JNK Inactivation Induces Polyploidy and Drug-Resistance in Coronarin D-Treated Osteosarcoma Cells
Abstract
:1. Introduction
2. Results
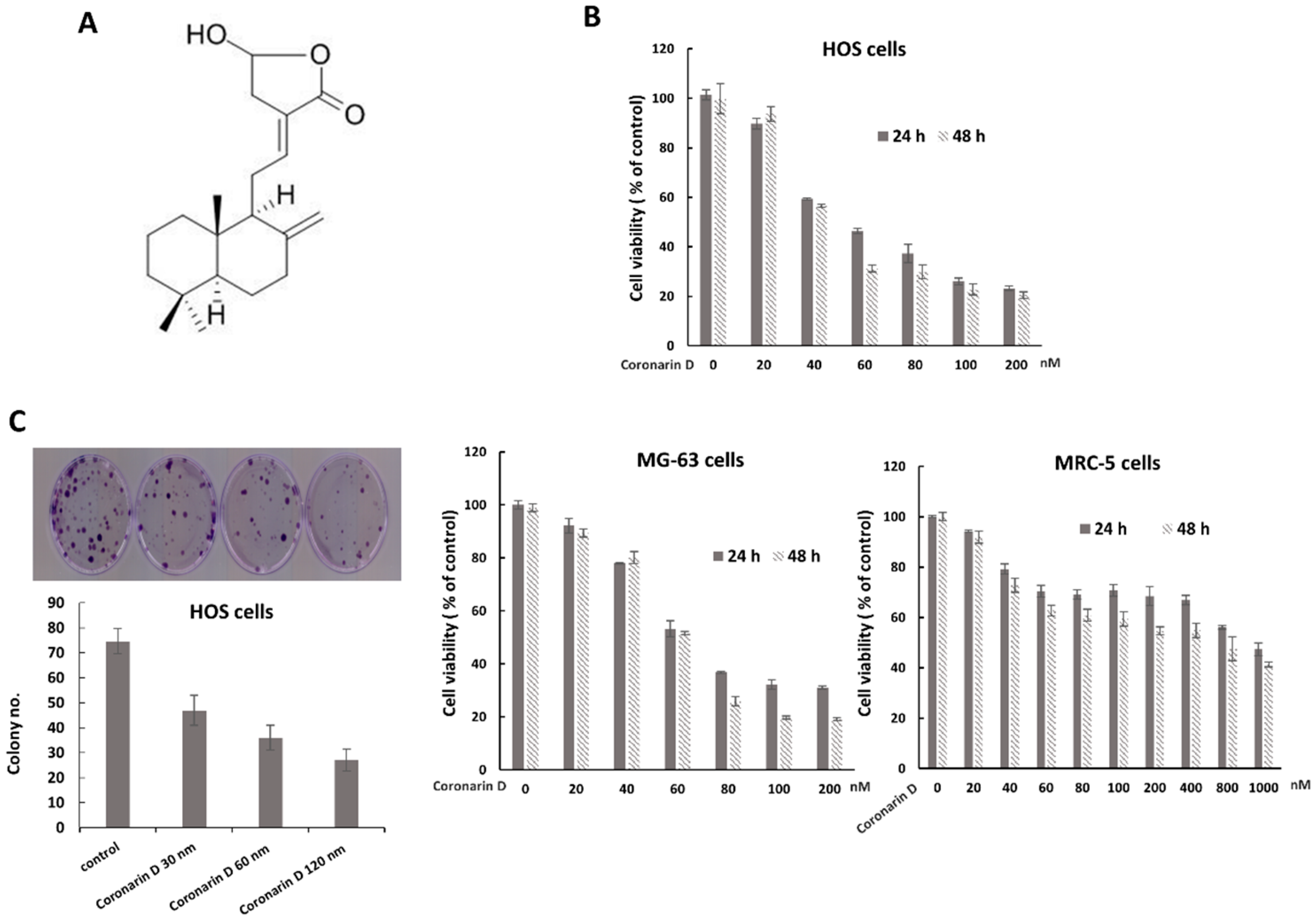
2.1. Coronarin D Reduced Osteosarcoma Cell Viability and Proliferation
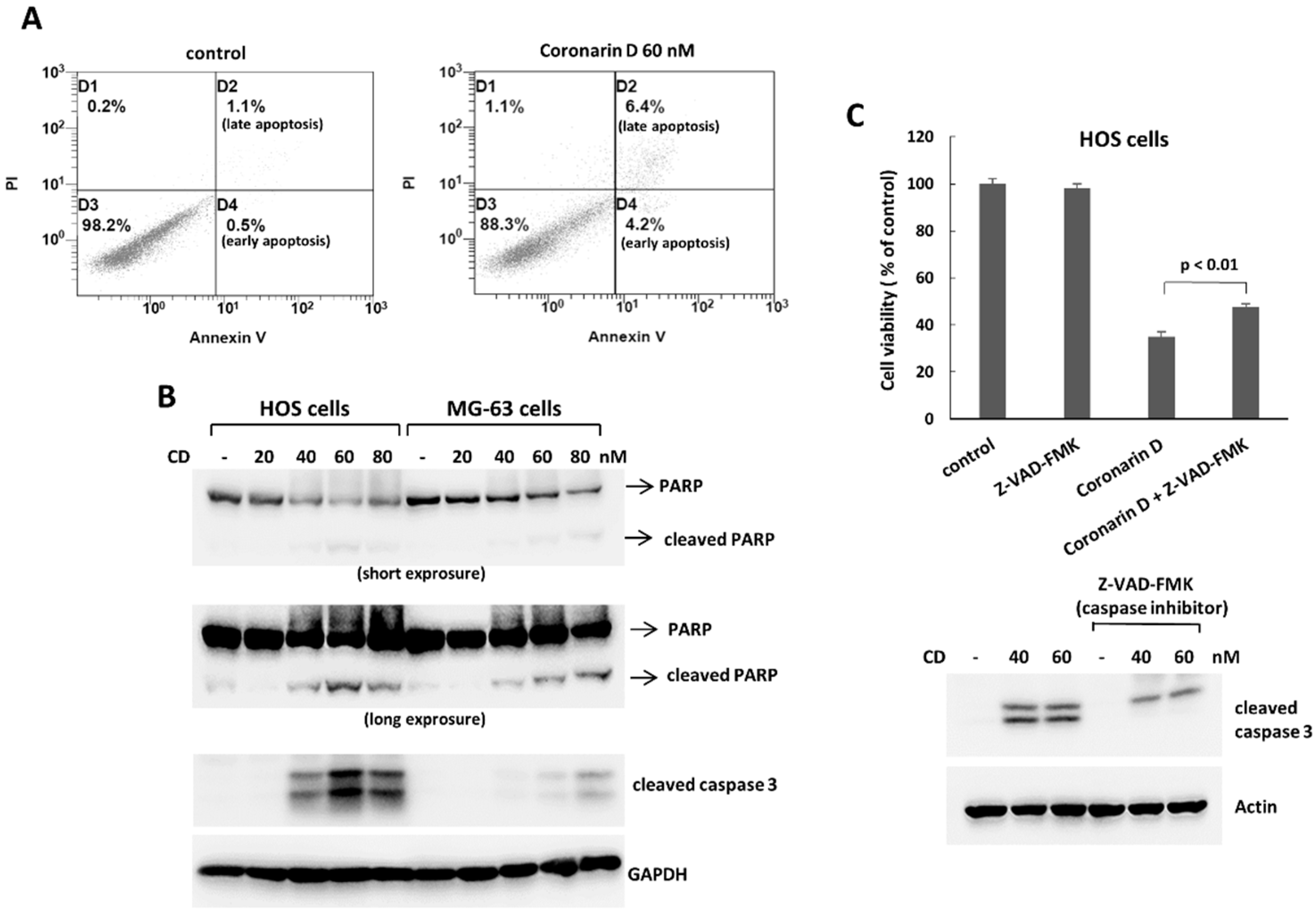
2.2. Coronarin D Induces Apoptosis and Cell Arrest
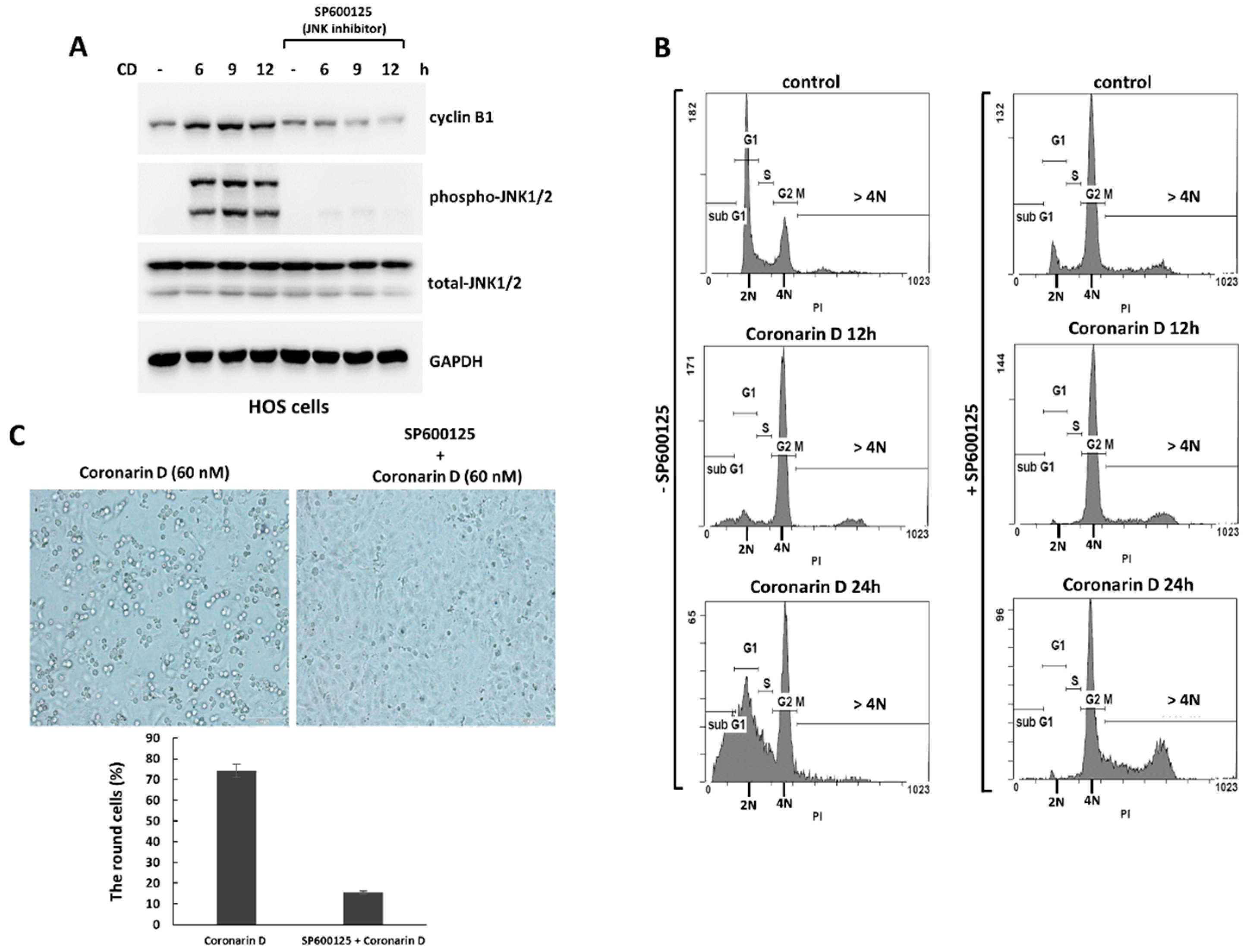
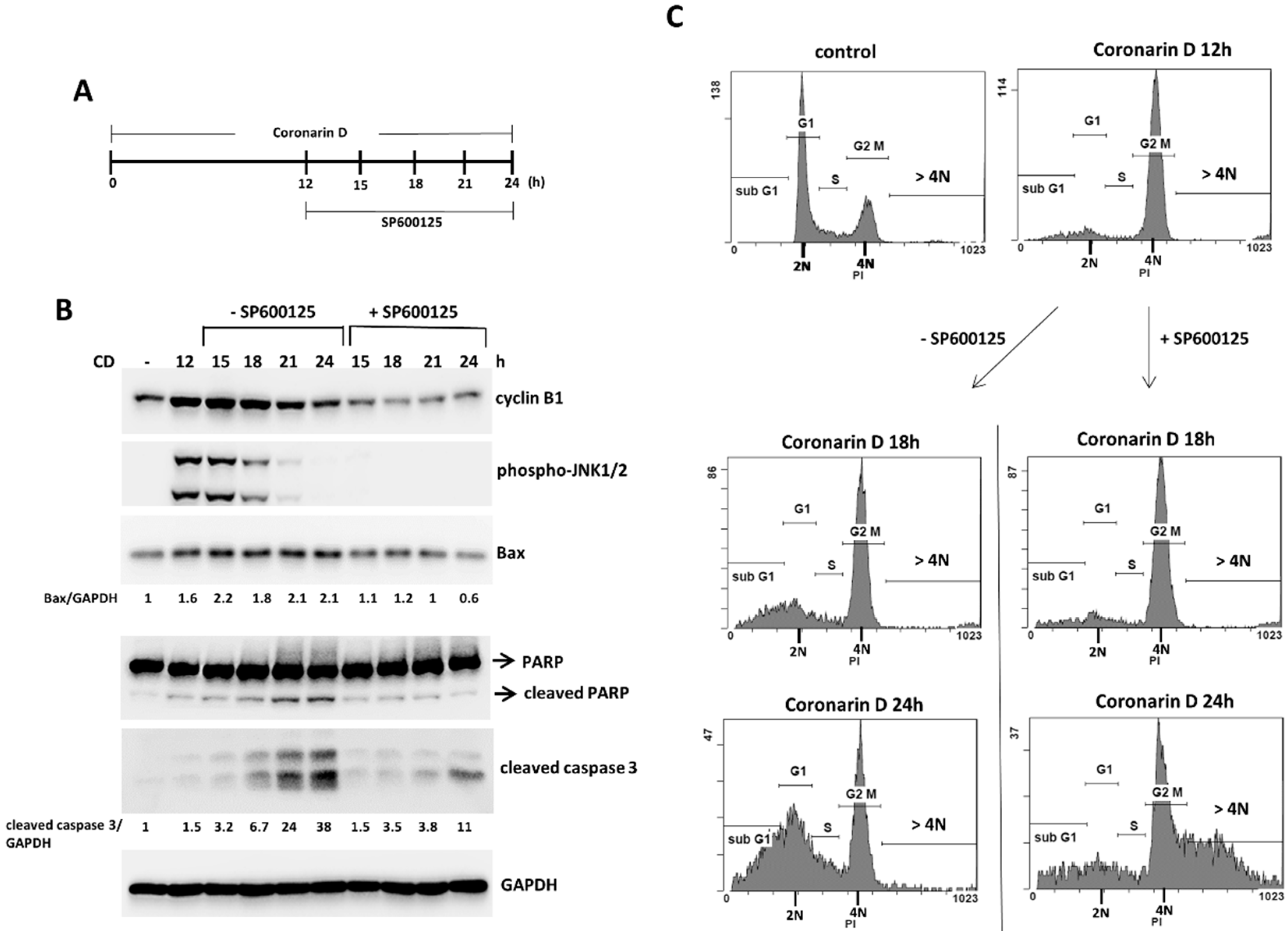
2.3. The Pretreatment with JNK Inhibitor Blocks the Mitotic Entry and Induces Polyploidy and Drug-Resistance in Coronarin D-Treated HOS Cells
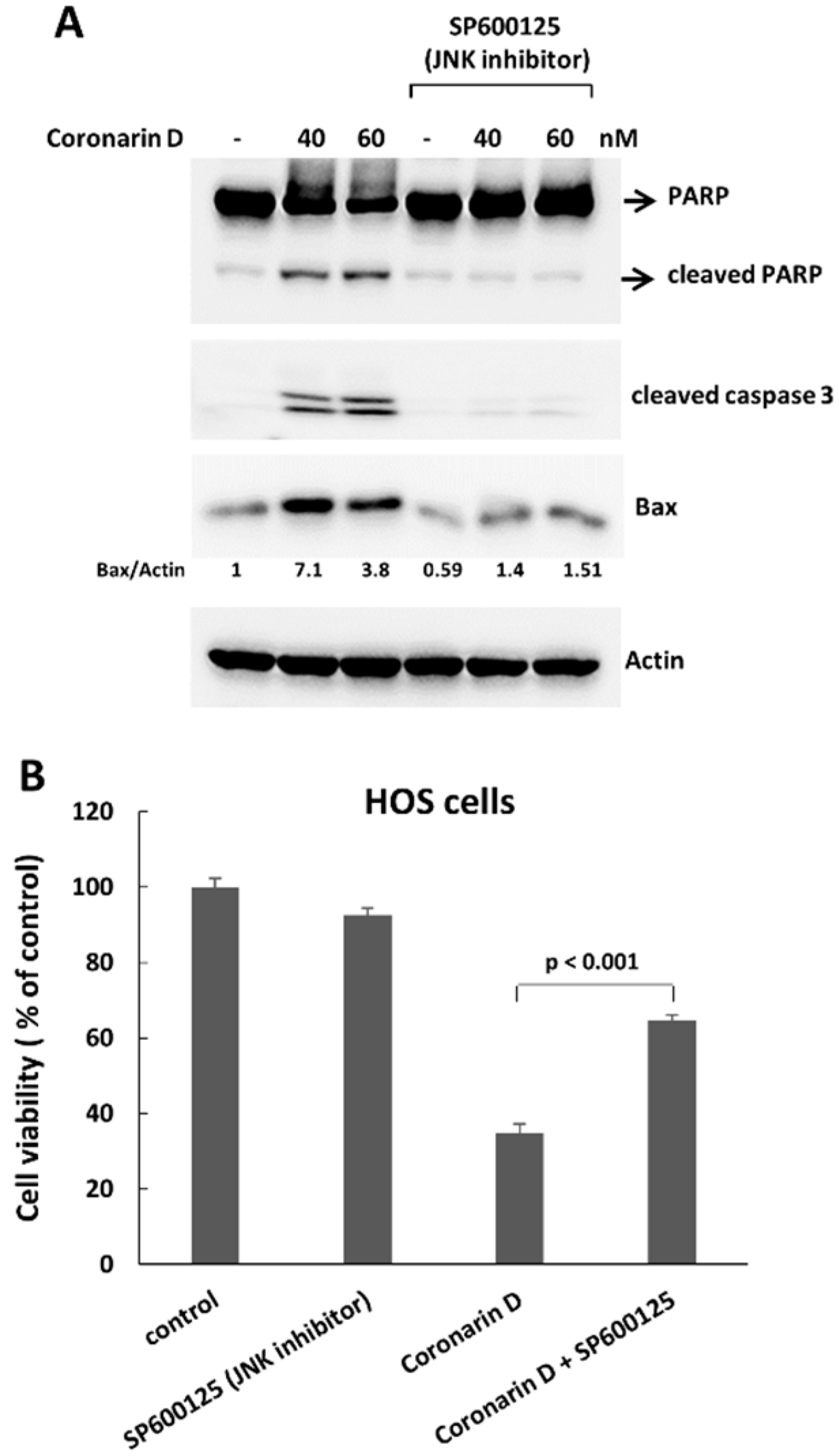
2.4. JNK Inactivation Eliminates the Accumulated Cyclin B1 and Induces Polyploidy in Coronarin D-Induced M Phase Arrest Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. MTT Assay
4.3. Flow Cytometry Analysis
4.4. Apoptosis Assay
4.5. Cell Lysis and Immunoblotting
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broadhead, M.L.; Clark, J.C.; Myers, D.E.; Dass, C.R.; Choong, P.F. The Molecular Pathogenesis of Osteosarcoma: A Review. Sarcoma 2011, 2011, e959248. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A Novel Benzimidazole Derivative, Mbic Inhibits Tumor Growth and Promotes Apoptosis Via Activation of Ros-Dependent Jnk Signaling Pathway in Hepatocellular Carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Basu, S.; Sarkar, N.; Ghosh, A.C. Advances in Cancer Therapy with Plant Based Natural Products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Kaomongkolgit, R.; Jamdee, K.; Wongnoi, S.; Chimnoi, N.; Techasakul, S. Antifungal Activity of Coronarin D against Candida Albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Reuk-ngam, N.; Chimnoi, N.; Khunnawutmanotham, N.; Techasakul, S. Antimicrobial Activity of Coronarin D and Its Synergistic Potential with Antibiotics. Biomed. Res. Int. 2014, 2014, 581985. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Ichikawa, H.; Anand, P.; Mohankumar, C.J.; Hema, P.S.; Nair, M.S.; Aggarwal, B.B. Coronarin D, a Labdane Diterpene, Inhibits Both Constitutive and Inducible Nuclear Factor-Kappa B Pathway Activation, Leading to Potentiation of Apoptosis, Inhibition of Invasion, and Suppression of Osteoclastogenesis. Mol. Cancer Ther. 2008, 7, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. Jnk-Signaling: A Multiplexing Hub in Programmed Cell Death. Genes Cancer 2017, 8, 682–694. [Google Scholar] [PubMed]
- Chen, J.C.; Hsieh, M.C.; Lin, S.H.; Lin, C.C.; Hsi, Y.T.; Lo, Y.S.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Coronarin D Induces Reactive Oxygen Species-Mediated Cell Death in Human Nasopharyngeal Cancer Cells through Inhibition of P38 Mapk and Activation of Jnk. Oncotarget 2017, 8, 108006–108019. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lyle, C.S.; Obey, T.B.; Gaarde, W.A.; Muir, J.A.; Bennett, B.L.; Chambers, T.C. Inhibition of Cell Proliferation and Cell Cycle Progression by Specific Inhibition of Basal Jnk Activity: Evidence That Mitotic Bcl-2 Phosphorylation Is Jnk-Independent. J. Biol. Chem. 2004, 279, 11957–11966. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.J.; Tsuji, T.; Cross, J.V.; Davis, R.J.; Templeton, D.J.; Jiang, W.; Ronai, Z.A. Jnk-Mediated Phosphorylation of Cdc25c Regulates Cell Cycle Entry and G(2)/M DNA Damage Checkpoint. J. Biol. Chem. 2010, 285, 14217–14228. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; An, S.; Yang, Y.; Liu, Y.; Hao, Q.; Tang, T.; Xu, T.R. Emerging Role of the Jun N-Terminal Kinase Interactome in Human Health. Cell Biol. Int. 2018, 42, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Deng, Z.H.; Zeng, C.; Lei, G.H. Jnk Pathway in Osteosarcoma: Pathogenesis and Therapeutics. J. Recept. Signal Transduct. 2016, 36, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Mingo-Sion, A.M.; Marietta, P.M.; Koller, E.; Wolf, D.M.; Van Den Berg, C.L. Inhibition of jnk reduces g2/m transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene 2004, 23, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.T.; Gonçalves, B.S.; Linden, R.; Chiarini, L.B. Activation of c-jun n-terminal kinase (jnk) during mitosis in retinal progenitor cells. PLoS ONE 2012, 7, e34483. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Buyuk, E.; Oktem, O.; Oktay, M.; Giancotti, F.G. The c-jun n-terminal kinase jnk functions upstream of aurora b to promote entry into mitosis. Cell Cycle 2008, 7, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Coquelle, A.; Vitale, I.; Vivet, S.; Mouhamad, S.; Viaud, S.; Zitvogel, L.; Kroemer, G. Selective resistance of tetraploid cancer cells against dna damage-induced apoptosis. Ann. N. Y. Acad. Sci. 2006, 1090, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Donthamsetty, S.; Kaur, R.; Yang, C.; Gupta, M.V.; Reid, M.D.; Choi, D.H.; Rida, P.C.G.; Aneja, R. Multinucleated Polyploidy Drives Resistance to Docetaxel Chemotherapy in Prostate Cancer. Br. J. Cancer 2017, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zeng, J.-Y.; Zhunag, C.; Zhang, R.; Wang, M. Abstract 921: Small-molecule inhibitor bms-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Cancer Res. 2013, 73, 921. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-T.; Huang, Y.-F.; Hsieh, C.-P.; Wu, C.-C.; Shen, T.-S. JNK Inactivation Induces Polyploidy and Drug-Resistance in Coronarin D-Treated Osteosarcoma Cells. Molecules 2018, 23, 2121. https://doi.org/10.3390/molecules23092121
Hsu C-T, Huang Y-F, Hsieh C-P, Wu C-C, Shen T-S. JNK Inactivation Induces Polyploidy and Drug-Resistance in Coronarin D-Treated Osteosarcoma Cells. Molecules. 2018; 23(9):2121. https://doi.org/10.3390/molecules23092121
Chicago/Turabian StyleHsu, Chang-Te, Yi-Fu Huang, Chen-Pu Hsieh, Chia-Chieh Wu, and Tai-Shan Shen. 2018. "JNK Inactivation Induces Polyploidy and Drug-Resistance in Coronarin D-Treated Osteosarcoma Cells" Molecules 23, no. 9: 2121. https://doi.org/10.3390/molecules23092121
APA StyleHsu, C.-T., Huang, Y.-F., Hsieh, C.-P., Wu, C.-C., & Shen, T.-S. (2018). JNK Inactivation Induces Polyploidy and Drug-Resistance in Coronarin D-Treated Osteosarcoma Cells. Molecules, 23(9), 2121. https://doi.org/10.3390/molecules23092121



