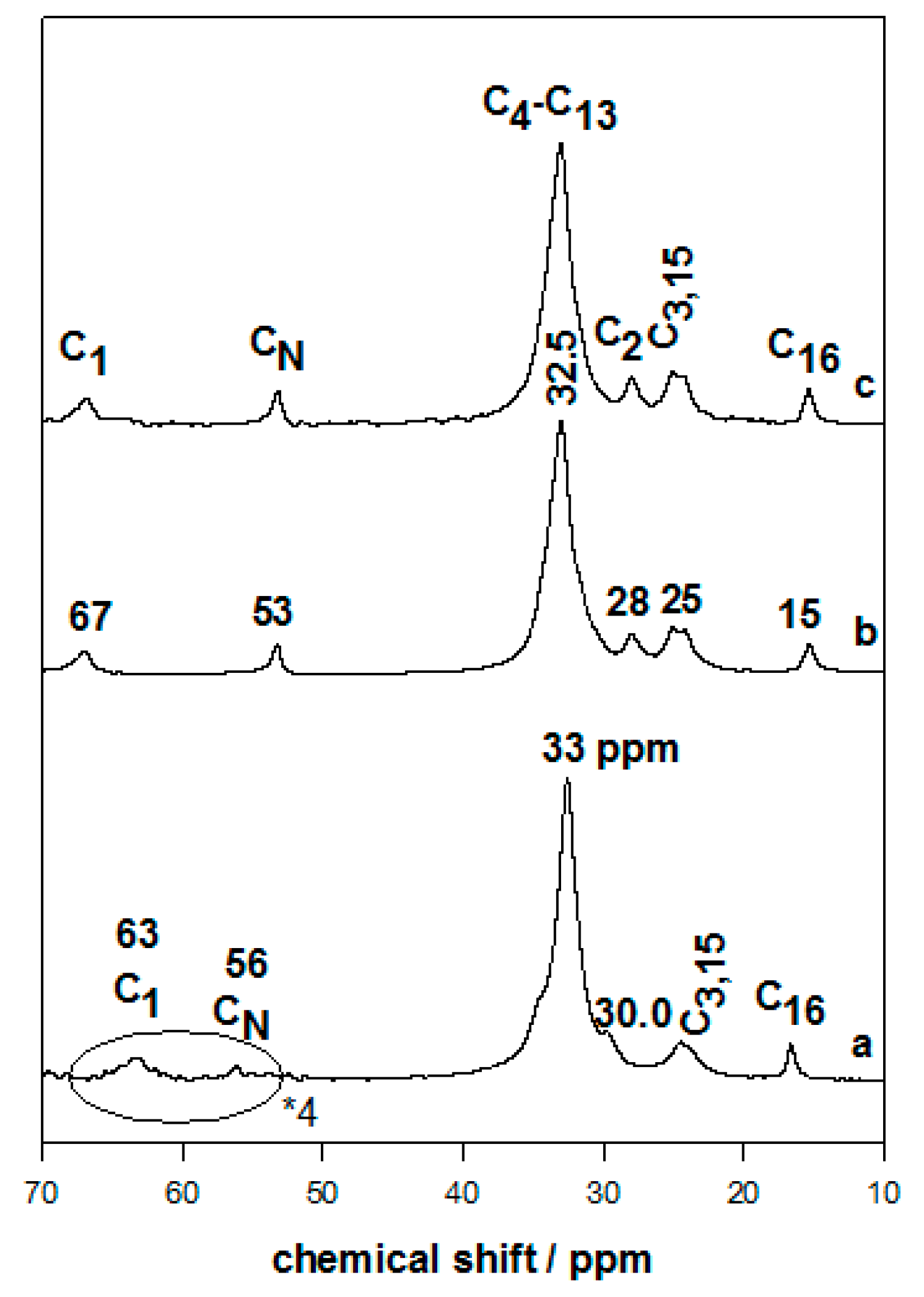
3.3. Solid-State NMR Studies
3.4. Microtextural Studies and Specific Surface Areas
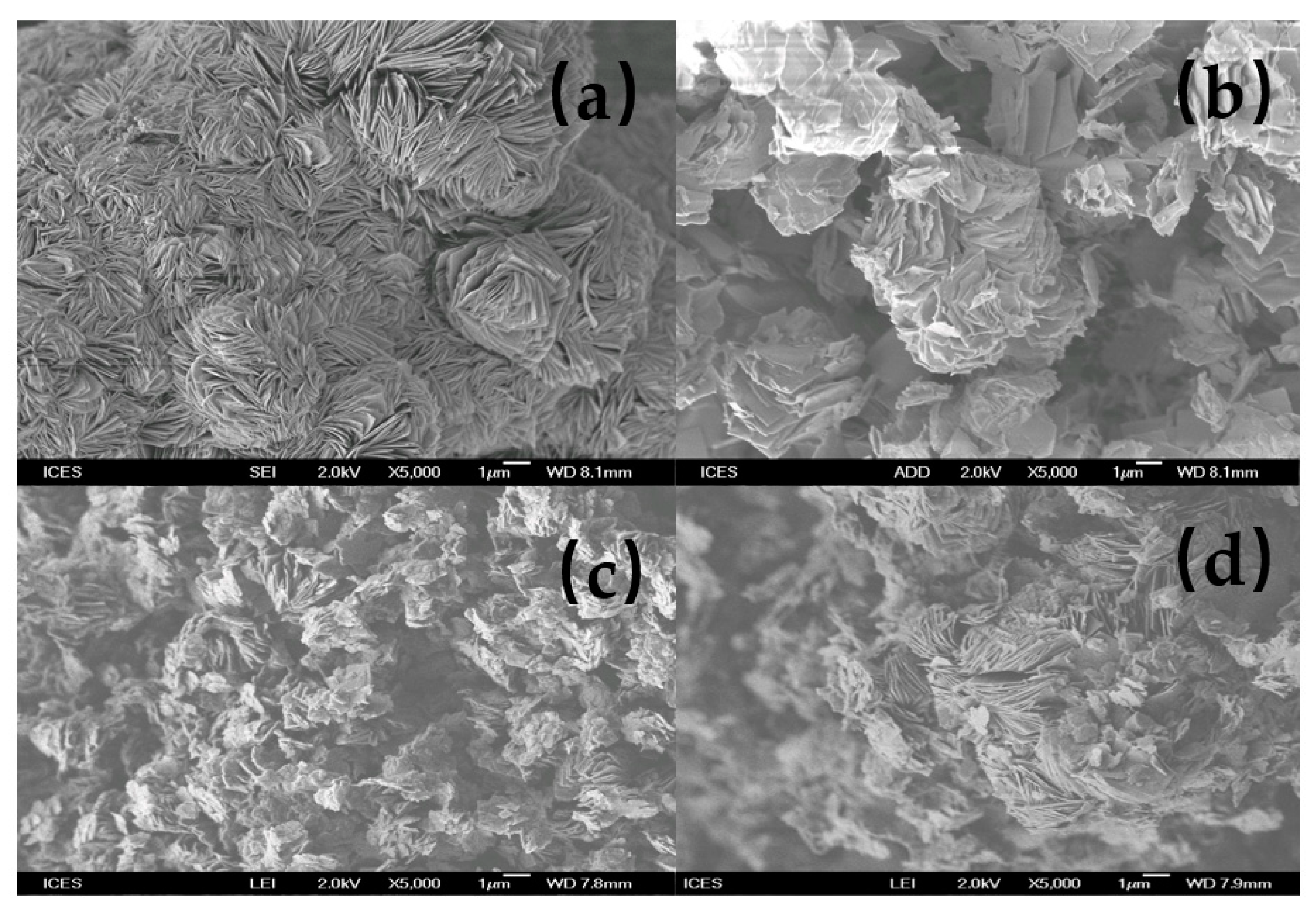
3.5. SEM Micrographs
3.6. Thermal Stability
3.6.1. Thermal Gravimetric Analysis (TGA)
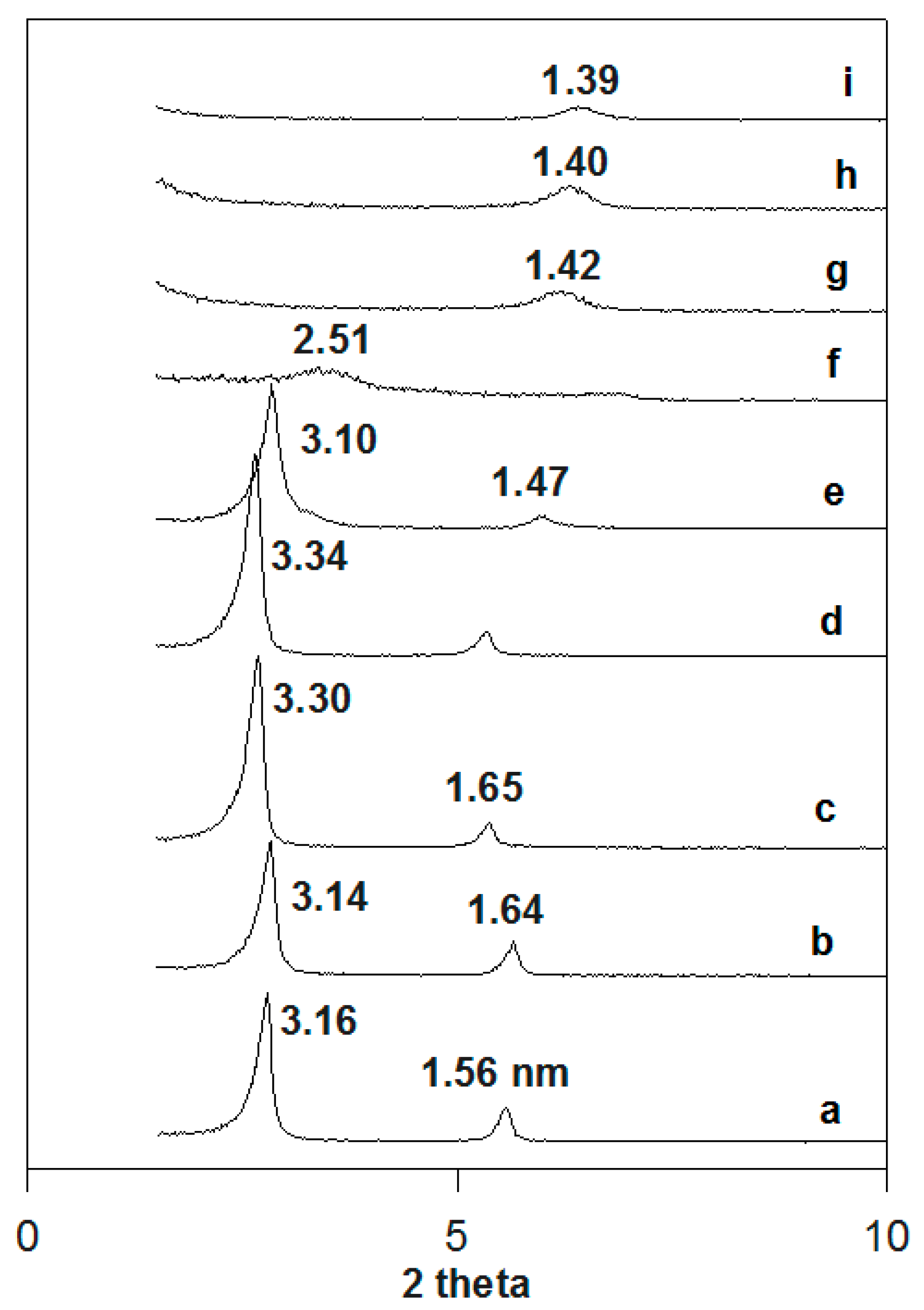
3.6.2. In Situ Powder XRD Studies
3.7. Removal of Eosin Studies
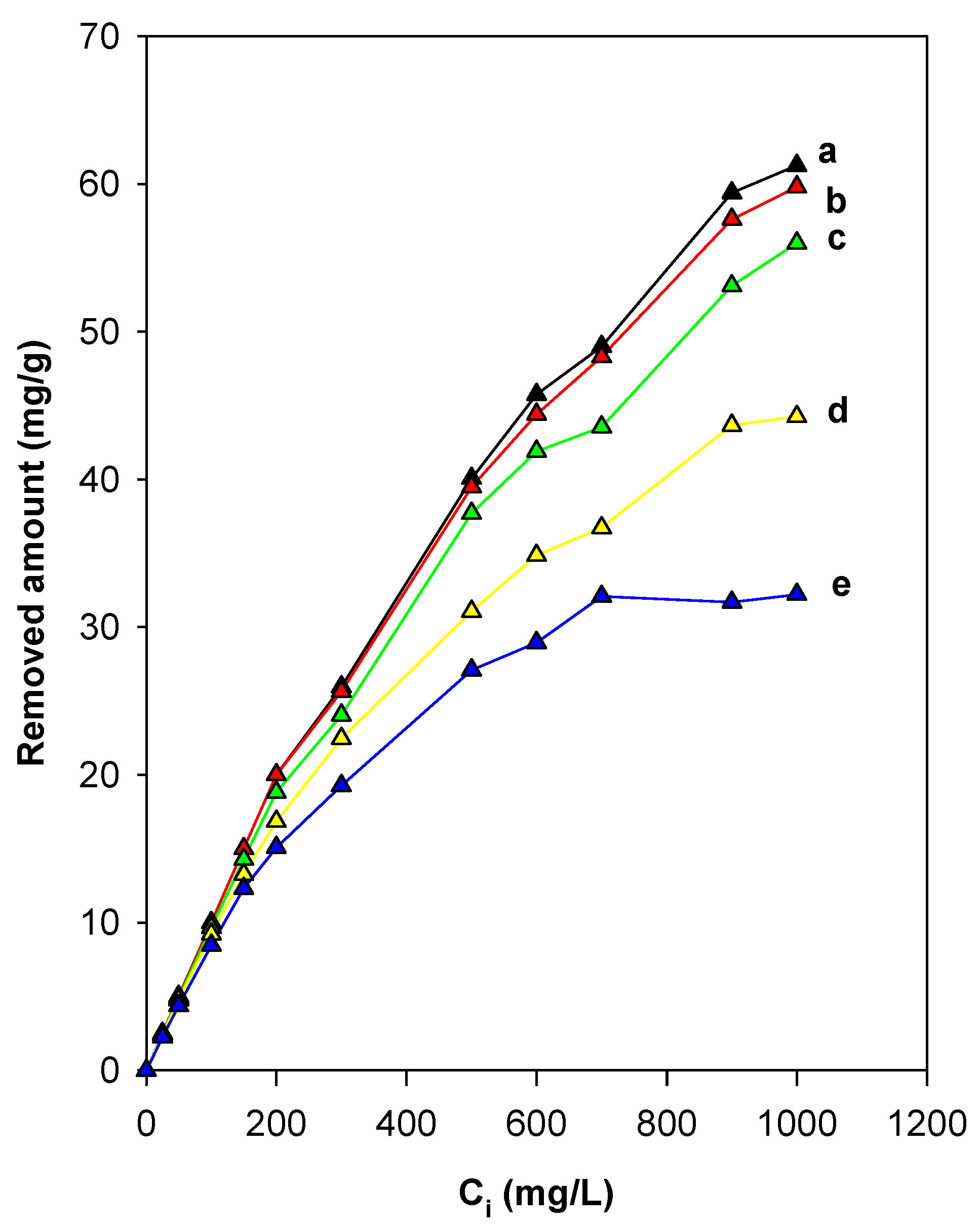
3.7.1. Effect of Initial Concentrations (Ci)
3.7.2. Effect of Organic Content
3.7.3. Effect of Removal Temperature
3.7.4. Effect of Preheated Temperature of Organo-Magadiite
3.7.5. Maximum Amount of Eosin Removed
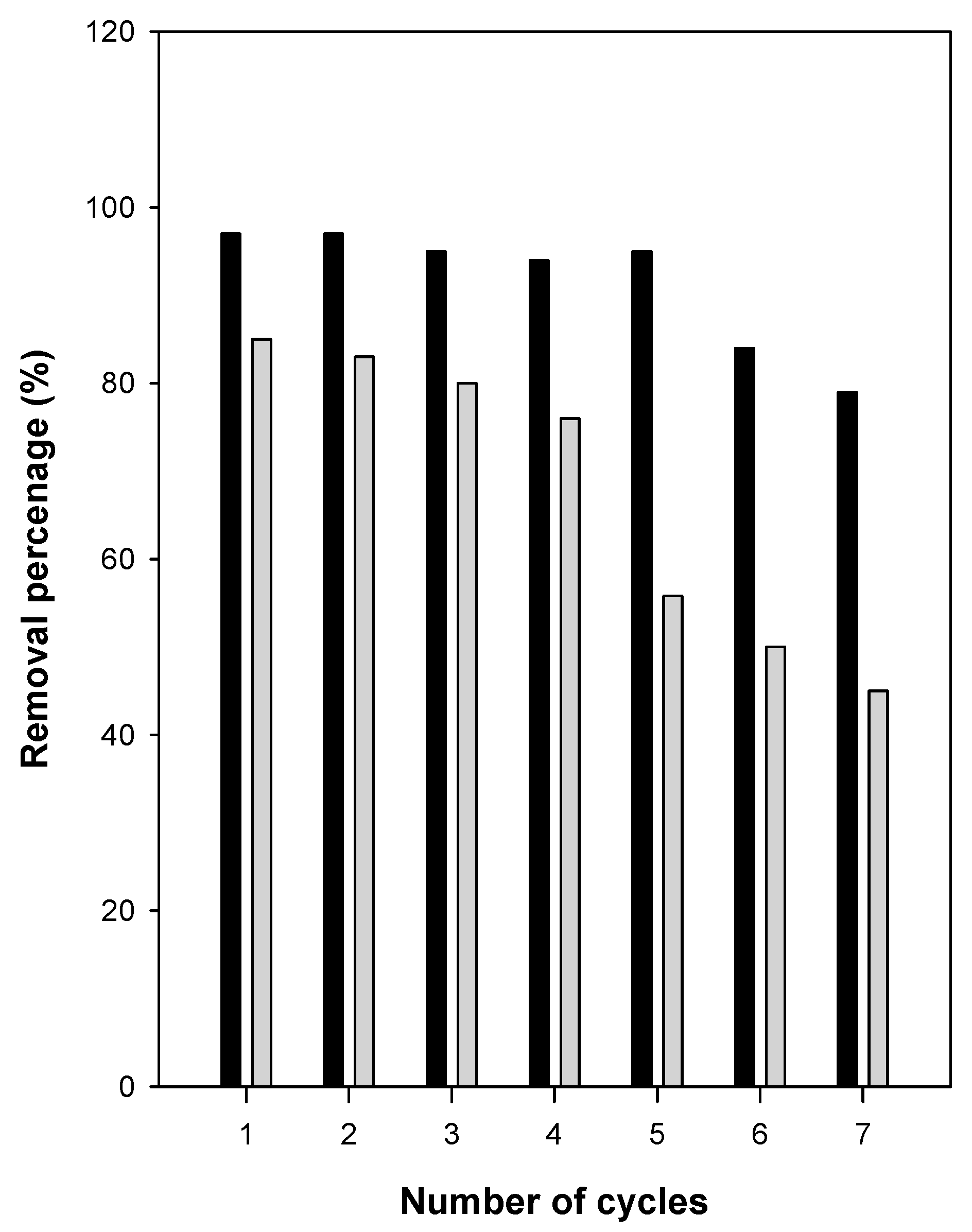
3.8. Removal/Regeneration Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| CEC | Cation exchange capacity |
| C16TMA | Cetyl trimethylammonium |
| C16TMAOH | Cetyl trimethylammonium hydroxide |
| C16TMACl | Cetyl trimethylammonium chloride |
| C16TMABr | Cetyl trimethylammonium bromide |
| C16Mag-20 | An organo-magadiite with a loaded amount of 20 mM C16TMA cations per 100 g |
| C16Mag-20 | An organo-magadiite with a loaded amount of 40 mM C16TMA cations per 100 g |
| C16Mag-80 | An organo-magadiite with a loaded amount of 80 mM C16TMA cations per 100 g |
| C16Mag-120 | An organo-magadiite with a loaded amount of 120 mM C16TMA cations per 100 g |
| UV | UV visible |
| Co(NO3)2·6H2O | Cobalt nitrate hexahydrate |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| NMR | Nuclear magnetic resonance |
| CP | Coupled proton |
| MAS | Magic Angle Spinning |
| EDX | Energy dispersive X-ray spectroscopy |
| T.P.V. | Total pore volume |
| A.P.D. | Average pore diameter |
| TGA | Thermal gravimetric Analysis |
| DTG | Derivative thermal gravimetric |
| DSC | Differential scanning calorimetry |
| qmax | Maximum adsorption capacity (mg/g) |
| KL | Langmuir constant (L/mg) |
| Ci | Initial concentration |
| RFA | Raw fly carbon ash |
| PAC | Powdered activated carbon |
| PPy/SD | Polymerpyrrole/sawdust |
| EDA-CS | Ethylenediamine modified chitosan |
| SC | Saccharomyces cerevisiae biosorbent |
References
- Selvam, T.; Inayat, A.; Schwieger, W. Reactivity and applications of layered silicates and layered double hydroxides. Dalton Trans. 2014, 43, 10365–10387. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Mianhui, L.; Plevert, J. Comparative studies on the synthesis of Na-magadiite, Na-kenyaite and RUB-18 phases. Clay Sci. 2006, 12, 25–30. [Google Scholar]
- Lagaly, G.; Beneke, K.; Weiss, A. Magadiite and H-magadiite: I. Sodium magadiite and some of its derivatives. Am. Mineral. 1975, 60, 642–649. [Google Scholar]
- Mokhtar, A.; Djelad, A.; Adjdir, M.; Zahraoui, M.; Bengueddach, A.; Sassi, M. Intercalation of hydrophilic antibiotic into the interlayer space of the layered silicate magadiite. J. Mol. Struct. 2018, 1171, 190–195. [Google Scholar] [CrossRef]
- Wang, S.F.; Lin, M.L.; Shieh, Y.N.; Wang, Y.R.; Wang, S. Organic modification of synthesized clay-magadiite. Ceram. Int. 2007, 33, 681–685. [Google Scholar] [CrossRef]
- Macedo, T.R.; Petrucelli, G.C.; Airoldi, C. Silicic acid magadiite as a host for N-alkyldiamine guest molecules and features related to the thermodynamics of intercalation. Clays Clay Miner. 2007, 55, 151–159. [Google Scholar] [CrossRef]
- Paz, G.L.E.; Munsignatti, C.O.; Pastore, H.O. Novel catalyst with layered structure: Metal substituted magadiite. J. Mol. Catal. A Chem. 2016, 422, 43–50. [Google Scholar] [CrossRef]
- Vieira, R.B.; Moura, P.A.S.; Vilarrasa-Garcia, E.; Azevedo, D.C.S.; Pastore, H.O. Polyamine-Grafted Magadiite: High CO2 Selectivity at Capture from CO2/N2 and CO2/CH4 Mixtures. J. CO2 Util. 2017, 23, 29–41. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, Q.S.; Gao, S.N.; Jiang, H.M.; Meng, C.G. Intercalation and in situ formation of coordination compounds with ligand 8-hydroxyquinoline-5-sulfonic acid in the interlayer space of layered silicate magadiite by solid-solid reactions. Microp. Mesop. Mater. 2018, 266, 14–23. [Google Scholar] [CrossRef]
- Yufeng, C.; Bao, Y.; Yan, Z. Intercalation of Tb into magadiite and characterization of Tb-intercalated magadiites. Clay Miner. 2016, 51, 697–706. [Google Scholar]
- Mokhtar, A.; Djelad, A.; Boudia, A.; Sassi, M.; Bengueddach, A. Preparation and characterization of layered silicate magadiite intercalated by Cu2+ and Zn2+ for antibacterial behavior. J. Porous Mater. 2017, 24, 1627–1636. [Google Scholar] [CrossRef]
- Kooli, F.; Kiyozumi, Y.; Mizukami, F. Novel Layered silicate and microporous silica materials in the Na-magadiite-H2O-(TMA)2O system. New J. Chem. 2001, 25, 1613–1620. [Google Scholar]
- Lv, T.M.; Zhang, S.L.; Feng, Z.; Wang, F.S.; Zhang, S.Q.; Zheng, J.Q.; Liu, X.; Meng, C.G.; Wang, Y. Synthesis of zeolite omega by the magadiite conversion method and insight into the changes of medium-range structure during crystallization. Cryst. Growth Des. 2017, 17, 3940–3947. [Google Scholar] [CrossRef]
- Pires, C.T.; Oliveira, N.G., Jr.; Airoldi, C. Structural incorporation of titanium and/or aluminum in layered silicate magadiite through direct synthesis. Mater. Chem. Phys. 2012, 135, 870–879. [Google Scholar] [CrossRef]
- Novodarszki, G.; Valyon, J.; Illes, A.; Dobe, S.; Mihalyi, M.R. Synthesis and characterization of Al-magadiite and its catalytic behavior in 1,4-pentanediol dehydration. React. Kinet. Mech. Catal. 2017, 121, 275–292. [Google Scholar] [CrossRef]
- Maeno, Z.; Mitsudomem, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Selective C–C Coupling Reaction of Dimethylphenol to Tetramethyldiphenoquinone Using Molecular Oxygen Catalyzed by Cu Complexes Immobilized in Nanospaces of Structurally-Ordered Materials. Molecules 2015, 20, 3089–3106. [Google Scholar] [CrossRef] [PubMed]
- Zebib, B.; Lambert, J.F.; Blanchard, J.; Breysse, M. LRS-1: A New Delaminated Phyllosilicate Material with High Acidity. Chem. Mater. 2006, 18, 34–40. [Google Scholar] [CrossRef]
- Moura, H.M.; Bonk, F.A.; Pastore, H.O. Pillaring cetyltrimethylammonium-magadiite: A stepwise method to mesoporous materials with controlled pores sizes and distribution. Eur. J. Miner. 2012, 24, 903–912. [Google Scholar] [CrossRef]
- Ma, J.; Cui, B.; Li, D. Mechanism of adsorption of anionic dye from aqueous solutions onto organobentonite. J. Hazard. Mater. 2011, 186, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Middea, A.; Spinelli, L.S.; Souza, F.G., Jr.; Neumann, R.; Fernandes, T.L.A.P.; Gomes, O.F.M. Preparation and characterization of an organo-palygorskite-Fe3O4 nanomaterial for removal of anionic dyes from wastewater. Appl. Clay Sci. 2017, 139, 45–53. [Google Scholar] [CrossRef]
- Ghavami, M.; Zhao, Q.; Javadi, S.; Jangam, J.S.D.; Jasinski, J.B.; Saraei, N. Change of organobentonite interlayer microstructure induced by sorption of aromatic and petroleum hydrocarbons—A combined study of laboratory characterization and molecular dynamics simulations. Colloids Surf. A 2017, 520, 324–334. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Sun, Z.; Zheng, S.; Xi, Y. Structures of nonionic surfactant modified montmorillonites and their enhanced adsorption capacities towards a cationic organic dye. Appl. Clay. Sci. 2017, 148, 1–10. [Google Scholar] [CrossRef]
- Hamoudi, S.; Yang, Y.; Moudrakovski, I.; Lang, S.; Sayari, A. Synthesis of Porous Organosilicates in the Presence of Alkytrimethylammonium Chlorides: Effect of the Alkyl Chain Length. J. Phys. Chem. B 2001, 105, 9118–9123. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.G.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Kooli, F.; Qin, L.S.; Kiat, Y.Y.; Weirong, Q.; Hian, P.C. Effect of hexadecyltrimethylammonium (C16TMA) counteranions on the intercalation properties of different montmorillonites. Clay Sci. 2006, 12, 325–330. [Google Scholar]
- Kooli, F.; Khimyak, Y.Z.; Alshahateet, S.F.; Chen, F. Effect of the acid activation levels of montmorillonite clay on the cetyltrimethylammonium cations adsorption. Langmuir 2005, 21, 8717–8723. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Mianhui, L.; Alshahateet, S.F.; Fengxi, C.; Zhu, Y. Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium (C16TMA) surfactants. J. Phys. Chem. Solids 2006, 67, 926–931. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L. Thermal stable cetyl trimethylammonium-magadiites: Influence of the surfactant solution type. J. Phys. Chem. C 2009, 113, 1947–1952. [Google Scholar] [CrossRef]
- Royer, B.; Natali, F.C.; Lima, E.C.; Macedo, T.R.; Airoldi, C. Sodic and Acidic Crystalline Lamellar Magadiite Adsorbents for the Removal of Methylene Blue from Aqueous Solutions: Kinetic and Equilibrium Studies. Sep. Sci. Technol. 2010, 45, 129–141. [Google Scholar] [CrossRef]
- Royer, N.F.; Cardoso, E.C.; Lima, T.R.; Macedo, C.; Airoldi, A. Useful organofunctionalized layered silicate for textile dye removal. J. Hazard. Mater. 2010, 181, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.L.; Pinto, A.A.J.; Souza, A.; Airoldi, C.; Viana, R.R. Kinetic and thermodynamic uranyl (II) adsorption process into modified Na-Magadiite and Na-Kanemite. J. Hazard. Mater. 2009, 166, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M. Application of synthetic layered sodium silicate magadiite nanosheets for environmental remediation of methylene blue dye in water. Materials 2017, 10, 760. [Google Scholar] [CrossRef] [PubMed]
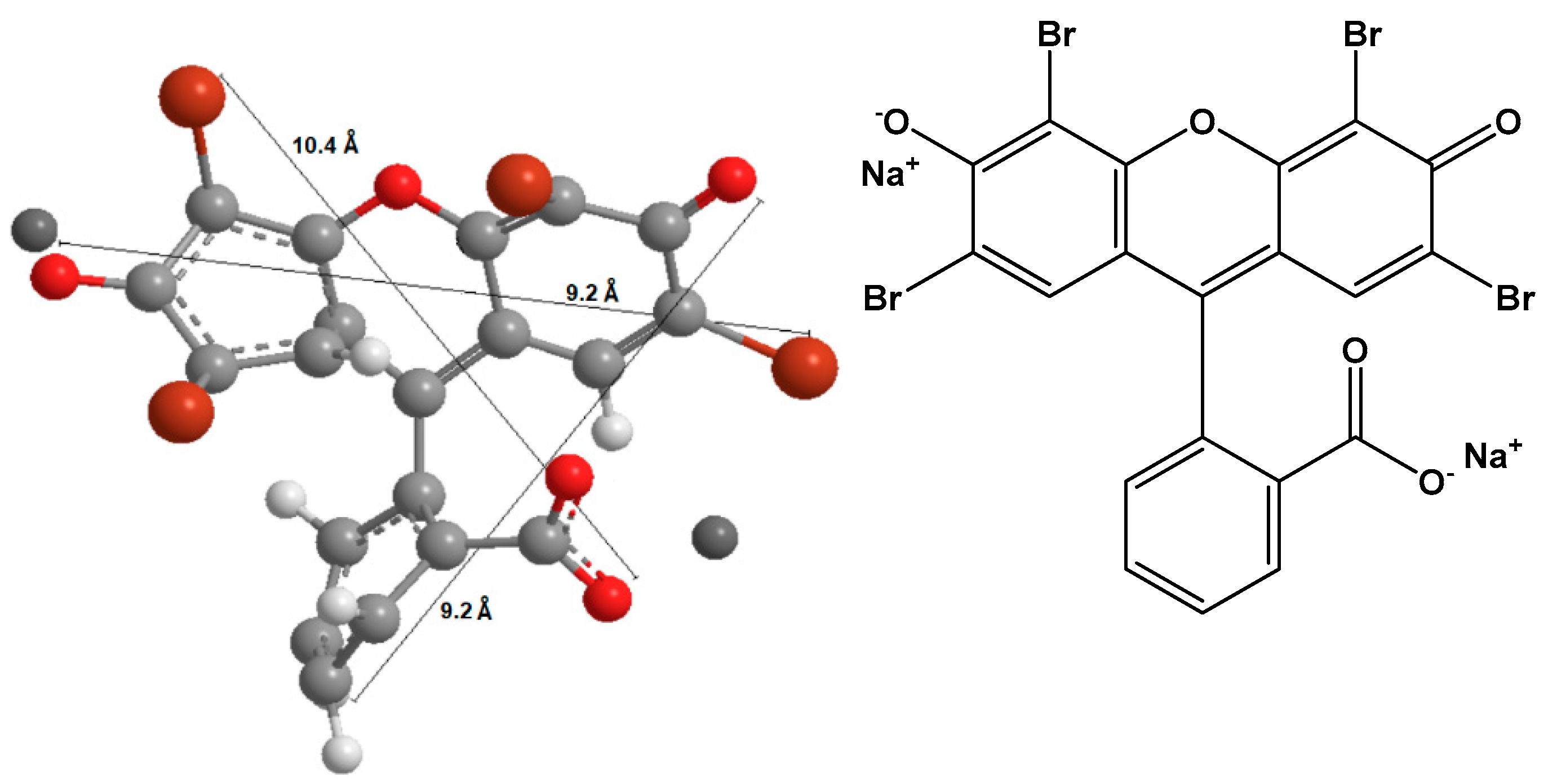
- Cooksey, C.J. Quirks of dye nomenclature. 10. Eosin Y and its close relatives. Biotech. Histochem. 2018, 93, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al-Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
- Ramos-Vianna, M.M.G.; Dweck, J.; Kozievitch, F.J.; Valenzuela-Diaz, F.R.; Buchler, P.M. Characterization and study of sorptive properties of differently prepared organoclays from a Brazilian natural bentonite. J. Therm. Anal. Calorim. 2005, 82, 595–602. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Characterization of organo-kenyaites: Thermal stability and their effects on eosin removal characteristics. Clay Miner. 2018, 53, 91–104. [Google Scholar] [CrossRef]
- Yukutake, H.; Kobayashi, M.; Otsuka, H.; Takahara, A. Thermal Degradation Behavior of Polystyrene/Magadiite Nanocomposites Prepared by Surface-initiated Nitroxide-Mediated Radical Polymerization. Polym. J. 2009, 41, 555–561. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, S.F.; Chang, L.C. Hydrothermal synthesis of magadiite. Appl. Clay Sci. 2006, 33, 73–77. [Google Scholar] [CrossRef]
- Moura, A.O.; Prado, A.G. Effect of thermal dehydration and rehydration on Na-magadiite structure. J. Colloid Interface Sci. 2009, 330, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, D.D.; Pabst, J.; Han, Z.; Wang, J.; Wilkie, C.A. Polystyrene magadiite nanocomposites. Polym. Eng. Sci. 2004, 44, 1122. [Google Scholar] [CrossRef]
- Kooli, F. Exfoliation Properties of acid-activated montmorillonites and their resulting organoclays. Langmuir 2009, 25, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y.; Hosaka, N.; Osada, S.; Terasawa, T.; Shimojima, A.; Kuroda, K. Interlayer Condensation of Protonated Layered Silicate Magadiite through Refluxing in N-Methylformamide. Bull. Chem. Soc. Jpn. 2015, 88, 1241–1249. [Google Scholar] [CrossRef]
- Vidal, N.; Volzone, C. Influence of organobentonite structure on toluene adsorption from water solution. Mater. Res. 2012, 15, 944–953. [Google Scholar] [CrossRef]
- Peng, S.; Gao, Q.; Wang, Q.; Shi, J. Layered Structural Heme Protein Magadiite Nanocomposites with High Enzyme-like Peroxidase Activity. Chem. Mater. 2004, 16, 2675–2684. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, R. Surface structure of CTMA+ modified bentonite and their sportive characteristics towards organic compounds. Colloids Surf. A 2008, 320, 19–24. [Google Scholar] [CrossRef]
- Thiesen, P.H.; Beneke, K.; Lagaly, G. Silylation of a crystalline silicic acid: An MAS NMR and porosity study. J. Mater. Chem. 2002, 13, 3010–3015. [Google Scholar] [CrossRef]
- Wang, L.Q.; Liu, J.; Exarhos, G.J.; Flanigan, K.Y.; Bordia, R. Conformation Heterogeneity and Mobility of Surfactant Molecules in Intercalated Clay Minerals Studied by Solid-State NMR. J. Phys. Chem. B 2000, 104, 2810–2816. [Google Scholar] [CrossRef]
- Gerstmans, A.; Urbanczyk, L.; Jérôme, R.; Robert, J.L.; Grandjean, J. XRD and NMR characterization of synthetic hectorites and the corresponding surfactant-exchanged clays. Clay Clays Miner. 2008, 43, 205–212. [Google Scholar] [CrossRef]
- He, H.; Frost, R.L.; Deng, F.; Zhu, J.; Wen, X.; Yuan, P. Conformation of surfactant molecules in the interlayer of montmorillonite studied by 13C MAS NMR. Clays Clay Miner. 2004, 52, 350–356. [Google Scholar] [CrossRef]
- Bi, Y.; Lambert, J.F.; Millot, Y.; Casale, S.; Blanchard, J.; Zeng, S.; Nie, H.; Li, D. Relevant parameters for obtaining high-surface area materials by delamination of magadiite, a layered sodium silicate. J. Mater. Chem. 2011, 45, 18403–18411. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Baskaralingam, P.; Pulikesi, M.; Elango, D.; Ramamurthi, V.; Sivanesan, S. Adsorption of acid dye onto organobentonite. J. Hazard. Mater. 2006, 128, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bezrodna, T.; Puchkovska, G.; Styopkin, V.; Baran, J.; Drozd, M.; Danchuk, V.; Kravchuk, V. IR-study of thermotropic phase transitions in cetyltrimethylammonium bromide powder and film. J. Mol. Struct. 2010, 973, 47–55. [Google Scholar] [CrossRef]
- Atar, N.; Olgun, A.; Colak, F. Thermodynamic, Equilibrium and Kinetic Study of the Biosorption of Basic Blue 41 using Bacillus maceran. Eng. Life Sci. 2008, 8, 499–506. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Al-Faze, R.; Kooli, F. Eosin removal properties of organo-local clay from aqueous solution. Orient. J. Chem. 2014, 30, 675–680. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Adsorption of Acid Blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite. J. Colloid Interface Sci. 2004, 280, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Ahmadishoar, J.; Bahrami, S.H.; Movassagh, B.; Amirshahi, S.H.; Arami, M. Removal of disperse blue 56 and disperse red 135 dyes from aqueous dispersions by modified montmorillonite nanoclay. Chem. Ind. Chem. Eng. Q 2017, 23, 21–29. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.; Frost, R.L. Characterisation of Organoclays and Adsorption of p-Nitrophenol: Environmental Application. J. Colloid Interface Sci. 2011, 360, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Jovic-Jovicic, N.; Milutinovic-Nikolic, A.; Grzetic, I.; Jovanovic, D. Organobentonite as efficient textile dye sorbent. Chem. Eng. Technol. 2008, 31, 567–574. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Appl. Clay Sci. 2002, 22, 55–63. [Google Scholar] [CrossRef]
- Onal, M.; Sarikaya, Y. Some physicochemical properties of partition nanophase formed in sportive organoclays. Colloids Surf. A 2007, 296, 216–221. [Google Scholar] [CrossRef]
- Hattacharyya, K.G.; Sarma, A. Adsorption characteristics of the dye, brilliant green, on neem leaf powder. Dyes Pigment. 2003, 57, 211–222. [Google Scholar] [CrossRef]
- Borisover, M.; Bukhanovsky, N.; Lapides, I.; Yariv., S. Mild pre-heating of organic cation-exchanged clays enhances their interactions with nitrobenzene in aqueous environment. Adsorption 2010, 16, 223–232. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bello, O.S.; Olusegun, O.A.; Njoku, V.O. Fly ash; an alternative to powdered activated carbon for the removal of eosin dye from aqueous solutions. Bull. Chem. Soc. Ethiop. 2013, 27, 191–204. [Google Scholar] [CrossRef]
- Thabet, M.S.; Ismaiel, A.M. Sol-gel γ-Al2O3 nanoparticles assessment of the removal of eosin yellow using: Adsorption, kinetic and thermodynamic parameters. J. Encapsulation Adsorpt. Sci. 2016, 6, 70–90. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Ikudayisi, V.A. The kinetics of eosin yellow removal from aqueous solution using pineapple peels. Edorium J. Waste Manag. 2017, 2, 5–11. [Google Scholar]
- Huang, X.Y.; Bin, J.P.; Bu, H.T.; Jiang, G.B.; Zeng, M.H. Removal of anionic dye eosin Y from aqueous solution usingethylenediamine modified chitosan. Carbohydr. Polym. 2011, 84, 1350–1356. [Google Scholar] [CrossRef]
- Ansari, R.; Mosayebzadeh, Z. Removal of Eosin Y, an anionic dye, from aqueous solutions using conducting electroactive polymers. Iran. Polym. J. 2010, 19, 541–551. [Google Scholar]
- Chatterjee, S.S.; Chatterjee, B.P.; Das, A.R.; Guha, A.R. Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. J. Colloid Interface Sci. 2005, 288, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bahramifar, N.; Tavasolli, M.; Younesi, H. Removal of eosin Y and eosin B dyes from polluted water through biosorption using Saccharomyces cerevisiae: Isotherm, kinetic and thermodynamic studies. J. Appl. Res. Water Wastewater 2015, 2, 108–114. [Google Scholar]
- Shahadat, M.M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| Sample | C% | H% | N% | Intercalated C16TMA Amount (mmol/g) |
|---|---|---|---|---|
| C16Mag-20 | 4.04 | 1.09 | 0.06 | 0.17 |
| C16Mag-40 | 8.82 | 2.78 | 0.21 | 0.38 |
| C16Mag-80 | 15.40 | 3.80 | 0.73 | 0.67 |
| C16Mag-120 | 22.20 | 4.82 | 1.11 | 0.97 |
| Samples | SBET (m2/g) | TPV * (cc/g) | APD + (nm) |
|---|---|---|---|
| Na-mag | 35 | 0.082 | 10.25 |
| C16Mag-20 | 23 | 0.068 | 11.80 |
| C16Mag-40 | 20 | 0.062 | 12.10 |
| C16Mag-80 | 15 | 0.054 | 13.09 |
| C16Mag-120 | 13 | 0.042 | 12.58 |
| Samples | qmax (mgg−1) | KL (Lg−1) | R2 |
|---|---|---|---|
| Na-Mag | 3.47 | 0.006 | 0.931 |
| C16Mag-20 | 25.06 | 0.116 | 0.9943 |
| C16Mag-40 | 42.54 | 0.093 | 0.9961 |
| C16Mag-80 | 63.06 | 0.0562 | 0.9943 |
| C16Mag-120 | 80.65 | 0.0712 | 0.9965 |
| C16Mag-80 (100) * | 62.12 | 0.0562 | 0.9913 |
| C16Mag-80 (150) * | 59.23 | 0.0551 | 0.9856 |
| C16Mag-80 (200) * | 54.00 | 0.0284 | 0.9946 |
| C16Mag-80 (215) * | 44.62 | 0.0216 | 0.9875 |
| C16Mag-80 (250) * | 33.43 | 0.0207 | 0.9665 |
| Samples | qmax (mg/g) | References |
|---|---|---|
| Organo-kenyaites | 48.01 | [36] |
| Organo-magadiites | 63.06–80.65 | [This study] |
| RFA | 43.48 | [67] |
| PAC | 62.28 | [67] |
| Alumina nanoparticles | 47.78 | [68] |
| Organo-local clays | 48.66 | [56] |
| Pineapple peels | 12.49 | [69] |
| EDA-CS | 294.12 | [70] |
| PPy/SD | 5.70 | [71] |
| Chitosan hydrobeads | 76.00 | [72] |
| SC | 200.00 | [73] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Hassani, H.O.; Ibrahim, S.M.; Al-Faze, R. Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration. Molecules 2018, 23, 2280. https://doi.org/10.3390/molecules23092280
Kooli F, Liu Y, Abboudi M, Rakass S, Hassani HO, Ibrahim SM, Al-Faze R. Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration. Molecules. 2018; 23(9):2280. https://doi.org/10.3390/molecules23092280
Chicago/Turabian StyleKooli, Fethi, Yan Liu, Mostafa Abboudi, Souad Rakass, Hicham Oudghiri Hassani, Sheikh Muhammad Ibrahim, and Rawan Al-Faze. 2018. "Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration" Molecules 23, no. 9: 2280. https://doi.org/10.3390/molecules23092280
APA StyleKooli, F., Liu, Y., Abboudi, M., Rakass, S., Hassani, H. O., Ibrahim, S. M., & Al-Faze, R. (2018). Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration. Molecules, 23(9), 2280. https://doi.org/10.3390/molecules23092280