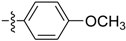
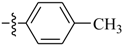
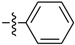
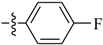
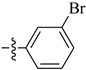
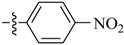
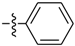
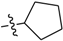
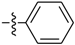
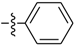
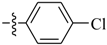
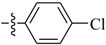
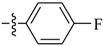
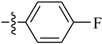
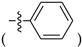
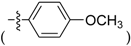
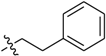
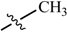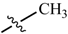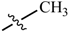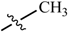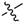Abstract
Beckmann rearrangement of ketoxime catalyzed by acidic ionic liquid-N-methyl-imidazolium hydrosulfate was studied. Rearrangement of benzophenone oxime gave the desirable product with 45% yield at 90 °C. When co-catalyst P2O5 was added, the yield could be improved to 91%. The catalyst could be reused three cycles with the same efficiency. Finally, reactions of other ketoximes were also investigated.
1. Introduction
Over the past years, amide derivatives have received much attention owing to their broad range of applications in many fields such as the pharmaceutical industry, chemical biology, the agrochemical industry, engineering plastics, and so on [1,2,3,4,5,6]. Various approaches have been developed for the synthesis of amide compounds including nucleophilic acyl substitution reactions with amines [7], Staudinger ligation [8], Schmidt reaction [9] and Beckmann rearrangement [10]. However, generations of large amounts of undesired by-products and corrosive phenomenon associated with common acid (H2SO4 and SOCl2) based on liquid phase protocols provide a challenging task for chemists to develop alternative methods [11,12]. A variety of alternative routes [13,14,15,16] based on organic and inorganic solid acids were developed. However, traditional methods often suffer from some drawbacks such as poor selectivity, harsh conditions, are not atom economic, or are not environmentally friendly.
From the point of view of atom conversion efficiency, Beckmann rearrangement is a perfect way for construction of amides, in general sulfuric acid is most commonly used rearrangement catalyst in commercial production of amides. However, it brings equipment corrosion and environmental pollution problems. Recently, ionic liquids [17] have emerged as potential green alternatives to organic solvents due to their unique properties of low volatility, high polarity, good thermal stability, and excellent solubility [18,19,20]. Further, there are more potential capabilities as effective catalysts and reagents [13], as chemical transformations have also been explored. In order to develop a green pathway of amide synthesis, we report here a Beckmann rearrangement reaction catalyzed by N-methyl-imidazolium hydrosulfate ([HMIm]HSO4) [21] under solvent free conditions (Scheme 1).
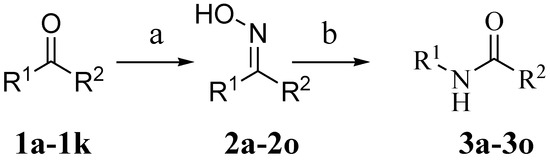
Scheme 1.
Synthesis of amides. Reagents and conditions: (a) NH2OH.HCl, NaOH, EtOH, H2O, reflux; (b) acidic ionic liquid, P2O5, N2, 90 °C, 6 h.
2. Results
Beckmann rearrangement of benzophenone oxime catalyzed by [HMIm]HSO4 was carried out at 120 °C over 6 h without any solvent, the desired product, benzanilide, was obtained in moderate yield (45%). The co-catalysts such as P2O5, FeCl3, ZnCl2, CuCl2.2H2O, and AlCl3 were investigated in this reaction system, the yield was improved significantly to 91% with P2O5. However, it has been shown in the literature that the conversion is around 20% only when P2O5 is used as the sole catalyst of Beckmann rearrangement [15]. When CuCl2.2H2O was added, the yield was reduced to 14%, and the reverse reaction of benzophenone oxime was observed, benzophenone was regenerated. The results are presented in Table 1.

Table 1.
Effect of co-catalyst on Beckmann rearrangement in ionic liquid systems.
The effect of the amount of co-catalyst P2O5 on reaction was investigated. The reaction yield was improved when more co-catalyst was added, and the best yield was around 90% when the amount of P2O5 was higher than 8 mol %. The results are presented in Figure 1.
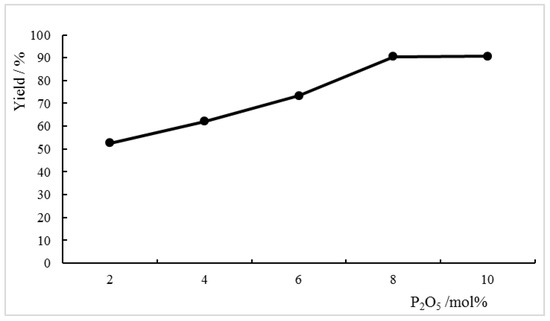
Figure 1.
Effect of the amount of co-catalyst P2O5 on Beckmann rearrangement of benzophenone oxime. Reaction conditions: Benzophenone oxime (9.5 mmol), [HMIm]HSO4 (11.4 mmol), 90 °C, 6 h.
The influence of the reaction temperature on the yield was investigated subsequently. It was found that 90 °C is the best reaction temperature. The results are presented in Figure 2.
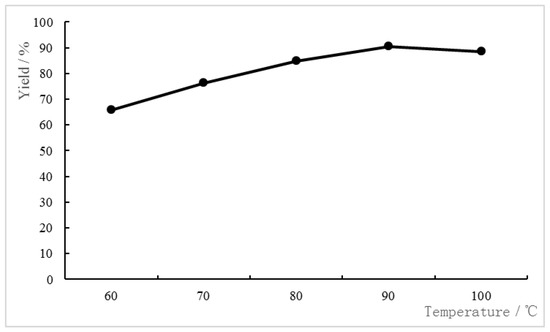
Figure 2.
Influence of the reaction temperature on Beckmann rearrangement of benzophenone oxime. Reaction conditions: Benzophenone oxime (9.5 mmol), [HMIm]HSO4 (11.4 mmol), 6 h, co-catalyst (P2O5 8%).
The recycling performance of ionic liquid has the most benefits from the point of view of environmental protection. During the reaction workup, the white product was precipitated out when ice water was added, after filtration, the mother liquid was evaporated in a vacuum, and ionic liquid was recovered and could be reused for three times. The results are presented in Table 2.

Table 2.
Effect of ionic liquid recycling on Beckmann rearrangement.
In order to explore the scope and limitations of this reaction, we extended the procedure to various aryl-substituted and alkyl-substituted ketoximes. In general, the reaction proceeded easily under the best conditions and the amide products were isolated in excellent yields and high purity. The results are presented in Table 3.

Table 3.
Formation of amides (3a–3o) from ketoxime (2a–2o) in the presence of ionic liquid and co-catalyst P2O5.
3. Experimental Section
All melting points were determined using a YRT-3 Digital Melting Point Apparatus (Tianjin, China). All melting points were uncorrected. All new compounds were characterized by HRMS-EI(M+), 1H and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker AV 600 MHz or Bruker AV 400 MHz instrument. HRMS spectra were obtained on an Agilent 6230 mass spectrometer.
3.1. Synthesis of N-methyl-imidazolium Hydrosulfate ([HMIm]HSO4)
N-Methylimidazole (8.2 g, 0.10 mol) was cooled down to 0 °C and concentrated sulfuric acid (10.0 g) was added dropwise. After addition, the solution was stirred 24 h at room temperature, a transparent viscous liquid (17.6 g) was obtained. Yield: 99%; IR (cm−1): 3345, 3150, 2870, 1447, 1337, 1221, 1048, 1082, 887.
3.2. General Procedures for Synthesis of Oxime Substrates 2a–2o
Ketone (0.027 mol) and hydroxylamine hydrochloride (3.0 g, 0.043 mol) were dissolved in EtOH (10 mL) and H2O (20 mL). To the mixture was added NaOH (5.5 g, 0.137 mol). The reaction mixture was heated under reflux and the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the reaction mixture was cooled down to room temperature, to the reaction mixture were added concentrated hydrochloric acid (15 mL) and water (100 mL). The solid was filtered off and recrystallized from EtOH, affording the products 2a–2o.
2a: White solid, Yield: 91%. m.p.: 87.6–88.7 °C; 1H-NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.9 Hz, 2H), 6.93 (d, J = 8.9 Hz, 2H), 3.85 (s, 3H), 2.30 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 160.5, 155.6, 129.0, 127.4, 113.9, 55.3, 13.3.
2b: White solid, Yield: 93%. m.p.: 88.0–89.0 °C; 1H-NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.2 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 2.39(s, 3H), 2.31 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 156.0, 139.3, 133.7, 129.3, 126.0, 21.3, 12.3.
2c: White solid, Yield: 90%. m.p.: 51.7–53.0 °C; 1H-NMR (600 MHz, CDCl3) δ 7.68–7.64 (m, 2H), 7.44–7.40 (m, 3H), 2.35(s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.1, 136.5, 129.3, 128.5, 126.1, 12.3.
2d: White solid, Yield: 70%. m.p.: 74.0–76.0 °C;1H-NMR (600 MHz, DMSO-d6) δ 11.21 (s, 1H), 7.76–7.59 (m, 2H), 7.22–7.19 (m, 2H), 2.14 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 162.4 (d, 1JCF = 245.5 Hz), 152.1, 133.5 (d, 4JCF = 3.0 Hz), 127.6 (d, 3JCF = 8.3 Hz), 115.2 (d, 2JCF = 21.5 Hz), 11.9.
2e: White solid, Yield: 91%. m.p.: 141.0–142.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.40 (s, 1H), 7.80 (t, J = 1.8 Hz, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.55 (d, J = 7.8 Hz, 1H), 7.34 (dd, J = 11.1, 4.6 Hz, 1H), 2.14 (s, 3H); 13C-NMR (150 MHz,DMSO-d6) δ 151.9, 139.3, 131.3, 130.6, 128.1, 124.6, 121.8, 11.4.
2f: Yellow solid, Yield: 90%. m.p.: 172.0–173.0 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.78 (s, 1H), 8.28—8.18 (m, 2H), 7.96–7.88 (m, 2H), 2.21 (s, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 157.1, 152.5, 148.3, 131.9, 131.8, 16.6.
2g: White solid, Yield: 95.6%. m.p.: 129.6–131.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.49 (s, 1H), 7.42–7.27 (m, 5H), 3.04–2.85 (m, 1H), 1.75–1.66 (m, 2H), 1.64–1.50 (m, 6H); 13C-NMR (150 MHz, DMSO-d6) δ 158.6, 135.8, 128.3, 128.2, 128.1, 45.2, 30.3, 24.9.
2h: White solid, Yield: 60%. m.p.: 92.0–93.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.40 (s, 1H), 7.48–7.44 (m, 2H), 7.43–7.40 (m, 1H), 7.387.37 (m, 5H), 7.30–7.25 (m, 2H); 13C-NMR (150 MHz, DMSO-d6) δ 155.2, 136.8, 133.5, 128.88, 128.86, 128.40, 128.36, 128.2, 127.0.
2i: White solid, Yield: 87.5%. m.p.: 134.0–136.0 °C; 1H-NMR (600 MHz, CDCl3) δ 7.48 (d, J = 8.6 Hz, 2H), 7.43–7.37 (m, 4H), 7.36–7.31 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 156.1, 135.9, 135.5, 134.3, 130.8, 130.4, 129.1, 128.8, 128.7.
2j: White solid, Yield: 88.5%. m.p.: 131.0–132.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.45 (s, 1H), 7.43–7.38 (m, 2H), 7.38–7.32 (m, 2H), 7.28 (dd, J = 12.3, 5.4 Hz, 2H), 7.19 (dd, J = 12.3, 5.4 Hz, 2H); 13C-NMR (150 MHz, DMSO-d6) δ 162.6 (d, 1JCF = 244.6 Hz), 161.9 (d, 1JCF = 244.6 Hz), 153.4, 133.2, 131.3 (d, 3JCF = 8.2 Hz), 129.5, 129.1 (d, 3JCF = 8.2 Hz), 115.4 (d, 2JCF = 22.6 Hz), 115.2 (d, 2JCF = 22.3 Hz).
2k: White solid, Yield: 87.5%. m.p.: 129.0–130.0 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.1 (s, 1H), 7.31 (d, J = 8.8 Hz, 2H), 7.24 (d, J = 8.8 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 6.91 (d, J = 8.8 Hz, 2H), 3.79 (s, 3H), 3.76 (s, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 159.8, 159.1, 154.4, 130.7, 129.7, 128.6, 125.6, 113.7, 113.4, 55.2, 55.1.
2l: Light yellow solid, Yield: 87.9%. m.p.:155.0–160.0 °C; (isomer 1): 1H-NMR (600 MHz, DMSO-d6) δ 11.27(s, 1H), 7.38–7.34 (m, 4H), 7.29–7.24 (m, 3H), 7.00 (d, J = 8.7 Hz, 2H), 3.79 (s, 3H); (isomer 2): 1H-NMR (600 MHz, DMSO-d6) δ 11.09 (s, 1H), 7.46–7.43 (m, 2H), 7.42–7.38 (m, 3H), 7.30 (d, J = 8.8 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H), 3.75 (s, 3H); (isomer 1): 13C-NMR (100 MHz, DMSO-d6) δ 159.85, 154.85, 137.32, 130.74, 129.22, 128.32, 128.29, 128.12, 113.80, 55.20; (isomer 2): 13C-NMR (100 MHz, DMSO-d6) δ 159.20, 154.80, 133.82, 128.87, 128.76, 128.29, 127.32, 125.37, 113.46, 55.15.
2m: Yield: 94.0%. m.p.: 84–86 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.81 (brs, 1H), 7.30–7.18 (m, 5H), 2.83 (t, J = 8.2 Hz, 2H), 2.51 (t, J = 8.2 Hz, 2H), 1.91 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 157.9, 141.0, 128.4, 128.3, 126.1, 37.7, 32.6,13.8.
2n: Yield: 90.0%. m.p.: 79–81 °C; 1H-NMR (600 MHz, DMSO-d6) δ 12.74 (s, 1H), 7.73–7.16 (m, 5H); 13C-NMR (150 MHz, DMSO-d6) δ145.2 (q, 2JCF = 30.0), 130.6, 129.0, 128.9, 127.2, 121.6 (q, 1JCF3 = 271.5).
2o: Yield: 85.5%. m.p.: 89–91 °C; 1H-NMR (600 MHz, DMSO-d6) δ 2.48 (dd, J = 6.8, 5.3, 2H), 2.48 (m, 2H), 1.76–1.45 (m, 6H); 13C-NMR (150 MHz, DMSO-d6) δ 157.1, 31.6, 26.6, 25.4, 25.2, 23.8.
3.3. General Procedures for the Synthesis of Amides 3a–3o
To a solution of the oxime substrates 2a–2o (9.50 mmol) in (HMIm)HSO4 (2.05 g, 11.4 mmol), the co-catalyst P2O5 (0.15 g, 1.0 mmol) was added. Then the solution was heated to 90 °C and the reaction was monitored by TLC. After completion of the reaction, the mixture was extracted with ethyl acetate (50 mL) twice, and the combined organic phase was washed with the aqueous solution of sodium bicarbonate and brine, dried over anhydrous Na2SO4 and concentrated in vacuo to afford a residue, which was purified by column (ethyl acetate: petroleum ether = 1:4) to afford the products 3a–3o.
3a [22]: White solid, Yield: 91%. m.p.: 127.0–128.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 9.80 (brs, 1H), 7.51–7.46 (m, 2H), 7.20 (d, J = 7.9 Hz, 1H), 6.89–6.84 (m, 2H), 3.71 (s, 3H), 2.01 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 168.2, 155.5, 133.0, 121.0, 114.2, 55.6, 24.3; HRMS(+): calcd. for C9H11NO2 [M + H]+ 166.0863, found 166.0859; calcd. for C9H11NO2Na [M + Na]+ 188.0682, found 188.0682.
3b [22]: White solid, Yield: 90%. m.p.: 149.0–150.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 9.84 (s, 1H), 7.46 (d, J = 8.3 Hz, 2H), 7.09 (d, J = 8.3 Hz, 2H), 2.24 (s, 3H), 2.02 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 173.2, 142.1, 137.0, 134.1, 124.2, 29.2, 25.6; HRMS(+): calcd. for C9H11NO [M + H]+ 150.0913, found 150.0912; calcd. for C9H11NONa [M + Na]+ 172.0733, found 172.0741.
3c [23]: White solid, Yield: 90%. m.p.: 108.5–110.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 9.93 (brs, 1H), 7.58 (dd, J = 1.0, 8.5 Hz, 2H), 7.29 (dd, J = 7.5, 8.4 Hz, 2H), 7.08–6.91 (m, 1H), 2.05 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 168.7, 139.8, 129.1, 123.4, 119.4, 24.5; HRMS(+): calcd. for C8H9NO [M + H]+ 136.0757, found 136.0755; calcd. for C8H9NONa [M + Na]+ 158.0576, found 158.0572.
3d [24]: Light yellow solid, Yield: 89%. m.p.: 153–155 °C; 1H-NMR (600 MHz, DMSO-d6) δ 9.99 (brs, 1H), 7.87–7.47 (m, 2H), 7.12 (t, J = 8.99 Hz, 2H), 2.04 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 168.6, 158.3.8 (d, 1JCF = 237.0 Hz), 136.2 (d, 4JCF = 1.5 Hz), 121.1 (d,3JCF = 7.5 Hz), 115.2 (d, 2JCF = 22.5 Hz), 24.3; HRMS(+): calcd. for C8H8FNO [M + H]+ 154.0663, found 154.0665; calcd. for C8H8FNONa [M + Na]+ 176.0482, found 176.0481.
3e [25]: White solid, Yield: 88%. m.p.: 87.0–89.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.11 (brs, 1H), 7.95 (s, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.27–7.23 (m, 1H), 7.22–7.19 (m, 1H), 2.05 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 169.1, 141.3, 131.1, 126.0, 122.0, 121.7, 118.1, 24.5; HRMS(+): calcd. for C8H8BrNO [M + H]+ 213.9862, found 213.9860; calcd. for C8H8BrNONa [M + Na]+ 235.9681, found 235.9681.
3f [25]: Yellow solid, Yield: 86%. m.p.: 214.0–215.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.57 (s, 1H), 8.21 (d, J = 9.2 Hz, 2H), 7.82 (d, J = 9.4 Hz, 2H), 2.12 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 169.8, 145.9, 142.3, 125.4, 119.0, 24.7; HRMS(+): calcd. for C8H8N2O3 [M + H]+ 181.0608, found181.0610; Calcd. for C8H8N2O3Na [M + Na]+ 203.0433, found 203.0437.
3g: White solid, Yield: 91%. m.p.: 160.0–161.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.29 (d, J = 7.0 Hz, 1H), 7.88–7.80 (m, 2H), 7.54–7.49 (m, 1H), 7.48–7.42 (m, 2H), 4.46–3.96 (m, 1H), 1.96–1.81 (m, 2H), 1.74–1.64 (m, 2H), 1.60–1.43 (m, 4H); 13C-NMR (150 MHz, DMSO-d6) δ 166.4, 135.3, 131.4, 128.6, 127.7, 51.4, 32.6, 24.1; HRMS(+): calcd. for C12H15NO [M + H]+ 190.1226, found 190.1228; calcd. for C12H15NO Na [M + Na]+ 212.1046, found 212.1044.
3h [23]: White solid, Yield: 89%. m.p.: 162.6–163.0 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 7.99–7.92 (m, 2H), 7.78 (d, J = 7.6 Hz, 2H), 7.63–7.58 (m, 1H), 7.57–7.51 (m, 2H), 7.36 (t, J = 7.9 Hz, 2H), 7.15–7.07 (m, 1H); 13C-NMR (100 MHz, DMSO-d6) δ 166.0, 139.6, 135.5, 132.0, 129.1, 128.9, 128.1, 124.1, 120.8; HRMS(+): calcd. for C13H11NO [M + H]+ 198.0913, found 198.0913; calcd. for C13H11NONa [M + Na]+ 220.0733, found 220.0733.
3i [25]: White solid, Yield: 85%. m.p.: 210.0–212.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.45 (s, 1H), 8.02–7.96 (m, 2H), 7.86–7.79 (m, 2H), 7.66–7.59 (m, 2H), 7.45–7.39 (m, 2H); 13C-NMR (150 MHz, DMSO-d6) δ 165.0, 138.4, 137.0, 133.8, 130.1, 129.0, 127.9, 122.4; HRMS(+): calcd. for C13H9Cl2NO [M + H]+ 266.0134, found 266.0129; calcd. for C13H9Cl2NONa [M + Na]+ 287.9953, found 287.9951.
3j [26]: Light yellow solid, Yield: 84%. m.p.: 183–185.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.31 (s, 1H), 8.04–8.02 (m, 2H), 7.82–7.72 (m, 2H), 7.37 (t, J = 8.8 Hz, 2H), 7.19 (t, J = 8.8 Hz, 2H); 13C-NMR (150 MHz, DMSO-d6) δ 164.3, 164.1 (d, 1JCF = 249.5 Hz), 158.3 (d, 1JCF = 240.3 Hz), 135.4, 131.2, 130.4 (d, 3JCF = 9.0 Hz), 122.2 (d, 3JCF = 7.8 Hz), 115.3 (d, 2JCF = 22.4 Hz), 115.2 (d, 2JCF = 22.8 Hz); HRMS(+): calcd. for C13H9F2NO [M + H]+ 234.0725, found 234.0727; calcd. for C13H9F2NONa [M + Na]+ 256.0544, found 256.0546.
3k [26]: Light yellow solid, Yield: 90%. m.p.: 204.0–205.0 °C; 1H-NMR (600 MHz, CDCl3) δ 7.83 (d, J = 8.8 Hz, 2H), 7.65 (s, 1H), 7.52 (d, J = 9.0 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 6.91 (d, J = 9.0 Hz, 2H), 3.87 (s, 3H), 3.81 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 164.5, 161.7, 155.4, 132.4, 129.4, 127.1, 121.9, 113.7, 113.5, 55.4, 55.2; HRMS(+): calcd. for C15H15NO3 [M + H]+ 258.1125, found 258.1127; calcd. for C15H15NO3Na [M + Na]+ 280.0944, found 280.0946.
3l [25] (N-(4-Methoxyphenyl)benzamide): Light yellow solid, Yield: 52%. M.p.: 156.5–159.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ 10.07 (s, 1H), 7.95 (d, J = 9.2 Hz, 2H), 7.74 (d, J = 7.6 Hz, 2H), 7.51 (t, J = 7.6 Hz, 1H), 7.33 (t, J = 7.9 Hz, 2H), 7.05 (d, J = 9.2 Hz, 2H), 3.84 (s, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 164.94, 161.91, 139.36, 131.40, 129.61, 128.58, 123.45, 120.37, 113.62, 55.45; HRMS(+): calcd. for C14H13NO2 [M + H]+ 228.1019, found 228.1020; calcd. for C14H13NO2Na [M + Na]+ 250.0838, found 250.0838; (4-Methoxy-N-phenylbenzamide): Light yellow solid, Yield: 28% 1H-NMR (600 MHz, DMSO-d6) δ 10.12 (s, 1H), 7.94 (d, J = 7.2 Hz, 2H), 7.67 (d, J = 9.0 Hz, 2H), 7.57 (t, J = 7.2 Hz, 1H), 7.09 (t, J = 8.8 Hz, 2H), 6.93 (d, J = 9.0 Hz, 2H), 3.74 (s, 3H); 13C-NMR (100 MHz, DMSO-d6) δ 165.14, 155.58, 135.07, 132.24, 128.37, 127.56, 127.00, 122.02, 113.76, 55.20. HRMS(+): calcd. for C14H13NO2 [M + H]+ 228.1019, found 228.1020; calcd. for C14H13NO2Na [M + Na]+ 250.0838, found 250.0838.
3m [27]: Yield: 89%. m.p.: 113–114 °C; 1H-NMR (600 MHz, DMSO-d6) δ 7.34–7.30 (m, 2H), 7.26–7.21 (m, 3H), 3.85–3.77 (m, 2H), 2.84–2.74 (m, 2H), 2.29 (s, 3H); 13C-NMR (150 MHz, DMSO-d6) δ 173.4, 139.0, 129.3, 129.0, 126.9, 46.4, 34.8, 26.6. HRMS(+): calcd. for C10H13NO [M + H]+ 164.1070, found 164.1071; calcd. for C10H13NONa [M + Na]+ 186.0889, found 186.0884.
3n [28]: Yield: 82%. m.p.: 84–87 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.26 (brs, 1H), 7.70 (dd, J = 0.83, 8.53 Hz, 2H), 7.43–7.38 (m, 2H), 7.24–7.20 (m, 1H), 13C-NMR (150 MHz, DMSO-d6) δ 155.0 (q, 2JCF = 36.0), 136.8, 129.4, 126.0, 121.5, 116.3 (q, 1JCF3 = 286.5). HRMS(+): calcd. for C8H6F3NO [M + H]+ 190.0474, found 190.0472; calcd. for C8H6F3NONa [M + Na]+ 212.0294, found 212.0296.
3o [29]: Yield: 85%. m.p.: 68–71 °C; 1H-NMR (600 MHz, DMSO-d6) δ 7.41 (brs, 1H), 3.05 (dd, J = 5.87, 10.09 Hz, 2H), 2.38–2.13 (m, 2H), 1.66 (q, J = 5.87 Hz, 2H), 1.56–1.46 (m, 4H); 13C-NMR (150 MHz, DMSO-d6) δ 177.4, 41.9, 36.9, 30.5, 30.3, 23.4, HRMS(+): calcd. for C6H11NO [M + H]+ 114.0913, found 114.0910; calcd. for C6H11NONa [M + Na]+ 136.0733, found136.0734.
4. Conclusions
In conclusion, we successfully demonstrated an efficient approach for the synthesis of amide derivatives via Beckmann rearrangement of ketoxime by using Brønsted acidic ionic liquid N-methyl-imidazolium hydrosulfate as an environmental friendly catalyst and solvent. The best reaction condition is: reaction temperature 90 °C, reaction time 6 h, solvent N-methyl-imidazolium hydrosulfate 10 grams, co-catalyst P2O5 8 mol %. Ionic liquid can be reused three times. The procedure can be extended to various symmetrical and unsymmetrical aryl-substituted and alkyl-substituted ketoxime substrates. The aryl group migration products are sole products for unsymmetrical aryl alkyl substituted amides.
Supplementary Materials
Supplementary File 1Author Contributions
H.H., X.Y., and S.Z. conceived and designed the experiments; X.C. and Z.X. performed the experiments; H.H. analyzed the data; and H.H. wrote the paper.
Funding
The project was supported by the Zhejiang province ecology first-class discipline and Zhejiang Provincial Department of Education general research projects (Y201636410).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghiaci, M.; Aghaei, H.; Oroojeni, M.; Aghabarari, B.; Rives, V.; Vicente, M.A.; Sobrados, I.; Sanz, J. Synthesis of paracetamol by liquid phase Beckmann rearrangement of 4-hydroxyacetophenone oxime over H3PO4/Al-MCM-41. Catal. Commun. 2009, 10, 1486–1492. [Google Scholar] [CrossRef]
- Murray, W.V.; Lalan, P.; Gill, A.; Addo, M.F.; Lewis, J.M.; Lee, D.K.H.; Rampulla, R.; Wachter, M.P.; Hsi, J.D.; Underwood, D.C. Substituted piperidin-2-one biphenyltetrazoles as angiotensin II antagonists. Bioorg. Med. Chem. Lett. 1992, 2, 1775–1779. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.J.L.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Chruma, J.J.; Cullen, D.J.; Bowman, L.; Toy, P.H. Polyunsaturated fatty acid amides from the Zanthoxylum genus—From culinary curiosities to probes for chemical biology. Nat. Prod. Rep. 2018, 35, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Z.-L.; Xiong, L.-X.; Wang, M.-Z.; Li, Y.-Q.; Li, Z.-M. Synthesis and Insecticidal Activities of Novel Anthranilic Diamides Containing Modified N-Pyridylpyrazoles. J. Agric. Food Chem. 2010, 58, 12327–12336. [Google Scholar] [CrossRef] [PubMed]
- Zhenyu, J.; Ziqing, C.; Jianwen, C.; Li, Z.; Fan, Y.; Xianlang, Q.; Haifeng, B. High efficiency toughness of aromatic sulfonamide in polyamide 6. J. Appl. Polym. Sci. 2018, 135, 46527. [Google Scholar]
- Nguyen, T.B.; Sorres, J.; Tran, M.Q.; Ermolenko, L.; Al-Mourabit, A. Boric Acid: A Highly Efficient Catalyst for Transamidation of Carboxamides with Amines. Org. Lett. 2012, 14, 3202–3205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Mampuys, P.; Sergeyev, S.; Ballet, S.; Maes, B.U.W. Amine Activation: N-Arylamino Acid Amide Synthesis from Isothioureas and Amino Acids. Adv. Synth. Catal. 2017, 359, 2481–2498. [Google Scholar] [CrossRef]
- Thigulla, Y.; Ranga, S.; Ghosal, S.; Subbalakshmi, J.; Bhattacharya, A. One-Pot Two Step Nazarov-Schmidt Rearrangement for the Synthesis of Fused δ-Lactam Systems. Chemistryselect 2017, 2, 9744–9750. [Google Scholar] [CrossRef]
- Gao, P.; Bai, Z. Carbon Tetrabromide/Triphenylphosphine-Activated Beckmann Rearrangement of Ketoximes for Synthesis of Amides. Chin. J. Chem. 2017, 35, 1673–1677. [Google Scholar] [CrossRef]
- Gregory, B.J.; Moodie, R.B.; Schofield, K. The Beckrnann Rearrangement of Acetophenone Oxirnes in Sulphuric Acid. Chem. Commun. 1968, 22, 1380–1381. [Google Scholar]
- Maia, A.; Albanese, D.C.M.; Landini, D. Cyanuric chloride catalyzed Beckmann rearrangement of ketoximes in biodegradable ionic liquids. Tetrahedron 2012, 68, 1947–1950. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A. Beckmann Rearrangement of Oximes under Very Mild Conditions. J. Org. Chem. 2002, 67, 6272–6274. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Ishihara, K.; Yamamoto, H. Cyanuric Chloride as a Mild and Active Beckmann Rearrangement Catalyst. J. Am. Chem. Soc. 2005, 127, 11240–11241. [Google Scholar] [CrossRef] [PubMed]
- Blasco, T.; Corma, A.; Iborra, S.; Lezcano-González, I.; Montón, R. In situ multinuclear solid-state NMR spectroscopy study of Beckmann rearrangement of cyclododecanone oxime in ionic liquids: The nature of catalytic sites. J. Catal. 2010, 275, 78–83. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Luo, C.; Yang, T.; Yang, L.; Suo, J. Sulfamic acid as a cost-effective and recyclable catalyst for liquid Beckmann rearrangement, a green process to produce amides from ketoximes without waste. Tetrahedron Lett. 2004, 45, 3369–3372. [Google Scholar] [CrossRef]
- Itoh, T. Activation of Lipase-Catalyzed Reactions Using Ionic Liquids for Organic Synthesis. Adv. Biochem. Eng. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, C.; Yu, S.; Liu, F. Dimerization of rosin using Brønsted–Lewis acidic ionic liquid as catalyst. Catal. Commun. 2008, 9, 2030–2034. [Google Scholar] [CrossRef]
- Alvarez de Cienfuegos, L.; Robles, R.; Miguel, D.; Justicia, J.; Cuerva, J.M. Reduction Reactions in Green Solvents: Water, Supercritical Carbon Dioxide, and Ionic Liquids. ChemSusChem 2011, 4, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, S.; Sapehiyia, V.; Singh, J.; Kad, G.L. Microwave accelerated preparation of [bmim][HSO4] ionic liquid: An acid catalyst for improved synthesis of coumarins. Catal. Commun. 2005, 6, 57–60. [Google Scholar] [CrossRef]
- Mo, X.; Morgan, T.D.R.; Ang, H.T.; Hall, D.G. Scope and Mechanism of a True Organocatalytic Beckmann Rearrangement with a Boronic Acid/Perfluoropinacol System under Ambient Conditions. J. Am. Chem. Soc. 2018, 140, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.; Srivastava, R. A simple, eco-friendly, and recyclable bi-functional acidic ionic liquid catalysts for Beckmann rearrangement. J. Mol. Catal. A Chem. 2013, 376, 90–97. [Google Scholar] [CrossRef]
- Jeong, T.-S.; Kim, M.J.; Yu, H.; Kim, K.S.; Choi, J.-K.; Kim, S.-S.; Lee, W.S. (E)-Phenyl- and -heteroaryl-substituted O-benzoyl-(or acyl)oximes as lipoprotein-associated phospholipase A2 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.K.; Du, C.; Pang, Y.D.; Lian, X.; Xue, C.T.; Chen, Y.Y.; Wang, X.F.; Cheng, M.S.; Guo, C.; Lin, B.; et al. Lewis acid-assisted N-fluorobenzenesulfonimide-based electrophilic fluorine catalysis in Beckmann rearrangement. Tetrahedron Lett. 2016, 57, 5820–5824. [Google Scholar] [CrossRef]
- Sharma, R.; Vishwakarma, R.A.; Bharate, S.B. Ligand-Free Copper-Manganese Spinel Oxide-Catalyzed Tandem One-Pot C–H Amidation and N-Arylation of Benzylamines: A Facile Access to 2-Arylquinazolin-4(3H)-ones. Adv. Synth. Catal. 2016, 358, 3027–3033. [Google Scholar] [CrossRef]
- Muraca, A.C.A.; Perecim, G.P.; Rodrigues, A.; Raminelli, C. Convergent Total Synthesis of (+/−)-Apomorphine via Benzyne Chemistry: Insights into the Mechanisms Involved in the Key Step. Synthesis 2017, 49, 3546–3557. [Google Scholar]
- Schmidt, E.Y.; Ushakov, I.A.; Zorina, N.V.; Mikhaleva, A.I.; Trofimov, B.A. The reaction of 2-arylazo-1-vinylpyrroles with trifluoroacetic anhydride: Unexpected formation of N-aryl-2,2,2-trifluoroacetamides and conjugated polymers. Mendeleev Commun. 2011, 21, 36–37. [Google Scholar] [CrossRef]
- Xing, S.; Han, Q.; Shi, Z.; Wang, S.; Yang, P.; Wu, Q.; Li, M. A hydrophilic inorganic framework based on a sandwich polyoxometalate: Unusual chemoselectivity for aldehydes/ketones with in situ generated hydroxylamine. Dalton Trans. 2017, 46, 11537–11541. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).