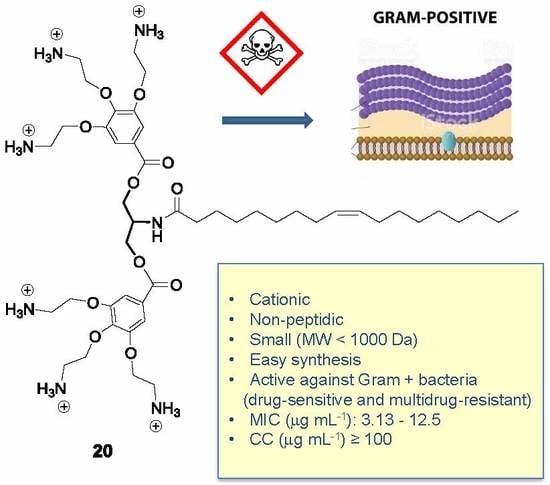
A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents
Abstract
1. Introduction
2. Results and Discussion
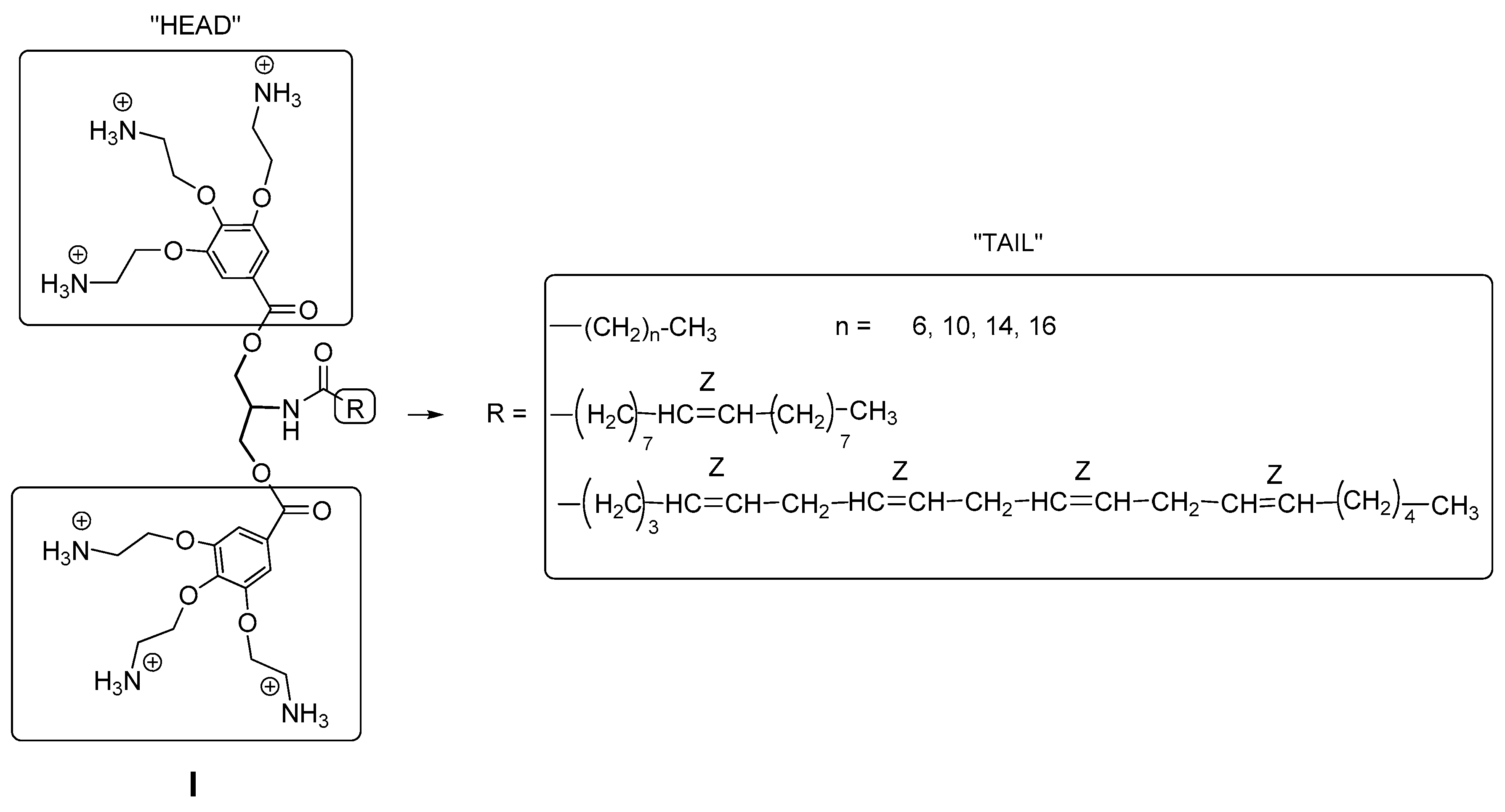
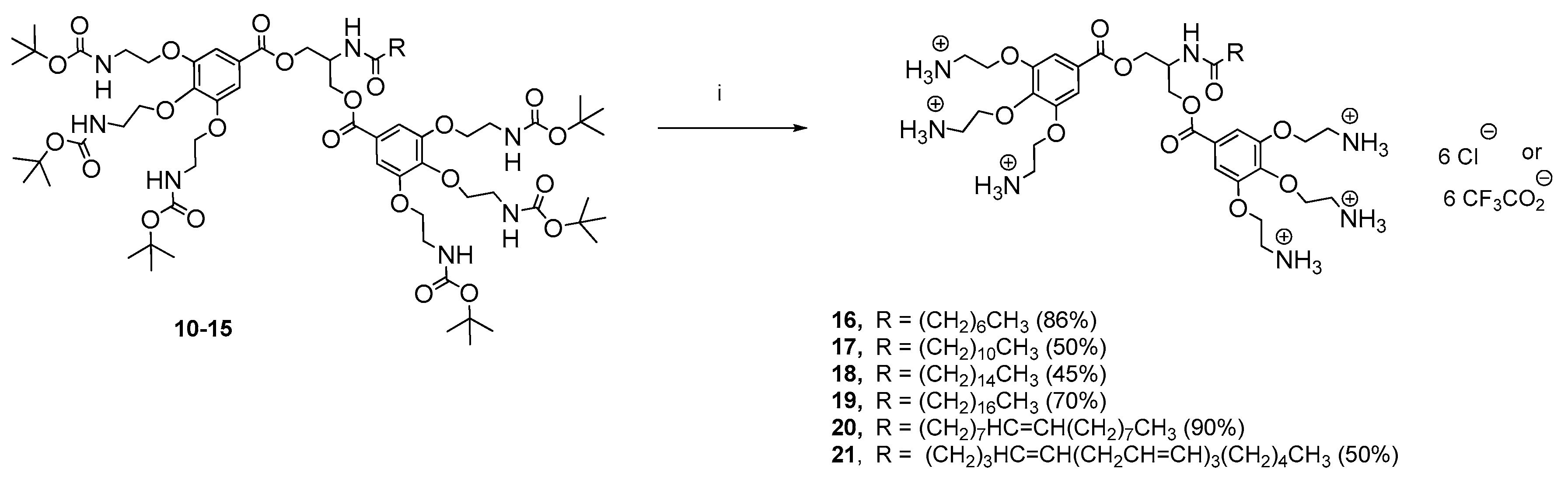
2.1. Chemical Results
2.2. Biological Results
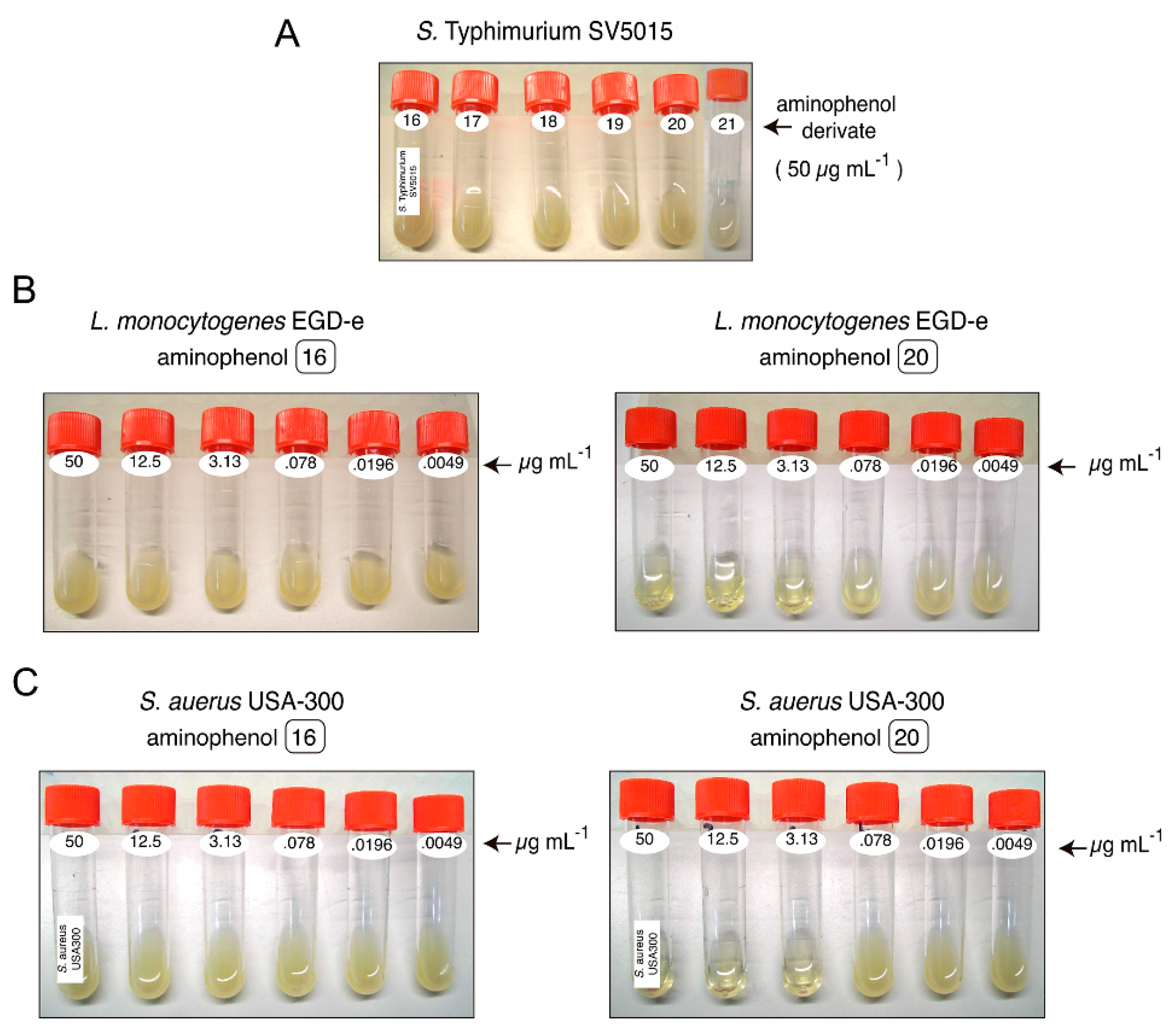
2.2.1. Antibacterial Activity
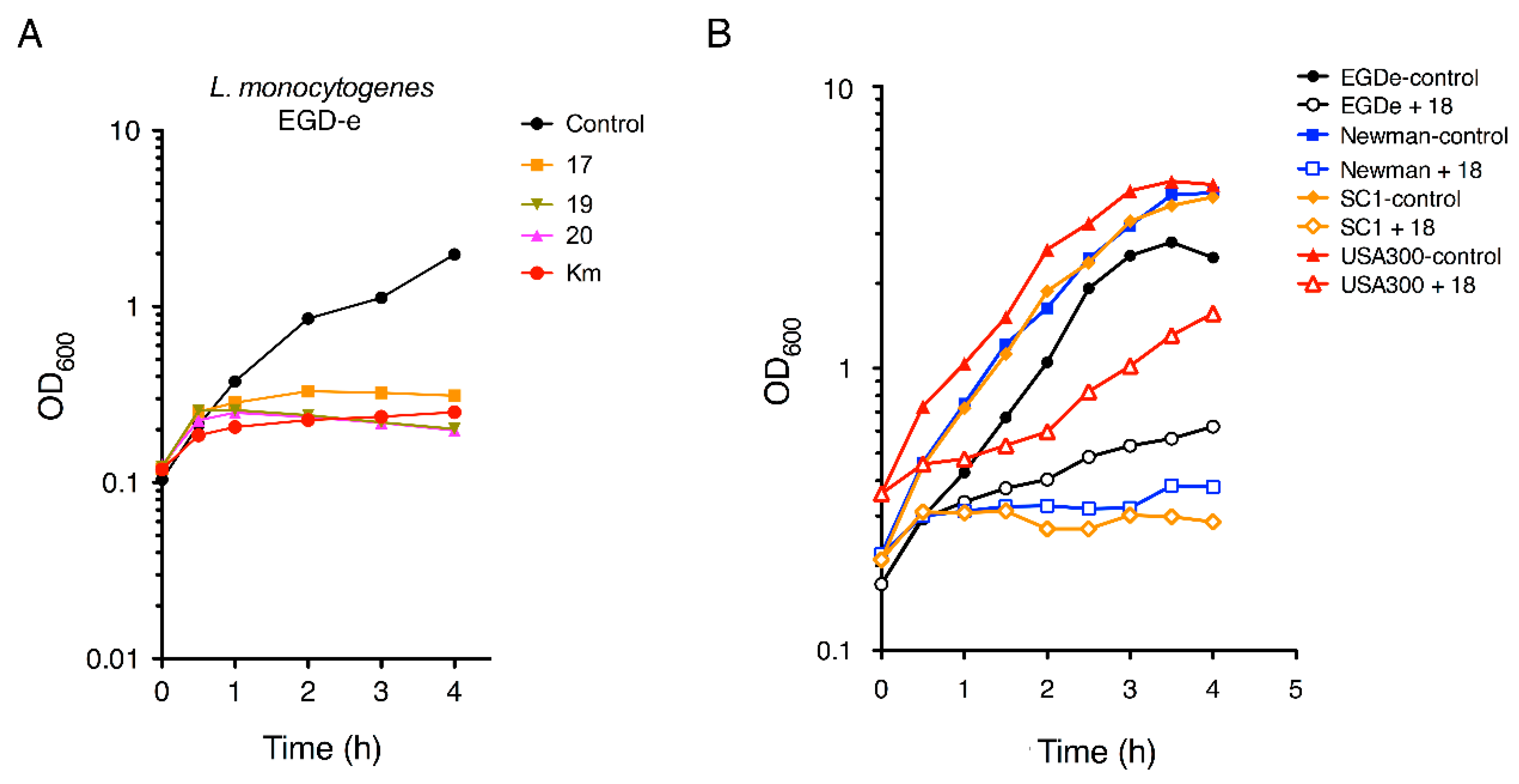
2.2.2. Antimicrobial Kinetics
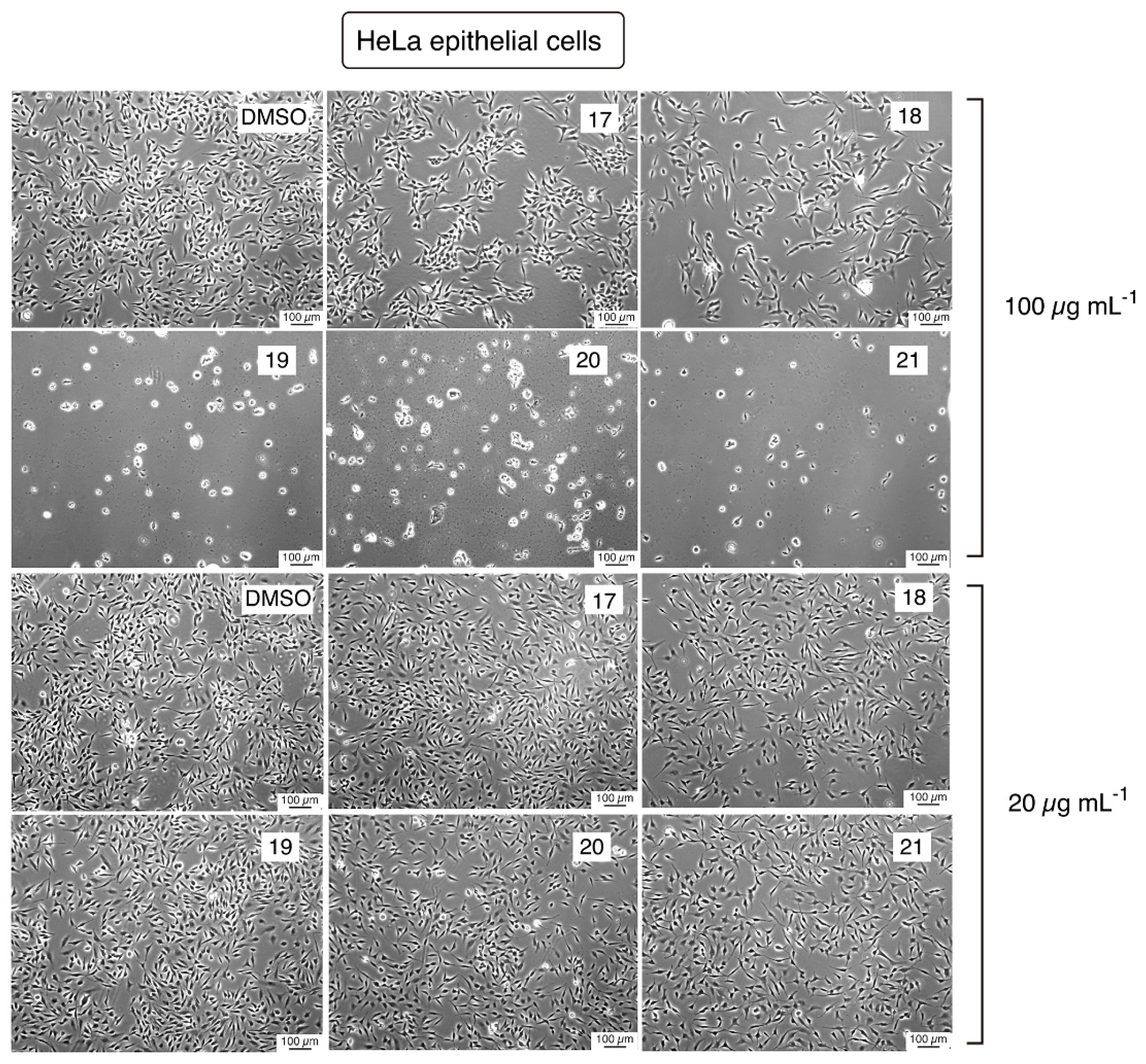
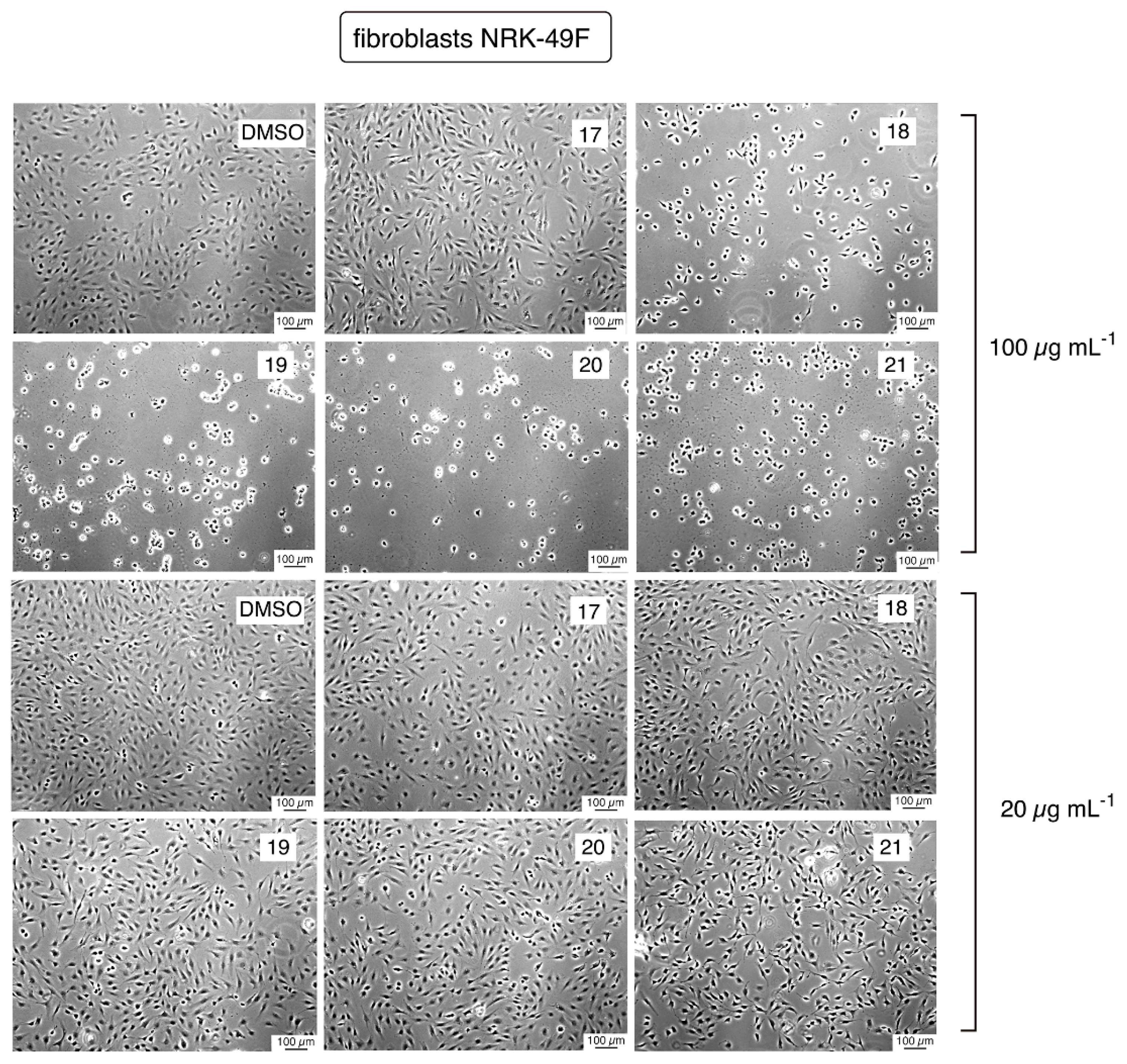
2.2.3. Cytotoxicity Evaluation
3. Conclusions
4. Materials and Methods
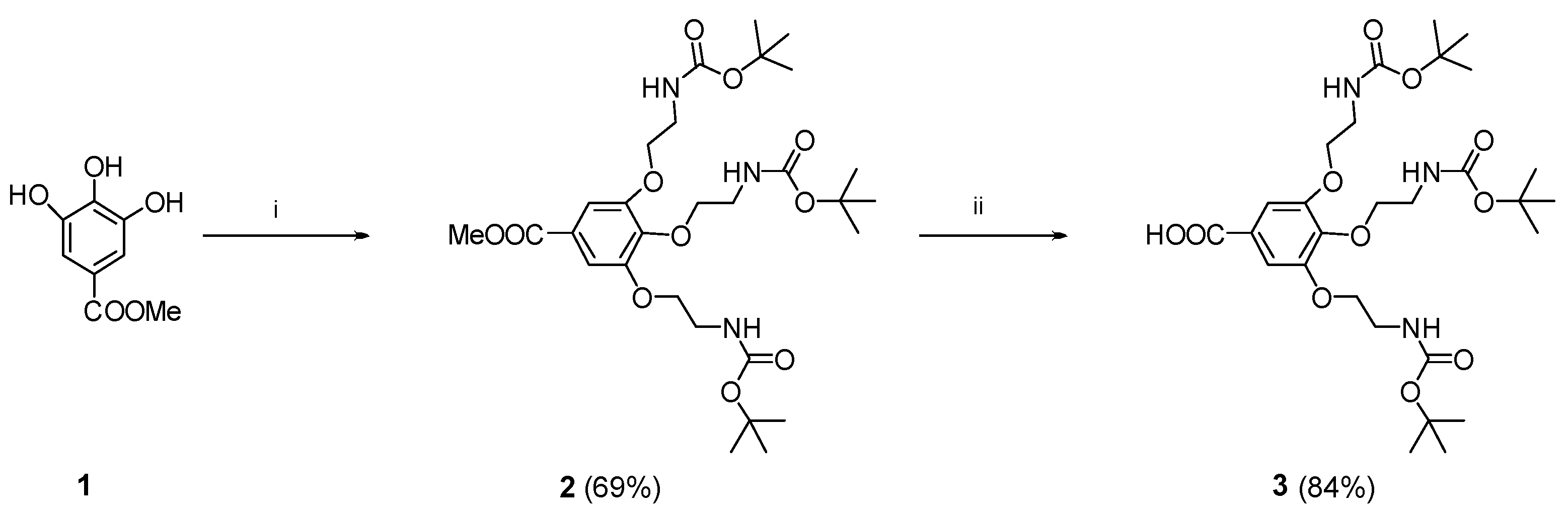
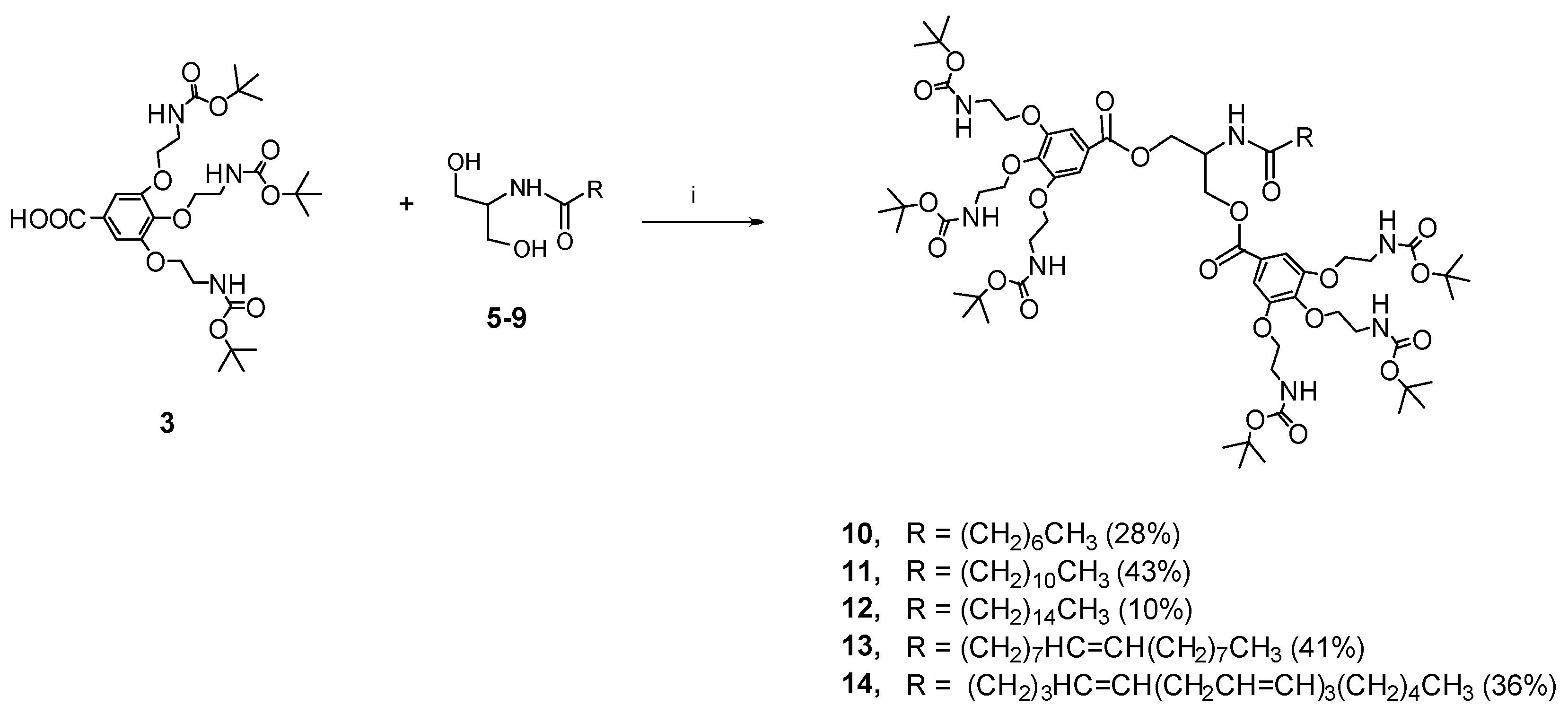
4.1. Synthesis
4.1.1. Methyl 3,4,5-tris[2-(Boc-amino)-1-ethoxy]benzoate (2)
4.1.2. 3,4,5-tris[2-(Boc-amino-1-ethoxy]benzoate (3)
4.1.3. General Procedure for the Synthesis of the N-acyl Serinol Derivatives 5–9
4.1.4. N-octanoyl Serinol (5)
4.1.5. N-dodecanoyl Odecanoyl Serinol (6)
4.1.6. N-hexadecanoyl Exadecanoyl Serinol (7)
4.1.7. N-oleoyl Serinol (8)
4.1.8. N-arachidonoyl Serinol (9)
4.1.9. General Coupling Procedure for the Synthesis of the Boc Protected Intermediates 10–14
4.1.10. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-octanoyl Serinol (10)
4.1.11. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-dodecanoyl Serinol (11)
4.1.12. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-hexadecanoyl Serinol (12)
4.1.13. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-oleoyl Serinol (13)
4.1.14. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-arachidonoyl Serinol (14)
4.1.15. Bis-O-[3,4,5-tris(2-N-Boc-amino-1-ethoxy)benzoyl]-N-octadecanoyl Serinol (15)
4.1.16. Synthesis of the Deprotected Serinol Derivatives 16–21
4.1.17. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-octanoyl Serinol Trifluoroacetate (16)
4.1.18. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-dodecanoyl Serinol Chloride (17)
4.1.19. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-hexadecanoyl Serinol Chloride (18)
4.1.20. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-octadecanoyl Serinol Trifluoroacetate (19)
4.1.21. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-oleoyl Serinol Trifluoroacetate (20)
4.1.22. Bis-O-[3,4,5-tris(2-ammonium-1-ethoxy)benzoyl]-N-octadecanoyl Serinol Trifluoroacetate (21)
4.2. Biological Methods
4.2.1. Bacterial Strains
4.2.2. Determination of Minimal Inhibitory Concentrations (MIC) of Aminophenol Compounds
4.2.3. Monitoring of Bacterial Growth in the Presence of Aminophenol Compounds
4.2.4. Toxicity Assays in Eukaryotic Cell Cultures
4.2.5. Phase-Contrast Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathew, A.; Cissell, R.; Liamthong, S. Antibiotic resistance in bacteria associated with food animals: A United States perspective of livestock production. Foodborne Pathog. Dis. 2007, 4, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.A. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Center For Disease Dynamics, Economics & Policy. State of the World’s Antibiotics; CDDEP: Washington, DC, USA, 2015. [Google Scholar]
- Antimicrobial Resistance: Global Report on Surveillance 2014. World Health Organization. Available online: www.who.int/drugresistance/documents/surveillancereport/en (accessed on 11 August 2017).
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Maloy, W.L.; Kari, U.P. Structure-activity on magainins and other host defense peptides. Biopolymers 1995, 37, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; López-Abarrategui, C.; Otero-González, A.J. Host defense peptides: An alternative as anti-infective and immunomodulatory therapeutics. Biopolymers 2012, 98, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by K-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochem. Biophys. Acta Biomembr. 1999, 1452, 55–70. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanism of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 2006, 8, 11–26. [Google Scholar] [PubMed]
- Easton, D.M.; Nijnik, A.; Mayer, M.L.; Hancock, R.E.W. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009, 27, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.E.; Davidson, D.J.; Hancock, R.E.W. A re-evaluation of the role of host defense peptides in mammalian immunity. Curr. Protein Pept. Sci. 2005, 6, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.T.; Cooper, M.A. Antibiotic in the clinical pipeline at the end of 2015. J. Antibiot. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Falla, T.J. Antimicrobial peptides: Therapeutic potential. Expert. Opin. Pharmacother. 2006, 7, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Madkour, A.E.; Tew, G.N. Antibacterial peptidomimetics: Polymeric synthetic mimics of antimicrobial peptides. Adv. Polym. Sci. 2013, 251, 141–172. [Google Scholar]
- Giuliani, A.; Pirri, G.; Bozzi, A.; Di Giulio, A.; Aschi, M.; Rinaldi, A.C. Antimicrobial peptides: Natural templates for synthetic membrane-active compounds. Cell. Mol. Life Sci. 2008, 65, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.H.; Haldar, J. Membrane-active small molecules: Designs inspired by antimicrobial peptides. ChemMedChem 2015, 10, 1606–1624. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Nimmagadda, A.; Su, M.; Hong, Y.; Shen, N.; Li, C.H.; Tsai, L.-Y.; Cao, J.; Li, Q.; Cai, J. Novel bis-cyclic guanidines as potent membrane-active antibacterial agents with therapeutic potential. Chem. Commun. 2017, 53, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Xia, D.; Teng, P.; Nimmagadda, A.; Zhang, C.H.; Odom, T.; Cao, A.; Hu, Y.; Cai, J. Membrane-active hydantoin derivatives as antibiotic agents. J. Med. Chem. 2017, 60, 8456–8465. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pachaiyappan, B.; Gruber, J.D.; Schmidt, M.G.; Zhang, Y.-M.; Woster, P.M. Antibacterial diamines targeting bacterial membranes. J. Med. Chem. 2016, 59, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Mensa, B.; Howella, G.L.; Scott, R.; DeGrado, W.F. Comparative Mechanistic Studies of Brilacidin, Daptomycin, and the Antimicrobial Peptide LL16. Antimicrob. Agents Chemother. 2014, 58, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.J.; Mulders, S.J.E.; Liskamp, R.M.J. Convergent synthesis and diversity of amino acid based dendrimers. Eur. J. Org. Chem. 2001, 10, 1903–1915. [Google Scholar] [CrossRef]
- Pindell, M.H. The pharmacology of kanamycin-a review and new developments. Ann. N. Y. Acad Sci. 1966, 132, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 Methicillin-Resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect Dis. 2015, 21, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Omardien, S.; Brul, S.; Zaat, S.A.J. Antimicrobial activity of cationic antimicrobial peptides against Gram-positives: Current progress made by understanding the mode of action and the response of bacteria. Front. Cell Dev. Biol. 2016, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Merg, A.D.; Slocik, J.; Blaber, M.G.; Schatz, G.C.; Naik, R.; Rosi, N.L. Adjusting the Metrics of 1-D Helical Gold Nanoparticle Superstructures Using Multivalent Peptide Conjugates. Langmuir 2015, 31, 9492–9501. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Cedrón, M.; Vargas, T.; Madrona, A.; Jiménez, A.; Pérez-Pérez, M.J.; Quintela, J.C.; Reglero, G.; San-Félix, A.; Ramírez de Molina, A. Novel polyphenols that inhibit colon cancer cell growth affecting cancer cell metabolism. J. Pharmacol. Exp. Ther. 2018, in press. [Google Scholar]
- Vivero, A.; Banos, R.C.; Mariscotti, J.F.; Oliveros, J.C.; Garcia-del Portillo, F.; Juarez, A.; Madrid, C. Modulation of horizontally acquired genes by the Hha-YdgT proteins in Salmonella enterica serovar Typhimurium. J. Bacteriol. 2008, 190, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative genomics of Listeria species. Science 2001, 294, 849–852. [Google Scholar] [PubMed]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal coagulase; mode of action and antigenicity. J Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Marques, E.; Zambrano, S.; Tierrez, A.; Bianchi, M.E.; Agresti, A.; Garcia-Del Portillo, F. Single-cell analyses reveal an attenuated NF-kappaB response in the Salmonella-infected fibroblast. Virulence 2017, 8, 719–740. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Montero, N.; Ramos-Marques, E.; Risco, C.; Garcia-Del Portillo, F. Intracellular Salmonella induces aggrephagy of host endomembranes in persistent infections. Autophagy 2016, 12, 1886–1901. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| MIC a (µg mL−1) | CC b (µg mL−1) | |||||
|---|---|---|---|---|---|---|
| Drug Sensitive Strains | Multidrug Resistant Strains | |||||
| Compound | S. Typhimurium SV5015 | L. monocytogenes EGD-e | S. aureus Newman | S. aureus SC-1 | S. aureus USA-300 | |
| 16 | >50 (*) | >50 | >50 | >50 | >50 | ≥100 |
| 17 | >50 | 12.5 | 50 | 50 | 12.5 | >100 (**) |
| 18 | >50 | 3.13 | 12.5 | 12.5 | 3.13 | ≥100 |
| 19 | >50 | 3.13 | 12.5 | 12.5 | 50 | ≥100 |
| 20 | >50 | 3.13 | 12.5 | 12.5 | 12.5 | ≥100 |
| 21 | >50 | 12.5 | 50 | 50 | 12.5 | ≥100 |
| Kanamycin | n/d | 2.34 | 9.4 | >150 | >150 | n/d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, A.; García, P.; De la Puente, S.; Madrona, A.; Camarasa, M.J.; Pérez-Pérez, M.-J.; Quintela, J.-C.; García-del Portillo, F.; San-Félix, A. A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents. Molecules 2018, 23, 1513. https://doi.org/10.3390/molecules23071513
Jiménez A, García P, De la Puente S, Madrona A, Camarasa MJ, Pérez-Pérez M-J, Quintela J-C, García-del Portillo F, San-Félix A. A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents. Molecules. 2018; 23(7):1513. https://doi.org/10.3390/molecules23071513
Chicago/Turabian StyleJiménez, Aranza, Pablo García, Sofia De la Puente, Andrés Madrona, María José Camarasa, María-Jesús Pérez-Pérez, José-Carlos Quintela, Francisco García-del Portillo, and Ana San-Félix. 2018. "A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents" Molecules 23, no. 7: 1513. https://doi.org/10.3390/molecules23071513
APA StyleJiménez, A., García, P., De la Puente, S., Madrona, A., Camarasa, M. J., Pérez-Pérez, M.-J., Quintela, J.-C., García-del Portillo, F., & San-Félix, A. (2018). A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents. Molecules, 23(7), 1513. https://doi.org/10.3390/molecules23071513