Effects of 200 Gy 60Co-γ Radiation on the Regulation of Antioxidant Enzymes, Hsp70 Genes, and Serum Molecules of Plutella xylostella (Linnaeus)
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of 60Co-γ Radiation on the Antioxidant Enzymes of Pupae Irradiated with 200 Gy
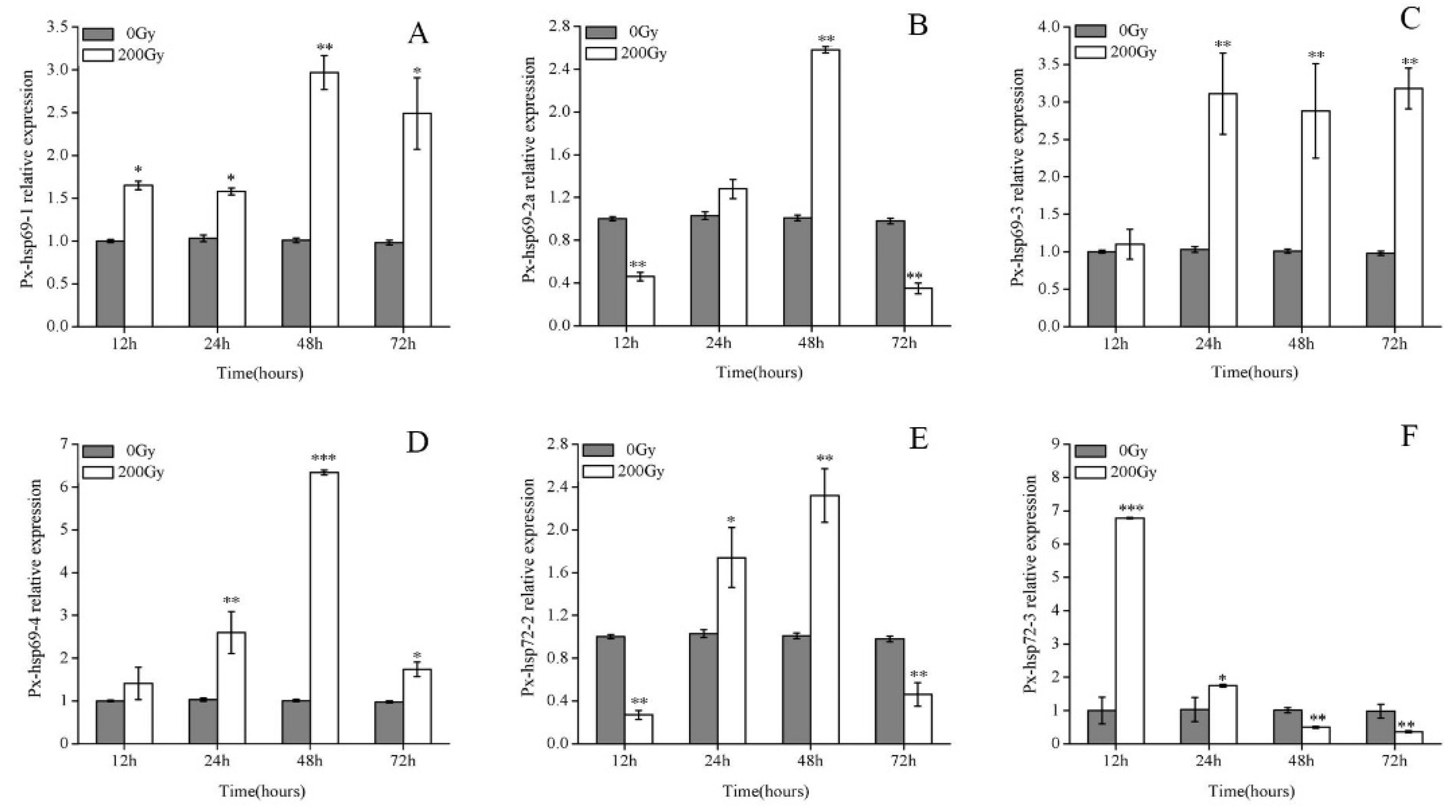
2.2. Effect of 60Co-γ Radiation on the Expression of Hsp70s Genes of Pupae Irradiated with 200 Gy
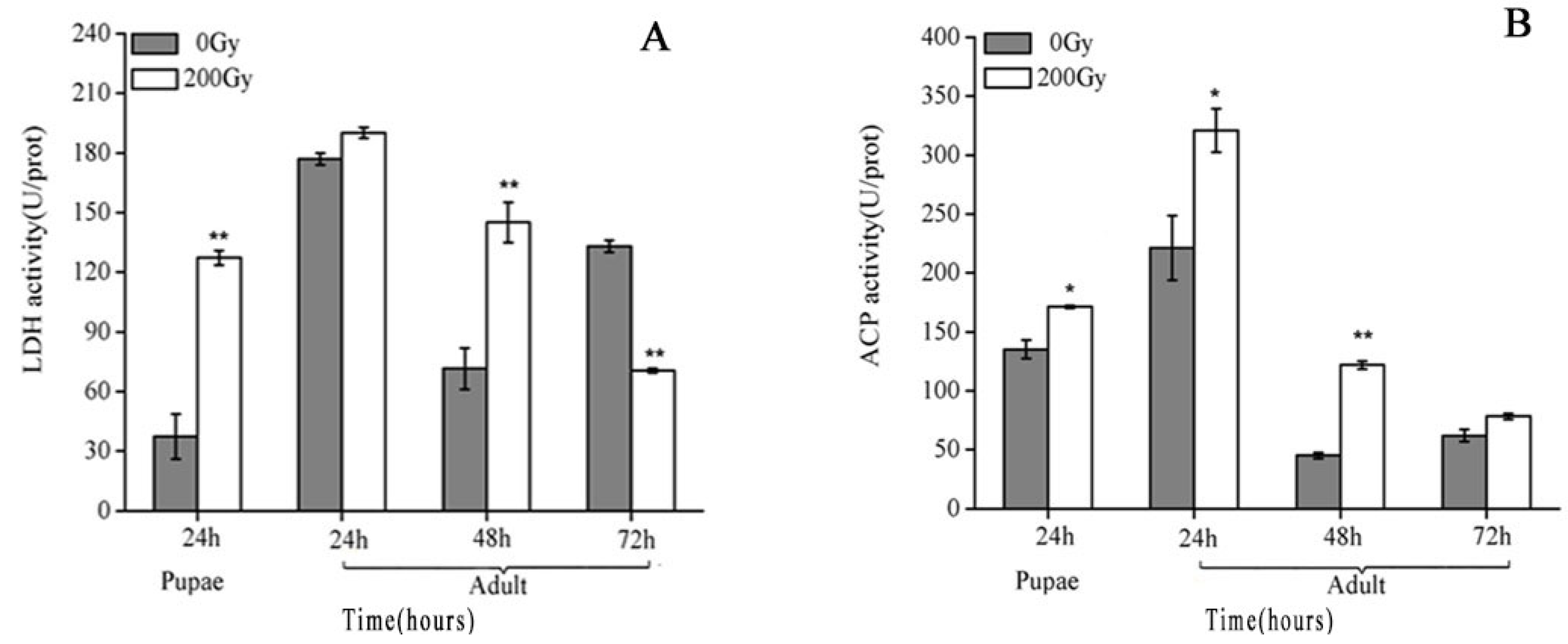
2.3. Effects of 60Co-γ Radiation on LDH and ACP Activity of Testes Irradiated with 200 Gy
3. Experimental
3.1. Insects
3.2. Irradiation
3.3. Enzyme Assay
3.4. Real-Time Quantitative PCR (qPCR) Analysis of Hsp70s Genes
3.5. Dissection of Pupae and Males
3.6. LDH and ACP Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Kfir, R. Biological Control of the Diamondback Moth Plutella xylostella in Africa; CAB International: London, UK, 2003. [Google Scholar]
- Kaur, H.; Garg, H. Pesticides: Environmental Impacts and Management Strategies. Pestic. Toxic Eff. 2014, 311–312. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, C.F.; Klassen, W.; Lance, D.R.; Mcinnis, D.O.; Robinson, A.S.; Carpenter, J.E.; Bloem, S.; Marec, F.; Barclay, H.J. Sterile Insect Technique; Springer: Berlin, Germany, 1989; pp. 3–36. [Google Scholar]
- Sonntag, C.V. The Chemical Basis of Radiation Biology; Taylor & Francis: Abingdon-on-Thames, UK, 1987; Volume 52, p. 976. [Google Scholar]
- Suman, S.; Khan, Z.; Zarin, M.; Chandna, S.; Seth, R.K. Radioresistant Sf9 insect cells display efficient antioxidant defence against high dose γ-radiation. Int. J. Radiat. Biol. 2015, 91, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Kuin, A.; Kruse, J.J.; Stewart, F.A. Proteinuria and vascular changes after renal irradiation: The role of reactive oxygen species (ROS) and vascular endothelial growth factor (Vegf) proteinuria and vascular changes after renal irradiation: The role of reactive oxygen species (os) A. Radiat. Res. 2003, 159, 174–181. [Google Scholar] [CrossRef]
- Bossi, O.; Gartsbein, M.; Leitges, M.; Kuroki, T.; Grossman, S.; Tennenbaum, T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J. Cell. Biochem. 2008, 105, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Buttemer, W.A.; Abele, D.; Costantini, D. From bivalves to birds: Oxidative stress and longevity. Funct. Ecol. 2010, 24, 971–983. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Alonso-Alvarez, C. Oxidative stress as a life-history constraint: The role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 2010, 24, 984–996. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 2, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Micheal, A.S.; Subramanyam, M.V.V. Antioxidant enzymes as defense mechanism against oxidative stress in midgut tissue and hemocytes of Bombyx mori larvae subjected to various stressors. Arch. Insect Biochem. Physiol. 2013, 4, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001, 11, 1254–1262. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, Y.; Cheng, Z.; Cheng, J.; Hu, R.; Cui, Y.; Gong, X.; Shen, W.; Hong, F. Effects of Added CeCl3 on Resistance of Fifth-Instar Larvae of Silkworm to Bombyx mori Nucleopolyhedrovirus Infection. Biol. Trace Elem. Res. 2012, 3, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Catalase and superoxide dismutase-2 enhance survival and protect ovaries during overwintering diapause in the mosquito. Culex pipiens. J. Insect Physiol. 2011, 5, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Calini, V.; Urani, C.; Camatini, M. Overexpression of HSP70 is induced by ionizing radiation in C3H 10T1/2 cells and protects from DNA damage. Toxicol. In Vitro 2003, 17, 561–566. [Google Scholar] [CrossRef]
- Zhao, L.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. Invertebr. Surviv. J. 2012, 9, 93–101. [Google Scholar]
- Shi, M.; Wang, Y.N.; Zhu, N.; Chen, X.X. Four Heat Shock Protein Genes of the Endoparasitoid Wasp, Cotesia vestalis, and Their Transcriptional Profiles in Relation to Developmental Stages and Temperature. PLoS ONE 2013, 8, e59721. [Google Scholar] [CrossRef] [PubMed]
- Mahroof, R.; Yan, Z.K.; Neven, L.; Subramanyam, B.; Bai, J. Expression patterns of three heat shock protein 70 genes among developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highlyhomologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dong, A. Effects of cadmium and lead on testicular enzymes of Bufo bufo gargarizns. Acta Ecol. Sin. 2004, 24, 2329–2333. [Google Scholar]
- Butts, I.A.; Litvak, M.K.; Trippel, E.A. Seasonal variations in seminal plasma and sperm characteristics of wild-caught and cultivated Atlantic cod, Gadus morhua. Theriogenology 2010, 73, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Pesch, S.; Bergmann, M.; Bostedt, H. Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology 2006, 66, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, S.; Hoedaya, M.S.; Sutardji, D.; Rahayu, A. Radiation Induced F1 Sterility in Diamondback Moth, Plutella xylostella L. and Tropical Army Worm, Spodoptera litura F.; Panel Proceedings; International Atomic Energy Agency: Vienna, Austria, 1993. [Google Scholar]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Ravenscroft, J.; Malik, S.; Gill, M.S.; Walker, D.W.; Clayton, P.E.; Wallace, D.C.; Malfroy, B.; Doctrow, S.R.; Lithgow, G.J. Extension of life-span with superoxide dismutase/catalase mimetics. Science 2000, 289, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, T.; Floyd, R.A. Susceptibility of Glutathione Peroxidase and Glutathione Reductase to Oxidative Damage and the Protective Effect of Spin Trapping Agents. Arch. Biochem. Biophys. 1994, 314, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Shishido, T. Distribution of Glutathione S-transferase Activity in Insect Tissues. Appl. Entomol. Zool. 1992, 27, 391–397. [Google Scholar] [CrossRef]
- Leu, J.I.; Julia, P.; Amanda, F.; Maureen, E.M.; Donna, L.G. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 2009, 36, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult drosophila melanogaster. FEBS J. 2010, 277, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Piano, A.; Franzellitti, S.; Tinti, F.; Fabbri, E. Sequencing and expression pattern of inducible heat shock gene products in the European flat oyster, Ostrea edulis. Gene 2005, 361, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Renner, T.; Waters, E.R. Comparative genomic analysis of the HSP70S from five diverse photosynthetic eukaryotes. Cell Stress Chaperones 2007, 12, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Udaka, H.; Ueda, C.; Goto, S.G. Survival rate and expression of heat-shock protein 70 and frost genes after temperature stress in drosophila melanogaster lines that are selected for recovery time from temperature coma. J. Insect Physiol. 2010, 56, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Ludewig, M.H.; Boshoff, A. Investigating the chaperone properties of a novel heat shock protein, HSP70.c, from Trypanosoma brucei. J. Parasitol. Res. 2014, 2014, 172582. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.J.; Chung, H.Y.; Kim, T.H.; Cho, C.K.; Yoo, S.Y.; Lee, Y. Inducible heat-shock protein 70 is involved in the radioadaptive response. Radiat. Res. 2000, 153, 318–326. [Google Scholar] [CrossRef]
- Mehlen, P.; Kretzremy, C.; Briolay, J.; Fostan, P.; Mirault, M.E.; Arrigo, A.P. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (HSP27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem. J. 1995, 312, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Preville, X.; Chareyron, P.; Briolay, J.; Klemenz, R.; Arrigo, A.P. Constitutive expression of human HSP27, drosophila HSP27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J. Immunol. 1995, 154, 363–374. [Google Scholar] [PubMed]
- Cui, X.H.; Xie, M.; Wan, F.H. Changes in expression level of heat shock protein 70 gene in Bemisia tabaci B-biotype (Homoptera:Aleyrodidae) under high temperature stress. Acta Entomol. Sin. 2007, 50, 1087–1091. [Google Scholar]
- Augustyniak, M.; Tarnawska, M.; Babczyńska, A.; Augustyniak, M. Hsp70 level in progeny of aging grasshoppers from variously polluted habitats and additionally exposed to zinc during diapause. J. Insect Physiol. 2009, 55, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shao-Guo, R.U.; Jiang, M. The influence of monocrotophos on microstructure, ultrastructure and characteristic enzyme in goldfish (Carassius auratus) testis. J. Fish. China 2007, 31, 568–574. [Google Scholar]
- Wang, X.-P.; Fang, Y.-L.; Zhang, Z.-N. Effects of delayed mating on the fecundity, fertility and longevity of females of diamondback moth, Plutella xylostella. Insect Sci. 2011, 18, 305–310. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, K.F.; Jing, Y.P.; Zhuang, H.M.; Wu, G. Identification of heat shock protein genes HSP70S and hsc70 and their associated mRNA expression under heat stress in insecticide-resistant and susceptible diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Eur. J. Entomol. 2015, 2, 215–226. [Google Scholar] [CrossRef]
- Larionov, A.; Krause, A.; Miller, W. A standard curve based method for relative real time PCR data processing. BMC Bioinform. 2005, 6, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.M.; Wang, K.F.; Zheng, L.; Wu, Z.J.; Miyata, T.; Wu, G. Identification and characterization of a cytochrome P450 CYP6CX1 putatively associated with insecticide resistance in Bemisia tabaci. Insect Sci. 2011, 18, 484–494. [Google Scholar] [CrossRef]
- Paladino, L.Z.C.; Ferrari, M.E.; Lauría, J.P.; Cagnotti, C.L.; Šíchová, J.; López, S.N. The effect of x-rays on cytological traits of tuta absoluta (lepidoptera: Gelechiidae). Fla. Entomol. 2016, 99, 43–53. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, N.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharm. 2007, 222, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Metcalf, N.B.; Monaghan, P. Ecological processes in a hormetic framework. Ecol. Lett. 2010, 13, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. Using Drosophila melanogaster to study the positive effets of mild stress on aging. Exp. Gerontol. 2011, 46, 345–348. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Luo, L.; Karthi, S.; Zhang, K.; Luo, J.; Hu, Q.; Weng, Q. Effects of 200 Gy 60Co-γ Radiation on the Regulation of Antioxidant Enzymes, Hsp70 Genes, and Serum Molecules of Plutella xylostella (Linnaeus). Molecules 2018, 23, 1011. https://doi.org/10.3390/molecules23051011
Li X, Luo L, Karthi S, Zhang K, Luo J, Hu Q, Weng Q. Effects of 200 Gy 60Co-γ Radiation on the Regulation of Antioxidant Enzymes, Hsp70 Genes, and Serum Molecules of Plutella xylostella (Linnaeus). Molecules. 2018; 23(5):1011. https://doi.org/10.3390/molecules23051011
Chicago/Turabian StyleLi, Xiaoxue, Lingyan Luo, Sengodan Karthi, Ke Zhang, Jianjun Luo, Qiongbo Hu, and Qunfang Weng. 2018. "Effects of 200 Gy 60Co-γ Radiation on the Regulation of Antioxidant Enzymes, Hsp70 Genes, and Serum Molecules of Plutella xylostella (Linnaeus)" Molecules 23, no. 5: 1011. https://doi.org/10.3390/molecules23051011
APA StyleLi, X., Luo, L., Karthi, S., Zhang, K., Luo, J., Hu, Q., & Weng, Q. (2018). Effects of 200 Gy 60Co-γ Radiation on the Regulation of Antioxidant Enzymes, Hsp70 Genes, and Serum Molecules of Plutella xylostella (Linnaeus). Molecules, 23(5), 1011. https://doi.org/10.3390/molecules23051011





