Study on the Quality Evaluation of Compound Danshen Preparations Based on the xCELLigence Real-Time Cell-Based Assay and Pharmacodynamic Authentication
Abstract
1. Introduction
2. Results
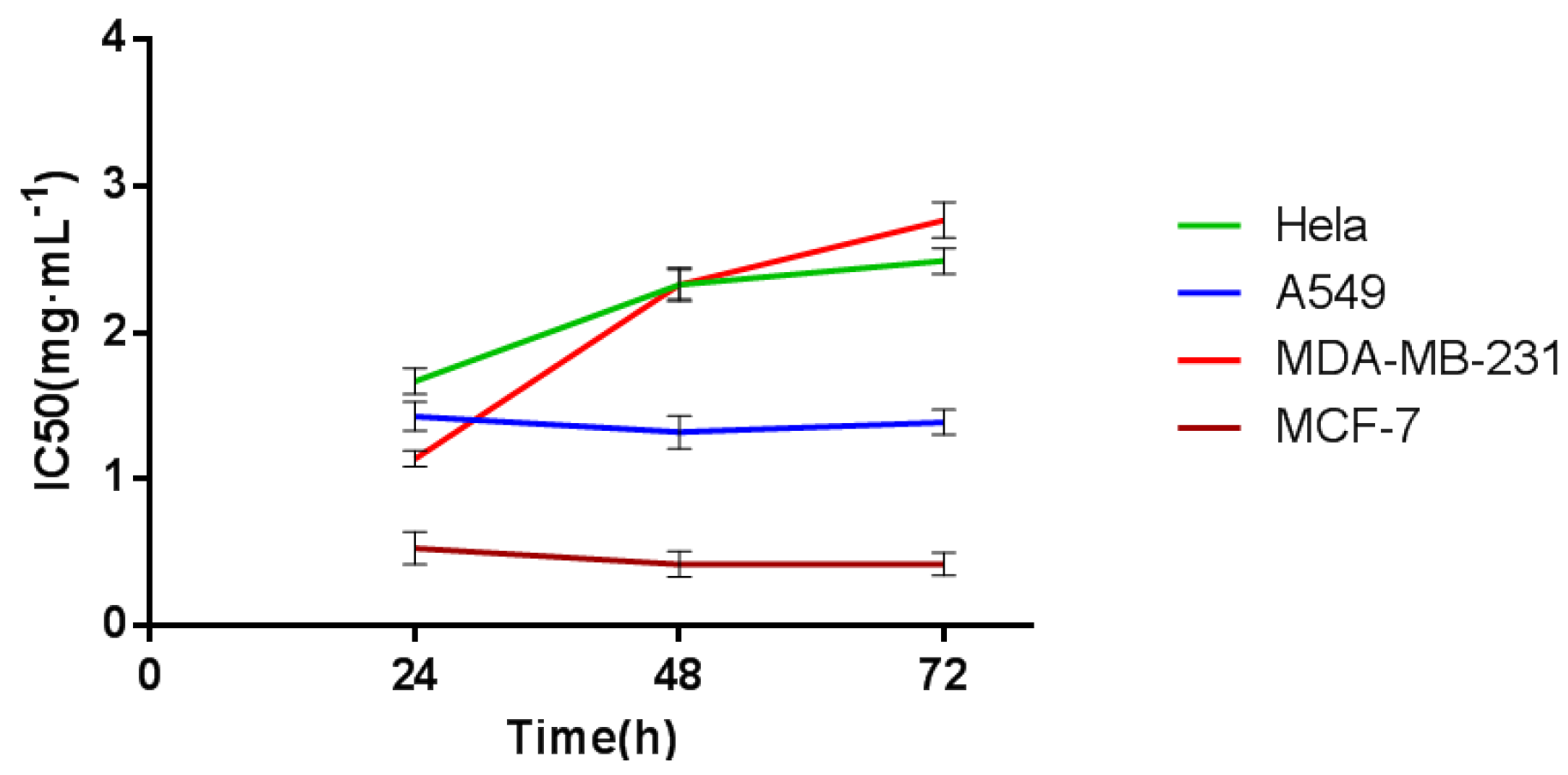
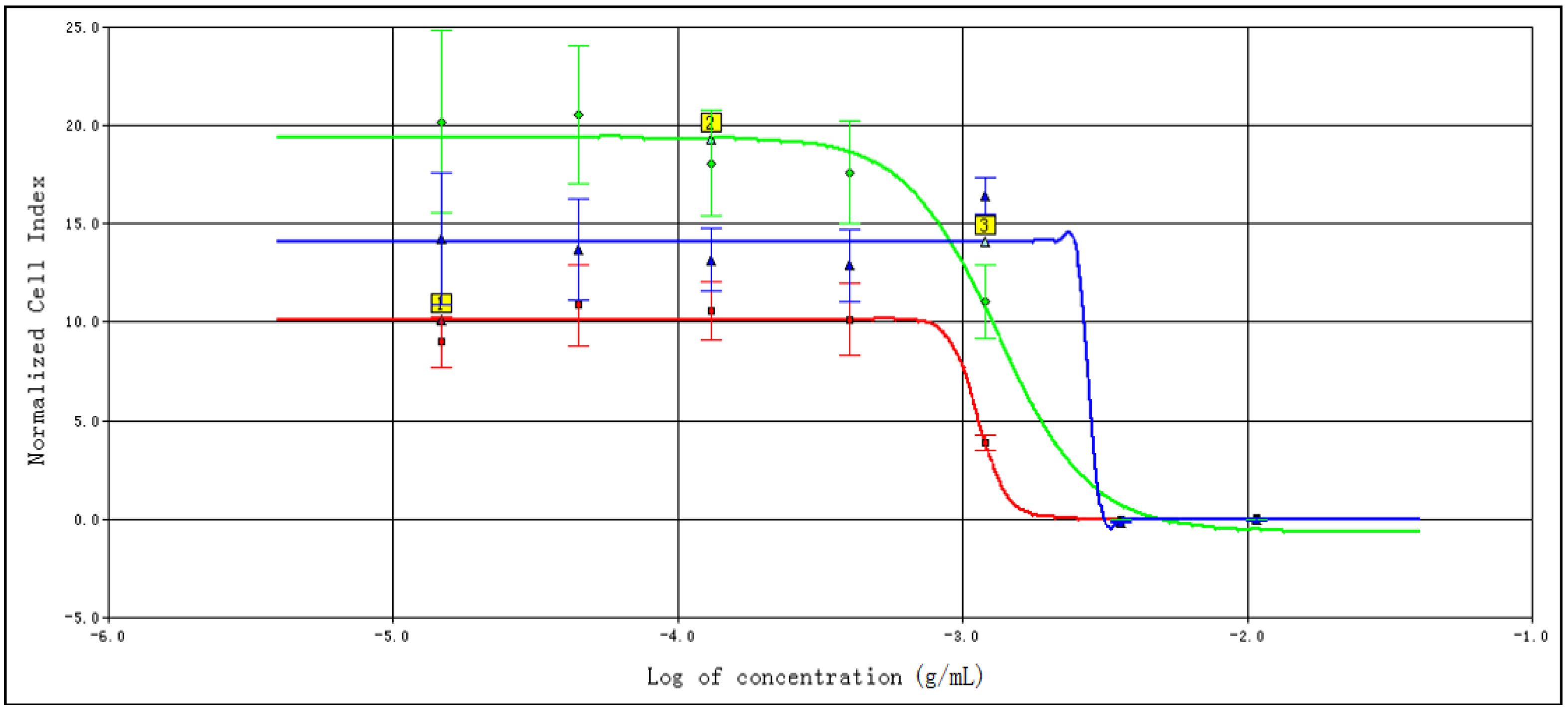
2.1. Screening of Specific Dependent Cell Line
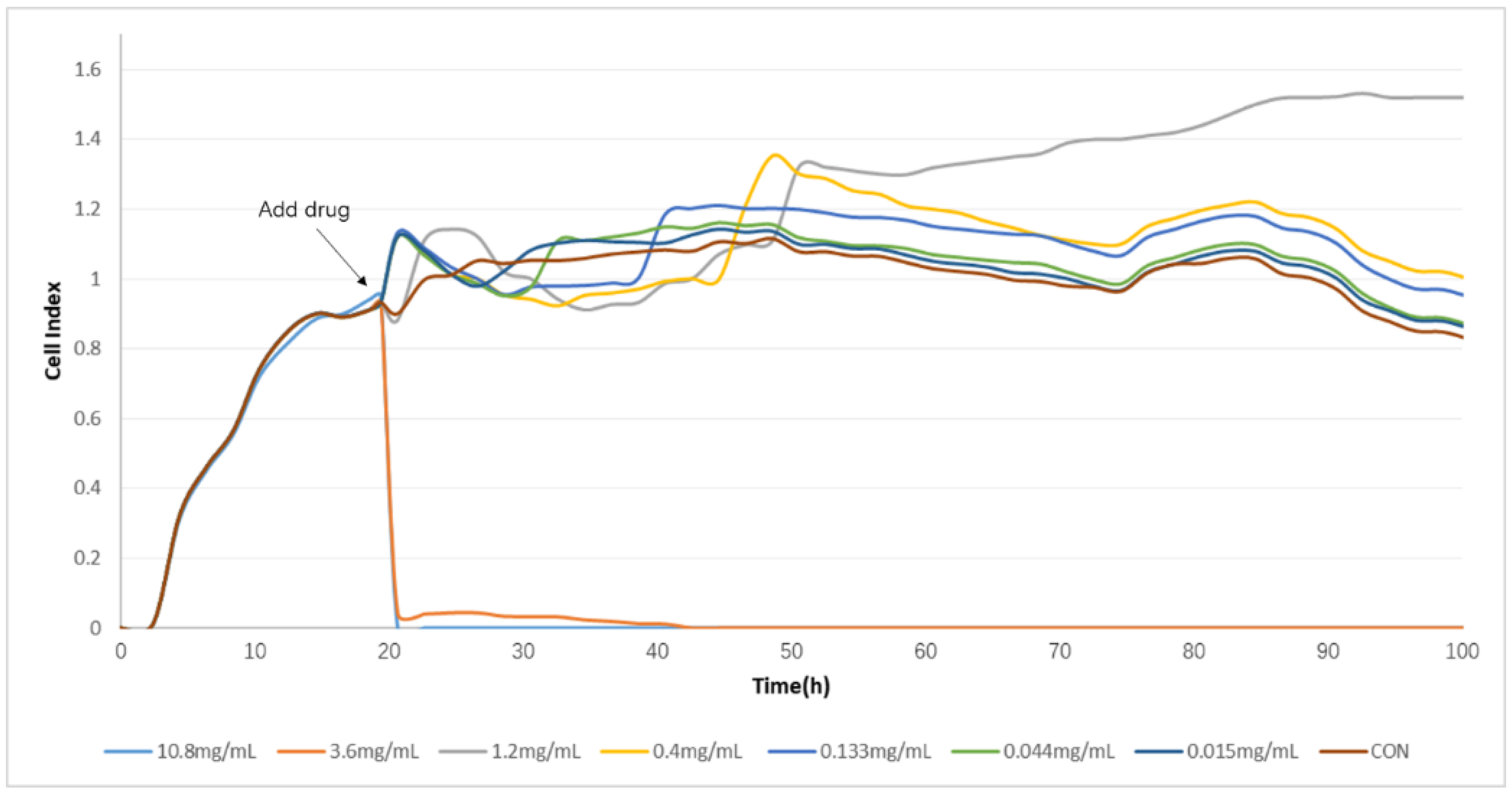
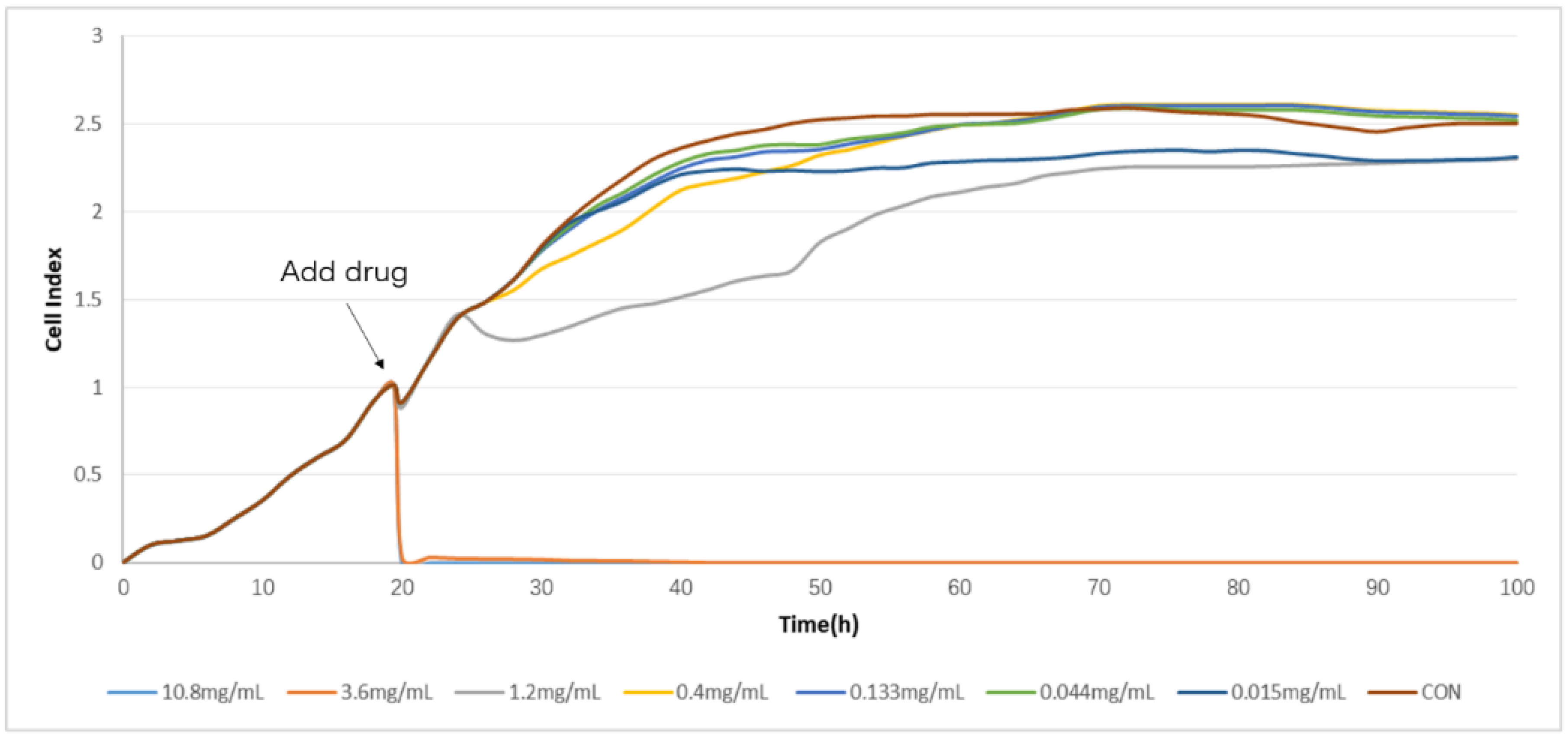
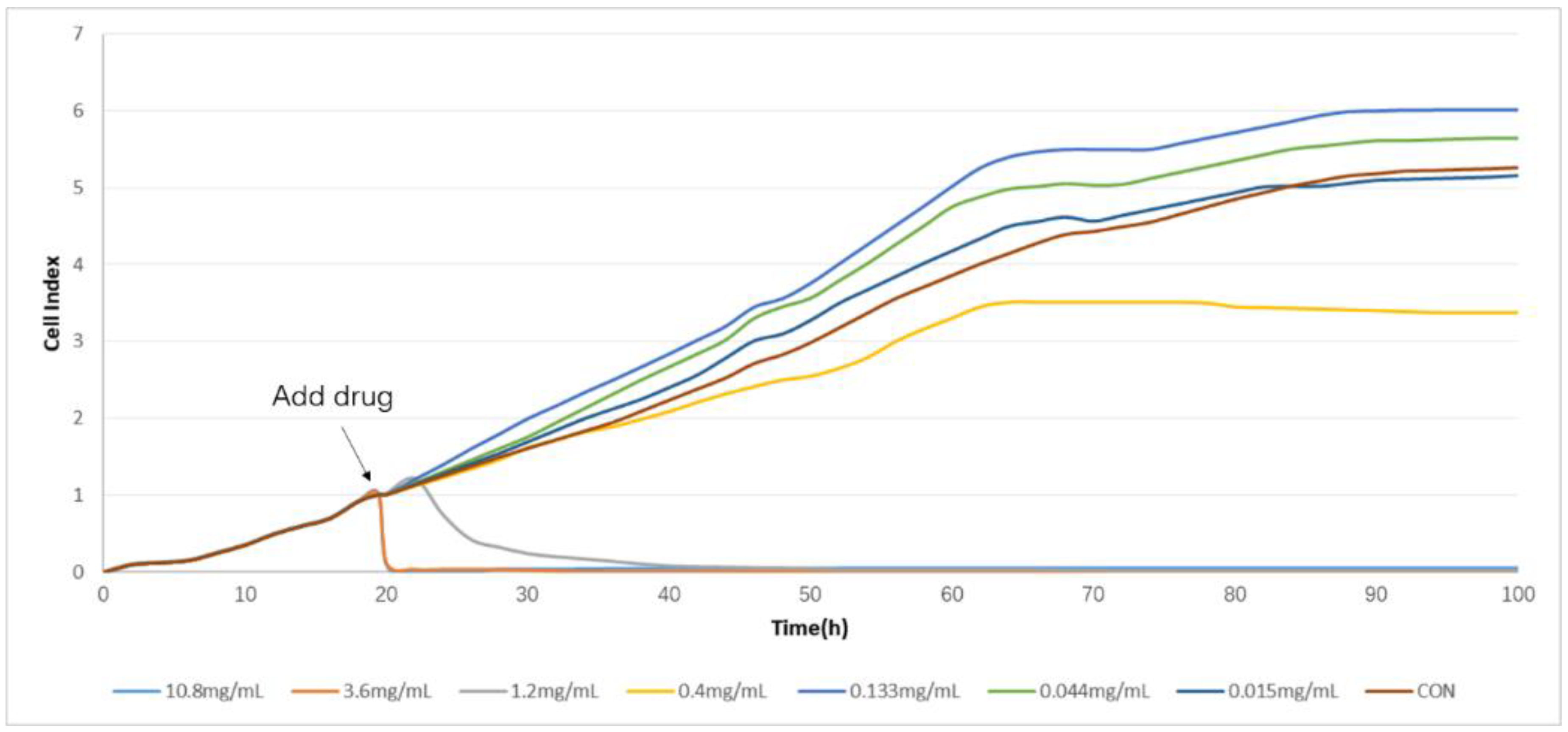
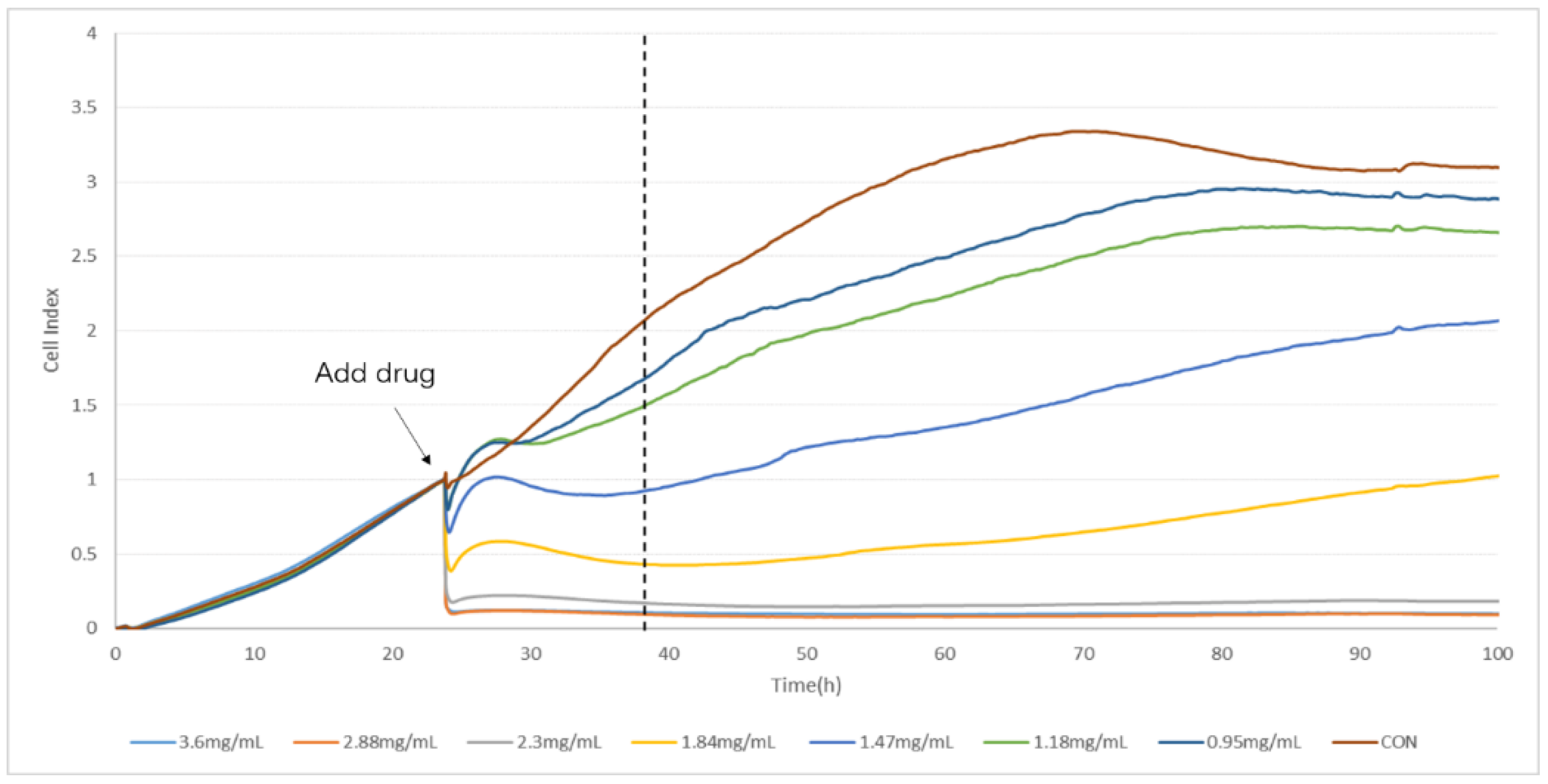
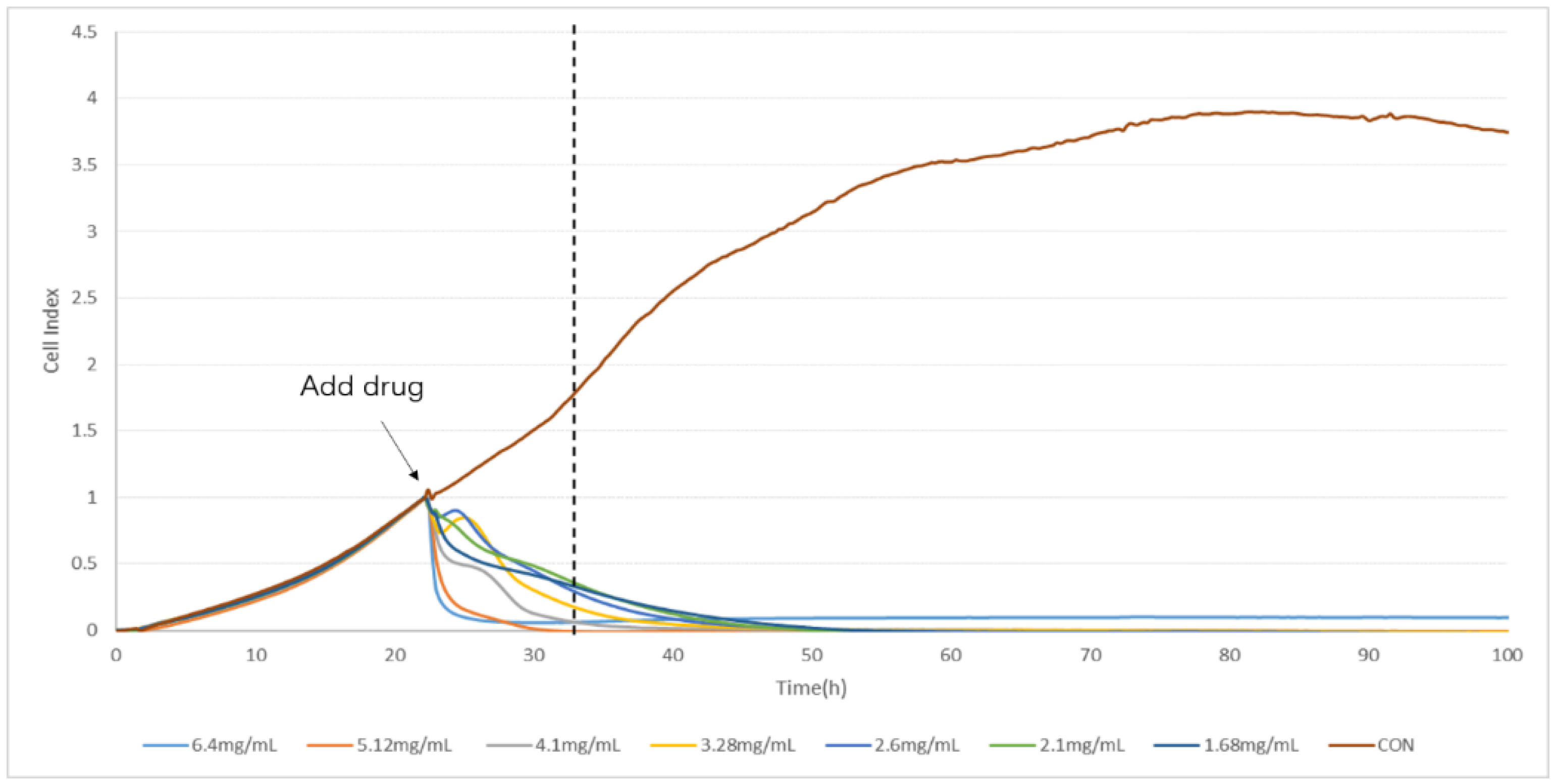
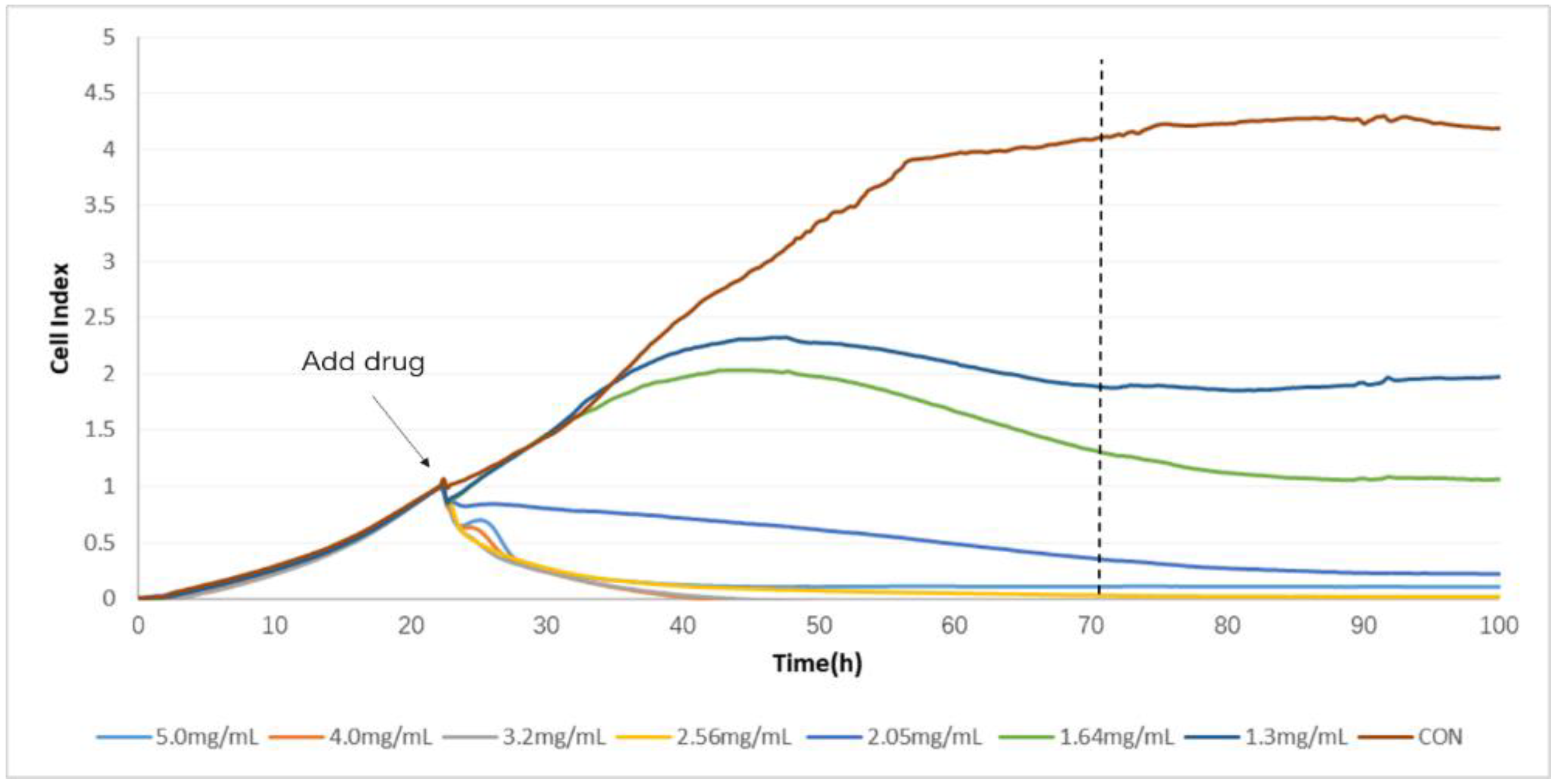
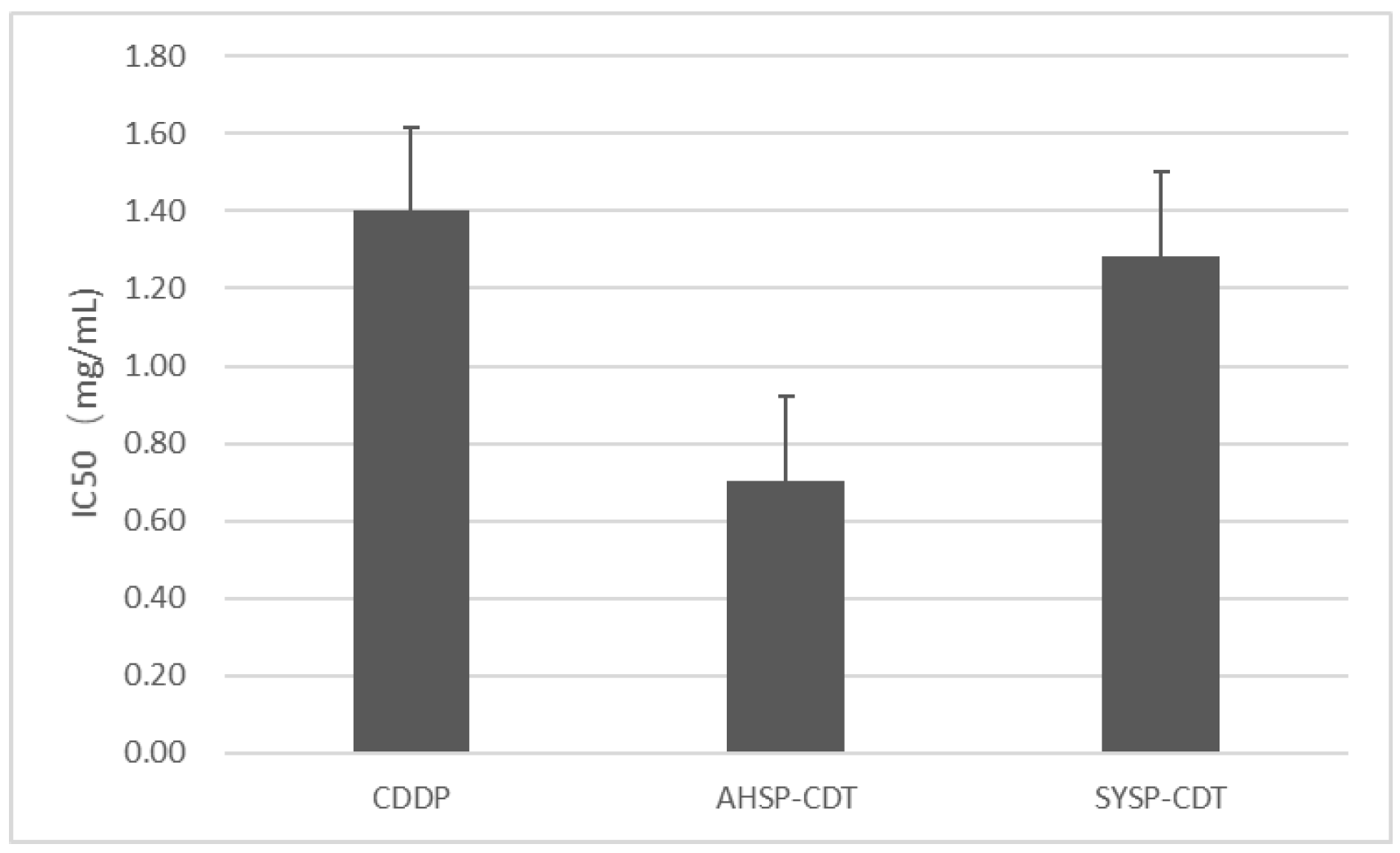
2.2. Determination of TCRPs of Compound Danshen Preparations
2.3. Results of Pharmacodynamic Authentication
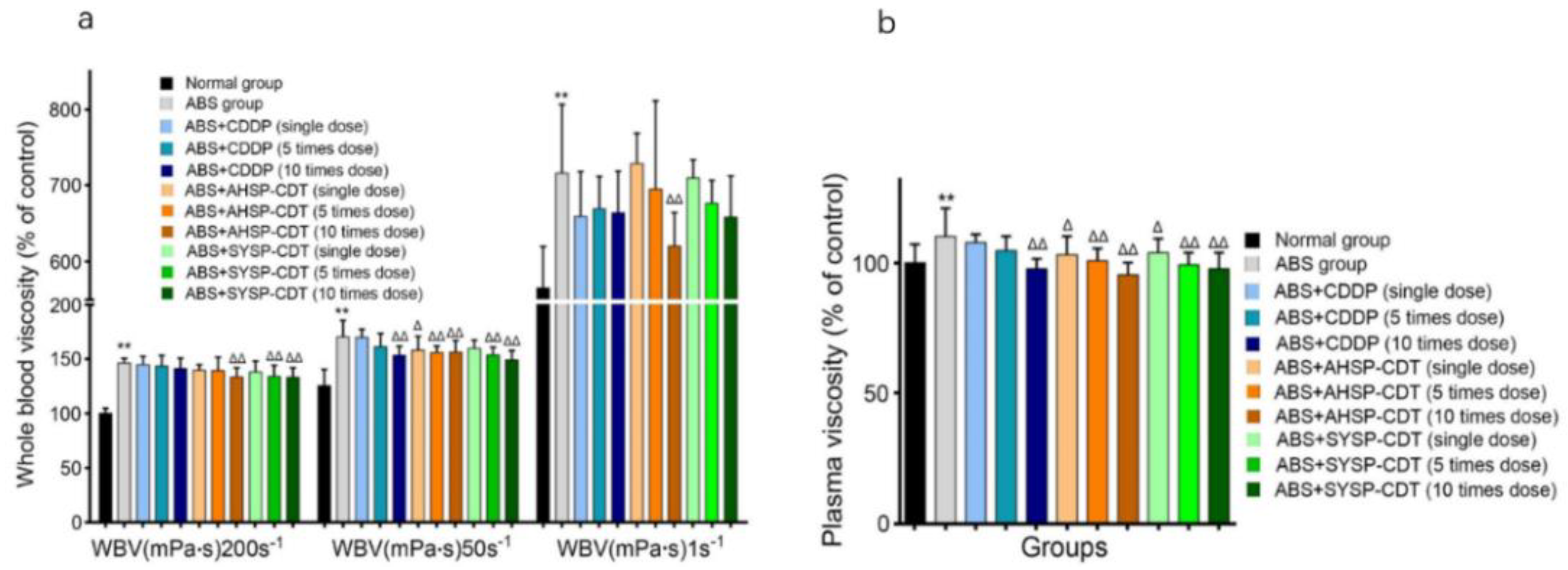
2.3.1. Effect of Danshen Preparations on Whole Blood Viscosity (WBV) and Plasma Viscosity (PV) of Acute Blood Stasis Rats
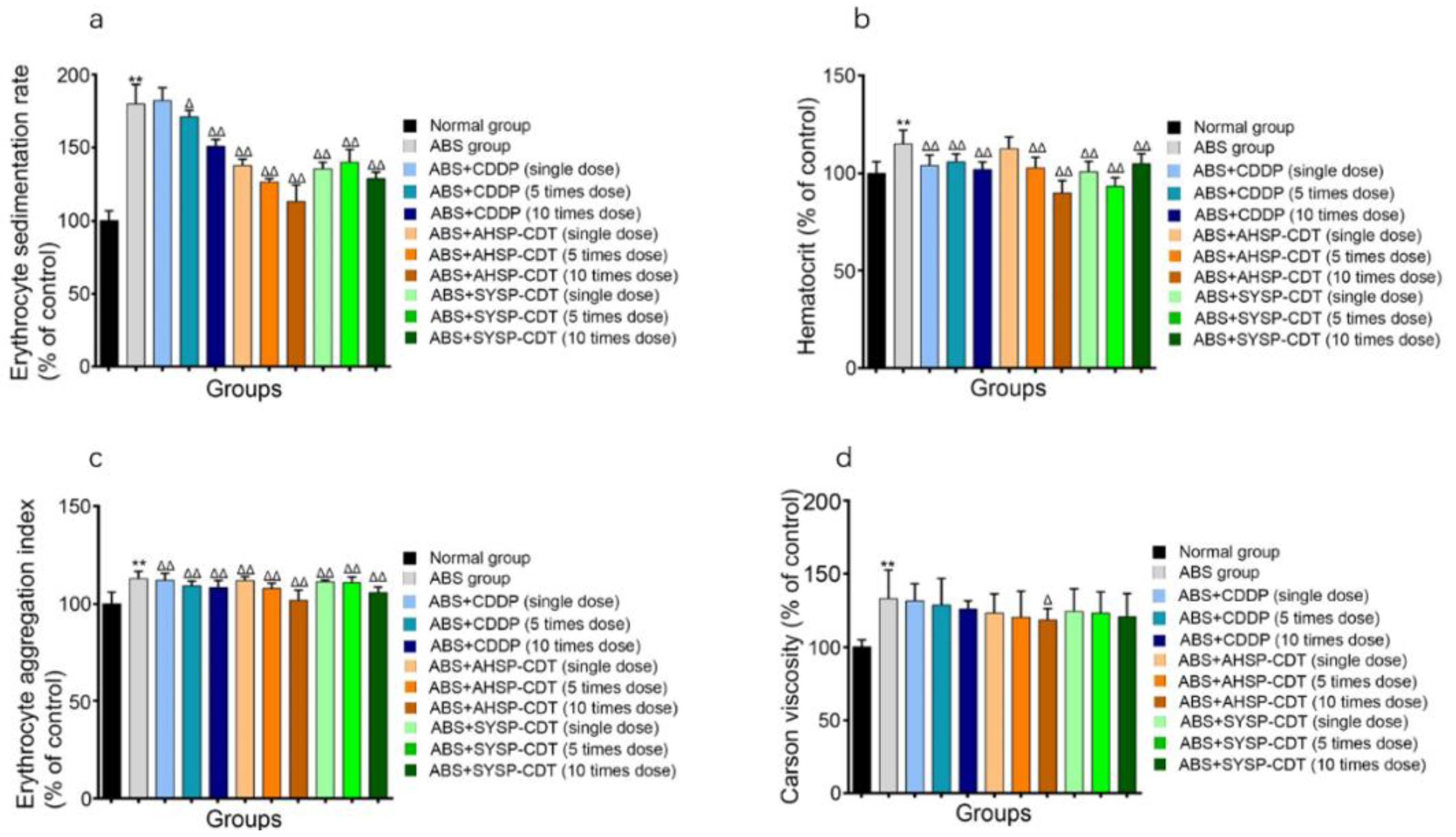
2.3.2. Effect of Danshen Preparations on the Erythrocyte Sedimentation Rate (ESR), Hematocrit (HCT), Erythrocyte Aggregation Index (EAI), and Carson Viscosity of Acute Blood Stasis Rats
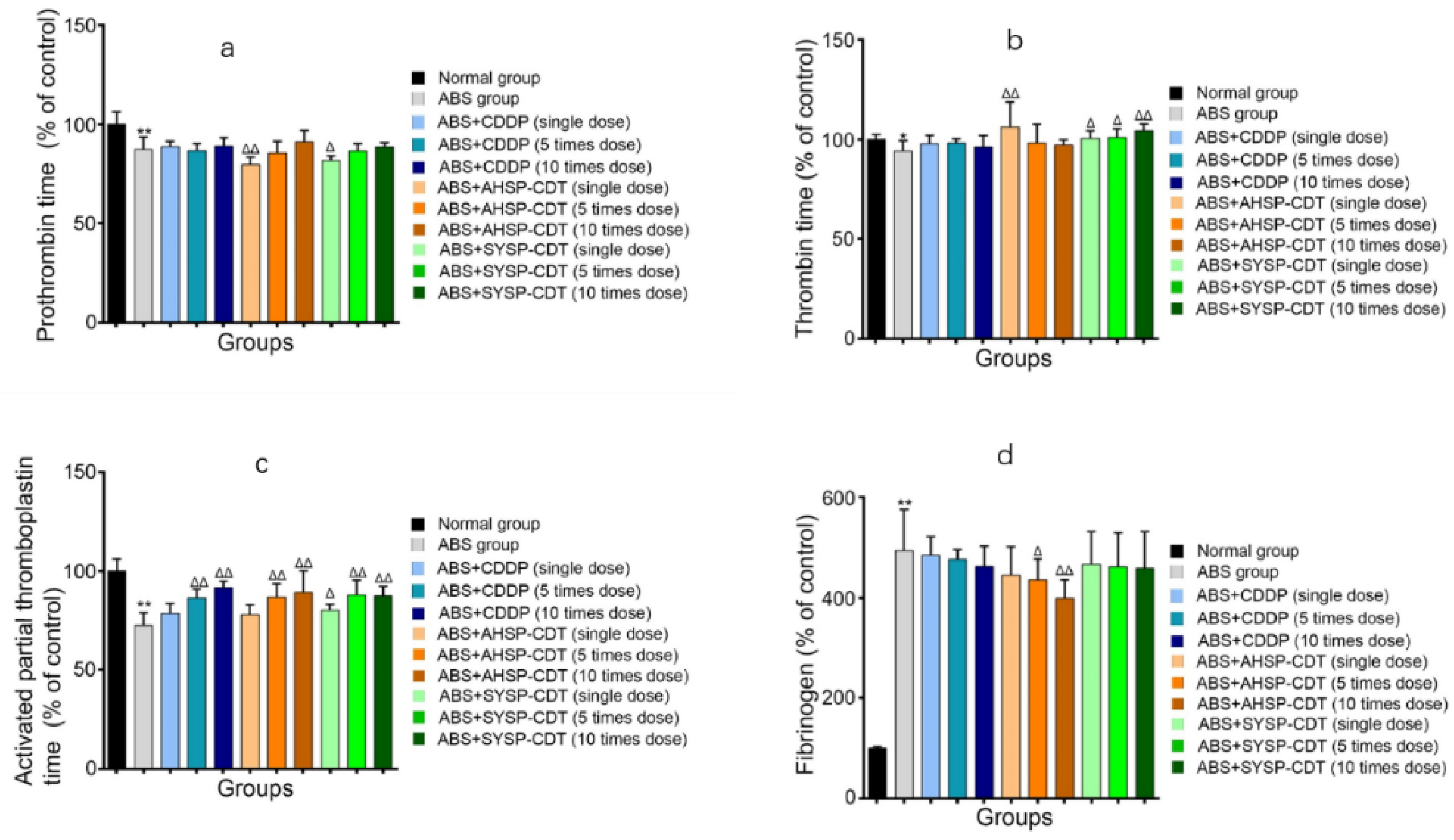
2.3.3. Effect of Danshen Preparations on the Prothrombin Time (PT), Thrombin Time (TT), Activated Partial Thromboplastin Time (APTT), and Fibrinogen (FIB) of Acute Blood Stasis Rats
2.3.4. The Total Value of the Blood Circulation Promotion Effect of Danshen Preparations Based on Multi-Index Comprehensive Index Value.
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Samples
4.3. Cell Line Screening
4.4. Determination of TCRPs of Compound Danshen Preparations
5. Pharmacodynamics Studies In Vivo of Compound Danshen Preparation
5.1. Animal
5.2. Reagent
5.3. Preparation of Acute Blood Stasis Model
5.4. Animal Grouping and Administration
5.5. Measuring Indicators of Blood Rheology and Coagulation
5.6. Statistical Method
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Geng, S.; Yi, X.; Chen, J.; Jiang, K.; Wu, Y. Recent Advances in Quality Analysis and Evaluation of Traditional Chinese Medicines. Phys. Test. Chem. Anal. 2015, 51, 1035–1042. [Google Scholar]
- Xiao, X.H.; Jin, C.; Zhao, Z.Z.; Xiao, P.G.; Wang, Y.Y. Probe into innovation and development of pattern of quality control and evaluation for Chinese medicine. China J. Chin. Mater. Med. 2007, 32, 1377–1381. [Google Scholar]
- Wang, P.; Qian, Z. The readable explanation of the editorial outline of Chinese Pharmacopoeia 2010 edition. Chin. J. Pharm. Anal. 2008, 28, 337–340. [Google Scholar]
- Chen, G.Y.; Wu, Q.N.; Wang, X.S.; Liang, Q.L.; He, X.X. Study on quality evaluation of Sparganii rhizoma by biopotency determination method. China J. Chin. Mater. Med. 2012, 37, 2913–2916. [Google Scholar]
- Zhou, D.L.; Yan, D.; Li, B.C.; Wu, Y.S.; Xiao, X.H. Investigation of metabolic action of Cordyceps sinensis and its cultured mycelia on Escherichia coli by microcalorimetry. Acta Pharm. Sin. 2009, 44, 640–644. [Google Scholar]
- Yan, G.; Wang, X.; Chen, Z.; Wu, X.; Pan, J.; Huang, Y.; Wan, G.; Yang, Z. In-silico ADME Studies for New Drug Discovery: From Chemical Compounds to Chinese Herbal Medicines. Curr. Drug Metab. 2017, 18, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Serra, J.; Gutierrez, A.; Munoz-Capo, S.; Navarro-Palou, M.; Ros, T.; Amat, J.C.; Lopez, B.; Marcus, T.F.; Fueyo, L.; Suquia, A.G.; et al. xCELLigence system for real-time label-free monitoring of growth and viability of cell lines from hematological malignancies. OncoTargets Ther. 2014, 7, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lei, K.F.; Yeh, W.L.; Chen, P.; Chan, Y.S.; Hsu, K.Y.; Chen, A.C. Comparison between xCELLigence biosensor technology and conventional cell culture system for real-time monitoring human tenocytes proliferation and drugs cytotoxicity screening. J. Orthop. Surg. Res. 2017, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Ma, L.-N.; Yan, D.; Zhang, C.-E.; Gao, D.; Xiong, Y.; Sheng, F.-Y.; Dong, X.-P.; Xiao, X.-H. Dynamic monitoring of the cytotoxic effects of protoberberine alkaloids from Rhizoma Coptidis on HepG2 cells using the xCELLigence system. Chin. J. Nat. Med. 2014, 12, 428–435. [Google Scholar] [CrossRef]
- Fu, H.; Fu, W.; Sun, M.; Shou, Q.; Zhai, Y.; Cheng, H.; Teng, L.; Mou, X.; Li, Y.; Wan, S.; et al. Kinetic Cellular Phenotypic Profiling: Prediction, Identification, and Analysis of Bioactive Natural Products. Anal. Chem. 2011, 83, 6518–6526. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-J.; Mei, X.; Yang, J.; Zhu, J.; Wu, J.-Y.; Pan, J.-H. A novel real-time cell electronic analysis technology for the authentication and quality control of natural medicines. Chin. Chem. Lett. 2013, 24, 1140–1144. [Google Scholar] [CrossRef]
- Mei, X.; Ge, S.; Pan, J. Preliminary Study on the Real-time Cell Electronic Analysis Technology in Quality Evaluation of Hirudo nipponica Whitman and Panax notoginseng. Chin. J. Pharm. 2013, 44, 872–877. [Google Scholar]
- Yan, G.; Sun, W.; Pei, Y.; Yang, Z.; Wang, X.; Sun, Y.; Yang, S.; Pan, J. A novel release kinetics evaluation of Chinese compound medicine: Application of the xCELLigence RTCA system to determine the release characteristics of Sedum sarmentosum compound sustained-release pellets. Saudi Pharm. J. 2018, 26, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Irelan, J.T.; Wu, M.-J.; Morgan, J.; Ke, N.; Xi, B.; Wang, X.; Xu, X.; Abassi, Y.A. Rapid and Quantitative Assessment of Cell Quality, Identity, and Functionality for Cell-Based Assays Using Real-Time Cellular Analysis. J. Biomol. Screen. 2011, 16, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Fang, P.; Hong, Z.; Ying, W.; Huo, P. Research Progress in Application of Real-Time Cell-based Assay(RTCA). Chin. Pharm. J. 2014, 49, 169–173. [Google Scholar]
- Zhu, J.; Wang, X.; Xu, X.; Abassi, Y.A. Dynamic and label-free monitoring of natural killer cell cytotoxic activity using electronic cell sensor arrays. J. Immunol. Methods 2006, 309, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiong, Z.; Lu, X.; Wen, J.; Liu, J. Advance in the Quality and Evaluation of Traditional Chinese Medicine and the Role of Systems Biology. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2009, 11, 120–126. [Google Scholar]
- Iwasaki, H.; Inafuku, M.; Taira, N.; Saito, S.; Oku, H. Tumor-Selective Cytotoxicity of Nitidine Results from Its Rapid Accumulation into Mitochondria. BioMed Res. Int. 2017, 2017, 2130594. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Nemade, H.; Chaudhari, U.; Acharya, A.; Hescheler, J.; Hengstler, J.G.; Papadopoulos, S.; Sachinidis, A. Cell death mechanisms of the anti-cancer drug etoposide on human cardiomyocytes isolated from pluripotent stem cells. Arch. Toxicol. 2018, 92, 1507–1524. [Google Scholar] [CrossRef] [PubMed]
- Chaousis, S.; Smout, M.; Wilson, D.; Loukas, A.; Mulvenna, J.; Seymour, J. Rapid short term and gradual permanent cardiotoxic effects of vertebrate toxins from Chironex fleckeri (Australian box jellyfish) venom. Toxicon 2014, 80, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, Y.; Zhou, J. Clinical Study of Promoting Blood Circulation and Removing Blood Stasis in Treating Malignant Tumor. J. Liaoning Univ. Tradit. Chin. Med. 2013, 15, 177–179. [Google Scholar]
- Zhou, Y.; Yang, H.; Guan, J.; Zhou, L.; Qin, S.; Xu, J.; Hu, Z. Effect of 5 Different Categories of Chinese Herbal Medicine on Proliferation of Human Lung Cancer A549 Cell. J. Chengdu Univ. Tradit. Chin. Med. 2013, 36, 15–17. [Google Scholar]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Control. Release 2016, 243, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, M.; Tang, Y.; Guo, J.; Shang, E.; Liu, X.; Qian, D.; Duan, J. Establishment and optimization of acute blood stasis rat model. Chin. Pharmacol. Bull. 2011, 27, 1761–1765. [Google Scholar]
- Zhang, J.; Feng, Y.; Li, S.; Liu, Y.; Zhang, Y.; Guo, Y.; Yang, M. Microvascular pathological features and changes in related injury factors in a rat acute blood stasis model. J. Tradit. Chin. Med. 2017, 37, 108–115. [Google Scholar] [PubMed]
- Liu, Z.; Xie, W.; Li, M.; Teng, N.; Liang, X.; Zhang, Z.; Yang, Z.; Wang, X. Oral Administration of Polaprezinc Attenuates Fluorouracil-induced Intestinal Mucositis in a Mouse Model. Basic Clin. Pharmacol. Toxicol. 2017, 121, 480–486. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds CDDP, CDT are available from the authors. |


| Group | Vs a | Vs b | Vs c | Vs d | Total Value of Blood Circulation Promotion Effect (4a + 3b + 2c + 1d) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBV200 | WBV50 | WBV1 | PV | HCT | FIB | ESR | PT | APTT | TT | EAI | Carson Viscosity | ||
| Normal | 0.32 | 0.26 | 0.21 | 0.09 | 0.13 | 0.80 | 0.44 | 0.14 | 0.38 | 0.06 | 0.11 | 0.25 | 8.78 |
| CDDP (single dose) | 0.01 | 0.00 | 0.08 | 0.02 | −0.01 | 0.02 | −0.01 | 0.01 | 0.08 | 0.04 | 0.01 | 0.01 | 0.68 |
| CDDP (5 times dose) | 0.02 | 0.05 | 0.06 | 0.05 | 0.05 | 0.03 | 0.05 | −0.01 | 0.19 | 0.04 | 0.03 | 0.03 | 1.57 |
| CDDP (10 times dose) | 0.03 | 0.10 | 0.07 | 0.11 | 0.16 | 0.06 | 0.16 | 0.02 | 0.26 | 0.02 | 0.04 | 0.05 | 3.05 |
| AHSP-CDT (single dose) | 0.04 | 0.07 | −0.02 | 0.06 | 0.23 | 0.10 | 0.23 | −0.09 | 0.07 | 0.13 | 0.01 | 0.07 | 2.45 |
| AHSP-CDT (5 times dose) | 0.05 | 0.08 | 0.03 | 0.08 | 0.11 | 0.12 | 0.30 | −0.02 | 0.20 | 0.05 | 0.04 | 0.09 | 2.9 |
| AHSP-CDT (10 times dose) | 0.09 | 0.08 | 0.13 | 0.13 | 0.22 | 0.20 | 0.37 | 0.04 | 0.23 | 0.03 | 0.10 | 0.11 | 4.72 |
| SYSP-CDT (single dose) | 0.05 | 0.06 | 0.01 | 0.06 | 0.12 | 0.05 | 0.25 | −0.06 | 0.11 | 0.07 | 0.01 | 0.06 | 2.09 |
| SYSP-CDT (5 times dose) | 0.08 | 0.10 | 0.06 | 0.10 | 0.19 | 0.06 | 0.22 | −0.01 | 0.21 | 0.07 | 0.02 | 0.07 | 3.3 |
| SYSP-CDT (10 times dose) | 0.09 | 0.12 | 0.08 | 0.11 | 0.09 | 0.07 | 0.28 | 0.01 | 0.20 | 0.11 | 0.06 | 0.09 | 3.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Zhu, Z.; Jin, L.; Chen, J.; Xie, H.; Miozzi, J.; Lei, F.; Wei, X.; Pan, J. Study on the Quality Evaluation of Compound Danshen Preparations Based on the xCELLigence Real-Time Cell-Based Assay and Pharmacodynamic Authentication. Molecules 2018, 23, 2090. https://doi.org/10.3390/molecules23092090
Yan G, Zhu Z, Jin L, Chen J, Xie H, Miozzi J, Lei F, Wei X, Pan J. Study on the Quality Evaluation of Compound Danshen Preparations Based on the xCELLigence Real-Time Cell-Based Assay and Pharmacodynamic Authentication. Molecules. 2018; 23(9):2090. https://doi.org/10.3390/molecules23092090
Chicago/Turabian StyleYan, Guojun, Zhitao Zhu, Liliang Jin, Jun Chen, Hui Xie, Jackelyn Miozzi, Feifei Lei, Xuchao Wei, and Jinhuo Pan. 2018. "Study on the Quality Evaluation of Compound Danshen Preparations Based on the xCELLigence Real-Time Cell-Based Assay and Pharmacodynamic Authentication" Molecules 23, no. 9: 2090. https://doi.org/10.3390/molecules23092090
APA StyleYan, G., Zhu, Z., Jin, L., Chen, J., Xie, H., Miozzi, J., Lei, F., Wei, X., & Pan, J. (2018). Study on the Quality Evaluation of Compound Danshen Preparations Based on the xCELLigence Real-Time Cell-Based Assay and Pharmacodynamic Authentication. Molecules, 23(9), 2090. https://doi.org/10.3390/molecules23092090





