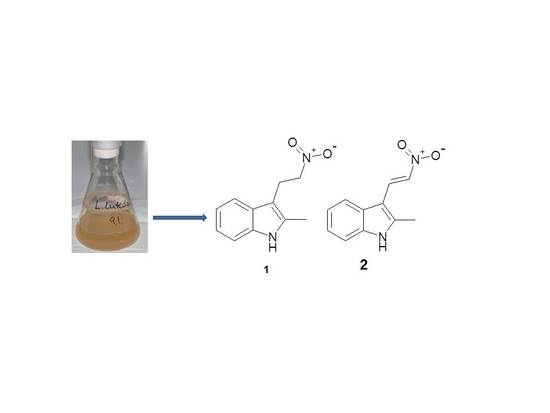
Six Heterocyclic Metabolites from the Myxobacterium Labilithrix luteola
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Cultivation of Labirithrix luteola
3.4. Isolation of Secondary Metabolites
3.5. Inhibitory Effects on HCV Infectivity
3.6. Cytotoxic Activity
3.7. Antimicrobial Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stadler, M.; Dersch, P. (Eds.) How to Overcome the Antibiotic Crisis–Facts, Challenges, Technologies and Future Perspectives; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- World Health Organization. Available online: http://www.who.int/ebola/en/ (accessed on 24 January 2018).
- Herrmann, J.; Fayad, A.A.; Müller, R. Natural products from myxobacteria: Novel metabolites and bioactivities. Nat. Prod. Rep. 2017, 34, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Karwehl, S.; Jansen, R.; Huch, V.; Stadler, M. Sorazolons, Carbazole Alkaloids from Sorangium cellulosum Strain Soce375. J. Nat. Prod. 2016, 79, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Irschik, H.; Huch, V.; Schummer, D.; Steinmetz, H.; Bock, M.; Schmidt, T.; Kirschning, A.; Müller, R. Carolactone—A macrolide ketocarbonic acid reducing biofilm formation by the caries- and endocarditis-associated bacterium Streptococcus mutans. Eur. J. Org. Chem. 2010, 7, 1284–1289. [Google Scholar] [CrossRef]
- Vollbrecht, L.; Steinmetz, H.; Schupp, T.; Petersen, F.; Memmert, F.; Hofmann, H.; Heusser, C.; Brinkmann, V.; von Matt, P.; Höfle, G.; et al. Argyrins, immunosuppressive cyclic peptides from Myxobacteria. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 2002, 55, 543–551. [Google Scholar]
- Sasse, F.; Steinmetz, H.; Heil, J.; Höfle, G.; Reichenbach, H. Tubulysins, new cytostatic peptides from Myxobacteria acting on microtubuli. Production, isolation, physico-chemical and biological properties. J. Antibiot. 2000, 53, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Garcia, R.; Bifulco, G.; Martinez, P.J.; Hüttel, S.; Sasse, F.; Meyerhans, A.; Stadler, M.; Müller, R. Aetheramides A and B, potent HIV-inhibitory depsipeptides from a Myxobacterium of the new genus “Aetherobacter”. Org. Lett. 2012, 14, 2854–2857. [Google Scholar] [CrossRef] [PubMed]
- Surup, F.; Viehrig, K.; Mohr, K.I.; Herrmann, J.; Jansen, R.; Müller, R. Disciformycins A and B: 12-membered macrolide glycoside antibiotics from the Myxobacterium Pyxidicoccus fallax active against multiresistant staphylococci. Angew. Chem. Int. Ed. 2014, 53, 13588–13591. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Stechling, M.; Wink, J.; Wilharm, E.; Stadler, M. Comparison of myxobacterial diversity and evaluation of isolation success in two niches: Kiritimati Island and German compost. MicrobiologyOpen 2015, 5, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Muramatsu, H.; Nagai, K. Vulgatibacter incomptus gen. nov., sp. nov. and Labilithrix luteola gen. nov., sp. nov., two myxobacteria isolated from soil in Yakushima Island, and the description of Vulgatibacteraceae fam. nov., Labilitrichaceae fam. nov. and Anaeromyxobacteraceae fam. Int. J. Syst. Evol. Microbiol. 2014, 64, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, H.; Höfle, G. Biologically active secondary metabolites from myxobacteria. Biotechnol. Adv. 1993, 11, 219–277. [Google Scholar] [PubMed]
- Sreevidya, N.; Mehrotra, S. Spectrophotometric method for estimation of Alkaloids precipitable with Dragendorff’s reagent in plant materials. J. AOAC Int. 2003, 86, 1124–1127. [Google Scholar] [PubMed]
- Sridhara, V.; Martin, M.A.; Menendez, J.C. Acid-free synthesis of carbazoles and carbazolequinones by intramolecular Pd-catalyzed, microwave-assisted oxidative biaryl coupling reactions–efficient syntheses of murrayafoline A, 2-methoxy-3-methylcarbazole, and glycozolidine. Eur. J. Org. Chem. 2009, 27, 4614–4621. [Google Scholar] [CrossRef]
- Chowdhury, B.K.; Mustapha, A.; Garba, M.; Bhattacharyya, P. Carbazole and 3-methylcarbazole from Glycosmis pentaphylla. Phytochemistry 1987, 26, 2138–2139. [Google Scholar]
- Luk, K.C.; Stern, L.; Weigele, M.; O’Brian, R.A.; Spirt, N. Isolation and identification of “diazepam-like” compounds from bovine urine. J. Nat. Prod. 1983, 46, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Böhlendorf, B.; Forche, E.; Bedorf, N.; Gerth, K.; Irschik, H.; Jansen, R.; Kunze, B.; Trowitzsch-Kienast, W.; Höfle, G. Antibiotics from gliding bacteria, LXXIII. Indole and quinoline derivatives as metabolites of tryptophan in Myxobacteria. Eur. J. Org. Chem. 1996, 1, 49–53. [Google Scholar]
- Abraham, W.R.; Spassov, G. 4-Hydroxymethyl-quinoline from Polyporus species. Phytochemistry 1991, 30, 371–372. [Google Scholar] [CrossRef]
- Guo, C.J.; Chang, F.-Y.; Wyche, T.P.; Backus, K.M.; Acker, T.M.; Funabashi, M.; Taketani, M.; Donia, M.S.; Nayfach, S.; Pollard, K.S.; et al. Discovery of reactive microbiota-derived metabolites that inhibit host proteases. Cell 2017, 168, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kawade, R.K.; Huang, P.-H.; Karad, S.N.; Liu, R.-S. Gold-catalyzed annulations of allenes with N-hydroxyanilines to form indole derivatives with benzaldehyde as a promoter. Org. Biomol. Chem. 2014, 12, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Sonar, V.N.; Crooks, P.A. Synthesis and antitubercular activity of a series of hydrazone and nitrovinyl analogs derived from heterocyclic aldehydes. J. Enzym. Inhib. Med. Chem. 2009, 24, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, T.; Zhou, P.; Floss, H.G. Formation of 2-methyltryptophan in the biosynthesis of thiostrepton: Isolation of S-adenosylmethionine: tryptophan 2-methyltransferase. Arch. Biochem. Biophys. 1990, 278, 35–40. [Google Scholar] [CrossRef]
- Gerth, K.; Trowitzsch, W.; Wray, V.; Höfle, G.; Irschik, H.; Reichenbach, H. Pyrrolnitrin from Myxococcus fulvus (Myxobacteriales). J. Antibiot. 1982, 35, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Elander, R.P.; Mabe, J.A.; Hamill, R.H.; Gorman, M. Metabolism of tryptophans by Pseudomonas aureofaciens VI. Production of pyrrolnitrin by selected Pseudomonas species. Appl. Microbiol. 1968, 16, 753–758. [Google Scholar] [PubMed]
- von Hahn, C.S.; Colpitts, T.; Schang, C.C.; Friesland, L.M.; Steinmann, M.; Manns, J.; Ott, M.P.; Wedemeyer, M.; Meuleman, H.; Pietschmann, T.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits Hepatitis C Virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Meth. 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Garozzo, A.; Pinizzotto, M.R.; Guerrera, F.; Tempera, G.; Castro, A.; Geremia, E. Antipoliovirus activity of isothiazole derivatives: Mode of action of 5,5′-diphenyl-3,3′-diisothiazole disulfide (DID). Arch. Virol. 1994, 135, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Sood, S.; Huch, V.; Kunze, B.; Stadler, M.; Müller, R. Pyrronazols, metabolites from the myxobacteria Nannocystis pusilla and N. exedens, are unusual chlorinated pyrone-oxazole-pyrroles. J. Nat. Prod. 2014, 77, 320–326. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |
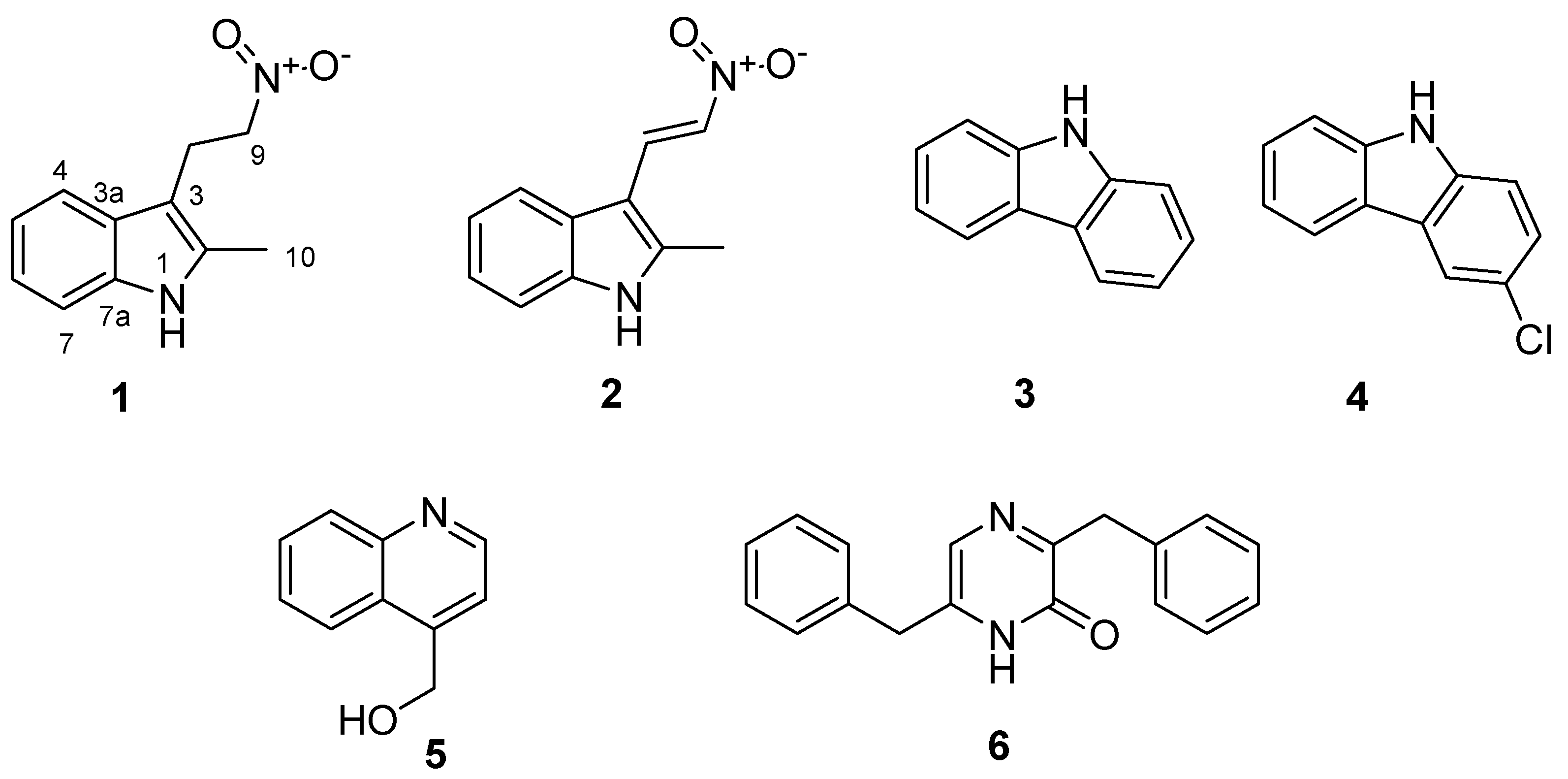

| Position | 1 a | 2 b | ||
|---|---|---|---|---|
| δC(N) | δH m (J [Hz]) | δC(N) | δH m (J [Hz]) | |
| 1 | (133.1) | (141.4) | 8.56 br. s | |
| 2 | 134.3 | 144.2 | ||
| 3 | 106.0 | 106.5 | ||
| 3a | 129.4 | 125.7 | ||
| 4 | 118.1 | 7.41 dt (7.7, 0.95) | 120.1 | 7.71 m |
| 5 | 120.0 | 6.97 ddd (7.8, 7.0, 1.4) | 122.6 | 7.30 m |
| 6 | 121.8 | 7.02 ddd (8.1, 7.0, 1.2) | 123.6 | 7.29 m |
| 7 | 111.6 | 7.23 dt (7.9, 0.9) | 111.4 | 7.38 m |
| 7a | 137.3 | 135.9 | ||
| 8 | 24.0 | 3.38 t (7.1) | 132.6 | 8.35 d (13.3) |
| 9 | 76.8 | 4.62 t (7.3) | 131.8 | 7.80 d (13.3) |
| 10 | 11.3 | 2.35 s | 12.5 | 2.66 s |
| 11 | (388.2) | (375.3) | ||
| Cell Line | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Mouse fibroblasts (L929) a | - | - | 45 | 44 | - | - |
| Human nasopharyngeal cells (KB3.1)a | - | - | 60 | 42 | 16 | - |
| Test Strain | 1 | 2 | 3 | 4 | 5 | 6 | Methanol |
|---|---|---|---|---|---|---|---|
| Fungi | |||||||
| Mucor hiemalis (DSM 2656) b | - | - | 16.6 | 33.3 | 33.3 | - | - |
| Candida albicans (DSM1665) b | - | - | 33.3 | 33.3 | 33.3 | - | - |
| Pichia anomala (DSM6766) b | - | - | 67 | 16.6 | - | - | - |
| Gram positive bacteria | |||||||
| Staphylococcus aureus (Newman) c | - | - | - | - | - | - | - |
| Bacillus subtilis (DSM 10) d | - | - | 6.7 | - | - | - | - |
| Micrococcus luteus (DSM 1790) e | - | - | - | - | - | - | - |
| Gram negative bacteria | |||||||
| Escherichia coli (ToLC) c | - | - | 33.3 | - | - | - | - |
| Escherichia coli (DSM1116) c | - | - | - | - | - | - | - |
| Chromobacter violaceum (DSM 30191) d | - | - | 6.7 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulwa, L.S.; Jansen, R.; Praditya, D.F.; Mohr, K.I.; Wink, J.; Steinmann, E.; Stadler, M. Six Heterocyclic Metabolites from the Myxobacterium Labilithrix luteola. Molecules 2018, 23, 542. https://doi.org/10.3390/molecules23030542
Mulwa LS, Jansen R, Praditya DF, Mohr KI, Wink J, Steinmann E, Stadler M. Six Heterocyclic Metabolites from the Myxobacterium Labilithrix luteola. Molecules. 2018; 23(3):542. https://doi.org/10.3390/molecules23030542
Chicago/Turabian StyleMulwa, Lucky S., Rolf Jansen, Dimas F. Praditya, Kathrin I. Mohr, Joachim Wink, Eike Steinmann, and Marc Stadler. 2018. "Six Heterocyclic Metabolites from the Myxobacterium Labilithrix luteola" Molecules 23, no. 3: 542. https://doi.org/10.3390/molecules23030542
APA StyleMulwa, L. S., Jansen, R., Praditya, D. F., Mohr, K. I., Wink, J., Steinmann, E., & Stadler, M. (2018). Six Heterocyclic Metabolites from the Myxobacterium Labilithrix luteola. Molecules, 23(3), 542. https://doi.org/10.3390/molecules23030542