Abstract
Flavokawain B (1) is a natural chalcone extracted from the roots of Piper methysticum, and has been proven to be a potential cytotoxic compound. Using the partial structure of flavokawain B (FKB), about 23 analogs have been synthesized. Among them, compounds 8, 13 and 23 were found in new FKB derivatives. All compounds were evaluated for their cytotoxic properties against two breast cancer cell lines, MCF-7 and MDA-MB-231, thus establishing the structure–activity relationship. The FKB derivatives 16 (IC50 = 6.50 ± 0.40 and 4.12 ± 0.20 μg/mL), 15 (IC50 = 5.50 ± 0.35 and 6.50 ± 1.40 μg/mL) and 13 (IC50 = 7.12 ± 0.80 and 4.04 ± 0.30 μg/mL) exhibited potential cytotoxic effects on the MCF-7 and MDA-MB-231 cell lines. However, the methoxy group substituted in position three and four in compound 2 (IC50 = 8.90 ± 0.60 and 6.80 ± 0.35 μg/mL) and 22 (IC50 = 8.80 ± 0.35 and 14.16 ± 1.10 μg/mL) exhibited good cytotoxicity. The lead compound FKB (1) showed potential cytotoxicity (IC50 = 7.70 ± 0.30 and 5.90 ± 0.30 μg/mL) against two proposed breast cancer cell lines. It is evident that the FKB skeleton is unique for anticancer agents, additionally, the presence of halogens (Cl and F) in position 2 and 3 also improved the cytotoxicity in FKB series. These findings could help to improve the future drug discovery process to treat breast cancer. A molecular dynamics study of active compounds revealed stable interactions within the active site of Janus kinase. The structures of all compounds were determined by 1H-NMR, EI-MS, IR and UV and X-ray crystallographic spectroscopy techniques.
1. Introduction
Breast cancer (BC) is a complex disease, composed of several subtype receptors both at the molecular and clinical level. BC is still the leading causes of death globally in women [1,2,3]. Chalcones are α,β-unsaturated carbonyl compounds with two aromatic rings (ring A and B), conjugated double bonds and a completely delocalized π-electron system [4,5]. Chalcones and their related analogues display several pharmacological properties, such as anticancer [6,7,8,9,10], anti-inflammatory [11,12,13,14], antimalarial [15,16], antileishmanial [17,18], antimicrobial [19], antifungal [20], antioxidant [21,22,23] and anticonvulsant activities [24]. Chalcone derivatives show acetylcholinesterase, tyrosinase [25,26], and monoamine oxidase (MAO) [27] enzyme inhibitory activities. Chalcones have gained the strong attention from scientists searching for new pharmacological active analogs.
Flavokawain B (FKB) (1), is a main bioactive chalcone isolated from the roots of Piper methysticum [28], which exhibited strong cytotoxicity against various cancer cell lines [29,30,31]. In this study, we synthesized 1–23 FKB analogs by manipulating ring B with the various substituted benzaldehydes. The main purpose when designing this proposal was to observe the effects of ring B in the breast cancer cell lines and to establish the structure–activity relationship, to eventually help future drug discovery. Previously, we investigated the antinociceptive, cytotoxic and anti-inflammatory activities of FKB (1) in vitro and in vivo [32,33,34,35,36].
2. Results and Discussion
2.1. Chemistry
Flavokawain B derivatives (1–23) were synthesized by Claisen–Schmidt condensation reaction using the template of FKB (1) (Scheme 1). FKB comprises ring A (2′-hydroxy-4′,6′-dimethoxyacetophenone) and B (benzaldehyde). Among FKB derivatives, three chalcones (E)-3-(2,5-dimethoxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (8), (E)-3-(2-fluorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (13) and (E)-3-(2-bromo-3-hydroxy-4-methoxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (23) were found to be new FKB analogs (Figure 1). Previously, compound 13 was synthesized by Bandgar and used as an intermediate for the preparation of nitrogen-containing chalcones, however, the data were not published [37]. The structure of FKB derivatives (1–23) were characterized by detailed spectroscopic techniques. The presence of characteristic signals appeared in 1H-NMR at δ 7.66–7.84 (J = 15–16 Hz) and 7.86–8.0 (J = 15–16 Hz) were assigned to α and β protons, respectively. The presence of carbonyl groups was supported by the signals at δ 191–193.45 in 13C-NMR and 1625–1634 cm−1 in the IR spectrum, respectively. The complete assignments and spectral data of compounds 8, 13 and 23 are given in Table 1, while the structure of compounds 7 and 9 was confirmed by the single X-ray crystallographic technique (Figure 2).
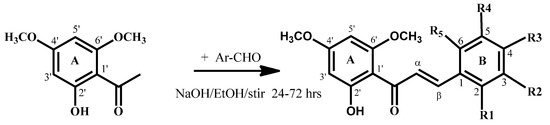
Scheme 1.
Synthesis of flavokawain B derivatives 1–23.
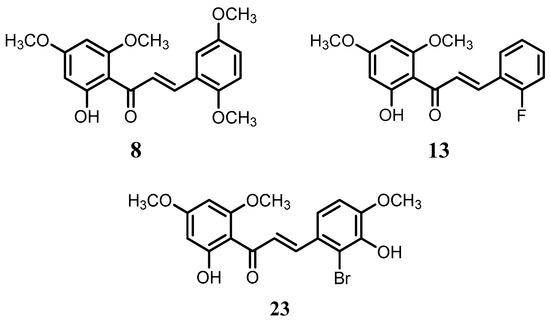
Figure 1.
The structure of compounds 8, 13 and 23.

Table 1.
1H- (600 MHz) and 13C- (150 MHz) NMR data for compound 8, 13 and 23.
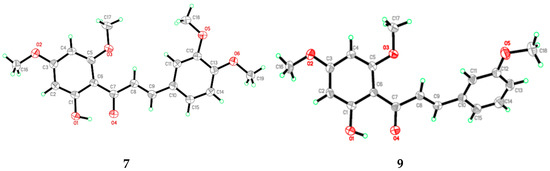
Figure 2.
ORTEP diagram of compound 7 and 9.
2.2. Structure–Activity Relationships
FKB derivatives 1–23 were screened against two breast cancer cell lines MCF-7 (human estrogen receptor positive breast cancer cells) and MDA-MB-231 (human estrogen receptor negative) breast cancer cell lines). FKB (1) still possessed greater cytotoxicity (IC50 = 7.70 ± 0.30 and 5.90 ± 0.30 μg/mL) compared to the majority of FKB derivatives. Of the 23 tested compounds, thirteen FKB derivatives recorded higher IC50 values than FKB (1). On the other hand, FKB derivatives 2–4, 7, 9, 13, 15, 16 and 22 were recorded as having potential cytotoxic effects on both MCF-7 and MDA-MB-231 cells, compared to FKB (1), see Table 2. Among the FKB derivatives, compounds 13, 15 and 16 showed slightly better cytotoxicity than FKB (1) and flavokawain A (FKA) (2) for MCF-7 cells.

Table 2.
IC50 values (μg/mL) of FKB derivatives after 72 h treatment.
Among the FKB derivatives with an IC50 value lower than 30 μg/mL, only compound 3 and 22 showed higher sensitivity to MCF-7 cells than MDA-MB-231, indicating that the modification of the FKB skeleton helps the selectivity between these two cell lines. In short, the FKB derivatives FKB-2F (13), FKB-3Cl (15) and FKB-2Cl (16) exhibited greater sensitivity to both tested cell lines, warranting further evaluation of their antitumor effects. It is well established that the anticancer properties of chalcones are due to the presence of α,β-unsaturated ketone moieties. Among the tested chalcones, the 2′ hydroxyl group in ring A is essential for the cytotoxicity, as has been studied against several biological assays, predominantly its anticancer properties [38,39]. The presence of electron-withdrawing and electron-donating groups effects the α,β-unsaturated system [38,40], which eventually effects the cytotoxicity. Generally, the electron-donating group in ring A improves the cytotoxic properties, as it affects the acidity of the 2′ hydroxyl group in ring A, while the electron-donating group in ring B, as in compound 5, 6, 8 and 10, did not improve the IC50 values at 30 μg/mL (Table 2), which possibly due to the effects of the electron-donating group on the α, β-unsaturated system. Since the α,β-unsaturated system becomes more nucleophile and the Michael receptor or protein cannot bind effectively thus activity decreases [38]. However, compounds 4 and 7 showed slightly better activity due to the selective position of the methoxy groups at 3, 4 and 2, 3, which contributed less to the α,β-unsaturated ketone. In contrast, compounds with electron-withdrawing groups in ring B possessing 2Cl (16), 3Cl (15) and 2F (13) exhibited better cytotoxicity, see Table 2. The presence of the electron-withdrawing group in ring B, especially at position 2 and 3, as in 13 (2F), 15 (3Cl) and 16 (2Cl), contributed more to the enhancement of cytotoxicity against breast cancer cell lines (38, 39). While the presence of electron-withdrawing groups in compounds 13, 15 and 16 pulling the electrons from the α,β-unsaturated ketone make it more electrophilic, so the receptor (nucleophile) possibly creates a strong interaction with the compound. While the electrons with the donating group, such as methoxy groups—especially at position 3, 4 or 3, 5 and 2, 4, 6, as in compounds 5, 6, 8, 10, 11, cause rich in nucleophilicity and resonate the α,β-unsaturated carbonyl [41], eventually, the structure become less effective and binding or the interaction with the Michael receptor (nucleophile), and the compounds reduce the cytotoxic effects [38,39].
2.3. Computational Studies
The potential FKB derivatives 1, 13, 15 and 16 were subjected to rigid receptor docking, and the resulting poses were analyzed visually. The crystal structure of JAK2 in complex with a potent quinoxaline inhibitor, PDB: 3KRR, was downloaded in MOE and subjected to protein preparation. The results of a PASS prediction highlight the probability of the activity of these compounds as a caspase-3 stimulant, JAK2 expression inhibitor, and apoptosis agonist, as shown in Table S1. Caspase-3 belongs to the cystine–aspartic-acid protease family of proteins [42], known to play a fundamental role in the execution phase of cell death in apoptosis [43]. The activators of caspase-3, such as MX-2060 [12,44], Smac [45] and Leachianone A [46], induce apoptosis in various types of cancers.
The high Pa values of the compounds (0.892–0.924) allowed us to conclude that our newly-synthesized chalcone derivatives may exhibit anticancer effects by activating the caspase-3-dependent apoptotic pathway. Moreover, the cell-specific response of the chalcone derivatives can be explained partly by the fact that the human MCF-7 breast carcinoma cells completely lack caspase-3 expression as a result of a frameshift mutation [43,47]. This loss-of-function mutation has contributed to chemotherapeutic resistance in breast cancer [12,44]. The Janus kinase-2 (JAK2) protein is targeted by many anti-cancer drugs to produce their response [45,48]. As presented in Table S1, our compounds may inhibit JAK2, with Pa values ranging from 0.821 to 0.878. We carried out a molecular docking simulation to establish the binding mode of the potent FKB derivatives with IC50 values in single digits, namely 1, 13, 15 and 16, with JAK2 (PDB:3KRR). All the compounds fit the active site of JAK2 wells, depicted in Figure 3. The docking results revealed that all the compounds mediated hydrophobic contacts with Val863 and Leu983. The methoxy group of the compounds interacted via an acceptor–donor hydrogen bonding motif to the Leu932 backbone. A hydrogen bond with Leu932 provided these compounds with anchorage in the cavity. In the case of compound 13 and 15, an additional hydrogen bond was observed with Leu855 and Asp944, respectively. Analysis of the average duty cycle revealed that the most stable hydrogen bond pair was Arg938 and O2, and Leu932 and O3 in the 13 and 16 complexes, respectively. The binding profile of 13-JAK2 shows equally high expression of three different host–guest pairs, Gly856, Leu855 and Lys857. Accurate characterization of protein–ligand interactions is indispensable for the drug design. Molecular dynamic simulation is a state-of-art technique that is used to characterize the time-dependent behavior for protein–ligand complex (See Supplementary Material).
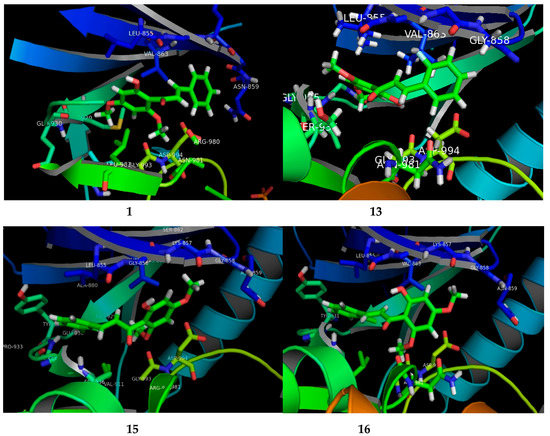
Figure 3.
The visual presenting the interaction of FKB derivatives (1, 13, 15 and 16) with the ATP binding site of human JAK2 tyrosine kinase.
3. Materials and Methods
3.1. Chemistry
Melting points were determined on a Fisher–Johns melting apparatus and are uncorrected. UV spectra were recorded on UV-VIS spectrophotometer model of type Genesys 10s and expressed in nm. FT-IR spectroscopic studies were carried out on a FTIR spectrophotometer 1000, model Perkin Elmer, at a room temperature of 25 °C. KBr pellets were dried in an oven and scanned for calibration purposes. 1H-NMR spectra of compounds were recorded on a Bruker Ascend TM 600 MHz machine. The chemical shifts (δ) are presented with reference to CDCl3 (δ: 7.25) and tetramethylsilane (TMS) (δ: 0.00) as the internal reference. Electron-spray ionization mass spectra in positive mode (ESI-MS) data were recorded on a Bruker Esquire 3000+ spectrometer. Column chromatography purifications were carried out on a Silica Gel 60 (Merck, 70–230 mesh). The purity of compounds was checked by thin-layer chromatography (TLC) and 1H-NMR. All the chemicals were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Other reagents were purchased from Sinopharm Chemical Reagent Co. Ltd., (Shanghai, China).
3.2. Docking Analysis Method
To rationalize the anti-cancer potential of FKB derivatives, the online web Prediction of Activity Spectra for Substances (PASS) server was used to predict the biological activity of potential FKB derivatives [49,50]. The information on the compounds was uploaded with the value of probability of activity (Pa) set to 0.7. The results were then analyzed critically to understand the molecular basis for the anti-cancer activity of the compounds. Molecular docking simulation of selected inhibitors against JAK2 was carried out using MOE [51]. The crystal structure of JAK2 in complex with a potent quinoxaline inhibitor, PDB:3KRR [52], was downloaded in MOE. The derivatives with potential IC50 values (Table 2.) for compound 1, 13, 15 and 16 were subjected to rigid receptor docking, and the resulting poses were analyzed visually. All the graphics were rendered using MOE and Chimera [53]. To study the dynamic behavior of the proposed ligand–protein complex, we have performed in a short production run of 10 ns using AMBER 14 [54]. The resulting trajectories were analyzed using the CPPTRAJ module in AMBERTOOLS 16 [55,56].
3.3. Synthesis of Flavokawain B derivatives
KOH (20% w/v aqueous solution) was added to a stirred solution of the appropriate acetophenone (1.0 eq) and a substituted benzaldehyde (1.25 eq.) in ethanol, and the mixture was stirred at room temperature for 48–72 h. The reaction mixture was cooled to 0 °C (ice-water bath) and acidified with HCl (10% v/v aqueous solution). In most cases, yellow precipitate was formed, filtered and washed with 10% aqueous HCl solution. In the cases where an orange-yellow layer was formed, the mixture was extracted with CH2Cl2 or ethyl acetate, the extracts was dried over Na2SO4 anhydrous and the solvent was evaporated and crystalized with methanol or ethanol.
3.4. Characterization Data
The data of flavokawain B (1) and flavokawain A (2) have been published previously by us [26,57].
(E)-1-(2′-Hydroxy-4′,6′-dimethoxyphenyl)-3-(4-(methylthio)phenyl)prop-2-en-1-one (3). Orange crystal (78.3% yield); m.p.: 129–131 °C; UV-Vis (CHCl3) λmax: 371–372 nm; IR (KBr, cm−1): 3469 (O-H), 2981–3014 (Ar C-H stretch), 1619 (C=O), 1548–1584 (C=C-C=O), 1431–1432 (Ar C=C), 1155–1218 (C-O) [36].
(E)-3-(2,3-Dimethoxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (4). Yellow crystal (83.4% yield); m.p.: 121–123 °C (lit. [58] 120–122 °C); UV-Vis (MeOH)λmax: 335–337 nm; IR (KBr, cm−1): 3458 (O-H), 2839–2985 (Ar C-H stretch), 1627 (C=O), 1556–1581 (C=C-C=O), 1350–1442 (C=C), 1213–1268 (C-O); 1H-NMR (600 MHz, CDCl3) δ: 14.34 (s, 1H, OH, C-2′), 8.08 (d, 1H, H-β, J = 15.78 Hz), 7.96 (d, 1H, H-α, J = 15.78 Hz), 7.24 (d, 1H, H-6, J = 7.74 Hz), 7.08 (t, 1H, H-5, J = 8.04, 7.98 Hz), 6.95 (d, 1H, H-4, J = 8.04 Hz), 6.11 (d, 1H, H-3′, J = 2.10 Hz), 5.96 (d, 1H, H-5′, J = 2.10 Hz), 3.91 (s, 3H, OCH3, C-6′), 3.90 (s, 3H, OCH3, C-2), 3.89 (s, 3H, OCH3, C-3), 3.84 (s, 3H, OCH3, C-4′); 13C-NMR (150 MHz, CDCl3) δ: 192.93 (C=O), 168.40 (C-2), 166.18 (C-6′), 162.55 (C-4′), 153.24 (C-3), 148.88 (C-1), 137.19 (C-2′), 129.79 (C-5), 128.95 (C-6), 124.12 (C-4), 119.80 (C-5′), 113.76 (C-3′), 106.47 (C-1′), 93.70 (C-β), 91.20 (C-α), 61.31 (OCH3), 55.92 (OCH3), 55.84 (OCH3), 55.58 (OCH3); EI-MS: m/z = 344 (60), 329 (7), 313 (100), 207 (30), 181 (24), 180 (87), 164 (70), 152 (37), 149 (55), 137 (51), 121 (52), 91 (25), 77 (21), 69 (13); (Molecular formula C19H20O6).
(E)-1-(2′-Hydroxy-4′,6′-dimethoxyphenyl)-3-(2,4,6-trimethoxyphenyl)prop-2-en-1-one (6). Orange crystal (82.8% yield); m.p.: 158–159 °C (lit. [59] 151–153 °C); UV-Vis (MeOH): λmax: 386 nm; IR (KBr, cm−1): 3464 (O-H), 2941–3010 (Ar C-H stretch), 1618 (C=O), 1414–1545 (C=C), 1122–1217 (C-O); 1H-NMR (600 MHz, CDCl3) δ; 14.76 (s, 1H, OH, C-2′), 8.32 (d, 1H, H-β, J = 15.78 Hz), 8.25 (d, 1H, H-α, J = 15.78 Hz), 6.13 (s, 2H, H-3 and H-5), 6.09 (d, 1H, H-3′, J = 2.40 Hz), 5.94 (d, 1H, H-5′, J = 2.40 Hz), 3.90 (s, 3H, OCH3, C-6 and C-6′), 3.89 (s, 3H, OCH3, C-2), 3.85 (s, 3H, OCH3, C-4′), 3.82 (s, 3H, OCH3, C-4); EI-MS: m/z = 374 (2), 344 (44), 329 (8), 313 (100), 281 (10), 207 (50), 181 (25), 180 (54), 164 (44), 152 (21), 149 (37), 137 (37), 121 (33), 105 (12), 91 (26), 77 (23), 69 (15); (Molecular formula C20H22O7).
(E)-3-(2,5-Dimethoxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (8). EI-MS: m/z = 344 (78), 327 (33), 282 (10), 207 (68), 191 (44), 181 (27), 164 (62), 152 (14), 151 (100), 137 (19); (Molecular formula C19H20O6).
(E)-3-(2-Fluorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (13). EI-MS: m/z = 302 (100), 301 (100), 285 (19), 282 (45), 207 (100), 181 (100), 166 (28), 152 (24), 149 (28), 137 (67), 121 (44), 101 (88), 95 (51), 69 (52); (Molecular formula C17H15FO4).
(E)-3-(4-Fluorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (14). Yellow flat crystal (80.7% yield); m.p.: 144–145 °C (lit. [17] 140–141 °C); UV-Vis (MeOH) λmax: 342–343 nm; IR (KBr, cm−1): 3460 (O-H), 2854–3118 (Ar C-H stretch), 1632 (C=O), 1573–1592 (C=C-C=O), 1345–1507 (C=C), 1115–1216 (C-O); 1H-NMR (600 MHz, CDCl3) δ: 14.28 (s, 1H, OH, C-2′), 7.82 (d, 1H, H-β, J = 15.60 Hz), 7.74 (d, 1H, H-α, J = 15.60 Hz), 7.59 (m, 1H, H-2), 7.58 (m, 1H, H-6), 7.10 (m, 1H, H-3), 7.09 (m, 1H, H-5), 6.11 (d, 1H, H-3′, J = 2.40 Hz), 5.96 (d, 1H, H-5′, J = 2.40 Hz), 3.92 (s, 3H, OCH3, C-6′), 3.84 (s, 3H, OCH3, C-4′); GC-MS: m/z = 302 (84), 301 (100), 285 (12), 274 (14), 207 (99), 181 (41), 153 (12), 137 (23), 121 (13), 101 (17), 69 (11); (Molecular formula C17H15FO4).
(E)-3-(3-Chlorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (15). Yellow solid (83.1% yield); m.p.: 105–107 °C (lit. [60] 104–106 °C); UV-Vis (MeOH) λmax: 338–339 nm; IR (KBr, cm−1): 3460 (O-H), 2945–3014 (Ar C-H stretch), 1634 (C=O), 1567–1585 (C=C-C=O), 1342–1443 (C=C), 1213–1215 (C-O); 1H-NMR (600 MHz, CDCl3) δ: 14.19 (s, 1H, OH, C-2′), 7.86 (d, 1H, H-β, J = 15.60 Hz), 7.68 (d, 1H, H-α, J = 15.60 Hz), 7.57 (brs, 1H, H-2), 7.46 (m, 1H, H-5) 7.35 (m, 2H, H-4 and H-6), 6.11 (d, 1H, H-3′, J = 2.34 Hz), 5.97 (d, 1H, H-5′, J = 2.34 Hz), 3.93 (s, 3H, OCH3, C-6′), 3.85 (s, 3H, OCH3, C-4′); 13C-NMR (150 MHz, CDCl3) δ: 192.26 (C=O), 168.47 (C-2′), 166.46 (C-4′), 162.50 (C-6′), 140.45 (C-β), 137.45 (C-1), 134.83 (C-3), 130.12 (C-2), 129.84 (C-4), 128.86 (C-5), 127.88 (C-6), 126.62 (C-α), 106.26 (C-1′), 93.81 (C-3′), 91.33 (C-5′), 55.95 (OCH3), 55.65 (OCH3); EI-MS: m/z = 317 (1), 314 (90), 297 (10), 283 (68), 267 (7), 207 (100), 181 (65), 167 (16), 152 (19), 137 (27), 121 (22); (Molecular formula C17H15ClO4).
(E)-3-(2-Chlorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (16). Yellow crystal (80.7% yield); m.p.: 132–134 °C (lit. [18] 135–136 °C); UV-Vis (MeOH) λmax: 341–342 nm; IR (KBr, cm−1): 3448 (O-H), 2957–3132 (Ar C-H stetch), 1632 (C=O), 1556–1585 (C=C-C=O), 1338–1435 (C=C), 1110–1219 (C-O), 745–748 (C-Cl); 1H-NMR (600 MHz, CDCl3) δ: 14.24 (s, 1H, OH, C-2′), 8.12 (d, 1H, H-β, J = 15.60 Hz), 7.85 (d, 1H, H-α, J = 15.60 Hz), 7.67 (m, 1H, H-6), 7.40 (m, 1H, H-3), 7.28 (m, 2H, H-4 and H-5), 6.08 (d, 1H, H-3′, J = 2.34 Hz), 5.93 (d, 1H, H-5′, J = 2.40 Hz), 3.88 (s, 3H, OCH3, C-6′), 3.81 (s, 3H, OCH3, C-4′); EI-MS: m/z = 318 (30), 301 (4), 283 (70), 267 (7), 207 (100), 181 (32), 165 (6), 152 (6), 137 (12), 101 (12), 69 (7); (Molecular formula C17H15ClO4).
(E)-3-(4-Chlorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (17). Yellow crystal (84.3% yield); m.p.: 171–173 °C (lit. [61] 173–175 °C); UV-Vis (MeOH) λmax: 342–343 nm; IR (KBr, cm−1): 3420 (O-H), 2981–3018 (Ar C-H stretch), 1630 (C=O), 1488–1591 (C=C), 1217–1219 (C-O), 820–822 (C-Cl); 1H-NMR (600 MHz, CDCl3) δ: 14.24 (s, 1H, OH, C-2′), 7.85 (d, 1H, H-β, J = 15.60 Hz), 7.72 (d, 1H, H-α, J = 15.60 Hz), 7.53 (d, 1H, H-2, J = 8.46 Hz), 7.52 (d, 1H, H-6, J = 8.46 Hz), 7.38 (d, 1H, H-3, J = 8.46 Hz), 7.37 (d, 1H, H-5, J = 8.46 Hz), 6.11 (d, 1H, H-3′, J = 2.40 Hz), 5.96 (d, 1H, H-5′, J = 2.40 Hz), 3.92 (s, 3H, OCH3, C-6′), 3.84 (s, 3H, OCH3, C-4′); EI-MS: m/z = 318 (57), 301 (7), 283 (2), 207 (100), 181 (34), 165 (13), 152 (19), 137 (25), 111 (6), 102 (20); (Molecular formula C17H15ClO4).
(E)-3-(4-Bromophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (18). Yellow flat crystal (80.5% yield); m.p.: 165.8–167.5 °C (lit. [17] 150–151 °C); UV-Vis (MeOH) λmax: 346 nm; IR (KBr, cm−1): 3447 (O-H), 2949–3010 (Ar C-H stretch), 1633 (C=O), 1568–1590 (C=C-C=O), 1338–1488 (C=C), 1215–1218 (C-O); 1H-NMR (500 MHz, CDCl3) δ: 7.85 (d, 1H, H-β, J = 15.50 Hz), 7.68 (d, 1H, H-α, J = 15.50 Hz), 7.53 (d, 1H, H-2, J = 8.50 Hz), 7.51 (d, 1H, H-6, J = 8.50 Hz), 7.45 (d, 1H, H-3, J = 8.50 Hz), 7.43 (d, 1H, H-5, J = 8.50 Hz), 6.09 (d, 1H, H-3′, J = 2.00 Hz), 5.95 (d, 1H, H-5′, J = 2.50 Hz), 3.90 (s, 3H, OCH3, C-6′), 3.83 (s, 3H, OCH3, C-4′); 13C-NMR (150 MHz, CDCl3) δ: 192.31 (C=O), 168.45 (C-2′), 166.40 (C-4′), 162.48 (C-6′), 140.78 (C-β), 134.53 (C-1), 132.10 (C-3), 132.10 (C-5), 129.68 (C-2), 129.68 (C-6), 128.15 (C-α), 124.19 (C-4), 106.29 (C-1′), 93.85 (C-3′), 91.32 (C-5′), 55.89 (OCH3), 55.61 (OCH3); EI-MS: m/z = 363 (48), 362 (48), 346 (5), 281 (5), 209 (16), 207 (100), 181 (25), 152 (9); (Molecular formula C17H15BrO4).
(E)-1-(2′-Hydroxy-4′,6′-dimethoxyphenyl)-3-(3-nitrophenyl)prop-2-en-1-one (20). Yellow cotton (82.6% yield); m.p.: 169–171 °C (lit. [61] 169–170 °C); UV-Vis (MeOH) λmax: 336 nm; IR (KBr, cm−1): 3460 (O-H), 2851–3092 (Ar C-H stretch), 1638 (C=O), 1583 (C=C-C=O), 1427–1525 (C=C), 1223–1225 (C-O); 1H-NMR (500 MHz, CDCl3) δ: 8.56 (brs, 1H, H-2), 8.30 (d, 1H, H-4, J = 8.20 Hz), 8.22 (d, 1H, H-6, J = 7.75 Hz), 8.15 (d, 1H, H-β, J = 15.70 Hz), 7.84 (d, 1H, H-α, J = 15.70 Hz), 7.78 (t, 1H, H-5, J = 7.95, 8.00 Hz), 6.17 (d, 1H, H-3′, J = 2.35 Hz), 6.14 (d, 1H, H-5′, J = 2.35 Hz), 4.04 (s, 3H, OCH3, C-6′), 3.91 (s, 3H, OCH3, C-4′); EI-MS: m/z = 329 (33), 301 (5), 281 (6), 254 (5), 207 (100), 181 (25), 152 (5), 95 (5), 73 (8); (Molecular formula C17H15NO6).
(E)-3-(5-Bromo-2-hydroxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (22). Yellow crystal (76.2% yield); m.p.: 166–168 °C (lit. [61] 177–178 °C); UV-Vis (MeOH) λmax: 359 nm; IR (KBr, cm−1): 3435 (O-H), 2945–2994 (Ar C-H stretch), 1623 (C=O), 1419–1590 (C=C), 1220–1221 (C-O); 1H-NMR (600 MHz, CDCl3) δ: 14.19 (s, 1H, OH, C-2′), 8.12 (d, 1H, H-β, J = 15.70 Hz), 8.01 (d, 1H, H-α, J = 15.70 Hz), 7.78 (brs, 1H, H-6), 7.39 (d, 1H, H-4, J = 8.70 Hz), 6.97 (d, 1H, H-3, J = 8.64 Hz), 6.13 (brs, 1H, H-3′), 6.12 (brs, 1H, H-5′), 3.98 (s, 3H, OCH3, C-6′), 3.88 (s, 3H, OCH3, C-4′); EI-MS: m/z = 379 (1), 364 (14), 333 (5), 305 (3), 281 (9), 251 (3), 227 (9), 214 (17), 207 (60), 191 (10), 142 (13), 115 (8), 95 (10), 73 (14); (Molecular formula C17H15BrO5).
(E)-3-(2-Bromo-3-hydroxy-4-methoxyphenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one (23). Orange crystal (85.6% yield); m.p.: 201–203 °C; UV-Vis (MeOH) λmax: 366–369 nm; IR (KBr, cm−1): 3425 (O-H), 2838–3014 (Ar C-H stretch), 1634 (C=O), 1496–1596 (C=C), 1213–1259 (C-O); EI-MS: m/z = 409 (4), 408 (4), 403 (3), 229 (3), 207 (3), 202 (3), 181 (2), 165 (3), 141 (4), 112 (5), 87 (4), 59 (4); (Molecular formula C18H17BrO6).
3.5. X-ray Crystallographic Analysis
X-ray analysis for all these samples was performed using Bruker APEX II DUO CCD diffractometer, employing MoKα radiation (λ = 0.71073 Å) with φ and ω scans, at room temperature. Data reduction and absorption correction were performed using the SAINT and SADABS programs [62]. All structures were solved by direct methods and refined by full-matrix, least-squares techniques on F2 using the SHELXTL software package [63]. Crystallographic data for the reported structures have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the CCDC deposition numbers of 1548733 and 1548734. Copies of available material can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (Fax: +44-(0)1223–336033 or e-mail: deposit@ccdc.cam.ac.uk). The ORTEP (Oak Ride Thermal Ellipsoid Plot Program) of compound 7 and 9 are shown in Figure 2 and X-ray crystallographic data presented in Table 3.

Table 3.
Crystal data and parameters for the structure refinement of 7 and 9.
3.6. Anticancer Activity
3.6.1. Sample preparation
Stock samples at 1 mg/mL of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) were prepared and kept at 4 °C.
3.6.2. MTT Cell Viability Assay
Breast cancer MCF-7 and MDA-MB-231 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured at 37 °C, 5% CO2 and 90% humidity using RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, USA). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] method cell viability assay was conducted according to our previous report [64]. In brief, MCF-7 and MDA-MB-231 were seeded overnight in 96-well plates at 8 × 104 cells/well. All compounds were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA) to get a stock solution of 1 mg/mL and stored at 4 °C. Then, the compounds were serially diluted into the seeded cells at concentrations ranging between 30–0.47 µg/mL. Cells treated with 3% DMSO (Sigma) were used as a negative control. After 72 h of incubation, 20 µL of MTT solution (5 mg/mL) was added into all the wells and the plates were further incubated for another 3 hrs. Then, 170 µL of solution from all the wells were discarded and 100 µL of DMSO (Sigma) were added to dissolve the purple crystals. The absorbance was then recorded using an ELISA plate reader (Biotek Instruments, Winooski, VT, USA) at the wavelength of 570 nm. All samples were tested for triplicates. The percentage of cell viability was calculated using following formula:
Cell viability (%) = [OD sample at 570 nm/OD negative control at 570 nm] × 100%
The IC50 value (concentration of compounds inhibited 50% of cell viability) was determined from the graph of cell viability vs absorbance.
4. Conclusions
Twenty-three FKB derivatives were synthesized by Claisen–Schmidt condensation reaction. Three FKB derivatives, namely 8, 13 and 23, were found to be new chalcones. The chalcones with electron-withdrawing and electron-donating substituents had effects on cytotoxicity. The FKB derivatives with electron-withdrawing groups in ring B, as in 13 (2F), 15 (3Cl) and 16 (2Cl), exhibited potential cytotoxic effects on breast cancer cell lines, while the presence of the substituted electron-donating groups, as in 4, 5, 9 and 10, showed lower cytotoxic effects on MCF-7 and MDA-MB-231 cell lines. The FKB derivatives 16 (IC50 = 6.50 ± 0.40 and 4.12 ± 0.20 μg/mL), 15 (IC50 = 5.52 ± 0.35 and 6.50 ± 1.40 μg/mL) and 13 (IC50 = 7.12 ± 0.80 and 4.04 ± 0.30 μg/mL) were found to be the most active compounds among the FKB series chalcones. The structure–activity relationship showed FKB chalcones with different substituents and effects on cytotoxicity. The FKB skeleton is unique for anticancer agents, additionally, the presence of halogens (Cl and F) in position two and three also improve the cytotoxicity of FKB (1). These findings could help to improve or find new drugs for future anti-cancer agents.
Supplementary Materials
The following docking study is available online, Figure S1: the graph presenting the trend of root mean square deviation (Ǻ) of all the four systems in the present study, Figure S2 and Table S1: the difference in the initial and final coordinates of FKB1-JAK2 complex. The cyan color illustrates the initial pose (1 ns) while the final pose (10 ns) is presented in magenta. The ligand presented significant deviation from the initial poses, which explains the abrupt hydrogen bonding profile of the system, Table S1: the selected biological activities and their probabilities of activity (Pa) and (Pi) as obtained from the PASS online server.
Acknowledgments
We are grateful to the Universiti Malaysia Pahang (www.ump.edu.my), Ministry of Education Malaysia FRGS [150109] and internal grant RDU 150349 and 150356. The authors thankful to USM for Fundamental Research Grant Scheme (FRGS) (203/PFIZIK/6711411) and RUPRGS grant (1001/PFIZIK/846076). For the analysis of HREI-MS, greatly thankful to HEJ Research Institute of Chemistry, University of Karachi, Pakistan.
Author Contributions
Addila Abu Bakar, Muhammad Nadeem Akhtar and Seema Zareen carried out the literature, design and synthesis of chalcones (synthesis and purification), Swee Keong Yeap, Norlaily Mohd Ali and Noorjahan Banu Alitheen contributed to the study of cancer cell lines, Ching Kheng Quah and Wan-Sin Loh contributed to the X-ray analysis of the compounds. Zaheer Ul-Haq and Syed Adnan Ali Shah contributed to the docking analysis. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.D.; Kumar, P.G.; Harika, G.; Pooja, B.; Rao, S.; Kumar, A.Y. Recent advances and potential pharmacological activities of chalcones and their heterocyclic derivatives a valuable insight. J. Chem. Pharm. Res. 2016, 8, 458–477. [Google Scholar]
- Patil, C.B.; Mahajan, S.K.; Katti, S.A. Chalcone: A Versatile Molecule. J. Pharm. Sci. Res. 2009, 1, 11–22. [Google Scholar]
- Dyrager, C.; Wickström, M.; Fridén-Saxin, M.; Friberg, A.; Dahlén, K.; Wallén, E.A.A.; Gullbo, J.; Grøtli, M.; Luthman, K. Inhibitors and promoters of tubulin polymerization: Synthesis and biological evaluation of chalcones and related dienones as potential anticancer agents. Bioorg. Med. Chem. 2011, 19, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Santibañez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural Antitumoral Activity Relationships of Synthetic Chalcones. Int. J. Mol. Sci. 2009, 10, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ilango, K.; Valentina, P.; Saluja, G.S. Synthesis and in vitro anticancer activity of some substituted chalcone derivatives. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 354–359. [Google Scholar]
- Kamal, A.; Ramakrishna, G.; Raju, P.; Viswanath, A.; Ramaiah, M.J.; Balakishan, G.; Pal-Bhadra, M. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorg. Med. Chem. Lett. 2010, 20, 4865–4869. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones Enhance TRAIL-Induced Apoptosis in Prostate Cancer Cells. Int. J. Mol. Sci. 2010, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Herencia, F.; Lo, M.P.; Ubeda, A.; Ferrándiz, M.L. Nitric oxide-scavenging properties of some chalcone derivatives. Nitric Oxide 2002, 6, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.L.; Gupta, P.; Pawar, R.S.; Singour, P.K.; Patil, U.K. Synthesis and biological evaluation of anti-inflammatory activity of 1,3 diphenyl propenone derivatives. Med. Chem. Res. 2010, 20, 461–465. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhao, D.H.; Quan, Y.C.; Sun, L.P.; Yin, X.M.; Guan, L.P. Synthesis and evaluation of antiinflammatory activity of substituted chalcone derivatives. Med. Chem. Res. 2010, 19, 403–412. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Mishra, N.; Kumar, B.; Sharma, M.; Bhattacharya, A.; Mishra, L.C.; Bhasin, V.K. Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Med. Chem. Res. 2009, 18, 407–420. [Google Scholar] [CrossRef]
- Hans, R.H.; Guantai, E.M.; Lategan, C.; Smith, P.J.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg. Med. Chem. Lett. 2010, 20, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Boeck, P.; Falcão, C.A.B.; Leal, P.C.; Yunes, R.A.; Filho, V.C.; Torres-Santos, E.C.; Rossi-Bergmann, B. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg. Med. Chem. 2006, 14, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Roussaki, M.; Lima, S.C.; Kypreou, A.M.; Kefalas, P.; Silva, A.C.D.; Detsi, A. Aurones: A promising heterocyclic scaffold for the development of potent antileishmanial agents. Int. J. Med. Chem. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zainuri, D.A.; Arshad, S.; Khalib, N.C.; Razak, I.A.; Pillai, R.R.; Sulaiman, S.F.; Hashim, N.S.; Ooi, K.L.; Armaković, S.; Armaković, S.J.; et al. Synthesis, XRD crystal structure, spectroscopic characterization (FT-IR, 1H and 13C NMR), DFT studies, chemical reactivity and bond dissociation energy studies using molecular dynamics simulations and evaluation of antimicrobial and antioxidant activities of a novel chalcone derivative, (E)-1-(4-bromophenyl)-3-(4-iodophenyl)prop-2-en-1-one. J. Mol. Struct. 2017, 1128, 520–533. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.N.; Tran, D.T. Synthesis, Antioxidant and Antimicrobial Activities of a Novel Series of Chalcones, Pyrazolic Chalcones, and Allylic Chalcones. Pharmacol. Pharm. 2011, 2, 282–288. [Google Scholar] [CrossRef]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: Structure-activity relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Prabhakar, P.K.; Doble, M. Synthesis, antioxidant evaluation, and quantitative structure-activity relationship studies of chalcones. Med. Chem. Res. 2011, 20, 482–492. [Google Scholar] [CrossRef]
- Kaushik, S.; Kumar, N.; Drabu, S. Synthesis and anticonvulsant activities of phenoxychalcones. Pharma Res. 2010, 3, 257–262. [Google Scholar]
- Sukumaran, S.D.; Chee, C.F.; Viswanathan, G.; Buckle, M.J.; Othman, R.; Abd Rahman, N.; Chung, L.Y. Synthesis, Biological Evaluation and Molecular Modelling of 2′-Hydroxychalcones as Acetylcholinesterase Inhibitors. Molecules 2016, 21, 955. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.N.; Sakeh, N.M.; Zareen, S.; Gul, S.; Lo, K.M.; Ul-Haq, Z.; Shah, S.A.A.; Ahmad, S. Design and synthesis of chalcone derivatives as potent tyrosinase inhibitors and their structural activity relationship. J. Mol. Struct. 2015, 1085, 97–103. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Chalcones: A valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009, 52, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, H.R.W.; Nanayakkara, N.P.D.; Khan, I.A. Kavalactones from Piper methysticum, and their 13C-NMR spectroscopic analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef]
- Abu, N.; Ho, W.Y.; Yeap, S.K.; Akhtar, M.N.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. The flavokawain: Uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 2015, 9, 1401–1417. [Google Scholar] [CrossRef]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complement. Altern. Med. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kamaldin, M.N.; Akhtar, M.N.; Mohamad, A.S.; Lajis, N.; Perimal, E.K.; Akira, A.; Ming-Tatt, L.; Israf, D.A.; Sulaiman, M.R. Peripheral antinociception of a chalcone, flavokawin B and possible involvement of the nitric oxide/cyclic guanosine monophosphate/potassium channels pathway. Molecules 2013, 18, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.S.; Akhtar, M.N.; Khalivulla, S.I.; Perimal, E.K.; Khalid, M.H.; Ong, H.M.; Zareen, S.; Akira, A.; Israf, D.A.; Lajis, N.; et al. Possible Participation of Nitric Oxide⁄Cyclic Guanosine Monophosphate⁄Protein Kinase C/ATP-Sensitive K+ Channels Pathway in the Systemic Antinociception of Flavokawin B. Basic Clin. Pharmacol. Toxicol. 2011, 108, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In Vivo Anti-Tumor Effects of Flavokawain A in 4T1 Breast Cancer Cell-Challenged Mice. Anticancer Agents Med. Chem. 2015, 15, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.M.; Akhtar, M.N.; Ky, H.; Lim, K.L.; Abu, N.; Zareen, S.; Ho, W.Y.; Alan-Ong, H.K.; Tan, S.W.; Alitheen, N.B.; et al. Flavokawain derivative FLS induced G2/M arrest and apoptosis on breast cancer MCF-7 cell line. Drug Des. Dev. Ther. 2016, 10, 1897–1907. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, S.A.; Gacche, R.N.; Korbad, B.L.; Hote, B.S.; Kinkar, S.N.; Jalde, S.S. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y.B.; Pichika, M.R. Chalcones with electron-withdrawing and electron-donating substituents: Anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014, 77, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Lauthier, F.; Simon, A.; Fagnere, C.; Basly, J.P.; Delage, C.; Chulia, A.J. Flavonoids: Structural Requirements for Antiproliferative Activity on Breast Cancer Cells. Bioorg. Med. Chem. Lett. 2001, 11, 3095–3097. [Google Scholar] [CrossRef]
- Jin, F.; Jin, X.Y.; Jin, Y.L.; Sohn, D.W.; Kim, S.A.; Sohn, D.H.; Kim, Y.C.; Kim, H.S. Structural Requirements of 2′,4′,6′-Tris(methoxymethoxy) chalcone Derivatives for Anti-inflammatory Activity: The Importance of a 2′-Hydroxy Moiety. Arch. Pharm. Res. 2007, 30, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Nakhjiri, M.; Safavi, M.; Alipour, E.; Emami, S.; Atash, A.F.; Jafari-Zavareh, M.; Ardestani, S.K.; Khoshneviszadeh, M.; Foroumadi, A.; Shafie, A. Asymmetrical 2,6-bis(benzylidene)cyclohexanones: Synthesis, cytotoxic activity and QSAR study. Eur. J. Med. Chem. 2012, 50, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Alnemri, E.S.; Livingston, D.J.; Nicholson, D.W.; Salvesen, G.; Thornberry, N.A.; Wong, W.W.; Yuan, J. Human ICE/CED-3 Protease Nomenclature. Cell 1996, 87, 171. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE). Available online: https://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm (accessed on 23 November 2017).
- Baffert, F.; Régnier, C.H.; De Pover, A.; Pissot-Soldermann, C.; Tavares, G.A.; Blasco, F.; Brueggen, J.; Chéne, P.; Drueckes, P.; Erdmann, D.; et al. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol. Cancer Ther. 2010, 9, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cheatham III, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham III, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; Izadi, S.; et al. AMBER 2016 Reference Manual; University of California: San Francisco, CA, USA, 2016; pp. 1–923. [Google Scholar]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. Hepatoprotective Role of Ethanolic Extract of Vitex negundo in Thioacetamide-Induced Liver Fibrosis in Male Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 Is Required for DNA Fragmentation and Morphological Changes Associated with Apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Sladek, T.L.; Liu, X.; Butler, B.R.; Froelich, C.J.; Thor, A.D. Reconstitution of Caspase 3 Sensitizes MCF-7 Breast Cancer Cells to Doxorubicin- and Etoposide-induced Apoptosis. Cancer Res. 2001, 61, 348–354. [Google Scholar] [PubMed]
- Fulda, S.; Debatin, K.M. Caspase activation in cancer therapy. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013; pp. 1–29. [Google Scholar]
- Sun, H.; Nikolovska-Coleska, Z.; Lu, J.; Qiu, S.; Yang, C.Y.; Gao, W.; Meagher, J.; Stuckey, J.; Wang, S. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J. Med. Chem. 2006, 49, 7916–7920. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.S.F.; Chung, K.K.W.; Lui, J.C.K.; Lau, C.P.; Hon, P.M.; Chan, J.Y.W.; Fung, K.P.; Au, S.W.N. Leachianone A as a potential anti-cancer drug by induction of apoptosis in human hepatoma HepG2 cells. Cancer Lett. 2007, 253, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.; Sayeski, P.P.; Keserű, G.M. Recent developments on JAK2 inhibitors: A patent review. Expert Opin. Ther. Pat. 2010, 20, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.S.; Akhtar, M.N.; Zakaria, Z.A.; Perimal, E.K.; Khalid, S.; Mohd, P.A.; Khalid, M.H.; Israf, D.A.; Lajis, N.H.; Sulaiman, M.R. Antinociceptive activity of a synthetic chalcone, flavokawain B on chemical and thermal models of nociception in mice. Eur. J. Pharmacol. 2010, 647, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.V.N.S.; Koteswara Rao, Y.; Mahender, I.; Das, B.; Rama Krishna, K.V.S.; Hara Kishore, K.; Murty, U.S.N. Flavanoids from Caesalpinia pulcherrima. Phytochemistry 2003, 63, 789–793. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Differential effects of synthesized 2′-oxygenated chalcone derivatives: modulation of human cell cycle phase distribution. Bioorg. Med. Chem. 2004, 12, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, L.D.; Mascarello, A.; Purificação, M.; Vernal, J.; Cordeiro, M.N.S.; Zenteno, M.E.; Villarino, A.; Nunes, R.J.; Yunes, R.A.; Terenzi, H. Synthetic chalcones as efficient inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase PtpA. Bioorg. Med. Chem. Lett. 2008, 18, 6227–6230. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, V.; Krishna, C.R.; Ramesh, M.; Parthasarathy, T. Synthesis, in vitro anticancer activity evaluation and docking investigations of novel aromatic chalcones. Mod. Chem. 2013, 1, 1–7. [Google Scholar] [CrossRef][Green Version]
- Bruker, S. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short History of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–23 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).