Abstract
In this study, a series of novel pyrazole-hydrazone derivatives containing an isoxazole moiety were synthesized. Antiviral bioassays indicated that some of the title compounds exhibited better in vivo antiviral activities against tobacco mosaic virus (TMV). In particular, compounds 6a, 6c and 6q exhibited the best curative activity, protection activity, and inactivation activity against TMV, respectively, which were superior to those of Ningnanmycin. This study demonstrated that this series of novel pyrazole-hydrazone derivatives containing an isoxazole amide moiety could effectively control TMV.
1. Introduction
Plant viruses cause diseases in a wide range of crop plant species and annually contribute to an estimated $600 billion in economic loss worldwide [1]. In recent years, progress has been made in screening for high-activity antiviral agents in natural products in China. For example, Ningnanmycin, isolated from Strepcomcesnoursei var. Xichangensis, was found to be more effective than existing products in the treatment of plant viruses such as tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV) [2]. Meanwhile, Ribavirin, a successful antiviral agent, is widely used to prevent TMV disease. However, the use of Ningnanmycin and Ribavirin in field trials is unsuitable because of its unsatisfactory curative rates and high control costs [3]. Therefore, identification of new effective antiviral agents remains a significant challenge [4].
In recent years, literature revealed that pyrazole derivatives had a wide range of biological activities, such as antibacterial [5,6,7], fungicidal [8,9,10], insecticidal [10,11,12,13], antiviral [14,15], and herbicidal [16,17] activities. In our previous work, we reported a series of pyrazole derivatives which had better insecticidal activity against Plutella xylostella [18]. Meanwhile, hydrazone, a highly efficient pharmacophore, has attracted more and more attention in the pesticides areas due to its broad-activities, such as antibacterial [19], fungicidal [20,21], insecticidal [20,22,23], and antiviral [20,24] activities. A few of the pyrazole-hydrazone derivatives have also been reported and possess various biological activities such as antibacterial [25], antifungal [21], anticancer [26], antioxidant [27], anti-inflammatory [28], antitumor and antiangiogenesis [29] and antiplatelet activities [30]. In addition, isoxazole, a five membered heterocyclic ring, was an important scaffold for synthesis of various natural compounds and their congeners and had a broad spectrum of pharmacological activities like antibacterial [31], antifungal [9,32,33], insecticide [34,35], and antiviral [36] activities.
Motivated by the above-mentioned findings and in continuation of our investigation, to discover new potentially active agents, in this study, a series of novel pyrazole-hydrazone derivatives containing an isoxazole moiety were designed and synthesized using a 3-substituted phenyl-5-methyl isoxazole-4-carboxylic acid as the starting material. The antiviral bioassay results indicated that most of the title compounds exhibited excellent in vivo antiviral activities against TMV. To the best of our knowledge, it is the first report on pyrazole-hydrazone derivatives containing an isoxazole moiety with potent antiviral activity against TMV.
2. Results and Discussion
2.1. Chemistry
Using 3-(2-chlorophenyl)-5-methyl isoxazole-4-carboxylic acid and 3-(2,6-dichlorophenyl)-5-methyl isoxazole-4-carboxylic acid as the starting materials, as shown in Scheme 1, the target compounds 6a–6r were obtained in five steps with yields of 85.9–91.2%. The physical characteristics, IR, 1H-NMR, 13C-NMR, and elemental analysis data for all the synthesized compounds are shown below. The 1H-NMR and 13C-NMR for all the synthesized compounds are shown in Supplementary Materials.
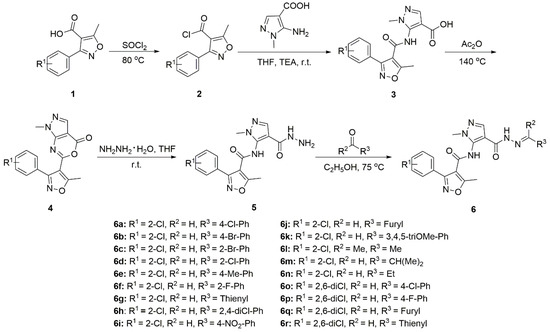
Scheme 1.
Synthetic route of the title compounds 6a–6r.
Data for N-(4-(2-(4-chlorobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-3-(2-chlorophenyl)-5-methylisoxazole-4-carboxamide (6a). White solid; m.p. 141–143 °C; yield 87.9%; 1H-NMR (500 MHz, Dimethylsulfoxide-d6 (DMSO-d6), ppm) δ: 11.54 (s, 1H, isoxazole–CONH–), 10.32 (s, 1H, pyrazole–CONH–), 8.31 (s, 1H, –N=CH–), 8.01 (s, 1H, pyrazole-H), 7.74–7.47 (m, 8H, Ar–H), 3.65 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.53, 160.66, 158.43, 147.02, 145.78, 144.08, 140.42, 140.78, 139.24, 137.88, 137.67, 132.84, 132.15, 130.25, 130.13, 129.97, 127.94, 127.50, 127.26, 113.72, 109.57, 36.66, 12.99. IR (KBr, cm−1) ν: 3404.36, 3282.84, 2933.73, 1716.65, 1699.29, 1683.86, 1653.00, 1635.64, 1627.92, 1595.13, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1458.18, 1436.97, 1411.89, 1375.25, 1303.88, 1253.75, 1219.01. Anal. Calc. for C23H18Cl2N6O3: C, 55.55%; H, 3.65%; N, 16.90%; Found: C, 55.81%; H, 3.79%; N, 17.08%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(4-bromobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6b). White solid; m.p. 236–237 °C; yield 90.1%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.57 (s, 1H, isoxazole–CONH–), 10.35 (s, 1H, pyrazole–CONH–), 8.29 (s, 1H, –N=CH–), 8.01 (s, 1H, pyrazole-H), 7.85–7.85 (d, 1H, J = 3.45 Hz, Ar–H), 7.66–7.45 (m, 7H, Ar–H), 3.66 (s, 3H, pyrazole–CH3), 2.86 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.46, 160.67, 158.38, 145.62, 137.80, 134.21, 132.87, 132.38, 132.36, 132.17, 132.16, 131.80, 130.14, 129.37, 129.19, 127.96, 127.93, 123.57, 113.57, 109.52, 36.55, 12.99. IR (KBr, cm−1) ν: 3444.87, 326.91, 2933.73, 1716.65, 1699.29, 1683.86, 1662.64, 1653.00, 1635.64, 1608.63, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1458.18, 1436.97, 1429.25, 1398.39, 1319.31, 1301.95. Anal. Calc. for C23H18ClBrN6O3: C, 50.99%; H, 3.35%; N, 15.51%; Found: C, 51.09%; H, 3.39%; N, 15.55%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(2-bromobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6c). White solid; m.p. 181–183 °C; yield 89.2%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.69 (s, 1H, isoxazole–CONH–), 10.30 (s, 1H, pyrazole–CONH–), 8.61 (s, 1H, –N=CH–), 7.97 (s, 1H, pyrazole–H), 7.91 (d, 1H, J = 7.45 Hz, Ar–H), 7.63 (d,1H, J = 8.00 Hz, Ar–H), 7.54–7.38 (m, 5H, A–H), 7.31 (t, 1H, J = 14.85 Hz, Ar–H), 3.59 (s, 3H, pyrazole–CH3), 2.79 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 160.56, 158.40, 158.18, 152.48, 147.87, 144.98, 137.96, 137.71, 133.63, 132.75, 132.10, 131.86, 130.07, 128.58, 127.88, 127.71, 127.63, 123.88, 113.69, 113.58, 112.30, 36.61, 12.91. IR (KBr, cm−1) ν: 3446.79, 3298.28, 2933.73, 1716.65, 1699.29, 1683.86, 1670.35, 1653.00, 1635.64, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1458.18, 1436.97, 1411.89, 1398.39, 1361.74, 1338.60, 1319.31, 1298.09, 1253.73, 1220.94. Anal. Calc. for C23H18ClBrN6O3: C, 50.99%; H, 3.35%; N, 15.51%; Found: C, 51.06%; H, 3.41%; N, 15.57%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(2-chlorobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6d). White solid; m.p. 170–171 °C; yield 88.7%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.64 (s, 1H, isoxazole–CONH–), 10.28 (s, 1H, pyrazole–CONH–), 8.66 (s, 1H, N=CH–), 7.97 (s, 1H, pyrazole–H), 7.53–7.37 (m, 8H, Ar–H), 3.59 (s, 3H, pyrazole–CH3), 2.79 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.52, 160.65, 158.51, 142.75, 140.37, 138.09, 137.70, 133.55, 132.82, 132.16, 131.88, 131.73, 130.46, 130.14, 128.17, 127.95, 127.84, 127.33, 127.14, 113.71, 109.36, 36.66, 12.97. IR (KBr, cm−1) ν: 3444.87, 3286.70, 2933.73, 1716.65, 1695.43, 1683.86, 1670.35, 1653.00, 1635.64, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1498.69, 1473.62, 1458.18, 1436.97, 1419.61, 1398.39, 1361.74, 1340.53, 1319.31, 1251.80, 1219.01. Anal. Calc. for C23H18Cl2N6O3: C, 55.55%; H, 3.65%; N, 16.90%; Found: C, 55.70%; H, 3.79%; N, 17.24%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(4-methylbenzylidene)hydrazinecarbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6e). White solid; m.p. 221–223 °C; yield 89.8%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.38 (s, 1H, isoxazole–CONH–), 10.27 (s, 1H, pyrazol–CONH–), 8.24 (s, 1H, –N=CH–), 7.97 (s, 1H, pyrazole–H), 7.55–7.42 (m, 6H, Ar–H), 7.22 (s, 1H, Ar–H), 7.20 (s, 1H, Ar–H), 3.62 (s, 3H, pyrazole–CH3), 2.81(s, 3H, –CH3), 2.28 (s, 3H, Ar–CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 193.16, 171.80, 171.53, 163.39, 160.66, 158.43, 147.02, 145.78, 144.08, 140.42, 140.28, 140.08, 139.24, 137.88, 137.67, 134.52, 132.84, 132.15, 130.25, 130.13, 129.97, 129.65, 127.94, 127.50, 127.26, 113.72, 109.57, 108.03, 36.66, 21.55, 12.99. IR (KBr, cm−1) ν: 3444.87, 3203.76, 3030.17, 2933.73, 1699.29, 1683.86, 1662.64, 1653.00, 1635.64, 1602.85, 1577.77, 1558.48, 1541.12, 1521.84, 1506.41, 1473.62, 1456.26, 1436.97, 1409.96, 1398.39, 1375.25, 1301.95, 1246.02, 1219.01. Anal. Calc. for C24H21ClN6O3: C, 60.44%; H, 4.44%; N, 17.62%; Found: C, 60.53%; H, 4.48%; N, 17.73%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(2-fluorobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6f). White solid; m.p. 180–182 °C; yield 88.3%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.62 (s, 1H, isoxazole–CONH–), 10.36 (s, 1H, pyrazole–CONH–), 8.57 (s, 1H, –N=CH–), 8.02 (s, 1H, pyrazole–H), 7.93–7.90 (t, 1H, J = 16.60 Hz, Ar–H), 7.60–7.28(m, 7H, Ar–H), 3.66 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.25, 160.15 (d, 1C, 2-F-Ph-C), 158.14, 139.27, 137.76, 137.41, 132.54, 132.04, 131.88, 129.86, 127.65, 127.54, 126.77, 126.49, 125.21, 122.11, 116.33, 116.18, 113.43, 109.10, 36.42, 12.69. IR (KBr, cm−1) ν: 3444.87, 3167.12, 2933.73, 1699.29, 1683.86, 1653.00, 1635.64, 1616.35, 1602.85, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1436.97, 1419.61, 1398.39, 1379.10, 1363.67, 1319.31, 1242.16, 1215.15. Anal. Calc. for C23H18ClFN6O3: C, 57.45%; H, 3.77%; N, 17.48%; Found: C, 57.51%; H, 3.96%; N, 17.53%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(thiophen-2-ylmethylene)hydrazine carbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6g). White solid; m.p. 225–227 °C; yield 89.6%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.44 (s, 1H, isoxazole–CONH–), 10.32 (s, 1H, pyrazole–CONH–), 8.54 (s, 1H, –N=CH–), 8.21 (s, 1H, pyrazole–H), 7.98 (s, 1H, Ar–H), 7.65–7.14 (m, 7H, Ar–H, thiophen–H), 3.65 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.34, 160.60, 158.32, 142.16, 139.72, 137.72, 137.62, 132.80, 132.16, 131.25, 130.75, 130.14, 129.26, 128.85, 128.57, 128.38, 127.96, 113.68, 109.11, 36.64, 12.99. IR (KBr, cm−1) ν: 3446.79, 3263.56, 2929.87, 1699.29, 1683.86, 1653.00, 1635.64, 1595.13, 1577.77, 1558.48, 1541.12, 1521.84, 1506.41, 1498.69, 1473.62, 1436.97, 1419.61, 1398.39, 1375.25, 1317.38, 1300.02, 1251.80, 1224.80. Anal. Calc. for C21H17ClN6O3S: C, 53.79%; H, 3.65%; N, 17.92%; Found: C, 54.08%; H, 4.02%; N, 17.96%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(2,4-dichlorobenzylidene)hydrazinecarbonyl)-1-methyl-1H- pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6h). White solid; m.p. 189–190 °C; yield 87.9%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.77 (s, 1H, isoxazole–CONH–), 10.39 (s, 1H, pyrazole–CONH–), 8.67 (s, 1H, –N=CH–), 8.03 (s, 1H, pyrazole–H), 7.80 (d, 1H, J = 8.00 Hz, Ar–H), 7.63–7.47 (m, 6H, Ar–H), 3.66 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.53, 160.76, 158.52, 141.59, 138.08, 137.68, 135.37, 135.20, 134.23, 132.76, 132.19, 132.08, 131.22, 130.17, 129.94, 128.61, 128.53, 127.98, 113.75, 109.32, 108.41, 36.62, 13.00. IR (KBr, cm−1) ν: 3444.87, 3284.77, 3186.40, 2929.87, 1699.29, 1683.86, 1674.21, 1653.00, 1635.64, 1581.63, 1570.06, 1543.05, 1521.84, 1506.41, 1489.05, 1473.62, 1458.18, 1436.97, 1419.61, 1398.39, 1386.82, 1363.67, 1319.31, 1300.02, 1251.80, 1222.87. Anal. Calc. for C23H17Cl3N6O3: C, 51.95%; H, 3.22%; N, 15.80%; Found: C, 51.99%; H, 3.48%; N, 15.93%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(4-nitrobenzylidene)hydrazinecarbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6i). White solid; m.p. 155–156 °C; yield 89.7%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.79 (s, 1H, isoxazole–CONH–), 10.37 (s, 1H, pyrazole–CONH–), 8.42 (s, 1H, –N=CH–), 8.31 (s, 1H, pyrazole–H), 8.29 (s, 1H, Ar–H), 8.14–7.92 (m, 2H, Ar–H), 7.61–7.46 (m, 5H, Ar–H), 3.67 (s, 3H, pyrazole–CH3), 2.86 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.53, 160.62, 158.46, 148.23, 144.36, 141.23, 137.77, 132.81, 132.17, 132.09, 130.15, 130.13, 129.40, 128.37, 128.24, 127.96, 127.83, 124.65, 124.56, 113.70, 109.35, 36.66, 12.97. IR (KBr, cm−1) ν: 3444.87, 3246.20, 3064.89, 2929.87, 1699.29, 1683.86, 1674.21, 1653.00, 1635.64, 1627.92, 1587.42, 1570.06, 1558.48, 1541.12, 1521.84, 1508.33, 1489.05, 1473.62, 1458.18, 1436.97, 1411.89, 1398.39, 1375.25, 1344.38, 1317.38, 1253.73, 1215.15. Anal. Calc. for C23H18ClN7O5: C, 54.39%; H, 3.57%; N, 19.30%; Found: C, 54.52%; H, 3.74%; N, 19.36%.
Data for 3-(2-chlorophenyl)-N-(4-(2-(furan-2-ylmethylene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6j). White solid; m.p. 147–149 °C; yield 86.4%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.45 (s, 1H, isoxazole–CONH–), 10.36 (s, 1H, pyrazole–CONH–), 8.21 (s, 1H, –N=CH–), 7.85 (s, 1H, pyrazole–H), 7.61–7.47 (m, 5H, Ar-H, furan–H), 6. 91 (t, 1H, J = 13.15 Hz, furan–H), 6.63 (s, 1H, furan–H), 3.65 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.48, 163.12, 160.69, 158.35, 149.97, 145.59, 137.85, 137.60, 136.76, 133.51, 132.81, 132.17, 130.15, 127.96, 127.84, 113.86, 113.72, 112.70, 109.55, 36.63, 12.96. IR (KBr, cm−1) ν: 3462.22, 3265.49, 2929.87, 1699.29, 1683.86, 1647.12, 1653.00, 1635.64, 1627.92, 1575.84, 1570.06, 1558.48, 1541.12, 1521.84, 1496.76, 1489.05, 1473.62, 1436.97, 1419.61, 1398.39, 1361.74, 1319.31, 1300.02, 1253.73, 1213.23. Anal. Calc. for C21H17ClN6O4: C, 55.70%; H, 3.78%; N, 18.56%; Found: C, 55.78%; H, 4.03%; N, 18.89%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(3,4,5-trimethoxybenzylidene)hydrazine carbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6k). White solid; m.p. 206–207 °C; yield 91.2%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.44 (s, 1H, isoxazole–CONH–), 10.28 (s, 1H, pyrazole–CONH–), 8.26 (s, 1H, –N=CH–), 7.80 (s, 1H, pyrazole–H), 7.60–7.47 (m, 4H, Ar–H), 6.99 (s, 1H, Ar–H), 6.97 (s, 1H, Ar–H), 3.83 (s, 6H, –OCH3), 3.70 (s, 3H, pyrazole–CH3), 3.66 (s, 3H, –OCH3), 2.85 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.58, 161.01, 160.59, 158.45, 158.23, 153.73, 153.71, 147.08, 146.67, 137.70, 136.96, 132.82, 132.17, 130.42, 130.15, 127.95, 127.81, 114.14, 110.70, 109.58, 104.72, 104.44, 60.65, 56.48, 36.68, 13.02. IR (KBr, cm−1) ν: 3444.87, 3226.91, 31867.40, 3066.82, 2929.87, 1699.29, 1683.86, 1668.43, 1647.21, 1616.35, 1577.77, 1558.48, 1541.12, 1527.62, 1506.41, 1489.05, 1473.62, 1448.54, 1417.68, 1381.03, 1354.03, 1317.38, 1301.95, 1238.30, 1203.58. Anal. Calc. for C26H25ClN6O6: C, 56.47%; H, 4.56%; N, 15.20%; Found: C, 56.86%; H, 4.84%; N, 15.47%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(propan-2-ylidene)hydrazinecarbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6l). White solid; m.p. 189–191 °C; yield 87.2%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 10.16 (s, 1H, isoxazole–CONH–), 9.28 (s, 1H, pyrazole–CONH–), 7.86 (s, 1H, pyrazole–H), 7.60–7.47 (m, 4H, Ar–H), 4.34 (s, 3H, –CH3), 3.61 (s, 3H, pyrazole–CH3), 2.85 (s, 3H, isoxazole–CH3), 2.50 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 170.80, 161.37, 159.91, 159.61, 136.57, 135.96, 132.09, 131.43, 131.38, 129.43, 129.38, 127.22, 127.15, 112.97, 108.85, 35.82, 24.84, 18.36, 12.22. IR (KBr, cm−1) ν: 3444.87, 3066.82, 1699.29, 1683.86, 1668.43, 1653.00, 1635.64, 1616.35, 1577.77, 1558.48, 1541.12, 1521.84, 1506.41, 1489.05, 1458.18, 1473.62, 1429.25, 1411.89, 1386.82, 1338.60, 1313.52, 1279.88, 1236.73. Anal. Calc. for C19H19ClN6O3: C, 55.01%; H, 4.62%; N, 20.26%; Found: C, 55.35%; H, 4.98%; N, 20.52%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-(2-methylpropylidene)hydrazinecarbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6m). White solid; m.p. 212–214 °C; yield 88.3%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.14 (s, 1H, isoxazole–CONH–), 9.27 (s, 1H, pyrazole–CONH–), 7.85 (s, 1H, pyrazole–H), 7.60–7.47 (m, 5H, –C=NH–, Ar–H), 4.32 (s, 3H, CH3), 3.61 (s, 3H, pyrazole–CH3), 2.85 (s, 4H, –CH–, –CH3), 2.50 (s, 3H, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.15, 166.20, 161.71, 160.25, 136.94, 136.32, 132.43, 131.76, 131.67, 129.74, 127.56, 127.47, 125.47, 119.61, 113.32, 109.20, 36.14, 30.18, 20.12, 12.57. IR (KBr, cm−1) ν: 3325.28, 3304.06, 3066.82, 1699.29, 1683.86, 1674.21, 1653.00, 1635.64, 1610.56, 1577.77, 1558.48, 1541.12, 1521.84, 1506.41, 1489.05, 1458.18, 1473.62, 1458.18, 1429.25, 1411.89, 1386.82, 1338.60, 1278.81, 1249.87, 1201.65. Anal. Calc. for C20H21ClN6O3: C, 56.01%; H, 4.94%; N, 19.60%; Found: C, 56.19%; H, 5.13%; N, 20.01%.
Data for 3-(2-chlorophenyl)-5-methyl-N-(1-methyl-4-(2-propylidenehydrazinecarbonyl)-1H-pyrazol-5-yl)isoxazole-4-carboxamide (6n). White solid; m.p. 199–201 °C; yield 89.6%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.01 (s, 1H, isoxazole–CONH–), 10.23 (s, 1H, pyrazole–CONH–), 7.91 (s, 1H, pyrazole–H), 7.60–7.39 (m, 5H, –C=NH–, Ar–H), 3.62 (s, 3H, pyrazole–CH3), 2.82 (s, 3H, isoxazole–CH3), 2.25 (q, 2H, J = 14.90 Hz, –CH2–), 1.05 (t, 3H, J = 14.90 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 172.81, 166.66, 161.98, 153.62, 146.78, 138.90, 137.94, 134.20, 133.51, 131.48, 129.28, 126.80, 121.48, 116.59, 38.00, 27.25, 14.25, 12.62. IR (KBr, cm−1) ν: 3325.28, 3304.06, 3066.82, 1699.29, 1683.86, 1674.21, 1653.00, 1635.64, 1615.63, 1577.77, 1558.48, 1541.12, 1521.84, 1506.41, 1489.05, 1473.62, 1456.26, 1436.97, 1417.68, 1398.39, 1338.60, 1309.31, 1269.81, 1238.74, 1207.29. Anal. Calc. for C19H19ClN6O3: C, 55.01%; H, 4.62%; N, 20.26%; Found: C, 55.36%; H, 4.87%; N, 20.54%.
Data for 3-(2,6-dichlorophenyl)-N-(4-(2-(4-chlorobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6o). White solid; m.p. 168–170 °C; yield 91.3%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.56 (s, 1H, isoxazole–CONH), 10.26 (s, 1H, pyrazole–CONH), 8.32 (s, 1H, –CH=N–), 8.03–8.01 (d, 1H, J = 9.70 Hz, pyrazole–H), 7.74–7.51 (m, 7H, Ar–H), 3.61 (s, 3H, pyrazole–CH3), 2.92 (s, 3H, isoxazole–CH3). 13C NMR (125 MHz, DMSO-d6, ppm) δ: 171.84, 163.47, 159.92, 158.61, 158.40, 145.62, 142.82, 140.37, 137.72, 134.95, 134.91, 133.86, 132.91, 129.47, 129.14, 128.96, 128.93, 127.28, 127.12, 113.60, 109.62, 36.42, 13.17. IR (KBr, cm−1) ν: 3444.87, 3176.76, 2951.09, 1699.29, 1683.86, 1653.00, 1635.64, 1616.35, 1605.89, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1448.54, 1411.89, 1398.39, 1319.31, 1301.95, 1215.15. Anal. Calc. for C23H17Cl3N6O3: C, 51.95% H, 3.22%; N, 15.80%; Found: C, 51.98%; H, 3.59; N, 15.96%.
Data for 3-(2,6-dichlorophenyl)-N-(4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6p). White solid; m.p. 154–156 °C; yield 90.6%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.51 (s, 1H, isoxazole–CONH–), 10.25 (s, 1H, pyrazol–CONH–), 8.33 (s, 1H, –CH=N–), 8.05 (d, 1H, J = 18.35 Hz, pyrazole–H), 7.78–7.57 (m, 5H, Ar–H), 7.31 (t, 2H, J = 17.75 Hz, Ar–H), 3.62 (s, 3H, pyrazole–CH3), 2.92 (s, 3H, isoxazole–CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.60, 171.21, 163.90, 162.81, 161.93, 159.29, 157.87 (d, 1C, 4-F-Ph-C), 145.21, 142.34, 139.74, 138.51, 137.08, 134.29, 132.26, 130.87, 129.00, 128.30, 126.66, 115.88, 115.71, 112.96, 109.02, 107.44, 35.81, 12.54. IR (KBr, cm−1) ν: 3446.79, 3068.75, 2951.09, 1699.29, 1683.86, 1653.00, 1635.64, 1627.92, 1602.85, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1448.54, 1419.61, 1398.39, 1319.31, 1298.09, 1234.44, 1213.23. Anal. Calc. for C23H17Cl2FN6O3: C, 53.61%; H, 3.33%; N, 16.31%; Found: C, 53.69%; H, 3.75%; N, 16.51%.
Data for 3-(2,6-dichlorophenyl)-N-(4-(2-(furan-2-ylmethylene)hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methylisoxazole-4-carboxamide (6q). White solid; m.p. 255–257 °C; yield 86.5%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.46 (s, 1H, isoxazole–CONH–), 10.29 (s, 1H, pyrazole–CONH–), 8.22 (s, 1H, –CH=N–), 7.99 (d, 1H, pyrazole–H), 7.85 (s, 1H, Ar–H), 7.64–7.52 (m, 3H, Ar–H, pyrazole–H, furan–H), 6.91 (d, 1H, J = 9.75 Hz, furan–H), 6.63 (s, 1H, furan–H), 3.62 (s, 3H, pyrazole–CH3), 2.93 (s, 3H, isoxazole–CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 171.82, 163.26, 159.95, 158.32, 149.98, 145.59, 140.80, 139.31, 137.64, 136.81, 134.92, 133.62, 132.90, 128.94, 127.28, 113.83, 112.70, 109.67, 107.80, 36.44, 13.16. IR (KBr, cm−1) ν: 3446.79, 3068.75, 2953.02, 1689.64, 1653.00, 1635.64, 1602.85, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1431.18, 1409.96, 1398.39, 1330.88, 1317.38, 1255.66, 1220.94. Anal. Calc. for C21H16Cl2N6O4: C, 51.76%; H, 3.31%; N, 17.25%; Found: C, 51.84%; H, 3.36%; N, 17.28%.
Data for 3-(2,6-dichlorophenyl)-5-methyl-N-(1-methyl-4-(2-(thiophen-2-ylmethylene)hydrazinecarbonyl) -1H-pyrazol-5-yl)isoxazole-4-carboxamide (6r). White solid; m.p. 248–251 oC; yield 85.9%; 1H-NMR (500 MHz, DMSO-d6, ppm) δ: 11.43 (s, 1H, isoxazole–CONH–), 10.22 (s, 1H, pyrazole–CONH–), 8.54 (s, 1H, –CH=N–), 7.98 (s, 1H, pyrazole–H), 7.65–7.49 (m, 5H, Ar–H, thiophen–H), 7.13 (s, 1H, thiophen–H), 3.61(s, 3H, pyrazole–CH3), 2.91 (s, 3H, isoxazole–CH3). 13C-NMR (125 MHz, DMSO-d6, ppm) δ: 172.35, 171.82, 163.26, 159.95, 158.64, 158.45, 158.32, 149.98, 149.87, 145.59, 140.80, 139.31, 137.69, 137.64, 136.81, 134.92, 133.62, 132.90, 128.94, 128.64, 127.28, 113.83, 113.61, 113.36, 112.70, 109.67, 107.80, 36.72, 13.16. IR (KBr, cm−1) ν: 3446.79, 3265.49, 2953.02, 1699.29, 1683.86, 1653.00, 1635.64, 1595.13, 1575.84, 1558.48, 1541.12, 1521.84, 1506.41, 1489.08, 1473.62, 1458.18, 1436.97, 1398.39, 1373.32, 1315.45, 1294.24, 1251.80, 1222.87. Anal. Calc. for C21H16Cl2N6O3S: C, 50.11%; H, 3.20%; N, 16.70%; Found: C, 50.28%; H, 3.43%; N, 16.76%.
2.2. Biological Evaluations
Table 1 shows that most of the title compounds exhibited good antiviral activity against TMV in vivo. Especially, among the title compounds evaluated, compounds 6a and 6h exhibited good curative activity against TMV, with inhibition rates of 56.2% and 55.6% at 500 μg/mL, respectively, which were similar to that of Ningnanmycin (54.6%). Meanwhile, compounds 6c, 6d, 6n, and 6k exhibited significant protection activity against TMV, with inhibition rates of 66.2%, 64.5%,64.2%, and 64.7% at 500 μg/mL, respectively, which were equal to that of Ningnanmycin (63.8%). In addition, compounds 6c, 6o, 6p, 6q, and 6r revealed excellent inactivation activities against TMV, with inhibition rates of 91.4%, 92.3%, 91.7%, 93.2%, and 92.6% at 500 μg/mL, respectively, which were similar to that of Ningnanmycin (92.5%).

Table 1.
Antiviral activity of the title compounds against TMV at 500 μg/mL in vivo.
As shown in Table 2, compounds 6a, 6h and 6k displayed excellent curative activities against TMV, with the 50% effective concentration (EC50) values of 240.8, 255.4 and 267.4 μg/mL, which were even better than that of Ningnanmycin (286.4 μg/mL). Meanwhile, as can be seen from Table 2, compounds 6c, 6d, 6h and 6j demonstrated excellent protection activities against TMV, with the EC50 values of 148.4, 184.9, 189.7 and 176.5 μg/mL, which were superior to that of Ningnanmycin (198.2 μg/mL). Furthermore, compounds 6q and 6r, as shown in Table 2, exhibited the best inactivation activity, with the EC50 values of 37.3 and 45.1 μg/mL, which were even better than that of Ningnanmycin (46.3 μg/mL).

Table 2.
The EC50 values of the testing compounds against TMV in vivo.
As an extension of this approach, the structure-activity relationships (SAR) were analyzed on the basis of the activity values in Table 1 and Table 2. First, when R1 and R2 substituent groups were 2-Cl and H, respectively, R3 was substituted with 4-Cl-Ph, 2,4-diCl-Ph, and 3,4,5-triMe-Ph groups, the corresponding target compounds 6a, 6h and 6k exhibited excellent curative activity against TMV, which was superior to those of Ningnanmycin and the other target compounds. Second, when R1 and R2 were substituted with 2-Cl and H groups, respectively, R3 was 2-Br-Ph, 2-Cl-Ph, 2,4-diCl-Ph, and Furyl groups, the corresponding target compounds 6c, 6d, 6h and 6j exhibited remarkable protection activity against TMV than those of Ningnanmycin and the other target compounds. Third, when R1, R2, and R3 substituent groups were 2,6-diCl, H, and Furyl, the corresponding compound 6q revealed the best inactivation activity which was even better than those of Ningnanmycin and the other target compounds. Finally, as shown in Table 1, compound 6 showed better anti-TMV activity than those of compound 3. Therefore, we found that, when the presence of the –Cl at 2-position or 2,6-positions of phenyl at R1 substituent group, the presence of small groups (–H) at R2 substituent group, the corresponding compounds presented good antiviral activities. We also found that introducing a hydrazine moiety into the target compounds could enhance the anti-TMV activity, which were also observed in previous studies of Li et al. and Yang [20,24].
3. Experimental
3.1. General Methods
The melting points of the products were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., Beijing, China) and were not corrected. The IR spectra were recorded on a Bruker VECTOR 22 spectrometer (Bruker Optics Inc. Billerica, MA, USA) in KBr disk. 1H-NMR and 13C-NMR (solvent DMSO-d6) spectral analyses were performed on a JEOL-ECX 500 NMR spectrometer (JEOL Ltd., Tokyo,Japan) at room temperature using tetramethylsilane (TMS) as an internal standard. Elemental analysis was performed on an Elementar Vario-III CHN analyser (Elementar, Frankfurt, Germany). Microwave experiments were carried out using a CEM Discover Labmate microwave apparatus (Discover®LabMate instrument, CEM Corporate Matthews, NC, USA). Analytical TLC was performed on silica gel GF254 (200–300 mesh). Column chromatographic purification was carried out using silica gel. Commercial reagents were used as received, unless otherwise indicated. All solvents were dried by standard methods in advance and distilled before use.
3.2. General Procedure for the Preparation of the Intermediate 5
As shown in Scheme 1, 3-substituted phenyl-5-methylisoxazole-4-carboxylic acid (0.02 mol) was added to distillated SOCl2 (50 mL) and reacted at 80 °C for 5 h to obtain intermediate 2. Then, intermediate 2 (0.02 mol) was added dropwise to a stirred solution of 5-amino-1-methyl-1H-pyrazole-4-carboxylic acid (0.02 mol) in anhydrous tetrahydrofuran (THF, 100 mL) and trimethylamine, the reaction mixture was stirred at room temperature for 2.0 h. The reaction mixture was poured into cold 5.0% dilute HCl solution (200 mL). The solid obtained was filtered, washed several times with water, dried to give the crude product which was further recrystallized and dried to give intermediate 3. A mixture of intermediate 3 (0.02 mol) and acetic anhydride (0.2 mol) was heated under reflux for 4.0 h. The solvent was removed under reduced pressure. The residue was washed with water. The separated solid was collected by filtration, washed with water, dried, and recrystallized and dried to give intermediate 4. To a solution of intermediate 4 (10 mmol) in THF (50 mL), 6 mL of 80% hydrazine hydrate was added slowly at room temperature. Then, the mixture was further reacted at room temperature for 2 h. The solvent was removed under reduced pressure, and the residue was washed with water and anhydrous ethanol to give the crude product, then recrystallized by ethanol and dried under vacuum to give the key intermediate 3-(2-chlorophenyl)-N-(4-(hydrazinecarbonyl)-1-methyl-1H-pyrazol-5-yl)-5-methyl isoxazole-4-carboxamide 5.
3.3. General Procedure for the Preparation of the Target Compounds 6a–6r
As shown in Scheme 1, different aldehydes and ketones (1 mmol) were added to a well-stirred solution of 5 (1 mmol) in ethanol (8 mL), and then a few drops of glacial acetic acid were added. The resulting mixture was refluxed for 2 h to afford a solid, and then filtered and recrystallized from a mixture of ethanol and DMF (1:1 in volume) to gain the title compounds 6a–6r.
3.4. In Vivo Antiviral Activity Test
3.4.1. Purification of TMV
Using the Gooding method [37], the virus was multiplied in N. tabacum cv. K326 and purified. The absorbance values were estimated at 260 nm using an ultraviolet spectrophotometer. The virus concentration was calculated by the following formula, where E represents the extinction coefficient for TMV, E0.1% 1cm 260nm is 3.1:
Virus concentration (mg/mL) = (A260 × dilution ratio)/E0.1% 1cm 260nm
3.4.2. Protection Activity of the Title Compounds against TMV in Vivo
The compound solution was smeared on the left side of growing N. tabacum L. leaves of the same age. The solvent without compound solution was smeared on the right side of the leaves to serve as the control. The leaves, which were previously scattered with silicon carbide, were inoculated with the virus after 12 h using a brush dipped in 6 × 10−3 mg/mL TMV and subsequently washed with water and rubbed softly along the nervature once or twice. After three to four days of the inoculation, the local lesions were counted. Three replications were reproduced for each compound.
3.4.3. Curative Activity of the Title Compounds against TMV in Vivo
The leaves were inoculated with TMV (concentration of 6 × 10−3 mg/mL) by dipping and brushing the whole leaves, which were previously scattered with silicon carbide. The leaves were then washed with water after inoculation for 0.5 h. The compound solution was smeared on the left side of the leaves, and the solvent without compound solution was smeared on the right side for control. The number of local lesions was counted and recorded three to four days after the inoculation. Three replications were reproduced for each compound.
3.4.4. Inactivation Activity of the Title Compounds against TMV in Vivo
The virus was inhibited after it was mixed with a compound solution of the same volume for 30 min. The right side of the N. tabacum L. leaves was then inoculated with the solvent and virus mixture for control. All of the leaves were previously scattered with silicon carbide. The number of local lesions was recorded three to four days after the inoculation. Three replications were reproduced for each compound.
The inhibition rates of the compounds were calculated according to the following formula:
Inhibition rate (%) = [(average local lesion number of control (not treated with compounds) − average local lesion number smeared with compounds)/average local lesion number of control (not treated with compounds)] × 100%
4. Conclusions
In summary, a series of novel pyrazole-hydrazone derivatives containing an isoxazole moiety were obtained with moderate yields and their antiviral activities against TMV were examined by a half-leaf method. Bioassay results revealed that some of the target compounds exhibited better antiviral activity against TMV in vivo than Ningnanmycin. Among the title compounds, compound 6a exhibited better curative activity against TMV than that of Ningnanmycin. Meanwhile, compound 6c showed remarkable protection activity against TMV, which was superior to that of Ningnanmycin. Moreover, compound 6q exhibited better inactivation activity against TMV which were superior to that of Ningnanmycin. The antibacterial tests showed that introducing a hydrazine moiety into the target compounds could enhance the anti-TMV activity; meanwhile, when the presence of the –Cl at 2-position or 2,6-positions of phenyl in the R1 substituent group, the presence of small groups (–H) in the R2 substituent group, the corresponding compounds presented good antiviral activities. Therefore, this study demonstrated that this series of pyrazole-hydrazone derivatives containing an isoxazole moiety can be considered for further development as a new class of tobacco protection agents.
Supplementary Materials
The 1H-NMR and 13C-NMR for all the synthesized compounds are available online.
Author Contributions
Writing—Original Draft Preparation, Z.Y.; Writing—Review and Editing, P.L. and X.G.; Project Administration, Z.Y.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 21466031).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, L.H.; Lin, Q.Y.; Wu, Z.J. Plant Virus; China Press: Beijing, China, 2009; pp. 1–5. [Google Scholar]
- Deng, G.B.; Wan, B.; Hu, H.Z.; Chen, J.R.; Yu, M.Q. Biological activity of ningnanmycin on tobacco mosaic virus. Chin. J. Appl. Environ. Biol. 2004, 10, 695–698. [Google Scholar]
- Wang, Z.W.; Wang, L.; Ma, S.; Liu, Y.X.; Wang, L.Z.; Wang, Q.M. Design, synthesis, antiviral activity, and SARs of 14-aminophenanthroindolizidines. J. Agric. Food Chem. 2012, 60, 5825–5831. [Google Scholar] [CrossRef] [PubMed]
- Song, B.A.; Yang, S.; Jin, L.H.; Bhadury, P.S. Environment-Friendly Antiviral Agents for Plants; Springer: Berlin, Germany, 2010; pp. 1–7. [Google Scholar]
- Yu, L.G.; Ni, T.F.; Gao, W.; He, Y.; Wang, Y.Y.; Cui, H.W.; Yang, C.G.; Qiu, W.W. The synthesis and antibacterial activity of pyrazole-fused tricyclic diterpene derivatives. Eur. J. Med. Chem. 2015, 90, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Al-Showiman, S.S.; Barakat, A.; Ghabbour, H.A.; Frey, W. Synthesis, in-vitro antibacterial, antifungal, and molecular modeling of potent anti-microbial agents with a combined pyrazole and thiophene pharmacophore. Molecules 2015, 20, 8712–8729. [Google Scholar] [CrossRef] [PubMed]
- Sangani, C.B.; Makwana, J.A.; Duan, Y.T.; Tarpada, U.P.; Patel, Y.S.; Patel, K.B.; Dave, V.N.; Zhu, H.L. Design, synthesis, and antibacterial evaluation of new Schiff’s base derivatives bearing nitroimidazole and pyrazole nuclei as potent E. coli FabH inhibitors. Res. Chem. Intermed. 2015, 41, 10137–10149. [Google Scholar] [CrossRef]
- Du, S.J.; Tian, Z.M.; Yang, D.Y.; Li, X.Y.; Li, H.; Jia, C.Q.; Che, C.L.; Wang, M.; Qin, Z.H. Synthesis, antifungal activity and structure-activity relationships of novel 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid amides. Molecules 2015, 20, 8395–8408. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Zhou, Y.M. Synthesis and antifungal activity of the derivatives of novel pyrazole carboxamide and isoxazolol pyrazole carboxylate. Molecules 2015, 20, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Shi, Y.J.; He, H.B.; Li, Y.; Li, Y.; Dai, H. Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring. Chin. Chem. Lett. 2015, 26, 672–674. [Google Scholar] [CrossRef]
- Dai, H.; Ye, L.Y.; Zhuang, H.Y.; Dai, B.J.; Fang, Y.; Shi, Y.J. Design, synthesis and bioactivities of novel dichloro-allyloxy-phenol-containing pyrazole oxime derivatives. Molecules 2015, 20, 21870–21880. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.H.; Xiao, J.J.; Ren, Z.L.; Chu, M.J.; Wang, P.; Meng, X.F.; Li, D.D.; Cao, H.Q. Design, synthesis and insecticidal activities of N-(4-cyano-1-phenyl-1H-pyrazol-5-yl)-1,3-diphenyl-1H-pyrazole-4-carboxamide derivatives. RSC Adv. 2015, 5, 55179–55185. [Google Scholar] [CrossRef]
- Deng, X.L.; Xie, J.; Li, Y.Q.; Yuan, D.K.; Hu, X.P.; Li, Z.; Wang, Q.M.; Chi, M.; Yang, X.L. Design, synthesis and biological activity of novel substituted pyrazole amide derivatives targeting EcR/USP receptor. Chin. Chem. Lett. 2016, 27, 566–570. [Google Scholar] [CrossRef]
- Singh Jadav, S.; Nayan Sinha, B.; Pastorino, B.; de Lamballerie, X.; Hilgenfeld, R.; Jayaprakash, V. Identification of pyrazole derivative as an antiviral agent against Chikungunya through HTVS. Lett. Drug Des. Discov. 2015, 12, 292–301. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Baraka, M.M.; Ibrahim, S.M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Rashad, A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009, 44, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Zhang, J.H.; Xia, X.D.; Kang, J.; Li, J.H. Design, synthesis and herbicidal evaluation of novel 4-(1H-pyrazol-1-yl)pyrimidine derivatives. Pest Manag. Sci. 2015, 71, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yue, X.L.; Chen, C.S.; Li, J.H.; Ma, H.J. Synthesis and herbicidal activity of 5-heterocycloxy-3-methyl-1-substituted-1H-pyrazoles. Molecules 2016, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Song, B.A.; Wu, J.; He, M.; Hu, D.Y.; Jin, L.H.; Zeng, S.; Xue, W.; Yang, S. Design, synthesis and insecticidal activities of novel acetamido derivatives containing N-pyridylpyrazole carboxamides. Eur. J. Med. Chem. 2013, 67, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, S.H.; Song, B.A.; Hu, D.Y.; He, M.; Jin, L.H.; Yang, S. Synthesis and antifungal activities of novel nicotinamide derivatives containing 1,3,4-oxadiazole. Chem. Cent. J. 2013, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.; Wang, K.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Synthesis and antiviral, insecticidal, and fungicidal activities of gossypol derivatives containing alkylimine, oxime or hydrazine moiety. Bioorg. Med. Chem. 2016, 24, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D. Synthesis and biological activity of hydrazone derivatives containing pyrazole. J. Chem. Res. 2008, 9, 489–491. [Google Scholar] [CrossRef]
- Yang, Z.B.; Hu, D.Y.; Zeng, S.; Song, B.A. Novel hydrazone derivatives containing pyridine amide moiety: Design, synthesis, and insecticidal activity. Bioorg. Med. Chem. Lett. 2016, 26, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, D.D.; Shan, W.L.; Zhao, Y.H.; Zhang, W.; Song, B.A.; Yang, S.; Ma, J. Synthesis and insecticidal activity of anthranilic diamides with hydrazone substructure. Chem. Pap. 2015, 69, 993–1003. [Google Scholar] [CrossRef]
- Liu, Y.X.; Song, H.J.; Huang, Y.Q.; Li, J.R.; Zhao, S.; Song, Y.C.; Yang, P.W.; Xiao, Z.X.; Liu, Y.X.; Li, Y.Q.; et al. Design, synthesis, and antiviral, fungicidal, and insecticidal activities of tetrahydro-β-carboline-3-carbohydrazide derivatives. J. Agric. Food Chem. 2014, 62, 9987–9999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, B.W.; Xin, J.R.; Li, J.F.; Wu, J.B.; Bao, X.R. Synthesis and antibacterial activities of N-[(1-aryl-3-phenyl-pyrazol-4-yl)methylene]-2-(halo-o-hydroxyphenyl)hydrazide derivatives. Chem. Res. Chin. Univ. 2013, 29, 449–453. [Google Scholar] [CrossRef]
- Xing, M.; Zhao, T.T.; Ren, Y.J.; Peng, N.N.; Yang, X.H.; Li, X.; Zhang, H.; Liu, G.Q.; Zhang, L.R.; Zhu, H.L. Synthesis, biological evaluation, and molecular docking studies of pyrazolyl-acylhydrazone derivatives as novel anticancer agents. Med. Chem. Res. 2014, 23, 3274–3286. [Google Scholar] [CrossRef]
- Nasrullah, M.; Khan, M.A.; Khan, M.N.; Humphrey, M.G.; Nasim, F.H.; Abidi, M.G.; Khan, M.N.; Farooq, U.; Munawar, M.A. Diaryl pyrazole-4-carbaldehyde benzoylhydrazones metal complexes: Synthesis and their antibacterial and antioxidant screening. Asian J. Chem. 2013, 25, 419–423. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Abdel-Aziz, N.I.; Abdel-Aziz, A.A.; El-Azab, A.S.; Asiri, Y.A.; ElTahir, K.E.H. Design, synthesis, and biological evaluation of substituted hydrazone and pyrazole derivatives as selective COX-2 inhibitors: Molecular docking study. Bioorg. Med. Chem. 2011, 19, 3416–3424. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.H.; Eissa, A.A.H.; Hassan, G.S. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. 2003, 51, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.G.M.; Rodrigues, C.R.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. Synthesis and pharmacological evaluation of novel heterotricyclic acylhydrazone derivatives, designed as PAF antagonists. Eur. J. Pharm. Sci. 2000, 11, 285–290. [Google Scholar] [CrossRef]
- Kang, Y.K.; Shin, K.J.; Yoo, K.H.; Seo, K.J.; Hong, C.Y.; Lee, C.S.; Park, S.Y.; Kim, D.J.; Park, S.W. Synthesis and antibacterial activity of new carbapenems containing isoxazole moiety. Bioorg. Med. Chem. Lett. 2000, 10, 95–99. [Google Scholar] [CrossRef]
- Santos, M.M.M.; Faria, N.; Lley, J.; Coles, S.J.; Hursthouse, M.B.; Martins, M.L.; Moreira, R. Reaction of naphthoquinones with substituted nitromethanes. Facile synthesis and antifungal activity of naphtho[2,3-d]isoxazole-4,9-diones. Bioorg. Med. Chem. Lett. 2010, 20, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Lin, F.; Li, B.C.; Liu, H.W.; Zhao, T.Q.; Zhang, Y.M.; Gu, Q. Efficient synthesis of bis-isoxazole ethers via 1,3-dipolar cycloaddition catalysed by Zn/Zn2+ and their antifungal activities. Chem. Pap. 2015, 69, 1500–1511. [Google Scholar] [CrossRef]
- Yu, X.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Design, synthesis, and acaricidal/insecticidal activities of oxazoline derivatives containing a sulfur ether moiety. J. Agric. Food Chem. 2015, 63, 9690–9695. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ozoe, F.; Furuta, K.; Ozoe, Y. 4,5-Substituted 3-isoxazolols with insecticidal activity act as competitive antagonists of housefly GABA receptors. J. Agric. Food Chem. 2015, 63, 6304–6312. [Google Scholar] [CrossRef] [PubMed]
- Daghigh, L.R.; Pordel, M.; Davoodnia, A.; Jajarmi, M. Synthesis, antiviral, and cytotoxic investigation of imidazo[4,5-a]acridones. Med. Chem. Res. 2015, 24, 3912–3919. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 6a–6r are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).