N-Butanol Subfraction of Brassica Rapa L. Promotes Reactive Oxygen Species Production and Induces Apoptosis of A549 Lung Adenocarcinoma Cells via Mitochondria-Dependent Pathway
Abstract
1. Introduction
2. Results
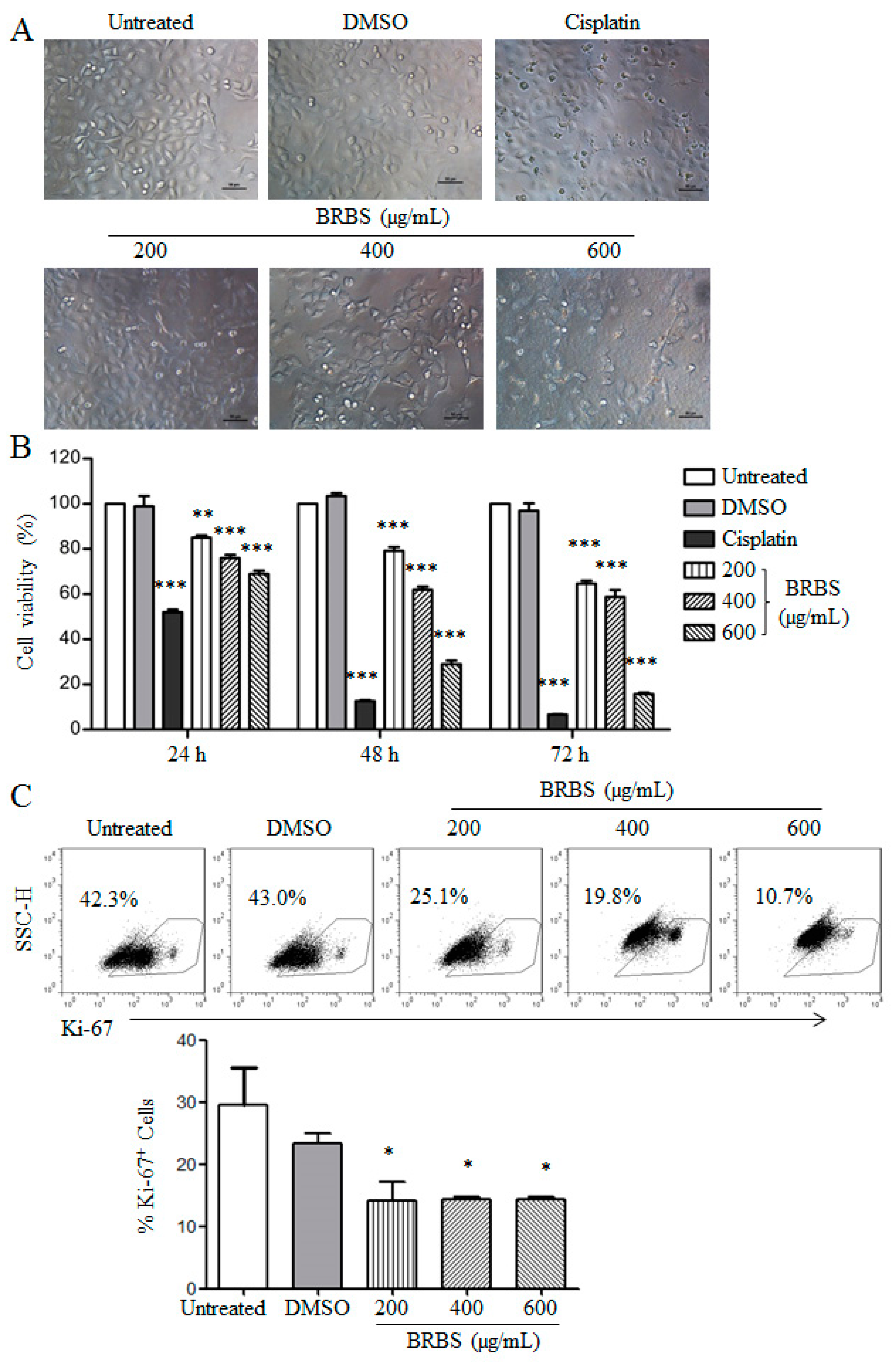
2.1. BRBS Inhibits the Proliferation of A549 Cells
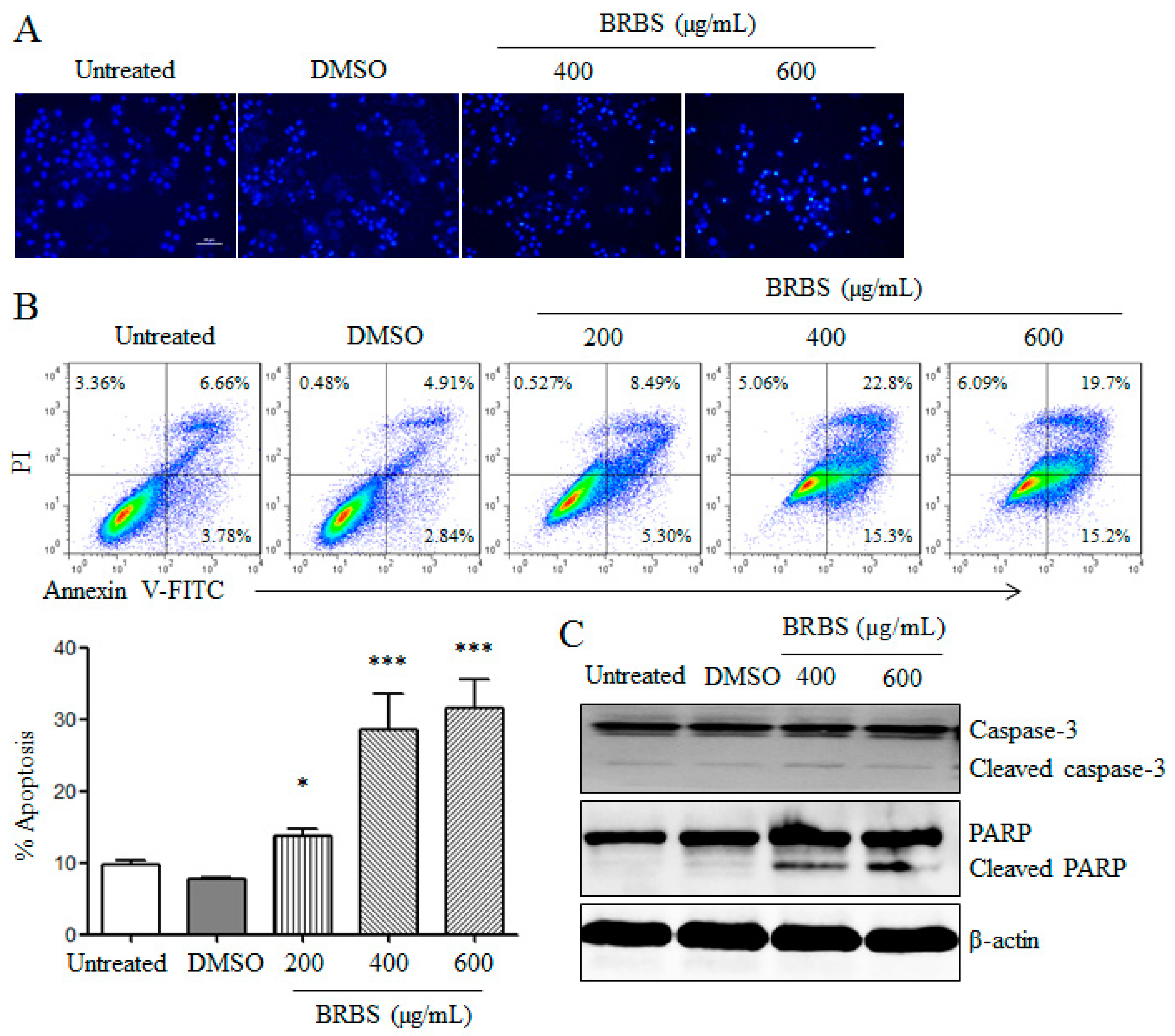
2.2. BRBS Induces Apoptosis and Cell Cycle Arrest in A549 Cells
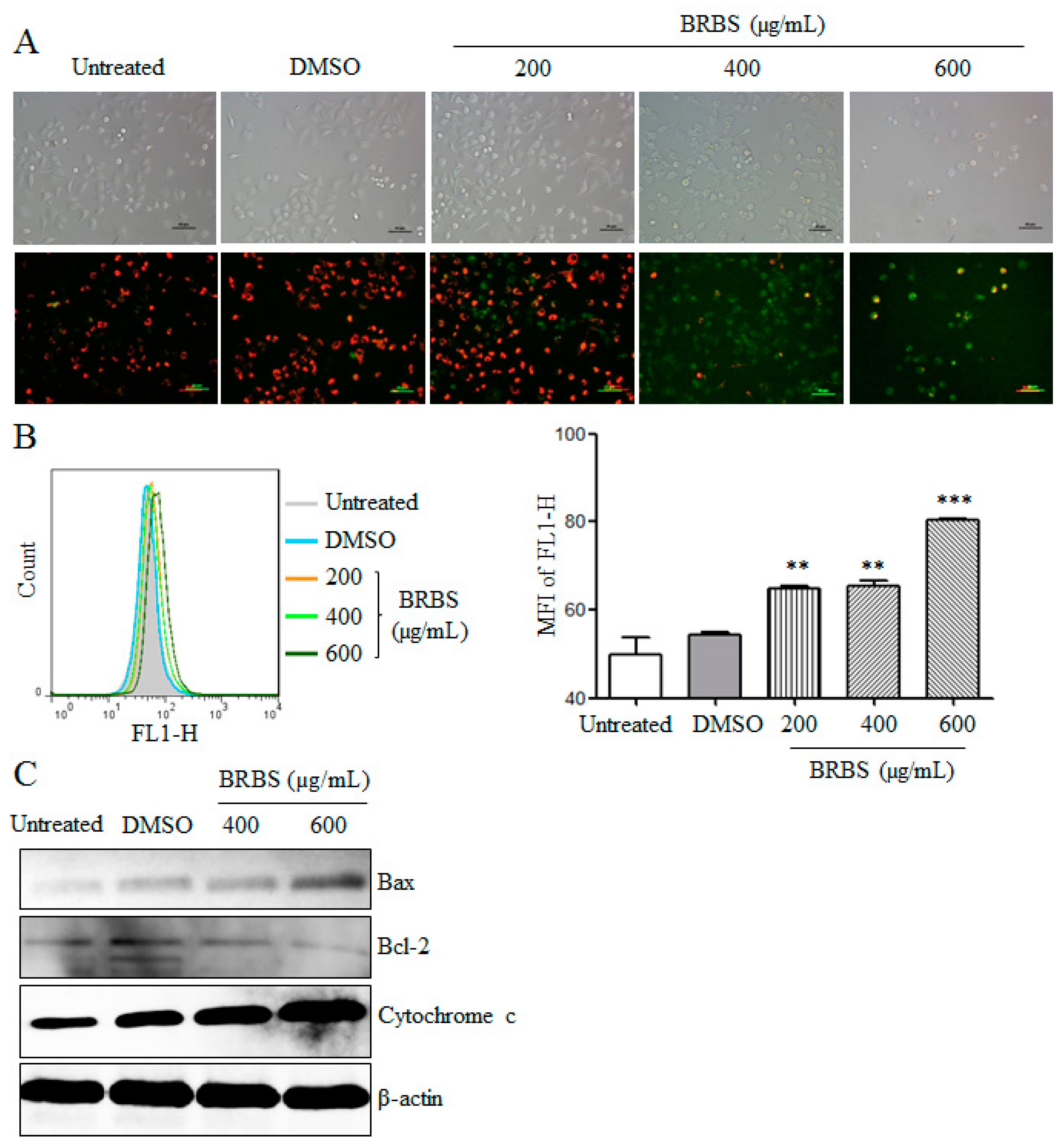
2.3. BRBS Reduces Mitochondrial Membrane Potential (MMP) of A549 Cells
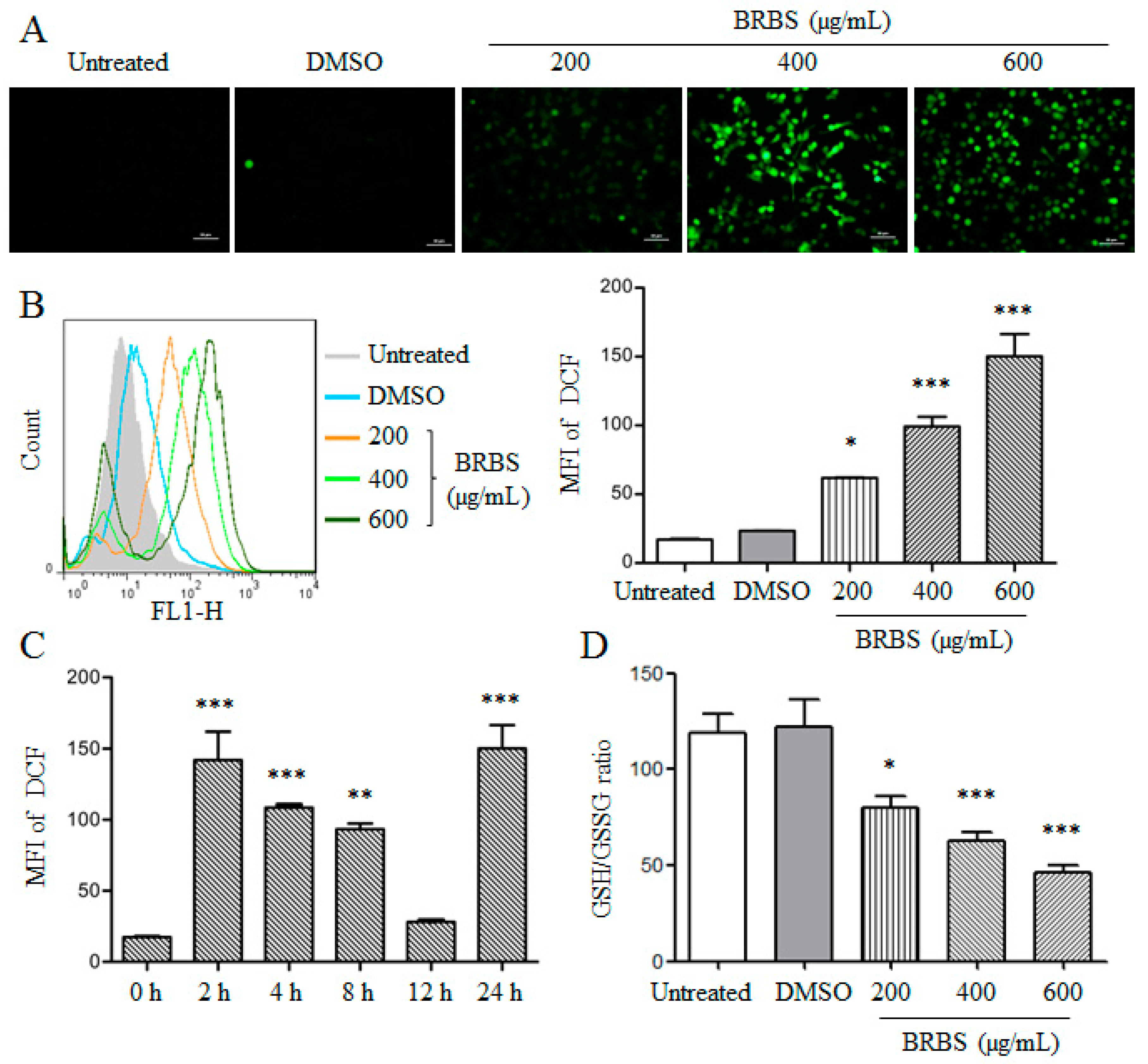
2.4. BRBS Increases ROS Production in A549 Cells
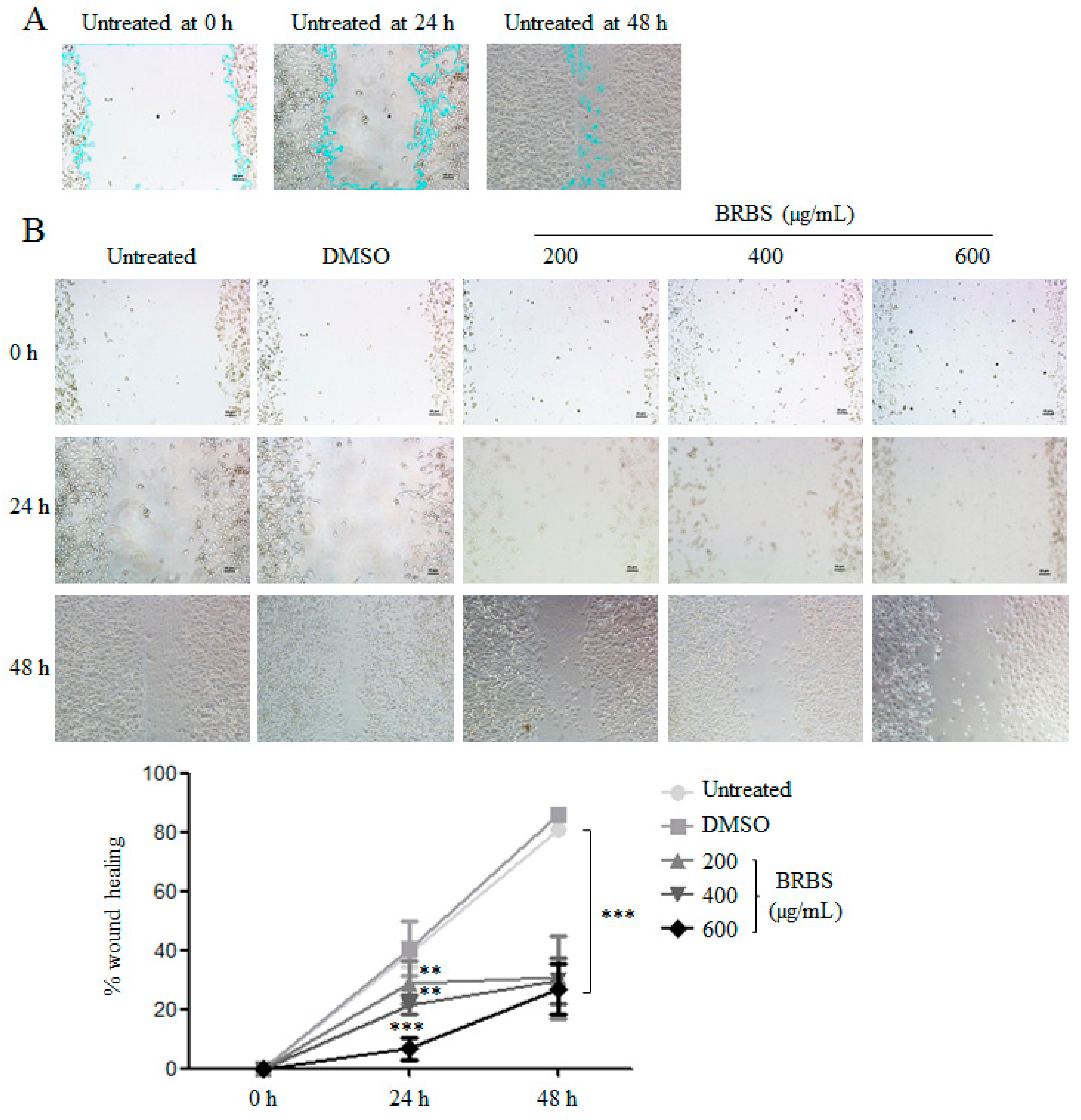
2.5. BRBS Inhibits A549 Cell Migration
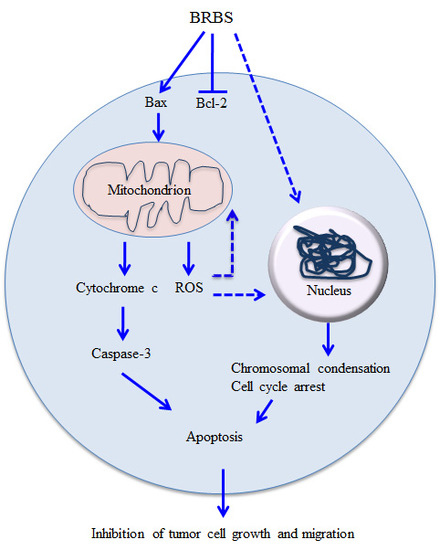
3. Discussion
4. Materials and Methods
4.1. N-Butanol Subfraction of B. rapa L. (BRBS)
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Observation of Cell Morphology
4.5. Determination of Intracellular Reactive Oxygen Species (ROS) and GSH/GSSG Ratio
4.6. Analysis of Apoptosis and Cell Cycle
4.7. Detection of Ki-67
4.8. Determination of Mitochondrial Membrane Potential
4.9. Wound Healing Assay
4.10. Hoechst 33258 Staining
4.11. Western Blot
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Dios, N.; Calvo, P.; Rico, M.; Martín, M.; Couñago, F.; Sotoca, A.; Taboada, B.; Rodríguez, A. Recent developments in radiotherapy for small-cell lung cancer: A review by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin. Transl. Oncol. 2017, 19, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Giovannetti, E.; Rolfo, C.; Karachaliou, N.; González-Cao, M.; Altavilla, G.; Rosell, R. Recent developments in the use of immunotherapy in non-small cell lung cancer. Expert. Rev. Respir. Med. 2016, 10, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Baize, N.; Monnet, I.; Greillier, L.; Quere, G.; Kerjouan, M.; Janicot, H.; Vergnenegre, A.; Auliac, J.B.; Chouaid, C. Second-line treatments of small-cell lung cancers. Expert Rev. Anticancer Ther. 2017, 17, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [PubMed]
- Han, S.Y.; Li, P.P. Progress of research in antitumor mechanisms with Chinese medicine. Chin. J. Integr. Med. 2009, 15, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Jia, J. Research advances on TCM anti-tumor effects and the molecular mechanisms. J. Cancer Res. Ther. 2014, 10, 8–13. [Google Scholar] [PubMed]
- Zhou, J.; Zhou, T.; Jiang, M.; Wang, X.; Liu, Q.; Zhan, Z.; Zhang, X. Research progress on synergistic anti-tumor mechanisms of compounds in traditional Chinese medicine. J. Tradit. Chin. Med. 2014, 34, 100–105. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules 2018, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Herz, C.; Schlotz, N.; Kupke, F.; Bartolomé Rodríguez, M.M.; Schreiner, M.; Rohn, S.; Lamy, E. The Brassica epithionitrile 1-cyano-2,3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol. Nutr. Food Res. 2015, 59, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Ye, H.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X.; Shen, W. Optimization of extraction, characterization and antioxidant activity of polysaccharides from Brassica rapa L. Int. J. Biol. Macromol. 2016, 82, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Noh, Y.S.; Lee, Y.S.; Cho, Y.W.; Baek, N.I.; Choi, M.S.; Jeong, T.S.; Kang, E.; Chung, H.G.; Lee, K.T. Arvelexin from Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene expression by inhibiting activation of IκB kinase. Br. J. Pharmacol. 2011, 164, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Ninomiya, M.; Nozawa, Y.; Koketsu, M. Chalcone glycosides isolated from aerial parts of Brassica rapa L. ‘hidabeni’ suppress antigen-stimulated degranulation in rat basophilic leukemia RBL-2H3 cells. Bioorg. Med. Chem. 2010, 18, 7052–7057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.E.; Wufuer, R.; Ji, J.H.; Li, J.F.; Cheng, Y.F.; Dong, C.X.; Taoerdahong, H. Structural Characterization and Immunostimulatory Activity of Polysaccharides from Brassica rapa L. J. Agric. Food Chem. 2017, 65, 9685–9692. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important brassica vegetables. Molecules 2011, 16, 6816–6832. [Google Scholar] [CrossRef] [PubMed]
- Vetterlein, M.W.; Roschinski, J.; Gild, P.; Marks, P.; Soave, A.; Doh, O.; Isbarn, H.; Höppner, W.; Wagner, W.; Shariat, S.F.; et al. Impact of the Ki-67 labeling index and p53 expression status on disease-free survival in pT1 urothelial carcinoma of the bladder. Transl. Androl. Urol. 2017, 6, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Mcilwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed]
- Virág, L.; Robaszkiewicz, A.; Rodriguez-Vargas, J.M.; Oliver, F.J. Poly(ADP-ribose) signaling in cell death. Mol. Aspects Med. 2013, 34, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, X.; Liu, X.; Xu, P.; Zhang, K.; Lin, X. Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des. Devel. Ther. 2015, 9, 2735–2744. [Google Scholar] [PubMed]
- Wang, Q.; Wang, H.; Jia, Y.; Pan, H.; Ding, H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017, 79, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. Chem. Biol. Interact. 2016, 245, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Li, T.; Ahmad Khan, M.K.; Rasul, A.; Nawaz, F.; Sun, M.; Zheng, Y.; Ma, T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed. Res. Int. 2013, 2013, 719858. [Google Scholar] [CrossRef] [PubMed]
- Maryam, A.; Mehmood, T.; Zhang, H.; Li, Y.; Khan, M.; Ma, T. Alantolactone induces apoptosis, promotes STAT3 glutathionylation and enhances chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin via oxidative stress. Sci. Rep. 2017, 7, 6242. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, X. Inhibitory effects of Broccolini leaf flavonoids on human cancer cells. Scanning 2012, 34, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, T.; Guo, X.; Ma, C.; Zhang, X. Purification, characterization, and biological activities of broccolini lectin. Biotechnol. Prog. 2015, 31, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Conde-Rioll, M.; Gajate, C.; Fernández, J.J.; Villa-Pulgarin, J.A.; Napolitano, J.G.; Norte, M.; Mollinedo, F. Antitumor activity of Lepidium latifolium and identification of the epithionitrile 1-cyano-2,3-epithiopropane as its major active component. Mol. Carcinog. 2018, 57, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Maryanovich, M.; Gross, A. A ROS rheostat for cell fate regulation. Trends Cell. Biol. 2013, 23, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Sohn, H.; Xue, L.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane is a novel mitochondrial H(+)-ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006, 66, 4880–4887. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aipire, A.; Chen, Q.; Cai, S.; Li, J.; Fu, C.; Ying, T.; Lu, J.; Li, J. N-Butanol Subfraction of Brassica Rapa L. Promotes Reactive Oxygen Species Production and Induces Apoptosis of A549 Lung Adenocarcinoma Cells via Mitochondria-Dependent Pathway. Molecules 2018, 23, 1687. https://doi.org/10.3390/molecules23071687
Aipire A, Chen Q, Cai S, Li J, Fu C, Ying T, Lu J, Li J. N-Butanol Subfraction of Brassica Rapa L. Promotes Reactive Oxygen Species Production and Induces Apoptosis of A549 Lung Adenocarcinoma Cells via Mitochondria-Dependent Pathway. Molecules. 2018; 23(7):1687. https://doi.org/10.3390/molecules23071687
Chicago/Turabian StyleAipire, Adila, Qiuyan Chen, Shanshan Cai, Jinyu Li, Changshuang Fu, Tianlei Ying, Jun Lu, and Jinyao Li. 2018. "N-Butanol Subfraction of Brassica Rapa L. Promotes Reactive Oxygen Species Production and Induces Apoptosis of A549 Lung Adenocarcinoma Cells via Mitochondria-Dependent Pathway" Molecules 23, no. 7: 1687. https://doi.org/10.3390/molecules23071687
APA StyleAipire, A., Chen, Q., Cai, S., Li, J., Fu, C., Ying, T., Lu, J., & Li, J. (2018). N-Butanol Subfraction of Brassica Rapa L. Promotes Reactive Oxygen Species Production and Induces Apoptosis of A549 Lung Adenocarcinoma Cells via Mitochondria-Dependent Pathway. Molecules, 23(7), 1687. https://doi.org/10.3390/molecules23071687