Abstract
Parkinson’s disease (PD) is a chronic, progressive, and age-related neurodegenerative disorder characterized by the loss of midbrain dopaminergic neurons caused by the accumulation of free radicals and oxidative stress. Based on the neuroprotective properties of 2-pyrazoline derivatives, in the current work, 1-(phenyl/4-substituted phenyl)-3-(2-furanyl/thienyl)-5-aryl-2-pyrazolines (3a–i, 4a–i) were synthesized via the cyclization of the chalcones (1, 2) with suitable phenylhydrazine hydrochloride derivatives. All these compounds were investigated for their neuroprotective effects using an in vitro 6-hydroxydopamine (6-OHDA)-induced neurotoxicity model of PD in the rat pheochromocytoma (PC-12) Adh cell line. In addition, some different pharmacokinetic parameters of all compounds were in silico predicted by the QikProp module of Schrödinger’s Maestro molecular modeling package. 4-Methylsulfonylphenyl substituted compounds 3h (20%) and 4h (23%) were determined as the most promising neuroprotective agents related to their inductive roles in cell viability when compared with the 6-OHDA-positive control group (43% and 42%, respectively). Moreover, in silico pharmacokinetic results indicated that all compounds were within the acceptable range intended for human use. According to both in vitro and in silico studies, compounds 3h and 4h draw attention as potential orally bioavailable therapeutic drug candidates against neurodegeneration in PD.
1. Introduction
Neurodegenerative diseases (NDDs) are rapidly rising in prevalence in many countries and are highly linked to the expected aging of the population, because these disorders mainly occur in the elderly. NDDs can be classified according to extrapyramidal and pyramidal movement problems or cognitive and behavioral problems. The most common forms of NDDs are Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and dementia with Lewy bodies (DLB) [1,2,3,4]. These diseases reveal common pathological properties such as the formation of insoluble protein-based aggregates in deteriorated neurons and glial cells, whereas there is a big difference in clinical features of patients suffering from them [5].
PD, firstly described as “paralysis agitans” by James Parkinson in 1817, is the second most common NDD affecting 2–3% of those >65 years of age [6,7]. The cause of PD is not clear, but the defects in mitochondrial functions and brain iron regulation, inflammation, and energy metabolism problems have been proven to be substantial in underlying mechanisms [8]. It is neuropathologically characterized by the loss of progressive dopaminergic neurons in the substantia nigra pars compacta and the accumulation of aggregated α-synuclein protein forming intracellular Lewy bodies in affected regions [8,9]. Moreover, the loss of dopaminergic neurons is associated with the decrease in the activity of catalase enzymes and the increase of monoamine oxidase-B (MAO-B) in glial cells. These enzymatic changes lead to emerging oxidative stress through the formation of higher levels of quinones, peroxides, and other reactive oxygen species (ROS), which then contribute to lipid and protein peroxidation and finally to neuronal death [8,9,10,11].
The cardinal clinical features of PD patients are motor impairment, including resting tremors, muscular rigidity, bradykinesia, and postural instability. Furthermore, these patients also mainly have speech and swallowing difficulties, a masklike facial expression, and micrographia [12,13]. Approved treatment procedures for PD to date, such as pharmacotherapy and neurosurgery, have mainly focused on the recovery of these clinical symptoms. However, recently a major approach for the treatment is to discover new beneficial, potential, and disease-modifying drugs targeting the underlying mechanisms related to the neurodegenerative process of PD [14,15,16].
The main aspect of oxidative stress in PD has been investigated with toxin-based models such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA). 6-OHDA (Figure 1) is a neurotoxin, which is capable of generating ROS in neurons, and is detected in the urine of PD patients treated with long-term L-3,4-dihydroxyphenylalanine (L-DOPA) (Figure 1), which is the natural precursor of dopamine (Figure 1) and the typically prescribed drug for the symptomatic treatment of PD. Neurodegeneration with a 6-OHDA-based model can mimic idiopathic PD pathogenesis in vitro, as well as in vivo. The primary advantages of this model are measurable motor deficit (rotation) and proven usage in the pharmacological screening of compounds effective on dopamine and its receptors [17,18,19,20].
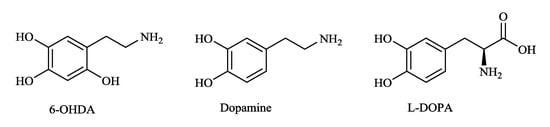
Figure 1.
Chemical structures of 6-hydroxydopamine (6-OHDA), dopamine, and L-3,4-dihydroxyphenylalanine (L-DOPA).
Rat pheochromocytoma (PC-12) cells have been widely used for in vitro studies exploring the mechanisms of NDDs. PC-12 Adh is a rat pheochromocytoma-derived cell-line, which responds to nerve growth factor (NGF) by switching from an immature chromaffin-cell-like phenotype to a sympathetic-neuron-like one. Therefore, this cell line has been widely used as an in vitro assay system for screening the neuroprotective effects of compounds [21,22,23,24].
Pyrazoline is a five-membered heterocyclic ring bearing two adjacent nitrogen atoms and one endocyclic double bond. It has three tautomeric forms called as 1-pyrazoline, 2-pyrazoline, and 3-pyrazoline, but among them, 2-pyrazoline is the most common one. 2-Pyrazoline-based compounds possess a wide range of pharmacological applications including antiepileptic, antidepressant, and anti-neurodegenerative activities. Moreover, these compounds can cross the blood–brain barrier (BBB) easily by means of their lipophilic characters [25,26,27,28].
Having the above aspects in mind, a series of 2-pyrazoline-based compounds 3a–i and 4a–i were designed and synthesized via the reaction of chalcones (1, 2). All the compounds were in vitro evaluted for their neuroprotective potentials against 6-OHDA-induced neurotoxicity in PC-12 Adh cells. In addition, in silico absorption, distribution, metabolism, and excretion (ADME) studies were performed to predict their physicochemical properties particularly their abilities to cross the BBB.
2. Results and Discussion
The synthesis of new pyrazoline derivatives (3a–i, 4a–i) followed the general pathway depicted in Scheme 1. Initially, 1,3-diaryl-substituted chalcones (1, 2) were synthesized via the base-catalyzed Claisen–Schmidt condensation of 2-acetylfuran/2-acetylthiophene with the appropriate aromatic aldehydes [29,30,31,32]. Then, the final compounds, 1-(phenyl/4-substituted phenyl)-3-(2-furanyl/thienyl)-5-aryl-2-pyrazolines (3a–i, 4a–i), were obtained via the cyclization of the chalcones (1, 2) with suitable phenylhydrazine hydrochloride derivatives in the presence of hot acetic acid [33].
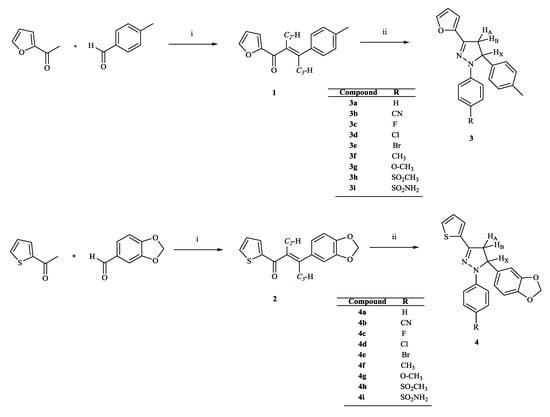
Scheme 1.
The synthetic route for the preparation of the compounds (1, 2, 3a–i, 4a–i). Reagents and conditions: (i) 40% (w/v) sodium hydroxide, ethanol, rt, 24 h; (ii) appropriate phenylhydrazine hydrochloride derivative, CH3COOH, reflux, 8 h.
The structures of all the compounds were elucidated by FTIR, 1H NMR, 13C NMR, mass spectral data, and elemental analyses. In the 1H NMR spectra of the compounds, 3a–i and 4a–i, the CH2 protons of the 2-pyrazoline ring resonated as a pair of doublets of doublets at 2.95–3.20 ppm (HA) (JAM = 17.13–17.64 Hz, JAX = 4.65–7.41 Hz) and 3.69–3.96 ppm (HM) (JMA = 17.18–17.64 Hz, JMX = 11.85–12.09 Hz). The CH proton appeared as a doublet of doublets at 5.29–5.59 ppm (HX) (JMX = 11.76–12.09 Hz, JAX = 4.68–7.38 Hz) owing to the vicinal coupling with two magnetically non-equivalent protons of the methylene group at the 4th position of the pyrazoline ring. All the other aromatic and aliphatic protons were observed in the expected regions. Mass spectral data and elemental analysis were also in agreement with the proposed structures of the compounds [33,34].
According to the IC50 values given in Table 1, apart from compound 1, treatment with the compounds at 100 μg/mL did not reduce cell viability significantly. The compounds demonstrated their protective effects against cell death in differentiated PC-12 Adh cells treated with 6-OHDA (150 μM) for 24 h. The reduction in cell viability was determined as 43% with 6-OHDA (150 μM) and 36, 29, 35, 37, 30, 31, 29, 20, 30% with compounds 3a–i (100 µg/mL), respectively (Figure 2; Table 2). However, compound 1 was found to be highly cytotoxic, and it reduced cell viability with the values of 97% (100 µg/mL) and 40% (10 µg/mL). In addition, the reduction in cell viability was evaluated as 34.1% with compound 2 and 59, 54, 76, 56, 41, 62, 69, 23, 87% with compounds 4a–i (100 µg/mL), respectively, when compared with 6-OHDA (150 μM) (42.13%) (Figure 2; Table 2). According to these results, all the compounds reduced cell viability compared with the control group after 24 h 6-OHDA exposure (* p < 0.05, *** p < 0.001, **** p < 0.0001 for compounds 1 and 3a–i; **** p < 0.0001 for compounds 2 and 4a–i). Compounds 3a–i, 2, and 4h might have neuroprotective potential against 6-OHDA induced neurotoxicity in PC-12 Adh cells but only the induction in cell viability percentage with compounds 3h and 4h was found significant compared with the 6-OHDA-positive control group related to the statistical analysis (** p < 0.01). This outcome pointed out that the presence of 4-methylsulfonylphenyl moiety enhanced the neuroprotective potency of the 2-pyrazoline ring as observed in both compounds 3h and 4h.

Table 1.
IC50 values of compounds 1, 2, 3a–i and 4a–i and 6-OHDA according to the cell viability assay.
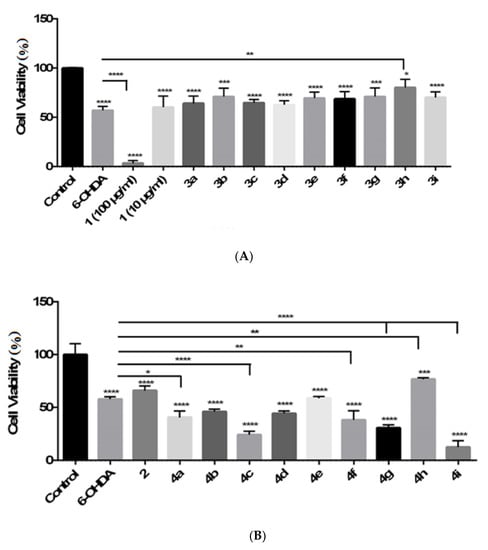
Figure 2.
Neuroprotective effects of compounds 1 and 3a–i (A); 2 and 4a–i (B) against 6-OHDA induced neurotoxicity. PC-12 Adh cells were treated with 100 µg/mL concentration of the compounds for 6 h before exposure to 150 µM 6-OHDA for 24 h. The cell viability was detected at the 24th hour. The results of the cell viability were presented as a % of the control (the O.D. value). Data are shown as mean ± SD of three experiments. ((n = 8), p > 0.05 n.s., * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001).

Table 2.
Cell viability values of 6-OHDA and compounds 1, 2, 3a–i, 4a–i according to the cell viability assay results. The results of the cell viability were presented as a % of the control (the O.D. value).
ADME properties of compounds 1, 2, 3a–i, and 4a–i were in silico assessed to enlighten the biological, pharmaceutical, and drug similarities of these compounds. The results given in Table 3 were found to be within the acceptable range intended for human use, making these derivatives promising drug candidates [35]. All these compounds were detected to exhibit excellent absorption % (92–100%) in human oral absorption on a 0–100% scale, and based on the parameters of the brain/blood partition coefficient (QPlogBB) (−1.08 to 0.78) and central nervous system (CNS) activity (−1 to 0), they were also found to pass through the BBB, which restrains drug entry from blood into brain by multiple mechanisms [36,37]. Solvent accessible surface area (SASA) is used to define the accessibility of the residue to the solvent; either it is between lipid or water accessibility, and it is essential to BBB permeability [38]. The SASA values of all the compounds were found within the range (467–680). The results also indicated that all the compounds obey Lipinski’s rule of five and Jorgensen’s rule of three; due to the fact that a potential orally active drug candidate should reveal no more than one violation of these rules [39,40].

Table 3.
Predicted ADME properties of compounds 1, 2, 3a–i, and 4a–i.
3. Materials and Methods
3.1. Chemistry
All reagents were purchased from commercial suppliers and were used without further purification. Melting points (M.p.) were determined on an Electrothermal 9100 melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. IR spectra were recorded on an IRPrestige-21 Fourier Transform Infrared spectrophotometer (Shimadzu, Tokyo, Japan). 1H NMR and 13C NMR spectra were recorded by a Bruker digital FT-NMR spectrometer (Bruker Bioscience, Billerica, MA, USA) in DMSO-d6. Mass spectra were recorded on an Agilent LC-MSD-Trap-SL Mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyzer (Perkin-Elmer, Norwalk, CT, USA) and the results were within ±0.4% of the theoretical values. Thin Layer Chromatography (TLC) was performed on TLC Silica gel 60 F254 aluminium sheets (Merck, Darmstadt, Germany) to check the purity of the compounds.
3.1.1. General Procedure for the Synthesis of the Compounds
1-(2-Furanyl/thienyl)-3-aryl-2-propen-1-one (1, 2)
2-Acetylfuran/2-acetylthiophene (0.02 mol), proper aromatic aldehyde (0.02 mol) and 40% (w/v) sodium hydroxide (5 mL) in ethanol (30 mL) were stirred at room temperature for 24 h. Then, the reaction mixture was poured into ice and the precipitated solid was filtered, washed with water, and dried. The product was crystallized from ethanol [29,30,31,32,33,34].
1-(2-Furanyl)-3-aryl-2-propen-1-one (1). Yield: 55%. M.p.: 111–113 °C. Lit. M.p.: 96–98 °C [41]. IR νmax (cm−1): 3126.61 (aromatic C-H stretching), 2927.94 (aliphatic C-H stretching), 1651.07 (C=O stretching), 1597.06, 1558.48, 1512.19, 1462.04 (C=C stretching), 1411.89, 1392.61, 1332.81, 1300.02, 1288.45, 1247.94, 1207.44, 1182.36, 1161.15, 1083.99, 1053.13, 1010.70 (C-O stretching and aromatic C-H in plane bending), 981.77, 927.76, 883.40, 864.11, 842.89, 813.96, 802.39, 765.74, 727.16 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.35 (3H, s, CH3), 6.79 (1H, dd, J = 3.57 Hz, 1.71 Hz aromatic proton), 7.28 (2H, d, J = 7.92 Hz, aromatic protons), 7.65 (1H, d, J = 15.72 Hz, C3-H), 7.70 (1H, d, J = 8.76 Hz, aromatic proton), 7.74–7.76 (2H, m, C2-H and aromatic proton), 7.81 (1H, dd, J = 3.60 Hz, 0.63 Hz, aromatic proton), 8.07 (1H, dd, J = 1.65 Hz, 0.66 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.54 (CH3), 113.16 (CH), 119.79 (CH), 121.37 (C2-H), 129.52 (2CH), 130.03 (2CH), 132.19 (C), 141.20 (C), 143.30 (C3-H), 148.74 (CH), 153.46 (C), 177.15 (C, C=O). Anal. Calcd. for C14H12O3: C, 79.22; H, 5.70; Found: C, 79.12; H, 5.64. MS (ESI) (m/z): [M + H]+ 212.08.
1-(2-Thienyl)-3-aryl-2-propen-1-one (2). Yield: 60%. M.p.: 126–128 °C. Lit. M.p.: 117–119 °C [35,42]. IR νmax (cm−1): 3105.39 (aromatic C-H stretching), 2989.66, 2906.73 (aliphatic C-H stretching), 1643.55 (C=O stretching), 1604.77, 1583.56, 1498.69, 1487.12, 1444.68 (C=C stretching), 1411.89, 1369.46, 1346.31, 1305.81, 1246.02, 1228.66, 1215.15, 1192.01, 1105.21, 1058.92, 1028.06 (C-O stretching and aromatic C-H in plane bending), 972.12, 925.83, 916.19, 860.25, 831.32, 800.46, 727.16, 700.16 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 6.12 (2H, s, O-CH2-O), 7.00 (1H, d, J = 8.01 Hz, aromatic proton), 7.30–7.36 (2H, m, aromatic protons), 7.64-7.69 (2H, m, C2-H and aromatic protons), 7.76 (1H, d, J = 15.51 Hz, C3-H), 8.04 (1H, dd, J = 4.92 Hz, 0.99 Hz, aromatic proton), 8.32 (1H, dd, J = 3.78 Hz, 0.99 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 102.15 (CH2), 107.42 (CH), 109.01 (CH), 120.35 (CH), 126.46 (C2-H), 129.29 (CH), 129.50 (C), 133.83 (CH), 135.70 (CH), 143.61 (C3-H), 146.26 (C), 148.58 (C), 150.10 (C), 182.00 (C, C=O). Anal. Calcd. for C14H10O3S: C, 65.10; H, 3.90; Found: C, 64.98; H, 3.84. MS (ESI) (m/z): [M + H]+ 258.04.
1-(Phenyl/4-Substituted phenyl)-3-(2-furanyl/thienyl)-5-aryl-2-pyrazolines (3a–i, 4a–i)
A mixture of appropriate chalcone (1, 2) (10.0 mmol) and phenylhydrazine hydrochloride derivative (20.0 mmol) was refluxed for 8 h in absolute ethanol (30 mL) in the presence of acetic acid (10 mL) to get 2-pyrazolines. Then the reaction mixture was poured into crushed ice. The precipitate was separated by filtration, washed with water and crystallized from methanol [33].
1-Phenyl-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3a) [43]. Yield: 67%. M.p.: 162–163 °C. IR νmax (cm−1): 3113.11, 3034.03 (aromatic C-H stretching), 2918.30, 2848.86 (aliphatic C-H stretching), 1595.13, 1498.69, 1487.12 (C=N and C=C stretching), 1413.82, 1365.60, 1334.74, 1319.31, 1247.94, 1176.58, 1128.36, 1072.42, 1051.20, 1033.85, 1022.27 (C-N, C-O stretching and aromatic C-H in plane bending), 997.20, 960.55, 923.90, 885.33, 869.90, 842.89, 813.96, 744.52, 731.02, 690.52, 673.16 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.98 (1H, dd, JAM = 17.28 Hz, JAX = 6.15 Hz, C4-HA pyrazoline), 3.82 (1H, dd, JMA = 17.28 Hz, JMX = 12.12 Hz, C4-HM pyrazoline), 5.40 (1H, dd, JMX = 12.09 Hz, JAX = 6.12 Hz, C5-HX pyrazoline), 6.61 (1H, dd, J = 3.42 Hz, 1.80 Hz, aromatic proton), 6.68–6.77 (2H, m, aromatic protons), 6.95 (2H, d, J = 8.72 Hz, aromatic protons), 7.10–7.16 (6H, m, aromatic protons), 7.80 (1H, dd, J = 1.71 Hz, 0.63 Hz). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 22.11 (CH3), 43.39 (CH2), 62.85 (CH), 111.12 (CH), 112.42 (CH), 113.45 (2CH), 119.05 (CH), 126.22 (2CH), 129.30 (2CH), 129.66 (C), 130.01 (2CH), 137.08 (CH), 139.66 (C, d, J = 3.75 Hz), 144.62 (2C, d, J = 6.00 Hz), 147.97 (C). Anal. Calcd. for C20H18N2O: C, 79.44; H, 6.00; N, 9.26; Found: C, 79.39; H, 6.04; N, 9.25. MS (ESI) (m/z): [M]+ 302.00, [M + H]+ 303.00.
1-(4-Cyanophenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3b). Yield: 55%. M.p.: 77–78 °C. IR νmax (cm−1): 3118.90 (aromatic C-H stretching), 2918.30 (aliphatic C-H stretching), 2212.35 (C≡N stretching), 1598.99, 1510.26 (C=N and C=C stretching), 1415.75, 1373.32, 1323.17, 1174.65, 1122.57, 1004.91 (C-N, C-O stretching and aromatic C-H in plane bending), 812.03, 740.67 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 3.06 (1H, dd, JAM = 17.58 Hz, JAX = 4.65 Hz, C4-HA pyrazoline), 3.90 (1H, dd, JMA = 17.49 Hz, JMX = 11.91 Hz, C4-HM pyrazoline), 5.58 (1H, dd, JMX = 11.76 Hz, JAX = 4.68 Hz, C5-HX pyrazoline), 6.64–6.65 (1H, m, aromatic proton), 6.90 (1H, d, J = 3.30 Hz, aromatic proton), 7.01 (2H, d, J = 8.73 Hz, aromatic protons), 7.07–7.16 (4H, m, aromatic protons), 7.54 (2H, d, J = 8.73 Hz, aromatic protons), 7.86 (1H, s, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.10 (CH3), 43.44 (CH2), 61.91 (CH), 99.40 (C), 112.66 (CH), 112.98 (CH), 113.25 (2CH), 120.39 (C), 126.04 (2CH), 130.19 (2CH), 133.76 (2CH), 137.50 (C), 138.56 (C), 142.89 (2C), 145.53 (CH), 146.89 (C). Anal. Calcd. for C21H17N3O: C, 77.04; H, 5.23; N, 12.84; Found: C, 77.06; H, 5.24; N, 12.82. MS (ESI) (m/z): [M + H]+ 327.90.
1-(4-Fluorophenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3c). Yield: 53%. M.p.: 87–89 °C. IR νmax (cm−1): 2987.74, 2900.94 (aliphatic C-H stretching), 1597.06, 1508.33 (C=N and C=C stretching), 1413.82, 1373.32, 1321.24, 1172.72, 1002.98 (C-N, C-O stretching and aromatic C-H in plane bending), 812.03, 742.59 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.98 (1H, dd, JAM = 17.25 Hz, JAX = 6.57 Hz, C4-HA pyrazoline), 3.82 (1H, dd, JMA = 17.25 Hz, JMX = 12.03 Hz, C4-HM pyrazoline), 5.37 (1H, dd, JMX = 11.97 Hz, JAX = 6.54 Hz, C5-HX pyrazoline), 6.61 (1H, dd, J = 3.36 Hz, 1.77 Hz, aromatic proton), 6.76 (1H, d, J = 3.33 Hz, aromatic proton), 6.93–6.99 (4H, m, aromatic protons), 7.15 (4H, s, aromatic protons), 7.80 (1H, d, J = 1.44 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.09 (CH3), 43.57 (CH2), 63.42 (CH), 111.20 (CH), 112.43 (CH), 114.65 (CH, d, J = 7.5 Hz), 115.84 (CH, d, J = 22.5 Hz), 126.32 (2CH), 130.04 (2CH), 137.18 (CH), 139.36 (CH), 139.93 (C), 141.56 (C), 144.70 (CH), 147.56 (C), 147.88 (C), 154.79 (C), 157.90 (C). Anal. Calcd. for C20H17FN2O: C, 74.98; H, 5.35; N, 8.74; Found: C, 74.94; H, 5.33; N, 8.76. MS (ESI) (m/z): [M + H]− 318.90, [M]+ 320.90, [M + H]+ 321.90.
1-(4-Chlorophenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3d). Yield: 83%. M.p.: 115–117 °C. IR νmax (cm−1): 2987.74, 2900.94 (aliphatic C-H stretching), 1598.99, 1492.90 (C=N and C=C stretching), 1375.25, 1076.28, 1051.20 (C-N, C-O stretching and aromatic C-H in plane bending), 817.82, 734.88 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 3.00 (1H, dd, JAM = 17.37 Hz, JAX = 5.85 Hz, C4-HA pyrazoline), 3.84 (1H, dd, JMA = 17.37 Hz, JMX = 12.06 Hz, C4-HM pyrazoline), 5.43 (1H, dd, JMX = 12.06 Hz, JAX = 5.82 Hz, C5-HX pyrazoline), 6.61 (1H, dd, J = 3.45 Hz, 1.80 Hz, aromatic proton), 6.79 (1H, d, J = 3.93 Hz, aromatic proton), 6.93 (2H, d, J = 7.94 Hz, aromatic protons), 7.13 (4H, s, aromatic protons), 7.17 (2H, d, J = 9.00 Hz, aromatic protons), 7.81 (1H, dd, J = 1.68 Hz, 0.63 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.08 (CH3), 43.49 (CH2), 62.75 (CH), 111.60 (CH), 112.48 (CH), 114.85 (2CH), 122.60 (C), 126.20 (2CH), 129.11 (2CH), 130.07 (2CH), 137.23 (C), 139.11 (C), 140.51 (C), 143.36 (C), 144.89 (CH), 147.73 (C). Anal. Calcd. for C20H17ClN2O: C, 71.32; H, 5.09; N, 8.32; Found: C, 71.28; H, 5.03; N, 8.35. MS (ESI) (m/z): [M + H]−− 334.90, [M]+ 336.90, [M + H]+ 337.90.
1-(4-Bromophenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3e). Yield: 79%. M.p.: 133–135 °C. IR νmax (cm−1): 2999.31, 2920.23 (aliphatic C-H stretching), 1593.20, 1512.19, 1492.90, 1483.26 (C=N and C=C stretching), 1365.60, 1319.31, 1122.57, 1087.85, 1074.35, 1051.20, 1004.91 (C-N, C-O stretching and aromatic C-H in plane bending), 883.40, 862.18, 815.89, 792.74, 732.95 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 3.00 (1H, dd, JAM = 17.37 Hz, JAX = 5.76 Hz, C4-HA pyrazoline), 3.83 (1H, dd, JMA = 17.34 Hz, JMX = 12.06 Hz, C4-HM pyrazoline), 5.43 (1H, dd, JMX = 12.03 Hz, JAX = 5.70 Hz, C5-HX pyrazoline), 6.61 (1H, dd, J = 3.42 Hz, 1.80 Hz, aromatic proton), 6.79 (1H, d, J = 3.39 Hz, aromatic proton), 6.88 (2H, d, J = 9.00 Hz, aromatic protons), 7.12 (4H, s, aromatic protons), 7.29 (2H, d, J = 8.97 Hz, aromatic protons), 7.81 (1H, t, J = 1.65 Hz, 1.08 Hz, 0.57 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.09 (CH3), 43.48 (CH2), 62.64 (CH), 110.21 (C), 111.65 (CH), 112.49 (CH), 115.35 (2CH), 126.19 (2CH), 130.07 (2CH), 131.94 (2CH), 137.24 (C), 139.05 (C), 140.57 (C), 143.67 (C), 144.91 (CH), 147.72 (C). Anal. Calcd. for C20H17BrN2O: C, 63.00; H, 4.49; N, 7.35; Found: C, 63.04; H, 4.53; N, 7.32. MS (ESI) (m/z): [M + H]−− 378.80, [M]+ 380.80, [M + H]+ 381.90, [M + H]+++ 383.00.
1-(4-Methylphenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3f). Yield: 53%; M.p. 67–68 °C. IR νmax (cm−1): 2972.31, 2900.94 (aliphatic C-H stretching), 1614.42, 1512.19 (C=N and C=C stretching), 1408.04, 1365.60, 1249.87, 1074.35, 1049.28 (C-N, C-O stretching and aromatic C-H in plane bending), 883.40, 804.32, 738.74 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.15 (3H, s, CH3), 2.24 (3H, s, CH3), 2.95 (1H, dd, JAM = 17.19 Hz, JAX = 6.36 Hz, C4-HA pyrazoline), 3.79 (1H, dd, JMA = 17.22 Hz, JMX = 12.09 Hz, C4-HM pyrazoline), 5.36 (1H, dd, JMX = 12.09 Hz, JAX = 6.33 Hz, C5-HX pyrazoline), 6.60 (1H, dd, J = 3.42 Hz, 1.80 Hz, aromatic proton), 6.72–6.74 (1H, m, aromatic proton), 6.83–6.86 (2H, m, aromatic protons), 6.94 (2H, d, J = 8.34 Hz, aromatic protons), 7.09–7.13 (4H, m, aromatic protons), 7.78 (1H, dd, J = 1.71 Hz, 0.63 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 20.54 (CH3), 21.08 (CH3), 43.34 (CH2), 63.13 (CH), 110.79 (CH), 112.39 (CH), 113.63 (2CH), 126.28 (2CH), 127.76 (CH), 128.80 (C), 129.73 (2CH, d, J = 16.50 Hz), 137.00 (C), 139.16 (C), 139.67 (CH), 142.51 (2C), 144.51 (CH), 148.08 (C). Anal. Calcd. for C21H20N2O: C, 79.72; H, 6.37; N, 8.85; Found: C, 79.66; H, 6.28; N, 8.89. MS (ESI) (m/z): [M]+ 316.90, [M + H]+ 318.00.
1-(4-Methoxyphenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3g). Yield: 27%; M.p. 70–72 °C. IR νmax (cm−1): 3118.90 (aromatic C-H stretching), 2918.30, 2831.50 (aliphatic C-H stretching), 1606.70, 1568.13, 1506.41, 1463.97 (C=N and C=C stretching), 1359.82, 1292.31, 1238.30, 1178.51, 1120.64, 1097.50, 1035.77 (C-N, C-O stretching and aromatic C-H in plane bending), 883.40, 873.75, 815.89, 746.45 (aromatic C-H out of plane bending). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.95 (1H, dd, JAM = 17.13 Hz, JAX = 7.14 Hz, C4-HA pyrazoline), 3.63 (3H, s, OCH3), 3.69 (1H, dd, JMA = 17.18 Hz, JMX = 12.00 Hz, C4-HM pyrazoline), 5.30 (1H, dd, JMX = 11.97 Hz, JAX = 7.17 Hz, C5-HX pyrazoline), 6.59 (1H, dd, J = 3.42 Hz, 1.80 Hz, aromatic proton), 6.71–6.77 (3H, m, aromatic protons), 6.86–6.90 (2H, m, aromatic protons), 7.12–7.18 (4H, m, aromatic protons), 7.78 (1H, dd, J = 1.71 Hz, 0.66 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.08 (CH3), 43.47 (CH2), 55.63 (CH3), 63.94 (CH), 106.80 (CH), 110.58 (CH), 112.36 (CH), 114.88 (2CH, d, J = 12.75 Hz), 126.42 (2CH), 127.34 (C), 130.01 (2CH, d, J= 8.25 Hz), 137.03 (C), 138.97 (C), 139.16 (C), 139.69 (CH), 144.41 (CH), 148.14 (C), 153.15 (C). Anal. Calcd. for C21H20N2O2: C, 75.88; H, 6.06; N, 8.43; Found: C, 75.90; H, 6.04; N, 8.50. MS (ESI) (m/z): [M]+ 332.00, [M + H]+ 333.00.
1-(4-Methylsulfonylphenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3h). Yield: 68%. M.p.: 162–163 °C. IR νmax (cm−1): 2985.81, 2900.94 (aliphatic C-H stretching), 1589.34, 1506.41 (C=N and C=C stretching), 1406.11, 1379.10, 1292.31, 1242.16, 1132.21, 1066.64 (C-N, C-O stretching and aromatic C-H in plane bending), 960.55, 883.40, 815.89, 769.60 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.24 (3H, s, CH3), 3.00 (1H, dd, JAM = 17.37 Hz, JAX = 5.76 Hz, C4-HA pyrazoline), 3.06 (3H, s, SO2CH3), 3.91 (1H, dd, JMA = 17.55 Hz, JMX = 11.97 Hz, C4-HM pyrazoline), 5.59 (1H, dd, JMX = 11.88 Hz, JAX = 4.74 Hz, C5-HX pyrazoline), 6.64 (1H, dd, J= 3.39 Hz, 1.77 Hz, aromatic proton), 6.89 (1H, d, J = 3.39 Hz, aromatic proton), 7.07 (2H, d, J = 8.88 Hz, aromatic protons), 7.14 (4H, s, aromatic protons), 7.65 (2H, d, J = 8.97 Hz, aromatic protons), 7.86 (1H, t, J = 1.59 Hz, 1.20 Hz, 0.39 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.10 (CH3), 43.45 (CH2), 44.62 (CH3), 61.98 (CH), 112.76 (2CH, d, J = 18.75 Hz), 113.67 (CH), 126.06 (2CH), 129.02 (2CH), 129.55 (CH), 129.85 (C), 130.20 (2CH), 137.46 (C), 139.63 (C), 142.64 (C), 145.45 (CH), 147.35 (2C, d, J = 8.25 Hz). Anal. Calcd. for C21H20N2O3S: C, 66.29; H, 5.30; N, 7.36; Found: C, 66.26; H, 5.34; N, 7.39. MS (ESI) (m/z): [M]+ 380.90, [M + H]+ 382.00.
1-(4-Sulfonamidophenyl)-3-(2-furanyl)-5-(4-methylphenyl)-2-pyrazoline (3i). Yield: 88%. M.p.: 139–140 °C. IR νmax (cm−1): 3369.64, 3257.77 (N-H stretching), 3120.82, (aromatic C-H stretching), 2920.23, 2852.72 (aliphatic C-H stretching), 1589.34, 1504.48 (C=N and C=C stretching), 1413.82, 1373.32, 1325.10, 1305.81, 1149.57, 1095.57, 1002.98 (C-N, C-O stretching and aromatic C-H in plane bending), 883.40, 866.04, 815.89, 738.74 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.24 (3H, s, CH3), 3.05 (1H, dd, JAM = 17.46 Hz, JAX = 4.92 Hz, C4-HA pyrazoline), 3.89 (1H, dd, JMA = 17.49 Hz, JMX = 12.00 Hz, C4-HM pyrazoline), 5.57 (1H, dd, JMX = 11.94 Hz, JAX = 4.83 Hz, C5-HX pyrazoline), 6.63 (1H, dd, J = 3.45 Hz, 1.80 Hz, aromatic proton), 6.85 (1H, d, J = 3.41 Hz, aromatic proton), 6.99–7.03 (4H, m, aromatic and NH2 protons), 7.12–7.13 (4H, m, aromatic protons), 7.57 (2H, d, J = 8.97 Hz, aromatic protons), 7.84 (1H, dd, J = 1.71 Hz, 0.66 Hz). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 21.08 (CH3), 43.33 (CH2), 62.06 (CH), 112.48 (2CH, d, J = 12.00 Hz), 126.12 (2CH), 127.59 (2CH), 130.11 (2CH), 133.51 (C), 137.35 (CH), 138.73 (2CH), 141.85 (C), 145.24 (2C), 146.22 (C), 147.47 (C). Anal. Calcd. for C20H19N3O3S: C, 62.97; H, 5.02; N, 11.02; Found: C, 62.94; H, 5.03; N, 11.05. MS (ESI) (m/z): [M]+ 381.90, [M + H]+ 382.90.
1-Phenyl-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4a) [44]. Yield: 83%. M.p.: 179–180 °C. IR νmax (cm−1): 3105.39, 3070.68 (aromatic C-H stretching), 2916.37 (aliphatic C-H stretching), 1593.20, 1498.69, 1481.33, 1442.75 (C=N and C=C stretching), 1379.10, 1317.38, 1238.30, 1109.07, 1037.70 (C-N, C-O stretching and aromatic C-H in plane bending), 937.40, 823.60, 748.38, 719.45 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.10 (1H, dd, JAM = 17.37 Hz, JAX = 6.33 Hz, C4-HA pyrazoline), 3.87 (1H, dd, JMA = 17.37 Hz, JMX = 12.09 Hz, C4-HM pyrazoline), 5.40 (1H, dd, JMX = 12.00 Hz, JAX = 6.30 Hz, C5-HX pyrazoline), 5.97 (2H, d, J = 0.57 Hz, O-CH2-O), 6.70–6.79 (3H, m, aromatic protons), 6.87 (1H, d, J = 8.13 Hz, aromatic proton), 6.95 (2H, d, J = 7.89 Hz, aromatic protons), 7.08–7.18 (3H, m, aromatic protons), 7.25 (1H, d, J = 3.51 Hz, aromatic proton), 7.59 (1H, d, J = 5.04 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.18 (CH2), 63.51 (CH), 101.52 (CH2), 106.54 (CH), 109.07 (CH), 113.46 (2CH), 119.16 (CH), 119.57 (CH), 127.90 (2CH), 128.28 (CH), 129.35 (2CH), 136.15 (C), 136.59 (C), 144.21 (C), 144.50 (C), 146.99 (C), 148.19 (C). Anal. Calcd. for C20H16N2O2S: C, 68.95; H, 4.63; N, 8.04; Found: C, 68.93; H, 4.64; N, 8.05. MS (ESI) (m/z): [M + H]− 346.90, [M]+ 347.90, [M + H]+ 348.90.
1-(4-Cyanophenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4b). Yield: 93%. M.p.: 156–157 °C. IR νmax (cm−1): 3105.39 (aromatic C-H stretching), 2916.37, 2848.86 (aliphatic C-H stretching), 2208.49 (C≡N stretching), 1600.92, 1510.26, 1481.33, 1442.75 (C=N and C=C stretching), 1396.46, 1325.10, 1242.16, 1174.65, 1095.57, 1035.77 (C-N, C-O stretching and aromatic C-H in plane bending), 935.48, 840.96, 821.68, 802.39, 727.16 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.20 (1H, dd, JAM = 17.64 Hz, JAX = 4.98 Hz, C4-HA pyrazoline), 3.95 (1H, dd, JMA = 17.64 Hz, JMX = 11.94 Hz, C4-HM pyrazoline), 5.57 (1H, dd, JMX = 11.82 Hz, JAX = 4.92 Hz, C5-HX pyrazoline), 5.99 (2H, d, J = 1.71 Hz, O-CH2-O), 6.73 (1H, dd, J = 7.98 Hz, 1.71 Hz, aromatic proton), 6.77 (1H, d, J = 1.56 Hz, aromatic proton), 6.87 (1H, d, J = 7.95 Hz, aromatic proton), 7.01 (2H, d, J = 8.91 Hz, aromatic protons), 7.14 (1H, dd, J = 5.04 Hz, 3.66 Hz, aromatic proton), 7.36 (1H, dd J = 3.60 Hz, 1.05 Hz, aromatic proton), 7.57 (2H, d, J = 8.94 Hz, aromatic protons), 7.69 (1H, dd, J = 5.04 Hz, 1.02 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.26 (CH2), 62.52 (CH), 99.45 (C), 101.65 (CH2), 106.41 (CH), 109.20 (CH), 113.21 (2CH), 119.42 (CH), 120.42 (C), 128.48 (CH), 129.18 (CH), 129.47 (CH), 133.80 (2CH), 135.24 (C), 135.46 (C), 146.75 (C), 147.24 (C), 147.51 (C), 148.33 (C). Anal. Calcd. for C21H15N3O2S: C, 67.54; H, 4.05; N, 11.25; Found: C, 67.56; H, 4.04; N, 11.24. MS (ESI) (m/z): [M]+ 373.90.
1-(4-Fluorophenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4c). Yield: 76%. M.p.: 108–110 °C. IR νmax (cm−1): 3086.11 (aromatic C-H stretching), 2970.38, 2916.37 (aliphatic C-H stretching), 1504.48, 1483.26, 1442.75 (C=N and C=C stretching), 1240.23, 1220.94, 1035.77 (C-N, C-O stretching and aromatic C-H in plane bending), 931.62, 802.39, 705.95 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.11 (1H, dd, JAM = 17.31 Hz, JAX = 6.81 Hz, C4-HA pyrazoline), 3.87 (1H, dd, JMA = 17.31 Hz, JMX = 11.97 Hz, C4-HM pyrazoline), 5.36 (1H, dd, JMX = 11.91 Hz, JAX = 6.75 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 0.78 Hz, O-CH2-O), 6.77–6.81 (2H, m, aromatic protons), 6.87 (1H, d, J = 7.83 Hz, aromatic proton), 6.91–6.94 (2H, m, aromatic protons), 6.95–7.02 (2H, m, aromatic protons), 7.04–7.11 (1H, m, aromatic proton), 7.24–7.26 (1H, m, aromatic proton), 7.60 (1H, dd, J = 5.07 Hz, 1.05 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.36 (CH2), 64.11 (CH), 101.55 (CH2), 106.62 (CH), 109.08 (CH), 114.65 and 114.75 (2CH), 115.75 and 116.05 (2CH), 119.70 (2CH), 127.98 and 128.28 (2CH), 136.04 and 136.31 (2C), 141.49 (C), 144.46 (C), 147.06 (C), 148.22 (C), 154.85 and 157.96 (C). Anal. Calcd. for C20H15FN2O2S: C, 65.56; H, 4.13; N, 7.65; Found: C, 65.54; H, 4.14; N, 7.66. MS (ESI) (m/z): [M + H]− 364.90, [M]+ 365.90, [M + H]+ 366.90.
1-(4-Chlorophenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4d). Yield: 92%. M.p.: 130–132 °C. IR νmax (cm−1): 3072.60 (aromatic C-H stretching), 2916.37, 2848.86 (aliphatic C-H stretching), 1595.13, 1492.90, 1481.33, 1442.75 (C=N and C=C stretching), 1382.96, 1319.31, 1244.09, 1136.07, 1093.64, 1037.70 (C-N, C-O stretching and aromatic C-H in plane bending), 933.55, 813.96, 796.60, 725.23, 707.88 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.13 (1H, dd, JAM = 17.40 Hz, JAX = 6.09 Hz, C4-HA pyrazoline), 3.89 (1H, dd, JMA = 17.43 Hz, JMX = 12.03 Hz, C4-HM pyrazoline), 5.42 (1H, dd, JMX = 11.94 Hz, JAX = 6.03 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 1.59 Hz, O-CH2-O), 6.74–6.78 (2H, m, aromatic protons), 6.87 (1H, d, J = 7.89 Hz, aromatic proton), 6.94 (2H, d, J = 9.03 Hz, aromatic protons), 7.11 (1H, dd, J = 5.07 Hz, 3.63 Hz, aromatic proton), 7.20 (2H, d, J = 9.00 Hz, aromatic protons), 7.27 (1H, dd, J = 3.57 Hz, 1.08 Hz, aromatic proton), 7.62 (1H, dd, J = 5.07 Hz, 1.05 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.30 (CH2), 63.41 (CH), 101.57 (CH2), 106.52 (CH), 109.10 (CH), 114.85 (2CH), 116.52 (C), 119.58 (CH), 122.70 (CH), 128.27 (2CH, d, J = 6.75 Hz), 129.21 (2CH, d, J = 6.75 Hz), 135.85 (C), 136.06 (C), 143.26 (C), 145.04 (C), 147.09 (C), 148.24 (C). Anal. Calcd. for C20H15ClN2O2S: C, 62.74; H, 3.95; N, 7.32; Found: C, 62.76; H, 3.94; N, 7.31. MS (ESI) (m/z): [M + H]−− 378.80, [M]+ 380.80, [M + H]+ 381.90, [M + H]++ 382.80, [M + H]+++ 383.90.
1-(4-Bromophenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4e). Yield: 90%. M.p.: 136–138 °C. IR νmax (cm−1): 3070.68 (aromatic C-H stretching), 2916.37 (aliphatic C-H stretching), 1589.34, 1481.33, 1442.75 (C=N and C=C stretching), 1382.96, 1319.31, 1244.09, 1128.36, 1091.71, 1035.77 (C-N, C-O stretching and aromatic C-H in plane bending), 935.48, 812.03, 707.88 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.13 (1H, dd, JAM = 17.40 Hz, JAX = 5.91 Hz, C4-HA pyrazoline), 3.88 (1H, dd, JMA = 17.43 Hz, JMX = 12.09 Hz, C4-HM pyrazoline), 5.42 (1H, dd, JMX = 11.88 Hz, JAX = 5.85 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 1.56 Hz, O-CH2-O), 6.73–6.77 (2H, m, aromatic protons), 6.83–6.94 (3H, m, aromatic protons), 7.10 (1H, dd, J = 4.89 Hz, 3.75 Hz, aromatic proton), 7.27–7.33 (3H, m, aromatic protons), 7.62 (1H, d, J = 4.95 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.29 (CH2), 63.29 (CH), 101.57 (CH2), 106.51 (CH), 109.11 (CH), 110.32 (CH), 115.34 (2CH), 119.56 (CH), 128.31 (2CH, d, J = 5.25 Hz), 132.00 (2CH), 135.82 (C), 136.01 (2C), 143.57 (C), 145.13 (C), 147.10 (C), 148.24 (C). Anal. Calcd. for C20H15BrN2O2S: C, 56.22; H, 3.54; N, 6.56; Found: C, 56.24; H, 3.53; N, 6.55. MS (ESI) (m/z): [M + H]— 426.80, [M]+ 427.80.
1-(4-Methylphenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4f). Yield: 47%. M.p.: 153–155 °C. IR νmax (cm−1): 3070.68 (aromatic C-H stretching), 2916.37, 2848.86 (aliphatic C-H stretching), 1593.20, 1481.33, 1442.75 (C=N and C=C stretching), 1382.96, 1319.31, 1244.09, 1130.29, 1091.71, 1035.77 (C-N, C-O stretching and aromatic C-H in plane bending), 933.55, 812.03, 802.39, 723.31, 707.88 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 2.16 (3H, s, CH3), 3.07 (1H, dd, JAM = 17.28 Hz, JAX = 6.57 Hz, C4-HA pyrazoline), 3.84 (1H, dd, JMA = 17.25 Hz, JMX = 12.03 Hz, C4-HM pyrazoline), 5.35 (1H, dd, JMX = 11.97 Hz, JAX = 6.54 Hz, C5-HX pyrazoline), 5.97 (2H, d, J = 1.26 Hz, O-CH2-O), 6.76 (2H, d, J = 6.84 Hz, aromatic protons), 6.86 (3H, d, J = 8.64 Hz, aromatic protons), 6.97 (2H, d, J = 8.43 Hz, aromatic protons), 7.09 (1H, dd, J = 5.04 Hz, 3.63 Hz, aromatic proton), 7.22 (1H, dd, J = 3.54 Hz, 0.99 Hz, aromatic proton), 7.57 (1H, dd, J = 5.04 Hz, 0.99 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 20.54 and 20.57 (CH3), 44.14 (CH2), 63.80 (CH), 101.50 (CH2), 106.57 (CH), 109.02 (CH), 113.65 (2CH), 119.63 (CH), 127.61 (CH), 127.88 (CH), 128.24 (CH), 129.78 (2CH), 136.29 (C), 136.63 (2C), 142.43 (C), 143.67 (C), 146.95 (C), 148.14 (C). Anal. Calcd. for C21H18N2O2S: C, 69.59; H, 5.01; N, 7.73; Found: C, 69.61; H, 5.00; N, 7.72. MS (ESI) (m/z): [M + H]−− 360.90, [M]+ 361.90, [M + H]+ 362.90.
1-(4-Methoxyphenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4g). Yield: 39%. M.p.: 140–142 °C. IR νmax (cm−1): 3107.32 (aromatic C-H stretching), 2916.37, 2848.86 (aliphatic C-H stretching), 1587.42, 1504.48, 1487.12, 1444.68 (C=N and C=C stretching), 1375.25, 1230.58, 1180.44, 1116.78, 1083.99, 1033.85 (C-N, C-O stretching and aromatic C-H in plane bending), 935.48, 829.39, 804.32, 707.88 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.07 (1H, dd, JAM = 17.16 Hz, JAX = 7.41 Hz, C4-HA pyrazoline), 3.65 (3H, s, OCH3), 3.83 (1H, dd, JMA = 17.19 Hz, JMX = 11.94 Hz, C4-HM pyrazoline), 5.29 (1H, dd, JMX = 11.85 Hz, JAX = 7.38 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 0.81 Hz, O-CH2-O), 6.76–6.81 (4H, m, aromatic protons), 6.85–6.91 (3H, m, aromatic protons), 7.09 (1H, dd, J = 5.04 Hz, 3.60 Hz, aromatic proton), 7.21 (1H, dd, J = 3.54 Hz, 1.02 Hz, aromatic proton), 7.56 (1H, dd, J = 5.04 Hz, 1.02 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.24 (CH2), 55.64 and 55.67 (CH3), 64.66 (CH), 101.50 (CH2), 106.71 (CH), 109.01 (CH), 114.83 (2CH), 115.05 (2CH), 119.82 (CH), 127.46 (2CH, d, J = 6.00 Hz), 128.22 (CH), 136.37 (C), 136.63 (C), 139.11 (C), 143.49 (C), 146.97 (C), 148.14 (C), 153.25 (C). Anal. Calcd. for C21H18N2O3S: C, 66.65; H, 4.79; N, 7.40; Found: C, 66.67; H, 4.78; N, 7.39. MS (ESI) (m/z): [M]+ 377.90, [M + H]+ 378.90.
1-(4-Methylsulfonylphenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4h). Yield: 63%. M.p.: 172–173 °C. IR νmax (cm−1): 3097.68 (aromatic C-H stretching), 2985.81, 2900.84 (aliphatic C-H stretching), 1589.34, 1502.55, 1483.26, 1442.75 (C=N and C=C stretching), 1392.61, 1381.03, 1296.16, 1247.94, 1078.21, 1051.20 (C-N, C-O stretching and aromatic C-H in plane bending), 821.68, 769.60, 705.95 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.07 (3H, s, SO2CH3), 3.20 (1H, dd, JAM = 17.64 Hz, JAX = 5.07 Hz, C4-HA pyrazoline), 3.96 (1H, dd, JMA = 17.61 Hz, JMX = 11.94 Hz, C4-HM pyrazoline), 5.57 (1H, dd, JMX = 11.85 Hz, JAX = 4.98 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 1.62 Hz, O-CH2-O), 6.75 (1H, dd, J = 7.95 Hz, 1.74 Hz, aromatic proton), 6.79 (1H, d, J = 1.56 Hz, aromatic proton), 6.88 (1H, d, J = 7.92 Hz, aromatic proton), 7.07 (2H, d, J = 8.94 Hz, aromatic protons), 7.13 (1H, dd, J = 5.07 Hz, 3.66 Hz, aromatic proton), 7.36 (1H, dd, J = 3.57 Hz, 1.05 Hz, aromatic proton), 7.65–7.69 (3H, m, aromatic protons). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.29 (CH2), 44.63 (CH3), 62.61 (CH), 101.64 (CH2), 106.43 (CH), 109.21 (CH), 112.61 (2CH), 119.45 (CH), 128.47 (CH), 129.05 (2CH), 129.38 (2CH), 129.61 (C), 135.30 (C), 135.56 (2C), 147.27 (2C), 148.32 (C). Anal. Calcd. for C21H18N2O4S2: C, 59.14; H, 4.25; N, 6.57; Found: C, 59.16; H, 4.24; N, 6.56. MS (ESI) (m/z): [M]+ 426.80, [M + H]+ 427.80.
1-(4-Sulfonamidophenyl)-3-(2-thienyl)-5-(1,3-benzodioxol-5-yl)-2-pyrazoline (4i) [45]. Yield: 83%. M.p.: 129–130 °C. IR νmax (cm−1): 3321.42, 3250.05 (N-H stretching), 3103.46, 3074.53 (aromatic C-H stretching), 2916.37, 2848.86 (aliphatic C-H stretching), 1591.27, 1502.55, 1485.19, 1442.75 (C=N and C=C stretching), 1394.53, 1323.17, 1307.74, 1238.30, 1151.10, 1095.57, 1035.77, 1001.06 (C-N, C-O stretching and aromatic C-H in plane bending), 933.55, 904.61, 860.25, 812.03, 713.66 (aromatic C-H out of plane bending and C-S stretching). 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 3.18 (1H, dd, JAM = 17.52 Hz, JAX= 5.10 Hz, C4-HA pyrazoline), 3.93 (1H, dd, JMA = 17.52 Hz, JMX = 11.94 Hz, C4-HM pyrazoline), 5.56 (1H, dd, JMX = 11.85 Hz, JAX = 5.01 Hz, C5-HX pyrazoline), 5.98 (2H, d, J = 2.04 Hz, O-CH2-O), 6.72–6.77 (2H, m, aromatic protons), 6.87 (1H, d, J = 7.86 Hz, aromatic proton), 7.01–7.04 (4H, m, aromatic and NH2 protons), 7.12 (1H, dd, J = 5.04 Hz, 3.63 Hz, aromatic proton), 7.33 (1H, dd, J = 3.57 Hz, 1.05 Hz, aromatic proton), 7.60 (2H, d, J = 8.94 Hz, aromatic protons), 7.66 (1H, dd, J = 5.04 Hz, 1.02 Hz, aromatic proton). 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 44.83 (CH2), 64.86 (CH), 101.66 (CH2), 106.85 (CH), 109.87 (CH), 115.65 (2CH), 119.87 (CH), 127.47 (CH), 127.96 (CH), 128.05 (CH), 131.88 (2CH), 135.77 (C), 136.45 (C), 146.03 (C), 147.49 (C), 148.90 (C), 159.36 (2C). Anal. Calcd. for C20H17N3O4S2: C, 56.19; H, 4.01; N, 9.83; Found: C, 56.24; H, 4.08; N, 9.80. MS (ESI) (m/z): [M]+ 427.80, [M + H]+ 428.80.
3.2. Pharmacology
3.2.1. Cell Culture
PC-12 Adh cells were cultured in 10% horse serum, 5% fetal bovine serum, and 1% penicillin-streptomycin containing DMEM growth medium, at 37 °C in a humidified incubator with 5% CO2. The proliferating cells were passaged 1:2 to new 25 and/or 75 cm2 flasks and cell stocks were prepared in order to use in future experiments. In order to induce PC-12 Adh cell differentiation into neuronal phenotype, growth medium was changed with 1% fetal bovine serum, 1% penicillin-streptomycin and 100 nM NGF containing DMEM differentiation medium.
Differentiated PC-12 Adh cells were stained with Trypan blue solution and counted by a cell counter device (Cedex XS, Innovatis, Malvern, PA, USA) in order to determine the appropriate cell numbers before the experiments.
3.2.2. Determination of Non-Cytotoxic Concentrations
In order to obtain non-cytotoxic concentrations of the compounds, the viability of neuronal cells was measured by using 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (WST-1) assay (Roche, Mannheim, Germany). The test is based on the cleavage of the tetrazolium salt WST-1 in formazan by mitochondrial dehydrogenases in viable cells. The formazan dye was quantified in a scanning multiwell spectrophotometer by measuring the absorbance of the dye at 420 nm. The differentiated PC-12 Adh cells were scratched and plated onto 96-well culture plates at 5 × 103 density per well. After 24 h, the cells were treated with 400, 200, 100, 50, and 25 µg/mL concentrations of compounds and 400, 200, 100, 50 and 25 µM 6-OHDA for 24 h. After the incubation period, the cell proliferation reagent WST-1 (10 μL per well) was added to the wells; and absorbances were measured after 3 h using a Cytation 3 cell imaging multi-mode reader at 420 nm (BioTek, Winooski, VT, USA). The measured absorbances directly correlated to the number of viable cells. The cell viability rates were expressed as a percentage of the controls, and IC50 values of the compounds and 6-OHDA were calculated according to the control group [46].
3.2.3. Determination of Neuroprotective Activity against 6-OHDA-Induced Neurodegeneration
In order to determine the in vitro neuroprotective potentials of the compounds, 6-OHDA-induced neurotoxicity model of PD was conducted [47,48]. The differentiated PC-12 Adh cells were scratched and plated onto 96-well culture plates at 5 × 103 density per well. After 24 h, the cells were treated with 100 µg/mL concentration (only 10 µg/mL and 100 µg/mL were tested for compound 1, which was found to be highly cytotoxic) of the compounds for 6 h. 100 µg/mL concentration of the compounds was determined as the non-cytotoxic concentration, and the IC50 value of the 6-OHDA (150 µM) was used as a positive control according to the WST-1 cell viability assay results. After a 6-h incubation period with the compounds, the medium was removed, and the cells were treated with 150 µM 6-OHDA in order to induce neurodegeneration via oxidative stress for 24 h. The control cells were cultured in differentiation medium containing 0.1% DMSO, and the cells cultured in 150 µM 6-OHDA were used as a positive control. After 24 h, the neuroprotective effects of the compounds were determined by WST-1 cell viability assay as explained above. The graphics were drawn according to the cell viability, which was expressed as percentage of the surviving control cells in the study.
3.2.4. Statistical Analysis
The graphics were drawn with Graphpad Prism 6.0 software and statistically analyzed using one-way ANOVA and Tukey’s post hoc test. The results are expressed as mean ± standard deviation and the means of three independent experiments (n = 8), n.s; p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 were considered significant compared with the control group and the 6-OHDA-positive control group.
3.3. Prediction of Pharmacokinetic Parameters
ADME properties of compounds 1, 2, 3a–i, and 4a–i were in silico predicted using QikProp program (Schrödinger Release 2016-2: QikProp, Schrödinger, LLC, New York, NY, USA, 2016). This program computes physically significant descriptors and pharmaceutically relevant properties, such as QPlogBB, CNS activity, SASA (in square angstroms using a probe with a 1.4 Å Radius) and a percentage of human oral absorption. The acceptability of compounds 1, 2, 3a–i and 4a–i, based on the Lipinski’s rule of five [39] and Jorgensen’s rule of three [40], was also determined.
4. Conclusions
In the recent work, 2-pyrazoline based compounds 3a–i and 4a–i were synthesized based on the reaction of chalcones (1, 2) and phenylhydrazine hydrochloride derivatives. All these compounds were evaluated for their neuroprotective effects against toxicity induced by 6-OHDA in rat pheochromocytoma (PC-12) cells. According to in vitro studies, 4-methylsulfonylphenyl containing compounds 3h and 4h induced cell viability percentage notably when compared with the 6-OHDA-positive control group. In silico ADME prediction also pointed out that all compounds were within the acceptable range for some pharmacokinetic parameters especially important for CNS activity. Consequently, compounds 3h and 4h stand out as potential orally bioavailable CNS acting drug candidates for further neuroprotective studies associated with PD.
Author Contributions
A.Ö., B.S., and M.D.A. designed the research, A.Ö., B.S., and M.D.A. performed the synthetic work. B.S. carried out the ADME studies and mainly wrote the manuscript. M.D. and E.K.T. performed the whole pharmacology part of this study. A.Ö. was also responsible for the correspondence of the manuscript. All the authors discussed, edited, and approved the final version of the manuscript.
Funding
This study was supported by Anadolu University Scientific Research Projects Commission under the grant no: 1805S208.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peden, A.H.; Ironside, J.W. Molecular pathology in neurodegenerative diseases. Curr. Drug Targets 2012, 13, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Yacoubian, T.A. Neurodegenerative disorders: Why do we need new therapies? In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders: Alzheimer’s Disease, 1st ed.; Adejare, A., Ed.; Academic Press: London, UK, 2017; Volume 1, pp. 1–16. [Google Scholar]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Worth, P.F. Parkinson’s disease. Medicine 2016, 44, 542–546. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Youdim, M.B.; Kupershmidt, L.; Amit, T.; Weinreb, O. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 132–136. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Standaert, D.G. Targets for neuroprotection in Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Newland, B.; Dunnett, S.B.; Dowd, E. Targeting delivery in Parkinson’s disease. Drug Discov. Today 2016, 21, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Radaideh, A.M.; Rababah, E.M. The role of magnetic resonance imaging in the diagnosis of Parkinson’s disease: A review. Clin. Imaging 2016, 40, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.L.; Petrucelli, L. Parkinson’s disease—Molecular mechanisms of disease. Drug Discov. Today Dis. Mech. 2004, 1, 399–405. [Google Scholar] [CrossRef]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Ciniglio Appiani, M.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Fell, M.J. Current approaches to the treatment of Parkinson’s disease. Bioorg. Med. Chem. Lett. 2017, 27, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Shimohama, S.; Sawada, H.; Kitamura, Y.; Taniguchi, T. Disease model: Parkinson’s disease. Trends Mol. Med. 2003, 9, 360–365. [Google Scholar] [CrossRef]
- Rezak, M. Current pharmacotherapeutic treatment options in Parkinson’s disease. Dis. Mon. 2007, 53, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A.I.; Negida, A.; Ahmed, H.; Abdel-Daim, M.M. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: Future applications in Parkinson’s disease. Biomed. Pharmacother. 2017, 85, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Francardo, V.; Schmitz, Y.; Sulzer, D.; Cenci, M.A. Neuroprotection and neurorestoration as experimental therapeutics for Parkinson’s disease. Exp. Neurol. 2017, 298, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, K.P.; Nandhu, M.S.; Paul, J.; Paulose, C.S. Oxidative stress mediated neuronal damage in the corpus striatum of 6-hydroxydopamine lesioned Parkinson’s rats: Neuroprotection by serotonin, GABA and bone marrow cells supplementation. J. Neurol. Sci. 2013, 331, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, M.; Martin, S.; Mitkovski, M.; Vozari, R.R.; Stühmer, W.; Bel, E.D. Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 2013, 61, 1084–1100. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective effects of flavonol isoquercitrin, against 6-hydroxy dopamine (6-OHDA)-induced toxicity in PC12 cells. BMC Res. Notes 2014, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Zhao, T.T.; Lee, K.S.; Lee, S.H.; Shin, K.S.; Park, K.H.; Choi, H.S.; Lee, M.K. Effects of (-)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson’s disease rat models. Neurochem. Int. 2015, 83, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.R.; Mayhoub, A.S.; Farag, A.M. Recent advances in the therapeutic applications of pyrazolines. Expert. Opin. Ther. Pat. 2012, 22, 253–291. [Google Scholar] [CrossRef] [PubMed]
- Marella, A.; Ali, M.R.; Alam, M.T.; Saha, R.; Tanwar, O.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Pyrazolines: A biological review. Mini. Rev. Med. Chem. 2013, 13, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Alex, J.M.; Kumar, R. 4,5-Dihydro-1H-pyrazole: An indispensable scaffold. J. Enzyme Inhib. Med. Chem. 2014, 29, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Upadhyay, S.; Paliwal, S.; Saraf, S.K. Privileged scaffolds as MAO inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018, 145, 445–497. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Altıntop, M.D.; Kaplancıklı, Z.A.; Turan-Zitouni, G.; Akalın Ciftçi, G.; Ulusoylar Yıldırım, Ş. Synthesis of 1-acetyl-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and evaluation of their anticancer activity. J. Enzyme Inhib. Med. Chem. 2013, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Altıntop, M.D.; Kaplancıklı, Z.A.; Turan-Zitouni, G.; Akalın Ciftçi, G.; Demirci, F. Synthesis and biological evaluation of a new series of pyrazolines as new anticandidal agents. Pharm. Chem. J. 2014, 48, 603–612. [Google Scholar] [CrossRef]
- Koçyiğit-Kaymakçıoğlu, B.; Beyhan, N.; Tabanca, N.; Ali, A.; Wedge, D.E.; Duke, S.O.; Bernier, U.R.; Khan, I.A. Discovery and structure activity relationships of 2-pyrazolines derived from chalcones from a pest management perspective. Med. Chem. Res. 2015, 24, 3632–3644. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Karaca Gencer, H.; Kapkac, H.A.; Atli, O.; Baysal, M.; Özdemir, A. Synthesis of new thiazolyl-pyrazoline derivatives and evaluation of their antimicrobial, cytotoxic and genotoxic effects. Lett. Drug Des. Discov. 2018, 15, 744–756. [Google Scholar] [CrossRef]
- Özdemir, A.; Altıntop, M.D.; Kaplancıklı, Z.A.; Can, Ö.D.; Demir Özkay, Ü.; Turan-Zitouni, G. Synthesis and evaluation of new 1,5-diaryl-3-[4-(methyl-sulfonyl)phenyl]-4,5-dihydro-1H-pyrazole derivatives as potential antidepressant agents. Molecules 2015, 20, 2668–2684. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Sever, B.; Altıntop, M.D. New benzodioxole-based pyrazoline derivatives: Synthesis and anticandidal, in silico ADME, molecular docking studies. Lett. Drug Des. Discov. 2018. [Google Scholar] [CrossRef]
- Frecer, V.; Berti, F.; Benedetti, F.; Miertus, S. Design of peptidomimetic inhibitors of aspartic protease of HIV-1 containing-PheψPr-core and displaying favourable ADME-related properties. J. Mol. Graph. Model. 2008, 27, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene delivery to the brain: The vascular route. Neuron 2002, 36, 555–558. [Google Scholar] [CrossRef]
- Ghose, A.K.; Herbertz, T.; Hudkins, R.L.; Dorsey, B.D.; Mallamo, J.P. Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem. Neurosci. 2012, 3, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.; Dorr, B.; Woetzel, N.; Staritzbichler, R.; Meiler, J. Solvent accessible surface area approximations for rapid and accurate protein structure prediction. J. Mol. Model. 2009, 15, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
- Zhang, M.; Xi, J.; Ruzi, R.; Li, N.; Wu, Z.; Li, W. Domino-fluorination-protodefluorination enables decarboxylative cross-coupling of α-oxocarboxylic acids with styrene via photoredox catalysis. J. Org. Chem. 2017, 82, 9305–9311. [Google Scholar] [CrossRef] [PubMed]
- Basaif, S.A.; Sobahi, T.R.; Khalil, A.K.; Hassan, M.A. Stereoselective crossed-aldol condensation of hetarylmethyl ketones with aromatic aldehydes in water: Synthesis of (2E)-3-aryl-1-hetarylprop-2-en-1-ones. Bull. Korean Chem. Soc. 2005, 26, 1677–1681. [Google Scholar] [CrossRef]
- Adibi, H.; Hajipour, A.R.; Jafari, H. Metal-free oxidative dehydrogenation of imidazolines and pyrazolines using silica-adsorbed peroxymonosulfate under aprotic and almost neutral conditions. Chem. Heterocycl. Comp. 2008, 44, 802. [Google Scholar] [CrossRef]
- Pragst, F.; Weber, F.G. Electrochemical behavior of N-aryl-Δ2-pyrazolines. VIII. Relations between the anodic and cathodic behavior and the absorption and fluorescence properties of N-aryl-Δ2-pyrazolines. J. Prakt. Chem. 1976, 318, 51–68. [Google Scholar] [CrossRef]
- Basaif, S.A.; Albar, H.A.; Faidallah, H.M. Synthesis of new pyrazoline and pyrazole derivatives. Indian J. Heterocycl. Chem. 1995, 5, 121–124. [Google Scholar]
- Engür, S.; Dikmen, M.; Öztürk, Y. Comparison of antiproliferative and apoptotic effects of a novel proteasome inhibitor MLN2238 with bortezomib on K562 chronic myeloid leukemia cells. Immunopharmacol. Immunotoxicol. 2016, 38, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mao, P.; Wang, J.; Wang, T.; Xie, C.H. Allicin protects PC12 cells against 6-OHDA-induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell. Physiol. Biochem. 2015, 36, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.D.; Guo, S.Q.; Hu, Z.W.; Li, W.L. NAMPT protects against 6-hydroxydopamine-induced neurotoxicity in PC12 cells through modulating SIRT1 activity. Mol. Med. Rep. 2016, 13, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2, 3a-i, 4a-i are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).