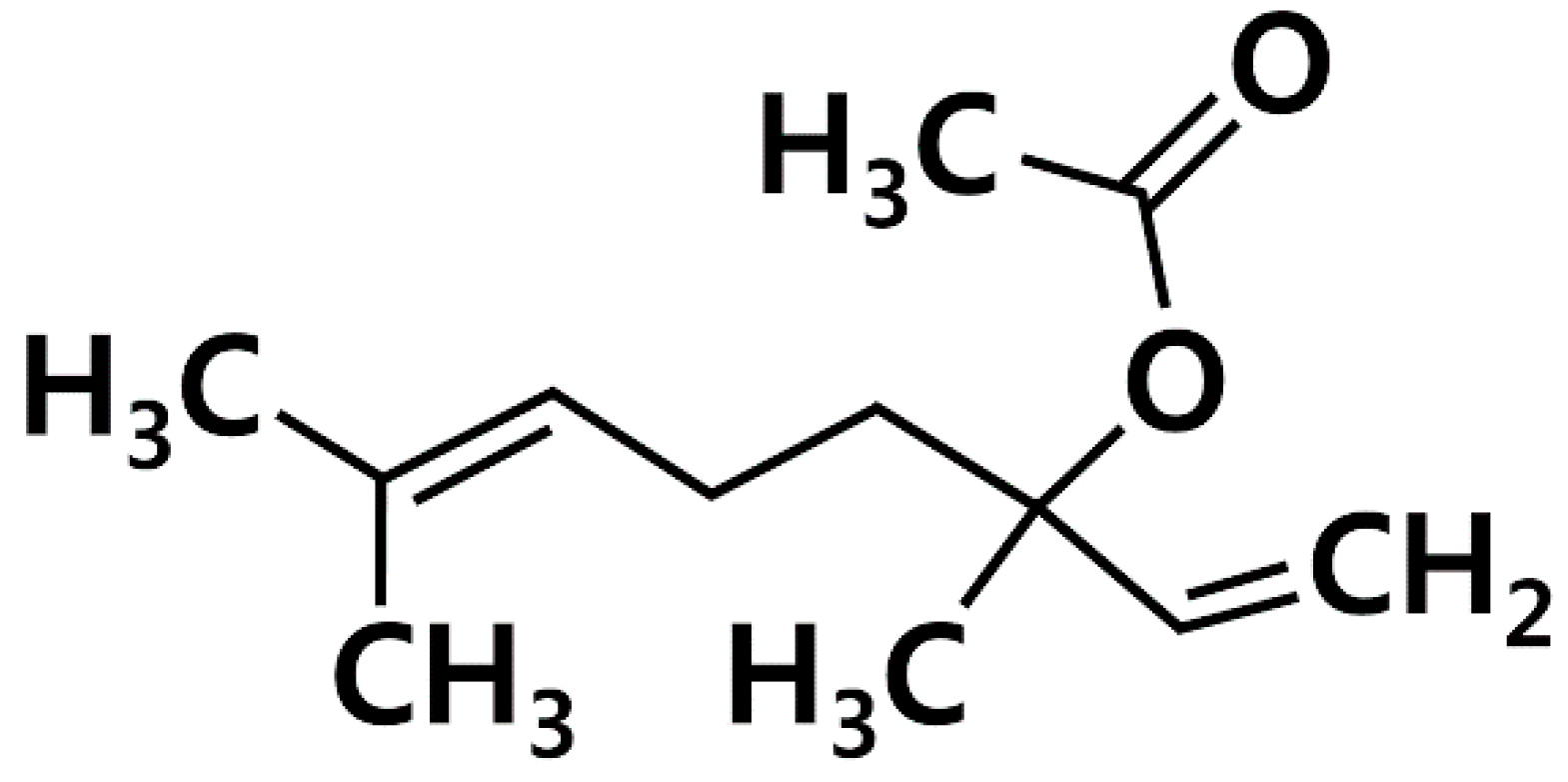
Effects of Linalyl Acetate on Thymic Stromal Lymphopoietin Production in Mast Cells
Abstract
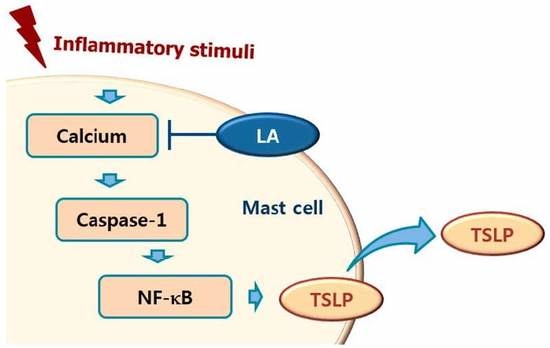
1. Introduction
2. Results
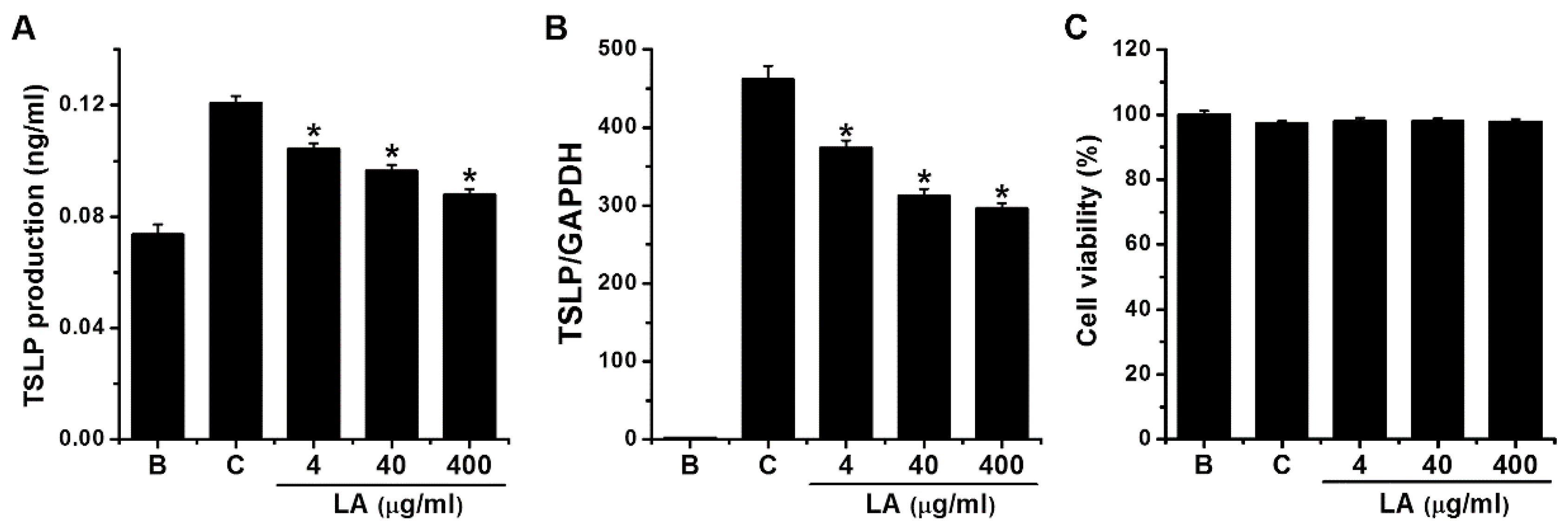
2.1. Effect of LA on TSLP Production in HMC-1 Cells
2.2. Effect of LA on TSLP mRNA Expression in HMC-1 Cells
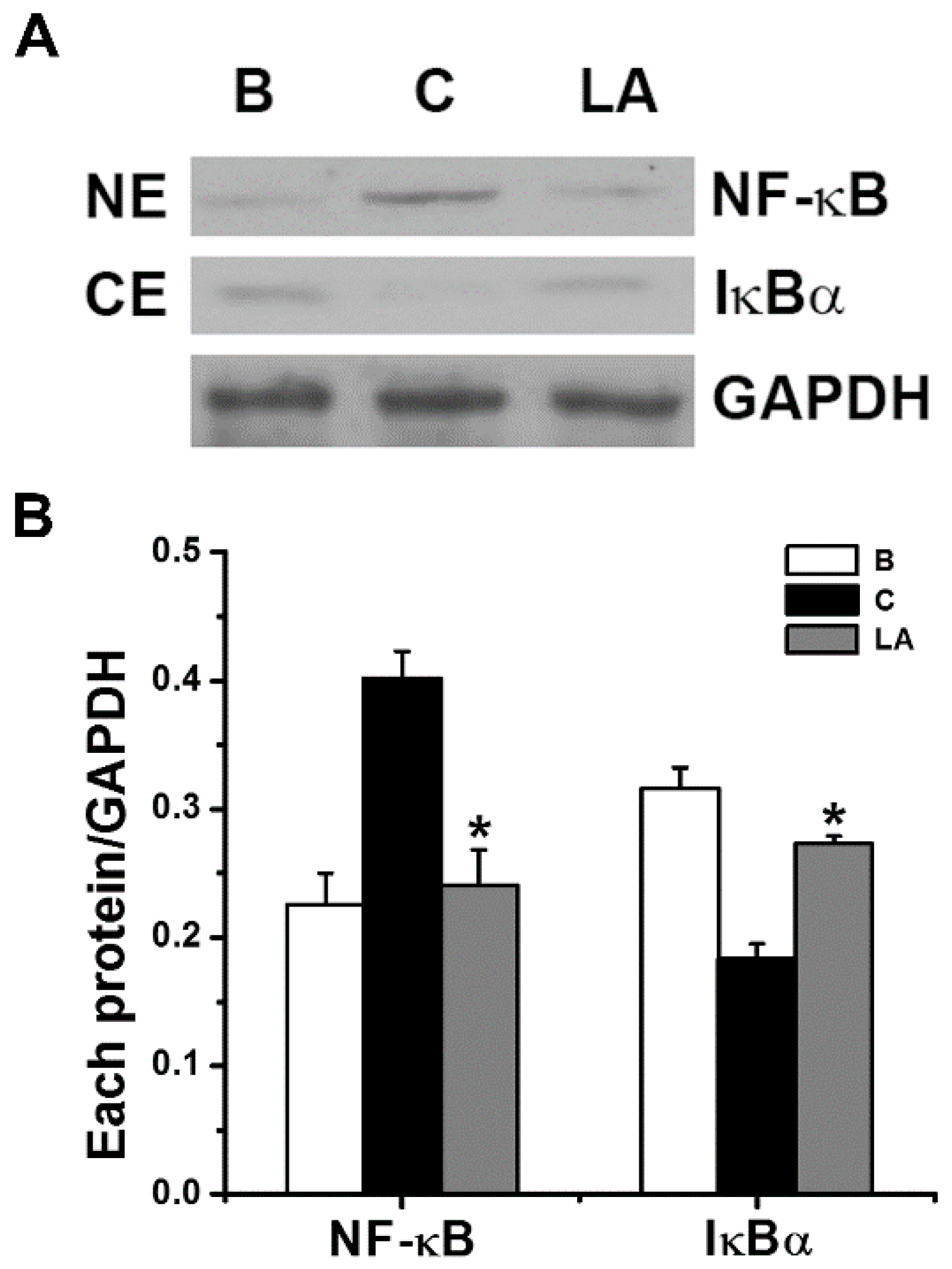
2.3. Effect of LA on NF-κB Activation and IκBα Degradation
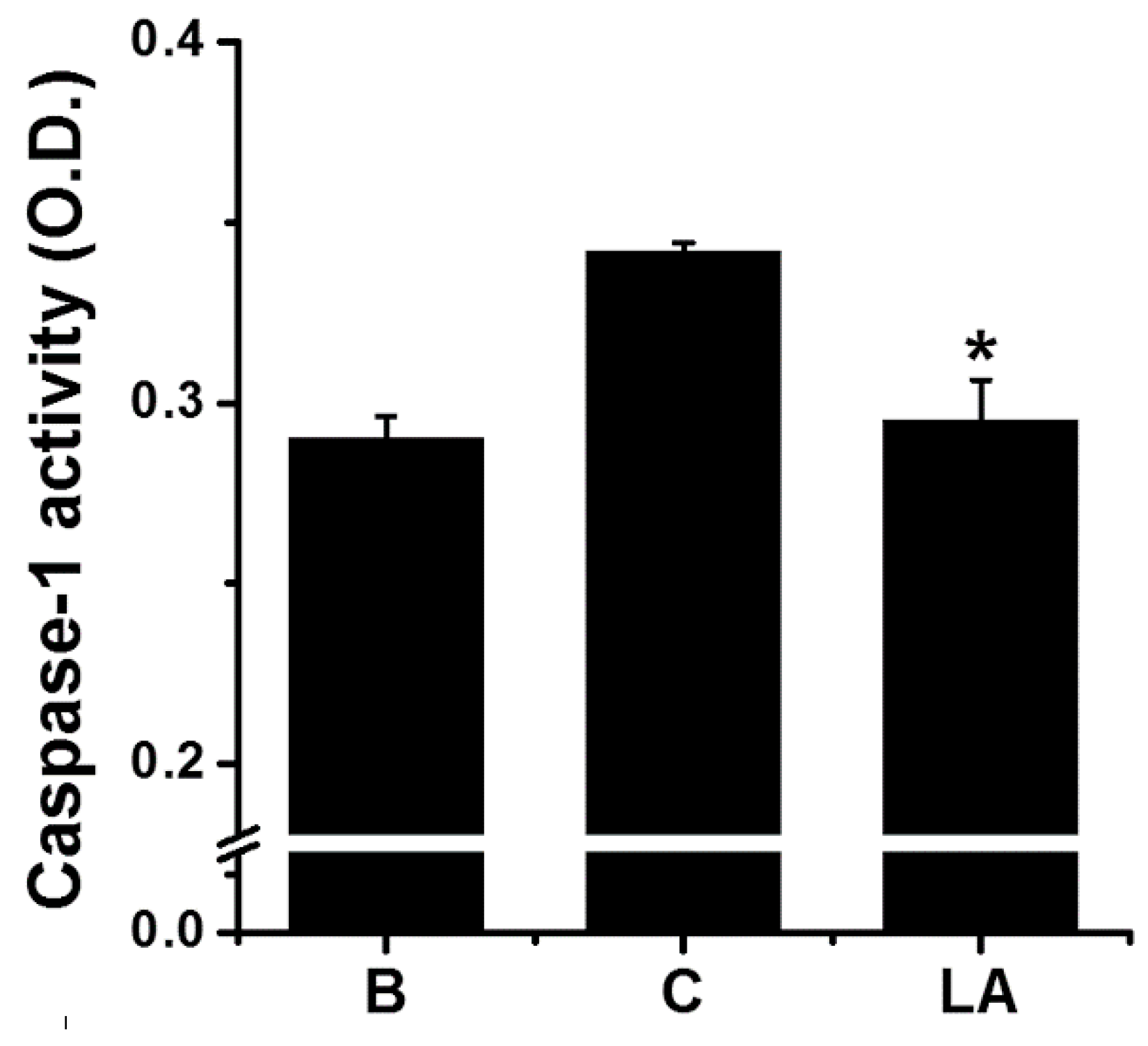
2.4. Effect of LA on Caspase-1 Activation in HMC-1 Cells
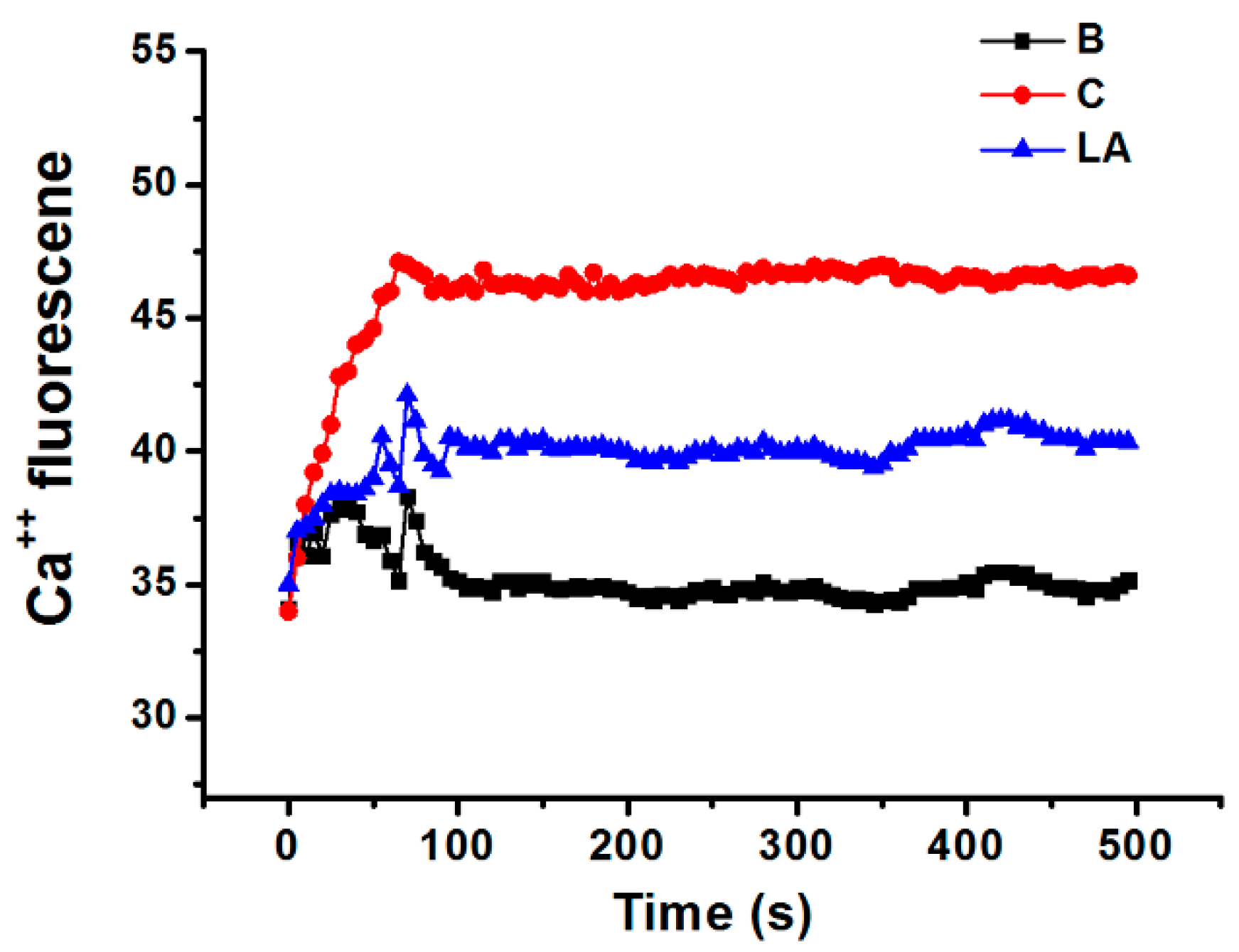
2.5. Effect of LA on Intracellular Calcium Level in HMC-1 Cells
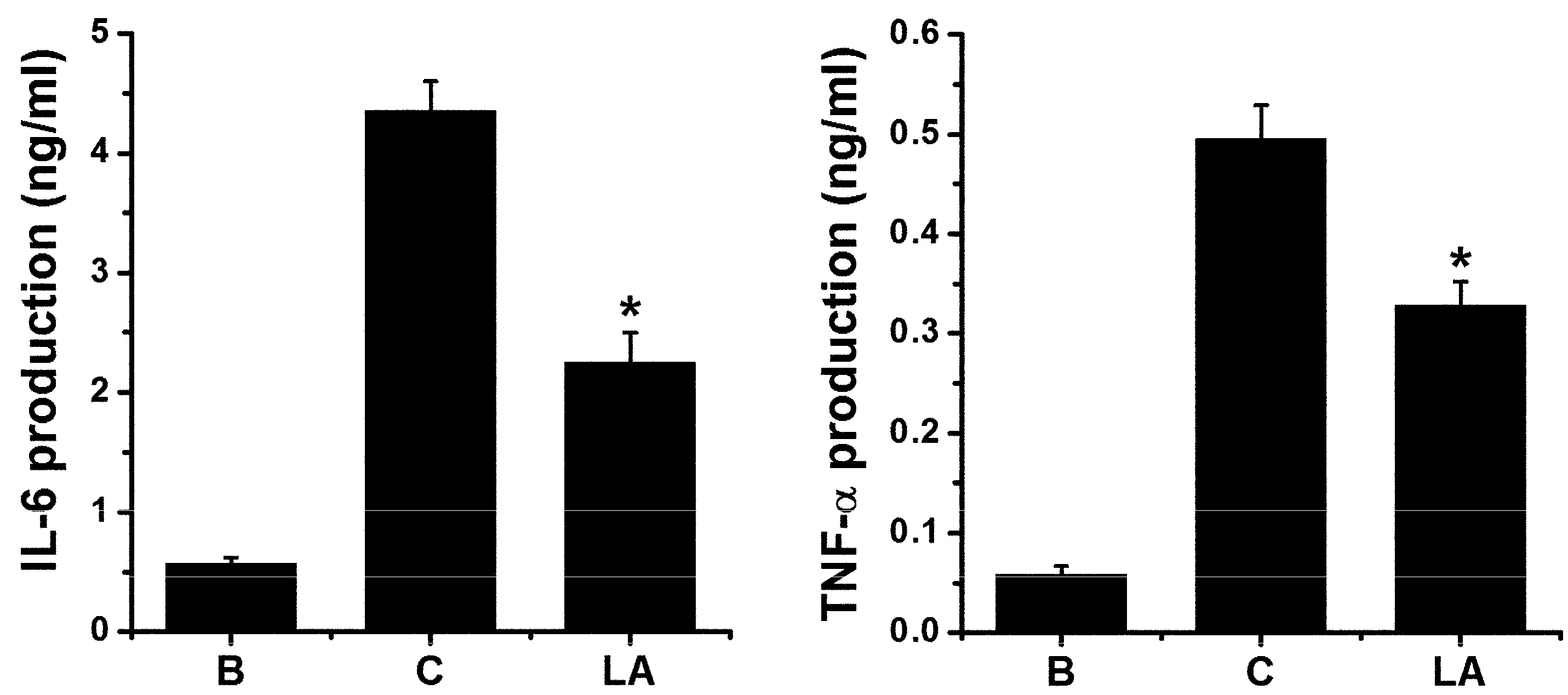
2.6. Effect of LA on Pro-inflammatory Cytokine Levels in HMC-1 Cells
2.7. Effect of LA in Animal Model
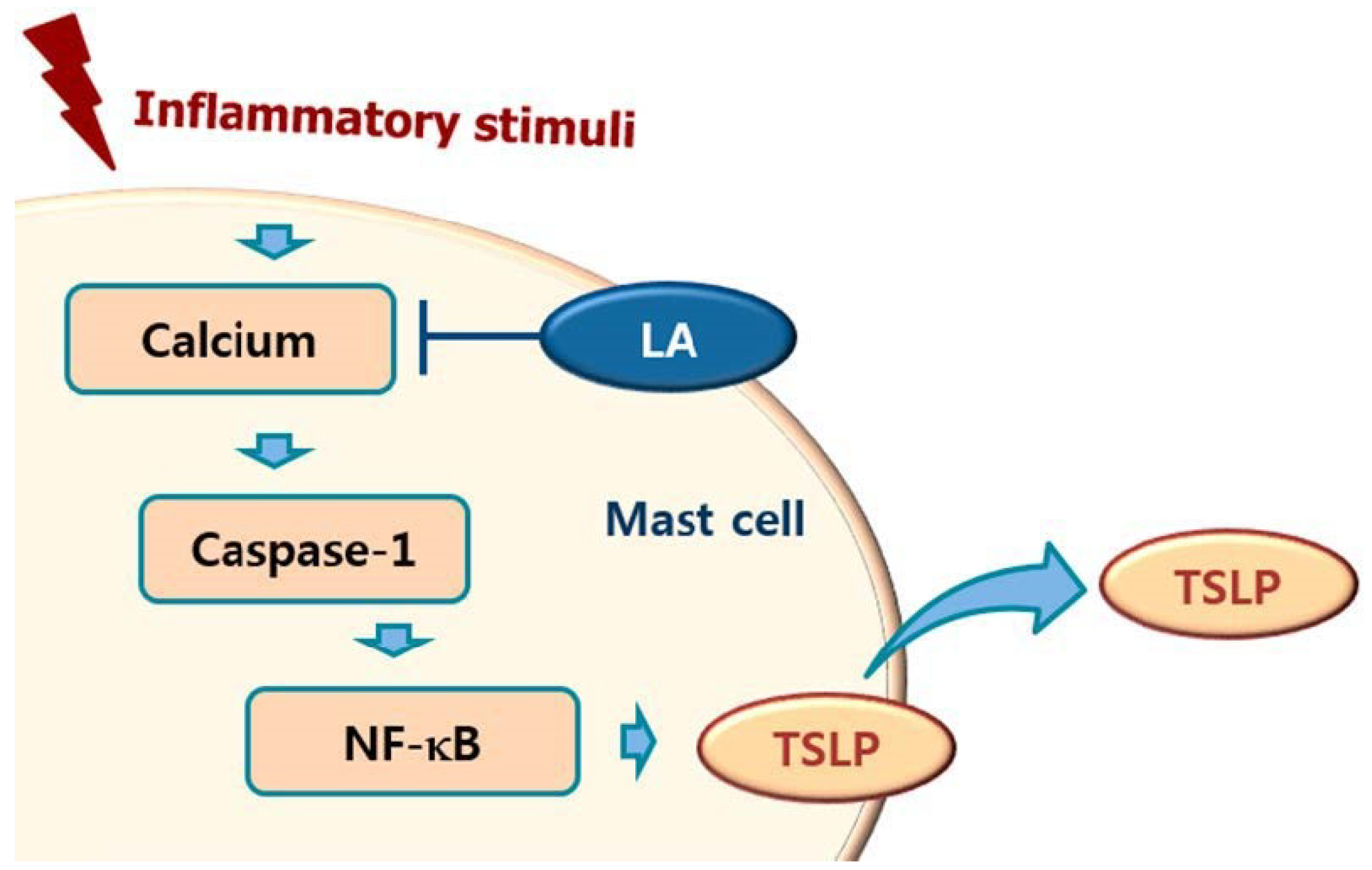
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells
4.3. MTT Assay
4.4. Cytokines Assay
4.5. Quantitative Polymerase Chain Reaction (qPCR)
4.6. Caspase-1 Assay
4.7. Preparation of Nuclear and Cytoplasmic Extracts
4.8. Western Blot Analysis
4.9. Fluorescent Measurements of the Intracellular Calcium Level
4.10. PMA-Induced Ear Edema in Mice
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arima, H.; Motoyama, K.; Higashi, T.; Fukushima, S.; Ihn, H. Anti-inflammatory Effect of Sacran on Atopic Dermatitis. Yakugaku Zasshi. 2018, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Löwa, A.; Jevtić, M.; Gorreja, F.; Hedtrich, S. Alternatives to animal testing in basic and preclinical research of atopic dermatitis. Exp. Dermatol. 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis. Ann. Dermatol. 2010, 22, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Plötz, S.G.; Ring, J. What’s new in atopic eczema? Expert Opin. Emerg. Drugs 2010, 15, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Landheer, J.; Giovannone, B.; Mattson, J.D.; Tjabringa, S.; Bruijnzeel-Koomen, C.A.; McClanahan, T.; de Waal Malefyt, R.; Knol, E.; Hijnen, D. Epicutaneous application of house dust mite induces thymic stromal lymphopoietin in nonlesional skin of patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, W.H.; Wang, X.R.; Liu, Y.; Chen, M.; Xu, X.G.; Liao, W.Q.; Hu, J.H. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharmacol. 2015, 28, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Döcke, W.D.; Zollner, T.M.; Röse, L. Chronic mouse model of TMA-induced contact hypersensitivity. J. Investig. Dermatol. 2009, 129, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. The suppression of thymic stromal lymphopoietin expression by selenium. Amino Acids 2012, 43, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Suppression of thymic stromal lymphopoietin production by rutin in mast cells. Food Chem. 2012, 133, 76–81. [Google Scholar] [CrossRef]
- Moon, P.D.; Choi, I.H.; Kim, H.M. Epigallocatechin-3-O-gallate inhibits the production of thymic stromal lymphopoietin by the blockade of caspase-1/NF-κB pathway in mast cells. Amino Acids 2012, 42, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Oh, H.A.; Nam, S.Y.; Moon, P.D.; Kim, D.W.; Kim, H.M.; Jeong, H.J. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J. Investig. Dermatol. 2014, 134, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.S.; Groß, C.J.; Dreier, R.F.; Saller, B.S.; Mishra, R.; Gorka, O.; Heilig, R.; Meunier, E.; Dick, M.S.; Ćiković, T.; et al. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 2017, 21, 3846–3859. [Google Scholar] [CrossRef] [PubMed]
- Boost, K.A.; Hoegl, S.; Hofstetter, C.; Flondor, M.; Stegewerth, K.; Platacis, I.; Pfeilschifter, J.; Muhl, H.; Zwissler, B. Targeting caspase-1 by inhalation-therapy: Effects of Ac-YVAD-CHO on IL-1 beta, IL-18 and downstream proinflammatory parameters as detected in rat endotoxaemia. Intensive Care Med. 2007, 33, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Kanneganti, T.D.; Franchi, L.; Núñez, G. Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 2007, 82, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Kim, N.R.; Kim, H.Y.; Jeong, H.J.; Kim, H.M. Schisandra chinensis and Its Main Constituent Schizandrin Attenuate Allergic Reactions by Down-Regulating Caspase-1 in Ovalbumin-Sensitized Mice. Am. J. Chin. Med. 2017, 45, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cells. Cytokine 2011, 54, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Hsieh, Y.S.; Shin, Y.K.; Kang, P.; Seol, G.H. Linalyl acetate prevents olmesartan-induced intestinal hypermotility mediated by interference of the sympathetic inhibitory pathway in hypertensive rat. Biomed. Pharmacother. 2018, 102, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baichwal, V.R. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997, 65, 111–137. [Google Scholar] [PubMed]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schöneberg, T.; Schaefer, M.; Krügel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Fujie, K.; Shinguh, Y.; Inamura, N.; Yasumitsu, R.; Okamoto, M.; Okuhara, M. Release of neutrophil elastase and its role in tissue injury in acute inflammation: Effect of the elastase inhibitor, FR134043. Eur. J. Pharmacol. 1999, 374, 117–125. [Google Scholar] [CrossRef]
- Li, G.; Lucas, J.J.; Gelfand, E.W. Protein kinase C alpha, betaI, and betaII isozymes regulate cytokine production in mast cells through MEKK2/ERK5-dependent and -independent pathways. Cell. Immunol. 2005, 238, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.S.; Al-Riyami, L.; Lumb, F.E.; Britton, G.J.; Poole, A.W.; Williams, C.M.; Braun, U.; Leitges, M.; Harnett, M.M.; Harnett, W. The role of individual protein kinase C isoforms in mouse mast cell function and their targeting by the immunomodulatory parasitic worm product, ES-62. Immunol. Lett. 2015, 168, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010, 22, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Venturelli, N.; Geha, R.S. Thymic stromal lymphopoietin and IL-33 promote skin inflammation and vaccinia virus replication in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Morizane, S.; Takiguchi, T.; Iwatsuki, K. Dexamethasone but not tacrolimus suppresses TNF-α-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J. Dermatol. Sci. 2015, 80, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Okumura, S.; Sagara, H.; Yuki, K.; Sasaki, T.; Watanabe, N.; Fueki, M.; Sugiyama, K.; Takeda, K.; Fukuda, T.; et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur. Respir. J. 2009, 34, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Luo, L.; Tian, H.; Feng, G.; Cai, Y.; Xu, R.; Wang, K.; Wang, Z. Study of the protective effects of dexamethasone on ileum mucosa injury in rats with severe acute pancreatitis. Pancreas 2008, 37, e74–e82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Herbert, C.; Thomas, P.S.; Wollin, L.; Beume, R.; Yang, M.; Webb, D.C.; Foster, P.S. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J. Pharmacol. Exp. Ther. 2003, 307, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA 2007, 104, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xie, X.; Zhu, Z.; Yu, X.; Liu, H.; Wang, H.; Fan, H.; Wang, D.; Jiang, G.; Hong, M. Screening active components from Yu-ping-feng-san for regulating initiative key factors in allergic sensitization. PLoS ONE 2014, 9, e107279. [Google Scholar] [CrossRef] [PubMed]
- Humke, E.W.; Shriver, S.K.; Starovasnik, M.A.; Fairbrother, W.J.; Dixit, V.M. ICEBERG: A novel inhibitor of interleukin-1beta generation. Cell 2000, 103, 99–111. [Google Scholar] [CrossRef]
- Moon, P.D.; Choi, I.H.; Kim, H.M. Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur. J. Pharmacol. 2011, 671, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Kim, H.M.; Jeong, H.J. Tryptanthrin ameliorates atopic dermatitis through down-regulation of TSLP. Arch. Biochem. Biophys. 2014, 542, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Han, N.R.; Ryu, K.J.; Kang, S.W.; Go, J.H.; Jang, J.B.; Choi, Y.; Kim, H.M.; Jeong, H.J. A novel compound 2-(4-{2-[(phenylthio)acetyl]carbonohydrazonoyl}phenoxy)acetamide downregulates TSLP through blocking of caspase-1/NF-κB pathways. Int. Immunopharmacol. 2016, 38, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Kim, H.M.; Jeong, H.J. Thymic stromal lymphopoietin is regulated by the intracellular calcium. Cytokine 2012, 59, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IκB/NF-κB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Anti-inflammatory effect of phenethyl isothiocyanate, an active ingredient of Raphanus sativus Linne. Food Chem. 2012, 131, 1332–1339. [Google Scholar] [CrossRef]
- Moon, P.D.; Choi, I.H.; Kim, H.M. Berberine inhibits the production of thymic stromal lymphopoietin by the blockade of caspase-1/NF-κB pathway in mast cells. Int. Immunopharmacol. 2011, 11, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Ben Trivedi, A.; Kitabatake, N.; Doi, E. Toxicity of dimethyl sulfoxide as a solvent in bioassay system with HeLa cells evaluated colorimetrically with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide. Agric. Biol. Chem. 1990, 54, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Jeong, H.J.; Kim, H.M. Down-regulation of thymic stromal lymphopoietin by curcumin. Pharmacol. Rep. 2013, 65, 525–531. [Google Scholar] [CrossRef]
- Moon, P.D.; Kim, K.Y.; Rew, K.H.; Kim, H.M.; Jeong, H.J. Anti-fatigue effects of porcine placenta and its amino acids in a behavioral test on mice. Can. J. Physiol. Pharmacol. 2014, 92, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Nam, S.Y.; Oh, H.A.; Han, N.R.; Kim, Y.S.; Moon, P.D.; Shin, S.Y.; Kim, M.H.; Kim, H.M. Interleukin-32-induced thymic stromal lymphopoietin plays a critical role in macrophage differentiation through the activation of caspase-1 in vitro. Arthritis Res. Ther. 2012, 14, R259. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Jeong, H.J.; Kim, H.M. Hydrogen sulfide diminishes the levels of thymic stromal lymphopoietin in activated mast cells. Arch. Dermatol. Res. 2016, 308, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Schoonbroodt, S.; Legrand-Poels, S.; Best-Belpomme, M.; Piette, J. Activation of the NF-κB transcription factor in a T-lymphocytic cell line by hypochlorous acid. Biochem. J. 1997, 321, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, M.H.; Oh, H.A.; Nam, S.Y.; Han, N.R.; Jeong, H.J.; Kim, H.M. Cysteine induces longitudinal bone growth in mice by upregulating IGF-I. Int. J. Mol. Med. 2015, 36, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, H.M. Antiinflammatory effects of traditional Korean medicine, JinPi-tang and its active ingredient, hesperidin in HaCaT cells. Phytother. Res. 2012, 26, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Nam, S.Y.; Ryu, K.J.; Yoou, M.S.; Choi, J.H.; Hwang, S.Y.; Kim, H.M.; Jeong, H.J. Inhibitory effects of atractylone on mast cell-mediated allergic reactions. Chem. Biol. Interact. 2016, 258, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Ryu, K.J.; Jang, J.B.; Kim, H.M.; Jeong, H.J. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 257–265. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Treatment | Dose (mg/kg) | Pre-Thickness (mm) | Post-Thickness (mm) | Increase (mm) |
|---|---|---|---|---|
| B | - | 0.3358 ± 0.0041 | 0.3390 ± 0.0035 | 0.0032 ± 0.0016 |
| C | - | 0.3335 ± 0.0062 | 0.4375 ± 0.0102 | 0.1040 ± 0.0072 |
| LA | 400 | 0.3342 ± 0.0066 | 0.3905 ± 0.0038 | 0.0563 ± 0.0049 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, P.-D.; Han, N.-R.; Lee, J.S.; Kim, H.-M.; Jeong, H.-J. Effects of Linalyl Acetate on Thymic Stromal Lymphopoietin Production in Mast Cells. Molecules 2018, 23, 1711. https://doi.org/10.3390/molecules23071711
Moon P-D, Han N-R, Lee JS, Kim H-M, Jeong H-J. Effects of Linalyl Acetate on Thymic Stromal Lymphopoietin Production in Mast Cells. Molecules. 2018; 23(7):1711. https://doi.org/10.3390/molecules23071711
Chicago/Turabian StyleMoon, Phil-Dong, Na-Ra Han, Jin Soo Lee, Hyung-Min Kim, and Hyun-Ja Jeong. 2018. "Effects of Linalyl Acetate on Thymic Stromal Lymphopoietin Production in Mast Cells" Molecules 23, no. 7: 1711. https://doi.org/10.3390/molecules23071711
APA StyleMoon, P.-D., Han, N.-R., Lee, J. S., Kim, H.-M., & Jeong, H.-J. (2018). Effects of Linalyl Acetate on Thymic Stromal Lymphopoietin Production in Mast Cells. Molecules, 23(7), 1711. https://doi.org/10.3390/molecules23071711