Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of Nanoemulsion Components Based on the Solubility of Quercetin
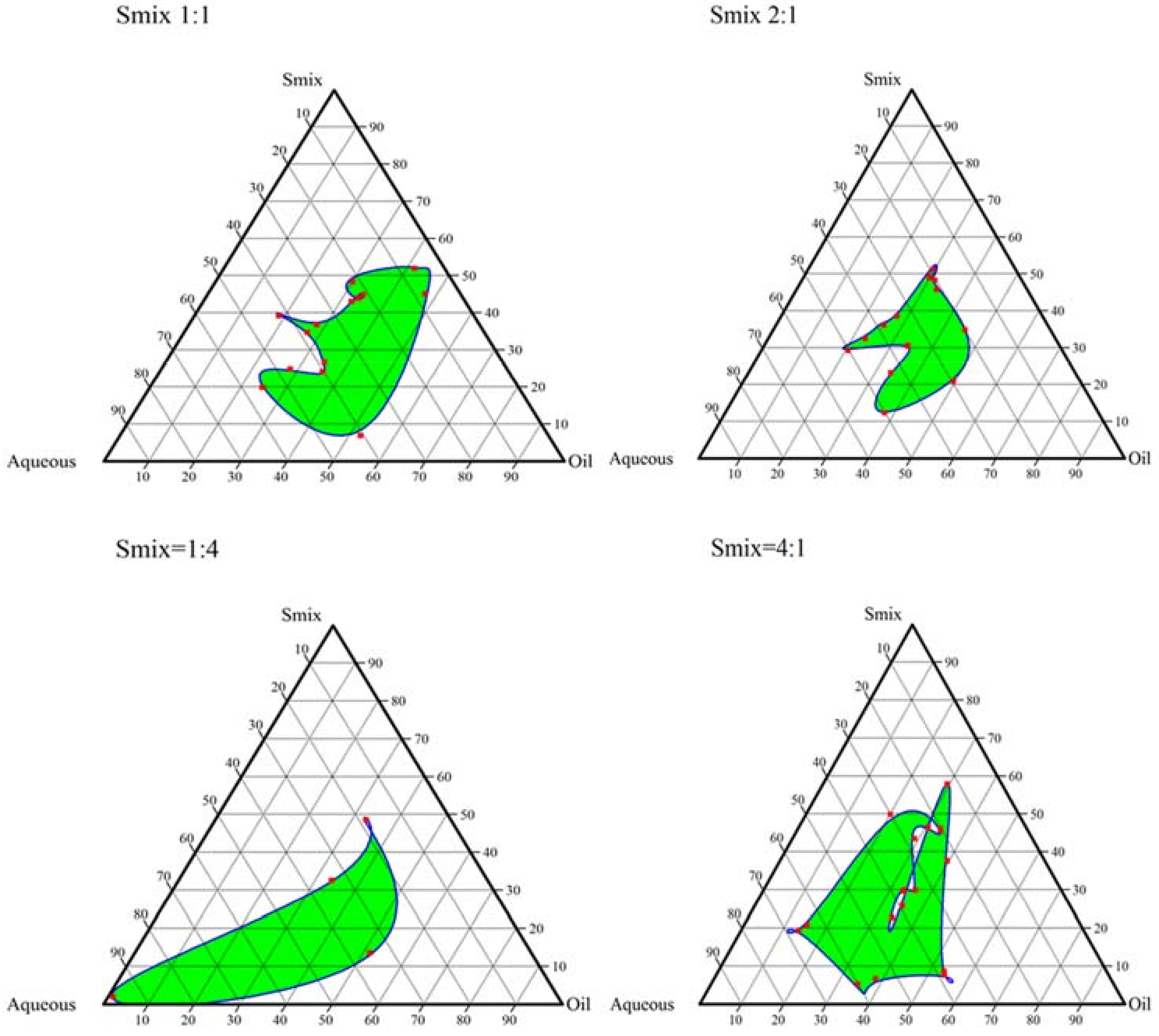
2.2. Construction of Pseudo-Ternary Phase Diagrams
2.3. Optimization of Quercetin Nanoemulsion
2.4. Thermodynamic Stability Testing of the Nanoemulsion
2.5. Characterization Results for Nanoemulsion
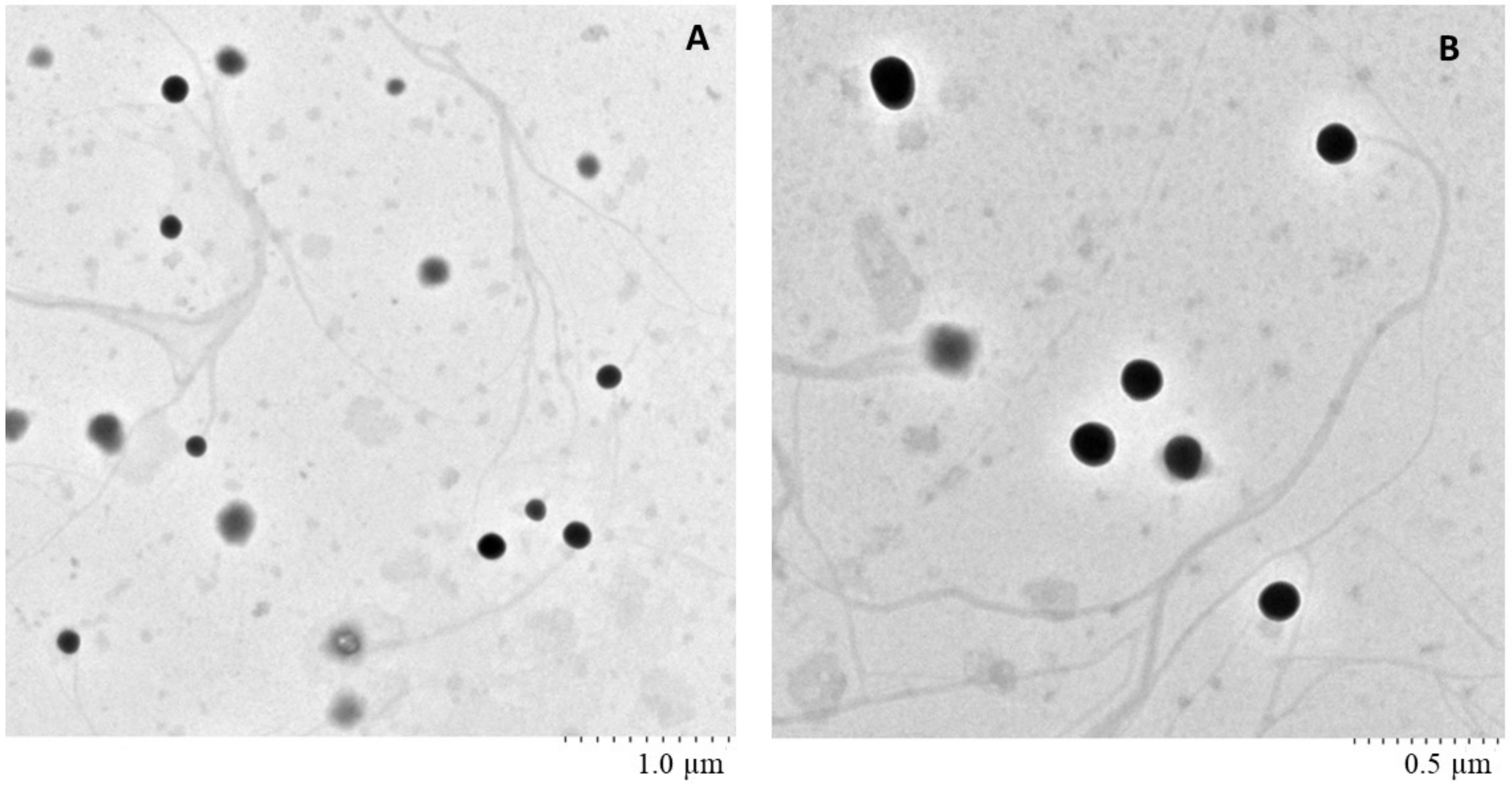
2.6. Surface Morphology of Nanoemulsion Using TEM
2.7. Preparation of Nanoemulgel
2.8. Characterization Results of Nanoemulgel
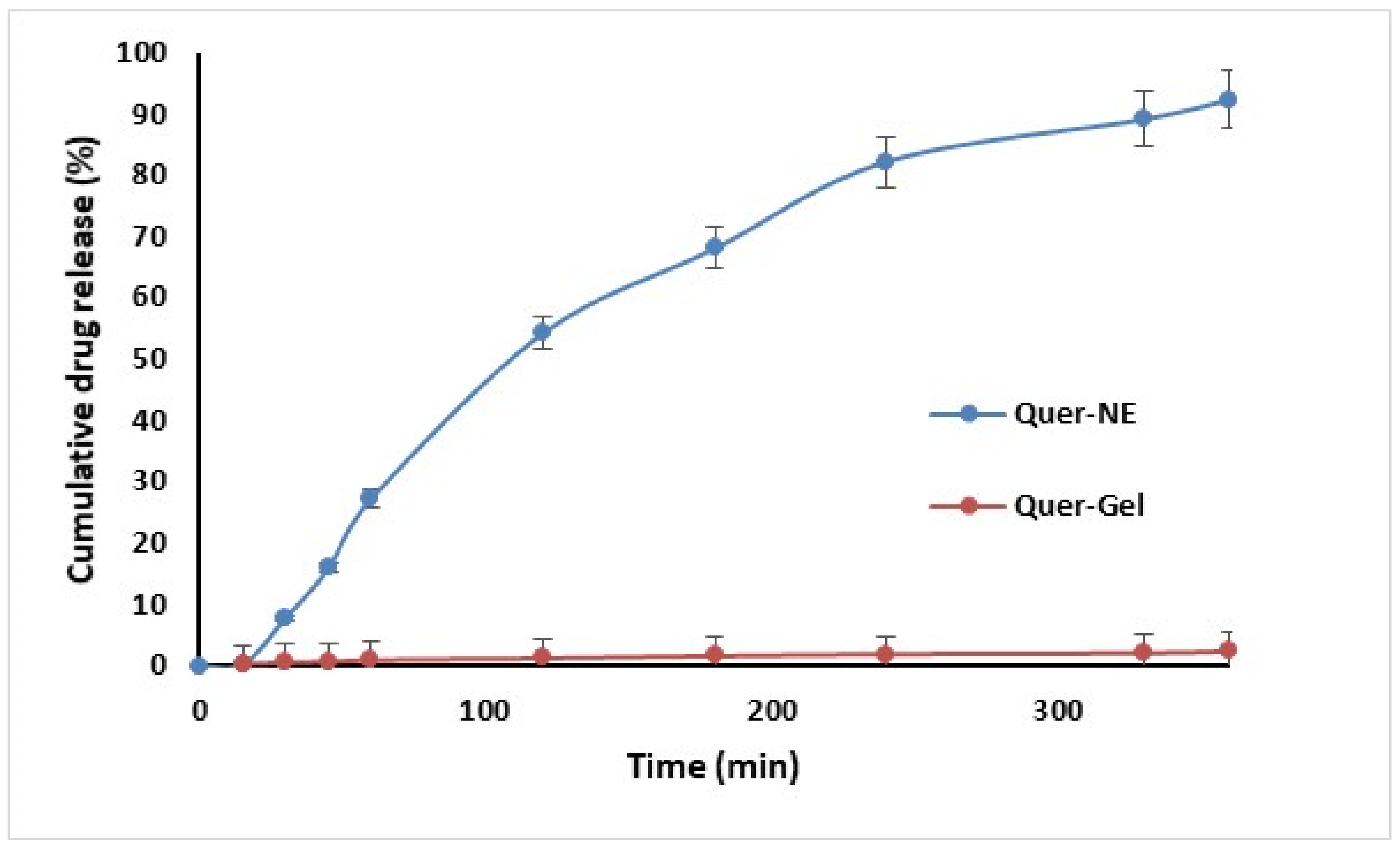
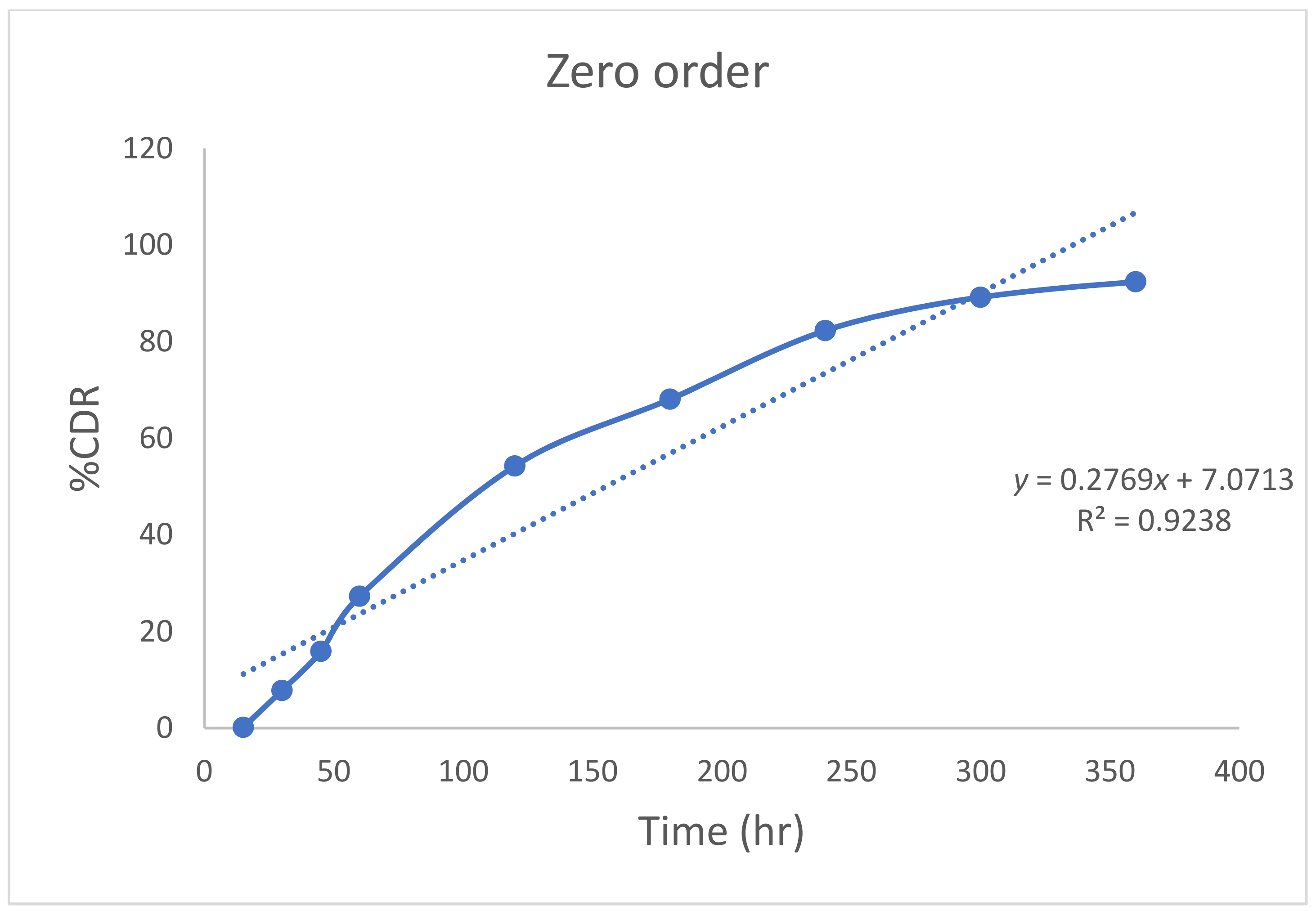
2.9. In Vitro Diffusion of Quercetin from Nanoemulgel
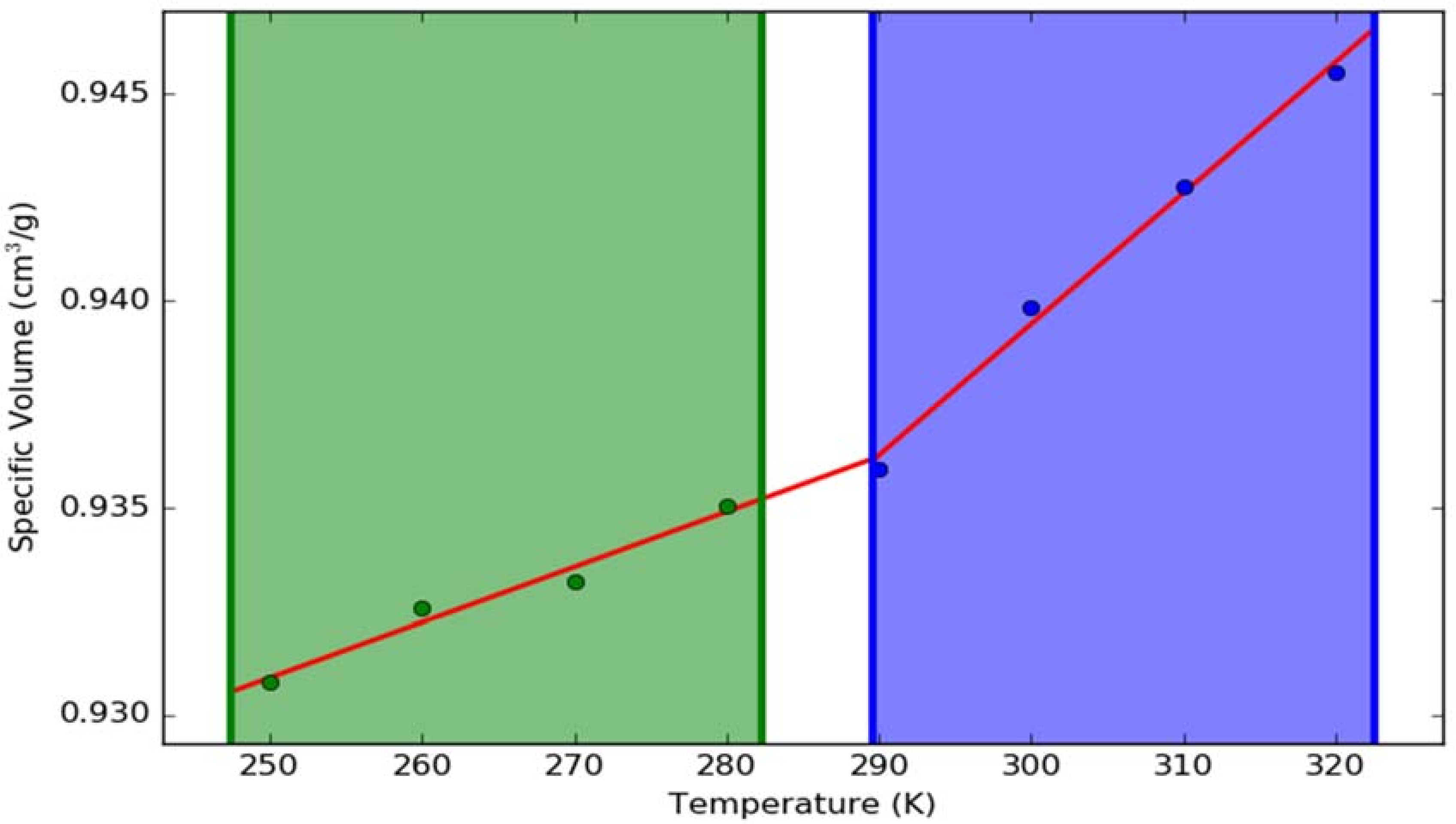
2.10. Stability Study Results
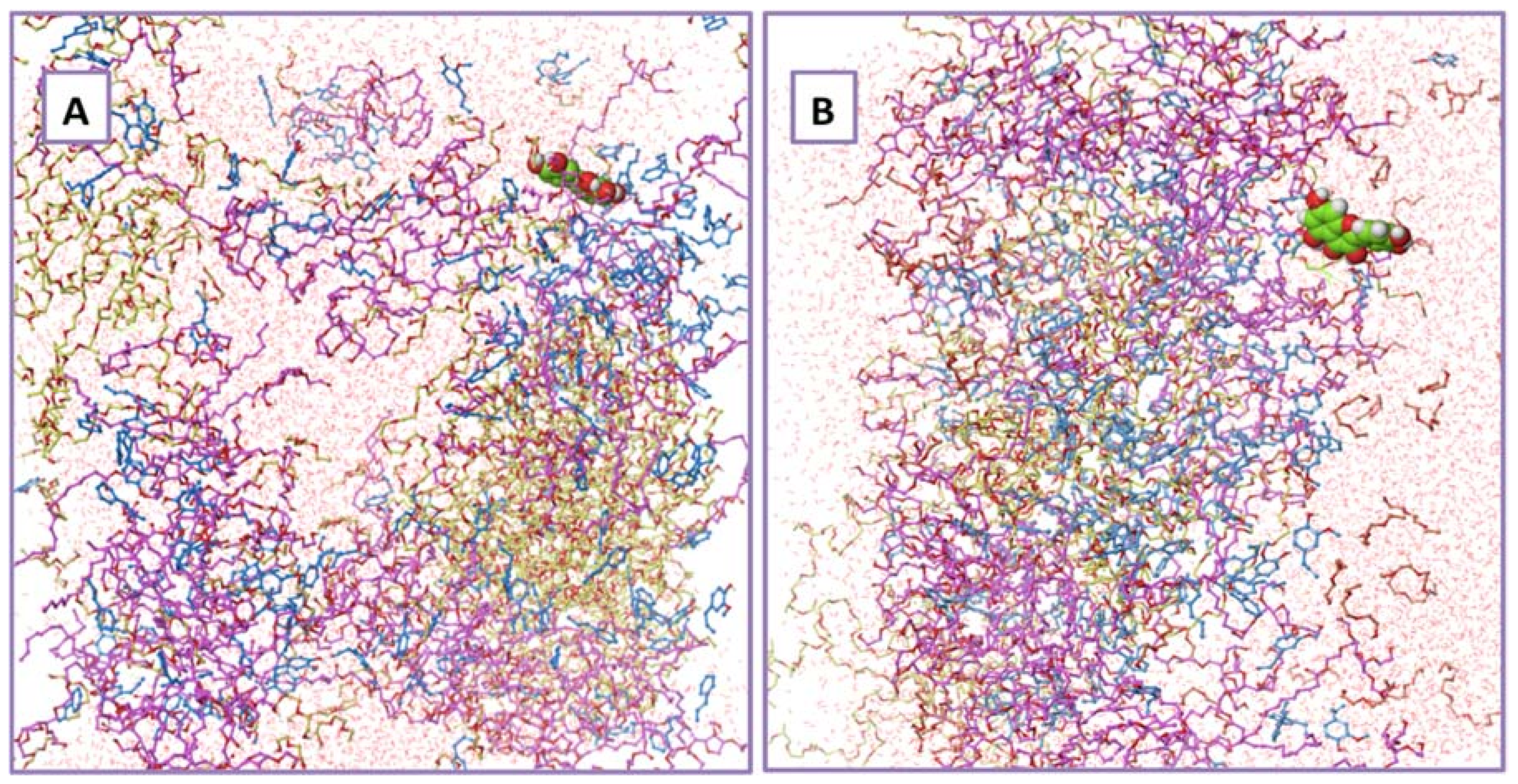
2.11. Results of Molecular Dynamic Simulations
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Screening of Nanoemulsion Components
3.2.2. Construction of Pseudo-Ternary Phase Diagrams
3.2.3. Preparation of Quercetin Nanoemulsion
3.2.4. Thermodynamic Stability Testing of the Nanoemulsion
3.2.5. Globule Size, Polydispersity Index (PDI) and Zeta Potential
3.2.6. Transmission Electron Microscopy (TEM)
3.2.7. Preparation of Nanoemulgel
3.2.8. Characterization of Nanoemulgel
- Sol-gel transition and syringeabilityThe nanoemulgel was observed for in situ gelation or sol-gel transition by the test-tube inverting method as per Srivastava et al. with slight modification [33]. 5 mL of sol stored at 2–8 °C was taken in a test tube, immersed in a water bath (Remi Equipment Ltd., Bangalore, India) maintained at a temperature of 37 °C. The sample was observed for gelation by tilting the test tubes at 90°. Gelation was said to have occurred when the meniscus would no longer move upon tilting and the time taken to gel was recorded. For testing syringeability, nanoemulsion stored at 2–8 °C was filled into a 1 mL syringe with a 22 gauge needle, and gentle force was applied by pressing the piston. The ease of syringeability was observed visually.
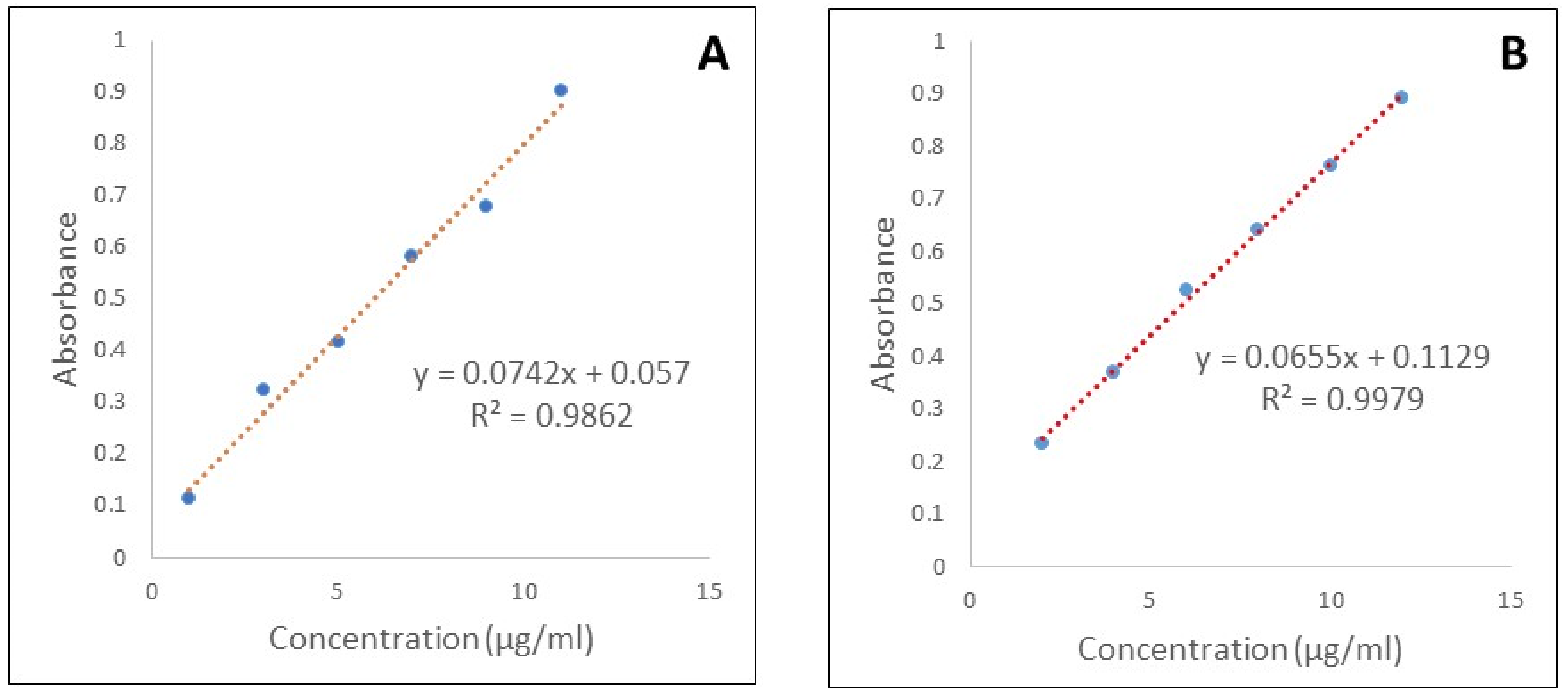
- Drug contentFor determining the drug content, 10 mg of the Quercetin nanoemulgel formulation was weighed and dissolved in phosphate buffer pH 7.4. The solution was filtered using Whatman filter paper (No. 41) and suitably diluted. The absorbance of the resulting solution was measured by UV spectrophotometry (UV-160 Shimadzu Corporation, Kyoto, Japan) at 269.2 nm and the drug content was determined.
- pH measurementThe pH of the nanoemulgel was read using a pH paper owing to the consistency of the formulation.
- Viscosity determinationThe viscosity was determined using Brookfield DV III ultra-programmable rheometer (Brookfield Engineering Laboratories, Middleboro, MA, USA). The viscosity of Quercetin nanoemulgel was measured at 26 °C and 37 °C, respectively. The instrument was calibrated using spindle 40 with viscosity standard fluid prior to the sample measurements.
3.2.9. In Vitro Diffusion Study and Release Kinetics
3.2.10. Stability Study
3.2.11. Molecular Dynamics (MD) Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Javed, S.; Kohli, K. Local delivery of minocycline hydrochloride: A therapeutic paradigm in periodontal diseases. Curr. Drug Deliv. 2010, 7, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Sihorkar, V.; Mishra, V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J. Clin. Pharm. Ther. 2000, 25, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 1994, 5, 78–111. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Novak, M.J. Host mediators in gingival crevicular fluid: Implications for the pathogenesis of periodontal disease. Crit. Rev. Oral Biol. Med. 1992, 3, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E.; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- De Pablo, P.; Chapple, I.L.C.; Buckley, C.D.; Dietrich, T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Devanoorkar, A.; Kathariya, R.; Guttiganur, N.; Gopalakrishnan, D.; Bagchi, P. Resistin: A potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis. Dis. Markers 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.-F.; Newman, M.G.; Kwok, Y.-Y.; Horikoshi, A.K. Effect of Chinese and western antimicrobial agents on selected oral bacteria. J. Dent. Res. 1982, 61, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Khalessi, A.M.; Pack, A.R.C.; Thomson, W.M.; Tompkins, G.R. An in vivo study of the plaque control efficacy of PersicaTM: A commercially available herbal mouthwash containing extracts of Salvadora persica. Int. Dent. J. 2004, 54, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Gulzar Ahmed, M.; Choudhari, R.; Acharya, A. Formulation and evaluation of in situ gel of atorvastatin for the treatment of periodontitis. RGUHS J. Pharm. Sci. 2015, 5, 53–60. [Google Scholar] [CrossRef]
- Ranjan, R.; Patil, S.R.; Veena, H.R. Effect of in-situ application of simvastatin gel in surgical management of osseous defects in chronic periodontitis—A randomized clinical trial. J. Oral Biol. Craniofac. Res. 2017, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Warade, S.; Lahoti, S. In situ gel formulation of ornidazole for the treatment of periodontal disease. Curr. Pharma Res. 2010, 1, 60–69. [Google Scholar]
- Sapra, P.; Patel, D.; Soniwala, M.; Chavda, J. Development and optimization of in situ periodontal gel containing Levofloxacin for the treatment of periodontal diseases. J. Sci. Innov. Res. JSIR 2013, 2, 607–626. [Google Scholar]
- Aiswarya, G.; Reza, K.; Rajan, R. Development, evaluation, and optimization of flurbiprofen nanoemulsions gel using quality by design concept. Asian J. Pharm. 2015, 9, 35–43. [Google Scholar] [CrossRef]
- Geoghegan, F.; Wong, R.W.K.; Rabie, A.B.M. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phyther. Res. 2009, 24, 817–820. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration: Effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [PubMed]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Chabane, M.N.; Ahmad, A.A.; Peluso, J.; Muller, C.D.; Ubeaud, G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2009, 61, 1473–1483. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Ma, Y.; Yu, A.; Cai, F.; Shao, W.; Zhai, G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf. B Biointerfaces 2009, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Choi, J.M.; Cho, E.; Jeong, D.; Shinde, V.V.; Kim, H.; Choi, Y.; Jung, S. Enhancement of solubility and bioavailability of quercetin by inclusion complexation with the cavity of mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Bull. Korean Chem. Soc. 2017, 38, 880–889. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J. Agric. Food Chem. 2002, 50, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Joshi, J.R. An overview on nanoemulsion: A novel approach. Int. J. Pharm. Sci. Res. 2012, 3, 4640–4650. [Google Scholar]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion complexation of etodolac with hydroxypropyl-beta-cyclodextrin and auxiliary agents: Formulation characterization and molecular modeling studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Thorat, S.; Nayak, U.; Sherje, A.; Murahari, M. Host-guest interaction study of Efavirenz with hydroxypropyl-β-cyclodextrin and l-arginine by computational simulation studies: Preparation and characterization of supramolecular complexes. J. Mol. Liquids 2018, 259, 55–64. [Google Scholar] [CrossRef]
- Ali, H.H.; Hussein, A.A. Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. AAPS Open 2017, 3, 4. [Google Scholar] [CrossRef]
- Chopra, M.; Nayak, U.Y.; Kumar Gurram, A.; Sreenivasa Reddy, M.; Koteshwara, K.B. Formulation, characterization and in vivo evaluation of self-nanoemulsifying drug delivery system for oral delivery of valsartan. Curr. Nanosci. 2014, 10, 263–270. [Google Scholar] [CrossRef]
- Ahmad, J.; Mir, S.R.; Kohli, K.; Chuttani, K.; Mishra, A.K.; Panda, A.K.; Amin, S. Solid-nanoemulsion preconcentrate for oral delivery of paclitaxel: formulation design, biodistribution, and γ scintigraphy imaging. Biomed. Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Kohli, K.; Ali, M. Formulation development of novel in situ nanoemulgel (NEG) of ketoprofen for the treatment of periodontitis. Drug Deliv. 2016, 23, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Neupane, Y.R.; Kumar, P.; Kohli, K. Nanoemulgel (NEG) of Ketoprofen with eugenol as oil phase for the treatment of ligature-induced experimental periodontitis in Wistar rats. Drug Deliv. 2016, 23, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Garala, K.; Joshi, P.; Patel, J.; Ramkishan, A.; Shah, M. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, S.; Sharma, S.; Deshpande, R.D.; Sharma, R.K. Preparation and optimization of nanoemulsions for targeting drug delivery. Int. J. Drug Dev. Res. 2013, 5, 37–48. [Google Scholar]
- Patil, A.; Mahale, S.; Joshi, C.; Karde, P.; Vaidya, P. Honey as a Potential Antimicrobial Agent against P. gingivalis. Int. J. Contemp. Med. Res. 2016, 3, 2697–2700. [Google Scholar]
- Nayak, U.Y.; Gopal, S.; Mutalik, S.; Ranjith, A.K.; Reddy, M.S.; Gupta, P.; Udupa, N. Glutaraldehyde cross-linked chitosan microspheres for controlled delivery of Zidovudine. J. Microencapsul. 2009, 26, 214–222. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Oil (mg) | Smix (mg) | Water (mg) | Water Added | Total (mg) | Appearance | Formulation | Oil (%) | Smix (%) | Water (%) |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 180 | 30 | 30 | 230 | Transparent, easily flowable | Nanoemulsion | 8.69 | 78.2 | 13.04 |
| 20 | 180 | 90 | 60 | 290 | Transparent, easily flowable | Nanoemulsion | 6.89 | 62.0 | 31.03 |
| 20 | 180 | 190 | 100 | 390 | Transparent, easily flowable | Nanoemulsion | 5.12 | 46.15 | 48.71 |
| 20 | 180 | 390 | 200 | 590 | Transparent, easily flowable | Nanoemulsion | 3.38 | 30.50 | 66.10 |
| 20 | 180 | 590 | 200 | 790 | Transparent, easily flowable | Nanoemulsion | 2.53 | 22.78 | 74.68 |
| 20 | 180 | 1090 | 500 | 1290 | Transparent, easily flowable | Nanoemulsion | 1.55 | 13.95 | 84.49 |
| 20 | 180 | 1590 | 500 | 1790 | Transparent, easily flowable | Nanoemulsion | 1.11 | 10.05 | 88.82 |
| Q (µg/mL) | 100 | 50 | 25 | 12.6 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tf | S | S | R | R | R | R | R | R | R | R |
| Pg | S | R | R | R | R | R | R | R | R | R |
| Sr. No. | Sample Name | Z Average Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| 1 | Smix ratio 5:1 Oil: Smix 1:7 | 153.51 ± 8.23 | 0.60 ± 0.03 | −3.03 ± 0.02 |
| 2 | Smix ratio 5:1 Oil: Smix 1:9 | 138.90 ± 10.14 | 0.56 ± 0.04 | −3.58 ± 0.01 |
| 3 | Smix ratio 4:1 Oil: Smix 1:7 | 136.05 ± 10.17 | 0.48 ± 0.07 | −9.20 ± 0.02 |
| 4 | Smix ratio 4:1 Oil: Smix 1:9 | 125.60 ± 9.42 | 0.53 ± 0.10 | −12.10 ± 0.07 |
| 5 | Smix ratio 3:1 Oil: Smix 1:7 | 115.20 ± 8.31 | 0.47 ± 0.05 | −3.16 ± 0.02 |
| 6 | Smix ratio 3:1 Oil: Smix 1:9 | 104.33 ± 6.28 | 0.39 ± 0.12 | −4.03 ± 0.04 |
| Days | Drug Content | pH | Colour |
|---|---|---|---|
| 0 | 99.98 ± 0.24 | 6.5 | Pale yellow |
| 5 | 99.72 ± 0.40 | 6.5 | Pale yellow |
| 30 | 98.98 ± 0.78 | 6.5 | Pale yellow |
| 60 | 98.12 ± 1.1 | 6.5 | Pale yellow |
| 90 | 97.0 ± 1.5 | 6.5 | Pale yellow |
| Property | At 275 K | At 313 K |
|---|---|---|
| Density | 1065 ± 4 kg/m3 | 1060 ± 6 kg/m3 |
| No. of Hydrogen bonds between formulation components and solvent | 991 ± 20 | 1200 ± 21 |
| Diffusion coefficient of solvent | 1.2504 × 10−9 m2/s | 2.8332 × 10−9 m2/s |
| Diffusion coefficient of formulation components | 1.0746 × 10−10 m2/s | 2.4066 × 10−10 m2/s |
| Radii of gyration of Poloxamer 407 molecules | 29.45 ± 2.2 | 31.72 ± 4.3 |
| Appearance | Attributed Formulation |
|---|---|
| Transparent and easily flowable | Nanoemulsion |
| Transparent and difficult to flow | Nanoemulgel |
| Milky/Cloudy and easily flowable | Emulsion |
| Milky/Cloudy and difficult to flow | Emulgel |
| Component | No. of Molecules | Molecular Mass | Composition in mg |
|---|---|---|---|
| Cinnamon Oil | 34 | 282.38 | 125.00 |
| Quercetin | 1 | 302.24 | 4.68 |
| Tween 80 | 55 | 1310.00 | 1125.00 |
| Carbitol® | 108 | 134.17 | 225.00 |
| Poloxamer 407 | 3 | 31,057.17 | 1380.00 |
| Water | 10,000 | 18.01 | 6000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules 2018, 23, 1363. https://doi.org/10.3390/molecules23061363
Aithal GC, Nayak UY, Mehta C, Narayan R, Gopalkrishna P, Pandiyan S, Garg S. Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules. 2018; 23(6):1363. https://doi.org/10.3390/molecules23061363
Chicago/Turabian StyleAithal, Gururaj C., Usha Yogendra Nayak, Chetan Mehta, Reema Narayan, Pratibha Gopalkrishna, Sudharsan Pandiyan, and Sanjay Garg. 2018. "Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations" Molecules 23, no. 6: 1363. https://doi.org/10.3390/molecules23061363
APA StyleAithal, G. C., Nayak, U. Y., Mehta, C., Narayan, R., Gopalkrishna, P., Pandiyan, S., & Garg, S. (2018). Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules, 23(6), 1363. https://doi.org/10.3390/molecules23061363








