LiGe(SiMe3)3: A New Substituent for the Synthesis of Metalloid Tin Clusters from Metastable Sn(I) Halide Solutions
Abstract
:1. Introduction
2. Results and Discussion
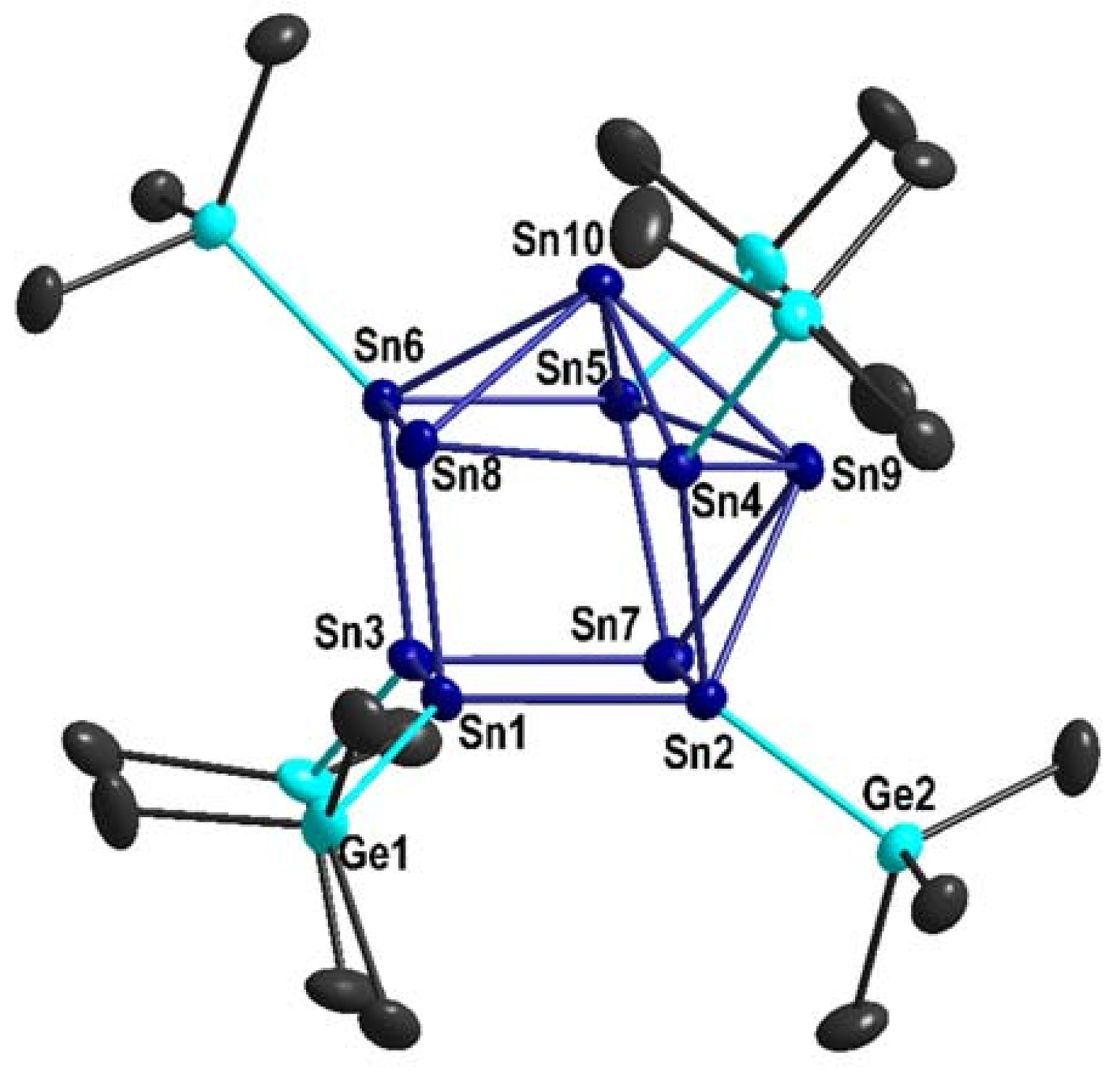
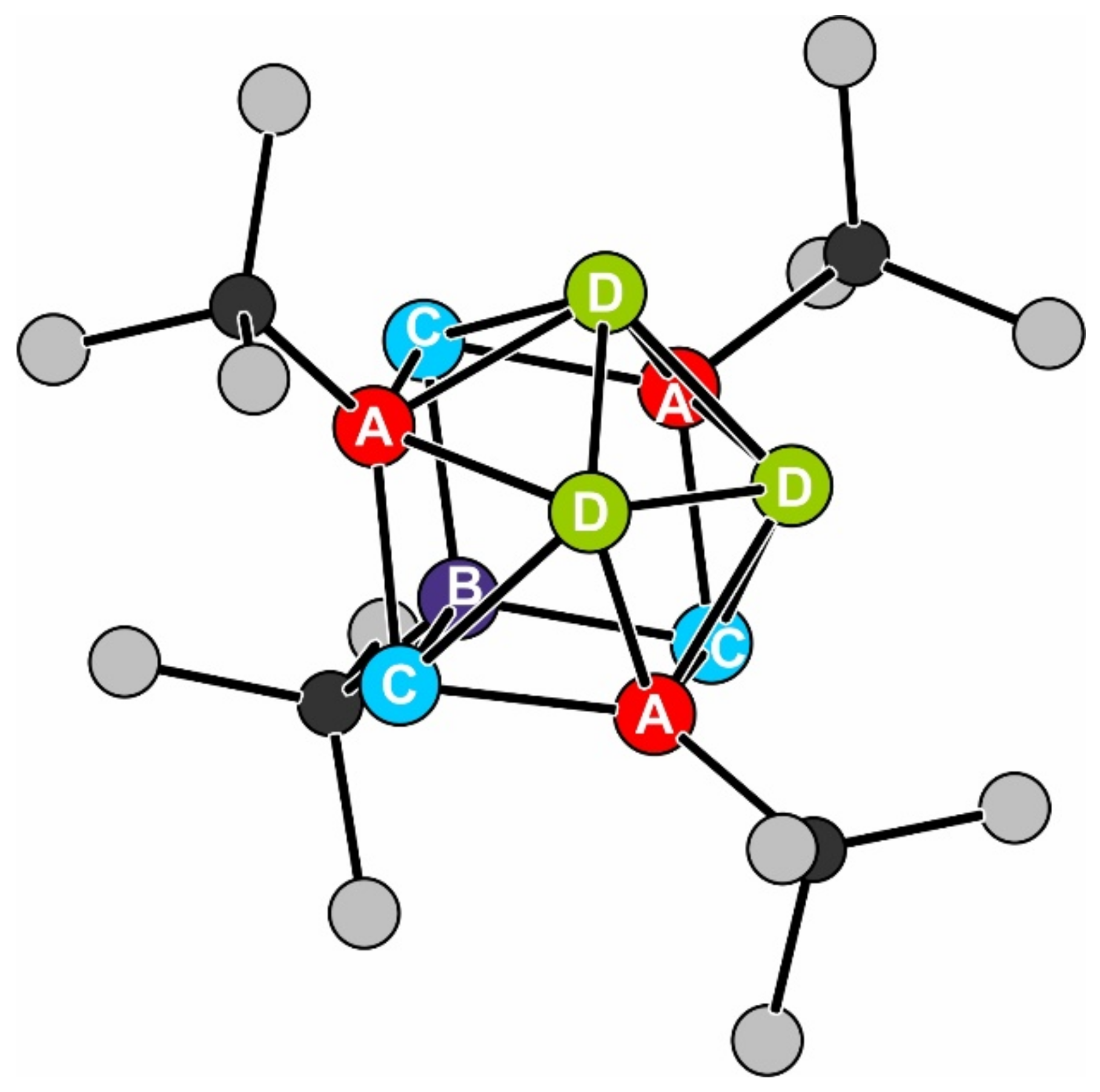
2.1. Sn10(Ge(SiMe3)3)6 1
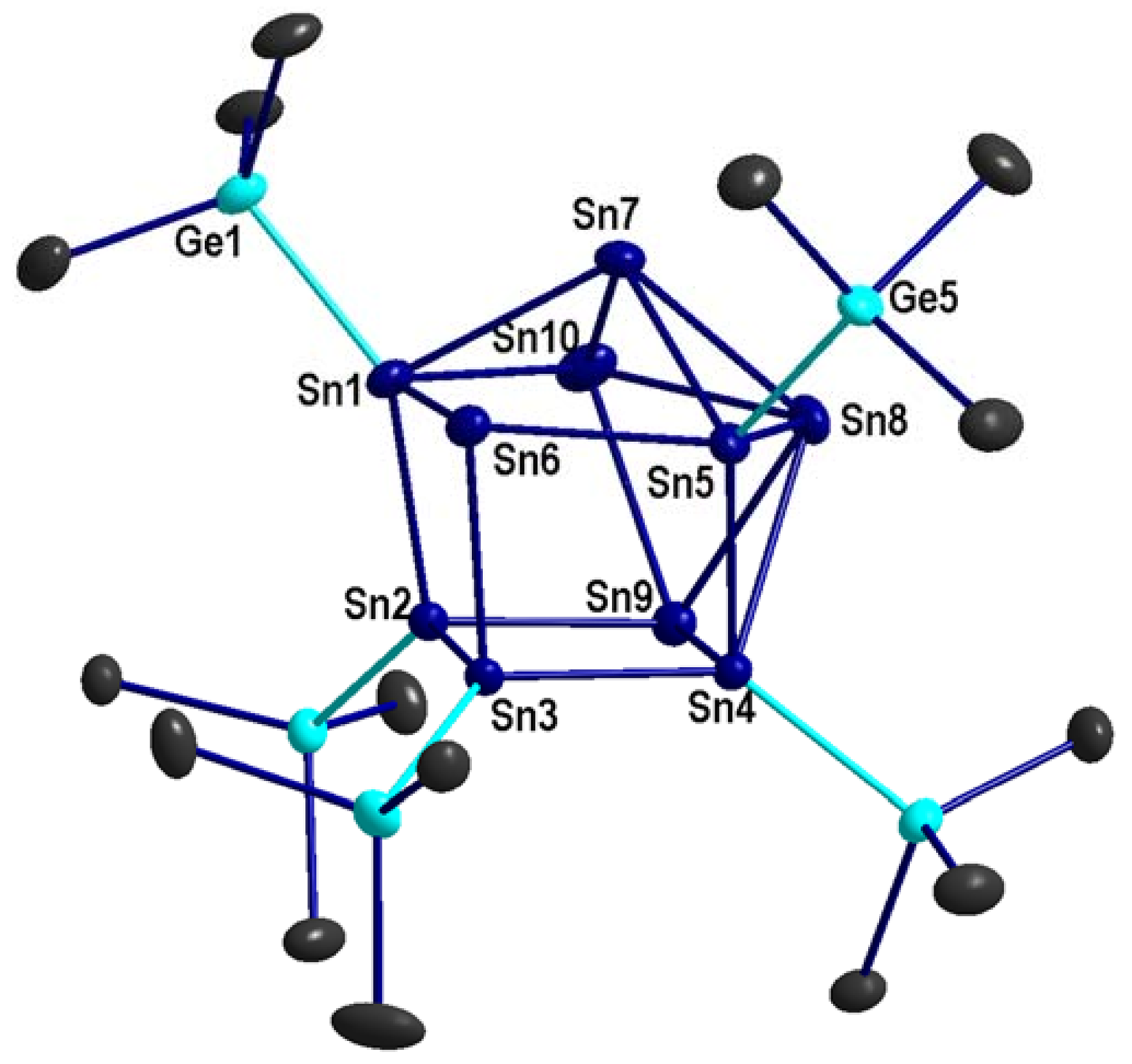
2.2. Li(thf)4Sn10[Ge(SiMe3)3]5 2
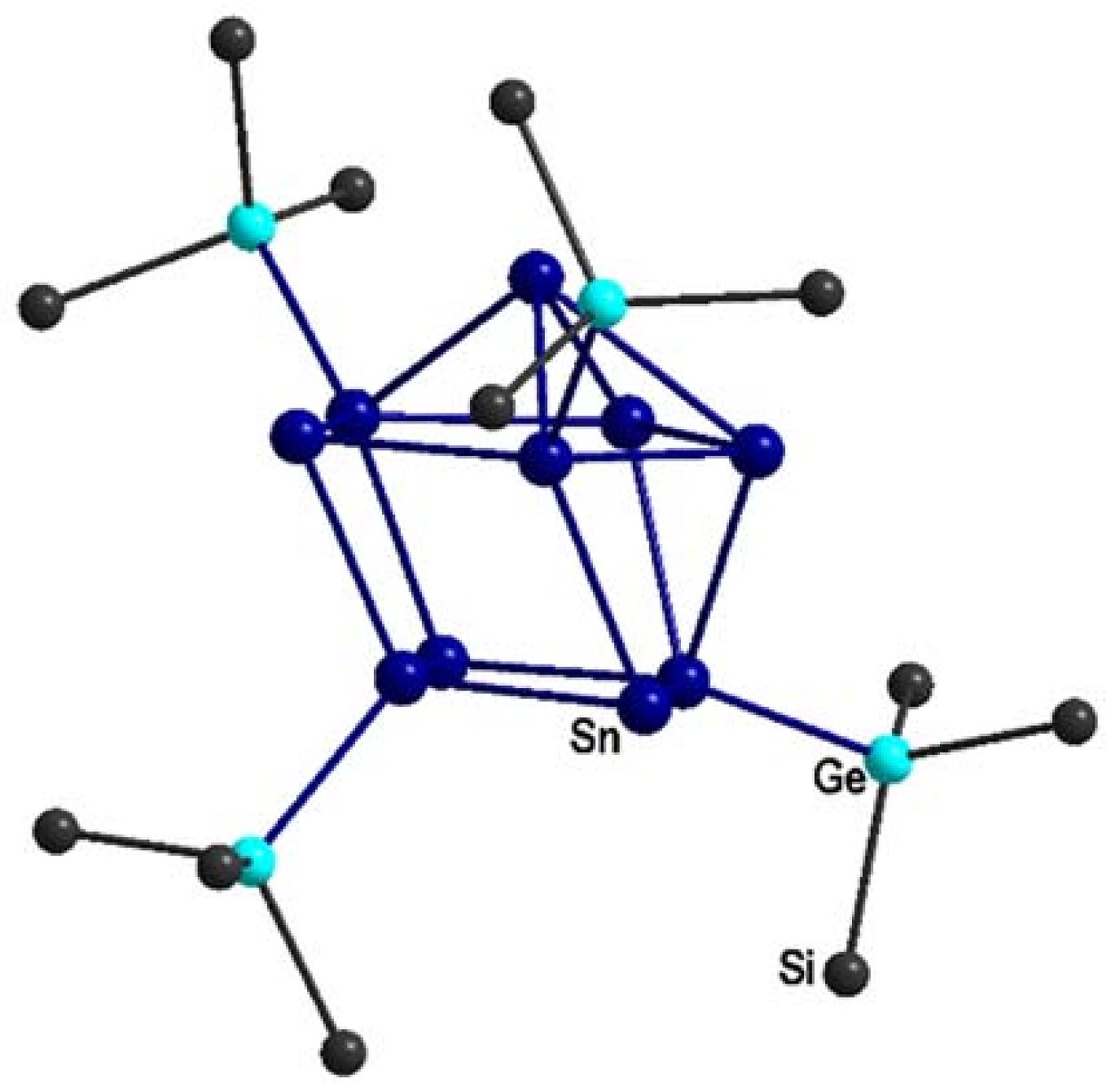
2.3. [Li(tmeda)2]2Sn10[Ge(SiMe3)3]4 3
3. Materials and Methods
3.1. LiGe(SiMe3)3
3.2. Sn10(Ge(SiMe3)3)6 (1)
3.3. [Li(thf)4]{Sn10[Ge(SiMe3)3]5} (2)
3.4. [Li(TMEDA)2]2{Sn10[Ge(SiMe3)3]4} (3)
3.5. X-ray Structural Characterization
3.5.1. Sn10[Ge(SiMe3)3]6
3.5.2. [Li(thf)4]{Sn10[Ge(SiMe3)3]5}
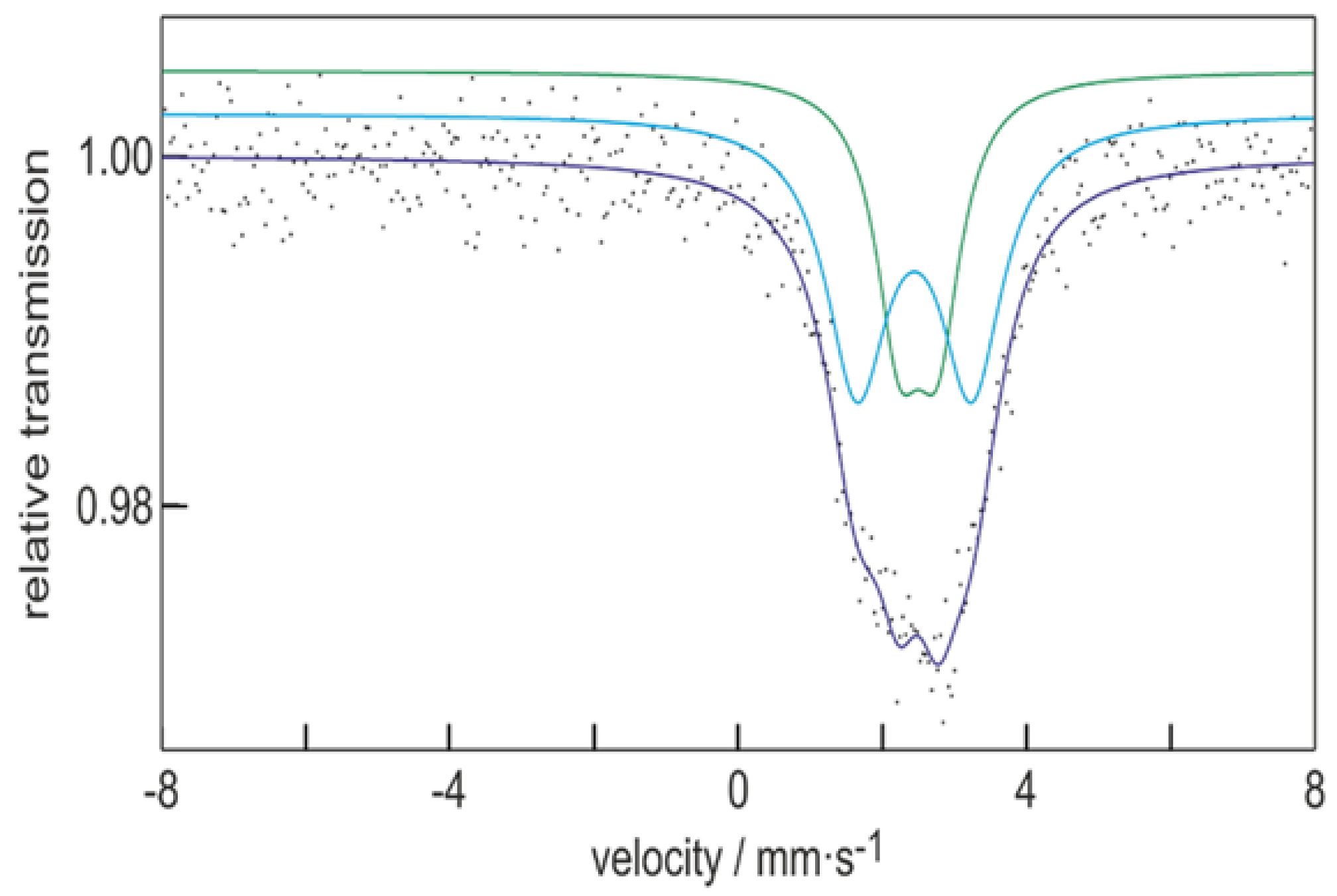
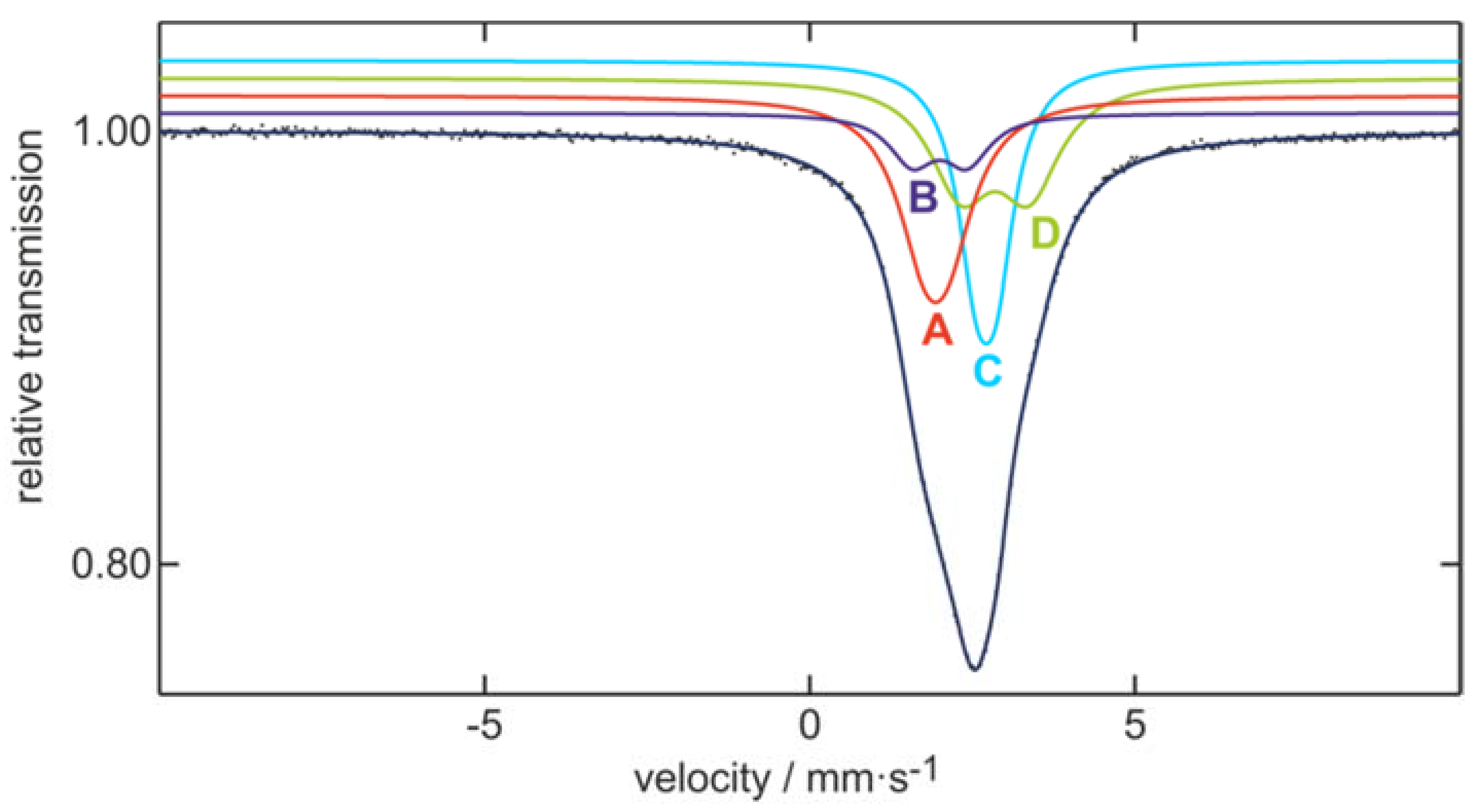
3.6. 119mSn-Mössbauer Spectroscopy
3.7. EDX
3.8. NMR
3.9. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References and Notes
- Goesmann, H.; Feldmann, C. Nanoparticulate Functional Materials. Angew. Chem. Int. Ed. 2010, 49, 1362–1397. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, A. Structure and Bonding. In Clusters—Contemporary Insight in Structure and Bonding; Dehnen, S., Ed.; Springer: Cham, Switzerland, 2017; Volume 174, pp. 135–200. [Google Scholar]
- Schnepf, A. Metalloid group 14 cluster compounds: An introduction and perspectives to this novel group of cluster compounds. Chem. Soc. Rev. 2007, 36, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Schnöckel, H. Structures and Properties of Metalloid Al and Ga Clusters Open Our Eyes to the Diversity and Complexity of Fundamental Chemical and Physical Processes during Formation and Dissolution of Metals. Chem. Rev. 2010, 110, 4125–4163. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Schnepf, A. Metalloid Sn clusters: Properties and the novel synthesis via a disproportionation reaction of a monohalide. Rev. Inorg. Chem. 2014, 36, 93–118. [Google Scholar] [CrossRef]
- Binder, M.; Schrenk, C.; Schnepf, A. The Synthesis of [Sn10(SiMe3)3)4]2− using a Metastable Sn(I) Halide Solution Synthesized via Co-condensation Technique. J. Vis. Exp. 2016, 117, e54498. [Google Scholar]
- Another fruitful route for the synthesis of metalloid tin clusters applies the reductive coupling of respective starting compounds giving metalloid tin clusters with up 17 tin atoms in the cluster core [9–14].
- Eichler, B.E.; Power, P.P. Synthesis and Characterization of [Sn8(2,6-Mes2C6H3)4] (Mes = 2,4,6-Me3C6H2): A Main Group Metal Cluster with a Unique Structure. Angew. Chem. Int. Ed. 2001, 40, 796–797. [Google Scholar] [CrossRef]
- Richardson, A.F.; Eichler, B.E.; Brynda, M.; Olmstead, M.M.; Power, P.P. Metal-Rich, Neutral and Cationic Organotin Cluster. Angew. Chem. Int. Ed. 2005, 44, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Brynda, M.; Herber, R.; Hitchcock, P.B.; Lappert, M.F.; Nowik, I.; Power, P.P.; Protchenko, A.V.; Ruzicka, A.; Steiner, J. Higher-Nuclearity Group 14 Metalloid Clusters: [Sn9{Sn(NRR’)}6]. Angew. Chem. Int. Ed. 2006, 45, 4333–4337. [Google Scholar] [CrossRef] [PubMed]
- Rivard, E.; Steiner, J.; Fettinger, J.C.; Giuliani, J.R.; Augustine, M.P.; Power, P.P. Convergent syntheses of [Sn7{C6H3-2,6-(C6H3-2,6-iPr2)2}2]: A cluster with a rare pentagonal bipyramidal motif. Chem. Commun. 2007, 4919–4921. [Google Scholar] [CrossRef]
- Prabusankar, G.; Kempter, A.; Gemel, C.; Schröter, M.-K.; Fischer, R.A. [Sn17{GaCl(ddp)}4]: A High-Nuclearity Metalloid Tin cluster Trapped by Electrophilic Gallium Ligands. Angew. Chem. Int. Ed. 2008, 47, 7234–7237. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, J.; Wölper, C.; Schulz, S. Synthesis and solid state structure of a metalloid tin cluster [Sn10(trip8)]. Chem. Commun. 2016, 52, 12282–12285. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Winter, F.; Pöttgen, R.; Schnepf, A. {Sn9[Si(SiMe3)3]2}2−: A Metalloid Tin Cluster Compound with a Sn9 Core of Oxidation State Zero. Inorg. Chem. 2012, 51, 8583–8588. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Neumaier, M.; Schnepf, A. {Sn9[Si(SiMe3)3]3}− and {Sn8Si[Si(SiMe3)3]3}−: Variations of the E9 Cage of Metalloid Group 14 Clusters. Inorg. Chem. 2012, 51, 3989–3995. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Winter, F.; Pöttgen, R.; Schnepf, A. {Sn10[Si(SiMe3)3]4}2−: A Highly Reactive Metalloid Tin Cluster with an Open Ligand Shell. Chem. Eur. J. 2015, 21, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Helmlinger, J.; Schnepf, A. {Sn10[Si(SiMe3)3]5}–: An Anionic Metalloid Tin Cluster from an Isolable SnI Halide Solution. Z. Anorg. Allg. Chem. 2012, 638, 589–593. [Google Scholar] [CrossRef]
- Schrenk, C.; Schellenberg, I.; Pöttgen, R.; Schnepf, A. The formation of a metalloid Sn10[Si(SiMe3)3]6 cluster compound and its relation to the α↔β tin phase transition. Dalton Trans. 2010, 39, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Kotsch, M.; Gienger, C.; Schrenk, C.; Schnepf, A. The Sterically Demanding Thiosilyl Group SSi(SiMe3)3 as a Ligand in Transition Metal Chemistry. Z. Anorg. Allg. Chem. 2016, 642, 670–675. [Google Scholar] [CrossRef]
- Binder, M.; Schrenk, C.; Block, T.; Pöttgen, R.; Schnepf, A. [Hyp-Au-Sn9(Hyp)3-Au-Sn9(Hyp)3-Au-Hyp]−: The longest intermetalloid chain compound of tin. Chem. Commun. 2017, 53, 11314–11317. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, C.; Gerke, B.; Pöttgen, R.; Clayborne, A.; Schnepf, A. Reactions with a Metalloid Tin Cluster {Sn10[Si(SiMe3)3]4}2–: Ligand Elimination versus Coordination Chemistry. Chem. Eur. J. 2015, 21, 8222–8228. [Google Scholar] [CrossRef] [PubMed]
- Gilman, H.; Smith, C.L. Tetrakis(trimethylsilyl)silane. J. Organomet. Chem. 1967, 8, 245–253. [Google Scholar] [CrossRef]
- Treutler, O.; Ahlrichs, R. Efficient molecular numerical integration schemes. J. Chem. Phys. 1995, 102, 346–354. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8884. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimize contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Davidson, E.R. Electronic population analysis of molecular Wavefunctions. J. Chem. Phys. 1967, 46, 3320–3324. [Google Scholar] [CrossRef]
- Roby, K.R. Quantum theory of chemical valence concepts. Mol. Phys. 1974, 27, 81–104. [Google Scholar] [CrossRef]
- Heinzmann, R.; Ahlrichs, R. Population analysis based on occupation numbers of modified atomic orbitals (MAOs). Theor. Chim. Acta 1976, 42, 33–45. [Google Scholar] [CrossRef]
- Erhardt, C.; Ahlrichs, R. Population analysis based on occupation numbers II. Relationship between shared electron numbers and bond energies and characterization of hypervalent contributions. Theor. Chim. Acta 1985, 68, 231–245. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX-A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- The Shared Electron Numbers (SENs) for Bonds are Reliable Measure of the Covalent Bonding Strength. For Example, the Sen for the Sn-Sn Single Bond in (SiH3)3Sn-Sn(SiH3)3 is 1.11; Whereas the SEN for a Double Bond in (SiH3)2Sn = Sn(SiH3)2 is 1.48.
- The molecular structure of [Li(tmeda)2]2Sn10[Ge(SiMe3)3]4 was rudimentarily solved in the orthorhombic crystal system with a = 16.2915(15), b = 30.669(3), c = 33.450(3) Å in space group Pbcm with half a molecule in the asymmetric unit.
- Shenoy, G.F.; Wagner, F.E. Mößbauer Isomer Shifts; North-Holland Publishing Company: Amsterdam, The Netherland, 1978. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Brand, R.A. Normos Mössbauer Fitting Program; University of Duisburg: Duisburg, Germany, 2002. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Sn10[Ge(SiMe3)3]6 1 | Sn10[Si(SiMe3)3]6 | |
|---|---|---|
| Sn1-Sn2 | 292.2 | 289.6 |
| Sn1-Sn8 | 288.5 | 288.1 |
| Sn2-Sn4 | 295.5 | 293.5 |
| Sn3-Sn6 | 291.3 | 290.9 |
| Sn4–Sn9 | 300.8 | 302.1 |
| Sn5-Sn7 | 293.0 | 292.5 |
| Sn5-Sn10 | 301.4 | 303.2 |
| Sn7-Sn9 | 310.8 | 312.9 |
| Sn1-Ge1 | 266.2 | Sn1–Si1: 264.0 |
| Compound | δ (mm∙s–1) | ΔEQ (mm∙s–1) | Γ (mm∙s–1) | Area (Fixed) |
|---|---|---|---|---|
| Sn10[Ge(SiMe3)3]6 | 2.45(2) | 1.59(5) | 1.05(4) | 60 |
| 2.51(2) | 0.54(4) | 0.79(6) | 40 |
| {Sn10[Ge(SiMe3)3]5}− | {Sn10[Si(SiMe3)3]5}− | |
|---|---|---|
| Sn1-Sn2 | 289.5 | 288.7 |
| Sn3-Sn4 | 291.8 | 293.3 |
| Sn3-Sn6 | 294.3 | 294.4 |
| Sn4-Sn5 | 292.1 | 292.7 |
| Sn5–Sn8 | 302.9 | 302.8 |
| Sn7-Sn10 | 298.1 | 297.3 |
| Sn8-Sn9 | 303.2 | 302.7 |
| Sn9-Sn10 | 301.9 | 302.4 |
| Signal | δ (mm∙s–1) | ΔEQ (mm∙s–1) | Γ (mm∙s–1) | Ratio (%) |
|---|---|---|---|---|
| A | 1.93(2) | 0.36(7) | 1.04(6) | 30 * |
| B | 2.00(7) | 0.8(1) | 0.9(1) | 10 * |
| C | 2.712(6) | 0.31(1) | 0.71(1) | 30 * |
| D | 2.85(4) | 1.05(9) | 1.16(2) | 30 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, M.; Schrenk, C.; Block, T.; Pöttgen, R.; Schnepf, A. LiGe(SiMe3)3: A New Substituent for the Synthesis of Metalloid Tin Clusters from Metastable Sn(I) Halide Solutions. Molecules 2018, 23, 1022. https://doi.org/10.3390/molecules23051022
Binder M, Schrenk C, Block T, Pöttgen R, Schnepf A. LiGe(SiMe3)3: A New Substituent for the Synthesis of Metalloid Tin Clusters from Metastable Sn(I) Halide Solutions. Molecules. 2018; 23(5):1022. https://doi.org/10.3390/molecules23051022
Chicago/Turabian StyleBinder, Mareike, Claudio Schrenk, Theresa Block, Rainer Pöttgen, and Andreas Schnepf. 2018. "LiGe(SiMe3)3: A New Substituent for the Synthesis of Metalloid Tin Clusters from Metastable Sn(I) Halide Solutions" Molecules 23, no. 5: 1022. https://doi.org/10.3390/molecules23051022
APA StyleBinder, M., Schrenk, C., Block, T., Pöttgen, R., & Schnepf, A. (2018). LiGe(SiMe3)3: A New Substituent for the Synthesis of Metalloid Tin Clusters from Metastable Sn(I) Halide Solutions. Molecules, 23(5), 1022. https://doi.org/10.3390/molecules23051022







