Tetragonia tetragonioides (Pall.) Kuntze Regulates Androgen Production in a Letrozole-Induced Polycystic Ovary Syndrome Model
Abstract
1. Introduction
2. Results
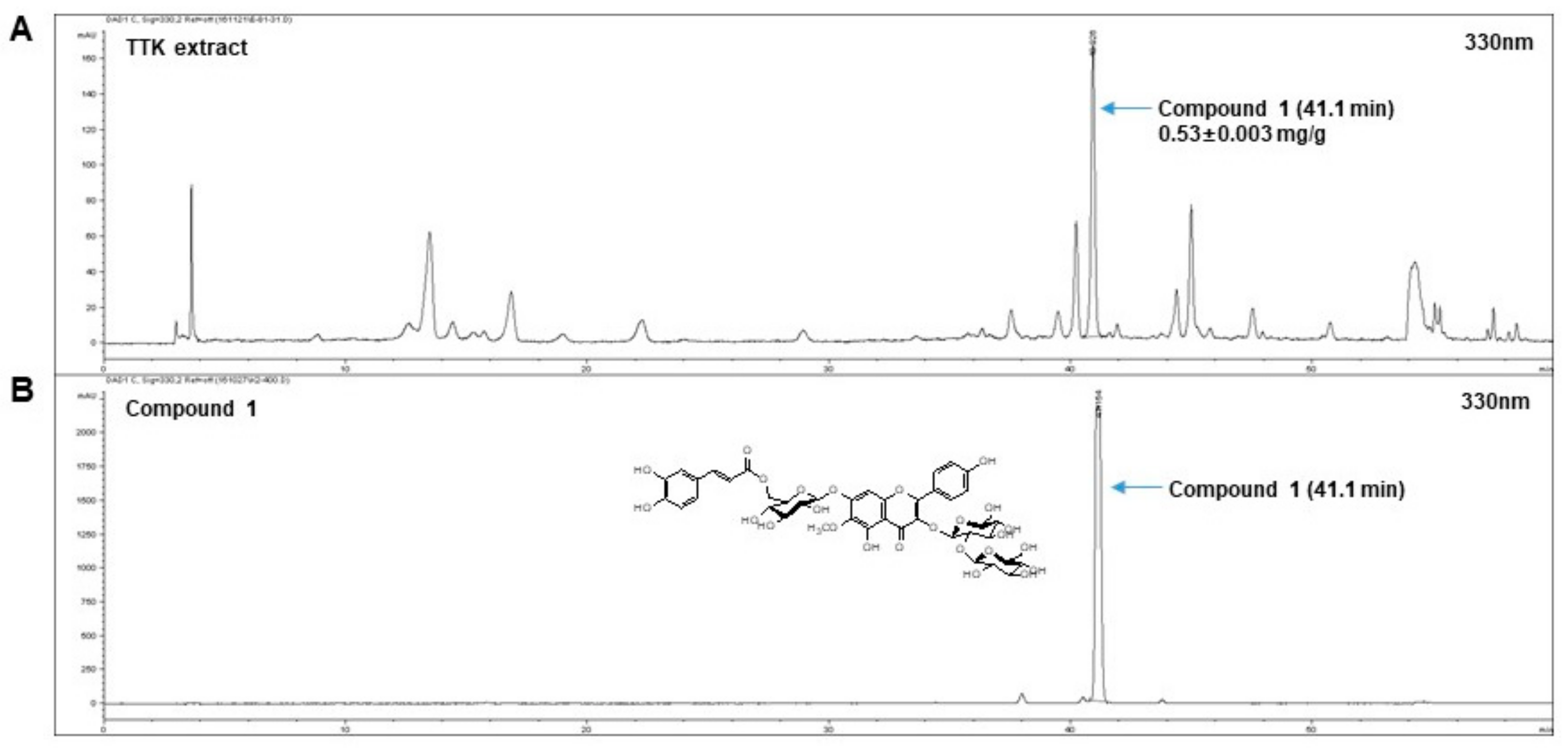
2.1. Quantitative Analysis of Marker Compounds in TTK Extract
2.2. Cytotoxicity on NCI-H295R Cells of FOR and TTK Extract
2.3. TTK Extract Inhibits FOR-Induced DHEA and Testosterone Production in NCI-H295R Cells
2.4. TTK Extract Inhibits Folliculogenesis in Letrozole-Induced PCOS Rats
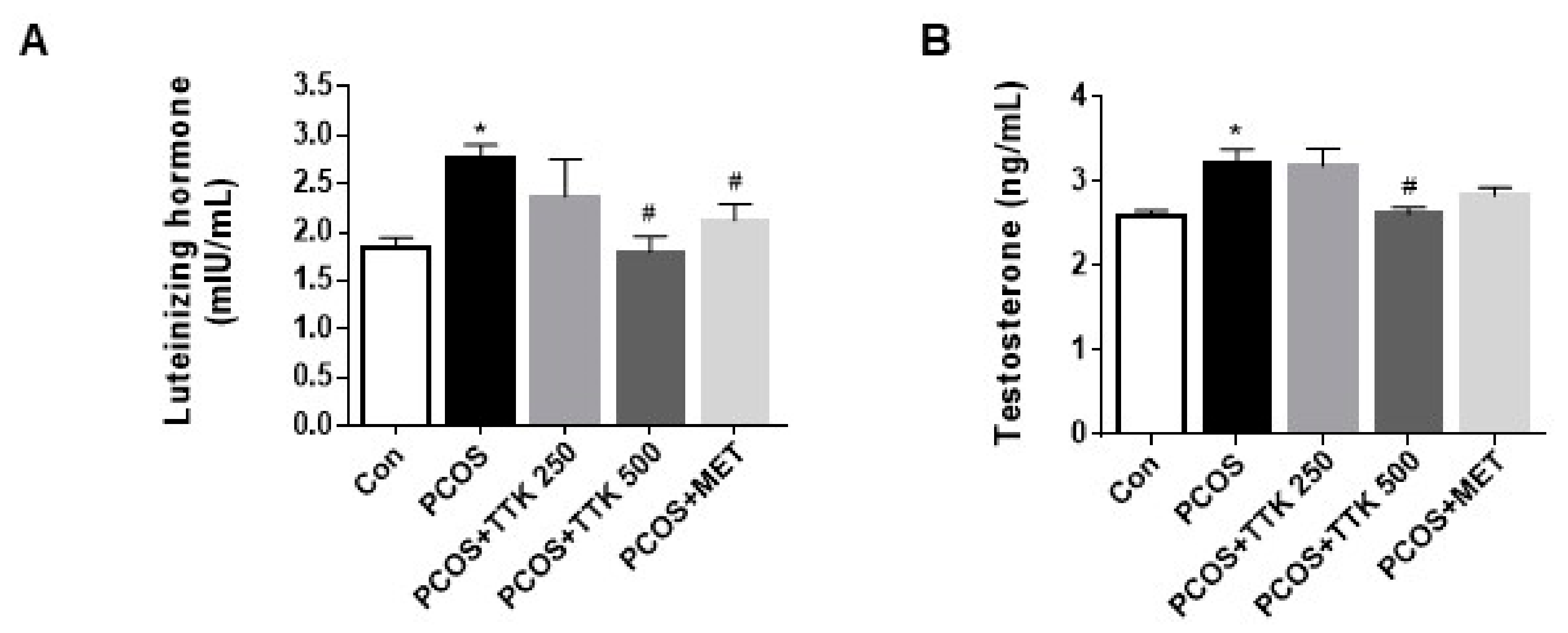
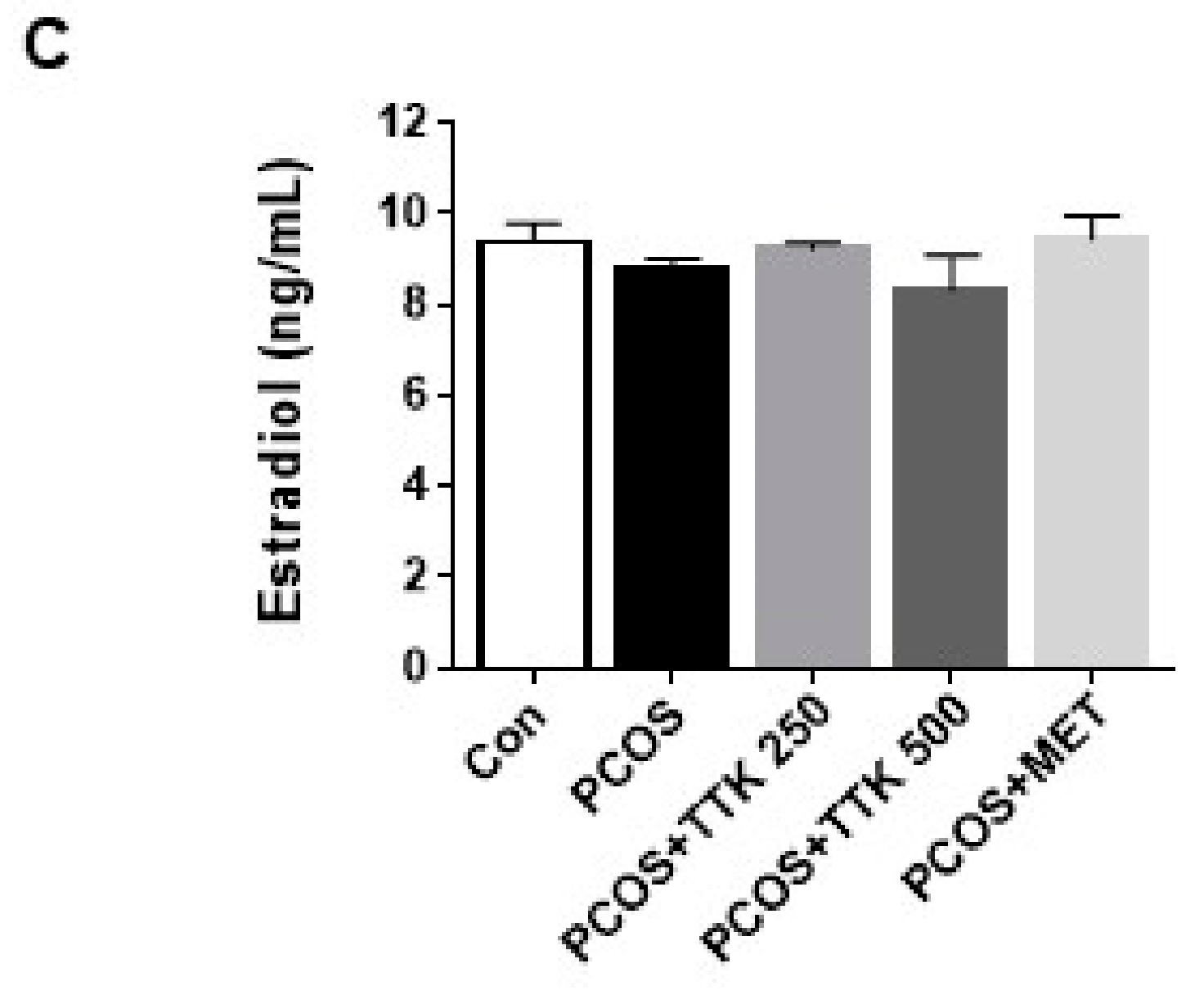
2.5. TTK Extract Inhibits Serum LH, Testosterone, and E2 Level(s) in Letrozole-Induced PCOS Rats
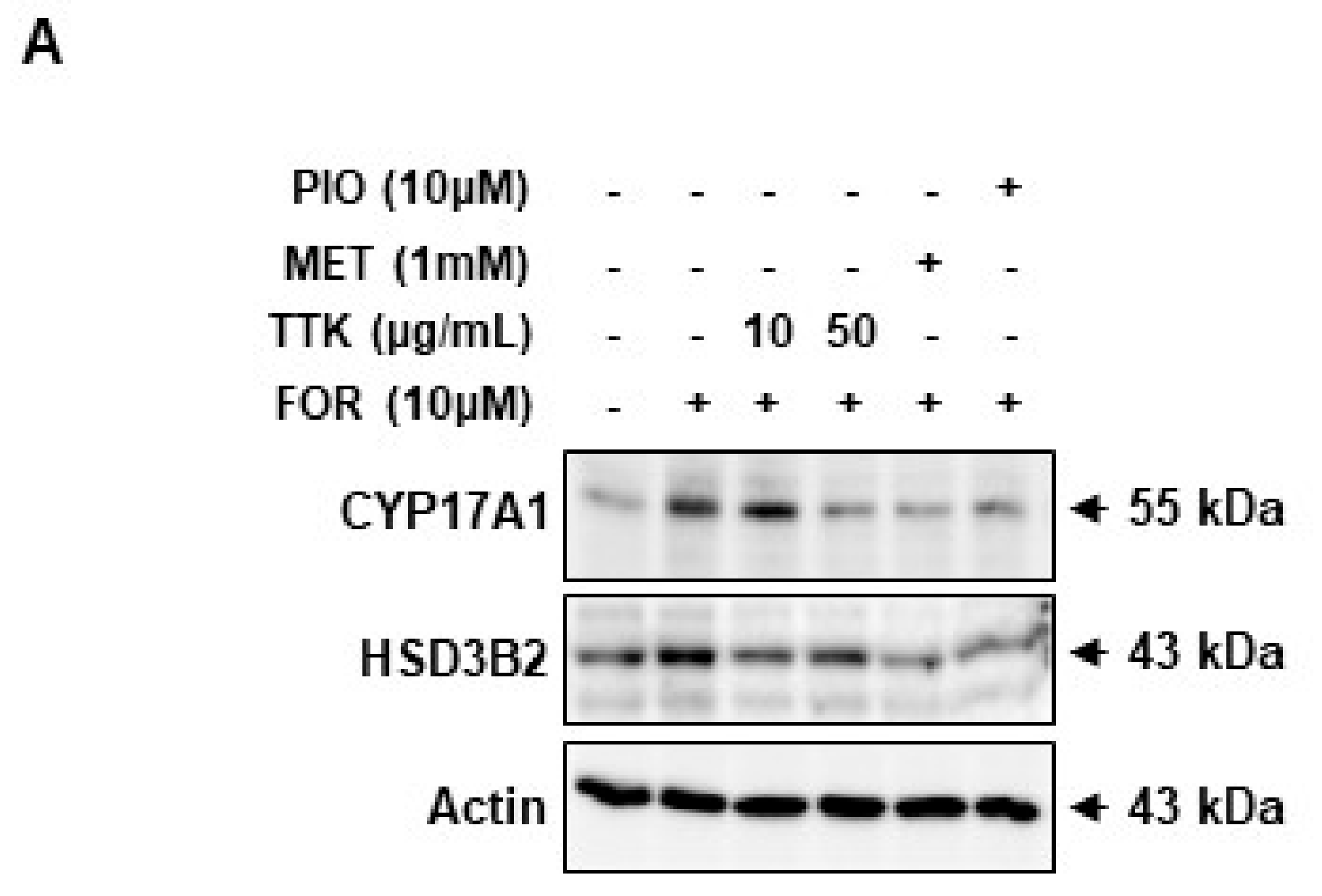
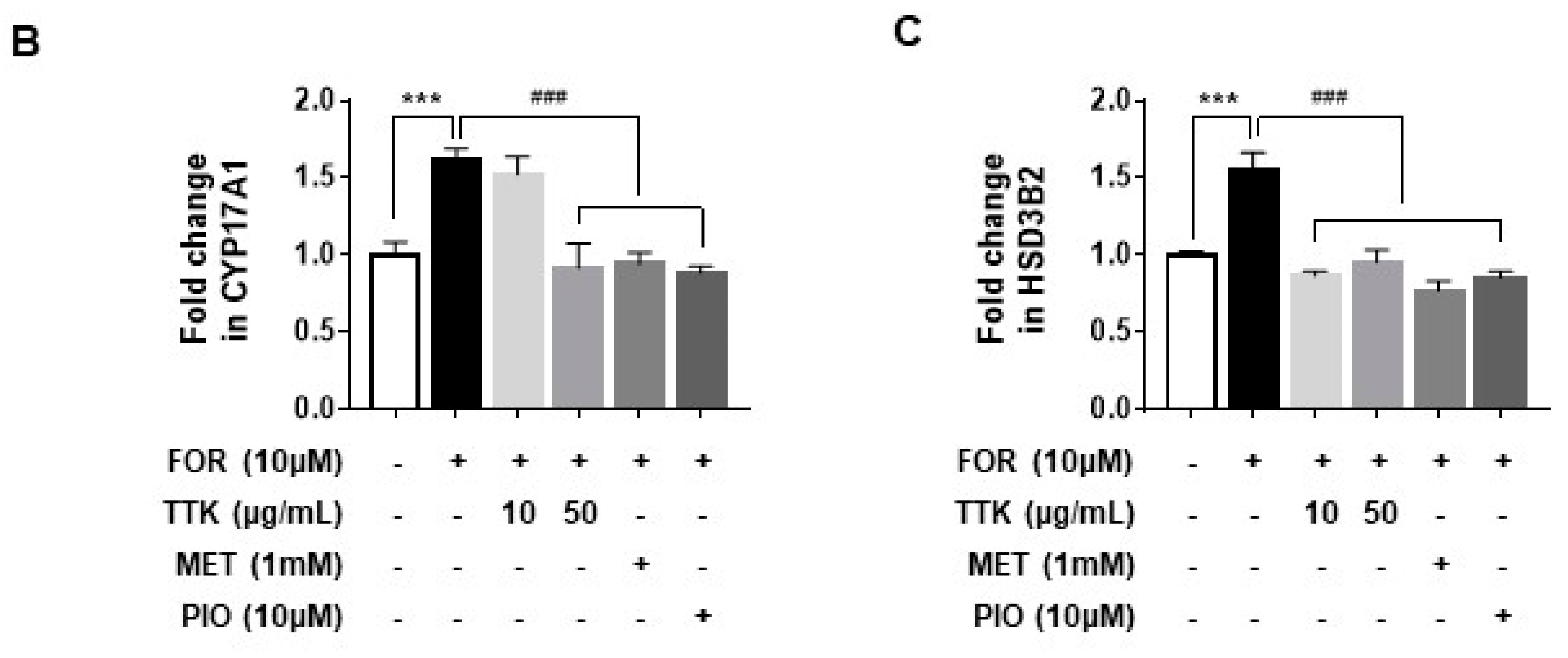
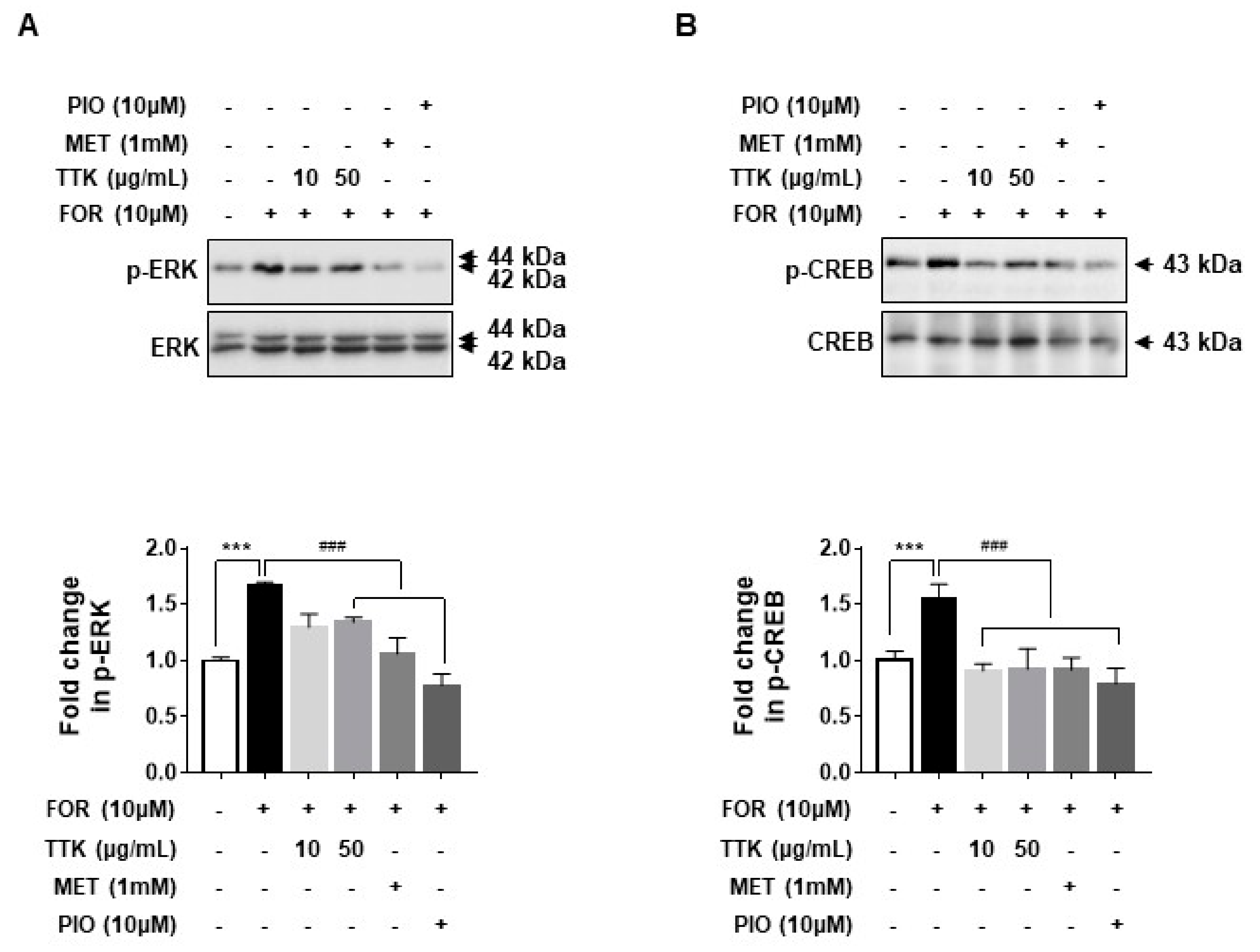
2.6. TTK Extract-Mediated Inhibition of FOR-Induced DHEA or Testosterone Production in NCI-H295R Cells Involves CYP17A1 and HSD3B2 Enzymes via the ERK-CREB Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation and Fingerprinting Analysis of TTK Extract
4.3. Cell Culture and Reagents
4.4. Cell Proliferation
4.5. DHEA and Testosterone Measurements
4.6. Experimental Animals and Treatments
4.7. Histopathological Analysis
4.8. Serum Hormone Analysis
4.9. Determination of Protein Levels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TTK | Tetragonia tetragonioides (Pall.) Kuntze; |
| PCOS | Polycystic ovary syndrome; |
| NCI-H295R cells | NCI-H295R human adrenocortical cells; |
| DHEA | dehydroepiandrosterone; |
| FOR | forskolin; |
| CYP17A1 | 17α-hydroxylase/17,20-lyase; |
| HSD3B2 | 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase type 2; |
| LH | luteinizing hormone; |
| E2 | 17β-estradiol; |
| ERK | extracellular signal-regulated kinase; |
| CREB | cAMP response element-binding protein |
References
- Lee, B.H.; Indran, I.R.; Tan, H.M.; Li, Y.; Zhang, Z.; Li, J.; Yong, E.L. A Dietary Medium-Chain Fatty Acid, Decanoic Acid, Inhibits Recruitment of Nur77 to the HSD3B2 Promoter In Vitro and Reverses Endocrine and Metabolic Abnormalities in a Rat Model of Polycystic Ovary Syndrome. Endocrinology 2016, 157, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Magoffin, D.; Munir, I.; Azziz, R. Effect of insulin and testosterone on androgen production and transcription of SULT2A1 in the NCI-H295R adrenocortical cell line. Fertil. Steril. 2009, 92, 793–797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Li, M.; Zhang, Y.; Wang, X.; Liu, H.; Wu, W.; Ma, W.; Quan, K.; Ng, E.H.; Wu, X.; et al. Cryptotanshinone Regulates Androgen Synthesis through the ERK/c-Fos/CYP17 Pathway in Porcine Granulosa Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 5985703. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Woods, K.S.; Bartolucci, A.A.; Azziz, R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2005, 62, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A.; Umstot, E.S.; Andersen, R.N.; Ranney, G.B.; Givens, J.R. Androgen parameters and their correlation with body weight in one hundred thirty-eight women thought to have hyperandrogenism. Am. J. Obstet. Gynecol. 1983, 146, 602–606. [Google Scholar] [CrossRef]
- Hoffman, D.I.; Klove, K.; Lobo, R.A. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil. Steril. 1984, 42, 76–81. [Google Scholar] [CrossRef]
- Carmina, E.; Koyama, T.; Chang, L.; Stanczyk, F.Z.; Lobo, R.A. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am. J. Obstet. Gynecol. 1992, 167, 1807–1812. [Google Scholar] [CrossRef]
- Meek, C.L.; Bravis, V.; Don, A.; Kaplan, F. Polycystic ovary syndrome and the differential diagnosis of hyperandrogenism. Obstet. Gynaecol. 2013, 15, 171–176. [Google Scholar] [CrossRef]
- Ehrmann, D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Schachter, M.E.; Montgomery, D.; Reid, R.W.; Jacobs, H.S. Polycystic ovaries are a common finding in untreated female to male transsexuals. Clin. Endocrinol. 1993, 38, 325–329. [Google Scholar] [CrossRef]
- Pache, T.D.; Fauser, B.C. Polycystic ovaries in female-to-male transsexuals. Clin. Endocrinol. 1993, 39, 702–703. [Google Scholar]
- Kafali, H.; Iriadam, M.; Ozardali, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012, 86, 149. [Google Scholar] [CrossRef] [PubMed]
- Liepa, G.U.; Sengupta, A.; Karsies, D. Polycystic ovary syndrome (PCOS) and other androgen excess-related conditions: Can changes in dietary intake make a difference? Nutr. Clin. Pract. 2008, 23, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Palomba, S.; Hart, R. Polycystic Ovary Syndrome, Obesity, and Pregnancy. Semin. Reprod. Med. 2016, 34, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kialka, M.; Milewicz, T.; Sztefko, K.; Rogatko, I.; Majewska, R. Metformin increases pressure pain threshold in lean women with polycystic ovary syndrome. Drug Des. Dev. Ther. 2016, 10, 2483–2490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Wickenheisser, J.K.; Nelson-Degrave, V.L.; McAllister, J.M. Dysregulation of cytochrome P450 17alpha-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Wickenheisser, J.K.; Biegler, J.M.; Nelson-Degrave, V.L.; Legro, R.S.; Strauss, J.F., 3rd; McAllister, J.M. Cholesterol side-chain cleavage gene expression in theca cells: Augmented transcriptional regulation and mRNA stability in polycystic ovary syndrome. PLoS ONE 2012, 7, e48963. [Google Scholar] [CrossRef] [PubMed]
- Nelson, V.L.; Qin, K.N.; Rosenfield, R.L.; Wood, J.R.; Penning, T.M.; Legro, R.S.; Strauss, J.F., 3rd; McAllister, J.M. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Harborne, L.; Fleming, R.; Lyall, H.; Norman, J.; Sattar, N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet 2003, 361, 1894–1901. [Google Scholar] [CrossRef]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, E.; Matteo, M.; Vizziello, G.; Schonauer, L.M.; Vitti, A.; D’Amato, G. Metformin pre-treatment followed by ovarian stimulation with GnRH antagonist is a valuable protocol for PCOS and obese women with previous ART failure, regardless of the presence of insulin resistance. Fertil. Steril. 2005, 84, S149. [Google Scholar] [CrossRef]
- Bonakdaran, S.; Mazloom Khorasani, Z.; Davachi, B.; Mazloom Khorasani, J. The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: A randomized placebo- controlled clinical trial. Iran. J. Reprod. Med. 2012, 10, 465–472. [Google Scholar] [PubMed]
- Valsamakis, G.; Lois, K.; Kumar, S.; Mastorakos, G. Metabolic and other effects of pioglitazone as an add-on therapy to metformin in the treatment of polycystic ovary syndrome (PCOS). Hormones 2013, 12, 363–378. [Google Scholar] [PubMed]
- Kempna, P.; Hofer, G.; Mullis, P.E.; Fluck, C.E. Pioglitazone inhibits androgen production in NCI-H295R cells by regulating gene expression of CYP17 and HSD3B2. Mol. Pharmacol. 2007, 71, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Tokunaga, K.; Kuboi, T. Isolation of a drought-responsive alkaline alpha-galactosidase gene from New Zealand spinach. Plant Biotechnol. 2008, 25, 497–501. [Google Scholar] [CrossRef]
- Ko, E.Y.; Cho, S.H.; Kang, K.; Kim, G.; Lee, J.H.; Jeon, Y.J.; Kim, D.; Ahn, G.; Kim, K.N. Anti-inflammatory activity of hydrosols from Tetragonia tetragonoides in LPS-induced RAW 264.7 cells. EXCLI J. 2017, 16, 521–530. [Google Scholar] [PubMed]
- Yousif, B.S.; Nguyen, N.T.; Fukuda, Y.; Hakata, H.; Okamoto, Y.; Masaoka, Y.; Saneoka, H. Effect of Salinity on Growth, Mineral Composition, Photosynthesis and Water Relations of Two Vegetable Crops; New Zealand Spinach (Tetragonia tetragonioides) and Water Spinach (Ipomoea aquatica). Int. J. Agric. Biol. 2010, 12, 211–216. [Google Scholar]
- Okuyama, E.; Yamazaki, M. The principles of Tetragonia tetragonioides having antiulcerogenic activity. II. Isolation and structure of cerebrosides. Chem. Pharm. Bull. 1983, 31, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Takeda, T.; Ogihara, Y.; Shimu, M.; Nomura, T.; Tomita, Y. Studies on the structure of polysaccharide from Tetragonia tetragonioides. Chem. Pharm. Bull. 1985, 33, 3675–3680. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, J.S.; Choi, Y.W.; Jeong, Y.K.; Joo, W.H. Inhibitory Activity on the Diabetes Related Enzymes of Tetragonia tetragonioides. Korean J. Biotechnol. Bioeng. 2008, 23, 419–424. [Google Scholar]
- Choi, H.J.; Yee, S.-T.; Kwon, G.-S.; Joo, W.H. Anti-inflammatory and Anti-tumor Effects of Tetragonia tetragonoides Extracts. Microbiol. Biotechnol. Lett. 2015, 43, 391–395. [Google Scholar]
- Lee, M.A.; Choi, H.J.; Kang, J.S.; Choi, Y.W.; Joo, W.H. Antioxidant Activities of the Solvent Extracts from Tetragonia tetragonioides. J. Life Sci. 2008, 18, 220–227. [Google Scholar] [CrossRef]
- Choi, H.S. Isolation and Structural Elucidation of Antioxidants from Tetragonia tetragonoides. Master’s Thesis, Department of Food Science & Technology, Chonnam National University, Gwangju, Korea, 2017. [Google Scholar]
- Lee, K.H.; Park, K.M.; Kim, K.R.; Hong, J.; Kwon, H.C.; Lee, K.R. Three new flavonol glycosides from the aerial parts of Tetragonia tetragonoides. Heterocycles 2008, 75, 419–426. [Google Scholar] [CrossRef]
- Mori, K.; Kinsho, T. Synthesis of sphingosine relatives, VII. Synthesis of anti-ulcergenic cerebroside isolated from Tetragonia tetragonioides. Eur. J. Org. Chem. 1988, 8, 807–814. [Google Scholar]
- Ryuk, J.A.; Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Lee, Y.H.; Park, S. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp. Biol. Med. 2017, 242, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kialka, M.; Milewicz, T.; Mrozinska, S.; Rogatko, I.; Sztefko, K.; Majewska, R. Pressure pain threshold and Beta-endorphins plasma level are higher in lean polycystic ovary syndrome women. Minerva Endocrinol. 2017, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, S. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Napoli, N.; Longo, R.A.; Rini, G.B.; Lobo, R.A. Metabolic syndrome in polycystic ovary syndrome (PCOS): Lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur. J. Endocrinol. 2006, 154, 141–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moger, W.H.; Anakwe, O.O. Effects of forskolin on androgen production by mouse interstitial cells in vitro. Interactions with luteinizing hormone and isoproterenol. Biol. Reprod. 1983, 29, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.R.; Rainey, W.E.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Margalioth, E.J.; Gal, M.; Ben-Chetrit, A.; Algur, N.; Zylber-Haran, E.; Brooks, B.; Huerta, M.; Spitz, I.M. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum. Reprod. 2005, 20, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Ghafurniyan, H.; Azarnia, M.; Nabiuni, M.; Karimzadeh, L. The Effect of Green Tea Extract on Reproductive Improvement in Estradiol Valerate-Induced Polycystic Ovarian Syndrome in Rat. Iran. J. Pharm. Res. 2015, 14, 1215–1233. [Google Scholar] [PubMed]
- Arentz, S.; Abbott, J.A.; Smith, C.A.; Bensoussan, A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement. Altern. Med. 2014, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Xiao, Z.; Ishida, J.; Nagai, M.; Wang, H.K.; Itokawa, H.; Su, C.Y.; Shih, C.; Chiang, T.; Chang, E.; et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002, 45, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Farideh, Z.Z.; Bagher, M.; Ashraf, A.; Akram, A.; Kazem, M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010, 11, 169–174. [Google Scholar] [PubMed]
- McGarvey, C.; Cates, P.A.; Brooks, A.; Swanson, I.A.; Milligan, S.R.; Coen, C.W.; O'Byrne, K.T. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology 2001, 142, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Polkowska, J.; Ridderstråle, Y.; Wańkowska, M.; Romanowicz, K.; Misztal, T.; Madej, A. Effects of intracerebroventricular infusion of genistein on gonadotrophin subunit mRNA and immunoreactivity of gonadotrophins and oestrogen receptor-alpha in the pituitary cells of the anoestrous ewe. J. Chem. Neuroanat. 2004, 28, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, S.R.; Haisenleder, D.J.; Lubahn, D.B.; Rissman, E.F. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine 1999, 11, 137–143. [Google Scholar] [CrossRef]
- Lindzey, J.; Jayes, F.L.; Yates, M.M.; Couse, J.F.; Korach, K.S. The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-alpha. J. Endocrinol. 2006, 191, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Biernacka-Lukanty, J.M.; Lehmann, T.P.; Trzeciak, W.H. Inhibition of CYP17 expression by adrenal androgens and transforming growth factor beta in adrenocortical cells. Acta Biochim. Pol. 2004, 51, 907–917. [Google Scholar] [PubMed]
- Turcu, A.F.; Auchus, R.J. Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol. Metab. Clin. N. Am. 2015, 44, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.W. Adrenocortical endocrine disruption. J. Steroid Biochem. Mol. Biol. 2016, 155, 199–206. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyun, B.-J.; Yang, H.; Sohn, E.; Yu, S.Y.; Lee, D.; Jung, D.H.; Ko, B.S.; Lee, H.W. Tetragonia tetragonioides (Pall.) Kuntze Regulates Androgen Production in a Letrozole-Induced Polycystic Ovary Syndrome Model. Molecules 2018, 23, 1173. https://doi.org/10.3390/molecules23051173
Pyun B-J, Yang H, Sohn E, Yu SY, Lee D, Jung DH, Ko BS, Lee HW. Tetragonia tetragonioides (Pall.) Kuntze Regulates Androgen Production in a Letrozole-Induced Polycystic Ovary Syndrome Model. Molecules. 2018; 23(5):1173. https://doi.org/10.3390/molecules23051173
Chicago/Turabian StylePyun, Bo-Jeong, Hyun Yang, Eunjin Sohn, Song Yi Yu, Dongoh Lee, Dong Ho Jung, Byoung Seob Ko, and Hye Won Lee. 2018. "Tetragonia tetragonioides (Pall.) Kuntze Regulates Androgen Production in a Letrozole-Induced Polycystic Ovary Syndrome Model" Molecules 23, no. 5: 1173. https://doi.org/10.3390/molecules23051173
APA StylePyun, B.-J., Yang, H., Sohn, E., Yu, S. Y., Lee, D., Jung, D. H., Ko, B. S., & Lee, H. W. (2018). Tetragonia tetragonioides (Pall.) Kuntze Regulates Androgen Production in a Letrozole-Induced Polycystic Ovary Syndrome Model. Molecules, 23(5), 1173. https://doi.org/10.3390/molecules23051173





