Synthesis of a Novel Fluorescent Ruthenium Complex with an Appended Ac4GlcNAc Moiety by Click Reaction
Abstract
1. Introduction
2. Results
3. Discussion
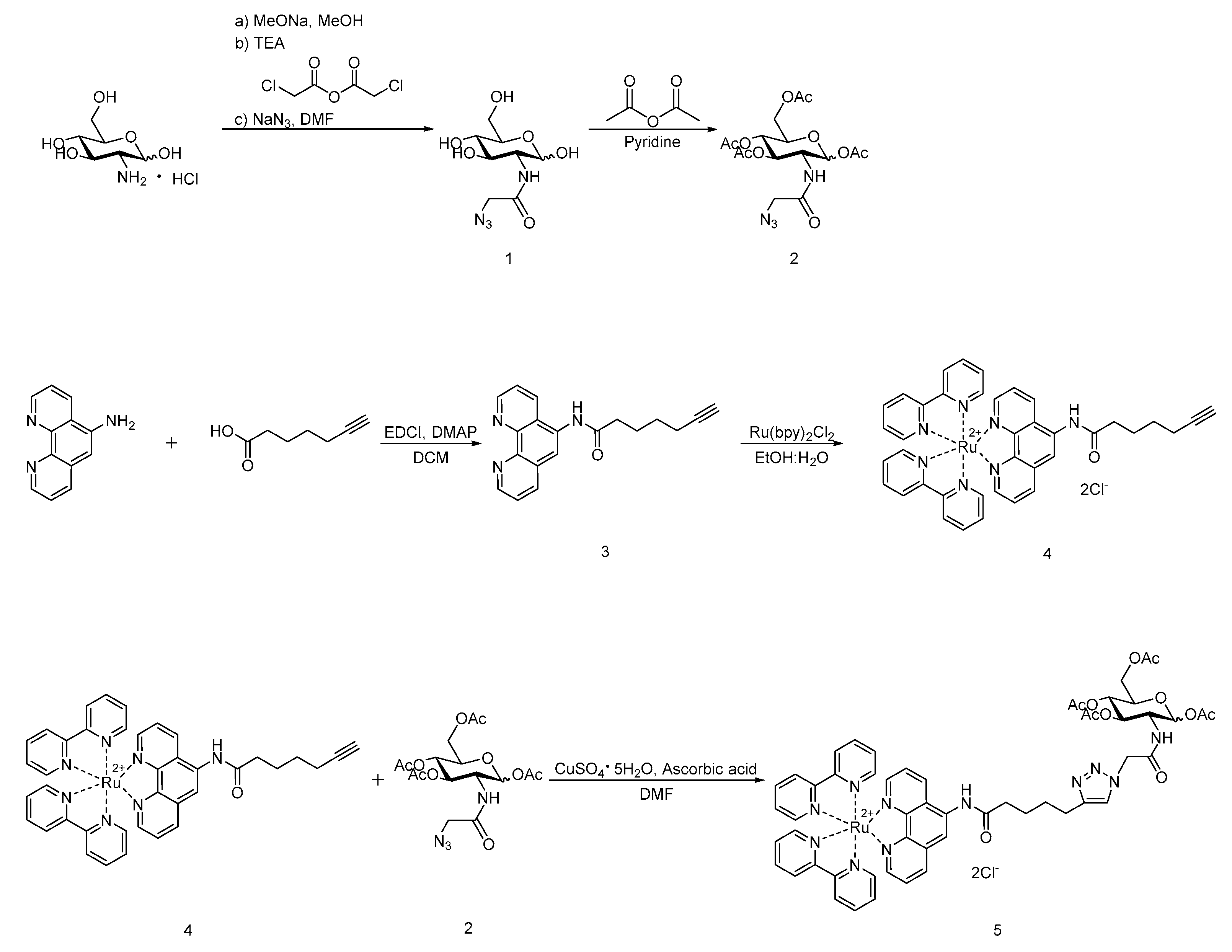
3.1. Synthesis of 2 and 3
3.2. Synthesis of Ruthenium Complexes
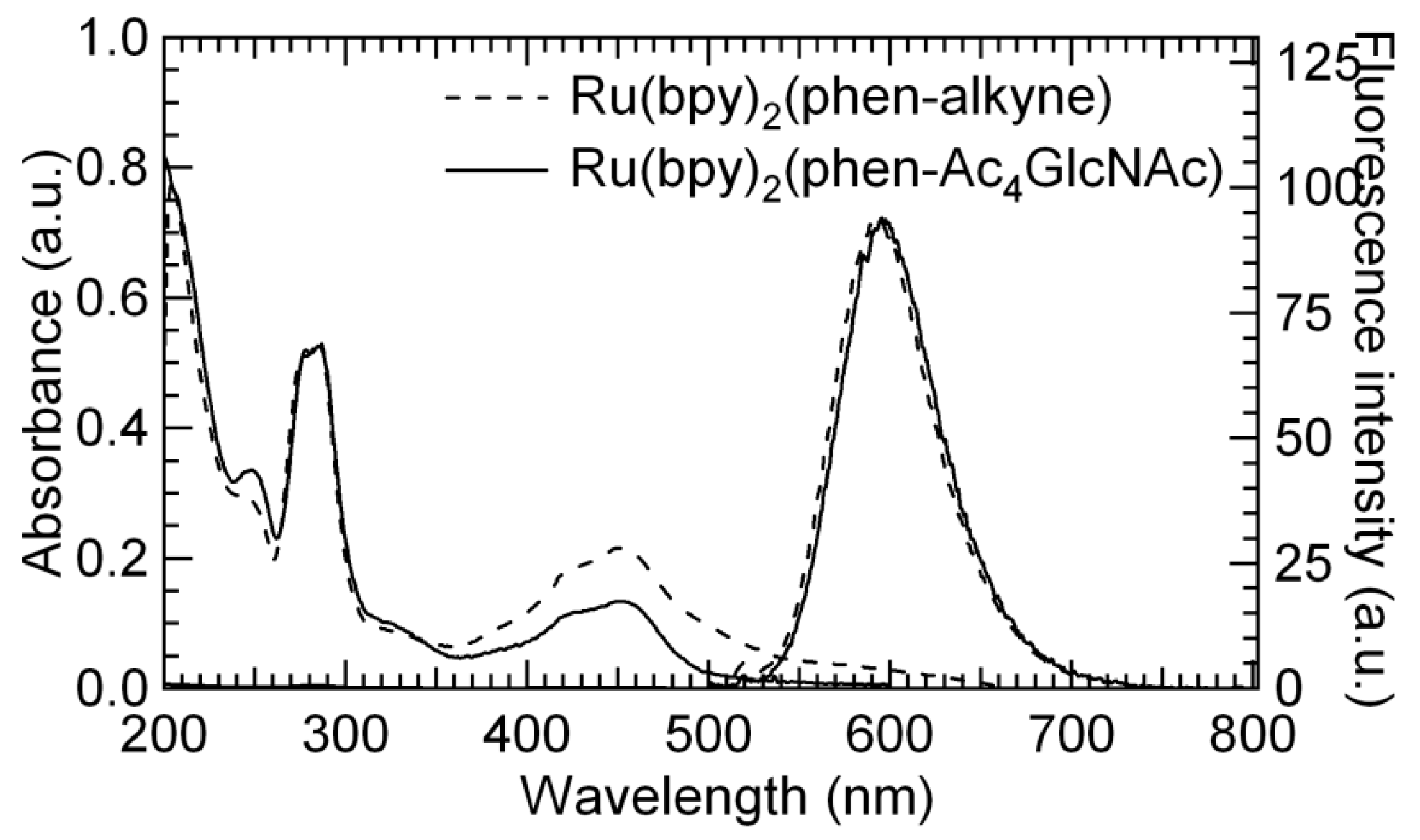
3.3. UV-Vis and Fluorescence Properties Studies of 4 and 5
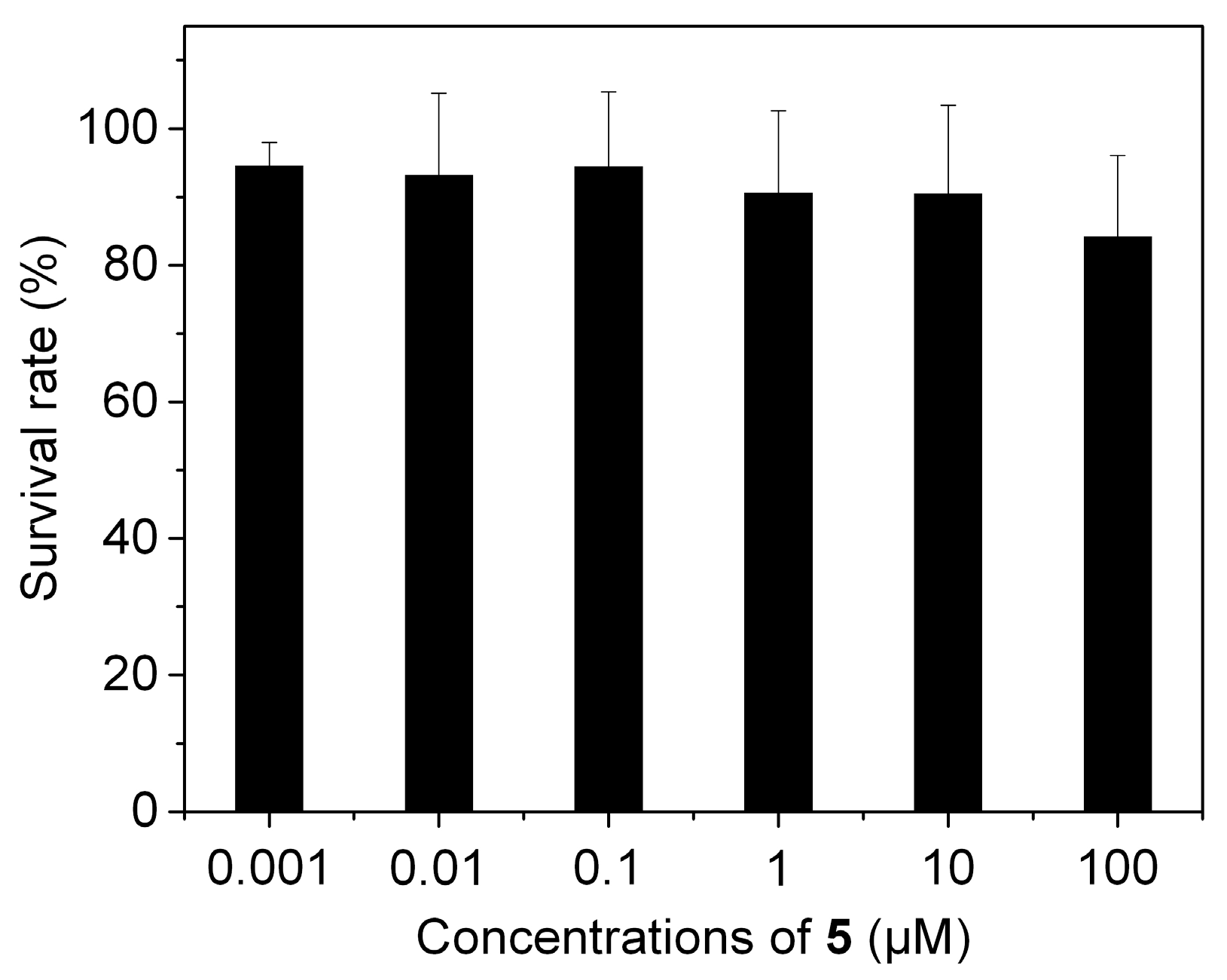
3.4. Cytotoxicity of 5 against Living Cells
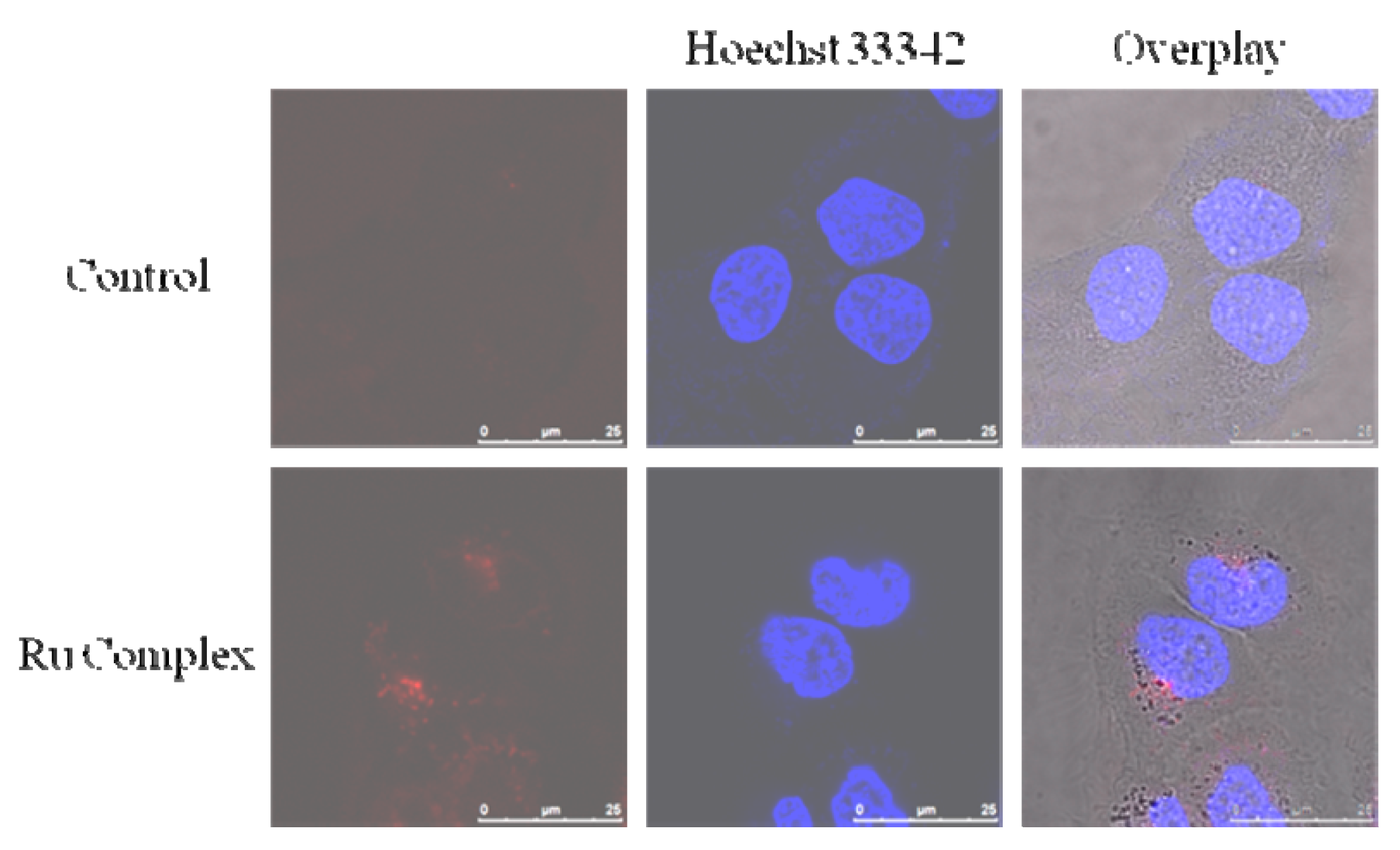
3.5. Imaging of 5 against Living MCF-7 Cells
4. Materials and Methods
4.1. Materials
4.2. Synthesis of 2-Azidoacetamido-2-deoxy-β-d-glucopyranose (GlcNAz, 1)
4.3. Synthesis of 1,3,4,6-Tetra-O-acetyl-2-N-azidoacetyl-2-deoxy-β-d-glucopyranose (Ac4GlcNAz, Mixture of Anomers, 2)
4.4. Synthesis of N-(1,10-phenanthrolin-5-yl)hept-6-ynamide (Phen-alkyne, 3)
4.5. Synthesis of Ru(bpy)2(Phen-alkyne)Cl2 (4)
4.6. Synthesis of Ru(bpy)2(Phen-Ac4GlcNAc)Cl2 (5)
4.7. Evaluation of the Cell Viability and Metabolic Activity
4.8. Imaging Cellular Uptake of 5
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jing, L.; Wang, J.; Wen, L.; He, Z.; Li, S.; Huang, K.; Jiang, K.; Xu, L.; Cheng, M.; Qu, J. An OGA-resistant probe allow specific visualization and accurate identification of O-GlcNAc-modified proteins in cells. ACS Chem. Biol. 2016, 11, 3002–3006. [Google Scholar] [CrossRef]
- Chuh, K.; Zaro, B.W.; Piller, F.; Piller, V.; Pratt, M.R. Changes in metabolic chemical reporter structure yielda selective probe of O-GlcNAc modification. J. Am. Chem. Soc. 2014, 136, 12283–12295. [Google Scholar] [CrossRef] [PubMed]
- Leturcq, M.; Lefebvre, T.; Vercoutter-Edouart, A.-S. O-GlcNAcylation and chromatin remodeling in mammals: An up-to-date overview. Biochem. Soc. Trans. 2017, 45, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Baldini, S.F.; Lefebvre, T. O-GlcNAcylation and the metabolic shift in high-proliferating cells: All the evidence suggests that sugars dictate the flux of lipid biogenesis in tumor processes. Front. Oncol. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, A.; Olivier-Van Stichelen, S.; Baldini, S.F.; Dehennaut, V.; Toillon, R.-A.; Le Bourhis, X.; El Yazidi-Belkoura, I.; Lefebvre, T. Silencing the nucleocytoplasmic O-GlcNAc transferase reduces proliferation, adhesion, and migration of cancer and fetal human colon cell lines. Front. Endocrinol. 2016, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Animesh, N.; Robert, S.; Deb, K.B.; Zhao, Y.X.; Sung, C.K.; John, R.F.; Zhao, Y.M. Global identification of O-GlcNAc-modified proteins. Anal. Chem. 2006, 78, 452–458. [Google Scholar] [CrossRef]
- Cecioni, S.; Vocadlo, D.J. Tools for probing and perturbing O-GlcNAc in cells and in vivo. Curr. Opin. Chem. Biol. 2013, 17, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Z.; Shen, A.; Tao, T.; Wan, C.; Zhu, X.; Huang, J.; Zhang, W.; Xia, N.; Wang, S. The role of ptp1bo-glcnacylation in hepatic insulin resistance. Int. J. Mol. Sci. 2015, 16, 22856–22869. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Haque, M.M.; Nam, G.; Ryoo, N.; Rhim, H.; Yun, K.K. Monitoring of intracellular tau aggregation regulated by oga/ogt inhibitors. Int. J. Mol. Sci. 2015, 16, 20212–20224. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.M.; Dweck, J.F.; Mason, D.E.; Hart, C.R.; Buck, S.B.; Peters, E.C.; Agnew, B.J.; Hsieh-Wilson, L.C. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 2008, 130, 11576–11577. [Google Scholar] [CrossRef] [PubMed]
- Mizanur, R.M.; Jaipuri, F.A.; Pohl, N.L. One-step synthesis of labeled sugar nucleotides for protein O-GlcNAc modification studies by chemical function analysis of an archaeal protein. J. Am. Chem. Soc. 2005, 127, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.-M.; Yip, A.M.-H.; Zhang, K.Y.; Liu, H.-W.; Wu, P.L.; Li, K.F.; Cheah, K.W.; Lo, K.K.-W. Bioorthogonal labeling, bioimaging, and photocytotoxicity studies of phosphorescent Ruthenium(II) polypyridine dibenzocyclooctyne complexes. Chem. Eur. J. 2015, 21, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, J.; Song, J.; Wang, Z.; Huang, L. An efficient method for selectively imaging and quantifying in situ the expression of sialylated glycoproteins on living cells. Glycobiology 2013, 23, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling live cells by copper-catalyzed alkyne−azide click chemistry. Bioconjugate Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Liu, Y.; Yao, W.; Sun, Y.; Zhang, P.; Huang, L.; Wang, Z. Fluorescein-5-thiosemicarbazide (FTSC) labeling for fluorescent imaging of pectin-derived oligogalacturonic acid transported in living cells by confocal microscopy. Eur. Food. Res. Technol. 2014, 239, 867–875. [Google Scholar] [CrossRef]
- Sawa, M.; Hsu, T.L.; Itoh, T.; Sugiyama, M.; Hanson, S.R.; Vogt, P.K.; Wong, C.H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Gao, L.; Chen, X. Protein-specific imaging of O-GlcNAcylation in single cells. Chembiochem 2015, 16, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Ostapchuk, P.; Hearing, P.; Carrico, I. Chemoselective attachment of small molecule effector functionality to human adenoviruses facilitates gene delivery to cancer cells. J. Am. Chem. Soc. 2010, 132, 13615–13617. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J. Chemical arsenal for the study of O-GlcNAc. Molecules 2011, 16, 1987–2022. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Bielski, R. Click chemistry strategies and decoupling. In Click Chemistry in Glycoscience: New Development and Strategies; Witczak, Z.J., Bielski, R., Eds.; John Wiley & Sons: New York, NY, USA, 2013; Volume I, pp. 3–32. ISBN 9781118275337. [Google Scholar]
- Zhu, Y.; Wu, J.; Chen, X. Metabolic labeling and imaging of N-linked glycans in arabidopsis thaliana. Angew. Chem. 2016, 128, 9447–9451. [Google Scholar] [CrossRef]
- Teo, C.F.; Wells, L. Monitoring protein O-linked β-N-acetylglucosamine status via metabolic labeling and copper-free click chemistry. Anal. Biochem. 2014, 464, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Zaro, B.W.; Batt, A.R.; Chuh, K.N.; Navarro, M.X.; Pratt, M.R. The small molecule 2-azido-2-deoxy-glucose is a metabolic chemical reporter of O-GlcNAc modifications in mammalian cells, revealing an unexpected promiscuity of O-GlcNAc transferase. ACS Chem. Biol. 2017, 12, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y. Applications of Azide-Based Bioorthogonal Click Chemistry in Glycobiology. Molecules 2013, 18, 7145–7159. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; An, X.; Song, B.; Zhang, W.; Dai, Z.; Yuan, J. A novel dinuclear ruthenium(II)-copper(II) complex-based luminescent probe for hydrogen sulfide. Dalton Trans. 2014, 43, 13055–13060. [Google Scholar] [CrossRef] [PubMed]
- Zaro, B.W.; Yang, Y.Y.; Hang, H.C.; Pratt, M.R. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc. Natl. Acad. Sci. USA 2011, 108, 8146–8151. [Google Scholar] [CrossRef] [PubMed]
- Gurcel, C.; Vercoutter-Edouart, A.S.; Fonbonne, C.; Mortuaire, M.; Salvador, A.; Michalski, J.C.; Lemoine, J. Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal. Bioanal. Chem. 2008, 390, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, K.; Zheng, Y.; Zhang, M.; Kondengaden, S.M.; Li, S.; Huang, K.; Li, J.; Song, J.; Wang, P.G. Two-Step chemoenzymatic detection of N-acetylneuraminic acid-α(2-3)-galactose glycans. J. Am. Chem. Soc. 2016, 138, 11473–11476. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Luchansky, S.J.; Hang, H.C.; Yu, C.; Lee, S.C.; Bertozzi, C.R. Investigating cellular metabolism of synthetic azidosugars with the staudinger ligation. J. Am. Chem. Soc. 2002, 124, 14893–14902. [Google Scholar] [CrossRef] [PubMed]
- Elmes, R.B.; Orange, K.N.; Cloonan, S.M.; Williams, D.C.; Gunnlaugsson, T. Luminescent ruthenium(II) polypyridyl functionalized gold nanoparticles; their DNA binding abilities and application as cellular imaging agents. J. Am. Chem. Soc. 2011, 133, 15862–15865. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4 and 5 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Cui, Y.; Xiao, N.; Lu, J.; Fang, C.-J. Synthesis of a Novel Fluorescent Ruthenium Complex with an Appended Ac4GlcNAc Moiety by Click Reaction. Molecules 2018, 23, 1649. https://doi.org/10.3390/molecules23071649
Cheng Q, Cui Y, Xiao N, Lu J, Fang C-J. Synthesis of a Novel Fluorescent Ruthenium Complex with an Appended Ac4GlcNAc Moiety by Click Reaction. Molecules. 2018; 23(7):1649. https://doi.org/10.3390/molecules23071649
Chicago/Turabian StyleCheng, Qi, Yalu Cui, Nao Xiao, Jishun Lu, and Chen-Jie Fang. 2018. "Synthesis of a Novel Fluorescent Ruthenium Complex with an Appended Ac4GlcNAc Moiety by Click Reaction" Molecules 23, no. 7: 1649. https://doi.org/10.3390/molecules23071649
APA StyleCheng, Q., Cui, Y., Xiao, N., Lu, J., & Fang, C.-J. (2018). Synthesis of a Novel Fluorescent Ruthenium Complex with an Appended Ac4GlcNAc Moiety by Click Reaction. Molecules, 23(7), 1649. https://doi.org/10.3390/molecules23071649





