Structure-Dependent Activity of Natural GABA(A) Receptor Modulators
Abstract
1. Introduction
2. Method
3. Natural GABA(A) Receptor Modulators
3.1. Alkaloids
3.2. Alkanes
3.3. Phenols
3.3.1. Flavones
- Hydroxylation in position 5 and 7 or in position 6 leads to increased receptor affinity.
- Methoxylation in position 6 or 8 also raises affinity of the compounds, even more when occurring in both positions.
- The affinity furthermore increases by hydroxylation in position 2’, while methoxylation in the same position leads to a loss of affinity.
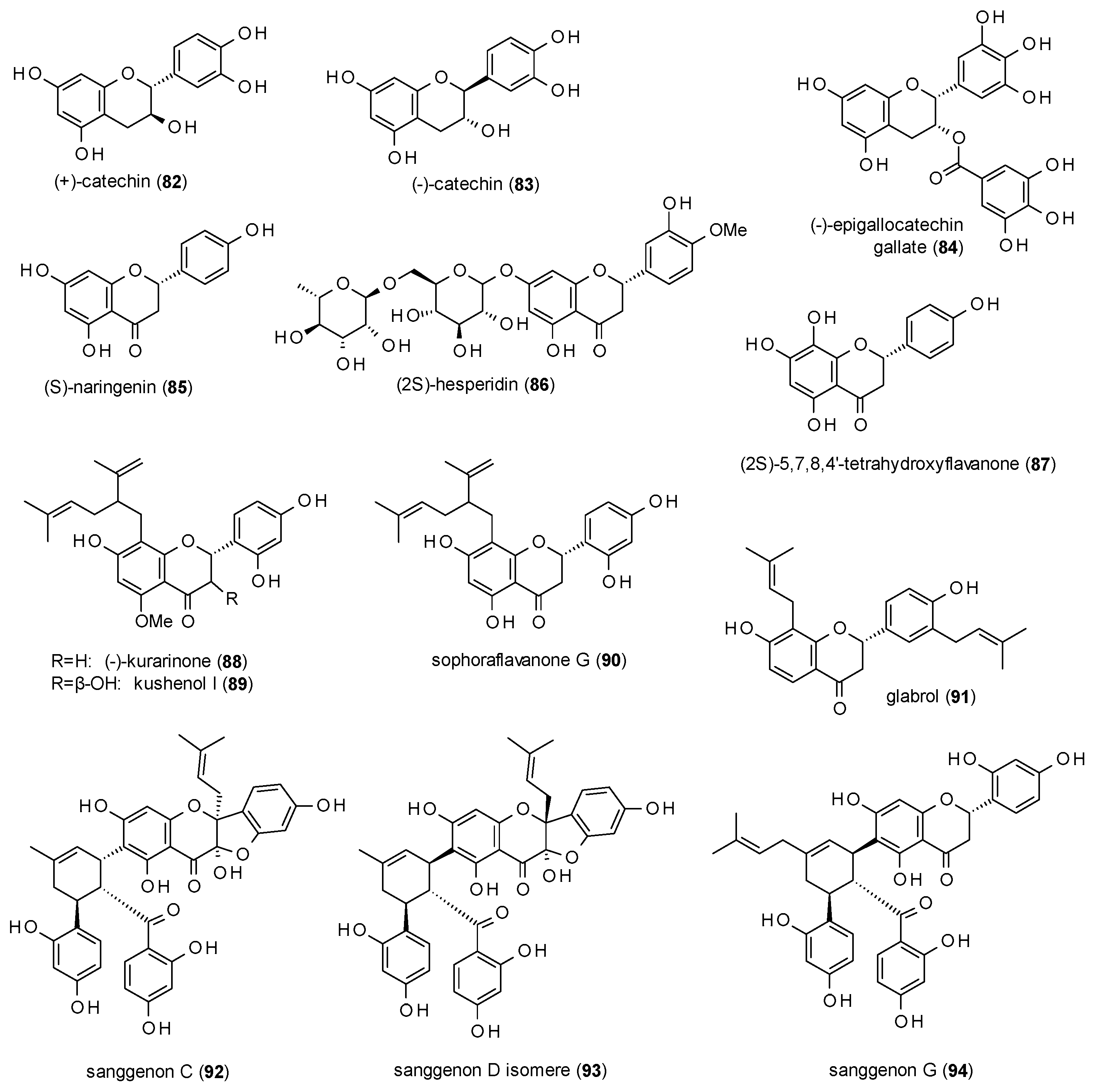
3.3.2. Flavanes
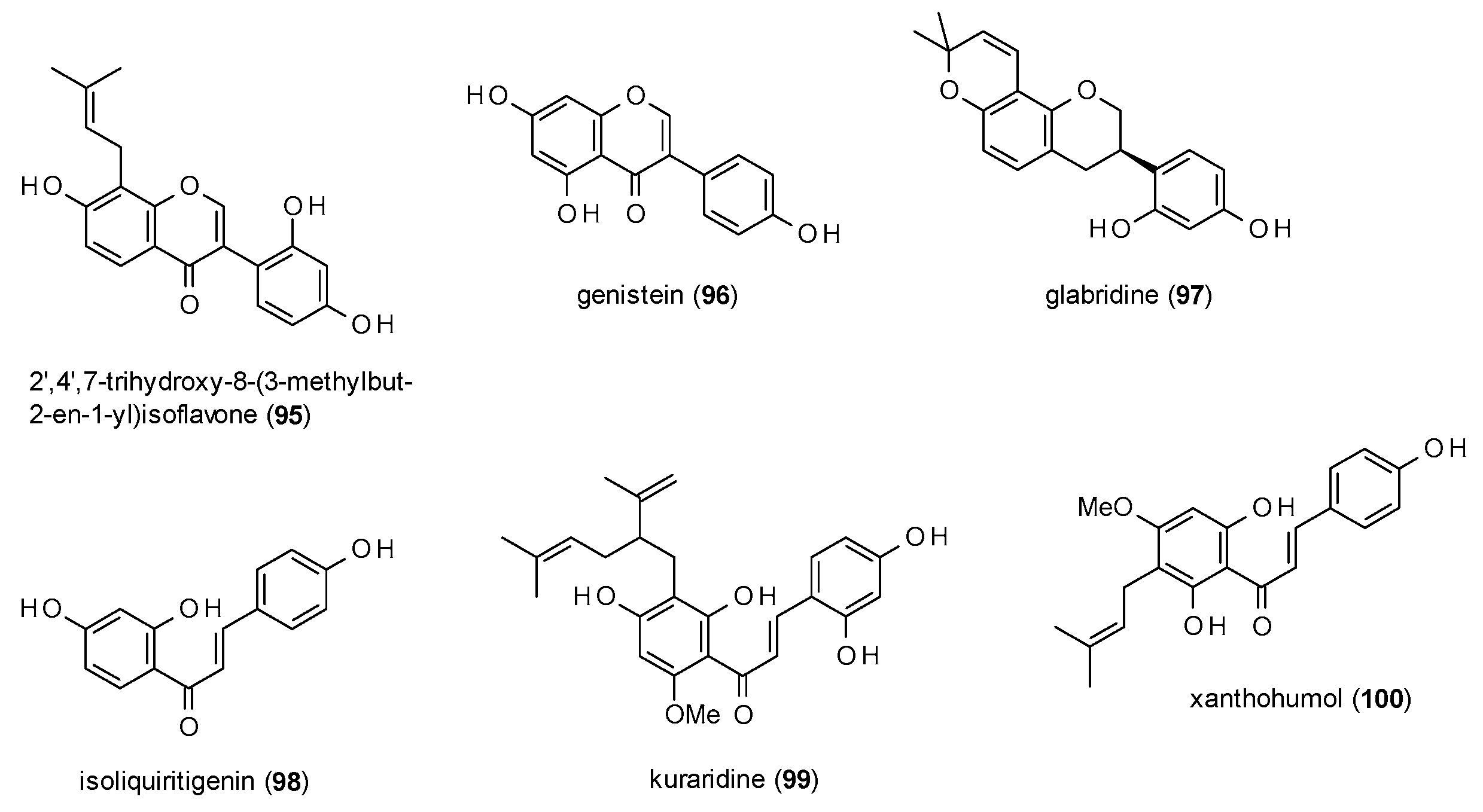
3.3.3. Isoflavonoids and Chalcones
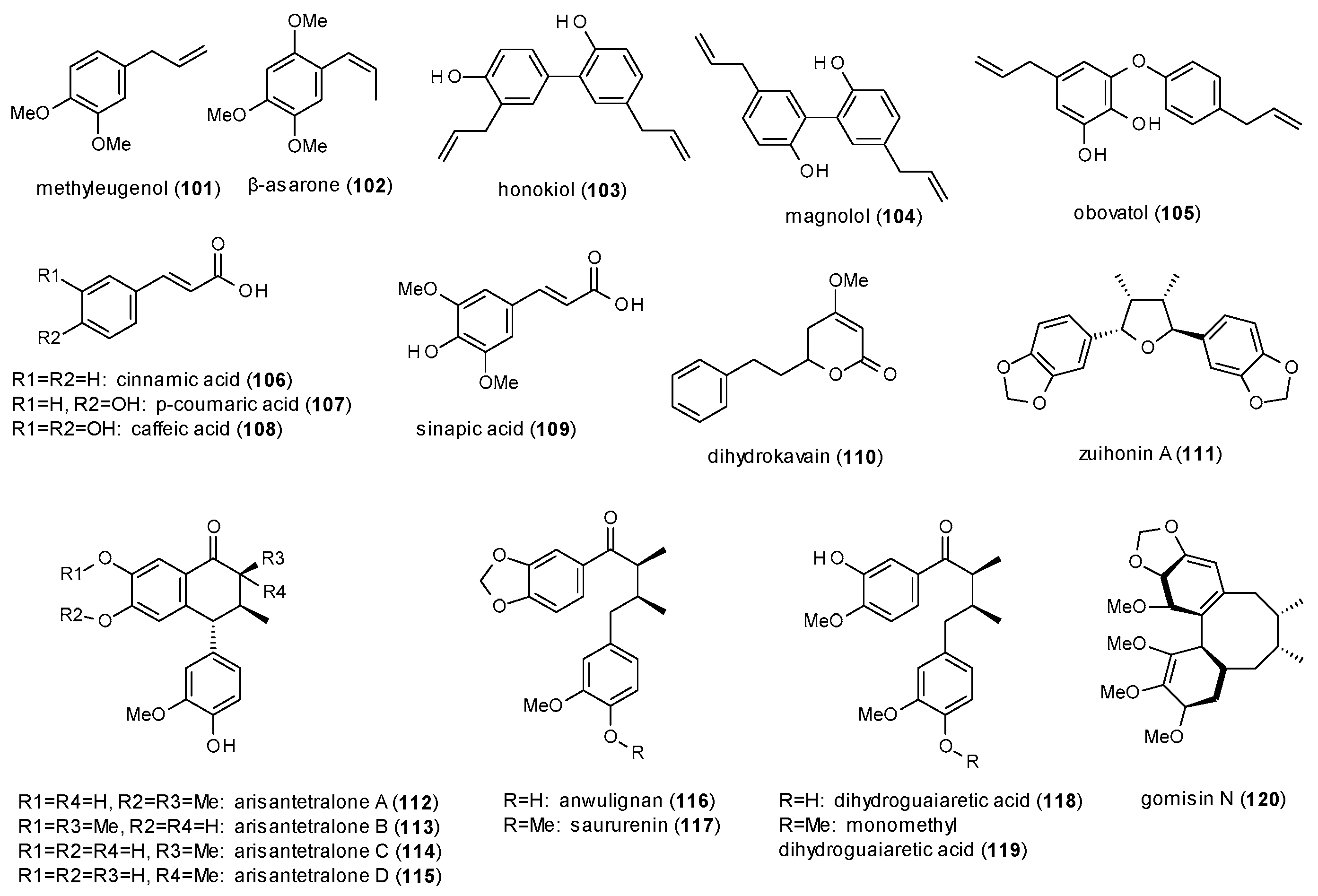
3.3.4. Phenylpropanes, Kavalactones and Lignans
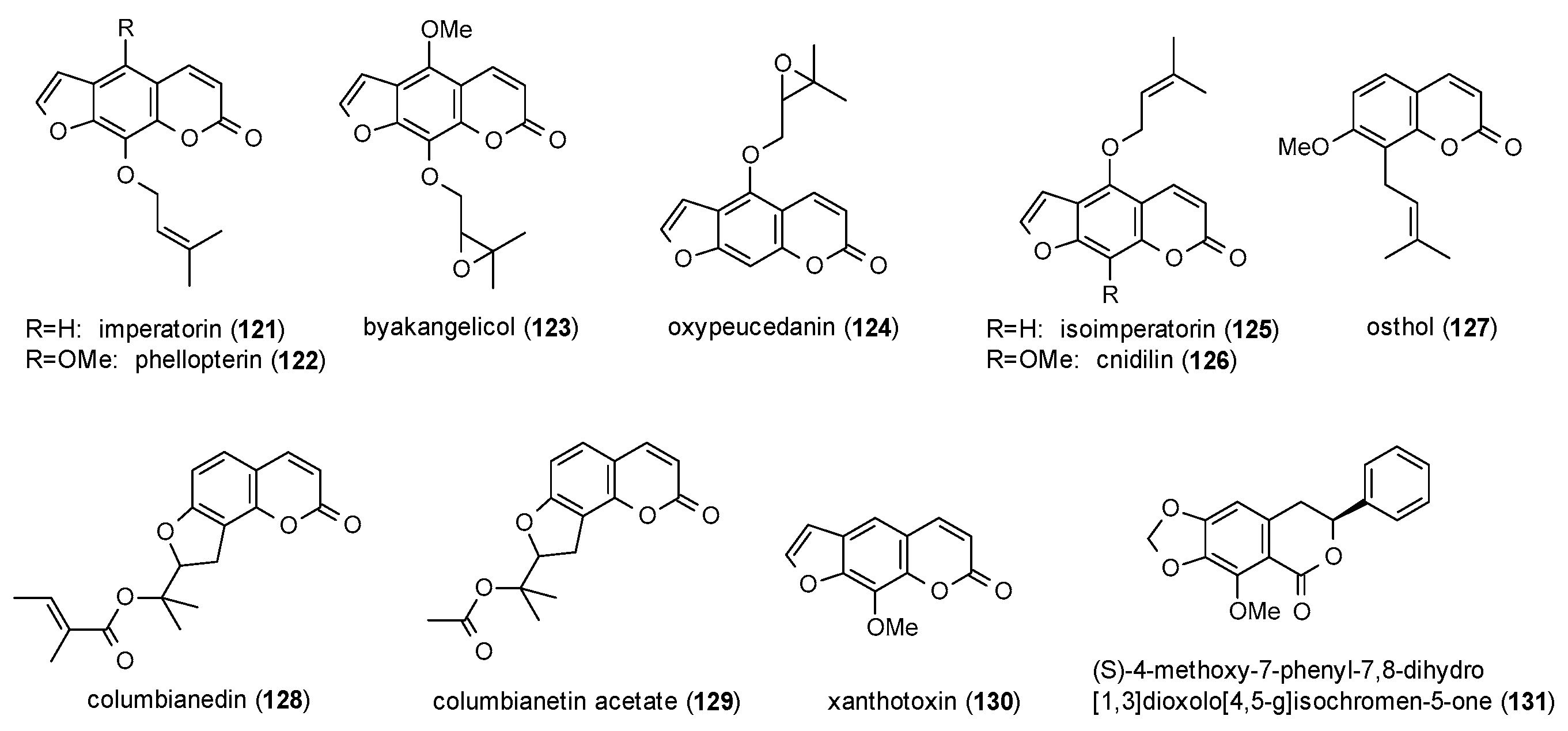
3.3.5. Coumarins
3.3.6. Diarylheptanoids, Stilbenes and Phenanthrenes
3.3.7. Simple Phenols and Polyphenols
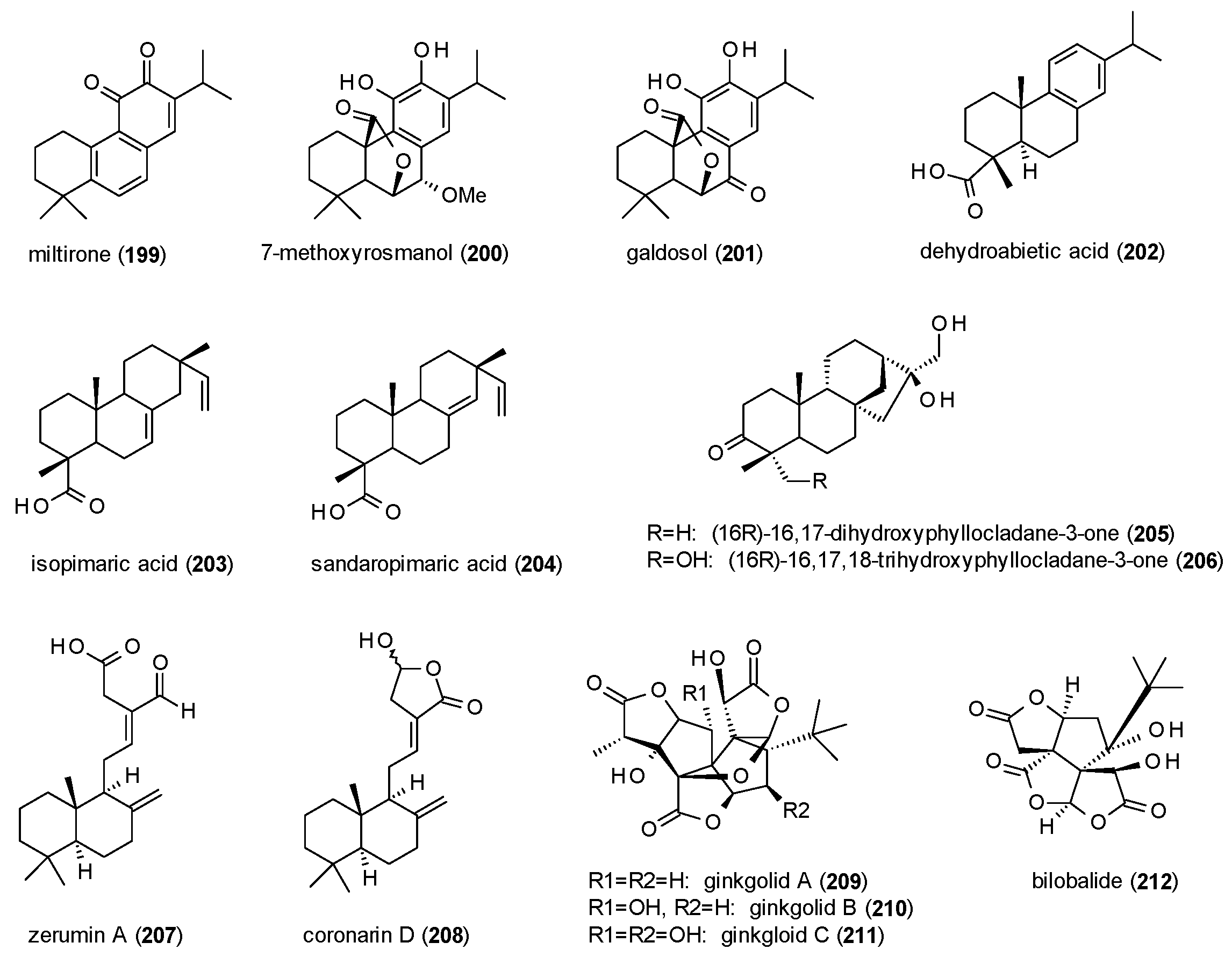
3.4. Terpenes
3.4.1. Monoterpenes
3.4.2. Sesquiterpenes
3.4.3. Diterpenes
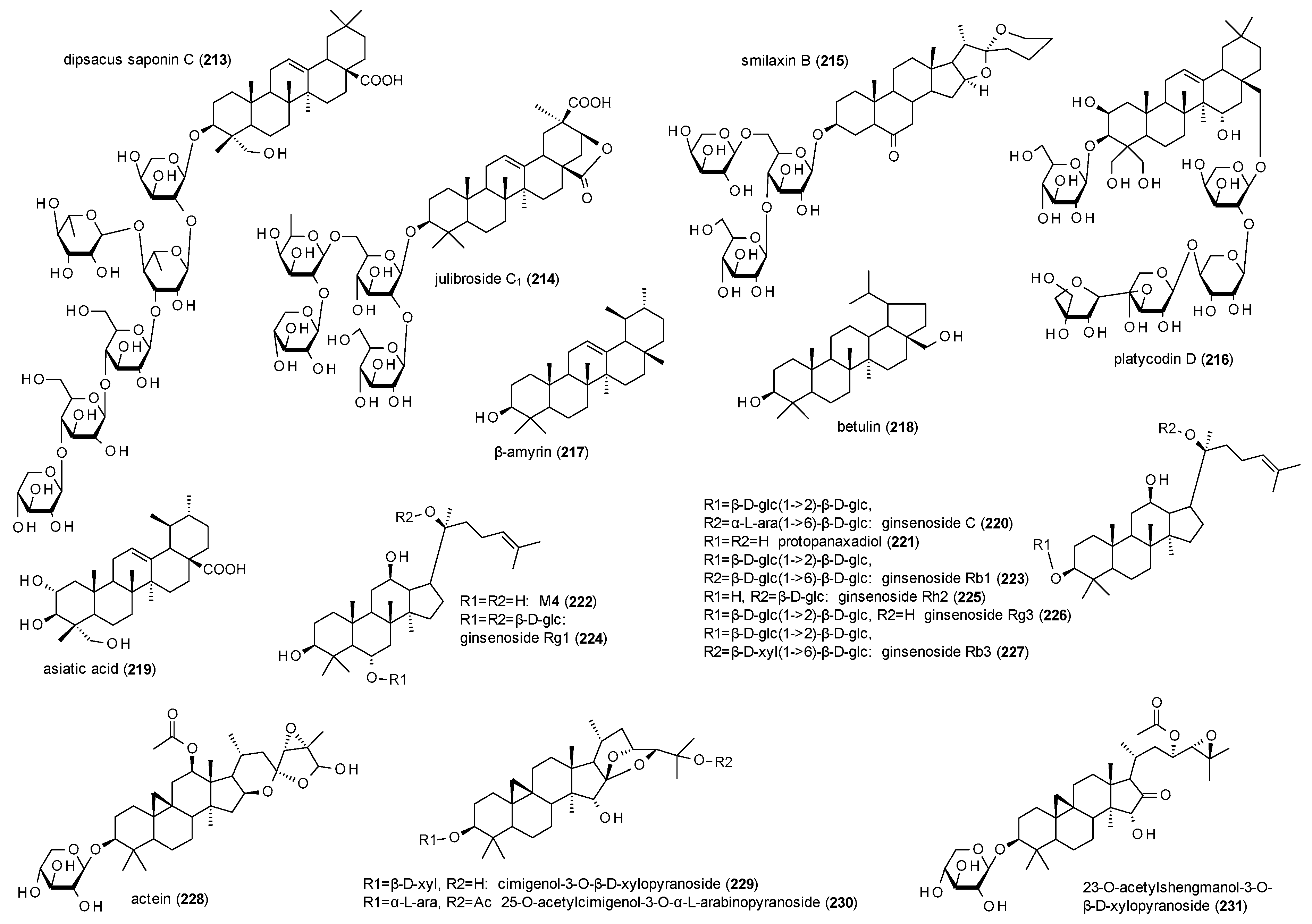
3.4.4. Triterpenes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABAA receptor trafficking and its role int the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.R. GABAA receptor channel pharmacology. Curr. Pharm. Des. 2005, 11, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Foerstermann, U.; Hofmann, F.B.; Starke, K. Allgemeine und Spezielle Pharmakologie und Toxikologie, 9th ed.; Elsevier GmbH: Munich, Germany, 2005; pp. 136–137. [Google Scholar]
- Johnston, G. GABAA receptor pharmacology. Pharmacol. Ther. 1996, 69, 173–198. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Moehler, H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006, 326, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Sterner, O. Modulation of GABAA receptors by natural products and the development of novel synthetic ligands for the benzodiazepine binding site. Curr. Drug Targets 2011, 12, 1674–1688. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M. Animal models for screening anxiolytic-like drugs: A perspective. Dialogues Clin. Neurosci. 2015, 17, 295–303. [Google Scholar] [PubMed]
- Fedotova, J.; Kubatka, P.; Büsselberg, D.; Shleikin, A.G.; Caprnda, M.; Dragasek, J.; Rodrigo, L.; Pohanka, M.; Gasparova, I.; Nosa, V.; et al. Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed. Pharmacother. 2017, 95, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Bouwknecht, J.A.; van der Gugten, J.; Groenink, L.; Olivier, B.; Paylor, R.E. Behavioural and physiological mouse models for anxiety: Effects of flesinoxan in 129S6/SvEvTac and C57BL/6J mice. Eur. J. Pharmacol. 2004, 494, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behavior in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Wilcox, K.S.; White, H.S. Discovery of antiepileptic drugs. Neurotherapeutics 2007, 4, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.J.; Pike, B.R.; O’Dell, D.M.; Lyeth, B.G.; Jenkins, L.W. The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 1994, 11, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Facklam, M.; Schoch, P.; Bonetti, E.P.; Jenck, F.; Martin, J.R.; Moreau, J.L. Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J. Pharmacol. Exp. Ther. 1992, 261, 1113–1121. [Google Scholar] [PubMed]
- Zhou, W.; Bao, Y.; Uhang, X.; Zeng, L.; Wang, L.; Wang, J.; Jiang, W. Optimal interval for hot water immersion tail-flick test in rats. Acta Neuropsychiatr. 2014, 26, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ramabadran, K.; Bansinath, M.; Turndorf, H.; Puig, M.M. Tail immersion test for the evaluation of a nociceptive reaction in mice. Methodological considerations. J. Pharmacol. Methods 1989, 21, 21–31. [Google Scholar] [CrossRef]
- Rudolph, U.; Möhler, H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006, 6, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; DeLorey, T.M. GABA and glycine. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uler, M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999; pp. 335–346. [Google Scholar]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 28 May 2018).
- Index Fungorum. Available online: http://www.indexfungorum.org/ (accessed on 28 May 2018).
- Alali, F.Q.; El-Elimat, T.; Li, C.; Qandil, A.; Alkofahi, A.; Tawaha, K.; Burgess, J.P.; Nakanishi, Y.; Kroll, D.J.; Navarro, H.A.; et al. New colchicinoids from a native Jordanian meadow saffron, Colchicum brachiphyllum: Isolation of the first naturally occurring dextrorotary colchicinoid. J. Nat. Prod. 2005, 68, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Bueno, O.F.; Leidenheimer, N.J. Colchicine inhibits GABAA receptors independently of microtubule depolymerization. Neuropharmacology 1998, 37, 383–390. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Savtschenko, A.; Merten, A.; Rusch, C.; Appel, K.; Kuchta, K. GABAA receptors binding assay of standardized Leonurus cardiaca and Leonurus japonicus extracts as well as their isolated constituents. Planta Med. 2015, 81, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Baburin, I.; Strommer, B.; Kim, H.J.; Hering, S.; Hamburger, M. HPLC-based activity profiling: Discovery of piperine as a positive GABAA receptor modulator targeting a benzodiazepine-independent binding site. J. Nat. Prod. 2010, 72, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Halbsguth, C.; Meißner, O.; Häberlein, H. Positive cooperation of protoberberin type 2 alkaloids from Corydalis cava on the GABAA binding site. Planta Med. 2003, 69, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Chung Leung, W.; Zheng, H.; Heun, M.; Lun Law, S.; Xue, H. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Progr. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 775–779. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Rejon-Orantes, J.C.; Gonzalez-Esquinca, A.R.; de la Mora, M.P. Annomontine, an alkaloid isolated from Annona purpurea, has anxiolytic-like effects in the elevated plus-maze. Planta Med. 2011, 77, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Schupp, P.; Proksch, P.; Wray, V.; Steube, K.; Müller, C.E.; Frobenius, W.; Herderich, M.; Soest, R.W.M. Bioactive pyridoacridine alkaloids from the Micronesian sponge Oceanapia sp. J. Nat. Prod. 1998, 61, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wang, Y.; Li, Y.; Chen, X.Q.; Yang, H.H.; Yue, J.M.; Hu, G.Y. Songorine, a diterpenoid alkaloid of the genus Aconitum, is a novel GABA receptor antagonist in rat brain. Neurosci. Lett. 2003, 337, 33–36. [Google Scholar] [CrossRef]
- Nesterova, Y.V.; Povet’eva, T.N.; Shults, E.E.; Ziuz’kov, G.N.; Aksinenko, S.G.; Afanas’eva, O.G.; Krapivin, A.V.; Kharina, T.G. Anxiolytic activity of diterpene alkaloid songorine. Pharmacol. Toxicol. 2015, 159, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.R.R.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. GABAA receptor modulation by terpenoids from Sideritis extract. Mol. Nutr. Food. Res. 2014, 58, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; Zaitone, S.A.; Gomaa, M.; ElSohly, M.A.; Hassanean, H.A.; Safwat, A.A. Mechanism of action of antiepileptic ceramide from Red Sea soft coral Sarcophyton aurtium. Bioorg. Med. Chem. Lett. 2015, 25, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Pollastro, F.; Verotta, L.; Ballero, M.; Romano, A.; Wyrembek, P.; Szczuraszek, K.; Mozrzymas, J.W.; Taglialatela-Scafati, O. Polyacetylenes from Sardinian Oenanthe fistulosa: A molecular clue to risus sardonicus. J. Nat. Prod. 2009, 72, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Wyrembek, P.; Lebida, K.; Mercik, K.; Szczuraszek, K.; Szczot, M.; Pollastro, F.; Appendino, G.; Mozrzymas, J.W. Block and allosteric modulation of GABAergic currents by oenanthotoxin in rat cultured hippocampal neurons. Br. J. Pharmacol. 2010, 160, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Uwai, K.; Ohashi, K.; Takaya, Y.; Ohta, T.; Tadano, T.; Kisara, K.; Shibusawa, K.; Sakakibara, R.; Oshima, Y. Exploring the structural basis of neurotoxicity in C17-polyacetylenes isolated from water hemlock. J. Med. Chem. 2000, 43, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Wyrembek, P.; Negri, R.; Kaczor, P.; Czyzewska, M.; Appendino, G.; Mozrzymas, J.W. Falcarindiol allosterically modulates GABAergic currents in cultured rat hippocampal neurons. J. Nat. Prod. 2012, 75, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Czyzewska, M.M.; Chrobok, L.; Kania, A.; Jatczak, M.; Pollastero, F.; Appendino, G.; Mozrzymas, J.W. Dietyry acetylenic oxylipin falcarindol differentially modulates GABAA receptors. J. Nat. Prod. 2014, 77, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Baur, R.; Simmen, U.; Senn, M.; Séquin, U.; Sigel, E. Novel plant substances acting as β subunit isoform-selective positive alloseric modulators of GABAA receptors. Mol. Pharmacol. 2005, 68, 787–792. [Google Scholar] [PubMed]
- Wang, H.; Hui, K.M.; Chen, Y.; Xu, S.; Wong, J.T.F.; Xue, H. Structure-activity relationships of flavonoids, isolated from Scutellaria baicalensis, binding to benzodiazepine site of GABAA receptor complex. Planta Med. 2002, 68, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.Y.; Xue, H. Development of effective therapeutics targeting the GABAA receptor: Naturally occurring alternatives. Curr. Pharm. Des. 2004, 10, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Hung, W.Y.; Chen, C.F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 2003, 464, 141–146. [Google Scholar] [CrossRef]
- De Carvalho, R.S.M.; Duarte, F.S.; de Lima, T.C.M. Involvement of GABAergic non-benzodiazepine sites in the axiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Yi, P.L.; Cheng, C.H.; Lu, C.Y.; Hsiao, Y.T.; Tsai, Y.F.; Li, C.L.; Chang, F.C. Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep-wake regulation. J. Ethnopharmacol. 2011, 135, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Lee, J.J.; Cho, Y.W.; Cheong, J.H.; Ko, K.H.; et al. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol. Learn. Mem. 2007, 87, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Huen, M.S.Y.; Leung, J.W.C.; Ng, W.; Lui, W.S.; Chan, M.N.S.; Wong, J.T.F.; Xue, H. 5,7-dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonist properties. Biochem. Pharmacol. 2003, 66, 125–132. [Google Scholar] [CrossRef]
- Hui, K.M.; Huen, M.S.Y.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.R.F.; Xue, H. Anxiolytic effect of wogonin, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef]
- Park, H.G.; Yoon, S.Y.; Choi, J.Y.; Lee, G.S.; Choi, J.H.; Shin, C.Y.; Son, K.H.; Lee, Y.S.; Kim, W.K.; Ryu, J.H.; et al. Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur. J. Pharmacol. 2007, 574, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.H.; Paladini, A.C.; Wolfman, C.; De Stein, M.L.; Calvo, D.; Diaz, L.E.; Pena, C. Chryin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990, 40, 2227–2232. [Google Scholar] [CrossRef]
- Wolfman, C.; Viola, H.; Paladini, A.; Dajas, F.; Medina, J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol. Biochem. Behav. 1994, 47, 1–4. [Google Scholar] [CrossRef]
- Zhai, K.; Hu, L.; Chen, J.; Fu, C.Y.; Chen, Q. Chrysin induces hyperagesia via the GABAA receptor. Planta Med. 2008, 74, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, J.B.; Ardenghi, P.; Dias, M.; Ferreira, M.B.C.; Izquierdo, I.; Medina, J.H. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effects on memory tasks in rats. Pharmacol. Biochem. Behav. 1997, 58, 887–891. [Google Scholar] [CrossRef]
- Goutman, J.D.; Waxemberg, M.D.; Donate-Oliver, F.; Pomata, P.E.; Calvo, D.J. Flavonoid modulation of ionic currents mediated by GABAA and GABAC receptors. Eur. J. Pharmacol. 2003, 461, 79–87. [Google Scholar] [CrossRef]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschmitz, M.; Schreier, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef]
- Campbell, E.L.; Chebib, M.; Johnston, G.A.R. The dietary flavonoids apigenin and (-)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochem. Pharmacol. 2004, 68, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Kavvadias, D.; Sand, P.; Youdim, K.A.; Zeeshan Qaiser, M.; Rice-Evans, C.; Baur, R.; Sigel, E.; Rausch, W.D.; Riederer, P.; Schreier, P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br. J. Pharmacol. 2004, 142, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.; Wasowski, C.; Levi de Stein, M.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.H.; Paladini, A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, A.B.; Madsen, K.D.; Liljefors, T.; Stafford, G.I.; van Staden, J.; Jäger, A.K. Biflavones from Rhus species with affinity for the GABAA/benzodiazepine receptor. J. Ethnopharmacol. 2006, 103, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Krydsfeldt, K.; Rasmussen, H.B. Bioassay-guided isolation of apigenin with GABA-benzodiazepine activity from Tanacetum parthenium. Phytother. Res. 2009, 23, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Gazola, A.C.; Costa, G.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; de Lima, T.C.M.; Schenkel, E.P. Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Rev. Bras. Farmacogn. 2015, 25, 158–163. [Google Scholar] [CrossRef]
- Shen, M.L.; Wang, C.H.; Chen, R.Y.T.; Zhou, N.; Kao, S.T.; Wu, D.C. Luteolin inhibits GABAA receptors in HED cells and brain slices. Sci. Rep. 2016, 6, 27695. [Google Scholar] [CrossRef] [PubMed]
- Wasowski, C.; Marder, M.; Viola, H.; Medina, J.H.; Paladini, A.C. Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABAA receptors, from Valeriana walichii. Planta Med. 2002, 68, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Marder, M.; Viola, H.; Wasowski, C.; Fernandez, S.; Medina, J.H.; Paladini, A.C. 6-methylapigenin and herperidin: New valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 2003, 75, 537–545. [Google Scholar] [CrossRef]
- Kavvadias, D.; Monschein, V.; Sand, P.; Riederer, P.; Schreier, P. Constituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptor. Planta Med. 2003, 69, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.M.; Jäger, A.K. Two flavonoids from Artemisia herba-alba Asso with in vitro GABAA-benzodiazepine receptor activity. J. Ethnopharmacol. 2005, 99, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.; Wasowski, C.; Marder, M.; Wolfman, C.; Pladini, A.C.; Medina, J.H. Sedative and hypnotic properties of Salvia guaranitica St Hil, and of its active principle, Cirsiliol. Phytomedicine 1997, 4, 47–52. [Google Scholar] [CrossRef]
- Shen, X.L.; Nielsen, M.; Witt, M.R. Inhibition of [methyl-3H]diazepam binding to rat brain membranes in vitro by dinatin and skrofulein. Acta Pharmacol. Sin. 1994, 15, 385–388. [Google Scholar]
- Grundmann, O.; Nakajima, J.I.; Kamata, K.; Seo, S.; Butterweck, V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine 2009, 16, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Shariati-Rad, S.; Zamansoltani, F. Anticonvulsive effects of intracerebroventricular administration of rutin in rats. Progr. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Nassiri-Asl, M.; Shafeei, M.; Sheikhi, M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012, 80, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhai, W.M.; Yang, Y.X.; Shi, J.L.; Liu, Q.T.; Liu, G.L.; Fang, N.; Li, J.; Guo, J.Y. GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. Pharmacol. Biochem. Behav. 2015, 128, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, H.; Bruggisser, R.; Schaffner, W.; Bogman, K.; Botomino, A.; Drewe, J. Transport of amentoflavone across the blood-brain barrier in vitro. Planta Med. 2002, 68, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Cho, S.; Yang, H.J.; Yong, J.I.; Yoon, M.S.; Han, D.S.; Baek, N.I. Rhus parviflora and its biflavonoid constituent, rhusflavone, induce sleep through the positive allosteric modulation of GABAA-benzodiazepine receptors. J. Ethnopharmacol. 2012, 142, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Eghorn, L.F.; Hoestgaard-Jensen, K.; Kongstad, K.T.; Bay, T.; Higgins, D.; Frolund, B.; Wellendorph, P. Positive allosteric modulation of the GHB high-affinity binding site by the GABAA receptor modulator monastrol and the flavonoid catechin. Eur. J. Pharmacol. 2014, 740, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Han, J.Y.; Moon, D.C.; Hong, J.T.; Oh, K.W. (−)-epigallocatechin-3-O-gallate augments pentobarbital-induced sleeping behaviour through Cl- channel activation. J. Med. Food 2011, 14, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.K.; Almqvist, J.P.; Vangsoe, S.A.K.; Stafford, G.I.; Adsersen, A.; Van Staden, J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- Yang, X.; Baburin, I.; Plitzko, I.; Hering, S.; Hamburger, M. HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root). Mol. Divers. 2011, 15, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, J.H.; Pae, A.N.; Han, D.; Kim, D.; Cho, N.C.; No, K.T.; Yang, H.; Yoon, M.; Lee, C.; et al. Hypnotic effects and GABAergic mechanism of licorice (Glycyrrhiza glabra) ethanol extract and its major flavonoid constituent glabrol. Bioorg. Med. Chem. 2012, 20, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Baburin, I.; Zaugg, J.; Ebrahimi, S.N.; Hering, S.; Hamburger, M. HPLC-based activity profiling—Discovery of sanggenons as GABAA receptor modulators in the traditional Chinese drug Sang bai pi (Morus alba root bark). Planta Med. 2012, 78, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Rueda, D.C.; De Mieri, M.; Hering, S.; Hamburger, M. HPLC-based activity profiling for GABAA receptor modulators in Adeoncarpus cincinnatus. J. Nat. Prod. 2014, 77, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Kim, S.; Cho, S.; Kim, I.H.; Han, D.; Jin, Y.H. Potentiating effect of glabridin on GABAA receptor-mediated responses in dorsal raphe neurons. Planta Med. 2013, 79, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N.I.; Jo, J.; Cho, C.W.; Park, J.H.; Shimizu, M.; et al. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem. Biophys. Res. Commun. 2011, 413, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Meissner, O.; Häberlein, H. Influence of xanthohumol on the binding behaviour of GABAA receptors and their lateral mobility at hippocampal neurons. Planta Med. 2006, 72, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, C.; Peng, Z.; Xie, Y.; Deng, S.; Nie, Y.Z.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Electrophysical characterization of methyleugenol: A novel agonist of GABA(A) receptors. ACS Chem. Neurosci. 2014, 5, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Eickmeier, E.; Ebrahimi, S.N.; Baburin, I.; Hering, S.; Hamburger, M. Positive GABAA receptor modulators from Acorus calamus and structural analysis of (+)-dioxosarcoguaiacol by 1D and 2D NMR and molecular modelling. J. Nat. Prod. 2011, 74, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Taferner, B.; Schuehly, W.; Huefner, A.; Baburin, I.; Wiesner, K.; Ecker, G.F.; Hering, S. Modulation of GABAA-receptors by honokiol and derivatives: Subtype selectivity and structure-activity relationship. J. Med. Chem. 2011, 54, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Kuribara, H.; Kishi, E.; Kimura, M.; Weintraub, S.T.; Maruyama, Y. Comparative assessment of the anxiolytic-like activities of honokiol and derivatives. Pharmacol. Biochem. Behav. 2000, 67, 597–601. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, H.; Jo, Y.J.; Kim, D.S.; Woo, S.S.; Li, R.; Hong, J.T.; Moon, D.C.; Oh, K.W.; Eun, J.S. Honokiol potentiates pentobarbital-induced sleeping behaviors through GABAA recptor Cl- channel activation. Biomol. Ther. 2008, 16, 328–335. [Google Scholar] [CrossRef]
- Alexeev, M.; Grosenbaugh, D.K.; Mott, D.D.; Fisher, J.L. The natural products magnolol and honokiol are positive allosteric modulators of both synaptic and extra-synaptic GABAA receptors. Neuropharmacology 2012, 62, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jo, Y.J.; Ma, Y.; Hong, J.T.; Kwon, B.M.; Oh, K.W. Obovatol isolated from Magnolia obovata enhances pentobarbital-induced sleeping time: Possible involvement of GABAA receptors/chloride channel activation. Phytomedicine 2009, 16, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Bisson, J.F.; Skinner, M. p-coumaric acid activates the GABA-A receptor in vitro and is orally anxiolytic in vivo. Phytother. Res. 2014, 28, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Jung, J.W.; Lee, J.J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Dey, L.; Wang, A.; Mehendale, S.; Xie, J.T.; Aung, H.H.; Ang-Lee, M.K. Kavalactones and dihydrokavain modulate GABAergic activity in a rat gastric-brainstem preparation. Planta Med. 2002, 68, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Ebrahimi, S.N.; Smiesko, M.; Baburin, I.; Hering, S.; Hamburger, M. Identification of GABA A receptor modulators in Kadsura longipedunculata and assignment of absolute configurations by quantum-chemical ECD calculations. Phytochemistry 2011, 72, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mao, X.; Zhao, X.; Liu, Z.; Liu, B.; Li, H.; Bi, K.; Jia, Y. Gomisin N isolated from Schisandra chinensis augments pentobarbital-induced sleep behaviors through the modification of the serotonergic and GABAergic system. Fitoterapia 2014, 96, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bergendorff, O.; Dekermandjian, K.; Nielsen, M.; Shan, R.; Witt, R.; Ai, J.; Sterner, O. Furanocoumarins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry 1997, 44, 1121–1124. [Google Scholar] [CrossRef]
- Singhuber, J.; Baburin, I.; Ecker, G.F.; Kopp, B.; Hering, S. Insights into structure-activity relationship of GABAA receptor modulating coumarins and furanocoumarins. Eur. J. Pharmacol. 2011, 668, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Eickmeier, E.; Rueda, D.C.; Hering, S.; Hamburger, M. HPLC-based activity profiling of Angelica pubescens roots for new positive GABAA receptor modulators in Xenopus oocytes. Fitoterapia 2011, 82, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Andres-Mach, M.; Cisowski, W.; Mazol, I.; Glowniak, K.; Czuczwar, S.J. Osthole suppresses seizures in the mouse maximal electroshock seizure model. Eur. J. Pharmacol. 2009, 607, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Andres-Mach, M.; Glensk, M.; Skalicka-Wozniak, K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: A comparative study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Coumarins from Angelica archangelica Linn. and their effects on anxiety-like behaviour. Prog. Neuropsychopharmacol. Biol. Psychatry 2013, 40, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Zagaja, M.; Glowniak, K.; Luszczki, J.J. Purification and anticonvulsant activity of xanthotoxin (8-methoxypsoralen). Cent. Eur. J. Biol. 2014, 9, 431–436. [Google Scholar] [CrossRef]
- Zagaja, M.; Pyrka, D.; Skalicka-Wozniak, K.; Glowniak, K.; Florek-Luszczki, M.; Glensk, M.; Luszczki, J.J. Effect of xanthotoxin (8-methoxypsoralen) on the anticonvulsant activity of classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Fitoterapia 2015, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Plitzko, I.; Zaugg, J.; Hering, S.; Hamburger, M. HPLC-based profiling for GABAA receptor modulators: A new dihydroisocoumarin from Haloxylon scoparium. J. Nat. Prod. 2010, 73, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, J.J.; Huang, C.; Wang, L.; Deng, S.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol from Rhizoma Curcumae suppresses epileptic seizure by facilitation of GABA(A) receptors. Neuropharmacology 2014, 81, 244. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.; Ng, I.; Tallapragada, V.J.; Hibbs, D.E.; Hanrahan, J.; Groundwater, P.W. The differential effects of resveratrol and trans-ε-viniferin on the GABA-induced current in GABAA receptor subtypes expressed in Xenopus laevis oocytes. J. Pharm. Pharm. Sci. 2015, 18, 328–338. [Google Scholar]
- Rueda, D.C.; Schöffmann, A.; De Mieri, M.; Raith, M.; Jähne, E.A.; Hering, S.; Hamburger, M. Identification of dihydrostilbenes in Pholidota chinensis as a new scaffold for GABAA receptor modulators. Bioorg. Med. Chem. 2014, 22, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Singhuber, J.; Baburin, I.; Khom, S.; Zehl, M.; Urban, E.; Hering, S.; Kopp, B. GABAA receptor modulators from the Chinese herbal drug Junci Medulla—The pith of Juncus effusus. Planta Med. 2012, 78, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Yoon, B.H.; Oh, H.R.; Ahn, J.H.; Kim, S.Y.; Park, S.Y.; Ryu, J.H. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol. Pharm. Bull. 2006, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, B.J.; Osasan, J.Y.; Olubiyi, O.O.; Oyemitan, I.A.; Atoyebi, S.A.M.; Elsegood, M.R.J.; Jones, R.C.F. Isolation of novel para-pentyl phenyl benzoate from Mondia whitei (Hook. F.) skeels (Periplocaeae), its structure, synthesis and neuropharmacological evaluation. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine 2011, 18, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Baek, N.I.; Kim, D.; Kang, S.M.; Yoon, M.; Yong, H.; Shimizu, M.; Han, D. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellmann induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A.R. (+)- and (−)-borneol: Efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Junior, L.J.; Guimaraes, A.G.; Araujo, B.E.S.; Geovana, F.O.; Santana, M.T.; Moreira, F.V.; Santos, M.R.V.; Cavalcanti, S.C.H.; De Lucca Junior, W.; Botelho, M.A.; et al. Carvacrol, (−)-borneol and citral reduce convulsant activity in rodents. Afr. J. Biotech. 2010, 9, 6466–6572. [Google Scholar]
- Höld, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. α-Thujone (the active component of absinthe): γ-Aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Czyzewska, M.M.; Mozrzymas, J.W. Monoterpene α-thujone exerts a differential inhibitory action on GABAA receptors implicated in phasic and tonic GABAergic inhibition. Eur. J. Pharmacol. 2013, 702, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Szczot, M.; Czyzewska, M.M.; Appendino, G.; Mozrzymas, J.W. Modulation of GABAergic synaptic currents and currents responses by α-thujone and dihydroumbellone. J. Nat. Prod. 2012, 75, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Borzone, M.; Delgado-Marin, L.; Garcia, D.A. Inhibitory effects of carvone isomers on GABAA receptor in primary cultures of rat cortical neurons. Chirality 2014, 26, 368–372. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.N.; de Sousa, D.P.; Nobrega, F.F.F.; Claudino, F.S.; Machado Araujo, D.A.; Leite, J.R.; Mattei, R. Anticonvulsant effect of a natural compound α,β-epoxy-carvone and its action on the nerve excitability. Neurosci. Lett. 2008, 443, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioid systems. Phytomedicine 2007, 14, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Bujons, J.; Vale, C.; Sunol, C. Allosteric positive interaction of thymol with the GABAA receptor in primary cultures of mouse cortical neurons. Neuropharmacology 2006, 50, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.G.; Neto, M.R.A.; Neto, P.F.T.; Moura, B.A.; Do Amaral, J.F.; De Sousa, D.P.; Vasconcelos, S.M.M.; De Sousa, F.C.F. Central nervous system activity of acute administration of isolpulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.F.S.C.; Da Silva, F.V.; Fernandes, H.B.; Oliveira, I.S.; Rao, V.S.; Oliveira, R.C.M.; Santos, F.A. Gastroprotective effect of (−)-myrtenol against ethanol-induced acute gastric lesions: Possible mechanisms. J. Pharm. Pharmacol. 2016, 68, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.A.C.; Costa, J.P.; De Carvalho, R.B.F.; De Sousa, D.P.; De Freitas, R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.A.C.; De Carvalho, R.B.F.; Silva, O.A.; De Sousa, D.P.; De Freitas, R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014, 118, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Silva, O.A.; De Almeida, A.A.C.; Cerqueira, G.S.; De Sousa, D.P.; De Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol, in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Goncalves, J.C.R.; Quintans-Junior, L.; Cruz, J.S.; Araujo, D.A.M.; De Almeida, R.N. Study of anticonvulsant effect of citronnellol, a monoterpene alcohol, in rodents. Neurosci. Lett. 2006, 401, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.C.; Turcotte, C.M.; Betts, B.A.; Yeung, W.Y.; Agyeman, A.S.; Burk, L.A. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur. J. Pharmacol. 2004, 506, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Watt, E.E.; Betts, B.A.; Kotey, F.O.; Humbert, D.J.; Griffith, T.N.; Kelly, E.W.; Veneskey, K.C.; Gill, N.; Rowan, K.C.; Jenkins, A.; et al. Menthol shares general anesthetic activity and sites of action on the GABAA receptor with the intravenous agent, propofol. Eur. J. Pharmacol. 2008, 590, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Fan, H.R.; Ding, J.; Huang, C.; Deng, S.; Zhu, T.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci. Rep. 2017, 7, 46654. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Lee, K.Y.; Choi, H.C.; Cho, J.; Kang, B.S.; Lim, J.C.; Lee, D.U. Modulation of radioligand binding to the GABAA-benzodiazepine receptor complex by a new component from Cyperus rotundus. Biol. Pharm. Bull. 2002, 25, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Khom, S.; Baburin, I.; Timin, E.; Hohaus, A.; Trauner, G.; Kopp, B.; Hering, S. Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunit specifity. Neuropharmacology 2007, 53, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Mehendale, S.; Xiao, Y.; Aung, H.H.; Xie, J.T.; Ang-Lee, M.K. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth. Analg. 2004, 98, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Kubin, Z.J.; Shepherd, J.N.; Ettinger, R.H. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine 2010, 17, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Garlet, Q.I.; Pires, L.C.; Milanesi, L.H.; Marafiga, J.R.; Baldisserotto, B.; Mello, C.F.; Heinzmann, B.M. (+)-Dehydrofukinone modulates membrane potential and delays seizure onset by GABAA receptor-mediated mechanism in mice. Toxicol. Appl. Pharmacol. 2017, 332, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, H.; Ito, M.; Asada, Y.; Kobayashi, Y. Inhalation administration of the sesquiterpenoid aristolen-1(10)-en-9-ol from Nardostachys chinensis has a sedative effect via the GABAergic system. Planta Med. 2015, 81, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Rueda, D.C.; Zaugg, J.; Quitschau, M.; Reich, E.; Hering, S.; Hamburger, M. Discovery of GABAA receptor modulator aristolactone in a commercial sample of the Chinese herbal drug “Chaihu” (Bupleurum chinense roots) unravels adulteration by nephrotoxic Aristolochia manshuriensis roots. Planta Med. 2012, 78, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Singhuber, J.; Baburin, I.; Kählig, H.; Urban, E.; Kopp, B.; Hering, S. GABAA receptor modulators from Chinese herbal medicines traditionally applied against insomnia and anxiety. Phytomedicine 2012, 19, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Oka, J.I.; Yamada, K. Anisatin, a potent GABA antagonist, isolated from Illicium anisatum. Neurosci. Lett. 1981, 25, 83–88. [Google Scholar] [CrossRef]
- Shinozaki, H.; Ishida, M.; Kudo, Y. Effects of anisatin on the GABA action in the crayfish neuromuscular junction. Brain Res. 1981, 222, 401–405. [Google Scholar] [CrossRef]
- Ikeda, T.; Ozoe, Y.; Okuyama, E.; Nagata, K.; Honda, H.; Shono, T.; Narahashi, T. Anisatin modulation of the γ-aminobutyric acid receptor-channel in rat dorsal ganglion neurons. Br. J. Pharmacol. 1999, 127, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sundaram, H.; Krishek, B.J.; Chazot, P.; Xie, X.; Bevan, P.; Brocchini, S.J.; Latham, C.J.; Charlton, P.; Moore, M.; et al. Regulation of neuronal and recombinant GABAA receptor ion channels by xenovulene A, a natural product isolated from Acremonium strictum. J. Pharmacol. Exp. Ther. 1997, 282, 513–520. [Google Scholar] [PubMed]
- Lee, C.M.; Wong, H.N.C.; Chui, K.Y.; Choang, T.F.; Hon, P.M.; Chang, H.M. Miltirone, a central benzodiazepine partial agonist from a Chinese medicinal herb Salvia miltiorriza. Neurosci. Lett. 1991, 127, 237–241. [Google Scholar] [CrossRef]
- Mostallino, M.C.; Mascia, M.P.; Pisu, M.G.; Busonero, F.; Talani, G.; Biggio, G. Inhibition of miltirone of up-regulation of GABAA receptor α4 subunit mRNA by ethanol withdrawal in hippocampal neurons. Eur. J. Pharmacol. 2004, 494, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rueda, D.C.; Raith, M.; De Mieri, M.; Schöffmann, A.; Hering, S.; Hamburger, M. Identification of dehydroabietic acid from Boswellia thurifera resin as a positive GABAA receptor modulator. Fitoterapia 2014, 99, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Khom, S.; Eigenmann, D.; Baburin, I.; Hamburger, M.; Hering, S. Identification and characterization of GABAA receptor modulatory diterpenes from Biota orientalis that decrease locomotor activity in mice. J. Nat. Prod. 2011, 74, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Wasowski, C.; Marder, M. Central nervous system activities of two diterpenes isolated form Aloysia virgata. Phytomedicine 2011, 18, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Ebrahimi, S.N.; Raith, M.; Zaugg, J.; Rueda, D.C.; Hering, S.; Hamburger, M. Phytochemical profiling of Curcuma kwangsiensis rhizome extract, and identification of labdane diterpenoids as positive GABAA receptor. Phytochemistry 2013, 96, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Duke, R.K.; Chebib, M.; Sasaki, K.; Wada, K.; Johnston, G.A.R. Ginkgolides, diterpene trilactones of Ginkgo biloba, as antagonists at recombinant α1β2γ2L GABAA receptors. Eur. J. Pharmacol. 2004, 494, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ivic, L.; Sands, T.T.J.; Fishkin, N.; Nakanishi, K.; Kriegstein, A.R. Terpene trilactones from Ginkgo biloba are antagonists of cortical glycine and GABAA receptors. J. Biol. Chem. 2003, 278, 49279–49285. [Google Scholar] [CrossRef] [PubMed]
- Kiewert, C.; Kumar, V.; Hildmann, O.; Rueda, M.; Hartmann, J.; Naik, R.S.; Klein, J. Role of GABAergic antagonism in the neuroprotective effects of bilobalide. Brain Res. 2007, 1128, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.W.; Song, D.K.; Huh, S.O.; Son, K.H.; Kim, Y.H. Antinociceptive mechanisms of Dipsacus saponin C administered intrathetically in mice. J. Ethnopharmacol. 2000, 71, 211–218. [Google Scholar] [CrossRef]
- Jung, Y.H.; Ha, R.R.; Kwon, S.H.; Hong, S.I.; Lee, K.H.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. Anxiolytic effects of Julibroside C isolated form Albizzia julibrissin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.W.M.; Song, D.K.; Son, K.H.; Woo, M.H.; Do, J.C.; Choi, Y.S.; Lee, K.H.; Kim, Y.H. Antinociceptive effect of smilaxin B administered intracerebroventricularly in the mouse. Planta Med. 1996, 62, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Han, E.J.; Lee, T.H.; Lee, J.K.; Han, K.J.; Lee, H.K.; Suh, H.W. Antinociceptive mechanisms of platycodin D administered intracerebroventricularly in the mouse. Planta Med. 2002, 68, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Park, H.J.; Gao, Q.; Lee, H.E.; Park, S.J.; Hong, E.; Jang, D.S.; Shin, C.Y.; Cheong, J.H.; Ryu, J.H. Positive effects of β-amyrin on pentobarbital-induced sleep in mice via GABAergic neurotransmitter system. Behav. Brain Res. 2015, 291, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Muceniece, R.; Saleniece, K.; Rumaks, J.; Krigere, L.; Dzirkale, Z.; Mezhapuke, R.; Zharkova, O.; Klusa, V. Betulin binds to γ-aminobutyric acid receptors and exerts anticonvulsant action in mice. Pharmacol. Biochem. Behav. 2008, 90, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Ceremuga, T.E.; Valdivieso, D.; Kenner, C.; Lucia, A.; Lathrop, K.; Stailey, O.; Bailey, H.; Criss, J.; Linton, J.; Fried, J.; et al. Evaluation of the anxiolytic and antidepressant effects of asiatic acid, a compound from Gotu Kola or Centella asiatica, in the male Sprague dawley rat. AANA J. 2015, 83, 91–98. [Google Scholar] [PubMed]
- Choi, S.E.; Choi, S.; Lee, J.H.; Whiting, P.J.; Lee, S.M.; Nah, S.Y. Effects of ginsenosides on GABAA receptor channels expressed in Xenopus oocytes. Arch. Pharm. Res. 2003, 26, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Choi, S.H.; Shin, T.J.; Hwang, S.H.; Kang, J.; Kim, H.J.; Kim, B.J.; Nah, S.Y. Effects of ginsenoside metabolites on GABAA receptor-mediated ion currents. J. Ginseng Res. 2012, 36, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.Y.; Park, J.H.; Hong, J.T.; Yoo, H.S.; Song, S.; Hwang, B.Y.; Eun, J.S.; Oh, K.W. Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biol. Pharm. Bull. 2005, 28, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, H.J.; Kim, N.J.; Kim, D.H. Anxiolytic-like effects of ginsenosides Rg3 and Rh22 from red ginseng in the elevated plus-maze model. Planta Med. 2009, 75, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Miao, B.; Song, X.; Jiang, Z. Inactivation of GABAA receptor reduces ginsenoside Rb3 neuroprotection in mouse hippocampal slices after oxygen-glucose deprivation. J. Ethnopharmacol. 2011, 133, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S.; Khom, S.; Taferner, B.; Hering, S.; Stuppner, H. Bioactivity-guided isolation of GABAA receptor modulating constituents from the rhizomes of Actaea racemosa. J. Nat. Prod. 2010, 73, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Strommer, B.; Khom, S.; Kastenberger, I.; Cicek, S.S.; Stuppner, H.; Schwarzer, C.; Hering, S. A cycloartane glycoside derived from Actaea racemosa L. modulates GABAA receptors and induces pronounced sedation in mice. J. Pharmacol. Exp. Ther. 2014, 351, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ferando, I.; Mody, I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsy. Epilepsia 2012, 53, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hosie, A.M.; Wilkins, M.E.; Smart, T.G. Neurosteroid binding sites on GABAA receptors. Pharmacol. Ther. 2007, 116, 7–19. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 228–231 are available from the author. |
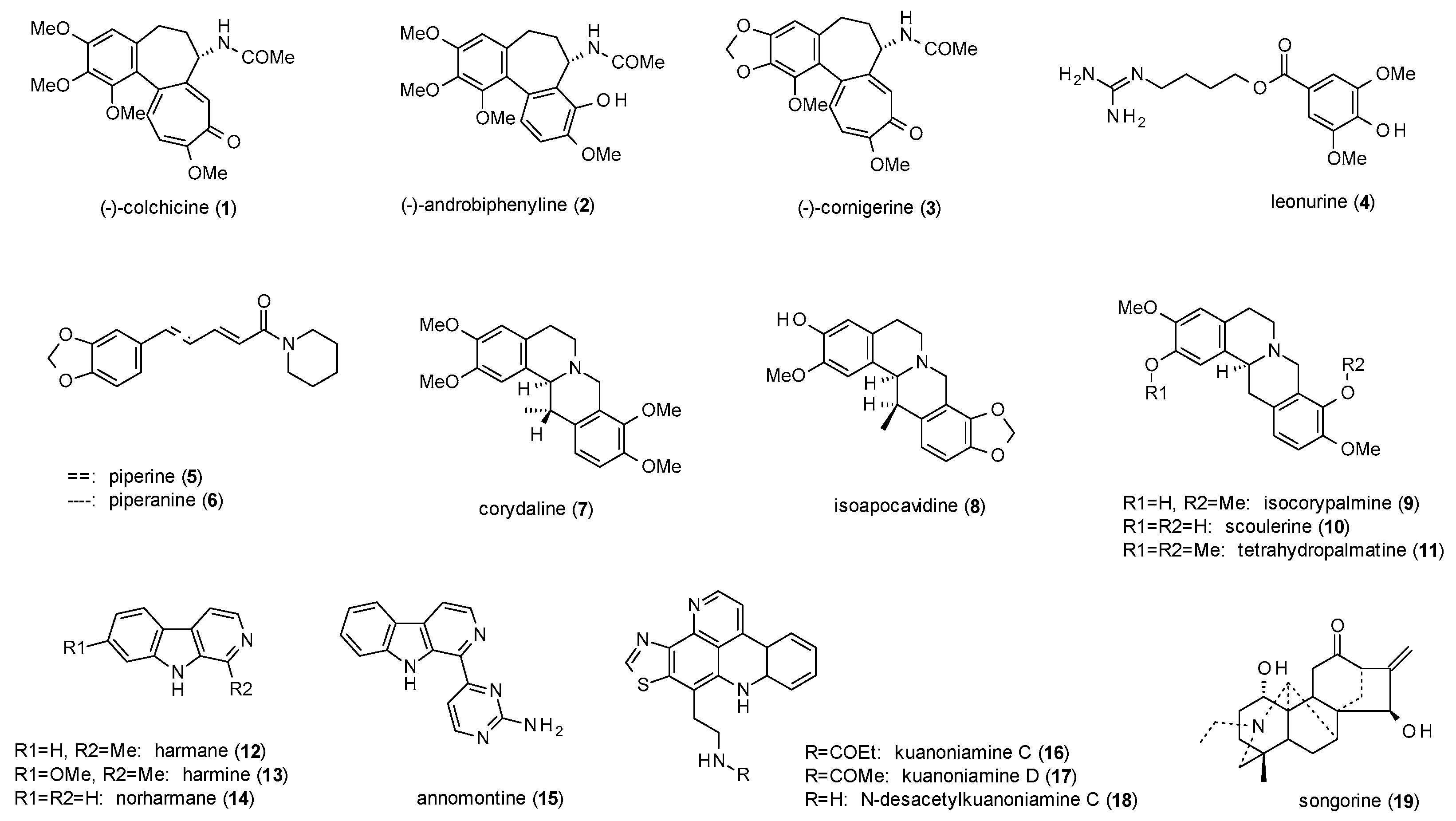
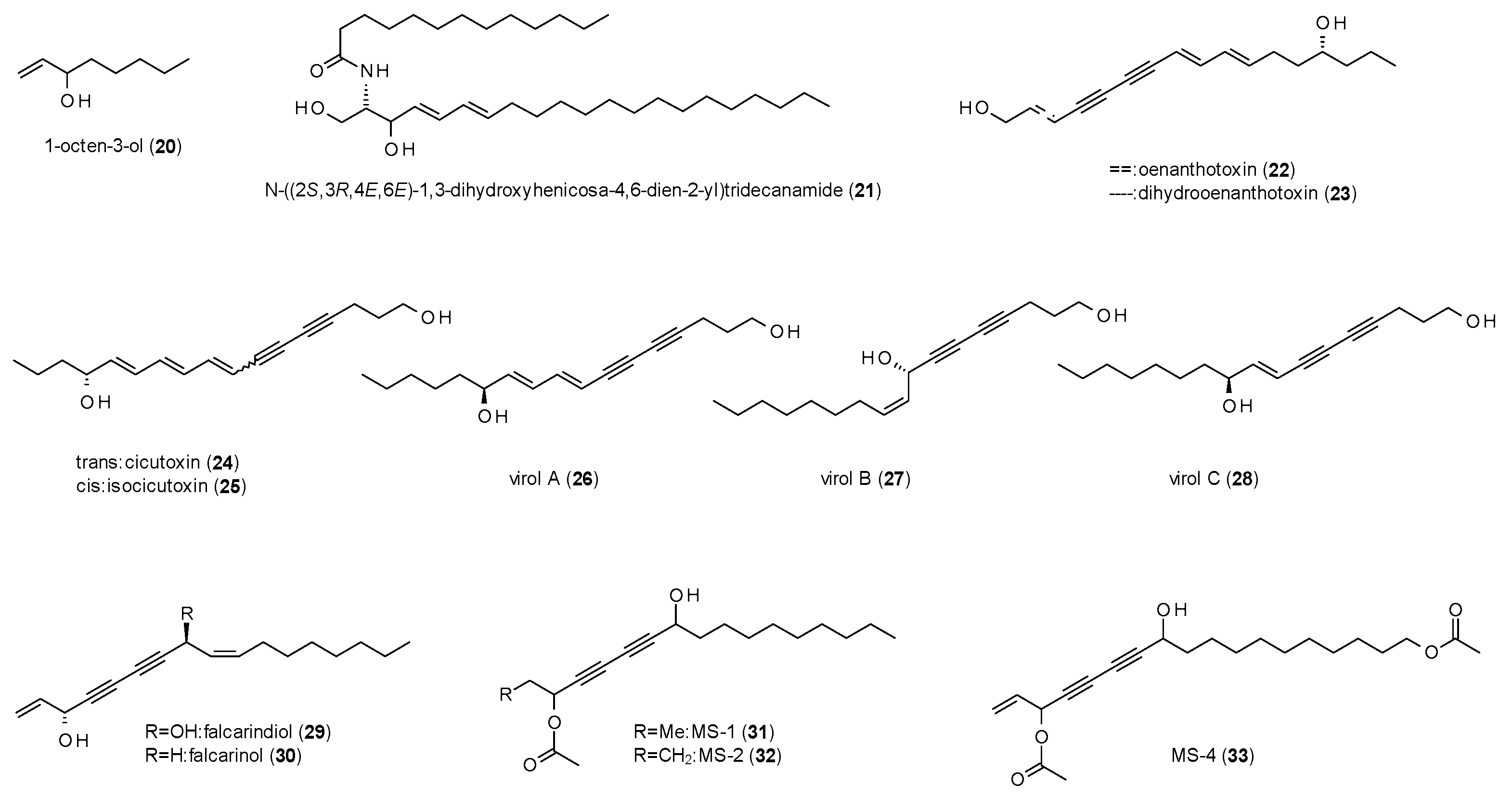
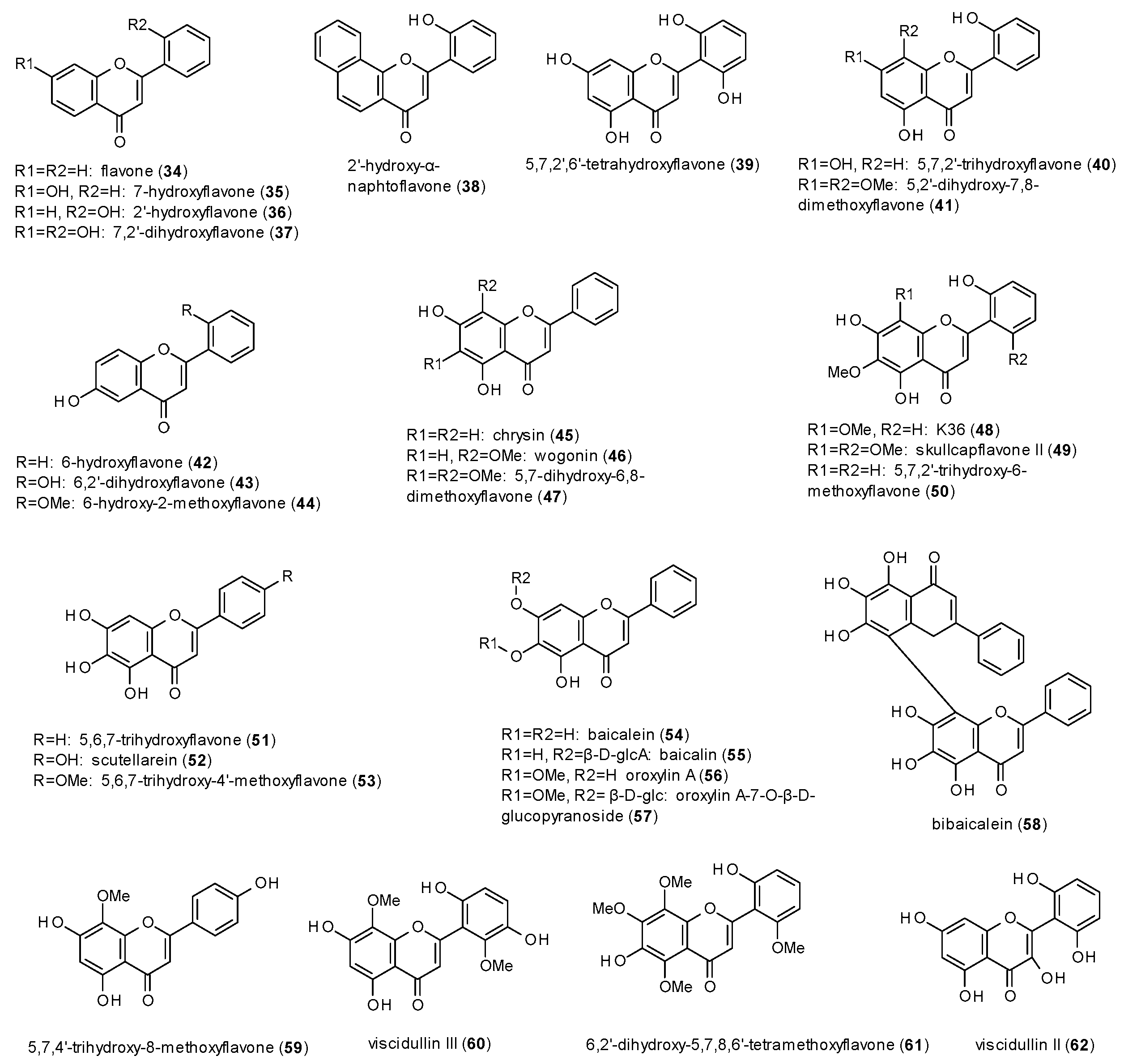
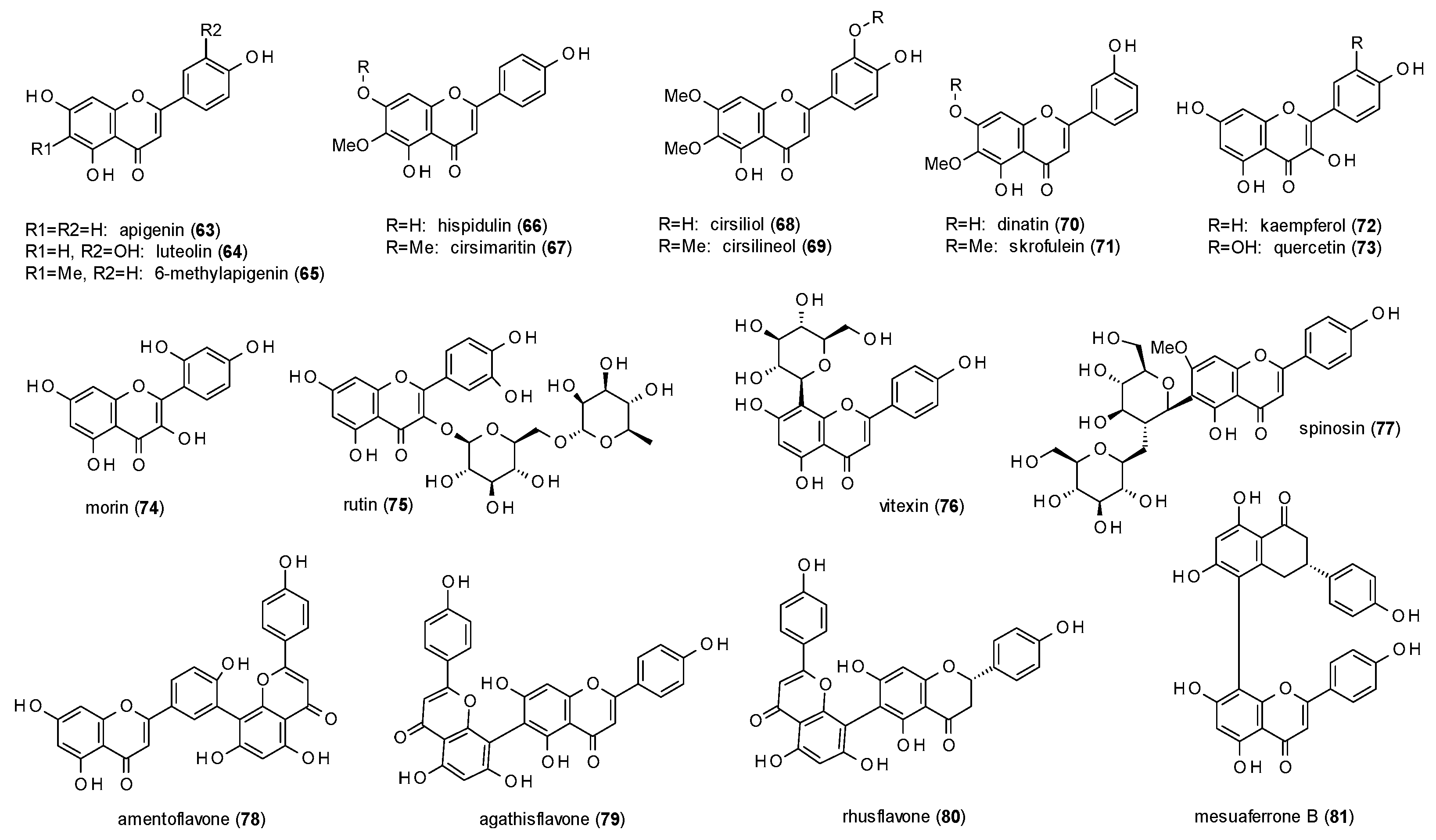
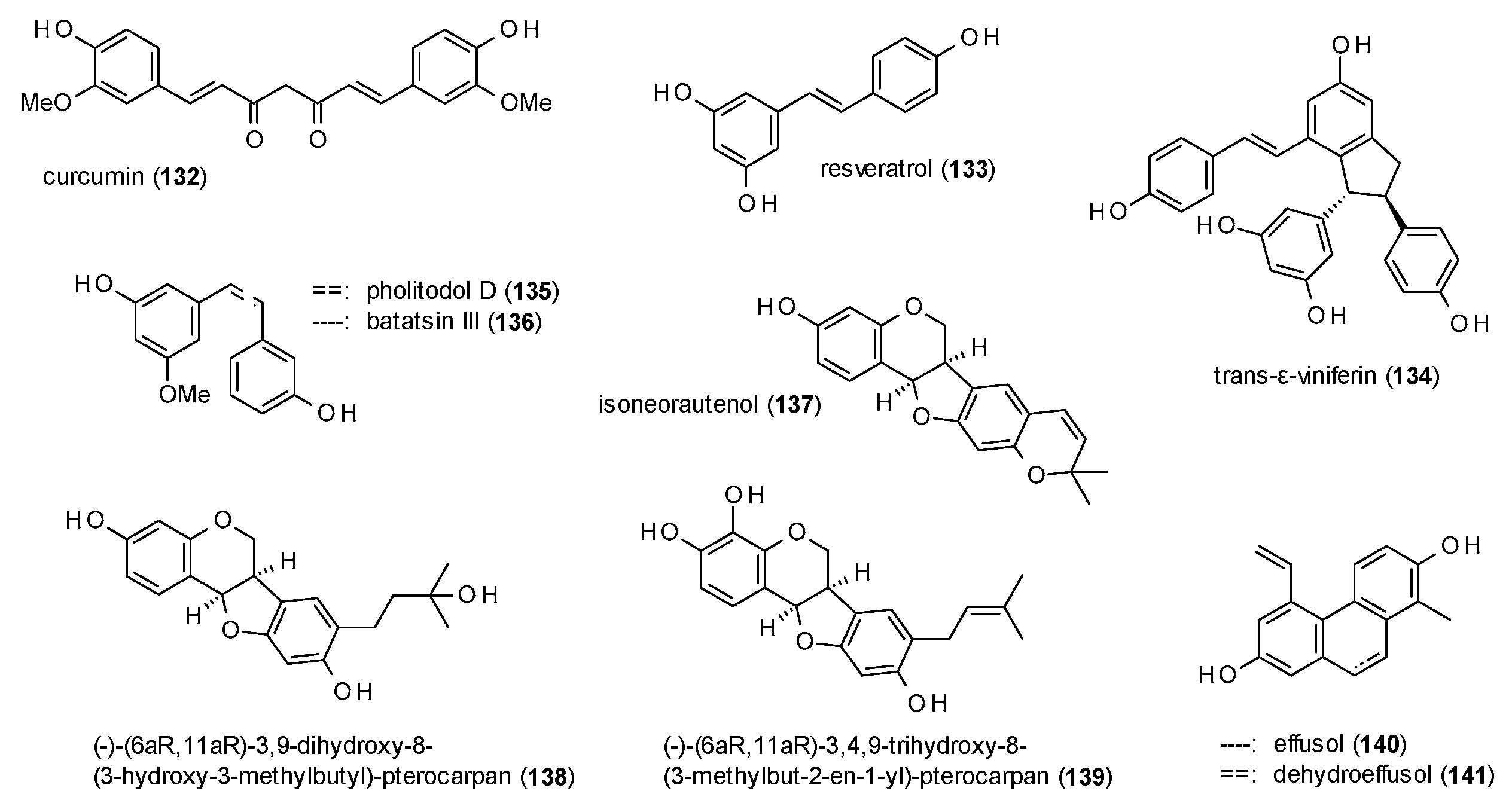
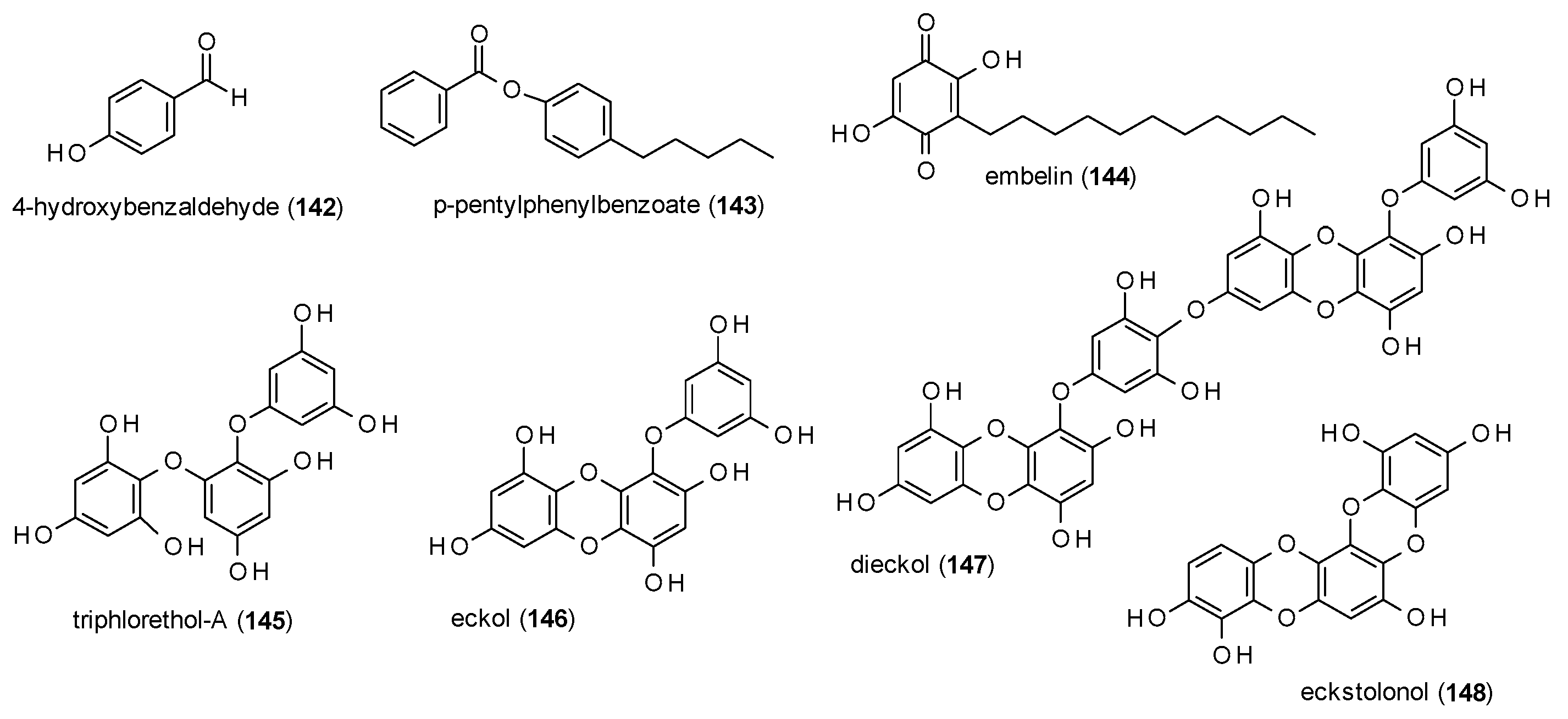
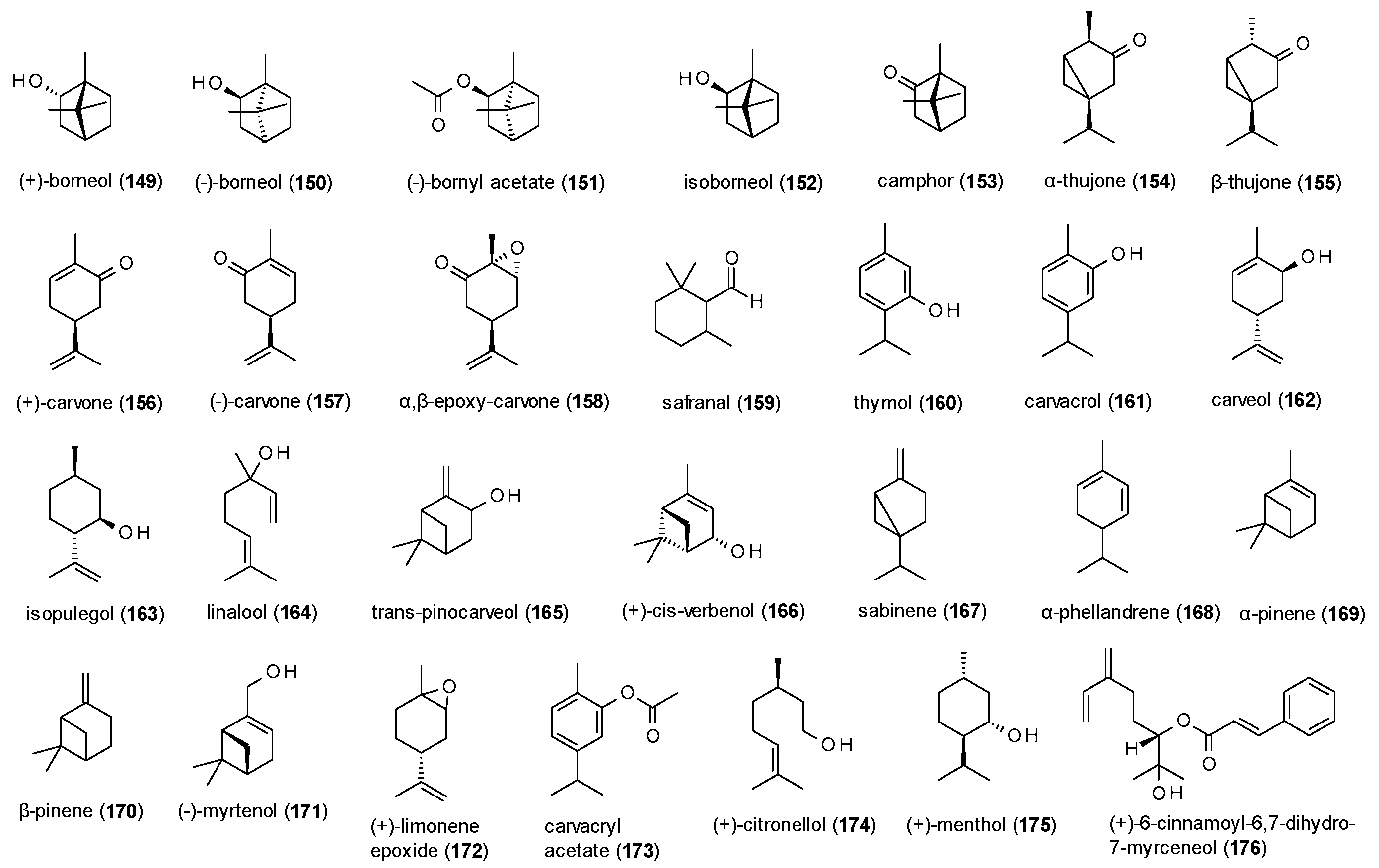
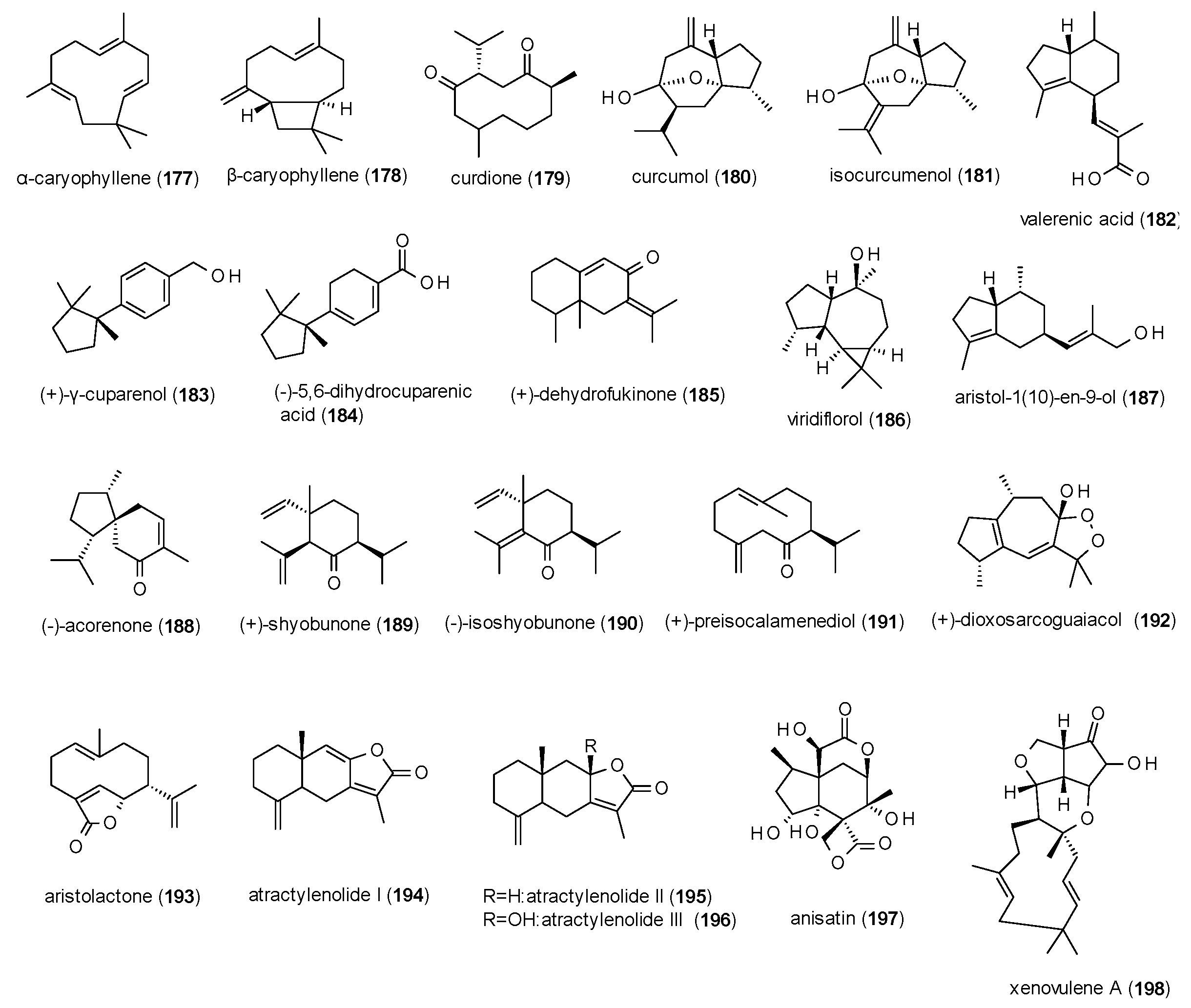
| Cmpd | Source | Assay | Subtype | c(GABA) | Imax [%] | c [µM] | IC50 [µM] | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Colchicum autumnale | Xenopus oocytes | α1β2γ2L | 10 µM | 59.9 ± 6.2 | 100 | [23] | |
| 19 | Aconitum leucostomum | Hippocampal neurons | 19.6 | [31] | ||||
| 22 | Oenanthe fistulosa | HEK-293T cells | α1β2γ2L | 1.39 | [35] | |||
| 23 | Oenanthe fistulosa | HEK-293T cells | α1β2γ2L | 0.835 | [35] | |||
| 26 | Cicuta virosa | Hippocampal neurons | 10 µM | 90 | 10 | 0.96 | [37] | |
| 34 | Xenopus oocytes | α1β2γ2S | 14.8 ± 0.8 | 30 | [54] | |||
| 45 | Xenopus oocytes | α1β2γ2S | 12.1 ± 0.5 | 30 | [54] | |||
| 56 | Scuttelaria baicalensis | CA1 neurons | 10 µM | 38.2 ± 6 | 100 | [46] | ||
| 63 | Xenopus oocytes | α1β2γ2S | 50.6 ± 0.8 | 30 | [54] | |||
| 63 | Matricaria recutita | Cerebellar granule cells | 10 µM | 32 ± 4 | 10 | [55] | ||
| 63 | Xenopus oocytes | α1β2γ2L | 40 µM | 49.6 | 100 | 8 | [56] | |
| 63 | Xenopus oocytes | α1β2γ2S | 6.9 ± 0.3 | [57] | ||||
| 64 | HEK-293T cells | α1β2γ2 | 66.5 ± 6.8 | 6.62 ± 2.11 | [62] | |||
| 73 | Xenopus oocytes | α1β2γ2S | 84.5 ± 4.9 | 30 | [54] | |||
| 74 | Xenopus oocytes | α1β2γ2S | 38.4 ± 4.8 | 30 | [54] | |||
| 84 | Xenopus oocytes | α1β2γ2L | 40/20 µM | 90.6/97 | 100 | 14.7/8.7 | [56] | |
| 96 | Xenopus oocytes | α1β2γ2L | 40/5 µM | 51/40 | 100 | 29.2/11.7 | [56] | |
| 134 | Xenopus oocytes | α1β2γ2L | 5.79 | [108] | ||||
| 154 | Dorsal root ganglia | 300 µM | 100 | 30 | 21 | [117] | ||
| 154 | HEK-293T cells | α1β2δ | 60 | 300 | [118] | |||
| 197 | Illicium anisatum | Dorsal root ganglia | 30 µM | 41.7 | 1 | 1.10 ± 1.40 | [144] | |
| 209 | Ginkgo biloba | Xenopus oocytes | α1β2γ2L | 300 µM | 11.9 ± 1.7 | [152] | ||
| 210 | Ginkgo biloba | Xenopus oocytes | α1β2γ2L | 300 µM | 10.1 ± 2.9 | [152] | ||
| 210 | Ginkgo biloba | Cortical neurons | α1β2γ2L | 30 µM | 63.2 ± 0.3 | 50 | 73 | [153] |
| 211 | Ginkgo biloba | Xenopus oocytes | α1β2γ2L | 300 µM | 12.0 ± 2.2 | [152] | ||
| 212 | Ginkgo biloba | Cortical neurons | α1β2γ2L | 30 µM | 46.8 ± 0.3 | 50 | 76 µM | [153] |
| Cmpd | Source | Assay | Subtype | c(GABA) | Pmax [%] | c [µM] | EC50 [µM] | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5 | Piper nigrum | Xenopus oocytes | α1β2γ2S | EC5–10 | 302 ± 26 | 300 | 52 ± 9 | [25] |
| 6 | Piper nigrum | Xenopus oocytes | α1β2γ2S | EC5–10 | 187 ± 10 | 300 | 56 ± 19 | [25] |
| 20 | Xenopus oocytes | α1β2γ2 | 1 µM | 295 ± 50 | 300 | [33] | ||
| 31–33 | Cussonia zimmermannii | Xenopus oocytes | α1β2γ2 | 110–440 | 0.6–3.5 | [40] | ||
| 46 | Scuttelaria baicalensis | Xenopus oocytes | α1β2γ2 | EC1 | 57 ± 6 | 30 | 3 | [48] |
| 66 | Xenopus oocytes | α1β2γ2 | EC2–5 | 47 ± 5 | 10 | [57] | ||
| 82 | Xenopus oocytes | α4β4δ | 68 ± 5 | 100 | [76] | |||
| 88 | Sophora flavescens | Xenopus oocytes | α1β2γ2S | EC3–10 | 578.5 ± 68.8 | 8.1 ± 1.4 | [79] | |
| 89 | Sophora flavescens | Xenopus oocytes | α1β2γ2S | EC3–10 | 267.6 ± 56.6 | 5.0 ± 2.3 | [79] | |
| 90 | Sophora flavescens | Xenopus oocytes | α1β2γ2S | EC3–10 | 604.9 ± 108.2 | 15.0 ± 3.6 | [79] | |
| 92 | Morus alba | Xenopus oocytes | α1β2γ2S | 730.4 ± 76.7 | 100 | 13.8 ± 1.5 | [81] | |
| 93 | Morus alba | Xenopus oocytes | α1β2γ2S | 715.8 ± 56.1 | 100 | 16.7 ± 2.0 | [81] | |
| 94 | Morus alba | Xenopus oocytes | α1β2γ2S | 719.3 ± 63.3 | 100 | 13.4 ± 1.6 | [81] | |
| 95 | Adenocarpus cinncinatus | Xenopus oocytes | α1β2γ2S | EC5–10 | 552.73 ± 84.07 | 500 | 2.8 ± 1.4 | [82] |
| 97 | Glycyrrhiza glabra | Dorsal raphe neurons | α2β2/3γ2L | 2 µM | 581 ± 91 | 3 | [83] | |
| 98 | Dorsal raphe neurons | EC10 | 151 | 10 | [84] | |||
| 99 | Sophora flavescens | Xenopus oocytes | α1β2γ2S | EC3–10 | 891.5 ± 163.0 | 4.0 ± 2.4 | [79] | |
| 101 | HEK-293T cells | α1β2γ2 | 290 ± 28 | [86] | ||||
| 102 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 1200 ± 163 | 500 | 171.5 ± 34.6 | [87] |
| 103 | Magnolia officinalis | Xenopus oocytes | α1β2γ2S | 1315 ± 281 | 300 | 36.2 ± 14.7 | [88] | |
| 106 | HEK-293T cells | 10.1 ± 10.5 | [93] | |||||
| 107 | HEK-293T cells | 16.9 ± 0.3 | [93] | |||||
| 108 | HEK-293T cells | 10.5 ± 2.3 | [93] | |||||
| 109 | Cortical neurons | 10 µM | 158 ± 20 | 1 | 0.04258 | [94] | ||
| 110 | Piper methysticum | Neonatal rat gastric-brainstem preparation | 31.5 ± 3.9 | 300 | 93 | [95] | ||
| 111 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 218.1 ± 20.8 | 21.8 ± 7.5 | [96] | |
| 112 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 245.0 ± 59.6 | 52.2 ± 24.8 | [96] | |
| 113 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 885.8 ± 291.2 | 135.6 ± 85.7 | [96] | |
| 114 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 168.7 ± 41.5 | 36.6 ± 16.4 | [96] | |
| 115 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 129.7 ± 36.8 | 118.7 ± 54.4 | [96] | |
| 116 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 395.6 ± 27.2 | 31.5 ± 7.1 | [96] | |
| 117 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 288.8 ± 23.7 | 12.8 ± 3.1 | [96] | |
| 118 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 793.4 ± 107.4 | 79.2 ± 19.4 | [96] | |
| 119 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 362.5 ± 87.1 | 54.6 ± 28.8 | [96] | |
| 121 | Cnidium monnieri | Xenopus oocytes | α1β2γ2S | 54 ± 13 | [99] | |||
| 121 | Angelica pubescens | Xenopus oocytes | α1β2γ2S | EC5–10 | 25.8 ± 12.7 | 300 | [100] | |
| 122 | Xenopus oocytes | α1β2γ2S | 57 ± 4 | [99] | ||||
| 124 | Xenopus oocytes | α1β2γ2S | 550 ± 71 | 100 | 25 ± 8 | [99] | ||
| 125 | Xenopus oocytes | α1β2γ2S | 34 ± 6 | [99] | ||||
| 126 | Angelica pubescens | Xenopus oocytes | α1β2γ2S | EC5–10 | 204.5 ± 33.2 | 300 | [89] | |
| 127 | Cnidium monnieri | Xenopus oocytes | α1β2γ2S | 124 ± 11 | 100 | 14 ± 1 | [99] | |
| 127 | Angelica pubescens | Xenopus oocytes | α1β2γ2S | EC5–10 | 273.6 ± 39.4 | 300 | [100] | |
| 128 | Angelica pubescens | Xenopus oocytes | α1β2γ2S | EC5–10 | 61.2 ± 20.2 | 300 | [100] | |
| 129 | Angelica pubescens | Xenopus oocytes | α1β2γ2S | EC5–10 | 38.0 ± 21.3 | 300 | [100] | |
| 131 | Haloxylon scoparium | Xenopus oocytes | α1β2γ2S | 144.6 ± 35.3 | 500 | 140.2 ± 51.2 | [106] | |
| 132 | Rhizoma curcumae oil | HEK-293T cells | α1β2γ2 | 1 µM | 120 ± 6 | 50 | [107] | |
| 133 | Xenopus oocytes | α1β2γ2L | EC10 (3 µM) | 126 ± 15 | 100 | 58.24 | [108] | |
| 135 | Pholidota chinensis | Xenopus oocytes | α1β2γ2S | EC3–10 | 786.8 ± 72.1 | 300 | 175.5 ± 25.5 | [109] |
| 136 | Pholidota chinensis | Xenopus oocytes | α1β2γ2S | EC3–10 | 1512.9 ± 176.5 | 300 | 52.5 ± 17.0 | [109] |
| 137 | Adenocarpus cinncinatus | Xenopus oocytes | α1β2γ2S | EC5–10 | 771.09 ± 57.94 | 500 | 40.7 ± 4.08 | [82] |
| 138 | Adenocarpus cinncinatus | Xenopus oocytes | α1β2γ2S | EC5–10 | 640.02 ± 53.56 | 500 | 8.6 ± 1.6 | [82] |
| 139 | Adenocarpus cinncinatus | Xenopus oocytes | α1β2γ2S | EC5–10 | 490.97 ± 22.34 | 500 | 18.8 ± 2.3 | [82] |
| 140 | Juncus effusus | Xenopus oocytes | α1β2γ2S | EC5–10 | 188 ± 20 | 300 | 31 ± 8 | [110] |
| 141 | Juncus effusus | Xenopus oocytes | α1β2γ2S | EC5–10 | 239 ± 18 | 300 | 27 ± 6 | [110] |
| 149 | Xenopus oocytes | α1β2γ2L | EC5–14 | 1251 ± 73 | 300 | 247.7 | [115] | |
| 150 | Xenopus oocytes | α1β2γ2L | EC15–24 | 1106 ± 73 | 300 | 236.9 | [115] | |
| 151 | Xenopus oocytes | α1β2γ2L | EC25–39 | 571 ± 123 | 300 | 111.2 | [115] | |
| 152 | Xenopus oocytes | α1β2γ2L | EC15–24 | 968 ± 88 | 300 | 190.5 | [115] | |
| 153 | Xenopus oocytes | α1β2γ2L | EC15–24 | 377 ± 156 | 300 | 469.1 | [115] | |
| 160 | Xenopus oocytes | α1β3γ2S | EC20 | 416 ± 72 | 100 | [123] | ||
| 161 | Xenopus oocytes | α1β2γ2 | 1 µM | 224 ± 85 | 300 | [33] | ||
| 162 | Xenopus oocytes | α1β2γ2 | 1 µM | 453 ± 176 | 300 | [33] | ||
| 163 | Xenopus oocytes | α1β2γ2 | 1 µM | 340 ± 70 | 300 | [33] | ||
| 164 | Xenopus oocytes | α1β2γ2 | 1 µM | 213 ± 105 | 300 | [33] | ||
| 165 | Xenopus oocytes | α1β2γ2 | 1 µM | 477 ± 68 | 300 | [33] | ||
| 166 | Xenopus oocytes | α1β2γ2 | 1 µM | 809 ± 118 | 300 | [33] | ||
| 167 | HEK-293T cells | α1β2γ2 | 1 µM | 156 ± 26 | 1000 | [33] | ||
| 168 | HEK-293T cells | α1β2γ2 | 1 µM | 168 ± 42 | 1000 | [33] | ||
| 169 | HEK-293T cells | α1β2γ2 | 1 µM | 116 ± 56 | 1000 | [33] | ||
| 170 | HEK-293T cells | α1β2γ2 | 1 µM | 179 ± 55 | 1000 | [33] | ||
| 171 | Xenopus oocytes | α1β2γ2 | 1 µM | 737 ± 234 | 300 | [33] | ||
| 175 | Xenopus oocytes | α1β2γ2S | EC20 | 496 ± 113 | 100 | [131] | ||
| 175 | Xenopus oocytes | α1β2γ2S | EC20 | 96.2 ± 3.8 | 50 | [132] | ||
| 176 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 834.6 ± 77.5 | 70.6 ± 12.2 | [96] | |
| 177 | HEK-293T cells | α1β2γ2 | 1 µM | 117 ± 57 | 1000 | [33] | ||
| 178 | HEK-293T cells | α1β2γ2 | 1 µM | 115 ± 52 | 1000 | [33] | ||
| 179 | Rhizoma curcumae oil | HEK-293T cells | α1β2γ2 | 1 µM | 133 ± 10 | 50 | [107] | |
| 180 | Rhizoma curcumae oil | HEK-293T cells | α1β2γ2 | 1 µM | 251 ± 16 | 300 | 34.4 ± 2.9 | [107] |
| 182 | Valeriana officinalis | Xenopus oocytes | α1β2γ2S | EC5–10 | 400.0 ± 77.6 | 100 | 13.6 ± 4.1 | [135] |
| 183 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 383.5 ± 89.3 | 57.3 ± 19.7 | [96] | |
| 184 | Kadsura longipedunculata | Xenopus oocytes | α1β2γ2S | EC5–10 | 413.4 ± 66.3 | 118.4 ± 29.9 | [96] | |
| 188 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 241 ± 23.1 | 500 | 34.0 ± 6.7 | [87] |
| 189 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 669 ± 112 | 1000 | 64.8 ± 19.8 | [87] |
| 190 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 164 ± 42.9 | 500 | 109.4 ± 46.6 | [87] |
| 191 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 886 ± 105 | 1000 | 135.1 ± 34.4 | [87] |
| 192 | Acorus calamus | Xenopus oocytes | α1β2γ2S | EC5–10 | 588 ± 126 | 300 | 65.3 ± 21.6 | [87] |
| 193 | Aristolochia manshuriensis | Xenopus oocytes | α1β2γ2S | EC5–10 | 70.7 ± 2.6 | 1000 | 56.02 ± 5.09 | [140] |
| 194 | Atractylodes macrocephala | Xenopus oocytes | α1β2γ2S | EC1–10 | 96 ± 3 | 300 | 12 ± 1 | [141] |
| 195 | Atractylodes macrocephala | Xenopus oocytes | α1β2γ2S | EC1–10 | 166 ± 122 | 300 | 70 ± 17 | [141] |
| 196 | Atractylodes macrocephala | Xenopus oocytes | α1β2γ2S | EC1–10 | 157 ± 12 | 300 | 99 ± 20 | [141] |
| 198 | Acremonium strictum | Xenopus oocytes | α1β1γ2S | EC20 | 180 | 1 | 0.05 ± 0.02 | [145] |
| 202 | Boswellia serrata | Xenopus oocytes | α1β2γ2S | EC5–10 | 397.5 ± 34.0 | 100 | 8.7 ± 1.3 | [148] |
| 203 | Platycladus orientalis | Xenopus oocytes | α1β2γ2S | EC5–10 | 425.2 ± 96.5 | 500 | 141.6 ± 68.0 | [149] |
| 204 | Platycladus orientalis | Xenopus oocytes | α1β2γ2S | EC5–10 | 855.7 ± 114.9 | 500 | 33.2 ± 8.7 | [149] |
| 207 | Curcuma kwangsiensis | Xenopus oocytes | α1β2γ2S | EC3–10 | 309.4 ± 35.6 | 300 | 24.9 ± 8.8 | [151] |
| 208 | Curcuma kwangsiensis | Xenopus oocytes | α1β2γ2S | EC3–10 | 211.0 ± 26.0 | 300 | 35.7 ± 8.8 | [151] |
| 220 | Panax ginseng | Xenopus oocytes | α1β2γ2S | 10 µM | 53.2 ± 12.3 | [162] | ||
| 221 | Panax ginseng | Xenopus oocytes | α1β2γ2S | 10 µM | 23.3 ± 1.4 | 100 | 23.1 ± 8.6 | [163] |
| 222 | Panaxginseng | Xenopus oocytes | α1β2γ2S | 10 µM | 54.1 ± 1.7 | 100 | 17.1 ± 2.2 | [163] |
| 228 | Actaea racemosa | Xenopus oocytes | α1β2γ2S | EC3–10 | 378 ± 64 | 300 | 36 ± 14 | [167] |
| 229 | Actaea racemosa | Xenopus oocytes | α1β2γ2S | EC3–10 | 256 ± 40 | 300 | 28 ± 17 | [167] |
| 230 | Actaea racemosa | Xenopus oocytes | α1β2γ2S | EC3–10 | 289 ± 45 | 300 | 26 ± 7 | [167] |
| 231 | Actaea racemosa | Xenopus oocytes | α1β2γ2S | EC3–10 | 1947 ± 185 | 300 | 27 ± 8 | [167] |
| Cmpd | Source | Binding Site | Ligand | IC50 [µM] | Ki [µM] | Stimulation [%] | c [µM] | Ref. |
|---|---|---|---|---|---|---|---|---|
| 4 | Leonurus japonicus | GABA/muscimol | [3H]gabazine | 15,000 | [24] | |||
| 19 | Aconitum leucostomum | GABA/muscimol | [3H]muscimol | 7.06 | [31] | |||
| 218 | GABA/muscimol | [3H]GABA | 64 ± 5 | [160] | ||||
| 2 | Colchicum szovitsii | benzodiazepine | [3H]flunitrazepam | 25% of 10 µM allopregnanolone | 10 | [22] | ||
| 3 | Colchicum szovitsii | benzodiazepine | [3H]flunitrazepam | 25% of 10 µM allopregnanolone | 10 | [22] | ||
| 4 | Leonurus japonicus | benzodiazepine | [3H]flumazenil | 123,000 | [24] | |||
| 16 | Oceanapia sp. | benzodiazepine | [3H]diazepam | 39 | 25 | [30] | ||
| 17 | Oceanapia sp. | benzodiazepine | [3H]diazepam | 32 | 25 | [30] | ||
| 18 | Oceanapia sp. | benzodiazepine | [3H]diazepam | 30 | 25 | [30] | ||
| 34 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 7.81 ± 1.81 | [42] | |||
| 35 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 4.20 ± 0.27 | [42] | |||
| 36 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.21 ± 0.10 | [42] | |||
| 37 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.56 ± 0.07 | [42] | |||
| 38 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.027 ± 0.003 | [42] | |||
| 39 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 6.42 ± 0.95 | [41] | |||
| 40 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.0075 ± 0.004 | [42] | |||
| 41 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.28 ± 0.076 | [41] | |||
| 42 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 2.64 ± 0.36 | [42] | |||
| 43 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.034 ± 0.001 | [42] | |||
| 44 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 9.46 ± 1.45 | [42] | |||
| 45 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.69 ± 0.12 | 0.64 ± 0.26 | [41,42] | ||
| 46 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 1.26 ± 0.24 | 1.52 ± 0.13 | [41,42] | ||
| 47 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.36 ± 0.095 | 0.20 ± 0.05 | [41,42] | ||
| 48 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.008 ± 0.0002 | 0.0061 ± 0.0001 | [41,42] | ||
| 49 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.31 ± 0.088 | [41] | |||
| 50 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.0038 ± 0.005 | [42] | |||
| 51 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 5.58 ± 0.02 | [42] | |||
| 52 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 21.0 ± 1.79 | [41] | |||
| 53 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 21.4 ± 1.42 | [41] | |||
| 54 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 10.1 ± 1.68 | [41] | |||
| 55 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | >100 | [41] | |||
| 56 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 0.14 ± 0.01 | 0.89 ± 0.06 | [41,42] | ||
| 57 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 22.6 ± 1.30 | [41] | |||
| 58 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 12.5 ± 1.58 | [41] | |||
| 59 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 1.27 ± 0.08 | [41] | |||
| 60 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 6.80 ± 1.18 | [41] | |||
| 61 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 32.8 ± 1.51 | [41] | |||
| 62 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 45.7 ± 2.48 | [41] | |||
| 63 | Tanacetum parthenium | benzodiazepine | [3H]flumazenil | 12 | 9 | [60] | ||
| 63 | Salvia officinalis | benzodiazepine | [3H]flumazenil | 30 ± 4 | [65] | |||
| 63 | Rhus pyroides | benzodiazepine | [3H]flumazenil | 10.0 ± 1.18 | [59] | |||
| 63 | Matricaria recutita | benzodiazepine | [3H]flunitrazepam | 4 | [58] | |||
| 66 | Artemisia herba-alba | benzodiazepine | [3H]flumazenil | 8 | [66] | |||
| 66 | Salvia officinalis | benzodiazepine | [3H]flumazenil | 1.3 ± 0.2 | [65] | |||
| 67 | Salvia officinalis | benzodiazepine | [3H]flumazenil | 350 ± 37 | [65] | |||
| 68 | Salvia coerulea | benzodiazepine | [3H]flunitrazepam | 200 | [67] | |||
| 68 | Salvia coerulea | benzodiazepine | [3H]zolpidem | 20 | [67] | |||
| 69 | Artemisia herba-alba | benzodiazepine | [3H]flumazenil | 100 | [66] | |||
| 70 | Artemisia herba-alba | benzodiazepine | [methyl-3H]DZP | 1.3 | [68] | |||
| 71 | Artemisia herba-alba | benzodiazepine | [methyl-3H]DZP | 22.7 | [68] | |||
| 78 | Hypericum perforatum | benzodiazepine | [3H]flunitrazepam | 0.0149 | [74] | |||
| 78 | Searsia pyroides | benzodiazepine | [3H]flunitrazepam | 37 | [59] | |||
| 79 | Searsia pyroides | benzodiazepine | [3H]flunitrazepam | 28 | [59] | |||
| 85 | Mentha aquatica | benzodiazepine | [3H]flumazenil | 2600 | [78] | |||
| 87 | Scuttelaria baicalensis | benzodiazepine | [3H]flunitrazepam | 21.4 ± 1.32 | [41] | |||
| 91 | Glycyrrhiza glabra | benzodiazepine | [3H]flumazenil | 1.63 | [80] | |||
| 98 | benzodiazepine | [3H]flunitrazepam | 0.453 | [84] | ||||
| 121 | Angelica dahurica | benzodiazepine | [3H]diazepam | 8.0 ± 0.8 | [98] | |||
| 122 | Angelica dahurica | benzodiazepine | [3H]diazepam | 0.36 ± 0.03 | [98] | |||
| 123 | Angelica dahurica | benzodiazepine | [3H]diazepam | 12 ± 3 | [98] | |||
| 154 | benzodiazepine | [3H]flunitrazepam | 74.44 | [120] | ||||
| 156 | benzodiazepine | [3H]flunitrazepam | 15.69 | [120] | ||||
| 157 | benzodiazepine | [3H]flunitrazepam | 90.02 | [120] | ||||
| 160 | benzodiazepine | [3H]flunitrazepam | 130.9 | [124] | ||||
| 181 | Cyperus rotundus | benzodiazepine | [3H]flunitrazepam | 105.0 ± 1.60 | [134] | |||
| 186 | Mentha aquatica | benzodiazepine | [3H]flumazenil | 190,000 | [78] | |||
| 199 | Salvia miltiorrhiza | benzodiazepine | [3H]flunitrazepam | 0.3 | [146] | |||
| 200 | Salvia officinalis | benzodiazepine | [3H]flumazenil | 7.2 ± 0.7 | [65] | |||
| 201 | Salvia officinalis | benzodiazepine | [3H]flumazenil | 0.8 ± 0.1 | [65] | |||
| 205 | Aloysia virgata | benzodiazepine | [3H]flumazenil | 111 ± 13 | [150] | |||
| 206 | Aloysia virgata | benzodiazepine | [3H]flumazenil | 56 ± 19 | [150] | |||
| 2 | Colchicum szovitsii | TBPS/picrotoxin | [35S]TBPS | 25% of 10 µM allopregnanolone | 10 | [22] | ||
| 3 | Colchicum szovitsii | TBPS/picrotoxin | [35S]TBPS | 25% of 10 µM allopregnanolone | 10 | [22] | ||
| 7 | Corydalis cava | TBPS/picrotoxin | [3H]bicuculline | 121 ± 2 | 0.1 | [26] | ||
| 8 | Corydalis cava | TBPS/picrotoxin | [3H]bicuculline | 149 ± 2 | 0.01 | [26] | ||
| 9 | Corydalis cava | TBPS/picrotoxin | [3H]bicuculline | 130 ± 3 | 0.1 | [26] | ||
| 10 | Corydalis cava | TBPS/picrotoxin | [3H]bicuculline | 146 ± 7 | 0.01 | [26] | ||
| 11 | Corydalis cava | TBPS/picrotoxin | [3H]bicuculline | 147 ± 3 | 0.1 | [26] | ||
| 24 | Cicuta virosa | TBPS/picrotoxin | [3H]EBOB | 0.54 ± 0.03 | [37] | |||
| 25 | Cicuta virosa | TBPS/picrotoxin | [3H]EBOB | 2.01 ± 0.09 | [37] | |||
| 26 | Cicuta virosa | TBPS/picrotoxin | [3H]EBOB | 1.15 ± 0.09 | [37] | |||
| 27 | Cicuta virosa | TBPS/picrotoxin | [3H]EBOB | 6.01 ± 0.29 | [37] | |||
| 28 | Cicuta virosa | TBPS/picrotoxin | [3H]EBOB | 7.87 ± 0.83 | [37] | |||
| 65 | Valeriana jatamansi | TBPS/picrotoxin | [35S]TBPS | 0.50 ± 0.17 | [63] | |||
| 79 | Rhus parviflora | TBPS/picrotoxin | [35S]TBPS | 0.149 | 0.091 | [75] | ||
| 80 | Rhus parviflora | TBPS/picrotoxin | [35S]TBPS | 0.073 | 0.045 | [75] | ||
| 81 | Rhus parviflora | TBPS/picrotoxin | [35S]TBPS | 0.455 | 0.280 | [75] | ||
| 145 | Ecklonia cava | TBPS/picrotoxin | [35S]TBPS | 7.180 | 4.419 | [80] | ||
| 146 | Ecklonia cava | TBPS/picrotoxin | [35S]TBPS | 1.739 | 1.070 | [80] | ||
| 147 | Ecklonia cava | TBPS/picrotoxin | [35S]TBPS | 4.991 | 3.072 | [80] | ||
| 148 | Ecklonia cava | TBPS/picrotoxin | [35S]TBPS | 2.422 | 1.491 | [80] | ||
| 154 | TBPS/picrotoxin | [35S]TBPS | 13 ± 4 | [117] | ||||
| 155 | TBPS/picrotoxin | [35S]TBPS | 37 ± 8 | [117] | ||||
| 197 | Illicium anisatum | TBPS/picrotoxin | [3H]EBOB | 0.43 | [144] | |||
| 212 | Ginkgo biloba | TBPS/picrotoxin | [35S]TBPS | 39 | [154] |
| Cmpd | Anxiety/Stress | Sedation | Convulsions | Myorelaxation | Analgesia | Ref. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC | FP | EPM | OF | HB | LD | MB | FS | TS | SIH | LMA | PIS | EIS | MES | PTZ | PTX | RR | HW | TF | TI | ||
| 11 | 0.5 | 50 | 50 | [27] | |||||||||||||||||
| 12 | 5 | [28] | |||||||||||||||||||
| 13 | 5 | [28] | |||||||||||||||||||
| 14 | 2.5 | [28] | |||||||||||||||||||
| 15 | 10 | – | – | – | [29] | ||||||||||||||||
| 19 | 0.25 | [32] | |||||||||||||||||||
| 21 | + | + | + | [34] | |||||||||||||||||
| 45 | 1 | – | 3 | + | – | – | 25 | [50,51,52,53] | |||||||||||||
| 46 | 7.5 | 7.5 | – | 5 | 5 | – | – | [48,49] | |||||||||||||
| 54 | 10 | + | + | – | [43,44] | ||||||||||||||||
| 55 | 10 | [43] | |||||||||||||||||||
| 56 | – | – | – | – | – | [47] | |||||||||||||||
| 63 | 0.5 | 50 | 30 | 25 | 0.6 | 25 | – | [55,58,61] | |||||||||||||
| 65 | 1 | – | [64] | ||||||||||||||||||
| 68 | 2 | [67] | |||||||||||||||||||
| 72 | 0.2 | – | [69] | ||||||||||||||||||
| 73 | 0.5 | [70] | |||||||||||||||||||
| 75 | 50 | [71] | |||||||||||||||||||
| 76 | 100 | [72] | |||||||||||||||||||
| 77 | 2.5 | 5 | 5 | [73] | |||||||||||||||||
| 80 | 12.5 | [75] | |||||||||||||||||||
| 84 | 5 | [77] | |||||||||||||||||||
| 86 | 1 | [64] | |||||||||||||||||||
| 98 | 25 | [84] | |||||||||||||||||||
| 103 | 20 | 0.1 | [89,90] | ||||||||||||||||||
| 105 | 0.05 | [92] | |||||||||||||||||||
| 107 | 3 | [93] | |||||||||||||||||||
| 109 | 4 | 4 | [94] | ||||||||||||||||||
| 120 | 5 | [97] | |||||||||||||||||||
| 121 | 5 | 5 | 5 | 300 | [102,103] | ||||||||||||||||
| 125 | 5 | 5 | 5 | [103] | |||||||||||||||||
| 127 | 259 | [101] | |||||||||||||||||||
| 130 | 150 | [105] | |||||||||||||||||||
| 142 | 50 | [111] | |||||||||||||||||||
| 143 | 300 | 100 | 100 | 100 | [112] | ||||||||||||||||
| 144 | 2.5 | 2.5 | 5 | [113] | |||||||||||||||||
| 149 | 5 | 50 | [116] | ||||||||||||||||||
| 158 | 200 | 300 | 200 | [121] | |||||||||||||||||
| 159 | 72.75 | [122] | |||||||||||||||||||
| 163 | 25 | 25 | 25 | 25 | [125] | ||||||||||||||||
| 171 | [126] | ||||||||||||||||||||
| 172 | 25 | 25 | 25 | 75 | [127,128] | ||||||||||||||||
| 173 | 25 | 25 | 25 | 25 | – | [129] | |||||||||||||||
| 174 | 400 | 100 | 200 | [130] | |||||||||||||||||
| 180 | 100 | [107] | |||||||||||||||||||
| 182 | 3 | [137] | |||||||||||||||||||
| 185 | 10 | [138] | |||||||||||||||||||
| 187 | + | [139] | |||||||||||||||||||
| 199 | 10 | – | [146] | ||||||||||||||||||
| 205 | 0.3 | 1 | [150] | ||||||||||||||||||
| 206 | 1 | 0.3 | 1 | [150] | |||||||||||||||||
| 213 | + | [157] | |||||||||||||||||||
| 214 | 0.5 | – | [156] | ||||||||||||||||||
| 215 | + | [157] | |||||||||||||||||||
| 216 | + | [158] | |||||||||||||||||||
| 217 | 10 | 3 | [159] | ||||||||||||||||||
| 219 | 30 | [161] | |||||||||||||||||||
| 223 | 10 | 50 | [164] | ||||||||||||||||||
| 224 | 10 | [164] | |||||||||||||||||||
| 225 | 10 | [165] | |||||||||||||||||||
| 226 | 10 | [165] | |||||||||||||||||||
| 231 | 0.6 | 6 | 0.2 | 20 | [168] | ||||||||||||||||
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çiçek, S.S. Structure-Dependent Activity of Natural GABA(A) Receptor Modulators. Molecules 2018, 23, 1512. https://doi.org/10.3390/molecules23071512
Çiçek SS. Structure-Dependent Activity of Natural GABA(A) Receptor Modulators. Molecules. 2018; 23(7):1512. https://doi.org/10.3390/molecules23071512
Chicago/Turabian StyleÇiçek, Serhat Sezai. 2018. "Structure-Dependent Activity of Natural GABA(A) Receptor Modulators" Molecules 23, no. 7: 1512. https://doi.org/10.3390/molecules23071512
APA StyleÇiçek, S. S. (2018). Structure-Dependent Activity of Natural GABA(A) Receptor Modulators. Molecules, 23(7), 1512. https://doi.org/10.3390/molecules23071512






