Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops
Abstract
1. Introduction
2. Prenylated Flavonoids
3. Mechanism of Action, Pharmacokinetics, and Biotransformation
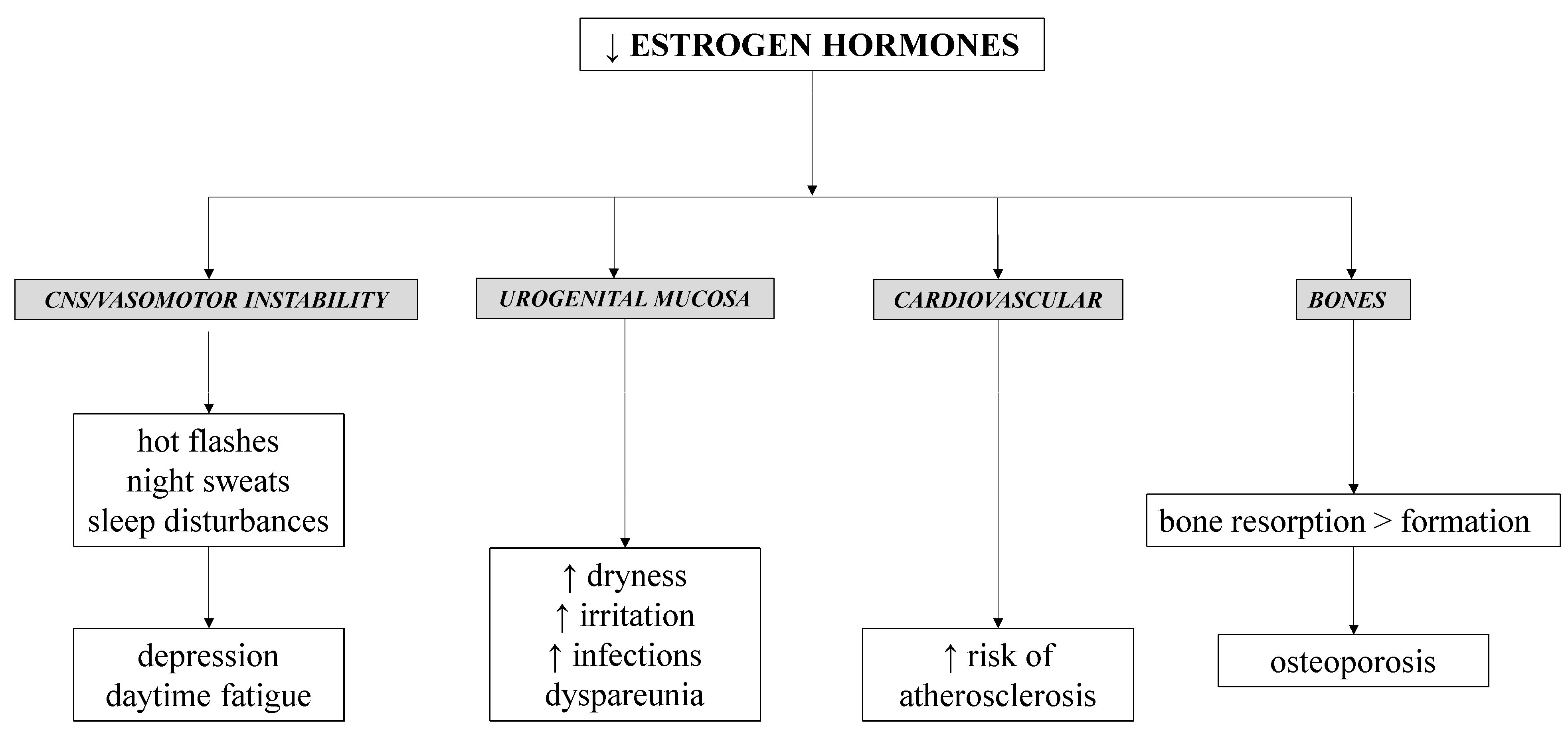
4. Menopause Therapy
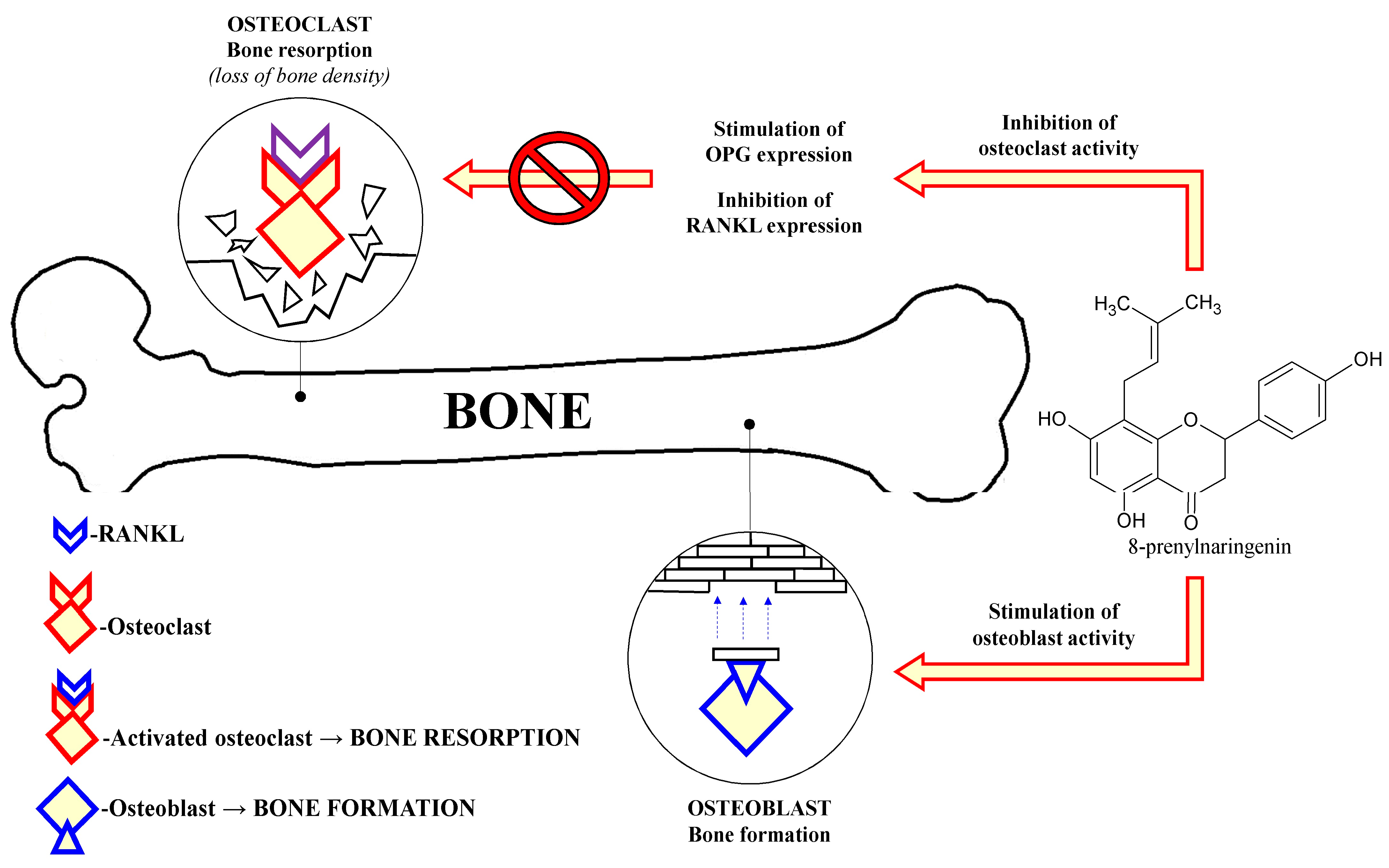
5. Prevention of Osteoporosis
6. Anticancer and Other Miscellaneous Effects of 8-PN
7. Safety Issues
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Bartmanska, A.; Tronina, T.; Poplonski, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, L.; Pauli, G.; Farnsworth, N. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Bolego, C.; Poli, A.; Cignarella, A.; Paoletti, R. Phytoestrogens: Pharmacological and therapeutic perspectives. Curr. Drug Targets 2003, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Mukai, R. Prenylation modulates the bioavailability and bioaccumulation of dietary flavonoids. Arch. Biochem. Biophys. 2014, 559, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ohn Mar, S.; Malhi, F.S.; Syed Rahim, S.H.; Soe, M.M. Chinese and Indian women's experience with alternative medications for menopause related symptoms: A qualitative analysis. Chin. J. Integr. Med. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women's health initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Anderson, J.P.; Ross, J.A. Estrogen replacement therapy and ovarian cancer. Epidemiology 2004, 15, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Heyerick, A.; Vervarcke, S.; Depypere, H.; Bracke, M.; De Keukeleire, D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas 2006, 54, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Aghamiri, V.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S.; Nazemiyeh, H. The effect of hop (Humulus lupulus L.) on early menopausal symptoms and hot flashes: A randomized placebo-controlled trial. Complement. Ther. Clin. Pract. 2016, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, R.; Vervarcke, S.; Vansteelandt, S.; Rompotti, P.; De Keukeleire, D.; Heyerick, A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine 2010, 17, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B.; Smeets, E.E.; Rijk, J.C.; Nijmeijer, S.M.; van den Berg, M. Phytoestrogens in menopausal supplements induce ER-dependent cell proliferation and overcome breast cancer treatment in an in vitro breast cancer model. Toxicol. Appl. Pharmacol. 2013, 269, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Uram, L.; Podgorski, R. In vitro effect of 8-prenylnaringenin and naringenin on fibroblasts and glioblastoma cells-cellular accumulation and cytotoxicity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Choi, H.S.; Choi, H.S.; Choi, Y.K.; Um, J.Y.; Choi, I.; Shin, Y.C.; Ko, S.G. Phytoestrogens induce apoptosis via extrinsic pathway, inhibiting nuclear factor-κB signaling in HER2-overexpressing breast cancer cells. Anticancer Res. 2011, 31, 3301–3313. [Google Scholar] [PubMed]
- Busch, C.; Noor, S.; Leischner, C.; Burkard, M.; Lauer, U.M.; Venturelli, S. Anti-proliferative activity of hop-derived prenylflavonoids against human cancer cell lines. Wien. Med. Wochenschr. 2015, 165, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Helle, J.; Bader, M.I.; Ehrhardt, T.; Nestler, K.; Kretzschmar, G.; Bernhardt, R.; Vollmer, G.; Nikolic, D.; Bolton, J.L.; et al. A standardized Humulus lupulus (L.) ethanol extract partially prevents ovariectomy-induced bone loss in the rat without induction of adverse effects in the uterus. Phytomedicine 2017, 34, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T. Význam chmelových prenylflavonoidů pro lidské zdraví. Chem. Listy 2012, 106, 1095–1103. [Google Scholar]
- Nikolic, D.; van Breemen, R.B. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013, 9, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Milligan, S.R. Reproductive and estrogenic effects of 8-prenylnaringenin in hops. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: San Diego, CA, USA, 2008; pp. 711–723. [Google Scholar]
- Koch, W.; Heim, G. Estrogens in hops and beer; preliminary report. Munch. Med. Wochenschr. 1953, 95, 845. [Google Scholar] [PubMed]
- Milligan, S.; Kalita, J.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 155–165. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, S.; Zarghi, A. Estrogen receptor ligands: A review (2013–2015). Sci. Pharm. 2016, 84, 409. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Gruppen, H.; Bovee, T.F.; Verbruggen, M.A.; Vincken, J.-P. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs). Food Funct. 2012, 3, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.D.; Shih, E.; Thacker, H.L. ERAAs for menopause treatment: Welcome the ‘designer estrogens’. Clevel. Clin. J. Med. 2017, 84, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric 2012, 15, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Besselink, E.; Henning, S.; Go, V.; Heber, D. Phytoestrogens induce differential estrogen receptor alpha-or beta-mediated responses in transfected breast cancer cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef]
- Roelens, F.; Heldring, N.; Dhooge, W.; Bengtsson, M.; Comhaire, F.; Gustafsson, J.A.; Treuter, E.; De Keukeleire, D. Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors α and β. J. Med. Chem. 2006, 49, 7357–7365. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, G.; Zierau, O.; Wober, J.; Tischer, S.; Metz, P.; Vollmer, G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem. Mol. Biol. 2010, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 58, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.; Hümpel, M.; Schaefer, O.; Schoemaker, R.; Schleuning, W.D.; Cohen, A.; Burggraaf, J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006, 62, 288–296. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.B. Pharmacokinetic and pharmacologic variation between different estrogen products. J. Clin. Pharmacol. 1995, 35, 18S–24S. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S. The oral bioavailability of 8-prenylnaringenin from hops (Humulus lupulus L.) in healthy women and men is significantly higher than that of its positional isomer 6-prenylnaringenin in a randomized crossover trial. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; Grubjesic, S.; Schwab, P.; Metz, P.; Van Breemen, R.B. Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes. Drug Metab. Dispos. 2004, 32, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.-H.; Kang, B.Y.; Lee, I.-S. Microbial metabolites of 8-prenylnaringenin, an estrogenic prenylflavanone. Arch. Pharm. Res. 2008, 31, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006, 136, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mobedi, H.; Roozbeh, N. Hops for menopausal vasomotor symptoms: Mechanisms of action. J. Menopausal Med. 2016, 22, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Lamuela-Raventós, R.M.; Estruch, R.; Sasot, G.; Doménech, M.; Tresserra-Rimbau, A. Beer polyphenols and menopause: Effects and mechanisms—A review of current knowledge. Oxid. Med. Cell. Longev. 2017, 2017, 4749131. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; Franks, R.B.; Fox, C. Review of efficacy of complementary and alternative medicine treatments for menopausal symptoms. J. Midwifery Womens Health 2017, 62, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mobedi, H.; Mosaffa, N.; Dolatian, M.; Ramezani Tehrani, F. Hormone therapy for relieving postmenopausal vasomotor symptoms: A systematic review. Arch. Iran. Med. 2016, 19, 141–146. [Google Scholar] [PubMed]
- Miller, V.M.; Harman, S.M. An update on hormone therapy in postmenopausal women: Mini-review for the basic scientist. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1013–H1021. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D. The evidence base for HRT: What can we believe? Climacteric 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Pickar, J.H.; Stevenson, J.C.; Mack, W.J.; Hodis, H.N. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016, 254, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. Complementary and alternative approaches to menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Tonob, D.; Melby, M.K. Broadening our perspectives on complementary and alternative medicine for menopause: A narrative review. Maturitas 2017, 99, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Locklear, T.D.; Doyle, B.J.; Perez, A.L.; Wicks, S.M.; Mahady, G.B. Menopause in Latin America: Symptoms, attitudes, treatments and future directions in Costa Rica. Maturitas 2017, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Depypere, H.T.; Comhaire, F.H. Herbal preparations for the menopause: Beyond isoflavones and black cohosh. Maturitas 2014, 77, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.; Li, X.F.; Kinsey-Jones, J.; Heyerick, A.; Brain, S.; Milligan, S.; O’Byrne, K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol. 2006, 191, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.A.; Potthoff, P.; Schneider, H.P. International versions of the menopause rating scale (MRS). Health Qual. Life Outcomes 2003, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Zierau, O.; Kretzschmar, G. Hop extracts and hop substances in treatment of menopausal complaints. Planta Med. 2013, 79, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Humpel, M.; Isaksson, P.; Schaefer, O.; Kaufmann, U.; Ciana, P.; Maggi, A.; Schleuning, W.D. Tissue specificity of 8-prenylnaringenin: Protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J. Steroid Biochem. Mol. Biol. 2005, 97, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Lv, X.; Ma, X.N.; Ge, B.F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Kang, L.; Ma, Y.; Chen, H.; Kuang, H.; Huang, Q.; He, M.; Peng, W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7. Food Sci. Nutr. 2014, 2, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hemachandra, L.; Madhubhani, P.; Chandrasena, R.; Esala, P.; Chen, S.-N.; Main, M.; Lankin, D.C.; Scism, R.A.; Dietz, B.M.; Pauli, G.F. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A). Cancer Prev. Res. 2012, 5, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodrigues, I.; Guardao, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhaes, J.; Ferreira-de-Almeida, M.; Negrao, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fugh-Berman, A. “Bust enhancing” herbal products. Obstet. Gynecol. 2003, 101, 1345–1349. [Google Scholar] [PubMed]
- Di Vito, C.; Bertoni, A.; Nalin, M.; Sampietro, S.; Zanfa, M.; Sinigaglia, F. The phytoestrogen 8-prenylnaringenin inhibits agonist-dependent activation of human platelets. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Kirichenko, T.V.; Melnichenko, A.A.; Orekhova, V.A.; Ravani, A.; Poggio, P.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Anti-atherosclerotic effects of a phytoestrogen-rich herbal preparation in postmenopausal women. Int. J. Mol. Sci. 2016, 17, 1318. [Google Scholar] [CrossRef] [PubMed]
- De Cremoux, P.; This, P.; Leclercq, G.; Jacquot, Y. Controversies concerning the use of phytoestrogens in menopause management: Bioavailability and metabolism. Maturitas 2010, 65, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; De Cremoux, P.; This, P.; Jacquot, Y. Lack of sufficient information on the specificity and selectivity of commercial phytoestrogens preparations for therapeutic purposes. Maturitas 2011, 68, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Dorfelt, P.; Chatterjee, N.; Helle, J.; Bader, M.I.; Vollmer, G.; Kretzschmar, G.; Kuhlee, F.; Thieme, D.; Zierau, O. Assessment of the effects of naringenin-type flavanones in uterus and vagina. J. Steroid Biochem. Mol. Biol. 2015, 145, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Helle, J.; Kraker, K.; Bader, M.I.; Keiler, A.M.; Zierau, O.; Vollmer, G.; Welsh, J.; Kretzschmar, G. Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol. Cell. Endocrinol. 2014, 392, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Macejova, D.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; Chen, S.N.; van Breemen, R.B.; Nikolic, D.; Goerl, F.; Muders, M.H.; et al. Evaluation of estrogenic potency of a standardized hops extract on mammary gland biology and on MNU-induced mammary tumor growth in rats. J. Steroid Biochem. Mol. Biol. 2017, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Li, J.; Nikolic, D.; Roche, N.; Blondeel, P.; Possemiers, S.; De Keukeleire, D.; Bracke, M.; Heyerick, A.; Van Breemen, R.B. Disposition of hop prenylflavonoids in human breast tissue. Mol. Nutr. Food Res. 2010, 54, S284–S294. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Jacquot, Y. Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer-considerations concerning related efficacy and safety. J. Steroid Biochem. Mol. Biol. 2014, 139, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Solak, K.A.; Santos, R.R.; van den Berg, M.; Blaauboer, B.J.; Roelen, B.A.; van Duursen, M.B. Naringenin (NAR) and 8-prenylnaringenin (8-PN) reduce the developmental competence of porcine oocytes in vitro. Reprod. Toxicol. 2014, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qiu, X.; Nikolic, D.; Chen, S.N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Phys. 2007, 76, 391–396. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules 2018, 23, 660. https://doi.org/10.3390/molecules23030660
Štulíková K, Karabín M, Nešpor J, Dostálek P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules. 2018; 23(3):660. https://doi.org/10.3390/molecules23030660
Chicago/Turabian StyleŠtulíková, Kateřina, Marcel Karabín, Jakub Nešpor, and Pavel Dostálek. 2018. "Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops" Molecules 23, no. 3: 660. https://doi.org/10.3390/molecules23030660
APA StyleŠtulíková, K., Karabín, M., Nešpor, J., & Dostálek, P. (2018). Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules, 23(3), 660. https://doi.org/10.3390/molecules23030660





